Highlights
-
•
In a large group of subjects without overt conduction system disease, there was a positive association between increasing BMI and electrocardiographic QRS duration that was independent of other covariates such as sex and age.
-
•
Females had narrower QRS complex than the males at similar age and in the similar BMI category.
-
•
Findings of this research should prompt further studies to explore the underlying mechanisms for these observations and potential reversibility of the conduction abnormality with weight loss
Keywords: ECG QRS duration, BMI, Sex, Age, Population health
Abbreviations: BMI, Body Mass Index; CRP, C Reactive Protein; CRT, Cardiac Resynchronisation therapy; Cx 43, Connexin 43; ECG, Electrocardiogram; EDV, End Diastolic Volume; ESV, End Systolic Volume; IQR, Interquartile range; QTc, Corrected QT interval; WHO, World Health Organisation
Abstract
Background
Electrocardiogram (ECG) measured QRS duration has been shown to influence cardiovascular outcomes. However, there is paucity of data on whether ECG QRS duration is influenced by obesity and sex in large populations.
Methods
All ECGs performed by a pathology provider over a 2-year period were included. ECGs with confounding factors and those not in sinus rhythm were excluded from the primary analysis.
Results
Of the 76,220 who met the inclusion criteria, 41,685 (55%) were females. The median age of the study cohort was 61 years (interquartile [IQR] range 48–71 years). The median QRS duration was 86 ms (IQR 80–94 ms). The median BMI was 27.6 kg/m2 (IQR 24.2–31.8 kg/m2). When stratified according to the World Health Organization classification of BMI < 18.50 kg/m2, 18.50–24.99 kg/m2, 25.00–29.99 kg/m2, and ≥ 30.00 kg/m2, the median QRS durations were 82 ms (IQR 76–88 ms), 86 ms (IQR 80–92 ms), 88 ms (IQR 80–94 ms) and 88 ms (IQR 82–94 ms), respectively (p < 0.001 for linear trend). Median QRS duration for females was 84 ms (IQR 78–88 ms); for males, it was 92 ms (IQR 86–98 ms), p < 0.001. Compared to males, females had narrower QRS complexes at similar age and similar BMI. In multiple linear regression analysis, BMI correlated positively with QRS duration (standardized beta 0.095, p < 0.001) independent of age, sex, and heart rate.
Conclusions
In this large cohort there was a positive association between increasing BMI and QRS duration. Females had narrower QRS duration than males at similar age and similar BMI.
1. Introduction
Obesity has been linked to an increased risk of arrhythmia, prolonged corrected QT interval (QTc) and increased risk of sudden cardiac death [1]. Various electrocardiographic measurements including R-R interval, and QTc have been studied in obese populations. A positive correlation between increased BMI and increased QTc has been documented, but was limited by small patient sample size [2]. A recent meta-analysis suggested correlation between QTc duration and increased risk of mortality [3]. P wave duration has also been observed to increase with general and central obesity, indicating a relationship between the development of obesity and atrial remodelling [4]. However, there is little data regarding potential relationship between obesity and QRS duration, which may be a marker of electrical remodelling in the conduction system and ventricle.
ECG measured QRS duration has been shown to influence cardiovascular outcomes in multiple clinical situations. Increased QRS duration has been associated with increased incidence of heart failure [5]. Increased QRS duration has been associated with increased mortality in diabetics [6] and predicted mortality in patients with non-ST segment elevation myocardial infarction [7].
Females have been noted to derive more benefit from cardiac resynchronisation therapy (CRT) therapy for similar entry QRS duration when compared to males [8], [9]. ECG QRS duration in females was also noted to be an independent predictor for development of atrial fibrillation [10]. However, there are no large studies available to compare QRS durations of females and males in the general population in different age groups and in different BMI categories.
In this study, we sought to determine the relationship between QRS duration BMI and sex.
2. Methods
2.1. Study population
Electrocardiographic (ECG) data and associated clinical information were obtained from a large pathology provider in Australia. All consecutive ECGs performed from 1 January 2017 to 31 December 2018 were included in the study. Additional routine clinical information collected during ECG performance included subject’s height, weight, medications, clinical indications, cardiologist’s interpretation, QRS duration and heart rate. ECGs were recorded on Schiller ECG machines (Schiller AG, Baar Switzerland Model AT 101). ECG intervals were computer generated. The QRS duration was measured on the superimposed 12 lead ECG QRS complexes on SEMA workstation (SchillerAG, Baar Switzerland) These values were verified by the reporting cardiologists and edited if required using electronic callipers.
