Abstract
Objective
To investigate the results of real-world application of non-myeloablative autologous HSCT for multiple sclerosis (MS).
Methods
Between July 2003 and October 2019 at a single center (Northwestern University), 414 patients with relapsing remitting MS (RRMS) and 93 patients with newly diagnosed secondary progressive MS (SPMS) underwent non-myeloablative HSCT.
Results
There was one treatment-related death (0.19%) due to hospital-acquired legionella pneumonia, and one patient developed neutropenic bacteremia (Klebsiella pneumonia) without sepsis. Overall 5-year survival was 98.8%. Post HSCT secondary autoimmune diseases (2nd ADs) were idiopathic thrombocytopenia (ITP) and hypo or hyperthyroidism. ITP was highest with alemtuzumab (14%) and 0 to 2.8% for the non-alemtuzumab regimens. After HSCT, 16 patients developed hypothyroidism (3.5%) and 15 developed hyperthyroidism / Grave’s disease (3.3%). Relapse free survival (RFS) at 5 years for RRMS and SPMS was 80.1% and 98.1%, respectively, while progression free survival (PFS) at 4 years for RRMS and SPMS was 95% versus 66%, respectively. For patients with RRMS, the EDSS significantly improved (p < 0.0001) at each follow-up from a pre-HSCT mean of 3.87 to 2.51, 2.50, 2.41, 2.33, and 2.19 at 1, 2, 3, 4, and 5 years, respectively. For SPMS, the EDSS improved significantly only at 1 year but not thereafter. For SPMS, the mean baseline EDSS of 5.09 changed post-HSCT to 4.85 (p = 0.04), 4.88 (p = 0.2), 4.92 (p = .27), 4.72 (p = 0.07), and 4.2 (p = 0.21) at 1, 2, 3, 4, 5 years, respectively.
Conclusion
In patients with RRMS, autologous non-myeloablative HSCT is an effective one-time therapy, while HSCT appears of less benefit for newly diagnosed SPMS.
Keywords: Hematopoietic stem cell transplantation (HSCT), Relapsing remitting multiple sclerosis (RRMS), Secondary progressive multiple sclerosis (SPMS), Active SPMS (aSPMS), Nonactive SPMS (naSPMS)
Introduction
Clinical trials of autologous hematopoietic stem cell transplantation (HSCT) were initiated in the 1990s [1]. Due to the unknown risks of this treatment and failure to appreciate that SPMS is predominately a neurodegenerative disease, early trials enrolled patients with SPMS, high EDSS disability scores, and in an attempt to completely destroy the pre-HSCT immune system, utilized intense myeloablative regimens originally developed for cancer [2–15]. Although myeloablative HSCT appeared effective in stopping acute attacks, new MRI lesion burden, and perhaps improving quality of life [16], patients with SPMS did not demonstrate post-HSCT neurological improvement, disease progression continued [2–15], and for at least 2 years after treatment, brain atrophy continued above normal age-related changes [17].
Gradually, the majority of HSCT trials reporting to the European Bone Marrow Transplant (EBMT) registry switched from treating SPMS to predominately treating RRMS with frequent relapses and using less intense immune specific non-myeloablative regimens [18, 19]. Since there is no clear demarcation in transition between RRMS and SPMS and the diagnosis is usually confirmed retrospectively [20], it is unknown whether HSCT of newly diagnosed active SPMS (aSPMS) with recent gadolinium enhancing lesions versus non-active SMPS (naSPMS) without recent MRI enhancing lesions will result in neurological improvement.
For RRMS, sustained reversal of neurologic disability as monitored by improvement in EDSS occurred after HSCT [21–27]. With one exception [26], trials were non-randomized, contained a relatively small number of subjects, and some incorporated the results of small numbers of SPMS patients in the outcomes [21, 22]. In the only randomized control trial for only RRMS [26], randomization (n = 55 in each arm) occurred between HSCT with a non-myeloablative regimen of cyclophosphamide and rabbit anti-thymocyte globulin (ATG) versus best available disease modifying treatment (DMT) for previously treated patients with 2 or more relapses in the preceding 12 months. There was no mortality and the EDSS improved in the transplant arm by more than 1.0 point but worsened, that is increased, in the DMT arm (P < 0.001) [26].
