Abstract
As important immunosuppressive viruses, chicken infectious anemia virus (CIAV) and subgroup J avian leukosis virus (ALV-J) have caused huge economic losses to the poultry industry globally. Recently, the co-infection of CIAV and ALV-J frequently occurred in the domestic chicken flocks in China. However, the synergistic pathogenesis of CIAV and ALV-J has not been fully investigated. Here, a co-infection study was performed to further understand the potential synergistic pathogenesis of CIAV and ALV-J. In vitro study showed that CIAV could promote the replication of ALV-J in HD11 cells, but ALV-J could not increase the replication of CIAV. Chicken infection study showed both CIAV and ALV-J with synergistic effects caused significant body weight loss to the infected chickens. Although ALV-J had no effect on CIAV viral shedding and tissue load, CIAV did significantly increase ALV-J viremia, viral shedding and tissue load in the co-infection group. Moreover, both CIAV and ALV-J could significantly inhibit the humoral immunity to H9N2 influenza virus and serotype 4 fowl adenovirus (FAdV-4). All these data demonstrate the synergistic pathogenesis for the co-infection of CIAV and ALV-J, and highlight the positive effect of CIAV on the pathogenesis of ALV-J.
Key words: chicken infectious anemia virus, subgroup J avian leukosis virus, co-infection, synergistic pathogenesis, immunosuppression
INTRODUCTION
Due to the large-scale and intensive development of Chinese poultry industry, and the lack of effective prevention and control strategies for vertically transmitted and immunosuppressive viral pathogens such as chicken infectious anemia virus (CIAV) and avian leukosis virus subgroup J (ALV-J), the infection and co-infection cases of CIAV and ALV-J were frequently identified in the domestic chicken flocks in China, which restricts the healthy and sustainable development of domestic poultry industry (Yin et al., 2007; Guo, 2010; Qin et al., 2010; Eltahir et al., 2011; Zhang et al., 2013; Chu, 2016; Feng et al., 2017; Li et al., 2017a, Li et al., 2017b; Zhang et al., 2017; Tan et al., 2020). Although the co-infections of CIAV with other pathogens such as H9N2 influenza virus (H9N2), infectious bronchitis virus (IBV), fowl adenovirus (FAdV), Marek's disease virus (MDV), reticuloendotheliosis virus (REV), avian reovirus (ARV), and infectious bursal disease virus (IBDV) have also been reported, the effects of CIAV infection on other pathogens have not been extensively or experimentally evaluated (Erfan et al., 2019; Li et al., 2020).
CIAV has recently been classified into genus Gyrovirus, the family Anelloviridae by the International Committee on Taxonomy of Viruses (ICTV) (Rosario et al., 2017). ALV-J belongs to the genus Alpharetrovirus, the family Retroviridae (Swayne et al., 2013). Both CIAV and ALV-J can transmit vertically and horizontally and result in immunosuppression in chicken flocks. CIAV generally causes aplastic anemia and systemic lymphoid tissue atrophy in chicks, and ALV-J infection mainly results in malignant proliferation of hematopoietic cells and induces myelocytoma and hemangioma (Cheng et al., 2010; Payne and Nair, 2012). Although the T cell and the myelocyte are the major target cells for CIAV and ALV-J, respectively, the hematopoietic cells are thought to be as co-target cells for both CIAV and ALV-J. In the clinical, the co-infection of CIAV and ALV-J causes higher immunosuppression than single infection (Yu, 2015). Thus, it is important to find out the mechanisms of interaction and synergism between CIAV and ALV-J. In this study, synergistic pathogenesis of the co-infection of CIAV and ALV-J was investigated in vitro and in vivo. Our results showed that CIAV could efficiently promote the replication and pathogenesis of ALV-J, but not vice versa.
MATERIALS AND METHODS
Cells and Viruses
The chicken macrophage cell line HD11 (kept in our laboratory) and the chicken fibroblast cell line DF-1 (from ATCC, kept in our laboratory) were cultured at 41°C and 37°C respectively in the cell incubator. The CIAV T1P6 strain was isolated from broiler (kept in our laboratory). The ALV-J GY03 strain isolated from commercial layers (GenBank accession number GU982308) was preserved in our laboratory.
Real-Time Quantitative PCR for Detection of CIAV
The primers for detecting the VP1 of CIAV were designed and synthesized according to the sequence of T1P6 (Table 1). The plasmid pcDNA3.1-VP1 was used for generating the standard curve. The quantitative PCR (q-PCR) detection method was established using TB Green Premix Ex Taq II of TaKaRa (Dalian, China). The samples were amplified and analyzed in ABI 7500 Real-time PCR system with the following procedure: 95°C for 30 s, followed by 40 cycles of denaturation at 95°C for 5 s, and annealing and extension at 60°C for 34 s.
Table 1.
Primers for q-PCR detection of CIAV.
| Name | Sequence |
|---|---|
| VP1-F | GCCCCGGTACGTATAGTGTG |
| VP1-R | CCCGTACATGTGGTCTGCAT |
Abbreviation: CIAV, chicken infectious anemia virus.
Virus Infection Study In Vitro
HD11 cells were infected with CIAV (106 copy numbers), ALV-J (0.001 MOI) or co-infected with CIAV (106 copy numbers) and ALV-J (0.001 MOI), respectively. All cells were collected and lysed at 8 days post-infection (dpi), and the Env protein of ALV-J was detected by Western blot. The genome DNA was also extracted from the supernatants at 8 dpi and the copy numbers of CIAV was determined by the q-PCR method as described above.
Animal Infection Study
A total of 144 one-day-old specific-pathogen-free (SPF) chicks were randomly divided into 4 groups (36 chickens per group). Chicks in group A were inoculated with 5 × 105 DNA copy numbers of the T1P6 in 0.2 mL PBS through leg muscle injection. Chicks in group B were inoculated with 104 TCID50 of the GY03 in 0.2 mL PBS through leg muscle injection. Chicks in group C were inoculated with 5 × 105 DNA copy numbers of the T1P6 and 104 TCID50 of the GY03 in 0.2 mL PBS through leg muscle injection. Chicks in group D were inoculated with 0.2 mL PBS through leg muscle injection. At 7, 14, 21, 28, 35, 49, and 63 dpi, the body weight of chickens were monitored, the blood and the cloacal swab samples were collected from the chickens for ALV-J detection. At each time points, 3 chickens from each group were randomly picked and euthanized, and the liver, spleen, and kidney from these chickens were collected for the detection of viral titers. To test the effects of the infection of CIAV and ALV-J on the immune response to the inactivated vaccine, these chickens were also immunized with 0.2 mL of an inactivated vaccine candidate NDV-H9N2-FAdV (provided by Sinopharm Yangzhou VAC Biological Engineering Co. Ltd., Yangzhou, China) through intramuscularly at 10 dpi. The sera from these chickens were collected at indicated time points and tested for antibodies against H9N2 influenza virus and serotype 4 fowl adenovirus (FAdV-4), respectively.
