Abstract
Food allergy carries high importance and responsibility, affecting an estimated 220 million people worldwide. It is a frequent cause of food-induced anaphylaxis, a life-threatening condition requiring a toll of about one death per 50 million people a year worldwide. In order to help patients to identify allergenic foods and thus avoid anaphylactic reactions, 66 countries over the 5 continents require by law that allergenic ingredients must be declared when used in prepackaged foods. Unfortunately, the mandatory allergen list is not uniform, but varies among different countries. The widespread adoption of Precautionary Allergen Labeling (PAL) results in a proliferation of unregulated PALs with different informative statements. In this situation, the need of a scientific consensus on the definition of food allergy and the identification of a tolerable risk with routinely used detection assays, considering not only the eliciting dose but also the food source, is urgent. The aim of this manuscript is: 1) to draw a picture of the global situation in terms of PALs, and 2) to highlight new approaches that could aid in tackling the problem of regulating the labeling of allergens. These include the Voluntary Incidental Trace Allergen Labelling (VITAL) system, which intersects reference doses and labelling decisions, and a direct quantification of trace amounts of allergens at lower limit of detection (LOD) levels in the food itself through proteomics. We here highlight how, although with some limitations, the steady advances in proteomic approaches possess higher sensitivity than the recommended VITAL reference doses, allowing the identification of allergens at much lower LOD levels than VITAL. Considering that each assay used to detect allergen in food products carries method-specific issues, a more comprehensive and harmonized approach implementing both quantitative and qualitative methods could help overcoming the risk stratification approach and the overuse of PALs, offering promise as the field moves forward towards improving consumers’ quality of life.
Keywords: Food labeling, Allergies, Allergens, VITAL, Analytical methods
Introduction
In the World Allergy Organization (WAO) International Scientific Conference (WISC), streamed live on July 16−18, 2020, participants from 62 countries discussed the latest news in terms of allergic diseases. Attendees included international researchers from a wide range of disciplines including clinicians, physicians, and health professionals. In the session “Food Labeling Issues for Severe Food Allergic Patients”, the topic of Precautionary Allergen Labeling (PAL) was tackled in a multidisciplinary and translational approach, contextualizing how food allergen labeling is increasingly subject to legislative and regulatory scrutiny nationally and internationally, with regulations varying broadly across countries. Many efforts have been made by many international entities, and scientific organizations have tried to harmonize food regulations among countries using both quantitative and qualitative detection methods. The aim of this manuscript is to draw a picture of the global situation in terms of PALs, also highlighting new approaches that could aid in tackling the problem of regulating the labeling of allergens to help allergic consumers to safely enjoy the widest possible array of foods, improving their overall quality of life.
The burden of food allergy
With an estimated 220 million people suffering worldwide from food allergies, food allergy carries high importance and responsibility.1 This condition, that affects 2%–10% of the world population, is a frequent cause of anaphylaxis, a life-threatening condition requiring a toll of about 1 death per 50 million people a year worldwide.2,3 Food allergy peaks in the pediatric age. Although children allergic to milk and egg may experience 1/2 reactions per year,4 older children and adolescents experience up to 1 reaction per month when suffering from severe and complex food allergies.5
Quality of life in people with severe food allergy
Living with severe food allergy can be described as an intricate pattern of "facts" and "feelings" interwoven into a child's developmental pathway from the time of diagnosis.6 Prevalence is rising across the world, as are hospital admissions for anaphylaxis with a case fatality rate of up to 1% for medically coded food anaphylaxis, although this varies according to the definition used.7 Reliable identification of patients at increased risk of fatal food anaphylaxis is not currently possible, since the majority of symptom scores have been empirically created and data-driven instruments are scarce. It has been suggested, therefore, that health related quality of life (HRQL) should play a central role in driving treatment decisions for people with food allergy.7