Individual publications on larger numbers of patients but less than 250 with MS undergoing HSCT have been reported by combining data from multiple centers or using registry data collected from multiple centers [21]. There have been little data published on the risk of post-HSCT infection or post-HSCT secondary autoimmune disease (2ndAD) [28–30] or on differences in EDSS outcome between RRMS, versus newly diagnosed aSPMS or newly diagnosed naSPMS. Herein, we report single-center real-world experience of non-myeloablative HSCT in 507 patients to help provide better informed judgement on the risks and role of non-myeloablative HSCT and selection of MS patients for HSCT.
Methods
Patient selection: This is an observational study of prospectively collected data on all patients (n = 511) who underwent HSCT at Northwestern University between July 2003 and October 2019 including on-study (n = 161) as well as all off-study (n = 350). One patient was evaluated but did not undergo HSCT due to death before mobilization apheresis related to sickle cell crises. Of 511 patients who underwent stem cell mobilization and HSCT during that time interval, four were excluded from outcome analysis due to a diagnosis other than RRMS or SPMS: 1 each for primary progressive MS, a single tumefactive demyelinating lesion, neuromyelitis optica (NMO) (originally misdiagnosed as MS), and coexistent spinal cerebellar ataxia, all of whom are alive except the NMO patient who died of acute respiratory failure from NMO that relapsed with brain stem lesions 5 years after HSCT.
Patients with RRMS met the same study criteria of 18 to 60 years old, an EDSS of between 2.0 and 6.5 (one patient while undergoing DMT washout on immune suppressive drugs had improvement of EDSS to 1.0), McDonald's 2010 diagnostic criteria, and failure on a first-generation DMT of either glatiramer acetate or an interferon defined as two acute relapses or one relapse with MRI evidence of disease activity at a separate time point, or failure on a second- or third-generation DMT defined as at least one documented clinical relapse within the prior year. Between studies RRMS were treated off-study using the latest approved study protocol. Patients with newly diagnosed SPMS (n = 93) or RRMS with an EDSS > 6.5 (n = 8) who had the presence of large or numerous enhancing lesions were treated on a compassionate basis. Newly diagnosed SPMS is defined as patients with a referral diagnosis of RRMS but who upon examination indicated a gradual change in baseline neurologic disability starting within 2 years of referral that was not associated with relapse or that was occurring between relapses but who were not yet diagnosed as SPMS.
All patients signed written consent prior to initiating treatment. There was no active recruitment or advertisement. After engraftment and hospital discharge patients remained in contact with local biweekly blood draws for 3 months and then monthly until 6 months. Thereafter, patients were encouraged to return at 6 months (optional), 1, 2, 3, 4, and 5 years. Patients who did not return were contacted by phone and questioned about any infections, hospitalizations, autoimmune diseases, new diagnoses, cancers, relapses, restarting DMTs, and current medications. The patient and family were asked to contact us immediately in the advent of relapse, hospitalization, or death for any reason. Patients who relapsed were requested to return or to obtain a local MRI to confirm relapse.
Patients were excluded if they had a pre-referral diagnosis of SPMS, primary progressive MS, a hereditary neurologic disease, or pulmonary, cardiac, renal or liver dysfunction, abnormal platelet or white blood cell counts, active infection, positive serology for HIV or hepatitis B or C, prior cancer other than localized cutaneous basal cell cancer, or were pregnant. John Cunningham virus (JCV) was checked pre-HSCT but results did not influence enrollment and JCV index was not monitored post-transplant. Although Epstein Barr virus (EBV) lymphoproliferative disease has been reported to occur after HSCT for MS when using more immunosuppressive non-myeloablative regimens [5, 31], EBV titer was not monitored herein due to the lower risk of EBV disease when using a the less intense immunosuppressive regimen with a lower cyclophosphamide mobilization dose, lower ATG dose (6.0 mg/kg), and without CD34 selection of the graft.
