Abstract
Introduction
Pembrolizumab plus chemotherapy significantly improved survival outcomes versus placebo plus chemotherapy in patients with previously untreated metastatic squamous NSCLC in the randomized, double-blind, phase 3 KEYNOTE-407 study. We present the results of Chinese patients enrolled in the KEYNOTE-407 global and China extension studies.
Methods
Patients enrolled from mainland China in the KEYNOTE-407 global (NCT02775435) and China extension studies (NCT03875092) were randomized 1:1 to 35 cycles of pembrolizumab or placebo plus four cycles of carboplatin and paclitaxel or nab-paclitaxel. Dual primary end points were overall survival (OS) and progression-free survival (PFS) (based on the Response Evaluation Criteria in Solid Tumors version 1.1 by blinded independent central review).
Results
A total of 125 patients were randomized (pembrolizumab–chemotherapy, n = 65; placebo–chemotherapy, n = 60). As of September 30, 2020, median (range) study follow-up was 28.1 (25.1‒40.9) months. Pembrolizumab–chemotherapy improved OS (hazard ratio [HR] = 0.44, 95% confidence interval [CI]: 0.28–0.70) and PFS (HR = 0.35, 95% CI: 0.24–0.52) versus placebo–chemotherapy. Two-year OS and PFS rates for pembrolizumab–chemotherapy versus placebo–chemotherapy were 56.9% versus 31.7% and 24.2% versus 3.3%, respectively. Treatment-related grade 3 to 5 adverse events occurred in 81.5% and 81.7%, respectively. Relative to baseline, pembrolizumab–chemotherapy improved global health status/quality of life scores at week 18 versus placebo–chemotherapy (difference in least squares means = 7.6, 95% CI: 1.5–13.7) and prolonged time to deterioration in cough, chest pain, or dyspnea (HR = 0.50, 95% CI: 0.28–0.89).
Conclusions
Pembrolizumab–chemotherapy prolonged survival versus placebo–chemotherapy with manageable toxicity and preserved or improved health-related quality of life in Chinese patients with metastatic squamous NSCLC. These findings support pembrolizumab–chemotherapy as first-line therapy in this population.
Keywords: Programmed death 1 blockade, Pembrolizumab, Chemotherapy, Squamous Non–small-cell lung cancer, China
Introduction
Lung cancer is the leading cause of cancer-related deaths in the People’s Republic of China.1 Inhibitors of programmed cell death-1 (PD-1) and its ligand, programmed death ligand 1 (PD-L1) have demonstrated significant improvements in clinical outcomes in patients with metastatic NSCLC.2, 3, 4, 5, 6, 7, 8, 9, 10, 11 In global studies, pembrolizumab, a highly selective monoclonal antibody that inhibits PD-1, has demonstrated substantial clinical benefit, including prolonged survival, as monotherapy in patients with NSCLC with PD-L1 tumor proportion score (TPS) greater than or equal to 1% without EGFR or ALK genetic aberrations6,8,12 and in combination with platinum-based chemotherapy, regardless of PD-L1 TPS in patients without EGFR or ALK genetic aberrations.2,5,7 Accordingly, pembrolizumab, as monotherapy and in combination with platinum-based chemotherapy, is approved for use in patients with NSCLC in multiple countries, including the People’s Republic of China.
Chinese patients are generally underrepresented in international randomized trials of anti–PD-(L)1 therapies.13 Because disease characteristics and treatment response may vary between Asian and White patients,14 evaluation of these treatments specifically in a Chinese population is warranted. In the KEYNOTE-042 China study, pembrolizumab monotherapy was found to prolong overall survival (OS) versus platinum-based chemotherapy in Chinese patients with locally advanced or metastatic NSCLC with PD-L1 TPS greater than or equal to 1% and without targetable EGFR or ALK alterations.15 Nevertheless, there are limited data evaluating pembrolizumab plus chemotherapy in Chinese patients with metastatic NSCLC.
KEYNOTE-407 was a global, randomized, double-blind, placebo-controlled, phase 3 study of pembrolizumab plus chemotherapy with carboplatin and paclitaxel or nab-paclitaxel (pembrolizumab–chemotherapy) compared with placebo plus chemotherapy (placebo–chemotherapy) in patients with previously untreated metastatic squamous NSCLC.7,16 In the global KEYNOTE-407 study, pembrolizumab–chemotherapy had significant improvements versus placebo–chemotherapy in the dual primary end points of OS (hazard ratio [HR] = 0.64, 95% confidence interval [CI]: 0.49–0.85, p < 0.001) and progression-free survival (PFS) (HR = 0.56, 95% CI: 0.45–0.70, p < 0.001).7 These improvements were maintained with longer follow-up.16 Pembrolizumab–chemotherapy was also found to improve health-related quality of life (HRQoL) versus placebo–chemotherapy.17 After enrollment in the global KEYNOTE-407 study was complete, the study continued to enroll patients in mainland China in an extension study to evaluate the consistency of outcomes in Chinese patients compared with the global study population.
Here, we report the outcomes of Chinese patients with previously untreated metastatic squamous NSCLC from the KEYNOTE-407 global and China extension studies. The key objective was to evaluate efficacy and safety of pembrolizumab–chemotherapy versus placebo–chemotherapy in Chinese patients in these studies. Patient-reported outcomes (PROs) were also evaluated.
Materials and Methods
Study Design and Patients
The study design of the global, randomized, placebo-controlled, double-blind, phase 3 KEYNOTE-407 study (ClinicalTrials.gov, NCT02775435) has been previously described.7 The KEYNOTE-407 China extension study (NCT03875092) was identical to the global KEYNOTE-407 study with the exception that it included only patients enrolled in mainland China after enrollment in the global study was completed.
Briefly, eligible patients were at least 18 years of age with histologically or cytologically confirmed diagnosis of stage IV squamous NSCLC, measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, an Eastern Cooperative Oncology Group performance status of 0 or 1, adequate organ function, and had provided a tumor tissue sample for PD-L1 evaluation and not received systemic treatment previously. Patients were excluded if they had major surgery within 3 weeks of treatment; received radiation therapy to the lung of greater than 30 Gy within 6 months of first study dose or completed palliative radiotherapy within 7 days before treatment; had a known history of previous malignancy, active central nervous system metastases, or carcinomatous meningitis; had peripheral neuropathy of grade 2 or higher (assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0); had active autoimmune disease; had received previous treatment with an anti–PD-(L)1 or anti–PD-L2 agent; or had interstitial lung disease or a history of pneumonitis that required steroid therapy.
