Abstract
It is now well-established that the therapy of type II diabetes mellitus has undergone a radical change in the past 15 years: countless innovative drugs, such as SGLT2I, able to guarantee an optimization of glycaemic control without causing hypoglycaemia, today represent real therapeutic cornerstones not only for the intrinsic ability of these molecules to ensure better glycaemic control but also for the effects they exert on the cardiovascular system. Several pioneering clinical trials, such as EMPA-REG, CANVAS, and DECLARE-TIMI-58, have demonstrated clear benefits of empagliflozin, canagliflozin, and dapagliflozin, respectively, in reducing cardiovascular risk and diabetes-associated macrovascular complications in the diabetic population. The promising results that emerged from these trials represent the spark that triggered a series of studies aimed at evaluating the efficacy of gliflozines in the treatment of patients with heart failure even in the absence of diabetes. Preliminary results confirm the efficacy of SGLT2I in the treatment of this population, representing a real therapeutic revolution.
Keywords: Heart failure, SGLT2I, Diabetes mellitus
Introduction
Heart failure (HF) is defined as a clinical syndrome caused by the insufficient capacity of the heart to maintain an adequate blood supply to meet the metabolic needs of the body or by the maintenance of a sufficient perfusion only at the cost of high filling pressures. In accordance with the last ESC guidelines, we can stratify patients with HF into three groups based on the ejection fraction (EF): HF with reduced ejection fraction (HFrEF) when the EF ≤40%; HF with preserved ejection fraction (HFpEF) when the EF ≥ 50% and HFmrEF when the EF is between 41% and 49%.1 It is estimated that 26 million people worldwide are affected by HF, with important socio-economic consequences.2 The prevalence of HF in the adult population is around 2% and this percentage exceeds 10% in patients >70 years old. Mortality at 12 months varies from 6.4% in case of chronic HF up to 23.6% in case of acute HF.2
With the exception of sacubitril-valsartan, in recent decades, no new molecule has become an integral part of the therapeutic armamentarium available to clinicians to treat patients with HFrEF, in addition to standard therapy with RAAS system inhibitors and beta blockers. The situation becomes even more dramatic in patients with preserved EF, in whom the therapies used in HFrEF, including sacubitril-valsartan (evaluated in the PARAGON-HF trial), have failed to demonstrate a benefit on hospitalization and mortality reduction. It is therefore essential to identify new effective therapeutic strategies.
The sodium–glucose co-transporter (SGLT2) inhibitor drugs, initially developed for exclusive use in patients with type II diabetes mellitus, have shown solid benefits since the first clinical trials in reducing the risk of death from cardiovascular events as well as a reduction in the rate of hospitalizations for HF.3 These promising results were the spark that has triggered a series of studies aimed at evaluating the efficacy of gliflozines in patients with HF even in the absence of diabetes.
SGLT2I drugs in the diabetic patient with known cardiovascular disease: the first evidences
There are currently four SGLT2Is approved by the European Medicines Agency (EMA) for the treatment of patients with type II diabetes mellitus: dapagliflozin, empagliflozin, canagliflozin, and ertugliflozin.
In the EMPA-REG OUTCOME study, the use of empagliflozin in diabetic patients with known cardiovascular disease reduced the primary composite endpoint [death from cardiovascular events, non-fatal acute myocardial infarction, and non-fatal stroke] by 14% in comparison with placebo. This is mainly due to a 38% reduction in death from cardiovascular events, associated with a reduction in the rate of hospitalization for HF.3
The benefits observed in HF patients were then confirmed for other glifozines: in the CANVAS study, the use of canagliflozin demonstrated a significant 33% reduction in hospitalization rates for HF in diabetic patients at high cardiovascular risk.
The use of dapagliflozin compared to placebo in the DECLARE-TIMI-58 study demonstrated, in line with other molecules of the same class, a reduction in hospitalization for HF in diabetic patients at high cardiovascular risk with and without reduced EF; however, the reduction in overall mortality and for cardiovascular events was observed only in patients with HF with an EF < 45%. In contrast with the other molecules of SGLT2i class, ertuglifozin in the VERTIS-CV trial did not demonstrate, despite having the same effects on glycaemic control, any benefit in reducing deaths from cardiovascular events and in the incidence of major cardiovascular events in diabetic patients, compared to placebo.4 A certain benefit has however emerged regarding the reduction of the risk of hospitalization for HF,5 further corroborating the hypothesis of a class effect of gliflozines in this category of patients. In Table 1, we summarized the above-mentioned trials, with the characteristics of the study populations and the main outcomes.