Immune suppression withdrawal: To minimize the potential risk of immune suppression-related JCV-mediated progressive multifocal encephalopathy (PML), patients had to be free of immune suppressive DMTs (except for interferons or glatiramer acetate) for various time intervals before transplantation: 12 months for alemtuzumab (lemtrada®), 5-6 months for natalizumab (tysabri®), 3 months for fingolimod (gilenya®) and dimethyl fumarate (tecfidera®), and 4–5 months for rituximab (rituxan®) or ocrelizumab (orevus®). Patients who had received teriflunomide (aubagio®) with plasma levels > 0.02 mg/L underwent either oral cholestyramine or activated charcoal clearance. While awaiting HSCT, active disease, if present, or beginning 4 months after stopping natalizumab was treated with monthly intravenous corticosteroids and if necessary intravenous immunoglobulins (IVIG) or intravenous cyclophosphamide 500–1000 mg. After HSCT, patients did not receive immune-based therapies unless they had a clinical relapse documented by either local neurologist or study team.
HSCT Procedures: Peripheral blood stem cells were collected as an outpatient 10 days after a 23-h admission for hydration (125 mL / hour), diuretics, and 2-h infusion of intravenous cyclophosphamide (2 g/m2) followed by 5–10 μg/kg per day of subcutaneous filgrastim beginning 5 days after cyclophosphamide.
All non-myeloablative regimens included cyclophosphamide, 200 mg /kg dosed 50 mg/kg/day at ideal weight plus 25% difference between actual and ideal weight if actual weight exceeded ideal weight, and one or more biologics. Cyclophosphamide dose was not allowed to exceed a total dose of 16 grams (4 grams per day). The first regimen of cyclophosphamide / alemtuzumab (20 mg) (n = 26) caused a high incidence of late autoimmune idiopathic thrombocytopenic purpura (ITP) [24]. To minimize risk of 2ndADS, alemtuzumab was subsequently substituted with ATG (6.0 mg/kg). The Cy/ATG regimen (n = 376) significantly reduced the risk of ITP [22, 26], but subsequently in an attempt to further diminish the risk of ITP, the same Cy/ATG regimen with intravenous immunoglobulin (IVIG) 400 mg/kg on day + 2 and + 8 (n = 46); or Cy/ATG with rituximab 500 mg day + 1 and or rituximab 500 mg day + 8 (n = 63) were utilized.
Blood products were irradiated, cytomegalovirus safe, and leukocyte depleted. Filgrastim (5–10 μg/kg per day) was started on day + 4 and continued until engraftment. Hydration (125–150 mL normal saline per hour), diuretics, and intravenous mesna were continued until 24 h after the last dose of cyclophosphamide. A Foley catheter was placed in patients with greater than 60 mL of post void urinary residual determined by bedside ultrasound. Vital signs were recorded every 4–6 h and orthostatic blood pressures was recorded BID while lying, sitting, and standing. If orthostatic, either a normal saline (NS) fluid bolus (500–1000 cc), NS continuous infusion (75–100 mL/hour) and or oral midodrine was initiated.
Prophylaxis for infections: On admission for HSCT, nasal swabs for methicillin resistant staphylococcus aureus (MRSA), and perianal surveillance swabs for vancomycin resistant enterococci (VRE) and extended spectrum beta-lactamase (ESBL) producing Escherichia coli were obtained. MRSA nasal colonization was treated with topical mupirocin (bactroban®) while stool VRE and ESBL colonization was not pre-emptively treated. Patients with respiratory symptoms (sore throat or sniffles) underwent a respiratory viral panel (RVP) screen and if positive, HSCT was delayed until recovery. Oral one-minute swish and spit with hydrogen peroxide was done 4 times a day as prophylactic oral hygiene.
Oral aciclovir or valacyclovir was started twice a day upon admission and continued for 1 year. Oral fluconazole (diflucan®) was started on day + 2 and continued for 3 months. Oral trimethoprim-sulfamethoxazole or monthly nebulized pentamidine was started after platelet engraftment and maintained for 3 months. Cytomegalovirus viral load was monitored for 90 days after discharge and was treated pre-emptively by switching from aciclovir to oral valganciclovir (900 mg twice daily) until testing negative by quantitative polymerase chain reaction. After a single patient developed legionella from the hospital water supply (showerhead), all patients received oral ciprofloxacin from admission until discharge. In 2017, due to a hospital-wide outbreak of mucormycosis among immune compromised cancer transplant and solid organ transplant recipients, fluconazole prophylaxis was switched to oral isavuconazonium (cresemba®) from day zero until hospital discharge and then switched back to fluconazole.