Patients were randomized 1:1 to receive pembrolizumab 200 mg every 3 weeks or saline placebo for up to 35 cycles. For the first four cycles, all patients received pembrolizumab or placebo intravenously on day 1 of each 21-day cycle and carboplatin (area under the concentration–time curve, 6 mg/mL/min) plus paclitaxel (200 mg/m2 on d 1 of each cycle) or nab-paclitaxel (100 mg/m2 on d 1, 8, and 15 of each cycle). Treatment with pembrolizumab or placebo continued until completion of 35 cycles, documented disease progression, unacceptable adverse events (AEs), intercurrent illness, investigator’s decision, or withdrawal of consent. Randomization was stratified by PD-L1 expression (TPS ≥1% versus <1%), by choice of taxane (paclitaxel versus nab-paclitaxel), and, in the global study, by geographic region (East Asia versus non-East Asia). Patients with unassessable PD-L1 status were included in the group with TPS less than 1%. Because positive results for PFS and OS (dual primary end points) were observed in the final analysis in the global study and in the interim analysis in the China extension study, this study was unblinded, allowing patients in the placebo–chemotherapy group with documented disease progression verified by blinded independent central review (BICR) per RECIST version 1.1 to cross over to receive open-label pembrolizumab monotherapy for up to 35 treatment cycles if they met all eligibility criteria. Patients in the pembrolizumab–chemotherapy group with confirmed radiographic progression (i.e., two scans at least 4 wk apart revealing progressive disease) on the basis of the immune-related RECIST, but were achieving a clinically meaningful benefit, and with no further increase in the tumor burden at the confirmatory tumor imaging visit, could continue treatment with pembrolizumab to complete a total of 35 treatment cycles.
The trial was conducted in compliance with local and national regulations and ethical requirements as outlined in the Declaration of Helsinki. The study protocol was approved by an institutional review board or ethics committee at each study site. All patients provided written informed consent before enrollment.
Assessments
Tumor PD-L1 expression was assessed before randomization at a central laboratory using the PD-L1 IHC 22C3 pharmDx Kit (Agilent Technologies, Carpinteria, CA) assay. Investigators and patients were blinded to PD-L1 status.
Tumor imaging was performed at weeks 6, 12, and 18 from the date of randomization. Subsequent tumor imaging was performed every 9 weeks until week 45 and then every 12 weeks thereafter. Response was assessed per RECIST version 1.1 by BICR. Patients were contacted every 12 weeks to assess survival during follow-up.
AEs were monitored from randomization to 30 days after discontinuation of treatment (90 days for serious AEs). All AEs were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
The European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire—Core 30 (EORTC QLQ-C30), EORTC QLQ—Lung Cancer Module 13 (EORTC QLQ-LC13), and the EuroQol 5-dimension 3-level questionnaire (EQ-5D-3L) were administered by trained personnel and completed electronically by the patient before treatment administration, AE evaluation, and disease status notification. PRO questionnaires were completed at cycles 1 to 7 and then every third cycle (every 9 wk) while on treatment up to 48 weeks, at the treatment discontinuation visit, and at the 30-day safety follow-up visit. The instruments were administered in the following order: EQ-5D-3L, EORTC QLQ-C30, and EORTC QLQ-LC13.17
End Points
The dual primary end points were OS and PFS assessed per RECIST version 1.1 by BICR. Secondary end points were objective response rate (ORR) and duration of response (DOR) assessed per RECIST version 1.1 by BICR and safety. Exploratory analyses included assessment of OS and PFS by PD-L1 status and PFS2 (defined as the time from randomization to objective tumor progression on next-line treatment, or death, whichever occurred first). PROs were evaluated as prespecified exploratory end points. As previously described,17 key PRO end points were the mean score change from baseline to week 9 and week 18 using the EORTC QLQ-C30 global health status (GHS)/quality of life (QoL) scale and the time to deterioration (TTD) in the composite end point of cough (EORTC QLQ-LC13-Q1), chest pain (EORTC QLQ-LC13-Q10), or dyspnea (EORTC QLQ-C30-Q8). TTD was defined as the time to first onset of greater than or equal to 10-point increase from baseline and confirmed by another adjacent greater than or equal to 10-point increase in any of the three symptoms under the right-censoring rule. Supportive PRO end points included mean score changes and the proportion of patients with “deteriorated,” “stable,” or “improved” scores from baseline to weeks 9 and 18 in the subscales of EORTC QLQ-C30 and EORTC QLQ-LC13. Patients’ postbaseline PRO scores were defined as “stable,” “improved,” or “deteriorated” according to a greater than or equal to 10-point change for each of the instruments or scales from baseline, as perceived to be clinically meaningful by patients.18
Statistical Analysis
This report includes data from the protocol-specified final analysis of the KEYNOTE-407 China extension study. Approximately 120 patients were planned for enrollment in mainland China in KEYNOTE-407. Statistical analyses for this trial have been previously described.7,16 Efficacy was assessed in the intention-to-treat population, which included all randomized patients. Safety was assessed in the as-treated population and included all patients who received at least one dose of study treatment. OS and PFS were estimated using the nonparametric Kaplan-Meier method. The magnitude of difference between the treatment groups (HRs and 95% CIs) was assessed using the stratified Cox proportional hazards model and the Efron method of tie handling. Between-group differences in OS and PFS were evaluated by the stratified log-rank test. For subgroup analysis of OS and PFS, patients with unassessable PD-L1 TPS were not included. ORRs for the two treatment groups were compared on the basis of the Miettinen and Nurminen method. DOR was summarized descriptively using the Kaplan-Meier method. No alpha was allocated to the China analysis. PRO analyses included all randomly assigned patients who received at least one dose of study treatment and completed at least 1 PRO assessment. Full statistical analysis methods for PRO analyses have been previously described; between-group comparisons were noted as differences in the least squares (LS) mean change from baseline with 95% CI.17
Results
Patients and Treatments
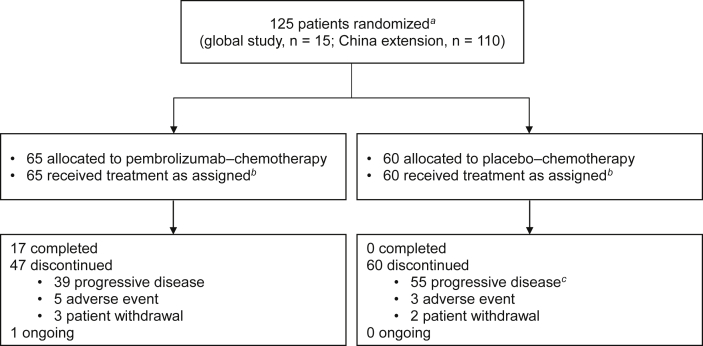
Of 182 patients screened at 18 sites in the People’s Republic of China between April 21, 2017, and August 28, 2018, a total of 125 eligible patients (15 in the global study; 110 in the extension study) were randomized to pembrolizumab–chemotherapy (n = 65) or placebo–chemotherapy (n = 60; Fig. 1 and Supplementary Table 1). All eligible patients received at least one dose of allocated treatment.