Table 1.
Summary of clinical trials on new antidiabetic drugs.
| Trial | Molecule | Dosage | Inclusion criteria | Primary CV outcome |
|---|---|---|---|---|
| EMPA-REG OUTCOME | Empagliflozin vs. placebo | 10 mg/day, incremental to 25 mg/day | Type II diabetes mellitus and high CV risk | Reduction in death rate from CV events [265/4687 patients (5.7%)] vs. placebo [198/2333 patients (8.5%), HR: 0.66 (95% CI: 0.55–0.79); P < 0.001] |
| Reduction in death rate and hospitalization for heart failure [104/4687 patients (4.5%)] vs. placebo [129/2333 (2.8), HR: 0.61 (95 % CI: 0.47–0.79); P < 0.001] | ||||
| CANVAS program | Canagliflozin vs. placebo | 100 mg/day, increased to 300 mg/day if GFR>60 mL/min/1.73 m2 | Poorly controlled type II diabetes mellitus with high risk of CV or previous CV events | Reduction of death rate from CV events, MI non-fatal, non-fatal stroke [reduction of 14% vs. placebo, HR: 0.86 (95% CI: 0.75–0.97)] |
| 33% reduction in hospitalization rate for heart failure vs. placebo [HR: 0.67 (95% CI: 0.52–0.87)] | ||||
| DECLARE-TIMI 58 | Dapagliflozin vs. placebo | 5 mg/day, which can be increased up to 10 mg/day | Type II diabetes mellitus and high CV risk or previous CV events | Reduction in hospitalization rate for heart failure by 27% vs. placebo [HR 0.73 (95 % CI: 0.61–0.88)] |
| VERTIS CV | Ertugliflozin vs. placebo | 5 mg/day, increased up to 15 mg/day | Type II diabetes mellitus and known CV disease | Reduction in risk of first hospitalization for heart failure vs. placebo [HR 0.70 (95% CI: 0.54–0.90); P = 0.006] |
| DAPA-HF | Dapagliflozin vs. placebo | 10 mg/die | Heart failure with reduced FE ≤40% + elevated NT-proBNP | Reduction of primary composite outcome (death from CV events, exacerbation of heart failure requiring hospitalization or urgent visit) by 26% [386 patients with dapagliflozin (16.3%) vs. 502 (21.2%) with placebo; HR: 0.74 (95% CI: 0.65–0.85); P < 0.001] |
| DELIVER | Dapagliflozin vs. placebo | 10 mg/die | Heart failure with preserved ejection fraction | Death from CV events, exacerbation of heart failure requiring hospitalization or urgent visit. Results not yet available |
| EMPEROR-Reduced | Empagliflozin vs. placebo | 10 mg/die | Heart failure with reduced EF ≤40% + elevated NT-proBNP | Reduced risk of death from CV events and heart failure exacerbation requiring hospitalization compared to placebo [19.4% vs. 24.7%; HR 0.75 (95% CI: 0.65–0.86) P < 0.001] |
| EMPEROR-Preserved | Empagliflozin vs. placebo | 10 mg/die | Heart failure with preserved ejection fraction | Death from CV events, exacerbation of heart failure with the need for hospitalization. Results not yet available |
CI, confidence interval.
SGLT2Is drugs in patients with HFrEF: what evidence
The advantages that the majority of SGLT2Is seem to confer in patients with HF have been investigated in two clinical trials designed to assess whether the benefits found were concrete even in non-diabetic patients: the DAPA-HF trial and the EMPEROR-Reduced trial.