Determination of ALV-J Viral Titer in Organs and ALV-J Viremia
The homogenates of 0.1 g liver, spleen, and kidney from the chickens were treated with 5 × penicillin and streptomycin for 45 min at 37°C, and the supernatants were obtained by centrifugation. The ALV-J-containing supernatants were inoculated into DF-1 cells and cultured for 6 d, and then the TCID50 of these supernatants were determined by immunofluorescence assay (IFA) and calculated by the Reed-Muench method. In addition, the collected blood was inoculated into DF-1 cells and cultured for 6 d. Then, the infected DF-1 cells were fixed and detected by IFA using mAb JE9 for the detection of ALV-J viremia.
Sandwich Enzyme-Linked Immunosorbent Assay for Detection of P27 Antigen of ALV
The collected cloacal swab samples were mixed with 800 μL of PBS. The samples with 3 cycles of repeated freeze-thaw and vortexing were directly subjected to a sandwich ELISA for detection of P27 of ALV as previously described (Li et al., 2018). The OD650 value was determined and the cut-off for the ELISA was 0.15 as previously described (Li et al., 2018).
Determination of DNA Copy Numbers of CIAV in Organs
The genome DNA of the homogenates of 0.1 g liver, spleen, and kidney from the chickens were extracted using TIANamp Genomic DNA Kit (Beijing, China). Then, the genome DNA was detected for CIAV by q-PCR as described above.
Western Blot
The HD11 cells infected with CIAV, ALV-J, and co-infected with CIAV and ALV-J, respectively, were lysed with RIPA buffer (CWbio, Beijing, China) containing protease inhibitors (CST, MA) at 8 dpi. The lysates were loaded with 4 × denaturing sample buffer and separated in 10% SDS-PAGE gel electrophoresis, and then were transferred onto 0.2 μm nitrocellulose (NC) membranes (GE Healthcare Life sciences, Freiburg, Germany). In Western blot, the mAb JE9 against ALV-J Gp85 (prepared and kept in our laboratory) and anti-GAPDH mAb (Abclonal, Wuhan, China) were used as primary antibodies, and HRP-conjugated goat anti-mouse IgG was served as the secondary antibody. The membrane was treated with Enhanced Chemiluminescent (Ncmbio, Suzhou, China) and the signals of proteins were detected with a chemiluminescence system (Tanon 5200).
Indirect ELISA for Detection of Antibody against FAdV-4
The chicken serum samples were diluted 1:400 in dilution buffer (phosphate-buffered saline with Tween 20, PBST) and then 100 μL of the diluted samples was then directly subjected to the indirect ELISA for the detection of the antibody against FAdV-4. The OD450 value was determined. The determine criteria of the indirect ELISA was: S (the OD450 value of sample-the average OD450 value of negative control) /P (the average OD450 value of positive control-the average OD450 value of negative control), if the above S/P ≥ 0.12, the sample was judged as positive, otherwise it was negative.
Hemagglutination Inhibition Assay
The hemagglutination inhibition (HI) assay was performed using 8 hemagglutination units of H9N2 and 0.5% chicken erythrocytes by standard methods as previously described (Lee et al., 2001).
Statistical Analysis
The statistical differences in this study were performed with a Student t test or one-way analysis of variance (ANOVA) using Prism 5.0 software package (GraphPad Software, La Jolla, CA). Means ± standard deviations were taken to present the results. A value of P < 0.05 was considered different and P < 0.01 was considered significantly different. P values of less than 0.05, 0.01, and 0.001 were indicated with *, ** and *** respectively.
Ethics Statement
All animal experiments complied with the institutional animal care guidelines and the protocol (SYXY-20), which was approved by the Animal Care Committee of Yangzhou University. At the end of the experiment, all the chickens were euthanized with CO2.
RESULTS
CIAV Could Promote the Replication of ALV-J In HD11 Cells
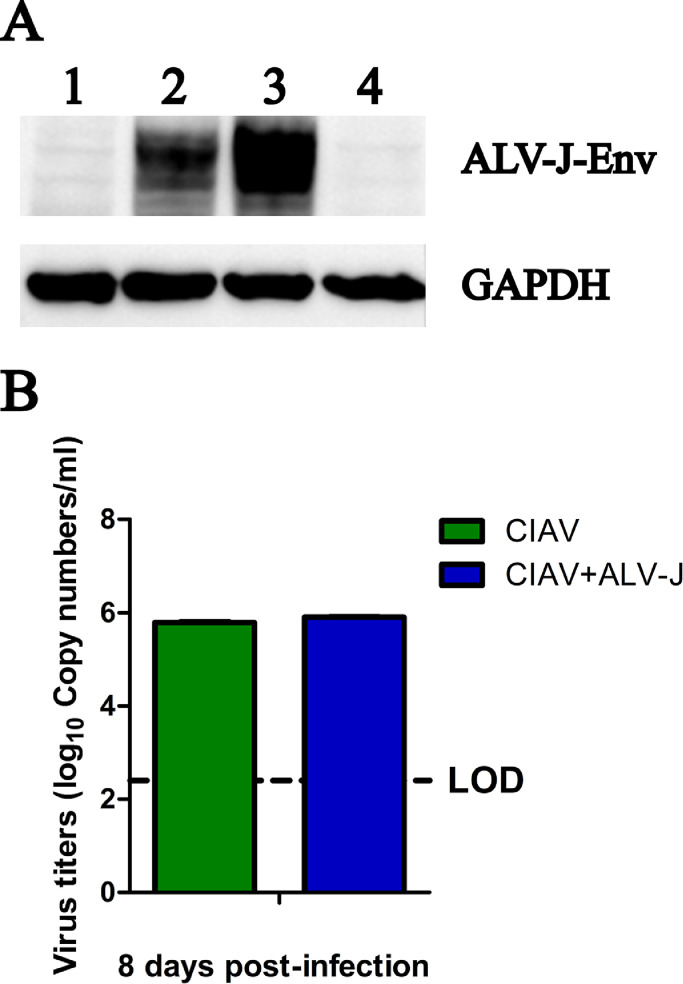
To explore whether ALV-J and CIAV could regulate the replication of each other in vitro, HD11 cells were infected with CIAV, ALV-J, or co-infected with CIAV and ALV-J, respectively. As shown in Figure 1A, the expression level of Env protein of ALV-J in HD11 cells in the co-infection group was significantly stronger than that in HD11 cells in ALV-J single infection group. As expected, the Env protein could not be detected in CIAV infection group and control group. Notably, the DNA copy numbers of CIAV in the supernatants collected at 8 dpi had no significantly difference between CIAV infection group and the co-infection group as described in Figure 1B. These data indicate that CIAV can promote the replication of ALV-J in chicken macrophage cells, but ALV-J cannot enhance the replication of CIAV.
