Abstract
Endodermal epithelial cells (EECs) within the yolk sac membrane (YSM) of avian embryos are responsible for the absorption and utilization of lipids. The lipids in the yolk are mostly composed of very low density lipoprotein (VLDL), uptake mainly depends on clathrin-mediated endocytosis (CME). The CME relies on vesicle formation through the regulation of dynamin (DNM). However, it is still unclear whether DNMs participate in avian embryonic development. We examined mRNA expression levels of several genes involved in lipid transportation and utilization in YSM during Japanese quail embryonic development using qPCR. The mRNA levels of DNM1 and DNM3 were elevated at incubation d 8 and 10 before the increase of SOAT1, CIDEA, CIDEC, and APOB mRNA's. The elevated gene expression suggested the increased demand for DNM activity might be prior to cholesteryl ester production, lipid storage, and VLDL transport. Hinted by the result, we further investigated the role of DNMs in the embryonic development of Japanese quail. A DNM inhibitor, dynasore, was injected into fertilized eggs at incubation d 3. At incubation d 10, the dynasore-injected embryo showed increased embryonic lethality compared to control groups. Thus, the activity of DNMs was essential for the embryonic development of Japanese quail. The activities of DNMs were also verified by the absorptions of fluorescent VLDL (DiI-yVLDL) in EECs. Fluorescent signals in EECs were decreased significantly after treatment with dynasore. Finally, EECs were pretreated with S-Nitroso-L-glutathione (GSNO), a DNM activator, for 30 min; this increased the uptake of DiI-yVLDL. In conclusion, DNMs serve a critical role in mediating lipid absorption in YSM. The activity of DNMs was an integral part of development in Japanese quail. Our results suggest enhancing lipid transportation through an increase of DNM activity may improve avian embryonic development.
Key words: Development, dynamin, endodermal epithelial cell, lipid, yolk sac
INTRODUCTION
Yolk serves as a main source of nutrients for embryonic growth in avian eggs (van der Wagt et al., 2020). Lipids in the yolk provide about 90% of the energy demand for the avian embryo (Speake et al., 1998; van der Wagt et al., 2020). The yolk lipids, primarily consisting of triacylglycerol, phospholipids, and cholesterol, are derived from livers lipids packaged in very low density lipoproteins (VLDLs). The VLDLs are then transported into growing oocytes (Burley et al., 1993; Schneider, 2016). During embryonic development, the yolk sac membrane (YSM) extends from the abdomen of the embryo to embrace and ingest the yolk progressively (Starck, 2020; Wong and Uni, 2021). The YSM play important roles in the uptake lipids from yolk and the release lipids into embryonic circulation (Schneider, 2016). The endodermal epithelial cells (EECs), within the YSM, in the area vasculosa are considered to be the most important cells for nutrient transportation in YSM (Lambson, 1970; Sheng and Foley, 2012; Yadgary et al., 2014). Facing the yolk, EECs are responsible for absorption of yolk lipids which are sequentially transported to the embryonic circulation (Schneider, 2016). Several proteins involved in the lipid utilization by YSM in avian embryos have been demonstrated. The lipoprotein receptors expressed on the surface of YSM's and EEC's, such as LR8, Cubilin, and LRP2, have been suggested to contribute to the import of VLDLs (Hermann et al., 2000; Bauer et al., 2013). In YSM, free cholesterol absorbed from yolk is esterified by acyl-CoA:cholesterol acyltransferase to form cholesteryl ester (CE), abundant in VLDL derived from yolk sac (Noble and Cocchi, 1990; Speake et al., 1998; Wang et al., 2017; Lin et al., 2020). Moreover, the proteins for VLDL formation can be detected during development of YSM (Hermann et al., 2000).
Dynamine (DNM) plays important roles in clathrin-mediated endocytosis (CME, also called receptor-mediated endocytosis) and clathrin-independent endocytic pathways (Ferguson and De Camilli, 2012). The CME, with the coordination of clathrin and other proteins, is the major cellular pathway to internalize low density lipoprotein (LDL) and VLDL (Goldstein et al., 1979; Goldstein et al., 1982; McMahon and Boucrot, 2011). With a GTPase domain, dynamin can be readily regulated (Warnock et al., 1996; McMahon and Boucrot, 2011; Ferguson and De Camilli, 2012; Cocucci et al., 2014). To mediate the membrane scission to form vesicles, DNM is recruited to the budding vesicles and assembled to form helical structures (Ferguson and De Camilli, 2012; Cocucci et al., 2014). In mammals, there are 3 isoforms of DNM (Cao et al., 1998). The DNM is composed of 4 main domains, 1) the G domain with GTPase activity, 2) a GTPase effector domain that interacts with the G domain, 3) a proline-rich domain, interacting with the protein having Src homology 3 (SH3) domain to regulate signal pathways and endocytosis, and 4) a pleckstrin homology (PH) domain, associating with the phospholipid of cell membranes (Warnock et al., 1996; McMahon and Boucrot, 2011; Ferguson and De Camilli, 2012; Cocucci et al., 2014; Krishnan et al., 2015).
EECs within the YSM play important roles in the transportation of yolk lipids and the production of cholesteryl ester during avian embryonic development. However, its mechanism has not been fully understood. In the current study, we identified 2 dynamin isoforms, DNM1 and DNM3, each was increased in EECs at incubation d 8 and 10, respectively. We hypothesized that DNMs may play a role in the embryonic development. We used Japanese quail to evaluate the involvement of DNMs in the embryonic development. Our results of experiments in vivo showed inhibiting dynamins resulted in embryonic lethality in Japanese quail embryos. Dynamins were required during the embryonic development of Japanese quail. Moreover, our results suggested that inhibition of dynamin activity decreased the uptake of VLDL in EECs. Activating dynamin activity promoted the uptake of VLDL in EECs. Our results showed that VLDL uptake was mediated by dynamins and was essential during embryonic development in Japanese quail embryos.
MATERIALS AND METHODS
Animals
The animal experiment protocol was approved by the Institutional Animal Care and Use Committee of National Taiwan University (NTU107-EL-00148). Birds had access to feed and water ad libitum. To obtained the fertilized eggs, each cage housed 1 male and 2 female adult Japanese quails at 25°C under a 14-h light: 10-h dark cycle. The fertilized eggs were incubated in an egg incubator at 37.5°C and relative humidity of 60%. The incubated eggs were automatically turned 6 times daily in the egg incubator.