DAPA-HF trial
In the DAPA-HF study (multi-centre, double-blind, and placebo controlled), 4744 diabetic and non-diabetic patients with HFrEF and a New York Heart Association (NYHA) class II, III, or IV were enrolled. All patients received optimal therapy for HFrEF and in addition received 10 mg of dapagliflozin or placebo once daily. Diabetic patients continued to take habitual hypoglycaemic therapy. The primary composite outcome was to determine whether dapagliflozin, in addition to standard therapy, was superior to placebo in reducing the incidence of HF exacerbation (requiring hospitalization or urgent visit to administer diuretic intravenous therapy) or death from cardiovascular causes. Secondary outcomes included hospitalization for HF, death from cardiovascular causes, symptoms at eight months based on the Kansas City Cardiomyopathy Questionnaire (KCCQ) score, worsening of renal function, and death from all causes. At a follow-up of 18.2 months, dapagliflozin showed to significantly reduce the primary composite endpoint [dapagliflozin 16.3% vs. placebo 21.2%; hazard ratio (HR) 0.74, P < 0.001], the rate of hospitalizations for HF (dapagliflozin 9.7% vs. placebo 13.4%; HR 0.70) and the number of deaths from cardiovascular causes (9.6% vs. 11.5%; HR 0.82), compared to placebo. Dapagliflozin has also shown to reduce the incidence of deaths from all causes (11.6% vs. 13.9%, HR 0.83) and the eight-month symptoms assessment based on the KCCQ score (P < 0.001), compared to placebo. The number of patients undergoing treatment needed to prevent a primary endpoint was 21. The benefits on the primary outcome were consistent in the various subgroups, including diabetic patients; however, patients in NYHA class III or IV experienced less benefits than patients in class II. There was no difference between the two groups in the rate of adverse events related to volume depletion, worsening of renal function, and hypoglycaemia.
EMPEROR-Reduced trial
In this multicentre, randomized and double-blind study, the efficacy and safety of empagliflozin were evaluated on 3730 patients with HF with an EF ≤40% and in NYHA class II, III, or IV.7 The protocol involved administration of empagliflozin 10 mg or placebo once daily in addition to the recommended optimal therapy. At a follow-up of 16 months, empagliflozin was effective compared to placebo in reducing cardiovascular deaths and HF hospitalization rate (19.4% vs. 24.7%; HR 0.75, P < 0.001) in patients with and without type II diabetes mellitus. Empagliflozin also had a favourable influence compared to placebo on secondary outcomes, represented by the total number of hospitalizations for HF (388 vs. 553 events; HR 0.70, P < 0.001) and by the rate of decline in renal function (−0.55 mL/min for 1.73 m2 annually vs. −2.28 mL/min for 1.73 m2 annually; P < 0.001). The number of treated patients needed to prevent a primary endpoint was 19. Uncomplicated urogenital infections were more frequent in patients treated with empagliflozin than placebo (1.7% vs. 0.6%), while the rates of hypoglycaemia, lower limb amputation, and bone fractures were not significantly different between the two groups.
SGLT2Is drugs in patients with HFpEF: what evidence
Two ongoing clinical trials are evaluating any benefits of gliflozine therapy in patients with HFpEF: the EMPEROR-Preserved and the DELIVER trial.
DELIVER trial
This is a multicentre, randomized, double-blind study with the aim of evaluating the effects of dapagliflozin on morbidity and mortality in patients with HFpEF with and without type II diabetes.8 The study started in August 2018 and will be completed in June 2021, with a total of ∼6100 participants. The primary outcomes were death from cardiovascular causes, exacerbation of HF requiring hospitalization or the need for an urgent visit. The study will determine whether the benefits observed in patients with HFrEF are also consistent in patients with HFpEF.
EMPEROR-Preserved trial
Similarly to the EMPEROR-Reduced study, this is a multicentre, randomized, double-blind trial with the aim of studying the effect of empagliflozin in addition to standard medical therapy in patients with HFpEF.9 A total of 5989 participants were enrolled. The primary outcome is death from cardiovascular causes or the need for hospitalization due to exacerbation of HF. The study was completed in April 2021 and the results are not yet available.
The scientific community is waiting for the results of these two Trials since to date no drug has shown any benefit in patients with HFpEF. Thus, if SGLT2Is will show to improve cardiovascular outcomes in this population, it would be the first innovative class of drugs suitable for patients with HFpEF.