Figure 1.
Summary of enrollment and disposition of Chinese patients in the KEYNOTE-407 China study. aRandomization occurred centrally using an interactive voice response system or integrated web response system. bAll patients who received treatment were included in the ITT, safety, and PRO population analyses. cIncludes patients with clinical progression or progressive disease. ITT, intention-to-treat; PRO, patient-reported outcome.
The baseline characteristics and demographics were well balanced between the treatment groups, with the exception of Eastern Cooperative Oncology Group performance status of 1 (pembrolizumab–chemotherapy, 69.2%; placebo–chemotherapy, 81.7%). All patients received paclitaxel as the taxane chemotherapy (Table 1).
Table 1.
Patient Baseline Demographic and Disease Characteristics in the Intention-to-Treat Population
| Characteristics | Pembrolizumab Plus Chemotherapy n = 65 | Placebo Plus Chemotherapy n = 60 |
|---|---|---|
| Age, y | ||
| Median (range) | 63.0 (31‒76) | 63.0 (39‒78) |
| Men, n (%) | 62 (95.4) | 57 (95.0) |
| ECOG PS, n (%) | ||
| 0 | 20 (30.8) | 11 (18.3) |
| 1 | 45 (69.2) | 49 (81.7) |
| Smoking status, n (%) | ||
| Current or former | 60 (92.3) | 54 (90.0) |
| Never | 5 (7.7) | 6 (10.0) |
| Histology, n (%) | ||
| Squamous | 64 (98.5) | 60 (100.0) |
| Adenosquamous | 1 (1.5) | 0 |
| Brain metastases, n (%) | 1 (1.5) | 3 (5.0) |
| PD-L1 TPS, n (%) | ||
| <1% | 25 (38.5) | 23 (38.3) |
| ≥1% | 37 (56.9) | 35 (58.3) |
| 1%–49% | 15 (23.1) | 20 (33.3) |
| ≥50% | 22 (33.8) | 15 (25.0) |
| Could not be evaluated | 3 (4.6) | 2 (3.3) |
| Taxane chemotherapy | ||
| Paclitaxel | 65 (100.0) | 60 (100.0) |
| Previous therapy, n (%) | ||
| Neoadjuvant or adjuvant therapy | 3 (4.6) | 2 (3.3) |
| Thoracic radiotherapy | 1 (1.5) | 1 (1.7) |
ECOG PS, Eastern Cooperative Oncology Group performance status; PD-L1, programmed death ligand 1; TPS, tumor proportion score.
As of the data cutoff of September 30, 2020, median (range) time from randomization to the date of database cutoff was 28.1 (25.1‒40.9) months. Median (range) duration of treatment was 11.0 months (1 d‒28.2 mo) in the pembrolizumab–chemotherapy group and 3.9 months (1 d‒17.3 mo) in the placebo–chemotherapy group. In the pembrolizumab–chemotherapy arm, 28 patients (43.1%) received subsequent therapy, including four (6.2%) who received at least one subsequent anti‒PD-(L)1 therapy. Of the 60 patients initially allocated to placebo–chemotherapy, 46 patients (76.7%) received subsequent therapy, including 39 (65.0%) who received subsequent anti‒PD-(L)1 therapy, 38 (63.3%) of whom received pembrolizumab in on-study crossover and one patient received anti‒PD-(L)1 therapy off-study. At the time of data cutoff, one patient in the pembrolizumab–chemotherapy group and no patients in the placebo–chemotherapy group remained on the study treatment (Fig. 1).
Efficacy in the Intention-to-Treat Population
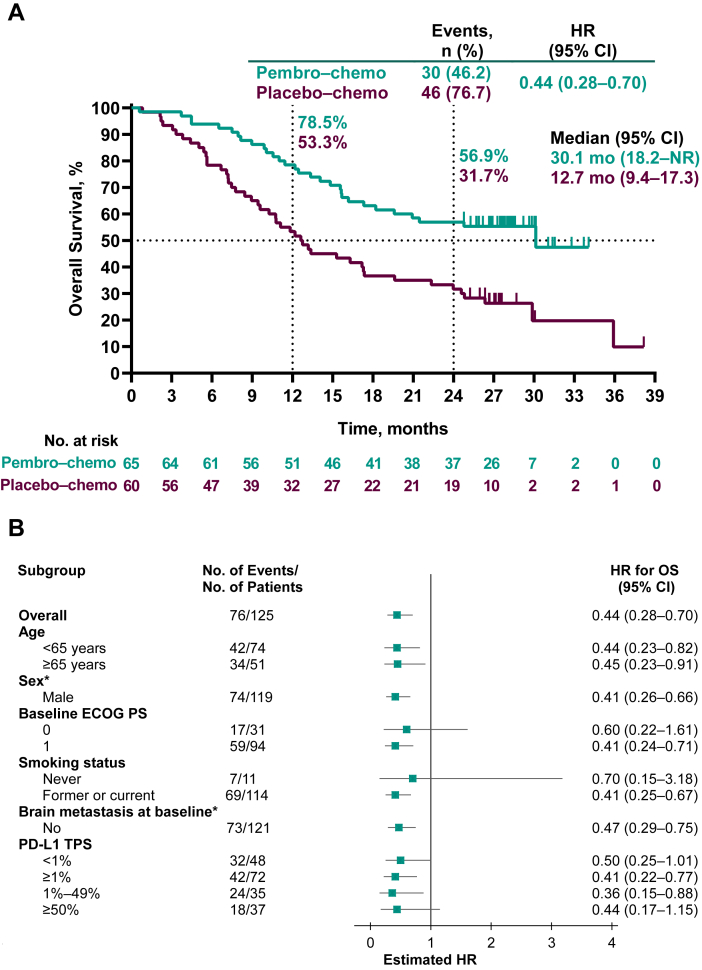
At the time of data cutoff, 30 deaths had occurred in the pembrolizumab–chemotherapy group and 46 in the placebo–chemotherapy group. Median (95% CI) OS was 30.1 (18.2–not reached [NR]) months in the pembrolizumab–chemotherapy group and 12.7 (9.4–17.3) months in the placebo–chemotherapy group (HR = 0.44, 95% CI: 0.28–0.70). The 2-year OS rates were 56.9% and 31.7%, respectively (Fig. 2A). HRs for OS favored pembrolizumab–chemotherapy across most patient subgroups, including patients with PD-L1 TPS greater than or equal to 1% and PD-L1 TPS less than 1% (Fig. 2B).