Subjects with age less than 20 and over 90, QRS duration > 120 ms and those with confounding causes for wide QRS duration such as conduction disease (complete and incomplete left and right bundle branch blocks, or non-specific intra-ventricular conduction delay), myocardial infarction (Q waves or evidence of acute ischaemia), left ventricular hypertrophy (Based on Sokolow-Lyon or Cornell criteria), medications that can affect QRS duration (e.g. anti-arrhythmic medications, anti-psychotic and antidepressant medications), non-sinus rhythm, and pre-excitation were excluded. Those subjects without recorded height and weights were also excluded. Duplicate ECGs for the same subjects were also removed from analysis. ECGs with noise interference were excluded.
The study was approved by the institutional ethics committee and was conducted in accordance with the Declaration of Helsinki.
2.2. Statistical analysis
All continuous variables were tested for Gaussian distribution based on the Kolmogorov-Smirnov (KS) test and Q-Q plot. Continuous variables with Gaussian distribution were presented as mean ± 1 standard deviation, or median with interquartile range (IQR) if not normally distributed. Categorical variables were presented as frequencies and percentages. Pairwise comparisons were performed using unpaired Student’s t-test and Mann-Whitney U test for continuous variables of Gaussian and non-Gaussian distribution respectively, and chi-square test for categorical variables. One-way ANOVA was used to compare continuous variables between multiple groups, with Bonferroni correction. Pearson correlation was used to determine the association between 2 continuous variables. Multiple linear regression analyses were performed to identify independent variables associated with QRS duration. In each multiple linear regression model, significant univariables with p < 0.05 were entered as covariates and independent variables were identified using the backward elimination method. A tolerance of > 0.4, equating to a variance inflation factor (VIF) of > 2.5 was set to avoid any potential multicollinearity. A 2-tailed p value of < 0.05 was considered significant. All statistical analyses were performed using SPSS V23.0 (IBM, USA).
3. Results
3.1. Baseline characteristics
A total of 121,162 records were initially found. Successive filters based on exclusion criteria were applied (Supplementary Fig. 1), leaving 79,465 records. Out of these records, 76,220 were in sinus rhythm and formed the primary cohort of the study (Table 1). The KS test (all p < 0.001) and Q-Q plot showed that age, height, weight, BMI, heart rate and QRS duration to be all not normally distributed, whether for the whole cohort or stratified by sex. Median age of the study cohort was 61 years (IQR 48 –71 years). For the total cohort, the median height, weight and BMI measurements were 168 cm (IQR 160 – 176 cm), 79 kg (IQR 67 – 93 kg), and 27.6 kg/m2 (IQR 24.2–31.8 kg/m2), respectively. The median heart rate of the total cohort was 66 beats per minute (bpm) (IQR 59–74 bpm), with a median QRS duration of 86 ms (ms) (IQR 80–94 ms). Table 1 also shows the study cohort’s baseline characteristics stratified by sex. The height, weight and BMI in male study participants were higher than female participants. The median heart rate was noted to be higher in females compared to males (68 bpm [61–75 bpm] vs 65 bpm [58–73 bpm] respectively, p < 0.001), while the median QRS duration was longer in males compared to females (92 ms [86–98 ms] vs 84 ms [78–88 ms], p < 0.001) (Table 1).
Table 1.
Baseline characteristics of cohort.