Dissection and Primary Culture of EECs From Japanese Quail Embryos
We followed the procedure from previous studies to isolate the primary EECs from Japanese quail embryos (Bauer et al., 2013; Lin et al., 2016; Wang et al., 2017). In brief, YSM were separated from the sinus terminals of mesoderm in Japanese quail embryos at incubation d 5 to obtain the endoderm. After digestion with collagenase type IV (17104019, Gibco, MA) at 37°C for 15 min, the endoderm of YSM from 6 embryos in the same group were collected and pooled as a single sample. The pooled samples were seeded in 24-well plates and cultured in DMEM/ F12 (pH 7.4, 12400–024, Gibco) with 10% new born calf serum (16010–159, Gibco) and 1% Pen-Strep Ampho. solution (03-033-1B, Biological Industries, CT). After culture for 2 d, EEC explants were formed and used for further experiment.
Gene Expression Was Determined by Real-Time PCR
Total RNA was extracted from Japanese quail embryonic YSM and EECs using the GENEzol Reagent (#GZR100, Geneaid, New Taipei, Taiwan). Two μg of total RNA of each sample was utilized to produce cDNA using a high capacity cDNA reverse-transcription kit (#4368814, Applied Biosystem, MA). The synthesized cDNA samples were used to analyze the mRNA levels using the SensiFAST SYBR Hi-ROX reagent (#BIO-92005, Bioline, London, UK) via the Step One Plus Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA). The real-time PCR program consisted of an initial denaturation for 7 min at 95°C, followed by 40 repeated cycles of denaturation for 10 s at 95°C, annealing for 30 seconds at 60°C, extension for 1 minute at 72°C, and a final extension for 10 min at 72°C. Relative gene expression was normalized by β-actin in the same sample and calculated using the following formula: 2−(Ct target genes- Ct β-actin) (Pfaffl, 2001). Primer sequences were shown in Table 1.
Table 1.
Primer set for real-time PCR.1
| Gene | Primer sequence (5’→3’) | Product size (bp) | NCBI reference number |
|---|---|---|---|
| SOAT1 | S 5’-GAAGGGGCCTATCTGGAACG-3’ A 5’-ATCTGCACGTGACATGACCA-3’ |
168 | XM_015290507.1 |
| APOB | S 5’-GGCCCTGATTCAGTGTGGAA-3’ A 5’-TCCCGGATTCTCTTCGGAGT-3’ |
144 | XM_015859545.1 |
| CIDEA | S 5’-CTCGGTGCTGCGATCTTTGG-3’ A 5’-GAGAGGAGGGAACAGGGTCT-3’ |
145 | XM_015855240.1 |
| CIDEC | S 5’-CCACGCTCTATGGCACCTAC-3’ A 5’-AGCGCTTCCTTCATGAGCC-3’ |
76 | XM_015874420.1 |
| DNM1 | S 5’-TTGTCCTGACAGCAGAGAAC-3’ A 5’-TGCTGGACATGAACCCCTTC-3’ |
120 | XM_015879114 |
| DNM3 | S 5’ - CATGGCAGATCGGATGGGAA-3’ A 5’ - GGAGTTTGCTCCTGAAGGCT-3’ |
101 | XM_015869656.1 |
| ACTB | S 5’-GTGATGGACTCTGGTGATGG-3’ A 5’-TGGTGAAGCTGTAGCCTCTC-3’ |
151 | NM_205518.1 |
SOAT1, sterol O-acyltransferase 1; APOB, apolipoprotein B; CIDEA, cell death inducing DFFA like effector A; CIDEC, cell death inducing DFFA like effector C; DNM1, dynamin 1; DNM2, dynamin 2; and ACTB, β-actin.
Dynasore Injection of Fertilized Japanese Quail Eggs Yolk
Eggs were incubated in an incubator at 37.5°C until d 3. Then, a candling inspection was implemented to exclude unfertilized eggs and early dysplasia of embryos. The eggs at incubation d 3 were randomly divided into 4 groups as follows: 1) a control group with no treatment (n = 11); 2) a sham group with a drilled hole and puncture by a 30 G needle into the egg yolk to simulate the dynasore (#14062, Cayman Chemical, MI) injection (n = 12); 3) a vehicle group injected with the same volume of dimethyl sulfoxide (DMSO) as the volume of injected dynasore (n = 15), and 4) a dynasore group injected with 35 µL of 225 mM dynasore, dissolved in DMSO, into the egg's yolk (final dosage = 2.538 mg / egg) (n = 15). The holes on the egg shells were filled by paper tape and wax. Then the eggs were incubated until incubation d 10. At d 10, the eggs were opened and the morphology of embryos was observed. To quantify the effects of dynasore injection on the fertilized eggs, ratio of embryonic lethality was calculated using the following formula: .
Isolation of Yolk VLDL and Labeling With 1,1′-Dioctadecyl-3,3,3′,3′ Tetramethylindocarbocyanine Perchlorate (DiI)
The isolation of yolk VLDL from the previous study was used in the present study following the published procedure (Bauer et al., 2013). Fertilized egg yolk at incubation d 5 was mixed with VLDL isolation buffer (20 mM Tris/HCl, 0.2 mM EDTA, 150 mM NaCl). The mixture of yolk and VLDL isolation buffer was centrifuged at 40,000 × g for 24 h at 4°C. After centrifugation, the yolk VLDL (yVLDL) was obtained from the top fraction and then dissolved in VLDL isolation buffer. Protein concentration of the isolated yVLDL was determined via the Bradford assay (#23238, Thermo Fisher Scientific). The isolated yVLDL sample containing 1 mg of protein was mixed with 50 µL of DiI solution (2.5 mg/mL in DMSO, #D3911, Thermo Fisher Scientific) The mixture of yVLDL and DiI was incubated and gently shaken for 16 h at 37°C in the dark. After centrifugation at 40,000 × g for 24 h at 4°C, DiI-labeled yVLDL (DiI-yVLDL) was collected from the top fraction of the tube. The DiI-yVLDL was diluted in DiI VLDL buffer (0.02 mM EDTA, 150 mM NaCl) to 150 mg/mL and stored at 4°C.