Either an intravenous cephalosporin cefepime (maxipime®) or piperacillin / tozobactam (zosyn®) was started on day 0 and continued until engraftment that occurred on day 9 or 10. For fever (≥ 100.4), methylprednisolone (500 mg) was infused to abate an ATG-related fever, and intravenous vancomycin was added to broaden gram-negative coverage. Initial fevers were evaluated with chest radiograph, urine analysis and culture and legionella antigen, and two blood cultures, one from the central line and one from a peripheral blood draw. If diarrhea was present, stool was sent for clostridium difficile toxin and culture. If, after 72 h, cultures remained negative, vancomycin was discontinued.
Outcomes: The outcomes were overall survival, treatment-related mortality (TRM), infections, secondary autoimmune diseases, relapse, progression, and change in EDSS performed by a study neurologist pre-HSCT and at 6 months (optional), 1, 2, 3, 4, and 5 years ± 3 months after transplantation. Disease progression, i.e., a sustained increase (worsening) in EDSS score not due to a non-MS disease process, is conservatively defined as at least a 0.5 point sustained increase in EDSS on 2 evaluations at least 6 months apart [32, 33]. Relapses are defined as neurologic symptoms lasting more than 24 h; not associated with infection, fever, medication, or heat intolerance; and deemed to require treatment by either the local neurologist or treatment team.
Statistical analysis: Measures of location and dispersion of data are presented as mean, median, range, standard deviation and 1st and 3rd quartiles. P values were determined by a 2 tailed paired student t-test. Survival rates were estimated via Kaplan–Meier curves with total number at risk and 95% confidence intervals. Analyses were conducted in R 4.0.3 and performed by a statistician (IBH).
Results
Demographics
Between July 2003 and October 2019, 511 patients (4 were subsequently excluded for wrong diagnosis) underwent HSCT for MS at Northwestern University in Chicago, Illinois (see Table 1 for median, range, standard deviation (STD). Of those 62% (317) were female and 38% (194) were male; 87% percent (n = 445) were Caucasian, 5% (n = 25) African American, 4% (n = 21) Hispanic, 4% (n = 19) Asian, and 0.2% (n = 1) self-described as mixed. The patients’ mean and median age at the time of the transplant were 36.7 and 37 years, respectively (range 17–59, standard deviation (STD) of 8.01) with a mean and median illness duration of 7.2 and 6 years, respectively (range 0.5–33 years, STD of 5.4). Pre-transplant mean and median EDSS scores were 4.1 and 4, respectively (range of 1–8, STD of 1.48). Prior DMTs, immune suppressive medications, and or cellular therapies are listed in Table 1. The mean and median follow-up was 2.7 and 3 years, respectively. The number of patients treated with each non-myeloablative regimen were: (1) Cy / ATG (n = 376), (2) Cy / ATG / rituximab 500 mg (n = 28), (3) Cy/ ATG / rituximab 1000 mg (n = 35), (4) Cy /ATG / IVIG (n = 46), (5) Cy / alemtuzumab (n = 26). The mean and median number of immune suppressive treatments before transplant were 3.7 and 4.0, respectively (arrange 1–11, STD of 1.49).
Table 1.