| Total cohort |
Female |
Male |
||
|---|---|---|---|---|
| Parameters | N = 76,220 | N = 41,685 (55%) | N = 34,535 (45%) | P value |
| Age, years | 58.7 ± 16.3 | 59.0 ± 16.6 | 58.5 ± 15.9 | |
| Median (IQR) | 61 (48–71) | 62 (48 – 72) | 61 (48–70) | <0.001 |
| Age by decade, years | ||||
| 20–29 | 4,806 (6.3) | 2,769 (6.6) | 2,037 (5.9) | <0.001 |
| 30–39 | 6,450 (8.5) | 3,477 (8.3) | 2,973 (8.6) | |
| 40–49 | 9,787 (12.8) | 5,251 (12.6) | 4,536 (13.1) | |
| 50–59 | 14,015 (18.4) | 7,486 (18.0) | 6,529 (18.9) | |
| 60–69 | 18,717 (24.6) | 9,872 (23.7) | 8,845 (25.6) | |
| 70–79 | 16,288 (21.4) | 9,054 (21.7) | 7,234 (20.9) | |
| 80–89 | 6,157 (8.1) | 3,776 (9.1) | 2,381 (6.9) | |
| Anthropometric measurements | ||||
| Height, cm | 168.2 ± 10.3 | 161.9 ± 7.4 | 175.8 ± 7.8 | |
| Median (IQR) | 168 (160–176) | 162 (157–167) | 176 (170–181) | <0.001 |
| Weight, kg | 81.0 ± 20.1 | 74.4 ± 19.1 | 89.0 ± 18.3 | |
| Median (IQR) | 79 (67–93) | 71 (61–85) | 87 (76–99) | <0.001 |
| Body mass index (BMI), kg/m2 | 28.6 ± 6.3 | 28.4 ± 6.9 | 28.7 ± 5.3 | |
| Median (IQR) | 27.6 (24.2–31.8) | 27.2 (23.4–32.2) | 28.0 (25.1–31.4) | <0.001 |
| BMI WHO categories | ||||
| <18.50 | 1,412 (1.9) | 1,179 (2.8) | 233 (0.7) | <0.001 |
| 18.50–24.99 | 21,683 (28.4) | 13,698 (32.9) | 7,985 (23.1) | |
| 25.00–29.99 | 26,721 (35.1) | 12,299 (29.5) | 14,422 (41.8) | |
| ≥30.00 | 26,404 (34.6) | 14,509 (34.8) | 11,895 (34.4) | |
| ECG measurements | ||||
| Heart rate recorded | 74,979 (98.4) | 40,913 (98.1) | 34,066 (98.6) | |
| Missing heart rate data | 1,241 (1.6) | 772 (1.9) | 469 (1.4) | |
| Heart rate*, beats/min | 67.3 ± 11.2 | 68.5 ± 10.8 | 65.8 ± 11.4 | |
| Median (IQR) | 66 (59–74) | 68 (61–75) | 65 (58–73) | <0.001 |
| QRS duration, ms | 87.0 ± 9.4 | 83.4 ± 8.2 | 91.3 ± 8.9 | |
| Median (IQR) | 86 (80–94) | 84 (78–88) | 92 (86–98) | <0.001 |
Plus-minus value represents mean ± standard deviation (SD); all others represent numbers of patients with values in brackets representing percentages, or otherwise stated.
ECG, electrocardiogram; IQR, interquartile range; WHO, World Health Organization
*Only in patients with heart rate recorded.
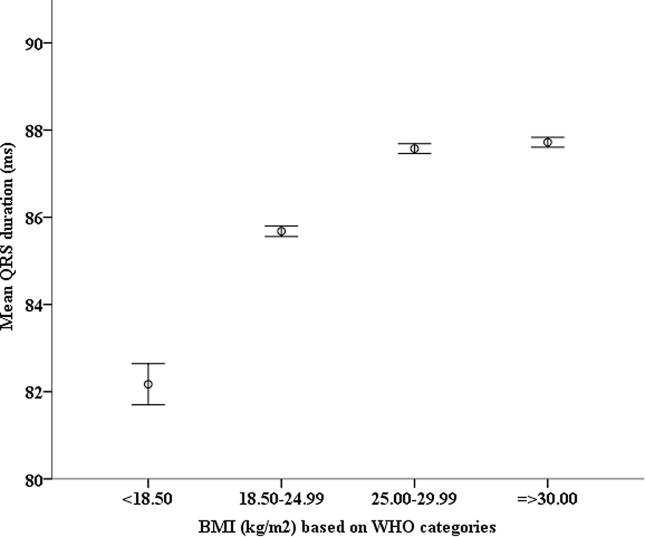
When the study cohort was stratified according to the World Health Organization classification of BMI < 18.50 kg/m2, 18.50–24.99 kg/m2, 25.00–29.99 kg/m2, and those with BMI ≥ 30.00 kg/m2, the median QRS durations were 82 ms (IQR 76–88 ms), 86 ms (IQR 80–92 ms), 88 ms (IQR 80–94 ms) and 88 ms (IQR 82–94 ms), respectively. The mean QRS durations were 82.2 ± 9.0 ms, 85.7 ± 9.1 ms, 87.6 ± 9.4 ms and 87.7 ± 9.4 ms respectively (p < 0.001 for linear trend) (Fig. 1).