Treatment of EECs With Dynasore, S-Nitroso-L-Glutathione, and DiI-yVLDL
EECs were seeded on cover slides. EECs were incubated in serum-free DMEM/F12 for 4 h prior to treatments. Then, the medium was replaced by serum-free DMEM/F12 containing dynasore (final concentration 80 µM) to treat EECs for various time periods. The DiI-yVLDL was added to treat EECs for 2 h. The EECs were pretreated with 100-µM S-Nitroso-L-Glutathione (GSNO) (#82240, Cayman Chemical, MI) for 30 min before exposure to DiI-yVLDL. To perform confocal microscopy, EECs were washed twice with PBS and fixed with 4% paraformaldehyde for 30 min. After removing the paraformaldehyde, the cells were washed twice with PBS. 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI) (#sc-24941, Santa Cruz Biotechnology, TX) was used to stain the nucleus. After DAPI staining, the cover slide with stained cells was mounted with nail polish. Images were captured from the Leica Confocal Microscope (TCS SP5 II, Leica, Nussloch, Germany) with 549 nm excitation and 565 nm emission.
Statistical Analysis
Fluorescent signal was quantified by ImageJ software. Statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc., CA). The values were mean ± SEM. The change of mRNA levels and the DiI/DAPI intensity ratio in the EECs treated with dynasore or GSNO for different times were analyzed using one-way ANOVA followed by Tukey's test to evaluate differences. The embryonic lethality was analyzed using a nonparametric method, Kruskal-Wallis one-way ANOVA, followed by Dunn's multiple comparison test. The DiI/DAPI intensity ratio in the EECs treated with dynasore at different concentrations was analyzed using a t test. A P-value ≤ 0.05 was considered statistically different.
RESULTS
The mRNA Expression Levels of Lipid Utilization Genes in YSM During Embryonic Developmental of Japanese Quail
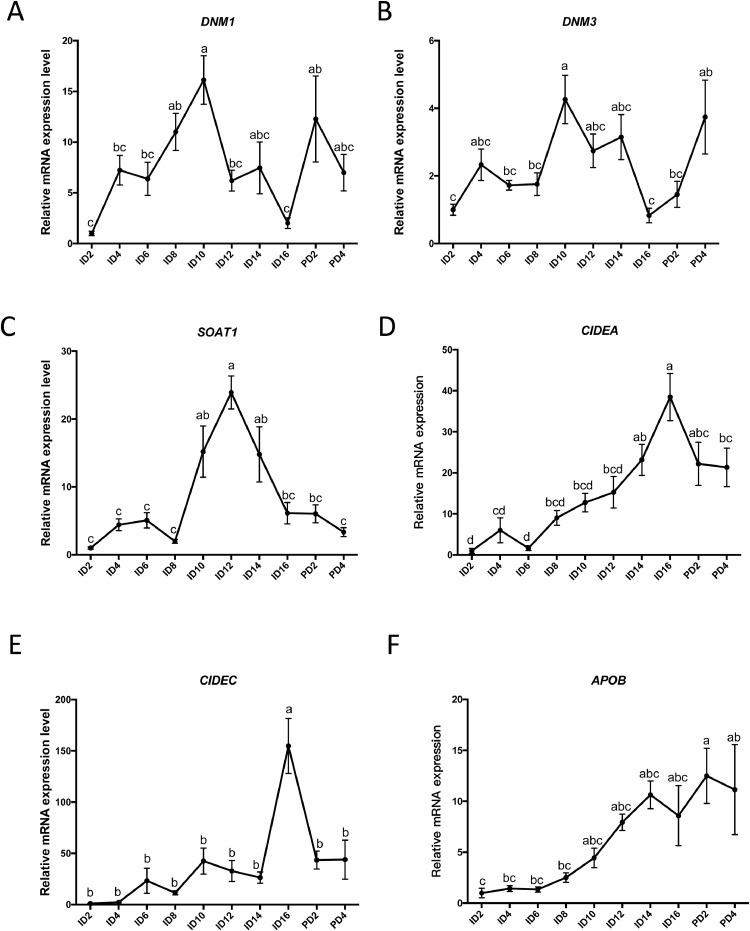
The changes of mRNA expression levels of DNM1, DNM3, Sterol O-acyltransferase 1 (SOAT1), Cell Death Inducing DFFA Like Effector A (CIDEA), Cell Death Inducing DFFA Like Effector C (CIDEC) and Apolipoprotein B (APOB) in the Japanese quail YSM were determined from incubation d 2 to post-hatch d 4 using real-time PCR analyses. Compared with incubation d 2, mRNA levels of DNM1 were increased from incubation d 8 to 10 and at post-hatch d 2 (Figure 1A). The mRNA levels of DNM3, the other isoform of dynamin, were increased at incubation d 10 and post-hatch d 4 (Figure 1B). The SOAT1 mRNA expression increased from incubation d 10 and reached a peak at d 12 of incubation (Figure 1C). The CIDEA mRNA expression was increased from incubation d 14 to post-hatch d 4 (Figure 1D). The expression of CIDEC was increased at incubation d 16 (Figure 1E). The APOB mRNA was increased from the d 2 to 4 after hatching (Figure 1F).
Figure 1.
The mRNA expression levels of lipid utilization genes in YSM during embryonic developmental of Japanese quail. (A) The mRNA levels of DNM1, (B) DNM3, (C) SOAT1, (D) CIDEA, (E) CIDEC, and (F) APOB in YSM were analyzed at the time points indicated. ID and PD represented incubation day and post-hatch day, respectively. The mRNA levels were detected by real-time PCR and normalized by β-actin in the same sample. The values were shown as mean ± SEM. SOAT1, n = 11 for each group; APOB, n = 3 for each group; CIDEA and CIDEC, n = 9 for each group; DNM1 and DNM3, n = 7 for each group. Values with different letters indicated significance at P ≤ 0.05 (one-way ANOVA). Abbreviations: APOB, apolipoprotein B; CIDEA, cell death inducing DFFA like effector A; CIDEC, cell death inducing DFFA like effector C; DNM1, dynamin 1; DNM3, dynamin 3; SOAT1, sterol O-acyltransferase 1.