Demographics of patients
| Parameter | All patients | RRMS | Newly diagnosed SPMS |
|---|---|---|---|
| Total number @ | 511@ | 414 | 93 |
| Female | 317 (62%) | 262 (63.3%) | 54 (58%) |
| Male | 194 (38%) | 152 (36.7%) | 39 (42%) |
| Caucasian | 445 (87%) | 366 (88%) | 85 (92%) |
| AA | 25 (5%) | 19 (4.5%) | 6 (6%) |
| Hispanic | 21 (4%) | 14 (3.4%) | 2 (2%) |
| Asian | 19 (4%) | 12 (2.9%) | 0 |
| Mixed | 1 (0.2%) | 3 (< 1%) | 0 |
| Age in years mean / median (range, STD) | 36.7 / 37 (17–59, 8.01) | 35.9 / 36 (17–60, 7.9) | 40 / 43 (24–56, 6.9) |
| Duration of MS years mean / median (range, STD) | 7.2 / 6 (0.5–33, 5.4) | 6.3 / 5 (0.5–25, 4.6) | 11 / 11 (2–33, 4.6) |
| Number prior different immune treatments | |||
| Mean / median (range, STD) | 3.7 / 4.0 (1–11, 1.49) | 3.6 / 3 (1–9, 1.43) | 4.15 / 4 (1–11, 1.68) |
| EDSS mean / median (range, STD) | 4.1 / 4 (1–8, 1.48) | 3.87 / 3.50 (1–8, 1.43) | 5.2 / 5.5 (3–8, 1.33) |
| Prior DMT | |||
| SQ glatiramer acetate | 293 (58%) | 235 (57%) | 58 (62%) |
| IM interferon beta-1a (avenox®) | 185 (45%) | 143 (35%) | 42 (45%) |
| SQ interferon beta-1a (rebif®) | 166 (41%) | 128 (31%) | 38 (41%) |
| SQ interferon beta-1b (betaseron®) | 107 (26%) | 76 (18%) | 31 (33%) |
| SQ peginterferon beta-1a (plegridy®) | 9 (2%) | 7 9 (< 0.2%) | 2 (2%) |
| IV natalizumab (tysabri®) | 170 (42%) | 126 (30%) | 44 (47%) |
| PO dimethyl fumarate (tecfidera®) | 159 (39%) | 128 (31%) | 31 (33%) |
| PO fingolimod (gilenya®) | 113 (28%) | 92 (22%) | 21 (23%) |
| PO teriflunomide (aubagio®) | 37 (7%) | 29 (7%) | 8 (8.6%) |
| IV mitoxantrone (novantrone®) | 18 (3.5%) | 11 (2.6%) | 7 (7.5%) |
| IV ocrelizumab (ocrevus®) | 15 (3.6%) | 10 (2.4%) | 5 (5.3%) |
| SQ daclizumab (zinbryta®) | 5 (1%) | 4 (< 1%) | 1 (1%) |
| IV alemtuzumab (lemtrada®) | 4 (0.8%) | 4 (< 1%) | 0 |
| PO cladribine (mavenclad®) | 1 (0.1%) | 1 (< 1%) | 0 |
| Other immune modulating drugs | |||
| Corticosteroids | 475 (94%) | 391 (94%) | 84 (90%) |
| Intravenous immunoglobulin (IVIG) | 32 (7.8%) | 27 (6.5%) | 5 (5.3%) |
| Plasmapheresis (PLEX) | 27 (7%) | 25 (6.0%) | 2 (2%) |
| IV Cyclophosphamide (cytoxan) | 25 (6.6%) | 21 (5.1%) | 4 (4.3%) |
| PO mycophenolate mofetil (cellcept®) | 6 (1.9%) | 6 (1.4%) | 0 |
| PO azathioprine (imuran) | 5 (1.5%) | 4 (< 1%) | 1 (1%) |
| PO methotrexate | 4 (< 1%) | 4 (< 1%) | 1 (1%) |
| Rituximab | 0 | 0 | |
| Prior cell therapy | |||
| HSC (IV)without chemotherapy | 3 (< 1%) | 2 (< 1%) | 1 (UCB) (1%) |
| Prior autologous HSCT | 2 (< 1%) | 2 (< 1%) | 0 |
| Mesenchymal stem cells (n = 1), | 1 (< 1%) | 1 (< 1%) | 0 |
@ Includes 1 patient each with primary progressive multiple sclerosis, tumefactive multiple sclerosis, neuromyelitis optica (NMO) initially misdiagnosed as MS, and coexistent spinal cerebellar ataxia,
EDSS Expanded Disability Status Scale, IM intramuscular, IV intravenous, HSC hematopoietic stem cells, HSCT hematopoietic stem cell transplantation, PO per os (oral), RRMS relapsing remitting multiple sclerosis, SPMS secondary progressive multiple sclerosis, SQ subcutaneous, STD standard deviation
A total of 414 patients had RRMS, and 93 patients had newly diagnosed secondary progressive MS (SPMS). As shown in Table 1 that includes median, range, STD, and 1st and 3rd quartiles, patients with RRMS compared to SPMS were on average younger 35.9 versus 40 years old, had a lower duration of disease 6.3 versus 11 years, lower number of prior immune suppressive treatments 3.6 versus 4.15, and lower baseline EDSS 3.87 versus 5.2. The only clinical symptom that differed between newly diagnosed SPMS and RRMS cases was lower extremity spasticity / stiffness that was present in 67% (62 of 93) of patients with SPMS versus no patients with RRMS. The number of patients who underwent HSCT but never returned for scheduled in-person follow-up evaluation that was required for EDSS documentation are 30 (7.2%) with RRMS and 5 (5.4%) with SPMS.
