Abstract
Platycosides, saponins contained in balloon flower, which have been used as food health supplements for respiratory diseases, have diverse pharmacological effects. Platycosides exhibit better pharmacological activity by hydrolyzing their own sugars. However, to date, there have been no studies on the production of deglucosylated platycodin D suitable for food applications. In this study, Pluszyme 2000P, which was derived from Aspergillus niger, a food-grade microorganism, was used to completely convert platycoside E into deglucosylated platycodin D. For an efficient and economical production of deglucosylated platycodin D, the productivity was improved approximately 2.4 times by application of high hydrostatic pressure and the discarded balloon flower leaf was used as a substrate. As a result, deglucosylated platycodin D was produced with the highest concentration (3.49 mg/mL) and productivity (581.7 mg/L/h) reported so far. Our results contribute to functional saponin production and the related food industries.
Keywords: Platycoside, Pluszyme 2000P, Food additive, Aspergillus niger, Balloon flower, Medicinal herb
Platycoside; Pluszyme 2000P; Food additive; Aspergillus niger; Balloon flower; Medicinal herb.
1. Introduction
Balloon flower roots have been used as medicinal herbs in Northeast Asia due to their effectiveness against respiratory diseases, including sore throat, bronchitis, tonsillitis, asthma, and tuberculosis (Zhang et al., 2015a). Over the last decade, the interest in platycosides, which are the saponins of the balloon flower root Platycodi radix, has increased due to their diverse pharmacological properties, such as immune stimulation (Noh et al., 2019; Xie et al., 2009), anti-oxidant (Luo et al., 2007; Ryu et al., 2012), anti-inflammatory (Lee et al., 2020; Zhang et al., 2021), anti-tumor (Li et al., 2016; Yim et al., 2016), anti-allergy (Oh et al., 2010; Zhang et al., 2015b), and anti-obesity properties (Kim et al., 2019b; Zhao et al., 2008). Platycoside is a pentacyclic triterpene aglycone with two-sided sugar chains that contain glucose residues at the C-3 position and an oligosaccharide consisting of arabinose, rhamnose, xylose, and apiose at the C-28 position in the aglycone. Platycoside E is a major platycoside that accounts for more than 20% of the total balloon flower root platycosides(Yoo et al., 2011). On the other hand, it is present in about 92% of the balloon flower leaf, which is discarded during the harvesting of the balloon flower root (Figure 1).(Shin et al., 2019)
Figure 1.
Chemical structures and contents of platycosides in balloon flower leaf. Platycosides contain glycosides at the C-3 and C-28 positions. The glycosides at the C-3 position are Glc and Glc-Glc-Glc. The glycosides at the C-28 position are Arap-Rha-Xyl and Arap-Rha(-Ac)-Xyl-Api. Glc, β-d-glucopyranosyl-; Arap, α-l-arabinopyranosyl-; Rha, α-l-rhamnopyranosyl-; Rha-Ac, 3′-O-acetyl-α-l-rhamnopyranosyl-; Xyl, β-d-xylopyranosyl-; and Api, β-d-apiofuranosyl-.
The biological activity of saponins is improved by their deglycosylation, since they are more readily absorbed in the human gastrointestinal tract due to the lower molecular weight and better hydrophobicity (Park et al., 2010; Shin and Oh, 2016). Therefore, various methods such as heating (Kim et al., 2000), acid treatment (Bae et al., 2004), and biotransformation (Kim et al., 2019a; Shin et al., 2018) have been used to deglycosylate saponins. Among these, the enzymatic biotransformation of platycosides shows the highest selectivity and productivity, which has been demonstrated in diverse studies using a crude enzyme from Aspergillus niger (Wie et al., 2007), β-galactosidase from Aspergillus oryzae (Ha et al., 2010), β-glucosidase from Aspergillus usamii (Ahn et al., 2018), recombinant β-glucosidases from Caldicellulosiruptor bescii (Kil et al., 2019), Caldicellulosiruptor owensensis (Shin et al., 2019), and Dictyoglomus turgidum (Kang et al., 2019), and commercial enzymes, such as cellulase (Ha et al., 2010), laminarinase (Jeong et al., 2014), snailase (Li et al., 2012), pectinase (Ju et al., 2020), and cytolase(Shin et al., 2020). β-Glucosidase from D. turgidum was the only enzyme that produced deglucosylated platycodin D and showed the highest lipoxygenase inhibitory activity among all platycosides. However, since a recombinant enzyme was used in production, it is difficult to apply this process to the food industry.
High hydrostatic pressure (HHP) has been applied in several food processes, including the inactivation of microorganisms, extraction of functional metabolites from plants, and increasing the content of certain food components, such as vitamin and gamma-amino butyric acid. In enzymatic biotransformation, HHP was effective in improving the activity and stability of several commercial enzymes, including viscozyme, naringinase, rhamnosidase, pectinase, cellulose, and amylase. (Palaniyandi et al., 2015). However, to date, all studies on the biotransformation of platycosides have been carried out only at atmospheric pressure (AP).
In this study, the biotransformation of platycoside E into deglucosylated platycodin D was accomplished using the commercial enzyme Pluszyme 2000P derived from A. niger, a food-grade microorganism. For a more efficient production of deglucosylated platycodin D, HHP was applied in the enzymatic biotransformation of platycosides. In addition, agricultural by-products of balloon flower leaf containing high levels of platycoside E were used as a substrate for the production of deglucosylated platycodin D.