Figure 1.
Detection for CIAV and ALV-J in the infected HD11 cells. The green and blue columns represent the CIAV and CIAV+ALV-J groups, respectively. (A) Comparison of ALV-J from infected HD11 cells at 8 dpi detected by Western blot using mAb JE9 against ALV-J Env and anti-GAPDH antibody. Lane 1 to 3: HD11 cells infected with CIAV, ALV-J, and CIAV+ALV-J at 8 dpi, respectively. Lane 4: Negative HD11 cells considered as control. (B) Comparison of genome DNA copy numbers of CIAV in the supernatants from HD11 cells infected with CIAV and CIAV+ALV-J at 8 dpi, respectively, determined by q-PCR. Abbreviations: ALV-J, J avian leukosis virus; CIAV, chicken infectious anemia virus.
Co-infection of CIAV and ALV-J Caused Significant Body Weight Loss for Chickens
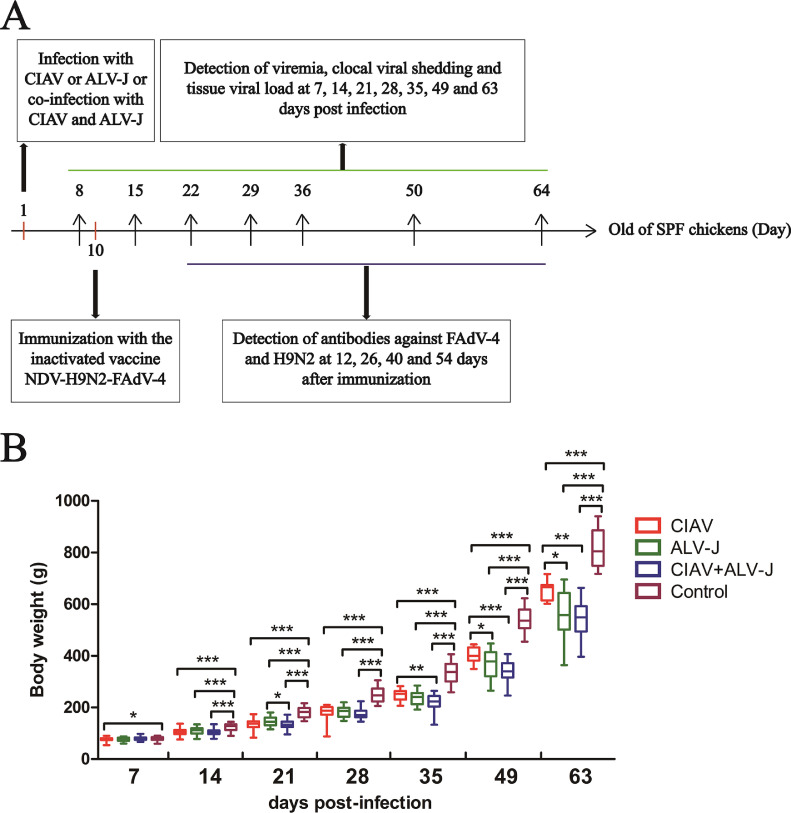
To further evaluate the pathogenesis of the co-infection of CIAV and ALV-J in vivo, 1-day-old SPF chickens were infected with ALV-J, CIAV or both of them. The chickens inoculated with PBS were set as a control. The detailed animal experiment process was shown in Figure 2A. To observe the growth status of the chickens, the body weights of the infected chickens were monitored at the indicated time points. As described in Figure 2B, the average body weight of chickens in the infection groups was much lower than that of chickens in the control group from 14 dpi to 63 dpi. Notably, the co-infection of CIAV and ALV-J caused more loss of body weight in comparison with the single infection groups. The average body weight of chickens in the control group was 814.22 g at 63 dpi whereas that of chickens in CIAV infection group, ALV-J infection group, and the co-infection of CIAV and ALV-J were only 653.4 g, 564.6 g, and 544.7 g, respectively. These data demonstrate that the infection of CIAV and ALV-J both can induce significant growth inhibition for chickens than the single infection of either CIAV or ALV-J.
Figure 2.
Schematic diagram of co-infection study in vivo and body weight loss in the infected chickens. The red, green, blue, and purple columns represent the CIAV, ALV-J, CIAV+ALV-J, and control groups, respectively. (A) The infection, immunization and detection of related data for chickens was showed at the indicated time points. (B) Comparison of body weights of chickens from different groups measured at different time points. The data were performed with one-way ANOVA using GraphPad 5 software. A P value of <0.05 was considered significant. P values of less than 0.05, 0.01 and 0.001 were indicated with *, ** and ***, respectively. Abbreviations: ALV-J, J avian leukosis virus ; CIAV, chicken infectious anemia virus.