Toxicity
Mortality: Transplant treatment-related mortality (TRM) defined as any death that is related to the transplant treatment or its complications was 0.19% (1 of 507) and was due to hospital-acquired legionella pneumonia. Overall survival was 98.8% as there were five late non-treatment-related deaths. Three died more than one year after HSCT from a cerebrovascular accident related to medication non-compliance, a myocardial infarction, and during an elective cholecystectomy (unknown cause); one patient died 3 years after HSCT from colon cancer. One patient who received alemtuzumab in the conditioning regimen died 10 years after transplantation from a T cell lymphoma. No patient developed myelodysplastic syndrome, leukemia, or bladder cancer.
Infections (Table 2): During the inpatient transplant interval, the main infections were stool clostridium difficile (1.1%), one bacteremia (0.19%) without hypotension or sepsis speciated as Klebsiella pneumoniae that was sensitive to cefepime. One patient died from legionella pneumonia on day 11 while engrafting. Subsequently, legionella species were isolated within the hospital water supply / shower head.
Table 2.
Infections during hospitalization and for the first year after autologous HSCT
| Site / pathogen | During hospitalization | From hospital discharge until day 100 | 100 days until one year post-HSCT |
|---|---|---|---|
| Mucosal surfaces total | 1 | 86 | 83 |
| Oral HSV | 1 | 1 | |
| Oral / vaginal candida | 10 | 2 | |
| Sinusitis bacteria | 12 | 17 | |
| Otitis media bacteria | 1 | 3 | |
| URTI non-specific, bacterial | 18 | 20 | |
| URTI—viral | |||
| RSV | 3 | 1 | |
| Rhinovirus | 1 | 1 | |
| Influenza A | 2 | 1 | |
| Influenza B | 3 | 3 | |
| Parainfluenza | 2 | ||
| Metapneumonia | 2 | ||
| Urinary tract infections total | 0 | 31 | 35 |
| UTIs- bacterial | 28 | 34 | |
| UTIs-viral | |||
| BK virus | 2 | 1 | |
| Adenovirus | 1 | ||
| Cutaneous total | 0 | 1 | 13 |
| Cutaneous Bacterial | 1 | 1 | |
| Cutaneous VZV- dermatomal | 12 | ||
| Blood bacteremia total | 1 | ||
| Blood cultures Gram positive | & 3 coagulase-negative staphylococcus aureus in one of two cultures / deemed skin contaminant | ||
| Blood cultures Gram negative | 1 Klebsiella pneumoniae | ||
| Stool clostridium difficile | 6 | 1 | 2 |
| Lung pneumonia | 1 legionella | 3 “walking” @ | 3- “walking”@ |
| Viral systemic total | 0 | 0 | 0 |
| CMV reactivation | 0 | 58 | Not monitored |
| CMV disease | 0 | 0 | 0 |
| EBV infection | 0 | 0 | 0 |
| PML | 0 | 0 | 0 |
@Walking pneumonia means treated as outpatient with oral antibiotics & Coagulase-negative staphylococcus from peripheral blood draws were deemed skin contaminants, because the simultaneously drawn cultures from the central line were negative
From time of hospital discharge (day 9 or 10) until day 100 after HSCT, the most common infections involved mucosal surfaces infections from viral (n = 13) or bacterial (n = 18) upper respiratory tract infections (URTI), sinusitis (n = 12), or oral or vaginal candidiasis, and bacterial (n = 28) or viral (n= 3) urinary tract infections (UTI) (Table 2). All patients recovered without need for hospitalization. Transient viral hemorrhagic cystitis occurred in 3 patients: 2 from BK virus, and one from adenovirus. One patient developed clostridium difficile diarrhea.