2. Materials and methods
2.1. Materials
Commercial enzymes such as Pluszyme 2000P, Cellosin AL8, Plantase UF, Sumizyme AC, Celluase KN, Plantase C150P, and Sumilact L was provided by the Bision Corporation (Seoul, Korea). Other commercial enzymes including Cellucalst 1.5L, Viscozyme L, Pectinex 5XL, Pectinex XXL, Pectinex Ultra Color, Novozyme 33095, and Novoprime B357 were purchased from Novozymes (Copenhagen, Denmark). The platycoside standards platycoside E, platycodin D3, and platycodin D were purchased from Ambo Laboratories (Daejeon, Republic of Korea). All the other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Deglucosylated platycodin D was purified from the reactants produced by Pluszyme 2000P. It was prepared by preparative high-performance liquid chromatography (Prep-HPLC, Agilent 1260; Agilent, Santa Clara, CA, USA) using a Hydrosphere C18 prep column (10 × 250 mm, 5 μm particle size; YMC, Kyoto, Japan). The column was eluted with distilled water at a flow rate of 4.5 mL/min at 30 °C and detection was conducted at 203 nm. The prepared deglucosylated platycodin D was used as a standard and a substrate.
2.2. Preparation of balloon flower leaf extract
Balloon flower leaf extract was prepared as previously reported. (Shin et al., 2019). A two-year-old balloon flower leaf harvested in Bonghwa-Gun was used to prepare the extract. The balloon flower leaf was dried for 72 h at 40 °C, and then ground using an electric grinder. A crude powder (100 g) as a particle size of about 300–700 μm was extracted and added to 1 L of 80% (v/v) methanol at 70 °C for 24 h without shaking. The methanol extract was filtered, the methanol was completely removed using a rotary evaporator, and the residue was dissolved in the same volume of distilled water. To prevent inhibition of the reactions, the sugars in the extract were removed using a Diaion HP-20 resin (Sigma-Aldrich, St. Louis, MO, USA) column. After loading 1 L of the extract onto the column, the resin that was adsorbed with platycosides was washed with 2 L of distilled water. The column was then eluted with 2 L of methanol at a flow rate of 0.5 mL/min. The methanol in the eluent was evaporated, and 1 L of distilled water was added to the residue. The resulting balloon flower leaf extract was used for the production of deglucosylated platycodin D.
2.3. Enzyme assay
Unless otherwise specified, the following reactions were performed at 60 °C in 100 mM citrate/phosphate buffer (pH 4.5) containing 0.05 mg/mL Pluszyme 2000P and 0.2 mM platycoside E for 10 min at AP (0.1 MPa) and HHP (50 MPa). The specific activities of Pluszyme 2000P for platycoside E, platycodin D3, platycodin D, and deglucosylated platycodin D were evaluated at various concentrations of the enzyme (0.05–0.5 mg/mL) and a 0.2 mM concentration of each platycoside for 10 min at 60 °C and pH 4.5. The effects of pH and temperature on the activity of Pluszyme 2000P were examined by varying the pH from 3.5 to 6.0 at 60 °C, and by varying the temperature from 40 to 70 °C at a pH of 4.5. The effect of the thermostability of Pluszyme 2000P was monitored as a function of the incubation time by maintaining the solution of enzymes at 45, 50, 55, 60, and 65 °C in citrate/phosphate buffer (pH 4.5). After incubation, the reaction samples were assayed with 0.2 mM platycoside E in citrate/phosphate buffer (pH 4.5) at 60 °C for 10 min. The effect of pressure was measured at AP (0.1 MPa) and HHP (0.1–400 MPa) using an HHP instrument (TFS-2L, Toyo-Koatsu Innoway Co. Ltd., Hiroshima, Japan). The mixtures for the reaction were immediately reacted in the HHP instrument set at each temperature and pressure, and the reaction samples were taken out from the HHP instrument and treated with an equal amount of n-butanol to terminate the reaction.
2.4. Biotransformation of platycoside E into deglucosylated platycodin D
The biotransformation of platycoside E into deglucosylated platycodin D was performed for 2 h at 60 °C in 50 mM citrate/phosphate buffer (pH 4.5) containing 0.2 mg/mL Pluszyme 2000P and 1 mM platycoside E at AP (0.1 MPa) and HHP (200 MPa). The optimal concentration of Pluszyme 2000P for the production of deglucosylated platycodin D from balloon flower leaf extract was determined by varying the concentration of the enzyme from 0.5 to 3 mg/mL using balloon flower leaf extract with 5 mg/mL platycoside E. The optimal concentration of platycoside E in the balloon flower leaf extract as a substrate was determined by varying the concentration of platycoside E from 1 to 10 mg/mL at a constant enzyme concentrations of 2.0 and 1.5 mg/mL at AP and HHP, respectively. The reactions were performed in 50 mM citrate/phosphate buffer (pH 4.5) for 3 h at 60 °C and HHP (200 MPa). The time-course reaction for converting platycoside E in balloon flower leaf extract to deglucosylated platycodin D was performed in 50 mM citrate/phosphate buffer (pH 4.5) with 2.0 and 1.5 mg/mL Pluszyme 2000P at AP and HHP, respectively, using 5 mg/mL platycoside E in balloon flower leaf extract (containing 5 mg/mL platycoside E and 0.07 mg/mL platycodin D) at 60 °C for 6 h.