CIAV Significantly Increased ALV-J Viremia, Viral Shedding, and Tissue Load
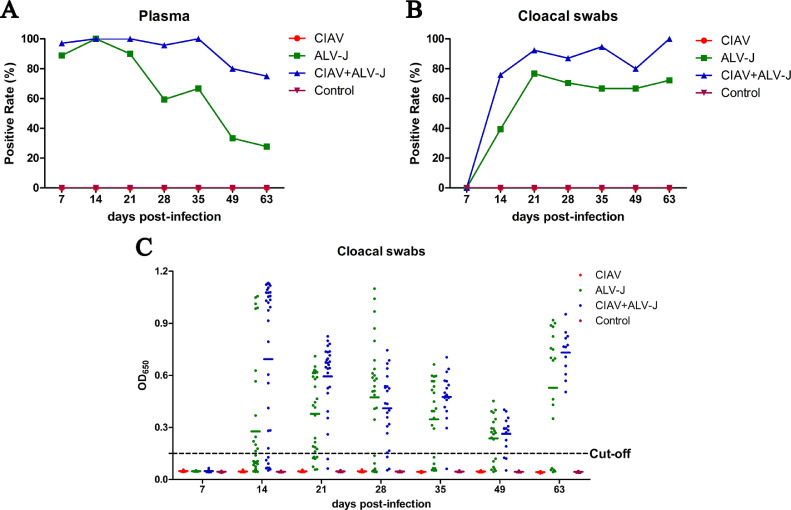
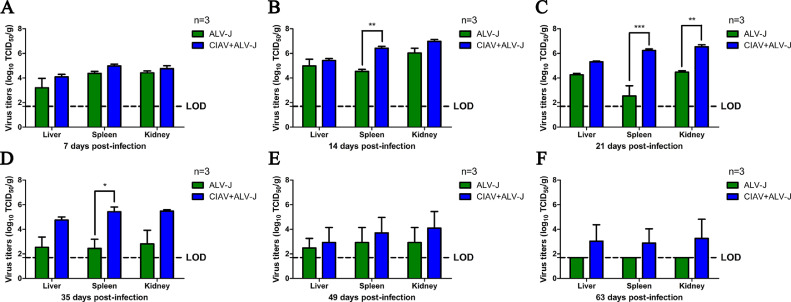
To investigate the effects of the infection of CIAV on the pathogenesis of ALV-J, the viremia, viral shedding, and tissue load of ALV-J were detected in the infected chickens. As shown in Figure 3A, the positive rate of ALV-J viremia in the chickens infected with ALV-J was 88.9 (32/36), 100 (32/32), 90 (27/30), 59.3 (16/27), 66.7 (16/24), 33.3 (7/21), and 27.8% (5/18) at 7, 14, 21, 28, 35, 49, and 63 dpi, respectively, whereas that in the chickens co-infected with CIAV and ALV-J was 97.1 (34/35), 100 (32/32), 100 (26/26), 95.7 (22/23), 100 (19/19), 80 (12/15), and 75% (9/12), respectively. For the viral shedding in the cloacas, as described in Figure 3B, the positive rate in the chickens infected with ALV-J was 0 (0/36), 39.4 (13/33), 76.7 (23/30), 70.4 (19/27), 66.7 (16/24), 66.7 (14/21), and 72.2% (13/18) at 7, 14, 21, 28, 35, 49, and 63dpi, respectively, whereas that in the chickens co-infected with CIAV and ALV-J was 0 (0/36), 75.8 (25/33), 92.3 (24/26), 87.0 (20/23), 94.7 (18/19), 80.0 (12/15), and 100% (12/12), respectively. The average ELISA OD650 values for the detection of P27 antigen in ALV-J single infection group were 0.277, 0.379, 0.474, 0.346, 0.236, and 0.528, at 14, 21, 28, 35, 49, and 63 dpi, respectively, whereas that in the co-infection group was 0.693, 0.594, 0.411, 0.476, 0.263, and 0.732, respectively, as described in Figure 3C. Moreover, for the viral tissue load, as described in Figure 4, the ALV-J viral titers in the liver, spleen and kidney from chickens co-infected with CIAV and ALV-J were generally higher than those in the chickens infected with ALV-J at all time points. Besides, the ALV-J viral titers in the spleen from chickens co-infected with CIAV and ALV-J were significantly higher than those in the chickens infected with ALV-J at 14, 21, and 35 dpi (Figures 4B–4D). Notably, ALV-J could not be detected in the liver, spleen, and kidney from the chickens only infected with ALV-J at 63 dpi, whereas ALV-J still could be detected in the chickens co-infected with CIAV and ALV-J (Figure 4F). All these data demonstrate that CIAV can significantly increase viremia, viral shedding and tissue load of ALV-J.
Figure 3.
ALV-J viremia and viral cloacal shedding in the infected chickens. The red, green, blue, and purple curves or spots represent the CIAV, ALV-J, CIAV+ALV-J, and control groups, respectively. (A) Comparison of the ALV-J viral positive rates in plasma from chickens in CIAV, ALV-J, CIAV+ALV-J, and control groups at different time points, determined by IFA. (B) Comparison of the positive rates for P27 antigen of ALV in cloacal swab from chickens in CIAV, ALV-J, CIAV+ALV-J, and control groups at different time points, determined by ELISA. (C) Comparison of P27 antigen levels of ALV in cloacal swab from chickens in CIAV, ALV-J, CIAV+ALV-J, and control groups at different time points, determined by ELISA. Abbreviations: ALV-J, J avian leukosis virus; CIAV, chicken infectious anemia virus.
Figure 4.
ALV-J tissue viral load in the infected chickens. The green and blue columns represent the ALV-J and CIAV+ALV-J groups, respectively. (A–E) Comparison of ALV-J viral loads in organs from chickens infected with ALV-J and CIAV+ALV-J at 7, 14, 21, 35, 49, and 63 dpi, respectively. The data were analyzed with a Student t test. A P value of <0.05 was considered significant. P values of less than 0.05, 0.01, and 0.001 were indicated with *, ** and ***, respectively. Abbreviations: ALV-J, J avian leukosis virus; CIAV, chicken infectious anemia virus; LOD, limit of detection.
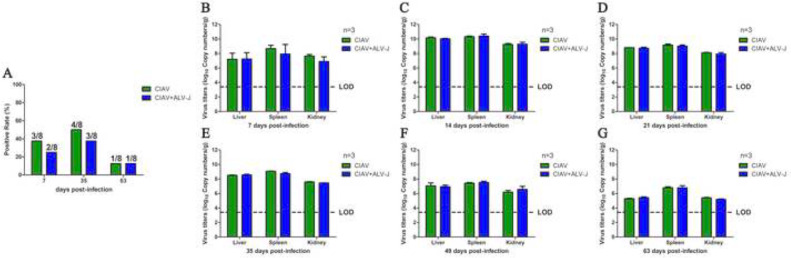
ALV-J Had No Effect on CIAV Viral Shedding and Tissue Load
To investigate the effects of the infection of ALV-J on the pathogenesis of CIAV, the CIAV viral shedding and tissue load were detected in the infected chickens. For the viral shedding in the cloacas, as described in Figure 5A, the positive rate in the chickens only infected with CIAV was 37.5 (3/8), 50 (4/8), and 12.5% (1/8) at 7, 35, and 63 dpi, respectively, and that in the chickens co-infected with CIAV and ALV-J was 25 (2/8), 37.5 (3/8), and 12.5% (1/8), respectively. In addition, the positive rate of the viral tissue load in CIAV single infection group and CIAV and ALV-J co-infection group was both 100% from 7 to 63 dpi, and there was no significant difference for the viral genome copy numbers of CIAV in the liver, spleen and kidney from the chickens co-infected with CIAV and ALV-J at all time-points tested when compared with the chickens only infected with CIAV (Figure 5B–5G). Notably, the viral genome copy numbers of CIAV in both groups reached to the peak with about 109 to 1010 copies/g at 14 dpi. These data demonstrate that the infection of ALV-J has no significant effects on viral shedding and tissue load of CIAV in the co-infected chickens.
Figure 5.