In the 265-day interlude from day 100 to 1-year post transplantation (Table 2), the most common infections were again URTI and UTI infections that resolved with oral antibiotics and occurred in 26 and 35 patients, respectively (Table 2). Two patients developed clostridium difficile diarrhea. One patient developed a cutaneous breast implant gram positive bacterial infection. Dermatomal varicella zoster virus (VZV) infections occurred for the first time in 12 patients. No patient at any time point after HSCT developed PML, CMV disease, Pneumocystis jiroveci pneumonia, or Epstein Barr virus (EBV) symptoms or lymphoproliferative disease. COVID 19 became a concern after this writing, but no patient has died from a COVID 19 infection.
2ndADS: New autoimmune diseases were ITP or thyroiditis. Post HSCT, 10 cases of ITP occurred, one at 6 months, eight between 1–2 years, and one at 3 years after transplantation. The incidence of ITP was highest with alemtuzumab (11.5%) and lower, approximately 2–3% for the non-alemtuzumab regimens. Except for one case at 3 years, all episodes of ITP occurred within 2 years after HSCT. ITP completely resolved after treatment with corticosteroids, IVIG and / or rituximab. The mean time to diagnosis post-HSCT of hypothyroidism was 1.7 years (range 1–3 years) and of hyperthyroidism/Grave’s disease was 3.6 years (range 1–9 years). The incidence of new hypo- or hyperthyroidism was roughly 10% with either an alemtuzumab or ATG containing regimens.
Neurologic outcome
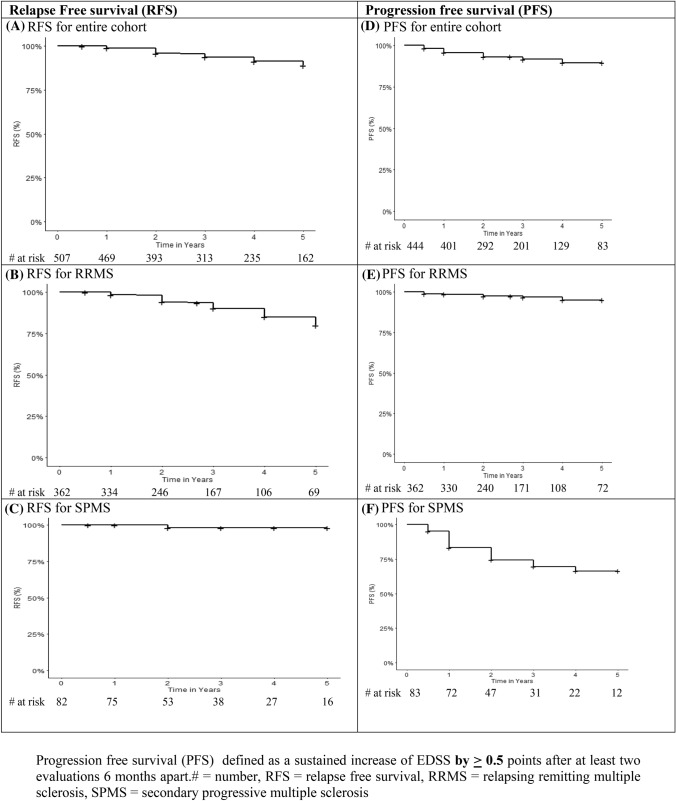
Relapse-free survival (Fig. 1A–C): Post HSCT no DMTs or immune modulating or suppressive drugs were given unless the patient had a clinical relapse determined by either the local neurologist or study team. A total of 34 patients relapsed. For the entire cohort, the relapse-free survival (RFS) with 95% confidence interval (CI) at 1, 2, 3,4, and 5 years was 98.9% (98–99.8), 95.9% (94–97.8), 93.7% (91.3–96.2), 91.3% (88.4–94.4), and 89.1% (85.5–92.8), respectively (Fig. 1A). For RRMS, 33 patients relapsed and the RFS with 95%CI at 1, 2, 3, 4, and 5 years was 98.5% (97.2–99.8), 94.1% (91.3–96.9), 90% (86.3–94.2), 85% (79.8–90.7), and 80.1% (73.5 to 87.4), respectively (Fig. 1B). A single patient with newly diagnosed SPMS relapsed for percent RFS with 95%CI of 100% at 1 year and 98.11% (94.5–100) at 2, 3, 4, and 5 years (Fig. 1C).
Fig. 1.