2.5. High-performance liquid chromatography analysis
An equal amount of n-butanol was added to the reaction mixture to quench the reaction and extract the platycosides. Of the separated fractions, the n-butanol soluble fraction of the extract was dried to completely evaporate the butanol. The dried residues containing platycosides were dissolved in methanol and subsequently analyzed using an HPLC system (Agilent 1100) at 203 nm using a hydrosphere C18 column (4.6 × 150 mm, 5 μm particle size; YMC, Kyoto, Japan). The column was eluted at 30 °C with a gradient of acetonitrile and water from 10:90 to 40:60, from 40:60 to 90:10, from 90:10 to 10:90, and at a constant ratio of 10:90 for 30, 15, 5, and 10 min, respectively, at a flow rate of 1 mL/min. The standard solutions of platycosides (0.2–1.0 mM) were used to construct the linear calibration curves relating the logarithmic value of the peak areas to the concentrations of platycosides, and the platycoside concentrations were determined based on the curves.
2.6. Ultra performance liquid chromatography quadrupole time-of-flight tandem mass spectrometry analysis
The platycosides were identified and confirmed using an ACQUITY Ultra performance liquid chromatography (UPLC) and SYNAPT G2-Si HDMS system (Waters Co. software; Taunton, MD, USA). An ACQUITY UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 mm, Waters, MD, USA) was used for the UPLC analysis at a column temperature of 40 °C. A gradient program was employed with the mobile phase combining solvent A (0.1% formic acid in H2O) and solvent B (0.1% formic acid in acetonitrile) as follows: 0 min (A:B = 100:0), 3 min (A:B = 75:25), 4 min (A:B = 35:65), 10 min (A:B = 0:100), and 12.5 min (A:B = 0:100). A subsequent re-equilibration step (1 min) was performed before the next injection. The injection volume and flow rate used were 3 μL and 0.4 mL/min, respectively.
Quadrupole time-of-flight tandem mass spectrometry (Q-TOF/MS) was operated in positive and negative electrospray ionization (ESI) modes. The operating parameters were set as follows: cone voltage of 30 V, capillary voltage of 2 kV, and a source temperature of 100 °C. Data were recorded in the mass-to-charge (m/z) range of 50–1,200 with a scan time of 0.25 s and an interscan time of 0.02 s for 12.5 min. Data acquisition and processing were performed using Analyst software (Masslynx V4.1, Waters, MD, USA).
2.7. Statistical analyses
The means and standard errors for every experiment were quantitatively calculated with one-way analysis of variance (ANOVA) from triplicates. ANOVA was conducted by Tukey's method with a significance level of p < 0.05 using SigmaPlot 10.0 (Systat Software, Chicago, IL).
3. Results and discussion
3.1. Hydrolytic pathway of platycoside E to deglucosyl platycodin D by pluszyme 2000P
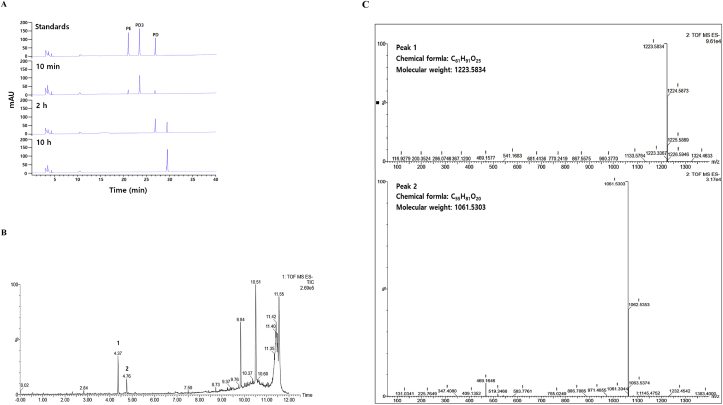
Fourteen commercial enzymes such as Pluszyme 2000P, Cellosin AL8, Plantase UF, Sumizyme AC, Celluase KN, Plantase C150P, Sumilact L, Cellucalst 1.5L, Viscozyme L, Pectinex 5XL, Pectinex XXL, Pectinex Ultra Color, Novozyme 33095, and Novoprime B357 were used to hydrolyze reagent-grade platycoside E as a substrate in this study. All enzymes except Pluszyme 2000P converted platycoside E to platycodin D3 or platycodin D without further hydrolysis (data not shown), whereas Pluszyme 2000P converted platycoside E as a substrate into an unknown platycoside via platycodin D3 and platycodin D, which were the intermediates (Figure 2A). A UPLC gradient elution with Q-TOF/MS detection of the sample that was reacted for 2 h was performed to examine the unknown platycoside. This compound was ionized in the negative mode to achieve the highest sensitivity for the chemical constituents. Figure 2B shows the total chromatogram ion (TIC) track with the unknown platycoside. Two peaks (tR = 4.37 min and 4.76 min) with the molecular formulas C61H91O25 and C55H81O20 showed similar deprotonated ions [M-H]- at m/z of 1,223.5834 and 1,061.5303, respectively (Figure 3C). The experimental mass data had a mass accuracy of less than 5 ppm compared to the theoretical value. Peaks 1 and 2 were identified as platycodin D and deglucosylated platycodin D, respectively, using an exact molecular formula that matches the fragmentation information. Thus, Pluszyme 2000P hydrolyzed the sugar moieties of platycosides via the following pathway: platycoside E → platycodin D3 → platycodin D → deglucosylated platycodin D (Figure 3).
Figure 2.
(A) HPLC profiles for the conversion of platycoside E into an unknown compound via platycodin D3 and platycodin D. (B) MS TIC chromatograms of deglucosylated platycodin D using UPLC-Q/TOF/MS in negative ion mode. (C) Extracted ion chromatogram of platycodin D (peak 1) and platycodin E (peak 2).
