Abstract
Dyslipidaemias and in particular elevated plasma low-density lipoprotein cholesterol (LDL-C) levels are major risk factors for atherosclerotic cardiovascular disease (ASCVD). Indeed, the more LDL-C is reduced the larger will be the ASCVD risk reduction. Although statins represent the first-line intervention to reduce the atherosclerotic burden driven by raised levels of LDL-C, adherence is not optimal and most patients do not follow guidelines and recommended doses. Thus, to achieve optimal LDL-C goals, especially in very high-risk patients, there is a need for new and safe agents, more tolerable than statins with low risk of myalgia. Thus, the present review will address the most recent clinical trials with bempedoic acid and inclisiran. Bempedoic acid is an oral drug acting at a biochemical step preceding hydroxymethylglutaryl-CoA reductase and not associated with muscular side effects. Inclisiran, the first-in-class small interfering RNA-based approach, has the ability to effectively reduce LDL-C by inhibiting the hepatic synthesis of proprotein convertase subtilisin/kexin type 9, with the advantage of requiring subcutaneous of a single dose on Day 1, Day 90, and every 6 months thereafter.
Keywords: Hypercholesterolemia, Statin side effect, Bempedoic acid, Inclisiran
Introduction
Atherosclerotic cardiovascular disease (ASCVD) encompassing pathologies caused by atherosclerosis in the coronary, cerebral, and peripheral arteries and the aorta is a leading cause of death and disability worldwide. Among risk factors accounting for this condition, low-density lipoprotein cholesterol (LDL-C) plays an unquestionably causal role in both the developed and the developing world.1 Indeed, the more LDL-C is lowered, the larger will be the ASCVD risk reduction: for each 38.7 mg/dL reduction in LDL-C, the risk of major cardiovascular events (MACEs) will decrease by 22%. This link is valid across different statin and non-statin trials with the relative risk reduction in major ASCVD events being similar for different lipid-lowering drug classes (statins, bile acid sequestrants, ezetimibe, fibrates, and PCSK9 inhibitors).2 However, considering that many patients do not achieve optimal LDL-C levels with statins alone and others are unable to tolerate statin therapy, to adhere to the guidelines’ recommendation, i.e. to achieve an over 50% reduction (or a target level of 55 mg/dL) in LDL-C in very high-risk patients, there is also a need for new and safe agents, more tolerable than statins with low risk of myalgia and new-onset diabetes. Studies on the use of statins in large patient series have generally indicated that most patients are not following guidelines and recommended doses.3
Thus, the present review will focus on the current status of two novel pharmacological lipid-lowering drugs (bempedoic acid and inclisiran), recently approved for the treatment of adults with established ASCVD who require additional lowering of LDL-C.
Bempedoic acid
Bempedoic acid is an oral, once daily, small molecule with an LDL lowering efficacy similar to that of ezetimibe and associated with a far lower percentage of muscular side effects. Approved by the FDA and EMA, bempedoic acid is indicated in patients with statin intolerance and who do not reach desired LDL-C levels with statins. It is prescribable as monotherapy (180 mg) or as a fixed-dose combination with ezetimibe (10 mg). Bempedoic acid is a prodrug converted exclusively in the liver to a coenzyme A derivative (bempedoyl-CoA) by an endogenous liver acyl-CoA-synthetase. The bempedoyl-CoA is the active metabolite responsible for the inhibition of ATP citrate lyase, the precursor of cytosolic acetyl-coenzyme A, the earlier step in the mevalonate pathway of cholesterol biosynthesis.
The safety and efficacy of the long-term use of bempedoic acid have been addressed in the CLEAR (Cholesterol Lowering via BEmpedoic Acid, an ACL-inhibiting Regimen) program comprising the following four phase 3 trials:
Tranquility (on statin-intolerant patients).
Harmony (patients with LDL-C of at least 70 mg/dL despite maximum tolerated statin therapy).
Wisdom [patients with ASCVD, heterozygous familial hypercholesterolaemia (HeFH), or both, on optimal statin treatment].
Serenity (statin-intolerant patients with ASCVD and inadequately controlled LDL-C).