CIAV viral cloacal shedding and tissue viral load in the infected chickens The green and blue columns represent the CIAV and CIAV+ALV-J groups, respectively. (A) Comparison of the positive rates of CIAV viral cloacal shedding in chickens infected with CIAV and CIAV+ALV-J at 7, 35, and 63 dpi. (B–G) Comparison of CIAV viral loads in organs from chickens infected with CIAV and CIAV+ALV-J at 7, 14, 21, 35, 49, and 63 dpi, respectively. The data were analyzed with a Student t test. A P value of <0.05 was considered significant. Abbreviations: ALV-J, J avian leukosis virus; CIAV, chicken infectious anemia virus; LOD, limit of detection.
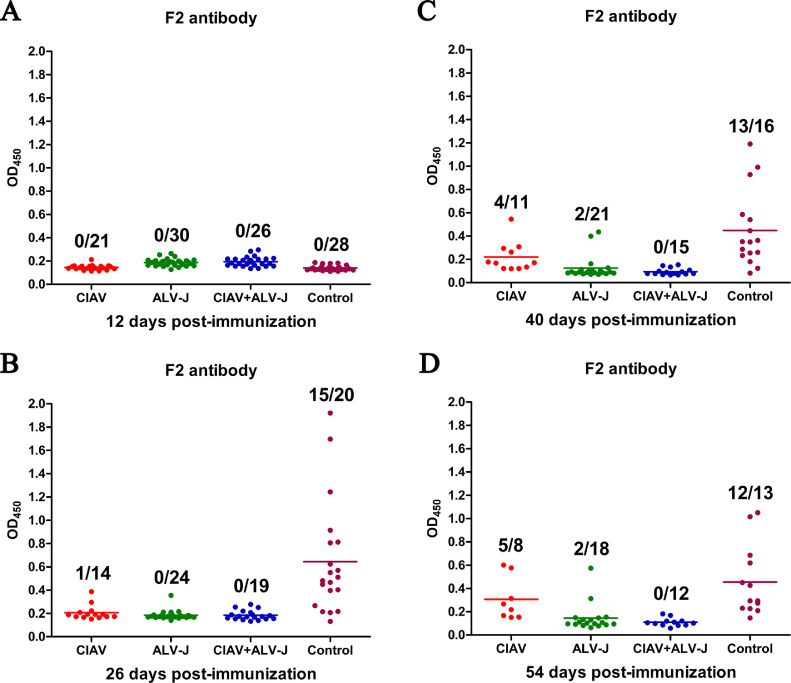
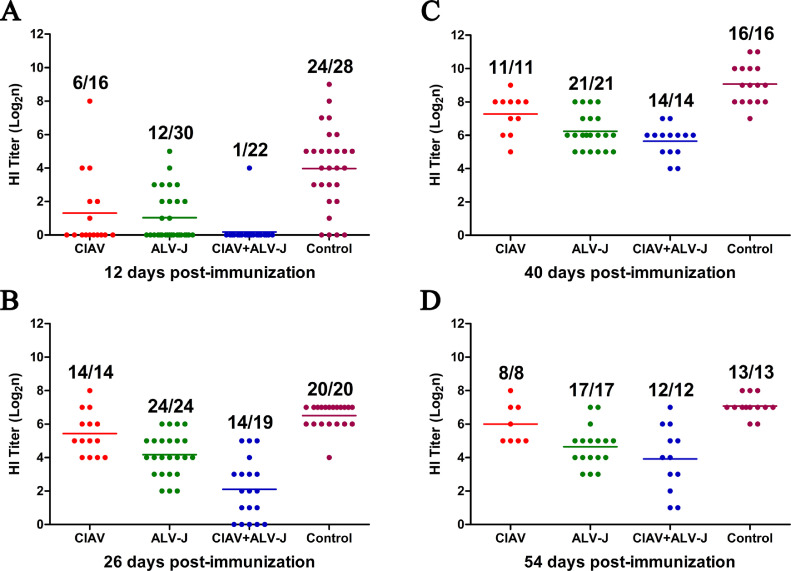
Co-infection of CIAV and ALV-J Significantly Inhibited the Humoral Immunity
To evaluate the effects of the infection of CIAV and ALV-J on the humoral immunity, all the chickens were immunized with the inactivated vaccine candidate NDV-H9N2-FAdV at 10 dpi. The sera of these chickens were collected at the indicated time points and tested for antibodies against FAdV-4 and H9N2, respectively. As described in Figure 6, the positive rate of the antibody against Fiber-2 protein of FAdV-4 in the control group was 0, 75, 81.3, and 92.3% with the average OD450 values of 0.140, 0.644, 0.448, and 0.456 at 12, 26, 40, and 54 days post vaccination (dpc), respectively, whereas the positive rate in CIAV infection group was 0, 7.1, 36.4, and 62.5% with the average OD450 values of 0.145, 0.206, 0.220, and 0.306, respectively, and that in ALV-J infection group was 0, 0, 9.5, and 11.1% with the average OD450 values of 0.187, 0.185, 0.124, and 0.145, respectively. Notably, the positive rate of the antibody against Fiber-2 protein of FAdV-4 in CIAV and ALV-J co-infection group was 0% at all time-points tested (Figure 6). For the detection of antibody against H9N2, the average HI titer in the control group was log2 3.964, log2 6.500, log2 9.063, and log2 7.077 at 12, 26, 40, and 54 dpc, respectively (Figure 7). The average HI titer in CIAV infection group was log2 1.313, log2 5.429, log2 7.237 and log2 6.000 at 12, 26, 40, and 54 dpc, respectively, and that in ALV-J infection group was log2 1.033, log2 4.167, log2 6.238 and log2 4.647, respectively (Figure 7). Notably, the average HI titer in the co-infection group was only log2 0.182, log2 2.105, log2 5.643 and log2 3.917 at 12, 26, 40, and 54 dpc, respectively (Figure 7). All these data demonstrate that the co-infection of CIAV and ALV-J can significantly inhibit the humoral immunity induced by the inactivated vaccine.
Figure 6.
Antibody against Fiber-2 of FAdV-4 in the infected chickens. The red, green, blue, and purple spots represent the CIAV, ALV-J, CIAV+ALV-J, and control groups, respectively. (A–D) Comparison of the levels of antibody against FAdV-4 from chickens immunized with the inactivated vaccine candidate NDV-H9N2-FAdV in CIAV, ALV-J, CIAV+ALV-J, and control groups at 12, 26, 40, and 54 dpc, determined by ELISA. Abbreviations: ALV-J, J avian leukosis virus; CIAV, chicken infectious anemia virus; FAdV-4, 4 fowl adenovirus.
Figure 7.