Compared to other allergies, such as seasonal allergies, in food allergy the risk is higher and constantly present. Questions such as "when", "what", "why", "how much", plague the sufferers' life leading to uncertainty, fear, and anxiety.8 A decrease of HRQL is common among patients with food allergy and their caregivers.9,10 The Food Allergy Quality of Life Questionnaire (FAQLQ) series of age-appropriate and disease specific measures have been used in general and treatment settings, cross-sectionally, and longitudinally.11,12 The measures consist of multi-dimensional items and subdomains, together with questions on demographics, symptoms, reaction history, diagnosis, prescription, and use of an epinephrine auto-injector (EAI). In FAQLQ, severity is typically defined as having a prescription for an EAI, or self-reported previous episodes of anaphylaxis (ie, the symptoms “difficulty breathing”, “inability to stand”, collapse, and/or loss of consciousness). The Food Allergy Independent Measure (FAIM), used in concert with FAQLQ, provides a measure of subjective perception of severity/chance of adverse outcome. A minimal clinical important difference (MCID) score of 0.45/0.5 has been reported for the questionnaires. The most significant impact of severe food allergy on FAQLQ, across age groups, is the persistent fear of an adverse reaction, and the restrictions, vigilance, and planning that are necessary to minimize the risk. Environmental factors (clinician and general public awareness, industry policy and practices, including PAL) also play an important and dynamic role in impacting Food Allergy Quality of Life (FAQL).13,14 Furthermore, disparities exist in the economic impact of food allergy based on socioeconomic status and anxiety may relate to the medical and nonmedical costs borne by families.15
Due to the wide array of factors that may amplify the impact of severe Food Allergy (FA) and provide a barrier to self-management, a thorough assessment of FAQL, subjective perceptions of FA severity, and environmental factors is necessary when initiating treatment and other decision making with FA patients and caregivers. This is particularly important since the need for psychosocial support is largely unmet and there is insufficient number of mental health clinicians equipped to work with FA families.
International requirements for allergenic ingredients labelling
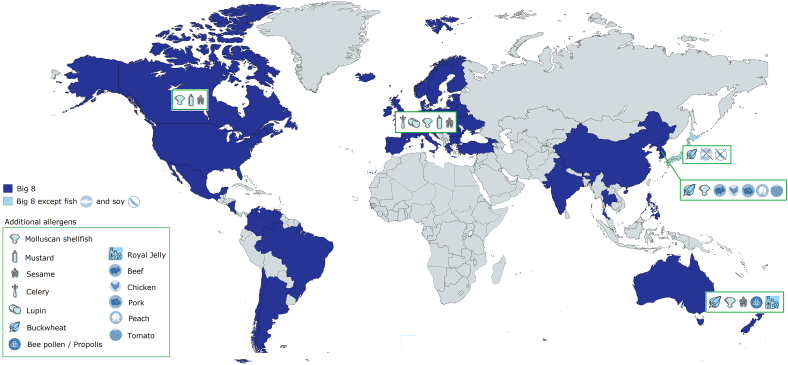
In order to help patients, identify allergenic foods and thus avoid anaphylactic reactions, 66 countries over the 5 continents require by law that allergenic ingredients have to be declared when used in prepackaged foods. Eight major food allergens (“The Big 8”) in addition to sulfites, have to be declared almost everywhere, namely eggs, milk, tree nuts, peanuts, fish, soy, wheat, and crustaceans shellfish, although some other countries such as Thailand, India, Philippines, and Hong Kong do not include specifically wheat but more generically cereals with gluten.16,17 Some other countries add cereals with gluten, molluscan shellfish, mustard, sesame (eg, Canada), plus lupin, and celery, (eg, Europe) to “The Big 8”. Korea has the most demanding legislation, adding to mandatory labeling buckwheat, molluscan shellfish, beef, chicken, peach, pork, and tomato. On the opposite, in Japan the food allergen labeling regulation has been in force for over 15 years and has been amended as needed, with currently fewer items on the mandatory allergens list (ie, crustacean shellfish, egg, milk, peanut, tree nuts, wheat, and buckwheat). In Australia and New Zealand, cereals with gluten, lupine, sesame, bee pollen/propolis, and Royal Jelly are added to the 8 main ones17,18 (Fig. 1). In South America, regulations on food allergen labeling range from "no show" (Belize, Antigua, Barbuda, Dominican Republic, Ecuador, Cuba, Jamaica) to a mandatory labeling (eg, Argentina, Brazil). Some countries adopt a “voluntary” food allergen labeling (eg, Mexico, Bolivia, and Peru). Other countries require a technical regulation on the labeling of prepackaged foods only (Nicaragua, Honduras, Guatemala, Panama, Costa Rica, Belize, El Salvador) (Table 1).17,18 The legislation, implemented 15 years ago in Europe and the United States, is a precious instrument of protection of food allergy sufferers. It eliminates the possibility of “conscious” omissions of the presence of allergens (complex ingredients, unintelligible definitions, etc.); so is the latest Mexican amendment on food labelling,19 as it includes several new allergens and a new allergen declaration for possible direct and cross contamination.