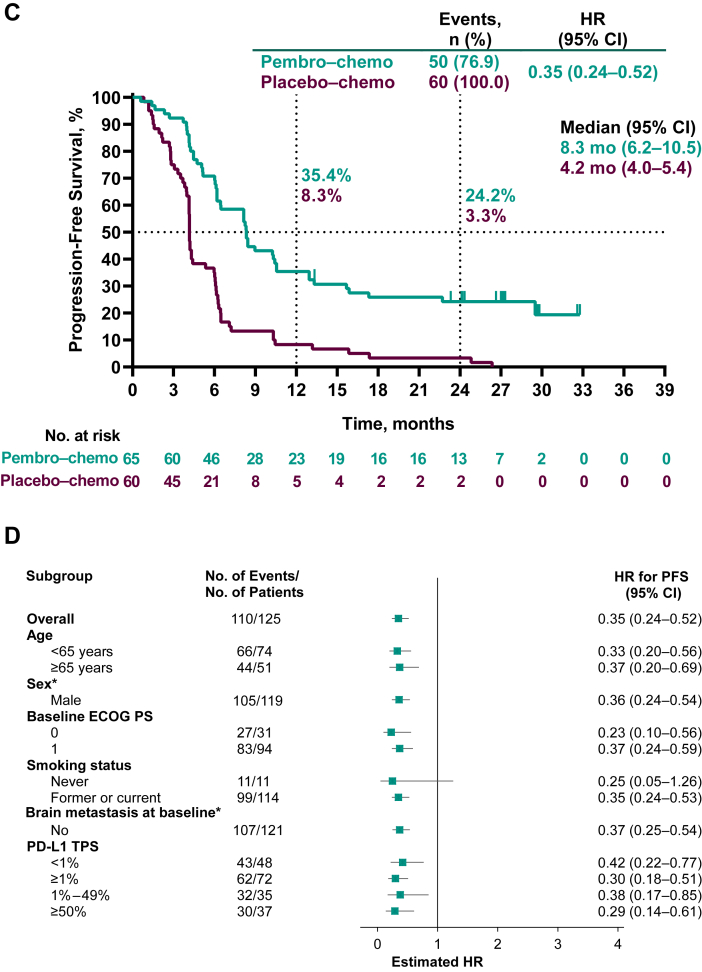
Figure 2.
Kaplan-Meier analysis of overall survival (A) in the intention-to-treat population and (B) in key patient subgroups and progression-free survival assessed by BICR per RECIST version 1.1 (C) in the intention-to-treat population and (D) in key patient subgroups. Patients with tumors who were not assessable for PD-L1 TPS were not included in the relevant subgroup analyses. ∗Data not presented for subgroups of “Female” (n = 6) and for patients with brain metastasis at baseline (n = 4) owing to very few patients which precludes any meaningful analysis. BICR, blinded independent central review; Chemo, chemotherapy; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; pembro, pembrolizumab; NR, not reached; OS, overall survival; PD-L1, programmed death-ligand 1; RECIST, Response Evaluation Criteria in Solid Tumors; TPS, tumor proportion score.
A total of 50 events of disease progression or death had occurred in the pembrolizumab–chemotherapy group and 60 events in the placebo–chemotherapy group at the time of data cutoff. Median (95% CI) PFS was 8.3 (6.2–10.5) months in the pembrolizumab–chemotherapy group and 4.2 (4.0–5.4) months in the placebo–chemotherapy group (HR = 0.35, 95% CI: 0.24–0.52, Fig. 2C). The 2-year PFS rates were 24.2% and 3.3%, respectively. HRs for PFS favored pembrolizumab–chemotherapy versus placebo–chemotherapy across most patient subgroups and regardless of PD-L1 TPS (Fig. 2D). In addition, median (95% CI) PFS2 was NR (15.6–NR) in the pembrolizumab–chemotherapy group and was 8.7 (7.4–10.5) months in the placebo–chemotherapy group (HR = 0.27, 95% CI: 0.18–0.43).
Overall, 52 patients in the pembrolizumab–chemotherapy group and 26 patients in the placebo–chemotherapy group achieved an objective response, for an ORR of 80.0% (95% CI: 68.2–88.9) versus 43.3% (95% CI: 30.6–56.8) (Table 2). Among the responders, two patients (3.1%) had complete response and 50 (76.9%) had partial responses in the pembrolizumab–chemotherapy group; no patient had a complete response and 26 patients (43.3%) had partial responses in the placebo–chemotherapy group. In addition, seven patients (10.8%) and 23 patients (38.3%) had stable disease in each respective treatment group. Median (range) DOR was 7.1 (1.7+ to 29.6+) months in the pembrolizumab–chemotherapy group and 3.5 (2.4+ to 9.0) months in the placebo–chemotherapy group. Overall, an estimated 33.0% of responders in the pembrolizumab–chemotherapy group had DOR greater than or equal to 18 months, whereas no patient in the placebo–chemotherapy group had DOR greater than or equal to 18 months (Table 2).
Table 2.