Mechanism of action of SGLT2I drugs and possible pharmacodynamic hypotheses
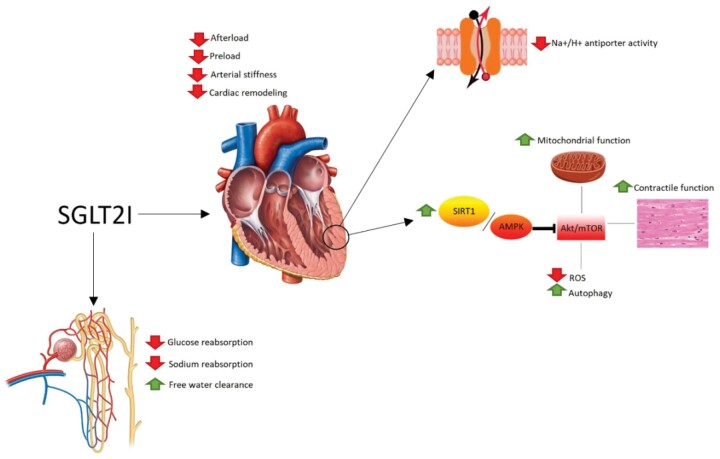
In a normoglycaemic individual, the concentration of glucose that reaches the nephron daily is equal to 162–180 g/day. This quantity is completely reabsorbed at the proximal convoluted tubule thanks to the presence, at this level, of SGLTs. Of these, the one responsible for the reabsorption of 90% of filtered glucose is represented by SGLT2, mainly expressed at the level of the S1 tract of the proximal convoluted tubule. The SGLT2 inhibitory drugs, called gliflozines, exert their effect by selective blocking these transporters, preventing the tubular reabsorption of glucose and promoting its renal excretion.3 Some mechanisms potentially underlying the benefits obtained in patients with HF, which cannot be explained only with a reduction in glycated haemoglobin and an increase in natriuresis, are discussed below (Figure 1).
Figure 1.
Main cardiovascular and renal effects of SGLT2 inhibitors.
‘Smart’ diuretic effect
The SGLT2Is drugs not only reduce the reabsorption of glucose but also that of sodium, resulting in a diuretic effect. The real peculiarity of these molecules that differentiates them from the canonical loop diuretics is the ability to stimulate an osmotic diuresis: the glucose not reabsorbed at the level of the proximal convoluted tubule reaches the distal nephron, causing an increase in tubular osmolarity and reducing the osmotic gradient between the tubular fluid and the interstitium; this results in a reduction in the passive reabsorption of water at the level of the collecting ducts and an increase in the free water clearance.10 Contrary to loop diuretics, the mechanism of action of SGLT2Is involves a greater reduction of fluids in the interstitial compartment compared to the intravascular one, resulting in a lower impact on the effective circulating volume and tissue perfusion.11 The maintenance of an adequate blood supply reduces the congestion pattern that characterizes HF and leads to a greater reduction in preload with a reverse cardiac remodelling.9
Promotion of favourable metabolic pathways
As a consequence of the glycosuria effect, SGLT2Is lead to a negative energy balance; at the metabolic level, this ‘fasting-mimicry’ condition induces cardio-protection by activating the SIRT1/AMPK signalling pathway, which ends in the suppression of Akt/mTOR.12 This mechanism induce in cardiomyocytes a metabolic state similar to that observed in the condition of prolonged fasting, which involves a series of advantages: reduction of oxidative stress, normalization of mitochondrial function, reduction of inflammation, increase in contractile activity, and increase in autophagy.12
Inhibition of the Na+/H+ antiport
It has been shown that SGLT2Is also down-regulate the Na+/H+ antiport, with a consequent reduction in the concentration of sodium and calcium in the cytoplasm of cardiomyocytes, and a consequent increase in mitochondrial calcium concentrations, thus causing an increase in synthesis of ATP and an improvement in cardiac contractile activity.13
The use of SGLT2Is in patients with HF in clinical practice
The benefits obtained in the various clinical trials led to the approval of the use of dapagliflozin in patients with HFrEF without diabetes by the EMA. However, the use of other SGLT2Is for the same indication is currently off-label in Europe. It seems reasonable to await a recommendation on the use of these molecules in the next ESC Guidelines on the management of HF. The last reference regarding the use of SGLT2Is dates back to 2019, when it was only suggested the possibility of adding canagliflozin or dapagliflozin in patients with type II diabetes mellitus in the presence of known CV disease or at high CV risk.