Several pooled analyses have been published demonstrating the superiority of bempedoic acid vs. placebo to reduce LDL-C when added to maximally tolerated statins, including moderate- or high-intensity statins or no background statin, in patients with hypercholesterolaemia. Among patients with ASCVD or HeFH or both, there was an 18% reduction in the LDL-C, an efficacy that reached a −24% in statin-intolerant patients.4
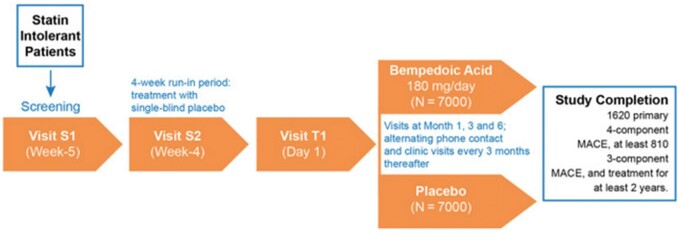
Going through the single trials, besides LDL-C lowering, bempedoic acid consistently showed a hsCRP lowering activity. In the CLEAR Tranquility, LDL-C and hsCRP were reduced by −28.5% and −34.6%, respectively (vs. placebo).5 In the CLEAR Harmony, there was a greater LDL-C lowering vs. placebo at Weeks 12 (difference, −18.1%), 24 (difference, −16.1%), and 52 (difference, −13.6%). Relative to hsCRP, the absolute difference was −25%, −19.1%, and −16.2% at Weeks 12, 24, and 52, respectively.6 In the CLEAR Wisdom, LDL-C levels were absolutely reduced by 17.4% at Week 12 and hsCRP by 8.7%.7 In the CLEAR Serenity, in patients on very low-dose statin, other lipid-modifying therapy or no therapy, LDL-C and hsCRP fell by 21.4% and 28%.8 Finally, the ongoing CLEAR Outcomes Study, which has randomized 14 014 patients, will test the superiority of bempedoic acid vs. placebo to prevent MACEs (cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, or coronary revascularization) in patients with (i) established ASCVD or at high risk of developing ASCVD, (ii) documented statin intolerance, and (iii) an LDL-C ≥100 mg/dL on maximally tolerated lipid-lowering therapy (Figure 1). The trial will continue until 1620 patients will experience a primary endpoint and minimum treatment duration of 36 months with a projected median treatment exposure of 42 months.9
Figure 1.
Study design of the CLEAR outcomes study. Reproduced with permission of Elsevier.9 MACE, major cardiovascular event.
Very recently, in order to evaluate whether bempedoic acid is effective in helping people to reach guideline-recommended LDL-C goals, a triple combination was tested. Bempedoic acid (180 mg), ezetimibe (10 mg), and atorvastatin (20 mg) significantly lowered LDL-C (−63.6% at Week 6), allowing more than 90% to reach LDL-C ≤70 mg/dL. hsCRP was reduced by 41.9%.10
Relative to safety,11 treatment with bempedoic acid neither increased the risk of all adverse events nor the incidence of myalgia vs. placebo. Conversely, a significant rise in serum uric acid and creatinine levels were found, as well as a small but significant increase in the incidence of gout. Finally, relative to drug–drug interactions, co-administration of simvastatin 20 mg with bempedoic acid 240 mg, or simvastatin 40 mg with bempedoic acid 180 mg, raised approximately by 2- and 1.5-fold the area under the curve (AUC) and Cmax of simvastatin (Table 1). The mechanism could be partly ascribed to the inhibition of the organic anion transporting polypeptide 1B1.12
Table 1.
Pharmacokinetic characteristics of bempedoic acid12
| Administration | Oral once daily |
|---|---|
| Adsorption | Concomitant food administration had no effect on the oral bioavailability |
| Tmax (180 mg) | 3.5 h |
| Volume of distribution | 18 L |
| Plasma binding proteins | 99% |
| Prodrug | Yes |
| Active metabolite | ESP15228 |
| Metabolism | Glucuronide (UGT2B7 mediated) |
| Transporter-mediated drug interactions | OATP1B1/3, OAT2, OAT3 |
| Half-life | 15–24 h |
| Drug–drug interactions |
|
OAT2/OAT3, organic anion transporter-2/3; OATP1B1/3, organic anion transporting polypeptide 1B1/3, UGT2B7, UDP Glucuronosyltransferase Family 2 Member B7.
Inclisiran
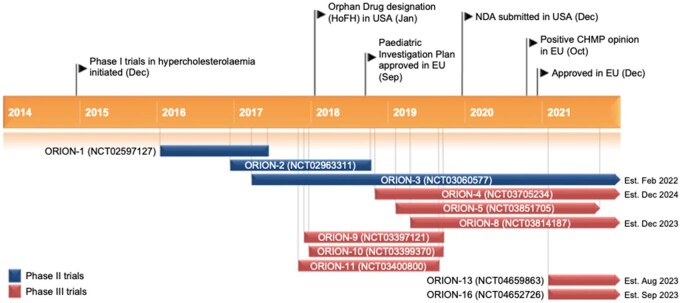
Inclisiran, designed to target the 3′ UTR of the PCSK9 mRNA, is a long-acting silencer RNA whose 3′ end of the passenger strand is functionalized with triantennary GalNAc, allowing a rapid and specific liver uptake.13 Inclisiran received its first approval in December 2020 in the EU (Figure 2). The indication is in adults with primary hypercholesterolaemia (HeFH and non-FH) or mixed dyslipidaemia, as an adjunct to diet: (i) in combination with a statin or statin with other lipid-lowering therapies in patients unable to reach LDL-C goals with the maximum tolerated statin dose or (ii) alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant, or for whom a statin is contraindicated. Inclisiran is not a substrate of common drug transporters and is not expected to be a cytochrome P450 substrate. It is not an inducer or an inhibitor of cytochrome P450 enzymes or common drug transporters.14 The recommended dose is 284 mg administered as a single subcutaneous (s.c.) injection (each mL contains inclisiran sodium equivalent to 189 mg inclisiran) on Day 1, Day 90, and every 6 months thereafter.