Inhibition of Dynamins Led to Embryonic Lethality in Japanese Quail
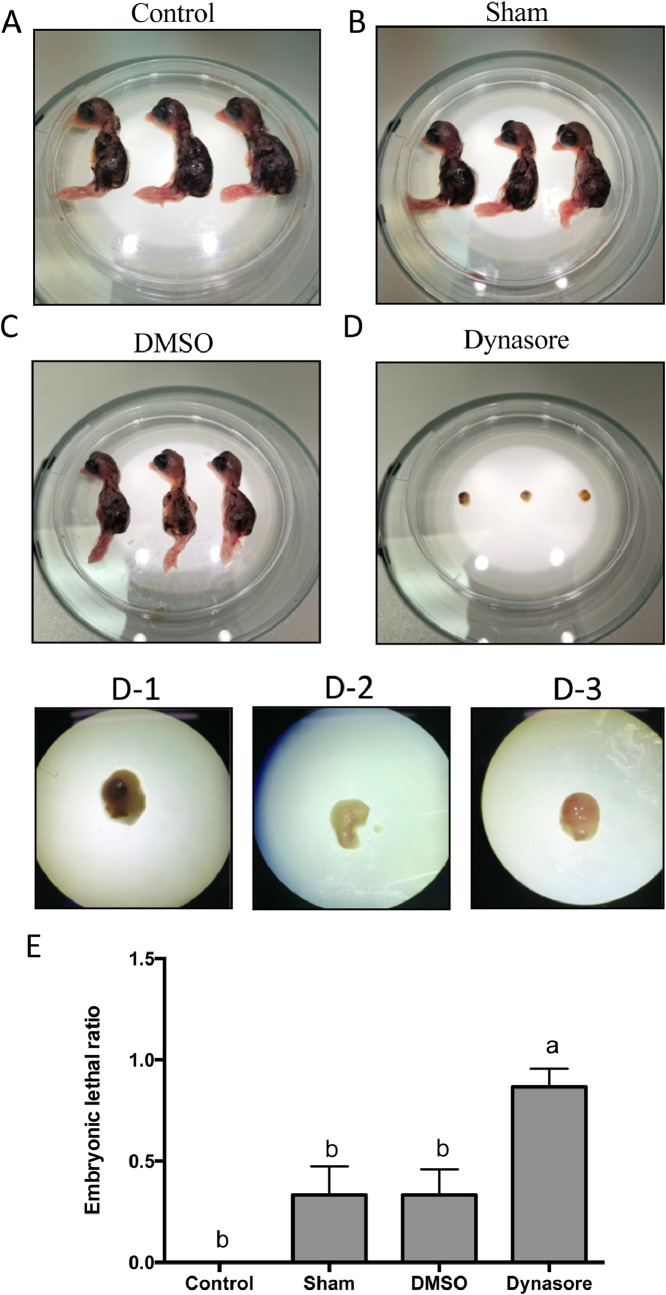
Among the lipid utilization related genes analyzed in the current study, mRNA levels of DNMs, particularly DNM1, were elevated prior to the changes of SOAT1, APOB, CIDEA, and CIDEC. The roles of DNMs in embryonic development of Japanese quail have not been determined. Dynasore, a dynamin inhibitor, was injected into yolks of fertilized eggs to examine whether the inhibition of dynamins affects its development. Dynasore (2.538 mg per egg) was injected at incubation d 3 with observation of embryo morphology at incubation d 10. The morphology of the embryos in the sham and DMSO groups was similar to the control group (Figures 2A–2C). However, the dynasore-injected embryos showed developmental defects (Figure 2D). Statistical results indicated that the ratio of early embryonic lethality in the dynasore-injected group was higher than in the control, sham, or DMSO groups (Figure 2E). The result indicates that functions of dynamins were essential for the embryonic development of Japanese quail.
Figure 2.
Inhibition of dynamins led to embryonic lethality in Japanese quail. (A) Appearance of the Japanese quail embryos at incubation d 10 from the eggs of control, (B) sham, (C) vehicle (DMSO), and (D) dynasore groups. (D-1 to D-3) Magnified pictures of embryos from D. After injection on incubation d 3, Japanese quail were incubated at 37°C until d 10. Thirty five µL DMSO (dimethyl sulfoxide) was injected into the DMSO group whereas the same amount of PBS was injected into the sham group. The dynasore group received dynasore (225 mM) in 35 µL DMSO. (E) The ratios of the embryonic lethality in control, sham, vehicle, and dynasore groups; the values were shown as mean ± SEM. Control group, n = 11; sham group, n = 12; DMSO group, n = 15, Dynasore group, n = 15. The statistical analysis used a nonparametric method, Kruskal-Wallis one-way ANOVA followed by Dunn's multiple comparison test. A P-value ≤ 0.05 was considered statistically different. The groups that share the same letters indicated no significant differences.
The Effect of Dynasore on the Uptake of DiI-yVLDL in EECs
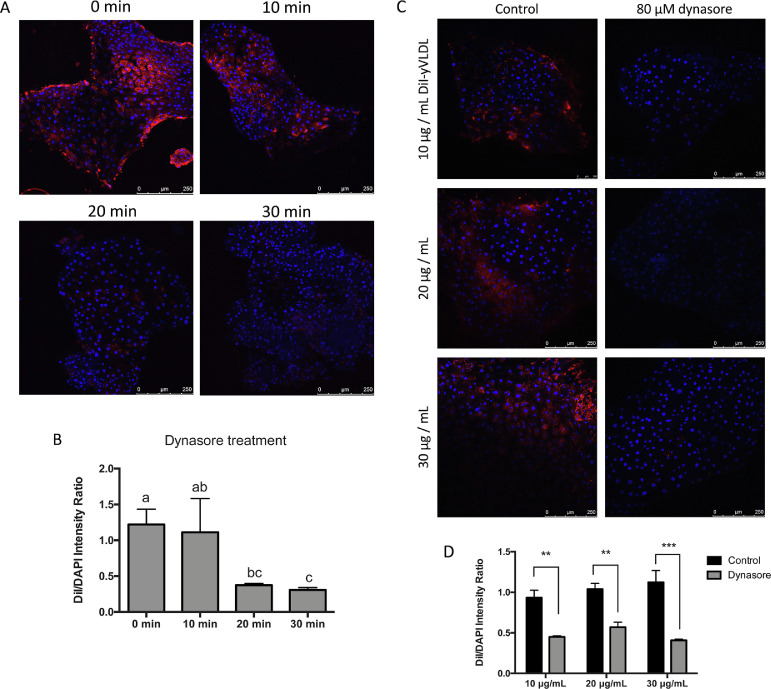
To verify whether dynamins were involved in VLDL uptake in EECs in the embryos of Japanese quail, we isolated primary EECs from the embryos at incubation d 5 to examine its ability of VLDL uptake. DiI-yVLDL, yolk VLDL labeled with red fluorescent, was utilized to detect the levels of VLDL internalized into EECs. After pretreating with 80 μM dynasore for 10 min, the red fluorescent signal in EECs exposed to DiI-yVLDL was not different from the control group (Figure 3A). Compared with control group, however, the fluorescent signals were decreased in the EECs pretreated with dynasore for 20 or 30 min (Figure 3A). Quantified results showed that the fluorescent signals in the cells pretreated with dynasore for 20 and 30 min were lower than the control groups (Figure 3B). Moreover, the pretreatments with 80 μM dynasore for 30 min decreased the imported DiI-yVLDL in the EECs exposed to 10, 20, or 30 μg/mL of DiI-yVLDL for two hours (Figures 3C and 3D). Thus, dynamins have a function in the uptake of VLDL into the EECs of Japanese quail embryos.