Summary of Confirmed Tumor Response per RECIST Version 1.1 by BICR in the Intention-to-Treat Population
| Tumor Response | Pembrolizumab Plus Chemotherapy n = 65 | Placebo Plus Chemotherapy n = 60 |
|---|---|---|
| Objective response rate,a % (95% CI) | 80.0 (68.2‒88.9) | 43.3 (30.6‒56.8) |
| Best overall response, n (%) | ||
| Complete response | 2 (3.1) | 0 |
| Partial response | 50 (76.9) | 26 (43.3) |
| Stable disease | 7 (10.8) | 23 (38.3) |
| Progressive disease | 3 (4.6) | 9 (15.0) |
| Not evaluable | 2 (3.1) | 2 (3.3) |
| No assessment | 1 (1.5) | 0 |
| Time to response, median (range), mo | 1.4 (1.1‒8.5) | 1.4 (0.8‒2.9) |
| DOR, median (range), mo | 7.1 (1.7+ to 29.6+) | 3.5 (2.4–9.0) |
| KM estimate of patients with extended DOR, % | ||
| ≥12 mo | 37.2 | 0 |
| ≥18 mo | 33.0 | 0 |
| ≥21 mo | 31.0 | 0 |
BICR, blinded independent central review; CI, confidence interval; DOR, duration of response; KM, Kaplan-Meier; RECIST, Response Evaluation Criteria in Solid Tumors.
Objective response rate consisted of complete response and partial response.
Safety Outcomes in the Intention-to-Treat Population
All patients experienced at least one treatment-related AE. Grade 3 to 5 treatment-related AEs occurred in 81.5% of patients in the pembrolizumab–chemotherapy group and 81.7% of patients in the placebo–chemotherapy group. The most frequently reported grade 3 to 5 treatment-related AEs in either treatment group were decreased neutrophil count (61.5% versus 56.7% for pembrolizumab–chemotherapy versus placebo–chemotherapy) and decreased white blood cell count (35.4% versus 20.0%; Table 3). Overall, two patients in the pembrolizumab–chemotherapy group and three in the placebo–chemotherapy group died owing to AEs (Supplementary Table 2), two of which were considered treatment-related—pneumonia in the pembrolizumab–chemotherapy group and gastrointestinal perforation in the placebo–chemotherapy group.
Table 3.
AE Summary in All Patients as Treated
| Events | Pembrolizumab Plus Chemotherapy n = 65 | Placebo Plus Chemotherapy n = 60 | ||
|---|---|---|---|---|
| Treatment-related AE, n (%) | 65 (100.0) | 60 (100.0) | ||
| Grades 3–5 | 53 (81.5) | 49 (81.7) | ||
| Leading to treatment discontinuation | ||||
| Any treatment | 8 (12.3) | 1 (1.7) | ||
| All treatmentsa | 1 (1.5) | 1 (1.7) | ||
| Led to deathb | 1 (1.5) | 1 (1.7) | ||
| Treatment-related AEs occurring in ≥20% of patients in either treatment group, n (%) | Any grade | Grades 3–5 | Any grade | Grades 3–5 |
| Decreased white blood cell count | 51 (78.5) | 23 (35.4) | 42 (70.0) | 12 (20.0) |
| Alopecia | 50 (76.9) | 0 | 39 (65.0) | 0 |
| Decreased neutrophil count | 50 (76.9) | 40 (61.5) | 43 (71.7) | 34 (56.7) |
| Anemia | 39 (60.0) | 3 (4.6) | 42 (70.0) | 6 (10.0) |
| Hypesthesia | 29 (44.6) | 0 | 22 (36.7) | 0 |
| Decreased appetite | 24 (36.9) | 0 | 18 (30.0) | 1 (1.7) |
| Increased alanine aminotransferase | 23 (35.4) | 0 | 15 (25.0) | 1 (1.7) |
| Nausea | 23 (35.4) | 0 | 10 (16.7) | 0 |
| Arthralgia | 19 (29.2) | 1 (1.5) | 11 (18.3) | 0 |
| Increased aspartate aminotransferase | 17 (26.2) | 0 | 11 (18.3) | 1 (1.7) |
| Decreased platelet count | 16 (24.6) | 5 (7.7) | 22 (36.7) | 4 (6.7) |
| Rash | 16 (24.6) | 0 | 3 (5.0) | 0 |
| Immune-mediated AEs and infusion reactions, n (%) | 22 (33.8) | 5 (7.7)c | 6 (10.0) | 1 (1.7)c |
| Hyperthyroidism | 11 (16.9) | 0 | 1 (1.7) | 0 |
| Hypothyroidism | 9 (13.8) | 1 (1.5) | 1 (1.7) | 0 |
| Pneumonitis | 6 (9.2) | 0 | 1 (1.7) | 0 |
| Type 1 diabetes mellitus | 3 (4.6) | 3 (4.6) | 0 | 0 |
| Infusion reactions | 2 (3.1) | 0 | 2 (3.3) | 1 (1.7) |
| Thyroiditis | 2 (3.1) | 0 | 0 | 0 |
| Colitis | 1 (1.5) | 1 (1.5) | 0 | 0 |
| Myositis | 0 | 0 | 1 (1.7) | 0 |
AE, adverse event.
Includes patients who discontinued pembrolizumab or placebo, carboplatin, and paclitaxel owing to an AE.
AEs leading to death that were attributed to the study treatment by the investigator(s) were pneumonia (n = 1) in the pembrolizumab–chemotherapy group and gastrointestinal perforation (n = 1) in the placebo–chemotherapy group.
There were no deaths owing to immune-mediated AEs and infusion reactions.
Immune-mediated AEs and infusion reactions occurred in 22 patients (33.8%) in the pembrolizumab–chemotherapy group and six (10.0%) in the placebo–chemotherapy group. There were no immune-mediated AEs or infusion reactions that led to death in either treatment group. Grade 3 or 4 immune-mediated AEs and infusion reactions occurred in five patients (7.7%) in the pembrolizumab–chemotherapy group (type 1 diabetes mellitus, n = 3; colitis and hypothyroidism, n = 1 each) and one patient (1.7%; infusion reaction) in the placebo–chemotherapy group (Table 3).
Outcomes in Patients Who Crossed Over to Pembrolizumab Monotherapy On-Study
A total of 38 patients crossed over from placebo–chemotherapy to pembrolizumab monotherapy on-study after disease progression. Of these, 34 patients (89.5%) had discontinued pembrolizumab and four (10.5%) were ongoing at the time of data cutoff. Median OS from the time of crossover to pembrolizumab was 12.7 (95% CI: 6.8‒17.7) months, and the 6-month OS rate was 76.3%. ORR (based on RECIST version 1.1 by investigator assessment) in patients who crossed over to on-study pembrolizumab was 10.5%; no patient had a complete response, and four patients had partial responses. Median DOR was NR (range: 3.7 to 16.6+ mo), and an estimated 75.0% had DOR greater than or equal to 12 months.