Figure 2.
Key milestones in the development of subcutaneous inclisiran for use in hypercholesterolaemia and mixed dyslipidaemia. CHMP, Committee for Medicinal Products for Human Use; HoFH, homozygous familial hypercholesterolaemia. Reproduced with permission of Springer Nature.14
The safety and efficacy of inclisiran have been and are being evaluated in the ORION program. Briefly, ORION-10 was carried out in the USA and ORION-11 in Europe and South Africa; the ORION-2, -5, and -9 trials have recruited familial hypercholesterolaemia (FH) patients, whereas ORION-3 and -8 were single-arm, open-label studies. ORION-7, conducted in New Zealand, is a phase 1, open-label study evaluating the effect of renal impairment on pharmacokinetics, pharmacodynamics. Data from ORION-1 and ORION-7 showed that there dose adjustments are not needed in patients with mild, moderate, or severe renal impairment or end-stage renal disease.15 ORION-13 and ORION-16 trials, not yet recruiting, will evaluate the safety and efficacy of inclisiran in adolescents (12–17 years old) with homozygous FH (HoFH) and HeFH, respectively, and elevated LDL-C on stable, standard of care background lipid-lowering therapy.
The first published trial, the phase 2 ORION-1 allowed to established that 300 mg on Day 1 and Day 90 and then every 180 days was the best dose regimen to be adopted. Interestingly, looking at waterfall plots of atherogenic lipoproteins, it is clear that inclisiran guarantees, at least for LDL-C and apoB levels, effects not found in statin trials.16
A pooled analysis, involving FH patients (ORION-9) or individuals with ASCVD (ORION-10 and -11), showed the superiority of inclisiran vs. placebo, i.e. LDL-C was lowered at Day 510 by −50.7%. Although safety was similar between groups, adverse events at the injection site were more frequent with inclisiran than placebo (5.0% vs. 0.7%).17 Awaiting the results of the long cardiovascular endpoint (CVOT) ORION-4 which will provide definitive evidence on the efficacy of inclisiran on MACE incidence, an exploratory analysis on MACEs has been conducted by using data of ORION-10 and -11. The estimation of MACEs reduction associated with LDL-C lowering indicate a linear trend in the risk of MACE and LDL-C reduction in line with previous conclusions on the activity of PCSK9 monoclonal antobodies.18
Conclusions
Bempedoic acid, besides efficiently lowering LDL-C, has an excellent tolerability with a very low incidence of myalgia. This is consequent to a non-skeletal muscle mediated conversion of inactive to active drug. Considering that statin discontinuation leads to a higher risk of coronary events, bempedoic acid may be chosen as a statin replacement (eventually in combination with ezetimibe) for patients needing LDL-C lowering and experiencing muscular side effects. Inclisiran has the advantage that a single s.c. injection dose leads to a long-lasting and durable LDL-C reduction, thus should guarantee a better compliance.
Conflict of interest: none declared.