Figure 3.
The effect of dynasore on the uptake of DiI-yVLDL into EECs. (A) The EECs were pretreated with 80 μM dynasore in DMSO for 10, 20, or 30 min, respectively; the EECs without any treatments were a control group (0 min). Then the EECs were treated with 20 μg/mL of yolk VLDL labeled with DiI (DiI-yVLDL) for 2 h. The EECs were photographed using confocal microscopy and the accumulated DiI-yVLDLs in the EECs were photographed using confocal microscopy. DAPI was used to stain the nucleus. Red signal, DiI-yVLDL; blue signal, DAPI. (B) The quantitative results of the fluorescence intensities of DiI/DAPI ratio in DiI-yVLDL-treated EECs with the pretreatments of dynasore for 0, 10, 20, or 30 min. Data presented as mean ± SEM (n = 3). Statistical analysis was performed by one-way ANOVA. Groups with different superscripts indicated a significant difference (P ≤ 0.05). (C) EECs were pretreated with 80 μM dynasore or vehicle (control) for 30 min and then treated with 10, 20, or 30 ng/mL of Dil-yVLDL for 2 h, respectively. Red signal, DiI-yVLDL; blue signal, DAPI. (D) The quantitative results of the fluorescence intensities of DiI/DAPI ratio in EECs with various treatments in (C). Data were analyzed by t test. ** P ≤ 0.01, *** P ≤ 0.001. (n = 3). Abbreviation: EEC, endodermal epithelial cells.
Uptake of DiI-yVLDL in EECs Was Enhanced by GSNO
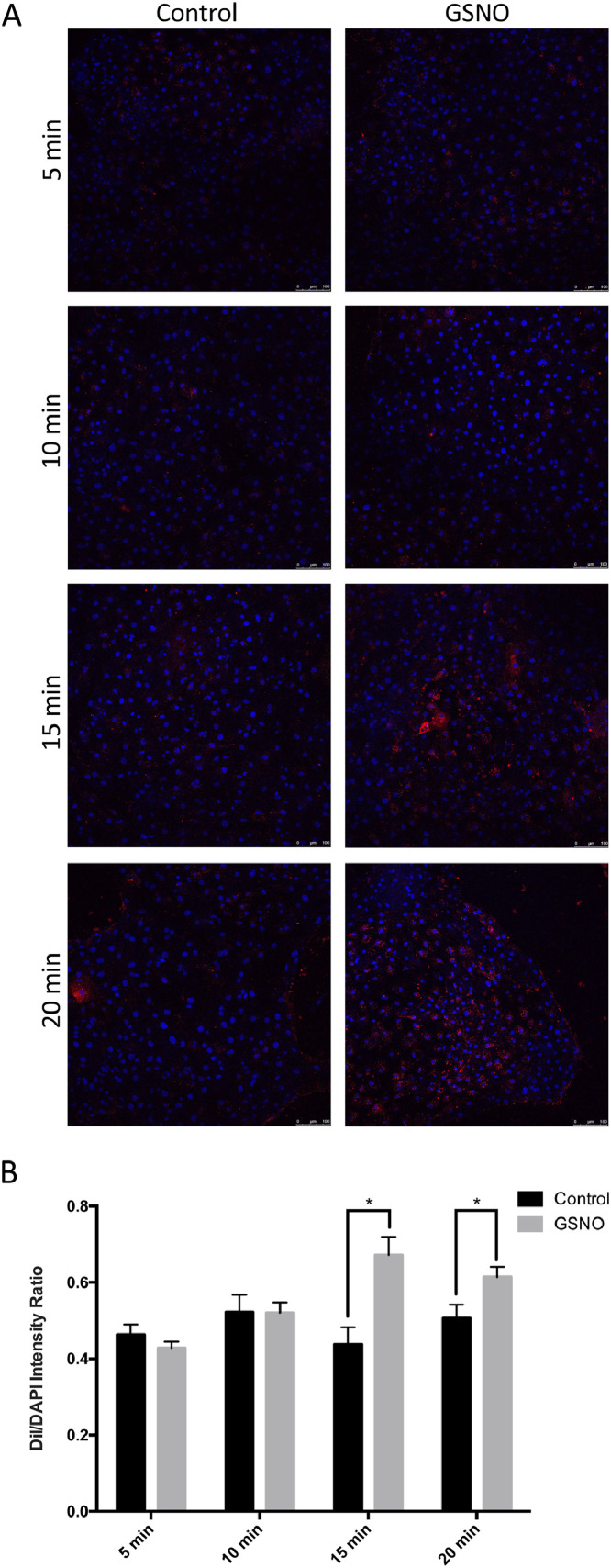
To further confirm the role of dynamins in the VLDL uptake into the EECs of Japanese quail embryos, we examined whether an increase of dynamins activity promotes VLDL uptake. After pretreatment with a nitric oxide (NO) donor, GSNO (100 μM), for 30 min to increase the GTPase activity of dynamins (Wang et al., 2006; Kang-Decker et al., 2007), the EECs exposed to DiI-yVLDL for 5 or 10 min showed comparable fluorescent signals compared to those without GSNO pretreatment (Figure 4A). After exposure to DiI-yVLDL for 15 or 20 min, however, EECs presented increased fluorescent signals in the GSNO-pretreated group (Figure 4B). Thus, GSNO enhanced the uptake of Dil-yVLDL and that dynamins play a role in VLDL uptake in Japanese quail embryos.
Figure 4.