AEs of any grade occurred in 32 of 38 patients (84.2%) who crossed over to pembrolizumab on-study, with grade 3 to 5 AEs occurring in nine patients (23.7%). Treatment-related AEs occurred in 25 patients (65.8%), with grade 3 to 5 treatment-related AEs occurring in three patients (7.9%; acquired tracheo-esophageal fistula, autoimmune hepatitis, pneumonia, and pneumonitis, n = 1 each). Immune-mediated and infusion reactions occurred in seven patients (18.4%), including two patients (5.3%) with grade 3 to 5 events. One patient died from an immune-mediated AE of pneumonitis.
PROs in the Intention-to-Treat Population
The PRO analysis population consisted of all 125 patients who received at least one study dose and completed at least 1 EORTC QLQ-C30 assessment or at least 1 EORTC QLQ-LC13 assessment. Completion and compliance rates for both EORTC QLQ-C30 and EORTC QLQ-LC13 were 98.5% to 100% at baseline and remained high at weeks 9 and 18 across both treatment groups (Supplementary Table 3).
Baseline mean (SD) EORTC QLQ-C30 GHS/QoL scale scores were similar between the pembrolizumab–chemotherapy group (74.3 [21.2]) and placebo–chemotherapy group (77.6 [15.1]). At week 9, the LS mean change in EORTC QLQ-C30 GHS/QoL scale scores from baseline was 1.8 (95% CI: –3.1 to 6.7) in the pembrolizumab–chemotherapy group and negative 4.9 (95% CI: –10.0 to 0.1) in the placebo–chemotherapy group. The LS mean difference at week 9 was 6.7 points (95% CI: 0.2–13.2) between the pembrolizumab–chemotherapy versus placebo–chemotherapy groups. At week 18, the LS mean change in GHS/QoL scale scores from baseline was 6.8 (95% CI: 2.0–11.7) in the pembrolizumab–chemotherapy group and negative 0.8 (95% CI: –6.1 to 4.5) in the placebo–chemotherapy group. The LS mean difference in scores between the treatment groups at week 18 was 7.6 points (95% CI: 1.5–13.7; Table 4 and Supplementary Fig. 1).
Table 4.
Mean Change From Baseline in the EORTC QLQ-C30 GHS/QoL Scale Score
| Assessment | Pembrolizumab Plus Chemotherapy n = 65 | Placebo Plus Chemotherapy n = 60 |
|---|---|---|
| Baseline | ||
| Completed questionnaire, n | 64 | 60 |
| Mean (SD) score | 74.3 (21.2) | 77.6 (15.1) |
| Week 9 | ||
| Completed questionnaire, n | 52 | 49 |
| Mean (SD) score | 77.7 (16.6) | 72.6 (20.2) |
| Change from baselinea | ||
| Included in analysis, n | 65 | 60 |
| LS mean score (95% CI) | 1.8 (–3.1 to 6.7) | –4.9 (–10.0 to 0.1) |
| Difference in LS mean (95% CI) | 6.7 (0.2‒13.2) | |
| Week 18 | ||
| Completed questionnaire, n | 55 | 42 |
| Mean (SD) score | 82.9 (13.7) | 76.2 (17.6) |
| Change from baselinea | ||
| Included in analysis, n | 65 | 60 |
| LS mean score (95% CI) | 6.8 (2.0‒11.7) | –0.8 (–6.1 to 4.5) |
| Difference in LS mean (95% CI) | 7.6 (1.5‒13.7) | |
CI, confidence interval; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30; GHS/QoL, global health status/quality of life; LS, least squares; PRO, patient-related outcome.
Based on constrained longitudinal data analysis model with the PRO scores as the response variable and treatment by study visit interaction as covariates.
Median TTD was NR in the pembrolizumab–chemotherapy group (95% CI: NR–NR) and 3.5 months (95% CI: 2.9–NR) in the placebo–chemotherapy group (HR = 0.50, 95% CI: 0.28–0.89). Furthermore, 20 patients (30.8%) in the pembrolizumab–chemotherapy group and 30 (50.0%) in the placebo–chemotherapy group experienced deterioration in the composite end point of cough, chest pain, or dyspnea from baseline to the time of data cutoff (Supplementary Fig. 2).
Minimal changes from baseline were observed in EORTC QLQ-C30 physical, cognitive, role, and emotional scale LS mean scores at weeks 9 and 18 in the pembrolizumab–chemotherapy group, but scores declined in social functioning at both time points. In the placebo–chemotherapy group, scores declined from baseline in physical and social functioning at both time points and declined in role functioning at week 18 (Supplementary Fig. 1). Similarly, there were minimal changes from baseline in all EORTC QLQ-C30 symptom scales at week 9 and improvement in symptoms of pain and dyspnea at week 18 in the pembrolizumab–chemotherapy group. In the placebo–chemotherapy group, there was an increase in fatigue and nausea and vomiting at week 9 and in dyspnea and appetite loss at week 18 (Supplementary Fig. 1). At all time points, changes in LS mean scores from baseline in symptom scales were more favorable in the pembrolizumab–chemotherapy group compared with the placebo–chemotherapy group; further illustrated in Supplementary Figure 3 depicting less deterioration in scores in pembrolizumab–chemotherapy group versus the placebo–chemotherapy group.
Fewer patients had deteriorated GHS/QoL scale scores in the pembrolizumab–chemotherapy group versus placebo–chemotherapy at week 9 (23.1% versus 28.3%) and week 18 (16.9% versus 35.0%) (Supplementary Fig. 3). More patients had improved GHS/QoL scale scores in the pembrolizumab–chemotherapy group versus placebo–chemotherapy at week 9 (29.2% versus 15.0%) and week 18 (36.9% versus 25.0%) (Supplementary Fig. 3). Overall, during 45 weeks of follow-up, mean EORTC QLQ-C30 GHS/QoL scale scores were generally maintained or improved from baseline in the pembrolizumab–chemotherapy group but worsened in the placebo–chemotherapy group after 27 weeks (Supplementary Fig. 4).