Fig. 1.
Figure illustrates the mean QRS duration, with 95% confidence interval, of study cohort stratified by BMI based on WHO categories. Linear trend P < 0.001 (See ANOVA analysis with Bonferroni correction regarding statistical differences between groups in supplementary data.) BMI, body mass index in kg/m2; WHO, World Health Organization.
Supplementary Fig. 2 shows the mean BMI of females and males participants stratified into their respective age groups by decade. One way ANOVA test showed differences between groups for both female and male cohorts were significant (p < 0.001). For females, post hoc test with Bonferroni correction showed no significant difference in mean BMI between age groups of 30–39 and 70–79, between age groups of 30–39 and 70–79, and between age groups of 40–49 and 50–59 and 60–69, while all other comparisons were significant. For males, there were no significant difference in mean BMI between age groups of 20–29 and 80–89, between age groups of 30–39 and 60–69, between age groups of 40–49 and 50–59, while all other comparisons were significant.
Analysis of univariable and multivariable determinants of QRS duration were conducted with BMI, and separately based on height and weight. BMI showed positive correlation with QRS duration and was independent of age, sex and heart rate (Supplementary Table 1A). Collinearity test suggests no collinearity between age, sex, BMI, heart rate and QRS duration.
When height and weight replaced BMI, both variables correlated positively and independently with QRS duration (Supplementary Table 1B). Collinearity test suggests no collinearity between age, sex, height, weight, heart rate and QRS duration.
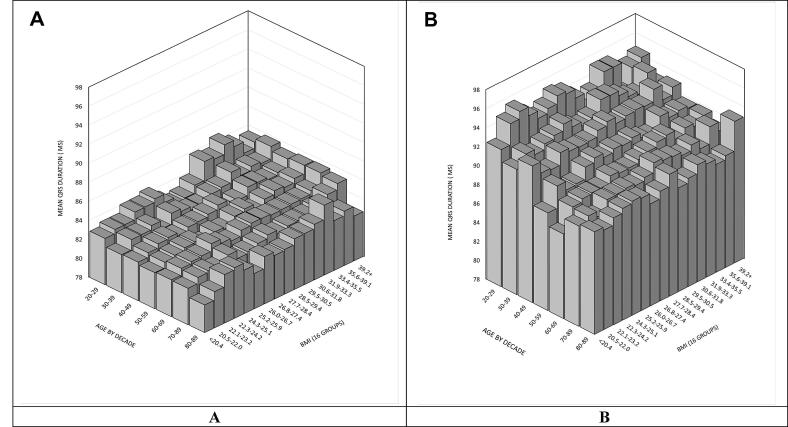
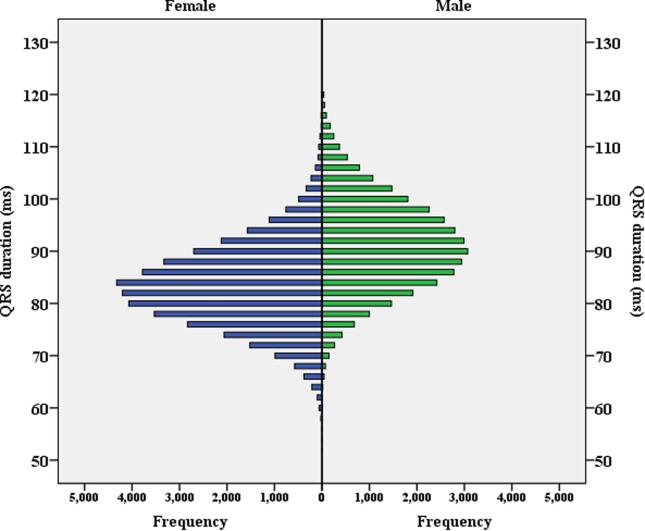
Fig. 2 shows mean QRS stratified by age (by decade) and BMI (17 groups) for females and males. These bar charts demonstrate the complex inter-relationship of QRS duration, age, BMI and sex. Fig. 3 illustrates the distribution of QRS duration of the study cohort stratified by sex.
Fig. 2.
Figure illustrates the mean QRS duration stratified by age and BMI. Figure A represents female cohort. Figure B represents male cohort.
Fig. 3.
Figure illustrates the distribution of QRS duration of study cohort stratified by sex.