Patients who may benefit from gliflozine therapy should have a GFR ≥30 mL/min, although the minimum GFR value for inclusion in the EMPEROR-Reduced study was 20 mL/min.7
Unlike other therapies for HFrEF, the recommended therapeutic dose, for both dapagliflozin and empagliflozin, is 10 mg once a day without titration. Urinary tract infections, and specifically fungal infections, represent the main complication associated with the use of these drugs, especially in women. These infections, which are generally mild, usually do not affect the continuation of therapy.3 It is also important to inform the patient about the possible risk of events related to volume depletion, such as orthostatic hypotension, especially if SGLT2Is are administered with loop diuretics. Finally, the increased risk for bone amputations and fractures, initially observed with canagliflozin, was not subsequently confirmed.14
A reasonable therapeutic approach could include the addition of an SGLT2I to a patient already on therapy with the maximum tolerated dose of RAAS inhibitor and beta blocker.
Elements in favour of an early introduction into therapy could be represented by the presence of type II diabetes mellitus with poor glycaemic control and/or blood pressure values that do not allow remaining therapy titration; this last point is of particular importance since, as demonstrated in the DAPA-HF study, it seems that SGLT2Is reduce systolic blood pressure values by only 1.3 mmHg when compared to placebo.15
Conclusions
SGLT2Is are a new effective therapeutic tool for the treatment of HFrEF that clinicians should start to prescribe in daily practice. The results of the DELIVER and EMPEROR-Preserved trials will allow us to understand if, eventually, it will be possible to have a safe and effective therapeutic option also for patients with HFpEF.
Conflict of interest: none declared.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Greenberg BH. Heart failure awareness 2002 –and beyond. J Card Fail 2002;8:6–7. [DOI] [PubMed] [Google Scholar]
- 3. Borghi C, Bragagni A.. The new type 2 diabetes mellitus therapy: comparison between the two classes of drugs GLPR (glucagon-like peptide receptor) agonists and SGLT2 (sodium-glucose cotransporter 2) inhibitors. Eur Heart J Suppl 2020;22:L28–L32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, Shih WJ, Gantz I, Terra SG, Cherney DZI, McGuire DK.. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020;383:1425–1435. [DOI] [PubMed] [Google Scholar]
- 5. Cosentino F, Cannon CP, Cherney DZI, Masiukiewicz U, Pratley R, Dagogo-Jack S, Frederich R, Charbonnel B, Mancuso J, Shih WJ, Terra SG, Cater NB, Gantz I, McGuire DK, On behalf of the VERTIS CV Investigators. Efficacy of ertugliflozin on heart failure-related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV Trial. Circulation 2020;142:2205–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang C-E, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde A-M.. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 7. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi D-J, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca H-P, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde M-F, Spinar J, Squire I, Taddei S, Wanner C, Zannad F.. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 8. Williams DM, Evans M.. Dapagliflozin for heart failure with preserved ejection fraction: will the DELIVER study deliver? Diabetes Ther 2020;11:2207–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams DM, Evans M.. Are SGLT-2 inhibitors the future of heart failure treatment? The EMPEROR-Preserved and EMPEROR-Reduced Trials. Diabetes Ther 2020;11:1925–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW.. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab 2018;20:479–487. [DOI] [PubMed] [Google Scholar]
- 11. Santos-Ferreira D, Gonçalves-Teixeira P, Fontes-Carvalho R.. SGLT-2 inhibitors in heart failure and type-2 diabetes: hitting two birds with one stone? Cardiology 2020;145:311–320. [DOI] [PubMed] [Google Scholar]
- 12. Packer M. SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care 2020;43:508–511. [DOI] [PubMed] [Google Scholar]
- 13. Kato ET, Kimura T.. Sodium-glucose co-transporters-2 inhibitors and heart failure: state of the art review and future potentials. Int J Heart Fail 2020;2:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Starr JA, Pinner NA, Lisenby KM, Osmonson A.. Impact of SGLT2 inhibitors on cardiovascular outcomes in patients with heart failure with reduced ejection fraction. Pharmacother J Hum Pharmacol Drug Ther 2021;2:1–11. [DOI] [PubMed] [Google Scholar]
- 15. Genuardi MV, Mather PJ.. The dawn of the four-drug era? SGLT2 inhibition in heart failure with reduced ejection fraction. Ther Adv Cardiovasc Dis 2021;15:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]