Uptake of DiI-yVLDL in EECs was enhanced by GSNO. (A) EECs were pretreated with 100 μM S-Nitroso-L-glutathione (GSNO, dynamin activator) or vehicle for 30 min and then exposed to 10 μg/mL of Dil-yVLDL for 5, 10, 15, or 20 min, respectively. DAPI was used to stain the nucleus. Red signal, DiI-yVLDL; blue signal, DAPI. (B) The quantitative results of the fluorescence intensities of DiI/DAPI ratio in EECs with various treatments in (A). Data were analyzed by t test. * P ≤ 0.05. (n = 4, each group). Abbreviation: EEC, endodermal epithelial cells.
DISCUSSION
Middle to late stages of incubation are critical periods for the avian embryo to utilize lipids from the yolk (Noble and Cocchi, 1990; Speake et al., 1998). In chicken embryos, the genes related to lipid transportation and lipid metabolism are upregulated in YSM from embryonic d 13 to 17 (Yadgary et al., 2014). This upregulation may enable YSM to actively absorb yolk TG and cholesterol. The increased activity of SOAT and inhibited activity of cholesteryl ester hydrolase in YSM enhanced the absorbed cholesterol converted to cholesteryl ester (Shand et al., 1993; Wang et al., 2017; Lin et al., 2020). The cholesterol esters are then transferred to embryos to support embryonic growth (Noble and Cocchi, 1990; Speake et al., 1998; Ye et al., 2009; Tiwari et al., 2013). Disrupted utilization of yolk lipids in YSM may lead to nutritional deficiencies resulting in embryonic mortality. EECs within YSM, functions to mediate lipid absorption, lipid remodeling, and lipoprotein assembly, are the predominate cells for transporting lipids to embryos during development (Bauer et al., 2013; Wong and Uni, 2021). Several enzymes and proteins, including SOAT1, lipoprotein lipase, APOB, and LR8, are involved in lipid transfer to avian embryos (Noble and Cocchi, 1990; Shand et al., 1993; Speake et al., 1998; Ding and Lilburn, 2000). In the current study, we demonstrated that DNMs were required for lipid transportation into EECs of the yolk sac membrane for embryonic growth.
DNM proteins play roles in the uptake of LDL and VLDL to transport nutrients (Goldstein et al., 1982; Ferguson and De Camilli, 2012; Cocucci et al., 2014). The activity of SOAT, functions in the conversion of cholesterols to cholesteryl esters, in YSM can be regulated by nutrients and hormones (Wang et al., 2017). Cholesteryl esters are actively produced in YSM at mid stages of avian embryonic development (Noble and Cocchi, 1990; Shand et al., 1993; Speake et al., 1998; Ding and Lilburn, 2000). Consistent with previous studies, our results showed expression levels of SOAT1 peaked at d 12 of incubation. Microsomal triglyceride transfer protein (MTP), a VLDL synthesizing enzyme, is highly expressed at 9, 11, and 16 incubation days in chicken YSM (Hermann et al., 2000). The CIDE protein controls lipid metabolism in the liver or adipose tissue (Xu et al., 2012) and interacts with APOB in the VLDL transport vesicles (Ye et al., 2009; Tiwari et al., 2013). Our results indicated that levels of APOB and CIDEA mRNA expression were elevated late and suggested that the synthesis of VLDL in YSM was primarily at late embryonic stages in Japanese quail. The expression level of DNM1 mRNA reached a peak earlier than those for SOAT1, APOB, and CIDEA do, suggesting that the machinery to transport lipids was present prior to the enzymes to produce the nutrients for transportation. The CME are important for the import of LDL and VLDL (Goldstein et al., 1979; Harding et al., 1983). The LDL receptor is known as a classical receptor in CME (McMahon and Boucrot, 2011); however, CME is also involved in mediating VLDL transportation in the avian embryonic yolk (Burley et al., 1993). Previous studies indicate that the mid-stage of incubation is an important period for the YSM to absorb lipids in chicken embryos (Yadgary et al., 2010; Uni et al., 2012; Yadgary and Uni, 2012). It was found that DNM1 and DNM3 mRNA was increased at incubation d 8 and 10, respectively. DNMs also function in the regulation of exocytosis (Jaiswal et al., 2009; Anantharam et al., 2011; Jackson et al., 2015). It is still not clear whether DNMs also play roles in the transport of the nutrients from the yolk to avian embryos. Based on the current data, we depicted a potential mechanism for lipid utilization in EECs (Figure 5); in short, DNMs were highly expressed during incubation d 8 to 10 and a large amount of VLDL was endocytosed into EECs. Thus, substrates were provided to the SOAT1 to esterify cholesterol in ER at the mid-stage of incubation. At late stages of incubation, APOB and CIDEA participate to synthesize VLDL and transport it to the embryonic circulation system.
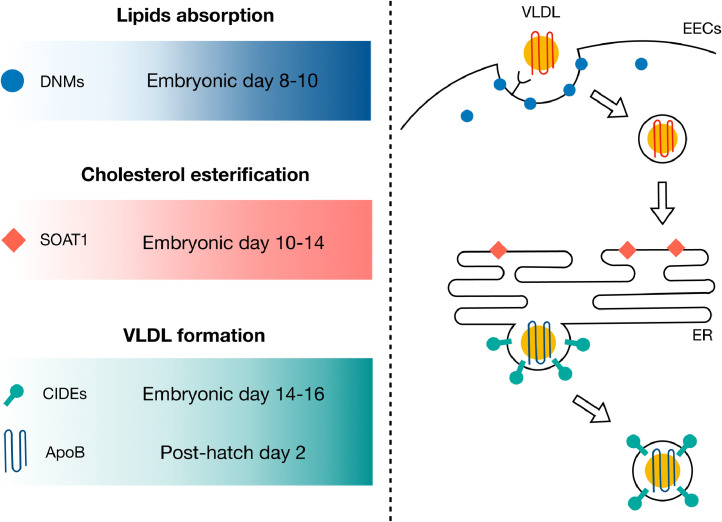
Figure 5.
Working model of the involvement of DNMs, SOAT1, CIDEs, and APOB in lipids absorption and utilization in EECs. During embryonic development of Japanese quail, the upregulation of DNMs during incubation d 8 to 10 may have contributed to the uptake of VLDL from yolk into EECs. During incubation d 10 to 14, SOAT1 was increased to convert the imported cholesterol, carried by yolk VLDL, to cholesteryl ester in endoplasmic reticulum (ER). At late stages of incubation, APOB and CIDEs were increased to cope with the demands for lipids in the developing embryos. Abbreviations: ApoB, apolipoprotein B; CIDE, cell death inducing DFFA like effector; DNM, dynamin; EEC, endodermal epithelial cells; ER, endoplasmic reticulum; SOAT1, sterol O-acyltransferase 1; VLDL, very low density lipoprotein.