Figure 3.
Deglucosylation pathway of the biotransformation of platycoside E to deglucosylated platycodin D via platycodin D3 and platycodin D by Pluszyme 2000P. Glc, β-d-glucopyranosyl-; Arap, α-l-arabinopyranosyl-; Rha, α-l-rhamnopyranosyl-; Xyl, β-d-xylopyranosyl-; and Api, β-d-apiofuranosyl-.
To date, no other enzymes derived from food-grade microorganisms have been able to convert platycoside E into deglucosylated platycodin D. β-Galactosidase from A. oryzae (Ha et al., 2010), cellulase from Trichoderma reesei (Ha et al., 2010), and laminarinase from Trichoderma sp.(Jeong et al., 2014) converted platycoside E into platycodin D, which indicates that these enzymes hydrolyze only the glucose moiety at the C-3 position of the platycosides. In contrast, crude enzyme and cytolase from A. niger (Shin et al., 2020), and pectinase from Aspergillus aculeatus (Ju et al., 2020) converted platycoside E into deapiose-dexylosylated platycodin D and glucosylated platyconic acid, respectively, hydrolyzing glycosides at the C-28 position. However, the enzyme that converts into deglucosylated platycodin D is only the recombinant β-glucosidase from D. turgidum (Kang et al., 2019).
3.2. Effect of pH and temperature on enzyme activity at atmospheric pressure and high hydrostatic pressure
The maximum hydrolytic activity of Pluszyme 2000P was observed at pH 4.5, 60 °C, and AP. A similar result was obtained at HHP (50 MPa), but the relative activity under HHP conditions was approximately 1.3-fold higher than under AP conditions (Figure 4A, B). The thermostability of Pluszyme 2000P was examined in the temperature range of 45–65 °C at AP and HHP (50 MPa) (Figure 4C, D), and first-order kinetics were displayed for the thermal inactivation by the enzyme. The half-lives of the enzyme at AP were 10.4, 8.3, 6.4, 4.9, and 1.5 h, and those at HHP were 35.9, 28.6, 19.9, 14.8, and 5.8 h at 45, 50, 55, 60, and 65 °C, respectively. These results indicate that the thermostability of Pluszyme 2000P was more than 3-fold higher under HHP than under AP conditions. β-Glucosidase from D. turgidum showed a half-life of 53 h at 70 °C (Kang et al., 2019), which means that Pluszyme 2000P has a lower thermostability than this enzyme. However, the hydrolytic activity of β-glucosidase from D. turgidum was highest at 80 °C, and the half-life at that temperature was 12 h, which indicates that Pluszyme 2000P at HPP has a slightly higher stability with half-life of 14.8 h at 60 °C where the hydrolytic activity is the highest.
Figure 4.
Effects of pH and temperature on Pluszyme 2000P activity under AP and HHP conditions. (A) Effects of pH and (B) temperature on Pluszyme 2000P activity. The symbols  and
and  represent AP and HHP, respectively. (C) Thermal inactivation of Pluszyme 2000P under AP conditions. (D) Thermal inactivation of Pluszyme 2000P under HHP conditions. The enzyme was incubated at 45 (
represent AP and HHP, respectively. (C) Thermal inactivation of Pluszyme 2000P under AP conditions. (D) Thermal inactivation of Pluszyme 2000P under HHP conditions. The enzyme was incubated at 45 ( ), 50 (
), 50 ( ), 55 (
), 55 ( ), 60 (
), 60 ( ), and 65 °C (
), and 65 °C ( ). The Y-axis of the graph for thermal inactivation is expressed on a logarithmic scale. Data are represented as the means of triplicate experiments, and error bars represent the standard deviation.
). The Y-axis of the graph for thermal inactivation is expressed on a logarithmic scale. Data are represented as the means of triplicate experiments, and error bars represent the standard deviation.
3.3. Effect of pressure level on enzyme activity
To determine the most suitable pressure level to maximize the hydrolytic activity of Pluszyme 2000P for platycosides, the activity of the enzyme was evaluated at 0–400 MPa (Figure 5). The relative activity increased to 342% as the pressure increased to 200 MPa, and then decreased to approximately 8% at 400 MPa. Therefore, the activity of Pluszyme 2000P for platycosides, such as platycoside E, platycodin D3, platycodin D, and deglucosylated platycodin D, were compared at AP and HHP (200 MPa) (Table 1). The specific activity for platycosides showed the following order, regardless of the pressure value: platycoside E > platycodin D3 > platycodin D. However, there was no activity for deglucosylated platycodin D at either AP or HHP, indicating that the enzyme was unable to hydrolyze the sugars at the C-28 position, even at the pressure value that yielded the highest hydrolytic activity. At HHP (200 MPa), the highest activity was observed for platycoside E, but the highest fold increase compared to that at AP was observed for platycodin D. These results indicated that the high pressure is more effective for sugars that are more difficult to hydrolyze.
Figure 5.
Effect of pressure on Pluszyme 2000P activity. The reactions were performed in 50 mM citrate/phosphate buffer (pH 4.5) containing 0.2 mM platycoside E at 60 °C for 10 min at 0.1–400 MPa. This pressure was induced using an HHP instrument. Data are represented as the means of triplicate experiments, and error bars represent the standard deviation.
Table 1.