Antibody against H9N2 in the infected chickens. The red, green, blue, and purple spots represent the CIAV, ALV-J, CIAV+ALV-J, and control groups, respectively. (A–D) Comparison of the levels of antibody against H9N2 (with HI titer) from chickens immunized with the inactivated vaccine candidate NDV-H9N2-FAdV in CIAV, ALV-J, CIAV+ALV-J, and control groups at 12, 26, 40, and 54 dpc, determined by HI assay. Abbreviations: ALV-J, J avian leukosis virus; CIAV, chicken infectious anemia virus; FAdV-4, 4 fowl adenovirus; HI, hemagglutination inhibition; H9N2, H9N2 influenza virus.
DISCUSSION
CIAV generally causes severe anemia and immunosuppression in young chicks, and impairs the immune system of the old chickens, which resulting in huge economic losses to the poultry industry worldwide (Adair, 2000). ALV-J not only induces hematopoiesis, hemangioma, and various kind of tumors in chickens but also causes immunosuppression by targeting different kinds of immune cells (Payne and Nair, 2012). Of note, in the past several years, the infection of CIAV or ALV-J occurred occasionally in China (Eltahir et al., 2011; Zhang et al., 2013; Feng et al., 2017; Li et al., 2017b; Zhang et al., 2017; Tan et al., 2020), and the co-infection of CIAV and ALV-J is currently circulating in some breeder flocks, layer flocks, and indigenous chicken flocks in China (Yin et al., 2007; Guo, 2010; Qin et al., 2010; Chu, 2016; Li et al., 2017a). It has been reported that the co-infection of CIAV and ALV-J could induce higher morbidity and mortality than the single infection of any of them (Yang, 2010). However, the pathogenic synergism of CIAV and ALV-J is not clear to date. It is critical to find out the underlying mechanisms of the co-infection of ALV-J and CIAV for providing novel strategies to solve such problem in poultry industry.
Although it is reported that CIAV could promote the viral infection of H9N2, IBV, and NDV (De Boer et al., 1994; Erfan et al., 2019), the impact of ALV-J and CIAV on each other's replication is still unknown. In this study, both in vitro and in vivo studies were performed to further understand the synergistic pathogenesis of ALV-J and CIAV. Chicken macrophage cell is one of the important immune cells for fighting the infections with various of viruses and can be as the target cell both for ALV-J and CIAV (McConnell et al., 1993a). Actually, we have tried to culture CIAV in DF-1, HD11, LMH, and MDCC-MSB-1 cells. However, the results showed that the CIAV strain used in this study was not susceptible to them except HD11 cells. Therefore, to explore whether ALV-J and CIAV can affect each other's the replication, HD11 cells was used in vitro study. Western blot and q-PCR results showed that only CIAV can promote the replication of ALV-J, but not vice versa. Thus, we provide a good co-infection model for ALV-J and CIAV in vitro study. Moreover, previous studies have reported that VP1, VP2, and VP3 all play vital roles in the replication or pathogenicity of CIAV (Douglas et al., 1995; Yamaguchi et al., 2001; Lai et al., 2017; Wang et al., 2017). Our primary data indicated that the VP3 protein might play a role in increasing the replication of ALV-J in HD11 cells. However, the the exact effects of the three viral proteins on ALV-J replication still need to be further elucidated by more comprehensive experiments.
To further evaluate the pathogenic synergism between CIAV and ALV-J in vivo, 1-day-old SPF chickens were infected with ALV-J, CIAV or both of them. Not surprisingly, the co-infection of ALV-J and CIAV could more significantly inhibits the growth of the chickens than those only infected with ALV-J or CIAV. Notably, consistently with the in vitro data, the in vivo results of ALV-J viremia, viral shedding, and tissue viral load also showed that CIAV could significantly promote ALV-J replication and pathogenicity in vivo, while ALV-J still could not benefit CIAV replication in vivo according to the data of CIAV viral shedding and tissue viral load. It should be mentioned that no tumor and typical histopathological symptoms (data not shown) were obseved in chickens infected with ALV-J or co-infected with CIAV and ALV-J possibly due to the short time of ALV-J infection. Besides, the inoculation method of ALV-J could also affect the formation of tumor and histopathology. Several studies have shown that the inoculation of chicken embryos with ALV is more likely to cause tumors (Wang et al., 2012; Justice et al., 2015). Since CIAV can inhibit innate immune response in vivo, CIAV may weaken the innate immune system or anti-ALV-J host factors in macrophages and other immune cells (Cloud et al., 1992; McConnell et al., 1993b; Bounous et al., 1995), which benefiting the replication of ALV-J. The reason that CIAV could not replicate more efficiently during ALV-J infection is possibly that the attenuation of the immune system caused by ALV-J infection could not be utilized by CIAV during its life-cycle. It is noteworthy that there were 8 and 6 chickens died in the CIAV single infection group and co-infection group during the study, respectively, but no chicken was dead in the ALV-J single infection group in the infection study. Although there was no significant difference between CIAV single infection group and co-infection group, however, the data of viremia, viral shedding and tissue viral load did clearly show the positive promotion effect of CIAV on the pathogenesis of ALV-J. Taken together, the important pathogenic synergism of CIAV and ALV-J is that CIAV can efficiently enhance the replication ability of ALV-J in chickens, which may lead to more severe immunosuppression.
Previous studies have reported that CIAV could affect the immune response for fowl adenovirus, MDV, and NDV (De Boer et al., 1994; Miles et al., 2001; Su et al., 2019). However, the effect of the co-infection of CIAV and ALV-J on humoral immunity against other pathogens is unknown. As 2 immunosuppressive pathogens, we speculate that the co-infection of ALV-J and CIAV can cause more severe immunosuppression, resulting in serious decreasing of host immune response. To further prove our hypothesis, the chickens were vaccinated with an inactivated vaccine candidate NDV-H9N2-FAdV at 10 dpi and the immune response of the infected chickens was evaluated by determining the humoral immunity against H9N2 and FAdV-4. Our result showed that the HI titers of the chickens co-infected with CIAV and ALV-J kept the lowest at all the detection time-points among the 4 groups. Notably, the co-infection of CIAV and ALV-J effectively inhibited the humoral immunity against H9N2 at early stage (12 dpc) since the positive rate for the chickens in the co-infection group possessed antibodies against H9N2 was at least 8 times lower than that in the other groups. Moreover, the antibody response against FAdV-4 of the chickens in the infection groups was significantly weaker compared with the control group. It is noteworthy that the co-infection of CIAV and ALV-J completely inhibited the production of the antibody against Fiber-2 of FAdV-4 at all the time-points we detected. All these data clearly demonstrate that the co-infection with CIAV and ALV-J can more efficiently inhibit the function of host immune system which severely affects the efficacy of vaccination for the chicken flocks and makes chickens more susceptible to other pathogens. Notably, we also detected the antibody against ALV with an indirect ELISA method as previously described (Qiu et al., 2011) and the antibody against CIAV was also evaluated using a kit from IDEXX. However, there were no significant differences between the ALV-J infection group and co-infection group or between the CIAV infection group and co-infection group. Also, the positive rate and the level of these antibodies were very low possibly due to the immune suppression or tolerance induced by the early infection.