Table 2 shows the mean QRS duration of males compared to females within each age groups, stratified by BMI based on WHO categories. The mean QRS duration was significantly longer for males compared to females across all groups and BMI ranges, except in the age group of 60–69 with BMI ≤ 18.50 kg/m2.
Table 2.
Comparing QRS duration of female vs male stratified by age groups and BMI
| BMI < 18.50 kg/m2 |
BMI 18.50–24.99 kg/m2 |
BMI 25.00–29.99 kg/m2 |
BMI ≥ 30.00 kg/m2 |
|||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Female | Male | Female | Male | Female | Male | Female | Male |
| Age by decade, yrs | Mean QRS duration, ms | |||||||
| 20–29 | 82.8 ± 7.7 | 91.3 ± 8.5* | 83.4 ± 7.9 | 94.4 ± 8.3* | 83.9 ± 7.9 | 94.0 ± 8.2* | 85.6 ± 7.5 | 94.3 ± 8.8* |
| 30–39 | 80.1 ± 10.6 | 87.7 ± 9.4* | 83.1 ± 7.4 | 92.3 ± 8.3* | 83.6 ± 8.0 | 93.2 ± 8.3* | 85.0 ± 7.6 | 93.1 ± 8.5* |
| 40–49 | 80.5 ± 8.1 | 90.5 ± 8.4* | 82.8 ± 7.6 | 91.8 ± 8.4* | 83.5 ± 7.6 | 92.4 ± 8.1* | 84.6 ± 7.9 | 92.9 ± 8.5* |
| 50–59 | 80.3 ± 8.5 | 86.5 ± 7.4* | 82.6 ± 7.6 | 90.2 ± 8.8* | 83.2 ± 7.8 | 91.5 ± 8.4* | 84.5 ± 8.1 | 91.8 ± 8.8* |
| 60–69 | 80.8 ± 8.5 | 83.0 ± 7.8 | 82.8 ± 7.8 | 89.5 ± 8.7* | 83.5 ± 8.0 | 90.7 ± 8.8* | 84.2 ± 8.5 | 91.4 ± 9.1* |
| 70–79 | 81.2 ± 8.4 | 86.1 ± 9.0* | 82.6 ± 8.3 | 89.4 ± 9.3* | 82.9 ± 8.4 | 90.5 ± 9.1* | 84.0 ± 8.9 | 90.9 ± 9.6* |
| 80–89 | 80.1 ± 9.3 | 88.5 ± 7.7* | 82.4 ± 8.4 | 89.5 ± 9.9* | 82.7 ± 8.8 | 89.6 ± 9.8* | 89.9 ± 9.5 | 89.9 ± 9.5* |
The plus-minus values represent mean ± 1 standard deviation.
*p < 0.05 comparing male to female mean QRS duration within each age group.
BMI, body mass index.
4. Discussion
There were two main findings in the present study. The first finding was a positive association between increasing BMI and QRS duration that was independent of other covariates such as sex and age. The other finding was females having a narrower QRS complex than the males at similar age and similar BMI category.
4.1. Association between BMI and QRS duration
The finding of a positive association between increasing BMI and QRS duration in our large study population, is consistent with prior smaller cohort studies [11], [12], [13], [14], [15], [16], [17]. For example, in an earlier study by Ishikawa et al, anthropometric measures such as chest diameter were measured in 300 Japanese men and was suggested to correlate with ECG measurements, however notably sex comparison could not be done and ECG changes with reference to validated indices of obesity (such as BMI) were not examined specifically (though simple height and weight measurements were correlated) [17]. Sun et al observed progressive increase in QRS duration with increasing BMI in an observational study of 5,556 children and adolescents [16]. Chi et al also reported association between increasing visceral adiposity burden on cardiac CT, and leftward deviation of P and QRS axes, longer PR interval and longer QRS duration in a cohort study of 3,411 adults. This study looked at a group younger than our study cohort and had only 28% females [12].
The present study which was a multiparametric analysis of the influences of age, sex and BMI, not only confirms the relationship between BMI and QRS duration in a large cohort but extends previous work by demonstrating that the association of BMI and QRS duration is independent of age in both females and males.