Specific activities of Pluszyme 2000P for platycosides in hydrolytic pathway of platycoside E into deglucosylated platycodin D under AP and HHP (200 MPa) conditions.
| Substrate | Product | Specific activity (nmol/min/mg) |
|
|---|---|---|---|
| AP | HHP | ||
| Platycoside E | Platycodin D3 | 467.5 ± 11.5 | 1598.9 ± 16.6 |
| Platycodin D3 | Platycodin D | 55.5 ± 5.1 | 216.7 ± 10.1 |
| Platycodin D | Deglucosylated platycodin D | 15.5 ± 1.5 | 89.1 ± 2.6 |
| Deglucosylated platycodin D | - | ND a | ND a |
The detection limit of deglucosylated platicodin D was calculated to be 1.64 μM.
ND, not detected.
3.4. Biotransformation of platycoside E into deglucosylated platycodin D under AP and HHP
Pluszyme 2000P converted 1 mM platycoside E into 0.39 and 1 mM deglucosylated platycodin D under AP and HHP (200 MPa) with molar conversion yields of 39 and 100%, respectively, after 2 h (Figure 6). An approximate 2.5-fold increase in productivity demonstrated that the high-pressure treatment is effective for the production of deglucosylated platycodin D from platycoside E. This result may be because activity of the enzyme was improved due to the high preference for the transition state with a smaller volume compared to the enzyme-substrate complex under high pressure(Czeslik et al., 2017). Platycodin D accumulated over time under AP conditions, but it was converted to deglucosylated platycodin D faster than the precursor platycodin D3 was converted to platycodin D under HHP conditions. As shown in Table 1, this result indicates that the activity of Pluszyme 2000P for platycodin D, in which the enzyme showed the lowest activity among the platycosides in the conversion pathway, increased with a greater fold under HHP conditions than the activities for other platycosides.
Figure 6.
Biotransformation of platycoside E ( ) into deglucosylated platycodin D (
) into deglucosylated platycodin D ( ) via platycodin D3 (
) via platycodin D3 ( ) and platycodin D (
) and platycodin D ( ) by Pluszyme 2000P at (A) AP and (B) HHP. The reactions were performed in 50 mM citrate/phosphate buffer (pH 4.5) containing 1 mM platycoside E at 60 °C for 2 h. Data are represented as the means of triplicate experiments, and error bars represent the standard deviation.
) by Pluszyme 2000P at (A) AP and (B) HHP. The reactions were performed in 50 mM citrate/phosphate buffer (pH 4.5) containing 1 mM platycoside E at 60 °C for 2 h. Data are represented as the means of triplicate experiments, and error bars represent the standard deviation.
3.5. Production of deglucosylated platycodin D from balloon flower leaf
The effect of Pluszyme 2000P concentration on the production of deglucosylated platycodin D at various concentrations (0.5–3 mg/mL) was measured using balloon flower leaf extract containing 5 mg/mL platycoside E under AP and HHP (200 MPa) conditions for 3 h (Figure 7A,B). Deglucosylated platycodin D was produced almost proportionally as the enzyme concentration increased up to 2.0 and 1.5 mg/mL at AP and HHP, respectively; however, above those concentrations, the increase in the production significantly decreased. To evaluate the effect of substrate concentration on the production of deglucosylated platycodin D, the concentration of platycoside E in the balloon flower leaf extract was adjusted from 1 to 10 mg/mL (Figure 7C, D). The reactions were performed with 2.0 and 1.5 mg/mL Pluszyme 2000P under AP and HHP (200 MPa) conditions, respectively, for 3 h. At both pressures, the production of deglucosylated platycodin D increased with an increasing substrate concentration up to 5 mg/mL, and then gradually decreased thereafter. Thus, 2.0 and 1.5 mg/mL Pluszyme 2000P at AP and HHP, respectively, and 5 mg/mL platycoside E in balloon flower leaf extract were selected as the enzyme and substrate concentrations, respectively, for the production of deglucosylated platycodin D.
Figure 7.
Effects of the enzyme and substrate concentrations on the production of deglucosylated platycodin D using balloon flower leaf extract. (A) Effect of the enzyme concentration at AP. (B) Effect of the enzyme concentration at HHP. (C) Effect of the substrate concentration at AP. (D) Effect of the substrate concentration at HHP.  , platycoside E;
, platycoside E;  , platycodin D3;
, platycodin D3;  , platycodin D;
, platycodin D;  , deglucosylated platycodin D; and ◊, molar conversion yield. Data are represented as the means of triplicate experiments, and error bars represent the standard deviation.
, deglucosylated platycodin D; and ◊, molar conversion yield. Data are represented as the means of triplicate experiments, and error bars represent the standard deviation.