In summary, this is the first demonstration that CIAV efficiently promotes the replication of ALV-J in vitro and in vivo, and the co-infection of CIAV and ALV-J induces severe immunosuppression for the chickens. Currently, as the only effective prevention and control strategy for ALV-J, the ALV-J eradication program has been efficiently conducted in China. Our data indicate that the co-infection of CIAV with ALV-J may affect the efficiency of the ALV-J eradication program. Notably, the effect of the attenuated CIAV vaccine on ALV-J pathogenicity needs to be tested. Although previous data showed that CIAV and NDV could promote the replication of each other in vivo, ALV-J did show limit effect on CIAV viral replication in vitro and in vivo. The reason that ALV-J could not help CIAV replication needs to be further elucidated. Since the single infection or co-infection of CIAV and ALV-J efficiently inhibited the humoral immunity induced by the inactivated vaccine NDV-H9N2-FAdV, it is important to exclude the contamination of CIAV and ALV-J in the live vaccines in clinic. Besides, the co-infection of CIAV and ALV-J did enhance their pathogenicity for the chickens, however, whether it is induced by the defect of the immune system caused by CIAV infection or by the increase of ALV-J replication efficiency remains to be further investigated.
ACKNOWLEDGMENTS
This study was supported by the National Key Research & Development (R&D) Plan (2018YFD0500106, 2016YFD0501605), the National Natural Science Foundation of China (31872491, 31972661), NCFC-RCUK-BBSRC (31761133002 and BB/R012865/1), the Key Laboratory of Prevention and Control of Biological Hazard Factors (Animal Origin) for Agrifood Safety and Quality (26116120), the Research Foundation for Talented Scholars in Yangzhou University and the Priority Academic Program Development of Jiangsu Higher Education Institutions
Authors’ contributions: HS, AQ, JY conceived and designed the experiments. JZ, LM, TL, LL, QK, XY, QX performed the experiments. JZ, LM, TL analyzed the data. TL, ZW, AQ contributed reagents/materials/analysis tools. JZ, JY, TL, HS and AQ wrote the paper. All of the authors have read and approved the final manuscript.
DISCLOSURES
All authors declare that they have no competing interests.
REFERENCES
- Adair B.M. Immunopathogenesis of chicken anemia virus infection. Dev. Comp. Immunol. 2000;24:247–255. doi: 10.1016/s0145-305x(99)00076-2. [DOI] [PubMed] [Google Scholar]
- Bounous D.I., Goodwin M.A., Brooks R.L., Jr, Lamichhane C.M., Campagnoli R.P., Brown J., Snyder D.B. Immunosuppression and intracellular calcium signaling in splenocytes from chicks infected with chicken anemia virus, CL-1 isolate. Avian Dis. 1995;39:135–140. [PubMed] [Google Scholar]
- Cheng Z., Liu J., Cui Z., Zhang L. Tumors associated with avian leukosis virus subgroup J in layer hens during 2007 to 2009 in China. J. Vet. Med. Sci. 2010;72:1027–1033. doi: 10.1292/jvms.09-0564. [DOI] [PubMed] [Google Scholar]
- Chu, H. 2016. Serological investigation, virus isolation and identification of CAV in sick chickens in parts of Anhui. Master. Anhui Agri. Univ., China. (In Chinese)
- Cloud S.S., Rosenberger J.K., Lillehoj H.S. Immune dysfunction following infection with chicken anemia agent and infectious bursal disease virus. II. Alterations of in vitro lymphoproliferation and in vivo immune responses. Vet. Immunol. Immunopathol. 1992;34:353–366. doi: 10.1016/0165-2427(92)90175-p. [DOI] [PubMed] [Google Scholar]
- De Boer G.F., Van Roozelaar D.J., Moormann R.J., Jeurissen S.H., Wijngaard J.C., Hilbink F., Koch G. Interaction between chicken anaemia virus and live Newcastle disease vaccine. Avian Pathol. 1994;23:263–275. doi: 10.1080/03079459408418994. [DOI] [PubMed] [Google Scholar]
- Douglas A.J., Phenix K., Mawhinney K.A., Todd D., Mackie D.P., Curran W.L. Identification of a 24 kDa protein expressed by chicken anaemia virus. J. Gen. Virol. 1995;76(Pt 7):1557–1562. doi: 10.1099/0022-1317-76-7-1557. [DOI] [PubMed] [Google Scholar]
- Eltahir Y.M., Qian K., Jin W., Wang P., Qin A. Molecular epidemiology of chicken anemia virus in commercial farms in China. Virol. J. 2011;8:145. doi: 10.1186/1743-422X-8-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erfan A.M., Selim A.A., Helmy S.A., Eriksson P., Naguib M.M. Chicken anaemia virus enhances and prolongs subsequent avian influenza (H9N2) and infectious bronchitis viral infections. Vet. Microbiol. 2019;230:123–129. doi: 10.1016/j.vetmic.2019.01.024. [DOI] [PubMed] [Google Scholar]
- Feng M., Dai M., Cao W., Tan Y., Li Z., Shi M., Zhang X. ALV-J strain SCAU-HN06 induces innate immune responses in chicken primary monocyte-derived macrophages. Poult. Sci. 2017;96:42–50. doi: 10.3382/ps/pew229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, W. 2010. Molecular epidemiological investigation and analysis of transmission characteristics of major immunosuppressive diseases in large-scale broiler farms. Master. Shandong Agric. Univ., China. (In Chinese)
- Justice J.F.t, Morgan R.W., Beemon K.L. Common viral integration sites identified in avian leukosis virus-induced B-cell lymphomas. mBio. 2015;6 doi: 10.1128/mBio.01863-15. e01863-01815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai G.H., Lien Y.Y., Lin M.K., Cheng J.H., Tzen J.T., Sun F.C., Lee M.S., Chen H.J., Lee M.S. VP2 of chicken anaemia virus interacts with apoptin for down-regulation of apoptosis through de-phosphorylated threonine 108 on Apoptin. Sci. Rep. 2017;7:14799. doi: 10.1038/s41598-017-14558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.S., Chang P.C., Shien J.H., Cheng M.C., Shieh H.K. Identification and subtyping of avian influenza viruses by reverse transcription-PCR. J. Virol. Methods. 2001;97:13–22. doi: 10.1016/s0166-0934(01)00301-9. [DOI] [PubMed] [Google Scholar]