There are several potential mechanisms to explain the finding of increased QRS duration in obese patients. Conduction velocity through the myocardium is influenced by properties of the cardiac myocytes (e.g. ion channel regulation, membrane capacitance and axial resistance) as well as factors affecting conduction between myocytes [18]. In a study of atrial myocardium, migration/infiltration of epicardial fat cells between myocytes in the atria has been implicated in the creation of electrically inert areas, that confound and delay electrical conduction [19]. Research investigating electrical and anatomical remodelling of the atria in obesity suggested that atrial fibrosis may be mediated by a pro-fibrotic cytokine activin A, which in turn may have several influences on myocytes and consequently conduction [20]. In addition, adipocytes may be a source of a vast range of clinically significant inflammatory mediators, including angiotensin II, fibrinogen, C-reactive protein (CRP), interlukin-6 and tumor necrosis factor-alpha [21]. Chronic inflammation driven by pro-inflammatory cytokines, may cause abnormal differentiation of fibroblasts into myofibroblasts [22] that could result in alteration in conduction by a number of mechanisms including disruption of cell-to-cell coupling, increased axial resistance and altered membrane capacitance [18].
4.2. Association between sex and QRS duration
In our study, we also observed that females had narrower QRS duration than males. This not only confirms observations from previous smaller studies, but importantly demonstrates that the association is independent of important confounders such as age and the increased body mass in males. McFarlane and colleagues studied ECG parameters in a heterogenous group of 1,555 adult white individuals, and 503 adult Chinese individuals and noted a statistically significant increase in QRS duration in males compared with females [23]. Randolph and colleagues studied 2,463 black and white patients with Heart failure and reduced ejection fraction (ejection fraction ≤ 35%) and found QRS duration was longest in white men (111 ms; IQR, 98–139) followed by white women (108 ms; IQR, 92–140), black men (100 ms; IQR, 91–120), and black women (94 ms; IQR, 86–118). Furthermore, the authors demonstrated in black patients, there was a 16% increase in risk of mortality for every 10 ms increase in QRS duration [24].
The narrower QRS in females may be related to reduced cardiac mass in females, as suggested in the Framingham heart offspring cohort study, where left ventricular mass was smaller in women, even after adjustment for height or body surface area [25], [26]. Animal studies have postulated that oestrogen may affect expression of gap junction protein Connexin43 (Cx43), and confer greater resilience to conduction abnormalities in female, suggesting a role for hormonal differences [27].
4.3. Study limitations
Although the findings of the present study were based on a large cohort, the influence of additional confounders such as structural heart disease and heart failure could not be evaluated because the medical history was variably recorded on the database. Future studies should explore linkage analysis based on our large ECG database to available population databases to obtain information including comorbidities, cardiac events leading to hospital admissions and death. Selection bias may also have confounded the results because the ECGs were collected for various indications including asymptomatic screening as well as symptomatic evaluation.
5. Conclusions
In this large group of subjects without overt conduction system disease, we found a positive association between QRS duration and increased body mass index which was independent of age and sex. We also found that females had narrower QRS complexes compared to the males at similar age and BMI category. These findings should prompt studies to further explore the underlying mechanisms, and potential reversibility of the conduction abnormality with weight loss and to see if gender differences in QRS duration may influence threshold for and response to therapies such as cardiac resynchronisation.