Fig. 1.
Selected examples of allergens subject to mandatory allergen labelling worldwide. In shades of blue are shown countries where the eight major allergens (“The Big 8”) must be declared if present. Country-specific allergens additionally subject to mandatory labeling are depicted with dedicated icons
Table 1.
Overview of national labeling regulations in Latin America
| Mandatory | Voluntary | Allergens Included |
|---|---|---|
| Argentina | Cuba | Cereals (containing wheat, oats, barley, rye, spelt) |
| Bolivia | Ecuador | Egg |
| Brazil | Jamaica | Crustaceans-shellfish |
| Chile | El Salvador | Fish |
| Colombia | Nicaragua | Peanut |
| Costa Rica | Honduras | Soy |
| Mexico | Guatemala | Milk |
| Venezuela | Peru | Tree nuts |
| Panama | Sulphites |
Unfortunately, the mandatory listed allergens are not uniform, but vary, in some cases greatly, among different countries. Such allergens are also identified based on different criteria: for example, in countries with well-developed food allergen labeling requirements, they are chosen based on specific health-related concerns, while in other countries they are derived from already recognized international regulations.16 Although several countries have a regulation in place governing mandatory labeling, 81 still do not have any allergen labeling requirements.18 On the other hand, a shared clinical threshold allowing the individuation of a legal limit on an allergen-by-allergen basis is not yet presently defined.20 There are also few official analytical methods and limits of determination that are accepted as analytical reference in specific countries, such in the case of polymerase chain reaction (PCR) methods for allergen detection in Germany and Japan.21 Overall, this poses a great risk for allergic people, as witnessed by the still observed reactions to food allergens, present even in countries with mandatory labeling.22
Precautionary allergen labelling
In this situation, the need of a scientific consensus on the definition of food allergy and the identification of a tolerable risk with standardized detection assays, considering not only the eliciting dose but also the food source, is urgent.23 An attempt to help with ensuring that packaged foods were as safe as possible for allergic consumers, by informing them about the possible presence of allergen contaminations in primary ingredients, preparation and storage, has been made with the voluntary use of PAL, intended as an “information on the possible and unintentional presence in food of substances or products causing allergies or intolerances, provided voluntarily by the food business operator”.24 Despite the good intentions, the widespread adoption of PAL has resulted in a proliferation of unregulated PALs with different informative statements that have generated confusion, uncertainty, and stress amongst consumers with severe allergies, further reducing their possible food choices.25,26 For these reasons, as also highlighted by other authors, PAL should be generally avoided or only used when a solid evidence-based risk management plan is present in place.16 Therefore, new approaches are required to tackle the problem of regulating the labeling of allergens to help allergic consumers to safely enjoy the widest possible array of foods. To this purpose, two possible solutions were brought up for discussion in the WISC in 2020.
The Voluntary Incidental Trace Allergen Labelling (VITAL) system
The VITAL system in intersecting reference doses and labelling decisions, allows thorough review of the allergen status of all the ingredients and the processing conditions that contribute towards the allergen status of the finished product, avoiding the indiscriminate use of PALs.25 Pioneers in this field have been the Australians, whose Allergen Bureau developed a two-grade approach intended to orient the choices of the industry in terms of labelling. According to the VITAL indications, food industry may choose to adopt in a label action level one (no precautionary statement) or two ( …. may be present). Every specific recommendation is based on reference doses for the specific food allergen, taken from the results of diagnostic Oral Food Challenges (OFCs). The labeling outcomes of the latest version of VITAL (ie, 3.0) are based on both reference doses and reference amounts of foods that, taken together, establish an action level underlying a cross-contact amount.27 Action level one is suggested when the food contains up to the eliciting dose 1% or 5% — depending on the food allergen. This is the dose expected to elicit objective symptoms in 1% or 5% of food allergy sufferers in OFCs. VITAL is not universally adopted but remains voluntary. Such an approach may provide a consistent risk assessment and labeling across the food industry, by guiding the review and management of allergen risk, providing a consistent and systematic framework to assess allergens and cross-contact allergens present in ingredients or generated during manufacturing processes. In addition, it could be of assistance demonstrating supply chain due diligence and reducing time and cost in responding to consumer complaints and product recall. However, the VITAL approach, by definition, is applicable only when the unintended allergen is distributed homogenously in the product; therefore, in situations where allergens are present in particulate forms, other approaches need to be identified to avoid PALs.16 Although further research is still needed to address the challenges in its implementation, a more quantitative approach than PAL, such as VITAL 3.0, could provide a more effective risk communication strategy, widening consumers’ food choices and improving their confidence in self-management. A proposal to achieve this extremely challenging goal, would be that an internationally recognized organization (such as Food and Agriculture Organization [FAO] or Codex Alimentarius Commission Committee on Food Labelling [Codex]), above regional regulators and Governments, would take the lead in trying to implement the VITAL system globally.