Discussion
In this analysis of Chinese patients enrolled in the KEYNOTE-407 global and extension studies, patients were generally found to derive similar treatment benefits as observed in the global KEYNOTE-407 study.7,16 Treatment with pembrolizumab plus carboplatin-paclitaxel substantially improved OS and PFS compared with placebo plus chemotherapy in Chinese patients with previously untreated metastatic squamous NSCLC, irrespective of PD-L1 TPS. Furthermore, pembrolizumab–chemotherapy was associated with higher ORR versus placebo–chemotherapy, and the median DOR with pembrolizumab–chemotherapy was more than twice that for placebo–chemotherapy. Pembrolizumab–chemotherapy had manageable toxicity, and no new safety signals were identified. HRQoL outcomes, relative to baseline, were improved or maintained in a greater proportion of patients in the pembrolizumab–chemotherapy group versus placebo–chemotherapy. Overall, these results support the use of pembrolizumab–chemotherapy as a standard-of-care first-line treatment in Chinese patients with metastatic squamous NSCLC.
The improvements in OS, PFS, ORR, and PFS2 observed with pembrolizumab–chemotherapy in the China study were generally consistent with those observed in the global KEYNOTE-407 study,7,16 despite differences in duration of median follow-up times and patient populations. In the China study, the HRs (95% CI) for OS and PFS were 0.44 (0.28–0.70) and 0.35 (0.24–0.52) and were 0.71 (0.58–0.88) and 0.57 (0.47–0.69) in the protocol-specified final analysis of the global KEYNOTE-407 study, respectively.16 The HRs were very similar in a longer-term follow-up analysis from the KEYNOTE-407 global study19 which had the same database cutoff date as the KEYNOTE-407 China study but with longer median follow-up time (40.1 mo versus 28.1 mo) because of earlier enrollment of patients, as per the protocol. Although the CIs were wider in the China study compared with the global study owing to the smaller sample size, the overall trends in outcomes were consistent between the studies. Together, these findings support the efficacy of pembrolizumab–chemotherapy in patients with previously untreated metastatic squamous NSCLC in the People’s Republic of China.
These findings are particularly encouraging in light of an effective crossover rate of 65.0%. At the time of data cutoff, 38 of 60 patients in the placebo–chemotherapy arm had crossed over to pembrolizumab on-study. Outcomes in these patients suggest antitumor activity of pembrolizumab monotherapy in the second line, with an ORR of 10.5% and median OS of 12.7 months; however, the survival outcomes observed in patients who received pembrolizumab–chemotherapy in this study suggest considerably greater benefit of receiving pembrolizumab in the first-line setting.
Pembrolizumab–chemotherapy was found to have manageable safety in the KEYNOTE-407 China study, and no new safety signals were identified. The frequency of AEs was similar between the treatment groups, including the incidence of immune-mediated AEs and infusion reactions, and was consistent with that reported in the global KEYNOTE-407 study.7,16
Pembrolizumab–chemotherapy maintained or improved HRQoL relative to baseline and improved HRQoL in comparison with placebo–chemotherapy at week 9 and week 18, as revealed by the LS mean differences of 6.7 and 7.6 in GHS/QoL scale scores, respectively. The differences were clinically meaningful at both time points, as supported by the findings that fewer patients reported deteriorated GHS/QoL scale scores at week 9 and week 18 in the pembrolizumab–chemotherapy group versus placebo–chemotherapy and more patients reported improved GHS/QoL scale scores at week 9 and week 18, respectively. Moreover, although a greater than or equal to 10-point change from baseline is generally perceived to be clinically meaningful,18 differences of up to 4 points have been noted as minimal important differences for GHS/QoL scale scores in patients with NSCLC,20 which further supports our findings.
The results of the symptom scales indicate a trend favoring the pembrolizumab–chemotherapy group as minimal changes from baseline were observed in almost all EORTC QLQ-C30 symptom scales in the pembrolizumab–chemotherapy group, whereas some worsening in fatigue, dyspnea, and appetite loss was reported in the placebo–chemotherapy group at week 9 or 18. Delay in symptom deterioration was further supported by the prolonged TTD for symptoms of cough, chest pain, or dyspnea in the pembrolizumab–chemotherapy group versus placebo–chemotherapy (HR = 0.50). The PRO findings from the KEYNOTE-407 China study are consistent with those from the KEYNOTE-407 global study17 and support improved efficacy of pembrolizumab–chemotherapy versus placebo–chemotherapy while maintaining HRQoL in this population.
In line with our study, similar benefits have been reported in the KEYNOTE-042 China study with pembrolizumab monotherapy in Chinese patients with previously untreated NSCLC with PD-L1 TPS greater than or equal to 1%, regardless of squamous or nonsquamous NSCLC.15 A recent phase 3 study of tislelizumab (anti‒PD-1 monoclonal antibody) plus platinum-based chemotherapy reported significantly longer PFS (p < 0.001) and higher ORR (∼75% versus 50%) versus chemotherapy alone in patients with locally advanced or metastatic squamous NSCLC. Because of short follow-up time (median follow-up = 8.6 mo), OS data were not reported in this study.21
Our study had certain limitations. As noted previously, the KEYNOTE-407 China study enrolled fewer patients than the global study and was not powered for formal comparisons in outcomes between the two studies and within key patient subgroups, including in patients with brain metastasis and by PD-L1 TPS. Nonetheless, analyses of OS and PFS in the intention-to-treat population and by PD-L1 TPS (≥1% and <1%) were consistent with those observed in the global KEYNOTE-407 study,16 albeit, the patient numbers were relatively small in some of the PD-L1 TPS subgroups and the confidence intervals were wider precluding more definitive conclusions.
Notably, the combination of pembrolizumab plus carboplatin and paclitaxel was approved in the People’s Republic of China for the first-line treatment of patients with metastatic squamous NSCLC on the basis of results from the global and China KEYNOTE-407 studies.22
In conclusion, pembrolizumab–chemotherapy as first-line treatment improved OS and PFS with durable long-term benefit compared with placebo–chemotherapy, regardless of PD-L1 expression, in patients with previously untreated metastatic squamous NSCLC enrolled in mainland China. The toxicity was manageable and HRQoL was preserved or improved relative to baseline with pembrolizumab–chemotherapy compared with placebo–chemotherapy. These findings are consistent with those from the global KEYNOTE-407 study and support the use of pembrolizumab–chemotherapy as a standard-of-care first-line therapy for patients with previously untreated metastatic squamous NSCLC in the People’s Republic of China.