In the dynasore injection study, all the embryos in the control groups developed normally, implying that the candling inspection at incubation d 3 can help us precisely detect unfertilized eggs or early dysplasia embryos. Injection of a large dose of DMSO into chick egg yolk affects the embryonic growth performance (Wyatt and Howarth, 1976), we made a similar observation. Since dynasore is able to bind to the lipid rafts to affect cell signaling and substrate recognition by their receptors (Preta et al., 2015), it is used as a DNM inhibitor to study cellular endocytosis (Macia et al., 2006; Mohanakrishnan et al., 2017). Because dynasore treatment of EECs reduced lipid accumulation and dynasore injection into embryos inhibited development, we speculated that dynasore inhibited DNM activity resulting in embryonic lethality in Japanese quail. NO stimulates the GTPase and self-assembly activity of DNMs by S-nitrosylation at a cysteine residue to promote endocytosis in endothelial cells ( Wang et al., 2006; Kang-Decker et al., 2007). The NO donated by GSNO does not target DNMs specifically; GSNO decreases the augmentation index and improves hemodynamics to alleviate pre-eclampsia (Root et al., 2004; Everett et al., 2014). In spite of these alternative possibilities, GSNO activated DNM activity and stimulated DiI-yVLDL uptake in EECs.
In conclusion, this is the first research to show that DNM serves a critical role in lipid absorption in YSM of avian embryos. In primary EECs, our results showed activating DNMs increased VLDL import; conversely, inhibiting DNMs suppressed VLDL import. The DNMs participated in the regulation of lipid transportation during embryonic development. Our results showed the injection of DNM inhibitors in fertilized eggs led to embryonic death. Thus, the activity of DNMs was vital during embryonic development. Accordingly, we hypothesized that the functions of DNMs in lipid transportation were required in avian embryonic development.
ACKNOWLEDGMENTS
We appreciate the supporting of Ministry of Science and Technology, Taiwan (Grant number 104-2313-B-002-039-MY3).
Consent for Publication: All authors consent to the publication of the manuscript.
DISCLOSURES
The authors declare no conflict of interests. This manuscript contains results only from pure scientific research project funded by Ministry of Science and Technology, Taiwan.
REFERENCES
- Anantharam A., Bittner M.A., Aikman R.L., Stuenkel E.L., Schmid S.L., Axelrod D., Holz R.W. A new role for the dynamin GTPase in the regulation of fusion pore expansion. Mol. Biol. Cell. 2011;22:1907–1918. doi: 10.1091/mbc.E11-02-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R., Plieschnig J.A., Finkes T., Riegler B., Hermann M., Schneider W.J. The developing chicken yolk sac acquires nutrient transport competence by an orchestrated differentiation process of its endodermal epithelial cells. J. Biol. Chem. 2013;288:1088–1098. doi: 10.1074/jbc.M112.393090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley R.W., Evans A.J., Pearson J.A. Molecular aspects of the synthesis and deposition of hens' egg yolk with special reference to low density lipoprotein. Poult. Sci. 1993;72:850–855. doi: 10.3382/ps.0720850. [DOI] [PubMed] [Google Scholar]
- Cao H., Garcia F., McNiven M.A. Differential distribution of dynamin isoforms in mammalian cells. Mol. Biol. Cell. 1998;9:2595–2609. doi: 10.1091/mbc.9.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci E., Gaudin R., Kirchhausen T. Dynamin recruitment and membrane scission at the neck of a clathrin-coated pit. Mol. Biol. Cell. 2014;25:3595–3609. doi: 10.1091/mbc.E14-07-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S.T., Lilburn M.S. The developmental expression of acyl-coenzyme A: cholesterol acyltransferase in the yolk sac membrane, liver, and intestine of developing embryos and posthatch turkeys. Poult. Sci. 2000;79:1460–1464. doi: 10.1093/ps/79.10.1460. [DOI] [PubMed] [Google Scholar]
- Everett T.R., Wilkinson I.B., Mahendru A.A., McEniery C.M., Garner S.F., Goodall A.H., Lees C.C. S-Nitrosoglutathione improves haemodynamics in early-onset pre-eclampsia. Br. J. Clin. Pharmacol. 2014;78:660–669. doi: 10.1111/bcp.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S.M., De Camilli P. Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Biol. 2012;13:75–88. doi: 10.1038/nrm3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J.L., Anderson R.G., Brown M.S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979;279:679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Goldstein J.L., Anderson R.G., Brown M.S. Receptor-mediated endocytosis and the cellular uptake of low density lipoprotein. Ciba Found. Symp. 1982:77–95. doi: 10.1002/9780470720745.ch5. [DOI] [PubMed] [Google Scholar]
- Harding C., Heuser J., Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann M., Mahon M.G., Lindstedt K.A., Nimpf J., Schneider W.J. Lipoprotein receptors in extraembryonic tissues of the chicken. J. Biol. Chem. 2000;275:16837–16844. doi: 10.1074/jbc.M000163200. [DOI] [PubMed] [Google Scholar]
- Jackson J., Papadopulos A., Meunier F.A., McCluskey A., Robinson P.J., Keating D.J. Small molecules demonstrate the role of dynamin as a bi-directional regulator of the exocytosis fusion pore and vesicle release. Mol. Psychiatry. 2015;20:810–819. doi: 10.1038/mp.2015.56. [DOI] [PubMed] [Google Scholar]
- Jaiswal J.K., Rivera V.M., Simon S.M. Exocytosis of post-Golgi vesicles is regulated by components of the endocytic machinery. Cell. 2009;137:1308–1319. doi: 10.1016/j.cell.2009.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang-Decker N., Cao S., Chatterjee S., Yao J., Egan L.J., Semela D., Mukhopadhyay D., Shah V. Nitric oxide promotes endothelial cell survival signaling through S-nitrosylation and activation of dynamin-2. J. Cell. Sci. 2007;120:492–501. doi: 10.1242/jcs.03361. [DOI] [PubMed] [Google Scholar]