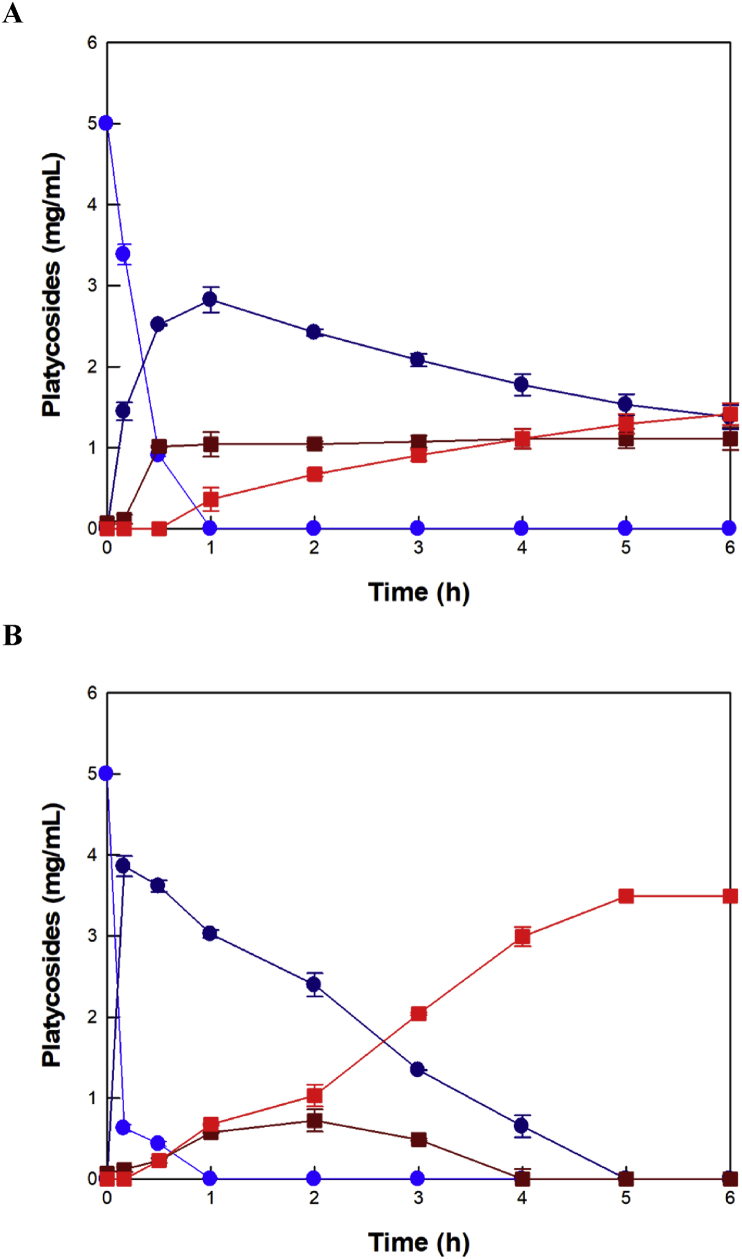
Under optimized reaction conditions, Pluszyme 2000P at HHP produced 3.49 mg/mL of deglucosylated platycodin D from 5 mg/mL platycoside E and 0.07 mg/mL platycodin D in balloon flower leaf extract in 6 h with a productivity of 581.7 mg/L/h and a molar yield of 100%, which were 2.5- and 2.4-fold higher than those at AP, respectively (Figure 8). β-Glucosidase from D. turgidum produced deglucosylated platycodin D from balloon flower root extract with a concentration of 0.96 mg/mL and a productivity of 47.8 mg/L/h (Kang et al., 2019), which were 3.6- and 12.2-fold, respectively, lower than those at HHP in this study (Table 2).
Figure 8.
Time-course reaction of the production of deglucosylated platycodin D from platycosides in balloon flower leaf extract by Pluszyme 2000P. (A) Time-course of the production of deglucosylated platycodin D at AP. (B) Time-course of the production of deglucosylated platycodin D at HHP.  , platycoside E;
, platycoside E;  , platycodin D3;
, platycodin D3;  , platycodin D; and
, platycodin D; and  , deglucosylated platycodin D. Data are represented as the means of triplicate experiments, and error bars represent the standard deviation.
, deglucosylated platycodin D. Data are represented as the means of triplicate experiments, and error bars represent the standard deviation.
Table 2.
Production of deglucosylated platycodin D from platycoside E and balloon flower extracts.
| Enzyme (Pressure) | Substrate | Concentration (mg/mL) | Productivity (mg/L/h) | Molar yield (%) | Reference |
|---|---|---|---|---|---|
| Pluszyme 2000P (AP) | Platycoside E | 0.41∗ | 207.3 | 39 | This study |
| Pluszyme 2000P (HHP) | Platycoside E | 1.06∗ | 531.5 | 100 | |
| Pluszyme 2000P (AP) | Balloon flower leaf extract | 1.41 | 235.6 | 41 | |
| Pluszyme 2000P (HHP) | Balloon flower leaf extract | 3.49 | 581.7 | 100 | |
| β-Glucosidase for D. turgidum | Platycoside E | 0.69∗ | 98.7 | 100 | (Kang et al., 2019) |
| β-Glucosidase for D. turgidum | Balloon flower root extract | 0.96∗ | 47.8 | 100 |
Value converted from molar concentration.
4. Conclusions
Pluszyme 2000P derived from A. niger completely converted platycoside E into deglucosylated platycodin D, which has been reported to have excellent anti-inflammatory effects. HHP was applied to this enzyme to improve the biotransformation process, resulting in a productivity of approximately 2.4 times that under AP conditions. In addition, deglucosylated platycodin D was produced with the highest concentration and productivity to date from balloon flower leaf extract, which can be recycled from discarded leaves. To the best of our knowledge, this is the first application of high-pressure treatment for the biotransformation of platycosides, which will greatly contribute to functional saponin production.
Declarations
Author contribution statement
Kyung-Chul Shin: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Dae Wook Kim: Performed the experiments; Analyzed and interpreted the data.
Yu Jin Oh, Min-Ju Seo: Performed the experiments.