The proteomic mass spectrometry based approach
A potentially alternative approach to PAL is the direct quantification of micro amounts of allergens in the food itself, to overcome the risk stratification approach. In this regard, a good sensitivity is demanded to the analytical method under development to be able to detect allergen traces in processed food products.28 Sensitivity of the method should comply with the reference doses recommended depending on the specific country. These values are often used as minimal target values for setting suitable Limit of Detection (LOD) and/or Limit of Quantification (LOQ) during development of a method for allergen quantification.
Targeted liquid chromatography–mass spectrometry (LC-MS/MS) methods have been proposed in alternative to enzyme-linked immunosorbent assay (ELISA) and DNA-based methods, thanks to the high accuracy and reliability offered as the case of milk and egg detection in cookies.29 Mass spectrometry (MS) proved to offer many advantages over the immunoassays, such as i) unambiguous identification of the allergen through the detection of signature peptides for each allergen, ii) multi-target analysis through detection of multiple peptide-markers in one run, and iii) allergen quantification through the quantification of the produced signature peptides.30 On the other hand, it should also be highlighted that MS approach suffers from some limitations being considered in general: (i) not a rapid method to be used along the food production lines for analytical verification of established sanitation procedures, allowing to minimize cross-contact and thus to minimize the use of PAL, (ii) expensive for the sophisticated instruments requiring specialized operators to run MS method, and (iii) subject to matrix issues. Other methods, such as ELISA and quantitative polymerase chain reaction (qPCR), while covering some of the above-mentioned limitations also carry method-specific issues, as illustrated by Holzhauser et al.21 Efforts have been directed at the European level to overcome issues encountered in food allergen analysis. The establishment of reference doses and the necessity for sensitive and harmonized analytical methods along with the production of allergen Reference Materials have been the key objectives of the past Integrated Approaches to Food Allergen and Allergy Management (IFAAM) project, are part of the objectives of the International Association for Monitoring and Quality Assurance in the Total Food Supply Chain (MoniQA), and are part of the current European Food Safety Authority (EFSA) funded Detection and Quantification of Allergens in Food and Minimum Eliciting Doses in Food Allergic Individuals (ThRAll) project. This latter is based on the application of a dual risk-based approach to food-allergen management.31 The first aims at developing a reference quantitative MS-based prototype reference method for the detection of multiple food allergens in complex standardized incurred food models by targeting 6 allergens including milk, egg, peanuts, hazelnuts, almond, and soy. Once stable and reliable markers have been identified in hard to analyze matrices, the method can be optimized and in-house validated.32 At last, an inter-laboratory comparison needs to be carried out to allow comparability of results among laboratories. Proteomic evaluations may in general offer a higher sensitivity than the recommended VITAL reference doses, allowing the identification of milk and egg allergens at a LOD levels 40 and 13 times lower than VITAL action level 1, respectively.29
The second objective of ThRAll aims at gathering and curating data derived by oral food challenge tests, to define thresholds and minimum eliciting doses for several allergens.31 The accomplishment of these objectives, using a more comprehensive and harmonized approach that implements both quantitative and qualitative methods, may pave the way to fill the current gaps in the food allergen field and help with tackling the problem of the risk stratification approach and the overuse of PALs.