- Krishnan S., Collett M., Robinson P.J. SH3 domains differentially stimulate distinct dynamin I assembly modes and G domain activity. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambson R.O. An electron microscopic study of the entodermal cells of the yolk sac of the chick during incubation and after hatching. Am. J. Anat. 1970;129:1–19. doi: 10.1002/aja.1001290102. [DOI] [PubMed] [Google Scholar]
- Lin H.J., Lin C.W., Mersmann H.J., Ding S.T. Sterol-O acyltransferase 1 is inhibited by gga-miR-181a-5p and gga-miR-429-3p through the TGFbeta pathway in endodermal epithelial cells of Japanese quail. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2020;240 doi: 10.1016/j.cbpb.2019.110376. [DOI] [PubMed] [Google Scholar]
- Lin H.J., Wang S.H., Pan Y.H., Ding S.T. Primary endodermal epithelial cell culture from the yolk sac membrane of Japanese quail embryos. J. Vis. Exp. 2016;109:e53624. doi: 10.3791/53624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia E., Ehrlich M., Massol R., Boucrot E., Brunner C., Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- McMahon H.T., Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- Mohanakrishnan A., Tran T.V.M., Kumar M., Chen H., Posner B.A., Schmid S.L. A highly-sensitive high throughput assay for dynamin's basal GTPase activity. PLoS One. 2017;12 doi: 10.1371/journal.pone.0185639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble R.C., Cocchi M. Lipid metabolism and the neonatal chicken. Prog. Lipid Res. 1990;29:107–140. doi: 10.1016/0163-7827(90)90014-c. [DOI] [PubMed] [Google Scholar]
- Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preta G., Cronin J.G., Sheldon I.M. Dynasore - not just a dynamin inhibitor. Cell Commun. Signal. 2015;13:24. doi: 10.1186/s12964-015-0102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root P., Sliskovic I., Mutus B. Platelet cell-surface protein disulphide-isomerase mediated S-nitrosoglutathione consumption. Biochem. J. 2004;382:575–580. doi: 10.1042/BJ20040759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W.J. Lipid transport to avian oocytes and to the developing embryo. J. Biomed. Res. 2016;30:174–180. doi: 10.7555/JBR.30.20150048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand J.H., West D.W., McCartney R.J., Noble R.C., Speake B.K. The esterification of cholesterol in the yolk sac membrane of the chick embryo. Lipids. 1993;28:621–625. doi: 10.1007/BF02536056. [DOI] [PubMed] [Google Scholar]
- Sheng G., Foley A.C. Diversification and conservation of the extraembryonic tissues in mediating nutrient uptake during amniote development. Ann. N. Y. Acad. Sci. 2012;1271:97–103. doi: 10.1111/j.1749-6632.2012.06726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speake B.K., Murray A.M., Noble R.C. Transport and transformations of yolk lipids during development of the avian embryo. Prog. Lipid Res. 1998;37:1–32. doi: 10.1016/s0163-7827(97)00012-x. [DOI] [PubMed] [Google Scholar]
- Starck J.M. Morphology of the avian yolk sac. J. Morphol. 2020;2020:1–14. doi: 10.1002/jmor.21262. [DOI] [PubMed] [Google Scholar]
- Tiwari S., Siddiqi S., Siddiqi S.A. CideB protein is required for the biogenesis of very low density lipoprotein (VLDL) transport vesicle. J. Biol. Chem. 2013;288:5157–5165. doi: 10.1074/jbc.M112.434258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uni Z., Yadgary L., Yair R. Nutritional limitations during poultry embryonic development. J. Appl. Poult. Res. 2012;21:175–184. [Google Scholar]
- van der Wagt I., de Jong I.C., Mitchell M.A., Molenaar R., van den Brand H. A review on yolk sac utilization in poultry. Poult. Sci. 2020;99:2162–2175. doi: 10.1016/j.psj.2019.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Moniri N.H., Ozawa K., Stamler J.S., Daaka Y. Nitric oxide regulates endocytosis by S-nitrosylation of dynamin. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1295–1300. doi: 10.1073/pnas.0508354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.H., Lin H.J., Lin Y.Y., Chen Y.J., Pan Y.H., Tung C.T., Mersmann H.J., Ding S.T. Embryonic cholesterol esterification is regulated by a cyclic AMP-dependent pathway in yolk sac membrane-derived endodermal epithelial cells. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock D.E., Hinshaw J.E., Schmid S.L. Dynamin self-assembly stimulates its GTPase activity. J. Biol. Chem. 1996;271:22310–22314. doi: 10.1074/jbc.271.37.22310. [DOI] [PubMed] [Google Scholar]
- Wong E.A., Uni Z. Centennial Review: the chicken yolk sac is a multifunctional organ. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R.D., Howarth B., Jr. Effect of dimethyl sulfoxide on embryonic survival and subsequent chick performance. Poult. Sci. 1976;55:579–582. doi: 10.3382/ps.0550579. [DOI] [PubMed] [Google Scholar]
- Xu L., Zhou L., Li P. CIDE proteins and lipid metabolism. Arterioscler. Thromb. Vasc. Biol. 2012;32:1094–1098. doi: 10.1161/ATVBAHA.111.241489. [DOI] [PubMed] [Google Scholar]
- Yadgary L., Cahaner A., Kedar O., Uni Z. Yolk sac nutrient composition and fat uptake in late-term embryos in eggs from young and old broiler breeder hens. Poult. Sci. 2010;89:2441–2452. doi: 10.3382/ps.2010-00681. [DOI] [PubMed] [Google Scholar]
- Yadgary L., Uni Z. Yolk sac carbohydrate levels and gene expression of key gluconeogenic and glycogenic enzymes during chick embryonic development. Poult. Sci. 2012;91:444–453. doi: 10.3382/ps.2011-01669. [DOI] [PubMed] [Google Scholar]
- Yadgary L., Wong E.A., Uni Z. Temporal transcriptome analysis of the chicken embryo yolk sac. BMC Genomics. 2014;15:690. doi: 10.1186/1471-2164-15-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Li J.Z., Liu Y., Li X., Yang T., Ma X., Li Q., Yao Z., Li P. Cideb, an ER- and lipid droplet-associated protein, mediates VLDL lipidation and maturation by interacting with apolipoprotein B. Cell Metab. 2009;9:177–190. doi: 10.1016/j.cmet.2008.12.013. [DOI] [PubMed] [Google Scholar]