6. Statement of Authorship
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation
7. Acknowledgment of grant support
The authors received nil financial aid/support/grants in the production of this work, and have nil financial interests to declare.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank the staff of the Department of Cardiology at Douglas Hanly Moir Pathology, Dr Annabelle Farnsworth, Ms Virginia Lloyd-Tait, Mrs Nichole Signorelli and Mr Peter Wilkins.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100884.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Finocchiaro G., Papadakis M., Dhutia H., Cole D., Behr E.R., Tome M., Sharma S., Sheppard M.N. Obesity and sudden cardiac death in the young: Clinical and pathological insights from a large national registry. Eur. J. Prev. Cardiol. 2018;25(4):395–401. doi: 10.1177/2047487317751291. [DOI] [PubMed] [Google Scholar]
- 2.El-Gamal A., Gallagher D., Nawras A., Gandhi P., Gomez J., Allison D.B., Steinberg J.S., Shumacher D., Blank R., Heymsfield S.B. Effects of obesity on QT, RR, and QTc intervals. Am. J. Cardiol. 1995;75(14):956–959. doi: 10.1016/s0002-9149(99)80700-0. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y., Post W.S., Blasco-Colmenares E., Dalal D., Tomaselli G.F., Guallar E. Electrocardiographic QT interval and mortality: a meta-analysis. Epidemiology. 2011;22(5):660–670. doi: 10.1097/EDE.0b013e318225768b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaidean G.D., Manczuk M., Magnani J.W. Atrial electrocardiography in obesity and hypertension: Clinical insights from the Polish-Norwegian Study (PONS) Obesity (Silver Spring). 2016;24(12):2608–2614. doi: 10.1002/oby.21678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhingra R., Pencina M.J., Wang T.J., Nam B.-H., Benjamin E.J., Levy D., Larson M.G., Kannel W.B., D’Agostino R.B., Vasan R.S. Electrocardiographic QRS duration and the risk of congestive heart failure: the Framingham Heart Study. Hypertension. 2006;47(5):861–867. doi: 10.1161/01.HYP.0000217141.20163.23. [DOI] [PubMed] [Google Scholar]
- 6.Singleton M.J., German C., Hari K.J., Saylor G., Herrington D.M., Soliman E.Z., Freedman B.I., Bowden D.W., Bhave P.D., Yeboah J. QRS duration is associated with all-cause mortality in type 2 diabetes: The diabetes heart study. J. Electrocardiol. 2020;58:150–154. doi: 10.1016/j.jelectrocard.2019.11.053. [DOI] [PubMed] [Google Scholar]
- 7.Brilakis E.S., Mavrogiorgos N.C., Kopecky S.L., Rihal C.C., Gersh B.J., Williams B.A., Clements I.P. Usefulness of QRS duration in the absence of bundle branch block as an early predictor of survival in non-ST elevation acute myocardial infarction. Am. J. Cardiol. 2002;89(9):1013–1018. doi: 10.1016/s0002-9149(02)02267-1. [DOI] [PubMed] [Google Scholar]
- 8.Linde C., Cleland J.G.F., Gold M.R., Claude Daubert J., Tang A.S.L., Young J.B., Sherfesee L., Abraham W.T. The interaction of sex, height, and QRS duration on the effects of cardiac resynchronization therapy on morbidity and mortality: an individual-patient data meta-analysis. Eur. J. Heart Fail. 2018;20(4):780–791. doi: 10.1002/ejhf.1133. [DOI] [PubMed] [Google Scholar]
- 9.Zusterzeel R., Curtis J.P., Caños D.A., Sanders W.E., Selzman K.A., Piña I.L., Spatz E.S., Bao H., Ponirakis A., Varosy P.D., Masoudi F.A., Strauss D.G. Sex-specific mortality risk by QRS morphology and duration in patients receiving CRT: results from the NCDR. J. Am. Coll. Cardiol. 2014;64(9):887–894. doi: 10.1016/j.jacc.2014.06.1162. [DOI] [PubMed] [Google Scholar]
- 10.Aeschbacher S., O'Neal W.T., Krisai P., Loehr L., Chen L.Y., Alonso A., Soliman E.Z., Conen D. Relationship between QRS duration and incident atrial fibrillation. Int. J. Cardiol. 2018;266:84–88. doi: 10.1016/j.ijcard.2018.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alpert M.A., Terry B.E., Cohen M.V., Fan T.M., Painter J.A., Massey C.V. The electrocardiogram in morbid obesity. Am. J. Cardiol. 2000;85(7):908–910. doi: 10.1016/s0002-9149(99)00894-2. [DOI] [PubMed] [Google Scholar]