Conclusions
Potential new approaches could help address the current food labeling issues for severe food allergic patients. Indeed, current gaps in the evidence include, among others, a need for harmonization in labelling activities, certified reference materials and standardized detection assays, a definition of acceptable risk level in food allergy, and best practices to support the food allergic consumer.16 A scientific consensus on the definition of food allergy that considers both the eliciting dose and the food source is therefore needed, and WAO is taking steps in that direction.33 In this context, measures of quality of life and economic burden of food allergy are essential. In the framework of the new WAO definition,34 we are currently in the preliminary stages of developing an integrative and intuitive website for all food allergy measures. Researchers, clinicians, and healthcare professionals (HCPs) will be able to download the FAQL and other questionnaires or use them online, and also access norms data, advice on use and scoring, papers and reports, and simple calculators. This will promote adherence to quality measures, consistency in quality measure reporting, and comparative and longitudinal research, with benefits for patients and families living with severe food allergy. Furthermore, the development of a dedicated website can help to create an on-line community, allowing for discussion of potential and ongoing projects in an interactive forum.
A more precise harmonization and regulation of the labeling of food allergens, together with a combination of both quantitative and qualitative methods of detection, will help to improve consumers’ quality of life. Although with some limitations, the steady advances in proteomic approaches have been shown to possess higher sensitivity than the recommended VITAL reference doses, allowing the identification of allergens at much lower LOD levels than the VITAL and potentially helping to tackle the problem of the risk stratification approach and the overuse of PALs. All in all, a tight collaboration among international organizations, regulators, and food industries should work towards implementing, as a first step, mandatory requirements for labelling of allergen in food products in all countries in a more coherent way, detected through harmonized laboratory assays and reference materials. On the opposite, PAL should only be applied with a solid technical rationale to avoid creating unnecessary confusion, uncertainty, and stress amongst consumers with severe allergies.
Abbreviations
EAI - epinephrine auto-injector; EFSA – European Food Safety Agency; FAIM - Food Allergy Independent Measure; FA – Food Allergy; FAQL – Food Allergy Quality of Life; FAQLQ – Food Allergy Quality of Life Questionnaire; HCP – Healthcare Professionals; HRQL – Health Related Quality of Life; IFAAM - Integrated Approaches to Food Allergen and Allergy Management; LC-MS - Liquid chromatography–mass spectrometry; MCID - Minimal Clinical Important Difference; MoniQA - Monitoring and Quality Assurance in the Total Food Supply Chain; MS – Mass Spectrometry; PAL - Precautionary Allergen Labelling WAO – World Allergy Organization.
Authors’ consent for publication
All authors of this paper have read and approved the final version submitted.
Author contributions
Conceptualization: AF, DR; data curation: AF, DR, LM, AD, SGD; project administration: AF; writing-original draft: AF, DR; writing-review & editing: AF, DR, AD, SGD, LM, VF, IJA; validation: AF, DR, AD, SGD, LM, VF, IJA; figures and tables: AF, DR; supervision: AF.
Availability of data and materials/Ethics approval
Being our work a review paper of the present literature, these two sections are not applicable.
Declaration of competing interest
Davide Risso is a full-time employee of Soremartec Italia Srl, Alba (CN, Italy).
Acknowledgements
The research was carried out thanks to the financial support of Soremartec Italia Srl, Alba (CN, Italy).
Footnotes
Full list of author information is available at the end of the article. https://doi.org/10.1016/j.waojou.2021.100598
References
- 1.Warren C.M., Jian J., Gupta R.S. Epidemiology and burden of food allergy. Curr Allergy Asthma Rep. 2020;14(20):6. doi: 10.1007/s11882-020-0898-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner P.J., Worm M., Ansotegui I.J., WAO Anaphylaxis Committee Time to revisit the definition and clinical criteria for anaphylaxis? World Allergy Organ J. 2019;12:100066. doi: 10.1016/j.waojou.2019.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.https://www.dailymail.co.uk/news/article-8517763/Bride-collapses-dies-nut-allergy-caused-desert-lavish-wedding-reception.html , accessed October 29th, 2020.