- Li H., Liao Z., Ren X., Yang R., Xie Q. Diagnosis and treatment of co-infection of chicken infectious anemia and subgroup J avian leukemia. Poult. Husbandr. Dis. Control. 2017;8:27–28. (In Chinese) [Google Scholar]
- Li T., Xie J., Lv L., Sun S., Dong X., Xie Q., Liang G., Xia C., Shao H., Qin A., Ye J. A chicken liver cell line efficiently supports the replication of ALV-J possibly through its high level viral receptor and efficient protein expression system. Vet. Res. 2018;49:41. doi: 10.1186/s13567-018-0537-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhang K., Pei Y., Xue J., Ruan S., Zhang G. Development and application of an MRT-qPCR assay for detecting coinfection of six vertically transmitted or immunosuppressive avian viruses. Front. Microbiol. 2020;11:1581. doi: 10.3389/fmicb.2020.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Fang L., Cui S., Fu J., Li X., Zhang H., Cui Z., Chang S., Shi W., Zhao P. Genomic characterization of recent chicken anemia virus isolates in China. Front. Microbiol. 2017;8:401. doi: 10.3389/fmicb.2017.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell C.D., Adair B.M., McNulty M.S. Effects of chicken anemia virus on cell-mediated immune function in chickens exposed to the virus by a natural route. Avian Dis. 1993;37:366–374. [PubMed] [Google Scholar]
- McConnell C.D., Adair B.M., McNulty M.S. Effects of chicken anemia virus on macrophage function in chickens. Avian Dis. 1993;37:358–365. [PubMed] [Google Scholar]
- Miles A.M., Reddy S.M., Morgan R.W. Coinfection of specific-pathogen-free chickens with Marek's disease virus (MDV) and chicken infectious anemia virus: effect of MDV pathotype. Avian Dis. 2001;45:9–18. [PubMed] [Google Scholar]
- Payne L.N., Nair V. The long view: 40 years of avian leukosis research. Avian Pathol. 2012;41:11–19. doi: 10.1080/03079457.2011.646237. [DOI] [PubMed] [Google Scholar]
- Qin L., Gao Y., Pan W., Deng X., Sun F., Li K., Qi X., Gao H., Liu C., Wang X. Investigation of co-infection of ALV-J with REV, MDV, CAV in layer chicken flocks in some regions of China. Chin. J. Prevent. Vet. Med. 2010;32:90–93. (In Chinese) [Google Scholar]
- Qiu Y., Qian K., Shen H., Jin W., Qin A. Development and validation of an indirect enzyme-linked immunosorbent assay for the detection of Avian leukosis virus antibodies based on a recombinant capsid protein. J. Vet. Diagn. Invest. 2011;23:991–993. doi: 10.1177/1040638711416966. [DOI] [PubMed] [Google Scholar]
- Rosario K., Breitbart M., Harrach B., Segales J., Delwart E., Biagini P., Varsani A. Revisiting the taxonomy of the family Circoviridae: establishment of the genus Cyclovirus and removal of the genus Gyrovirus. Arch. Virol. 2017;162:1447–1463. doi: 10.1007/s00705-017-3247-y. [DOI] [PubMed] [Google Scholar]
- Su Q., Meng F., Li Y., Zhang Y., Zhang Z., Cui Z., Chang S., Zhao P. Chicken infectious anemia virus helps fowl adenovirus break the protection of maternal antibody and cause inclusion body hepatitis-hydropericardium syndrome in layers after using co-contaminated Newcastle disease virus-attenuated vaccine. Poult. Sci. 2019;98:621–628. doi: 10.3382/ps/pey153. [DOI] [PubMed] [Google Scholar]
- Swayne D.E., Glisson J.R., Mcdougald L.R., Nolan L.K., Suarez D.L., V.L.J.D.o.P. Nair . 13th ed. Wiley-Blackwell; Ames, IA: 2013. Diseases of Poultry. [Google Scholar]
- Tan C., Wang Z., Lei X., Lu J., Yan Z., Qin J., Chen F., Xie Q., Lin W. Epidemiology, molecular characterization, and recombination analysis of chicken anemia virus in Guangdong province. China Arch. Virol. 2020;165:1409–1417. doi: 10.1007/s00705-020-04604-8. [DOI] [PubMed] [Google Scholar]
- Wang Q., Gao Y., Wang Y., Qin L., Qi X., Qu Y., Gao H., Wang X. A 205-nucleotide deletion in the 3′ untranslated region of avian leukosis virus subgroup J, currently emergent in China, contributes to its pathogenicity. J. Virol. 2012;86:12849–12860. doi: 10.1128/JVI.01113-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Song X., Gao H., Wang X., Hu Y., Gao Y., Qi X., Qin L., Lin H., Gao L., Yao S., Han C., Wang X., Chen H. C-terminal region of apoptin affects chicken anemia virus replication and virulence. Virol. J. 2017;14:38. doi: 10.1186/s12985-017-0713-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Imada T., Kaji N., Mase M., Tsukamoto K., Tanimura N., Yuasa N. Identification of a genetic determinant of pathogenicity in chicken anaemia virus. J. Gen. Virol. 2001;82:1233–1238. doi: 10.1099/0022-1317-82-5-1233. [DOI] [PubMed] [Google Scholar]
- Yang, M. 2010. Interaction of co-infection of chicken infectious anemia virus and other immunosuppressive diseases. Master. Shandong Agric. Univ., China. (In Chinese)
- Yin X., Sun Q., Li T., Xu S., Yi T. Detection of CIAV and ALV-J in embryos of AA broilers and breeders and chicks at 1-day-old by PCR. Chin. J. Vet. Sci. 2007:636–639. (In Chinese) [Google Scholar]
- Yu, S. 2015. Molecular epidemiological investigation of CIAV in some areas of Sichuan and the effect of co-infection of CIAV and ALV-J on vaccine immunity. Master. Sichuan Agric. Univ., China. (In Chinese)
- Zhang X., Liu Y., Wu B., Sun B., Chen F., Ji J., Ma J., Xie Q. Phylogenetic and molecular characterization of chicken anemia virus in southern China from 2011 to 2012. Sci. Rep. 2013;3:3519. doi: 10.1038/srep03519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Cui N., Han N., Wu J., Cui Z., Su S. Depression of vaccinal immunity to Marek's disease by infection with chicken infectious anemia virus. Front. Microbiol. 2017;8:1863. doi: 10.3389/fmicb.2017.01863. [DOI] [PMC free article] [PubMed] [Google Scholar]