- 12.Chi P.-C., Chang S.-C., Yun C.-H., Kuo J.-Y., Hung C.-L., Hou C.-Y., Liu C.-Y., Yang F.-S., Wu T.-H., Bezerra H.G., Yeh H.-I., Pizzi C. The Associations between Various Ectopic Visceral Adiposity and Body Surface Electrocardiographic Alterations: Potential Differences between Local and Remote Systemic Effects. PLoS ONE. 2016;11(7):e0158300. doi: 10.1371/journal.pone.0158300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraley M.A., Birchem J.A., Senkottaiyan N., Alpert M.A. Obesity and the electrocardiogram. Obes. Rev. 2005;6(4):275–281. doi: 10.1111/j.1467-789X.2005.00199.x. [DOI] [PubMed] [Google Scholar]
- 14.Frank S., Colliver J.A., Frank A. The electrocardiogram in obesity: statistical analysis of 1,029 patients. J. Am. Coll. Cardiol. 1986;7(2):295–299. doi: 10.1016/s0735-1097(86)80494-6. [DOI] [PubMed] [Google Scholar]
- 15.Lalani A.P., Kanna B., John J., Ferrick K.J., Huber M.S., Shapiro L.E. Abnormal signal-averaged electrocardiogram (SAECG) in obesity. Obes. Res. 2000;8(1):20–28. doi: 10.1038/oby.2000.4. [DOI] [PubMed] [Google Scholar]
- 16.Sun G.Z., Li Y., Zhou X.H., Guo X.F., Zhang X.G., Zheng L.Q. Association between obesity and ECG variables in children and adolescents: A cross-sectional study. Exp Ther Med. 2013;6(6):1455–1462. doi: 10.3892/etm.2013.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa K. Correlation coefficients for electrocardiographic and constitutional variables. Am. Heart J. 1976;92(2):152–161. doi: 10.1016/s0002-8703(76)80250-5. [DOI] [PubMed] [Google Scholar]
- 18.King J.H., Huang C.L., Fraser J.A. Determinants of myocardial conduction velocity: implications for arrhythmogenesis. Front. Physiol. 2013;4:154. doi: 10.3389/fphys.2013.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahajan R., Lau D.H., Brooks A.G., Shipp N.J., Manavis J., Wood J.P.M., Finnie J.W., Samuel C.S., Royce S.G., Twomey D.J., Thanigaimani S., Kalman J.M., Sanders P. Electrophysiological, Electroanatomical, and Structural Remodeling of the Atria as Consequences of Sustained Obesity. J. Am. Coll. Cardiol. 2015;66(1):1–11. doi: 10.1016/j.jacc.2015.04.058. [DOI] [PubMed] [Google Scholar]
- 20.Mahajan R., Nelson A., Pathak R.K., Middeldorp M.E., Wong C.X., Twomey D.J. Electroanatomical Remodeling of the Atria in Obesity: Impact of Adjacent Epicardial Fat. JACC Clin. Electrophysiol. 2018;4(12):1529–1540. doi: 10.1016/j.jacep.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Poirier P., Giles T.D., Bray G.A., Hong Y., Stern J.S., Pi-Sunyer F.X., Eckel R.H. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 22.Weber K.T., Sun Y., Tyagi S.C., Cleutjens J.P.M. Collagen network of the myocardium: function, structural remodeling and regulatory mechanisms. J. Mol. Cell. Cardiol. 1994;26(3):279–292. doi: 10.1006/jmcc.1994.1036. [DOI] [PubMed] [Google Scholar]
- 23.Macfarlane P.W., McLaughlin S.C., Devine B., Yang T.F. Effects of age, sex, and race on ECG interval measurements. J. Electrocardiol. 1994;27(Suppl):14–19. doi: 10.1016/s0022-0736(94)80039-1. [DOI] [PubMed] [Google Scholar]
- 24.Randolph T.C., Broderick S., Shaw L.K., Chiswell K., Mentz R.J., Kutyifa V., Velazquez E.J., Gilliam F.R., Thomas K.L. Race and Sex Differences in QRS Interval and Associated Outcome Among Patients with Left Ventricular Systolic Dysfunction. J. Am. Heart Assoc. 2017;6(3) doi: 10.1161/JAHA.116.004381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salton C.J., Chuang M.L., O’Donnell C.J., Kupka M.J., Larson M.G., Kissinger K.V., Edelman R.R., Levy D., Manning W.J. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J. Am. Coll. Cardiol. 2002;39(6):1055–1060. doi: 10.1016/s0735-1097(02)01712-6. [DOI] [PubMed] [Google Scholar]
- 26.van Hout M.J.P., Dekkers I.A., Westenberg J.J.M., Schalij M.J., Scholte A., Lamb H.J. The impact of visceral and general obesity on vascular and left ventricular function and geometry: a cross-sectional magnetic resonance imaging study of the UK Biobank. Eur. Heart J. Cardiovasc. Imaging. 2020;21(3):273–281. doi: 10.1093/ehjci/jez279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimoni Y., Emmett T., Schmidt R., Nygren A., Kargacin G. Sex-dependent impairment of cardiac action potential conduction in type 1 diabetic rats. Am. J. Physiol. Heart Circ. Physiol. 2009;296(5):H1442–H1450. doi: 10.1152/ajpheart.01150.2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.