- 4.Fleischer D.M., Perry T.T., Atkins D. Allergic reactions to foods in preschool-aged children in a prospective observational food allergy study. Pediatrics. 2012;130:e25–32. doi: 10.1542/peds.2011-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiocchi A., Artesani M.C., Riccardi C. Impact of omalizumab on food allergy in patients treated for asthma: a real-life study. J Allergy Clin Immunol Pract. 2019;7:1901–1909. doi: 10.1016/j.jaip.2019.01.023. e5. [DOI] [PubMed] [Google Scholar]
- 6.Herbert L., DunnGalvin A. Psychotherapeutic treatment for psychosocial concerns related to food allergy: current treatment approaches and unmet needs. J Allergy ClinImmunol: In Pract. 2021;9:101–108. doi: 10.1016/j.jaip.2020.10.037. [DOI] [PubMed] [Google Scholar]
- 7.Turner P.J., Campbell D.E. Epidemiology of severe anaphylaxis: can we use population-based data to understand anaphylaxis? Curr Opin Allergy Clin Immunol. 2016;16:441–450. doi: 10.1097/ACI.0000000000000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arasi S., Nurmatov U., Dunn-Galvin A. Consensus on DEfinition of Food Allergy SEverity (DEFASE) an integrated mixed methods systematic review. World Allergy Organ J. 2021;14:100503. doi: 10.1016/j.waojou.2020.100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polloni L., Muraro A. Anxiety and food allergy: a review of the last two decades. Clin Exp Allergy. 2020;50:420–441. doi: 10.1111/cea.13548. [DOI] [PubMed] [Google Scholar]
- 10.DunnGalvin A., Polloni L., Le Bovidge J. Preliminary development of the food allergy coping and emotions questionnaires for children, adolescents, and young people: qualitative analysis of data on IgE-mediated food allergy from five countries. J Allergy Clin Immunol Pract. 2018;6:506–513. doi: 10.1016/j.jaip.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 11.DunnGalvin A., Koman E., Raver E. An examination of the food allergy quality of life questionnaire performance in a countrywide American sample of children: cross-cultural differences in age and impact in the United States and Europe. J Allergy Clin Immunol Pract. 2017;5:363–368. doi: 10.1016/j.jaip.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 12.Dunn Galvin A., Hourihane J.O. Psychosocial mediators of change and patient selection factors in oral immunotherapy trials. Clin Rev Allergy Immunol. 2018;55:217–236. doi: 10.1007/s12016-018-8700-5. [DOI] [PubMed] [Google Scholar]
- 13.DunnGalvin A. In: Health Claims and Food Labelling. Astley S., editor. Royal Society of Chemistry; 2019. The impact of "labelling" on the beliefs, attitudes, and behaviours of consumers with food allergy: a multilevel perspective; pp. 127–140. [Google Scholar]
- 14.Marchisotto M.J., Harada L., Kamdar O. Food allergen labeling and purchasing habits in the United States and Canada. J Allergy Clin Immunol Pract. 2017;5:345–351. doi: 10.1016/j.jaip.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Bilaver L.A., Kester K.M., Smith B.M., Gupta R.S. Socioeconomic disparities in the economic impact of childhood food allergy. Pediatrics. 2016;137 doi: 10.1542/peds.2015-3678. [DOI] [PubMed] [Google Scholar]
- 16.Muraro A., Hoffmann-Sommergruber K., Holzhauser T. EAACI Food Allergy and Anaphylaxis Guidelines Group. EAACI Food Allergy and Anaphylaxis Guidelines. Protecting consumers with food allergies: understanding food consumption, meeting regulations and identifying unmet needs. Allergy. 2014;69(11):1464–1472. doi: 10.1111/all.12453. [DOI] [PubMed] [Google Scholar]
- 17.https://farrp.unl.edu/documents/Regulatory/FARRP-International-Regulatory-Chart-110619.pdf, accessed May 18th, 2021.
- 18.Diao X. 2017. An Update on Food Allergen Management and Global Labeling Regulations.https://conservancy.umn.edu/bitstream/handle/11299/194287/Food%20allergen%20management%20and%20global%20labeling%20regulations.pdf [Google Scholar]
- 19.Modification to the Official Mexican Standard NOM-051-SCFI/SSA1-2010, General labeling specifications for prepackaged foods and non-alcoholic beverages-Commercial and health information, published on April 5, 2010. http://www.dof.gob.mx/2020/SEECO/NOM_051.pdf , accessed May 4th, 2020.