References
- 1. Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL.. Global epidemiology of dyslipidaemias. Nat Rev Cardiol 2021;doi: 10.1038/s41569-021-00541-4. [DOI] [PubMed] [Google Scholar]
- 2. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS.. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 2016;316:1289–1297. [DOI] [PubMed] [Google Scholar]
- 3. Ray KK, Molemans B, Schoonen WM, Giovas P, Bray S, Kiru G, Murphy J, Banach M, De Servi S, Gaita D, Gouni-Berthold I, Hovingh GK, Jozwiak JJ, Jukema JW, Kiss RG, Kownator S, Iversen HK, Maher V, Masana L, Parkhomenko A, Peeters A, Clifford P, Raslova K, Siostrzonek P, Romeo S, Tousoulis D, Vlachopoulos C, Vrablik M, Catapano AL, Poulter NR; DA VINCI study. EU-Wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: the DA VINCI study. Eur J Prev Cardiol 2020;doi: 10.1093/eurjpc/zwaa04. [DOI] [PubMed] [Google Scholar]
- 4. Banach M, Duell PB, Gotto AM, Laufs U, Leiter LA, Mancini GBJ, Ray KK, Flaim JA, Ye Z, Catapano AL Jr. Association of bempedoic acid administration with atherogenic lipid levels in phase 3 randomized clinical trials of patients with hypercholesterolemia. JAMA Cardiol 2020;5:1124–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ballantyne CM, Banach M, Mancini GBJ, Lepor NE, Hanselman JC, Zhao X, Leiter LA.. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis 2018;277:195–203. [DOI] [PubMed] [Google Scholar]
- 6. Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, Robinson PL, Ballantyne CM.. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med 2019;380:1022–1032. [DOI] [PubMed] [Google Scholar]
- 7. Goldberg AC, Leiter LA, Stroes ESG, Baum SJ, Hanselman JC, Bloedon LT, Lalwani ND, Patel PM, Zhao X, Duell PB.. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR wisdom randomized clinical trial. JAMA 2019;322:1780–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laufs U, Banach M, Mancini GBJ, Gaudet D, Bloedon LT, Sterling LR, Kelly S, Stroes ESG.. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc 2019;8:e011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nicholls SJ, Lincoff AM, Bays HE, Cho L, Grobbee DE, Kastelein JJ, Libby P, Moriarty PM, Plutzky J, Ray KK, Thompson PD, Sasiela W, Mason D, McCluskey J, Davey D, Wolski K, Nissen SE.. Rationale and design of the CLEAR-outcomes trial: evaluating the effect of bempedoic acid on cardiovascular events in patients with statin intolerance. Am Heart J 2021;235:104–112. [DOI] [PubMed] [Google Scholar]
- 10. Rubino J, MacDougall DE, Sterling LR, Hanselman JC, Nicholls SJ.. Combination of bempedoic acid, ezetimibe, and atorvastatin in patients with hypercholesterolemia: a randomized clinical trial. Atherosclerosis 2021;320:122–128. [DOI] [PubMed] [Google Scholar]
- 11. Bays HE, Banach M, Catapano AL, Duell PB, Gotto AM, Laufs U, Leiter LA, Mancini GBJ, Ray KK, Bloedon LT, Sasiela WJ, Ye Z, Ballantyne CM.. Bempedoic acid safety analysis: pooled data from four phase 3 clinical trials. J Clin Lipidol 2020;14:649–659.e6. [DOI] [PubMed] [Google Scholar]
- 12.European Medicines Agency. Summary of Product Characteristics - Nilemdohttps://www.google.com/search?client=firefox-b-d&q=EMA+bempedoic+acid.
- 13. Macchi C, Sirtori CR, Corsini A, Santos RD, Watts GF, Ruscica M.. A new dawn for managing dyslipidemias: the era of RNA-based therapies. Pharmacol Res 2019;150:104413. [DOI] [PubMed] [Google Scholar]
- 14. Lamb YN. Inclisiran: first approval. Drugs 2021;81:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wright RS, Collins MG, Stoekenbroek RM, Robson R, Wijngaard PLJ, Landmesser U, Leiter LA, Kastelein JJP, Ray KK, Kallend D.. Effects of renal impairment on the pharmacokinetics, efficacy, and safety of inclisiran: an analysis of the ORION-7 and ORION-1 studies. Mayo Clin Proc 2020;95:77–89. [DOI] [PubMed] [Google Scholar]
- 16. Ray KK, Stoekenbroek RM, Kallend D, Leiter LA, Landmesser U, Wright RS, Wijngaard P, Kastelein JJP.. Effect of an siRNA therapeutic targeting PCSK9 on atherogenic lipoproteins: prespecified secondary end points in ORION 1. Circulation 2018;138:1304–1316. [DOI] [PubMed] [Google Scholar]
- 17. Wright RS, Ray KK, Raal FJ, Kallend DG, Jaros M, Koenig W, Leiter LA, Landmesser U, Schwartz GG, Friedman A, Wijngaard PLJ, Garcia Conde L, Kastelein JJP.. Pooled patient-level analysis of inclisiran trials in patients with familial hypercholesterolemia or atherosclerosis. J Am Coll Cardiol 2021;77:1182–1193. [DOI] [PubMed] [Google Scholar]
- 18. Cordero A, Santos-Gallego CG, Fácila L, Rodríguez-Mañero M, Bertomeu-González V, Castellano JM, Seijas-Amigo J, Núñez J, Zuazola P, González-Juanatey JR, Badimon JJ.. Estimation of the major cardiovascular events prevention with Inclisiran. Atherosclerosis 2020;313:76–80. [DOI] [PubMed] [Google Scholar]