Chae Sun Na: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Yeong-Su Kim: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by R&D Program for Forest Science Technology, Korea Forest Service (Korea Forestry Promotion Institute) (Project No. 2021400A00-2125-CA02), and Konkuk University (KU Research Professor Program).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Ahn H.J., You H.J., Park M.S., Johnston T.V., Ku S., Ji G.E. Biocatalysis of platycoside E and platycodin D3 using fungal extracellular β-glucosidase responsible for rapid platycodin D production. Int. J. Mol. Sci. 2018;19:2671. doi: 10.3390/ijms19092671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae E.A., Han M.J., Kim E.J., Kim D.H. Transformation of ginseng saponins to ginsenoside Rh2 by acids and human intestinal bacteria and biological activities of their transformants. Arch Pharm. Res. (Seoul) 2004;27:61–67. doi: 10.1007/BF02980048. [DOI] [PubMed] [Google Scholar]
- Czeslik C., Luong T.Q., Winter R. Enzymatic activity under pressure. MRS Bull. 2017;42:738–742. [Google Scholar]
- Ha I.J., Ha Y.W., Kang M., Lee J., Park D., Kim Y.S. Enzymatic transformation of platycosides and one-step separation of platycodin D by high-speed countercurrent chromatography. J. Separ. Sci. 2010;33:1916–1922. doi: 10.1002/jssc.200900842. [DOI] [PubMed] [Google Scholar]
- Jeong E.K., Ha I.J., Kim Y.S., Na Y.C. Glycosylated platycosides: identification by enzymatic hydrolysis and structural determination by LC-MS/MS. J. Separ. Sci. 2014;37:61–68. doi: 10.1002/jssc.201300918. [DOI] [PubMed] [Google Scholar]
- Ju J.H., Kang S.H., Kim T.H., Shin K.C., Oh D.K. Biotransformation of glycosylated saponins in balloon flower root extract into 3-O-beta-D-glucopyranosyl platycosides by deglycosylation of pectinase from Aspergillus aculeatus. J. Microbiol. Biotechnol. 2020;30:946–954. doi: 10.4014/jmb.2001.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.H., Kim T.H., Shin K.C., Ko Y.J., Oh D.K. Biotransformation of food-derived saponins, platycosides, into deglucosylated saponins including deglucosylated platycodin D and their anti-inflammatory activities. J. Agric. Food Chem. 2019;67:1470–1477. doi: 10.1021/acs.jafc.8b06399. [DOI] [PubMed] [Google Scholar]
- Kil T.G., Kang S.H., Kim T.H., Shin K.C., Oh D.K. Enzymatic biotransformation of balloon flower root saponins into bioactive platycodin D by deglucosylation with Caldicellulosiruptor bescii β-glucosidase. Int. J. Mol. Sci. 2019;20:3854. doi: 10.3390/ijms20163854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.A., Shin K.C., Oh D.K. Complete biotransformation of protopanaxadiol-type ginsenosides into 20-O-beta-glucopyranosyl-20(S)-protopanaxadiol by permeabilized recombinant Escherichia coli cells coexpressing beta-glucosidase and chaperone genes. J. Agric. Food Chem. 2019;67:8393–8401. doi: 10.1021/acs.jafc.9b02592. [DOI] [PubMed] [Google Scholar]
- Kim W.Y., Kim J.M., Han S.B., Lee S.K., Kim N.D., Park M.K., Kim C.K., Park J.H. Steaming of ginseng at high temperature enhances biological activity. J. Nat. Prod. 2000;63:1702–1704. doi: 10.1021/np990152b. [DOI] [PubMed] [Google Scholar]
- Kim H.L., Park J., Jung Y., Ahn K.S., Um J.Y. Platycodin D, a novel activator of AMP-activated protein kinase, attenuates obesity in db/db mice via regulation of adipogenesis and thermogenesis. Phytomedicine. 2019;52:254–263. doi: 10.1016/j.phymed.2018.09.227. [DOI] [PubMed] [Google Scholar]
- Lee S., Han E.H., Lim M.K., Lee S.H., Yu H.J., Lim Y.H. Kang, S. Fermented Platycodon grandiflorum extracts relieve airway inflammation and cough reflex sensitivity in vivo. J. Med. Food. 2020;23:1060–1069. doi: 10.1089/jmf.2019.4595. [DOI] [PubMed] [Google Scholar]
- Li W., Zhao L.C., Wang Z., Zheng Y.N., Liang J., Wang H. Response surface methodology to optimize enzymatic preparation of deapio-platycodin D and platycodin D from radix platycodi. Int. J. Mol. Sci. 2012;13:4089–4100. doi: 10.3390/ijms13044089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Tian Y.H., Liu Y., Wang Z., Tang S., Zhang J., Wang Y.P. Platycodin D exerts anti-tumor efficacy in H22 tumor-bearing mice via improving immune function and inducing apoptosis. J. Toxicol. Sci. 2016;41:417–428. doi: 10.2131/jts.41.417. [DOI] [PubMed] [Google Scholar]
- Luo H., Lin S., Ren F., Wu L., Chen L., Sun Y. Antioxidant and antimicrobial capacity of Chinese medicinal herb extracts in raw sheep meat. J. Food Protect. 2007;70:1440–1445. doi: 10.4315/0362-028x-70.6.1440. [DOI] [PubMed] [Google Scholar]