- 20.Fierro V., Di Girolamo F., Marzano V., Dahdah L., Mennini M. Food labeling issues in patients with severe food allergies: solving a hamlet-like doubt. Curr Opin Allergy Clin Immunol. 2017;17:204–211. doi: 10.1097/ACI.0000000000000362. [DOI] [PubMed] [Google Scholar]
- 21.Holzhauser T., Johnson P., Hindley J.P. Are current analytical methods suitable to verify VITAL® 2.0/3.0 allergen reference doses for EU allergens in foods? Food Chem Toxicol. 2020;145:111709. doi: 10.1016/j.fct.2020.111709. [DOI] [PubMed] [Google Scholar]
- 22.Blom W.M., Michelsen-Huisman A.D., van Os-Medendorp H. Accidental food allergy reactions: products and undeclared ingredients. J Allergy Clin Immunol. 2018;142:865–875. doi: 10.1016/j.jaci.2018.04.041. [DOI] [PubMed] [Google Scholar]
- 23.Muraro A., Werfel T., Hoffmann-Sommergruber K. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014;69:1008–1025. doi: 10.1111/all.12429. [DOI] [PubMed] [Google Scholar]
- 24.Turner P.J., Gowland M.H. Precautionary allergen labelling: no more traces! Allergy. 2016;71:1505–1507. doi: 10.1111/all.12961. [DOI] [PubMed] [Google Scholar]
- 25.Allen K.J., Remington B.C., Baumert J.L. Allergen reference doses for precautionary labeling (VITAL 2.0): clinical implications. J Allergy Clin Immunol. 2014;133:156–164. doi: 10.1016/j.jaci.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 26.DunnGalvin A., Chan C.H., Crevel R. Precautionary allergen labelling: perspectives from key stakeholder groups. Allergy. 2015;70:1039–1051. doi: 10.1111/all.12614. [DOI] [PubMed] [Google Scholar]
- 27.Allergen Bureau . 2019. Summary of the 2019 VITAL Scientific Expert Panel Recommendations.http://allergenbureau.net/wp-content/uploads/2019/09/VSEP-2019-Summary-Recommendations_FINAL_Sept2019.pdf [Google Scholar]
- 28.Pilolli R., Nitride C., Gillard N. Critical review on proteotypic peptide marker tracing for six allergenic ingredients in incurred foods by mass spectrometry. Food Res Int. 2020;128:108747. doi: 10.1016/j.foodres.2019.108747. [DOI] [PubMed] [Google Scholar]
- 29.Monaci L., De Angelis E., Guagnano R. Validation of a MS based proteomics method for milk and egg quantification in cookies at the lowest VITAL levels: an alternative to the use of precautionary labeling. Foods. 2020;9:E1489. doi: 10.3390/foods9101489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monaci L., Pilolli R., De Angelis E., Crespo J.F., Novak N., Cabanillas B. Food allergens: classification, molecular properties, characterization, and detection in food sources. Adv Food Nutr Res. 2020;93:113–146. doi: 10.1016/bs.afnr.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Mills E.N.C., Adel-Patient K., Bernard H. Detection and quantification of allergens in foods and minimum eliciting doses in food-allergic individuals (ThRAll) J AOAC Int. 2019;102:1346–1353. doi: 10.5740/jaoacint.19-0063. [DOI] [PubMed] [Google Scholar]
- 32.Pilolli R., Van Poucke C., De Angelis E. Discovery based high resolution MS/MS analysis for selection of allergen markers in chocolate and broth powder matrices. Food Chem. 2021;343:128533. doi: 10.1016/j.foodchem.2020.128533. [DOI] [PubMed] [Google Scholar]
- 33.Fiocchi A., Ebisawa M. Severe food allergies: can they be considered rare diseases? Curr Opin Allergy Clin Immunol. 2017;17:201–203. doi: 10.1097/ACI.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 34.Arasi S., Nurmatov U., Turner P.J. Consensus on DEfinition of food allergy SEverity (DEFASE): protocol for a systematic review. World Allergy Organ J. 2020 Dec 19;13:100493. doi: 10.1016/j.waojou.2020.100493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Being our work a review paper of the present literature, these two sections are not applicable.