CRediT Authorship Contribution Statement
Ying Cheng, Li Zhang, Jie Hu, Donglin Wang, ChengPing Hu, Jianying Zhou, Lin Wu, Lejie Cao, Jiwei Liu, Helong Zhang, Hong Sun, Ziping Wang, Hongjun Gao, Luis Paz-Ares: Investigation, Writing - review & editing.
Yuping Sun, Xiaohan Hu: Formal analysis, Writing - review & editing.
Ben Li, Paul Schwarzenberger: Data curation, Formal analysis, Writing - review & editing.
Data Sharing Statement
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Acknowledgments
This work was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey. Representatives of the funder participated in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The authors thank the patients and their families and caregivers for participating in the studies, along with all investigators and site personnel. Study support was provided by Hui Wang, MA, Yu Tian, Master, Clinical Medicine, and Zhe Ya Sun Bachelor, Accounting and Veterinary Medicine, of MSD China; Diana Chirovsky, PhD, and Nabeegha Shinwari, PharmD, of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey. Medical writing and editorial assistance were provided by Christabel Wilson, MSc, of ICON plc (North Wales, PA); this assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey.
Footnotes
Disclosure: Drs. Sun and Li are employees of MSD China. Drs. Hu and Schwarzenberger are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, and stockholders of Merck & Co., Inc., Kenilworth, New Jersey. Dr. Paz-Ares reports honoraria to self/spouse for scientific advice or as a speaker for Adacap, Amgen, AstraZeneca, Bayer, Blueprint Medicines, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Incyte, Ipsen, Lilly, Merck, MSD, Novartis, PharmaMar, Pfizer, Roche, Sanofi, Servier, and Sysmex; being a board member for Genómica; and receipt of grants to institution from AstraZeneca, Bristol-Myers Squibb, MSD, and Pfizer. The remaining authors declare no conflict of interest.
Previous presentation: A portion of the data from this analysis have been accepted for an oral presentation at the 2021 Chinese Society of Clinical Oncology Annual Meeting, Beijing, People’s Republic of China; September 25 to 29, 2021 (abstract identification 10128). A portion of these data was also presented at the International Association for the Study of Lung Cancer 2021 World Conference on Lung Cancer; Worldwide Virtual Event, September 8 to 14, 2021 (abstract number 650; P18).
Cite this article as: Cheng Y, Zhang L, Hu J, et al. Pembrolizumab plus chemotherapy for Chinese patients with metastatic squamous NSCLC in KEYNOTE-407. JTO Clin Res Rep. XXXX;X:XXXXXX.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2021.100225.
Supplementary Data
References
- 1.Cao M., Li H., Sun D., Chen W. Cancer burden of major cancers in China: a need for sustainable actions. Cancer Commun (Lond) 2020;40:205–210. doi: 10.1002/cac2.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borghaei H., Langer C.J., Gadgeel S. 24-month overall survival from KEYNOTE-021 cohort G: pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced nonsquamous non-small cell lung cancer. J Thorac Oncol. 2019;14:124–129. doi: 10.1016/j.jtho.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Borghaei H., Paz-Ares L., Horn L. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer J., Reckamp K.L., Baas P. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi L., Rodriguez-Abreu D., Gadgeel S. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 6.Mok T.S.K., Wu Y.L., Kudaba I. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 7.Paz-Ares L., Luft A., Vicente D. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 8.Reck M., Rodríguez-Abreu D., Robinson A.G. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 9.Rittmeyer A., Barlesi F., Waterkamp D. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Socinski M.A., Jotte R.M., Cappuzzo F. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 11.West H., McCleod M., Hussein M. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 12.Reck M., Rodríguez-Abreu D., Robinson A.G. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37:537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 13.Peng L., Wu Y.L. Immunotherapy in the Asiatic population: any differences from Caucasian population? J Thorac Dis. 2018;10(suppl 13):S1482–S1493. doi: 10.21037/jtd.2018.05.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soo R.A., Kawaguchi T., Loh M. Differences in outcome and toxicity between Asian and Caucasian patients with lung cancer treated with systemic therapy. Future Oncol. 2012;8:451–462. doi: 10.2217/fon.12.25. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y.L., Zhang L., Fan Y. Randomized clinical trial of pembrolizumab vs chemotherapy for previously untreated Chinese patients with PD-L1-positive locally advanced or metastatic non-small-cell lung cancer: KEYNOTE-042 China study. Int J Cancer. 2021;148:2313–2320. doi: 10.1002/ijc.33399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paz-Ares L., Vicente D., Tafreshi A. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. 2020;15:1657–1669. doi: 10.1016/j.jtho.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Mazieres J., Kowalski D., Luft A. Health-related quality of life with carboplatin-paclitaxel or nab-paclitaxel with or without pembrolizumab in patients with metastatic squamous non-small-cell lung cancer. J Clin Oncol. 2020;38:271–280. doi: 10.1200/JCO.19.01348. [DOI] [PubMed] [Google Scholar]
- 18.Osoba D., Rodrigues G., Myles J., Zee B., Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 19.Robinson A.G., Vicente D., Tafreshi A. First-line pembrolizumab plus chemotherapy for patients with advanced squamous NSCLC: 3-year follow-up from KEYNOTE-407. J Thorac Oncol. 2021;16(suppl):S748–S749. [Google Scholar]
- 20.Maringwa J.T., Quinten C., King M. Minimal important differences for interpreting health-related quality of life scores from the EORTC QLQ-C30 in lung cancer patients participating in randomized controlled trials. Support Care Cancer. 2011;19:1753–1760. doi: 10.1007/s00520-010-1016-5. [DOI] [PubMed] [Google Scholar]
- 21.Wang J., Lu S., Yu X. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7:709–717. doi: 10.1001/jamaoncol.2021.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Businesswire Merck’s KEYTRUDA® (pembrolizumab) now approved in China for first-Line treatment of metastatic squamous non-small cell lung cancer (NSCLC) in combination with chemotherapy. https://www.businesswire.com/news/home/20191126005394/en/Merck%E2%80%99s-KEYTRUDA%C2%AE-pembrolizumab-Now-Approved-in-China-for-First-Line-Treatment-of-Metastatic-Squamous-Non-Small-Cell-Lung-Cancer-NSCLC-in-Combination-with-Chemotherapy
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.