- Noh E.M., Kim J.M., Lee H.Y., Song H.K., Joung S.O., Yang H.J., Kim M.J., Kim K.S., Lee Y.R. Immuno-enhancement effects of Platycodon grandiflorum extracts in splenocytes and a cyclophosphamide-induced immunosuppressed rat model. BMC Comp. Med. Ther. 2019;19:322. doi: 10.1186/s12906-019-2724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y.C., Kang O.H., Choi J.G., Lee Y.S., Brice O.O., Jung H.J., Hong S.H., Lee Y.M., Shin D.W., Kim Y.S., Kwon D.Y. Anti-allergic activity of a platycodon root ethanol extract. Int. J. Mol. Sci. 2010;11:2746–2758. doi: 10.3390/ijms11072746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyandi S.A., Damodharan K., Lee K.W., Yang S.H., Suh J.W. Enrichment of ginsenoside Rd in Panax ginseng extract with combination of enzyme treatment and high hydrostatic pressure. Biotechnol. Bioproc. Eng. 2015;20:608–613. [Google Scholar]
- Park C.S., Yoo M.H., Noh K.H., Oh D.K. Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl. Microbiol. Biotechnol. 2010;87:9–19. doi: 10.1007/s00253-010-2567-6. [DOI] [PubMed] [Google Scholar]
- Ryu C.S., Kim C.H., Lee S.Y., Lee K.S., Choung K.J., Song G.Y., Kim B.H., Ryu S.Y., Lee H.S., Kim S.K. Evaluation of the total oxidant scavenging capacity of saponins isolated from Platycodon grandiflorum. Food Chem. 2012;132:333–337. doi: 10.1016/j.foodchem.2011.10.086. [DOI] [PubMed] [Google Scholar]
- Shin K.C., Kim D.W., Woo H.S., Oh D.K., Kim Y.S. Conversion of glycosylated platycoside E to deapiose-xylosylated platycodin D by cytolase PCL5. Int. J. Mol. Sci. 2020;21:1207. doi: 10.3390/ijms21041207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K.C., Seo M.J., Kim D.W., Yeom S.J., Kim Y.S. Characterization of β-glycosidase from Caldicellulosiruptor owensensis and its application in the production of platycodin D from balloon flower leaf. Catalysts. 2019;9:1025. [Google Scholar]
- Shin K.C., Kim T.H., Choi J.H., Oh D.K. Complete biotransformation of protopanaxadiol-type ginsenosides to 20-O-beta-glucopyranosyl-20(S)-protopanaxadiol using a novel and thermostable beta-glucosidase. J. Agric. Food Chem. 2018;66:2822–2829. doi: 10.1021/acs.jafc.7b06108. [DOI] [PubMed] [Google Scholar]
- Shin K.C., Oh D.K. Classification of glycosidases that hydrolyze the specific positions and types of sugar moieties in ginsenosides. Crit. Rev. Biotechnol. 2016;36:1036–1049. doi: 10.3109/07388551.2015.1083942. [DOI] [PubMed] [Google Scholar]
- Wie H.J., Zhao H.L., Chang J.H., Kim Y.S., Hwang I.K., Ji G.E. Enzymatic modification of saponins from Platycodon grandiflorum with Aspergillus niger. J. Agric. Food Chem. 2007;55:8908–8913. doi: 10.1021/jf0716937. [DOI] [PubMed] [Google Scholar]
- Xie Y., Sun H.X., Li D. Platycodin D is a potent adjuvant of specific cellular and humoral immune responses against recombinant hepatitis B antigen. Vaccine. 2009;27:757–764. doi: 10.1016/j.vaccine.2008.11.029. [DOI] [PubMed] [Google Scholar]
- Yim N.H., Hwang Y.H., Liang C., Ma J.Y. A platycoside-rich fraction from the root of Platycodon grandiflorum enhances cell death in A549 human lung carcinoma cells via mainly AMPK/mTOR/AKT signal-mediated autophagy induction. J. Ethnopharmacol. 2016;194:1060–1068. doi: 10.1016/j.jep.2016.10.078. [DOI] [PubMed] [Google Scholar]
- Yoo D.S., Choi Y.H., Cha M.R., Lee B.H., Kim S.J., Yon G.H., Hong K.S., Jang Y.S., Lee H.S., Kim Y.S., Ryu S.Y., Kang J.S. HPLC-ELSD analysis of 18 platycosides from balloon flower roots (Platycodi Radix) sourced from various regions in Korea and geographical clustering of the cultivation areas. Food Chem. 2011;129:645–651. doi: 10.1016/j.foodchem.2011.04.106. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wang Y., Yang D., Zhang C., Zhang N., Li M., Liu Y. Platycodon grandiflorus - an ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015;164:147–161. doi: 10.1016/j.jep.2015.01.052. [DOI] [PubMed] [Google Scholar]
- Zhang J., Song N., Liu Y., Guo J. Platycodin D inhibits beta-amyloid-induced inflammation and oxidative stress in BV-2 cells via suppressing TLR4/NF-kappaB signaling pathway and activating Nrf2/HO-1 signaling pathway. Neurochem. Res. 2021;46:638–647. doi: 10.1007/s11064-020-03198-6. [DOI] [PubMed] [Google Scholar]
- Zhang T., Yang S., Du J., Jinfu Y., Shumin W. Platycodin D attenuates airway inflammation in a mouse model of allergic asthma by regulation NF-kappaB pathway. Inflammation. 2015;38:1221–1228. doi: 10.1007/s10753-014-0089-6. [DOI] [PubMed] [Google Scholar]
- Zhao H.L., Harding S.V., Marinangeli C.P., Kim Y.S., Jones P.J. Hypocholesterolemic and anti-obesity effects of saponins from Platycodon grandiflorum in hamsters fed atherogenic diets. J. Food Sci. 2008;73:H195–H200. doi: 10.1111/j.1750-3841.2008.00915.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.