Relapse-free survival (RFS) and Progression-Free Survival (PFS). Progression-free survival (PFS) defined as a sustained increase of EDSS by ≥ 0.5 points after at least two evaluations 6 months apart. # = number, RFS relapse-free survival, RRMS relapsing remitting multiple sclerosis, SPMS secondary progressive multiple sclerosis
Progression-free survival (Fig. 1D–F): Progression defined as a sustained 0.5 or more increase in the EDSS occurred in a total of 32 patients. For the entire cohort PFS (95%CI) at 1, 2, 3, and 4 years was 95.7% (93.8–97.6), 93.13% (90.5–95.7), 91.7% (88.7–93.4), and 89.6% (85.9–93.4) at 4 years (Fig. 1D). For RRMS, 11 patients progressed with a PFS (95%CI) at 1, 2, 3, and 4 years of 98.6% (97.3–99.8), 97.3% (95.6–99.2), 96.8% (94.7–98.9), and 95% (91.8–98.3), respectively (Fig. 1E). For SPMS, 21 patients progressed with a PFS (95%CI) at1, 2, 3, and 4 years of 83.2% (75.3–92), 74.4 (64.6–85.6), 69.4% (54.8–80.4), and 66% (54.8–80.4), respectively (Fig. 1F).
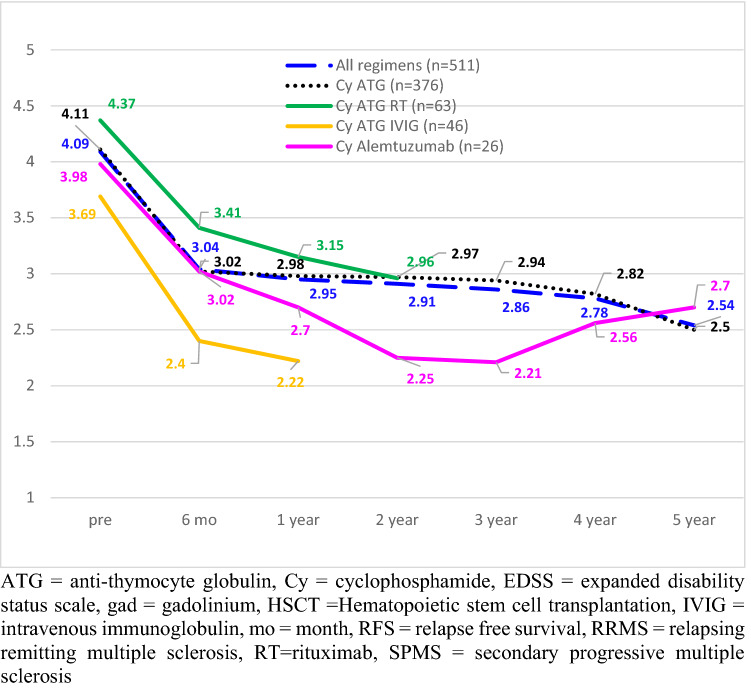
Neurologic disability (EDSS): The pre- and post-HSCT EDSS dispersion of data, that is mean, median, range, standard deviation (STD), and 1st and 3rd quartiles are presented in Tables 3 and 4. Disability measured by the Kurtzke EDSS improved for the entire group from a mean before HSCT of 4.09, to 3.04 at 6 months post (p < 0.0001), 2.95 at one year (p < 0.0001), 2.91 at 2 years (p < 0.0001), 2.86 at 3 years (p < 0.0001), 2.78 at 4 years (p < 0.0001), and 2.54 at 5 years (p < 0.0001) after transplantation (Fig. 2; Table 3). Patients treated with different non-myeloablative conditioning regimens had similar post-transplant improvements in baseline EDSS (Table 3; Fig. 2). For example, when compared to baseline, improvement (decline) in EDSS at 1 year was – 1.14 (all regimens), – 1.13 (Cy / ATG), – 1.27 (Cy Alemtuzumab), – 1.22 (Cy / ATG / rituximab) and – 1.47 (Cy / ATG / IVIG). For the three regimens with 5-year follow-up, the decline (improvement) in EDSS compared to baseline was – 1.55, – 1.61, and – 1.26 for all regimens, Cy / ATG, and Cy / alemtuzumab, respectively.
Table 3.
EDSS for all patients according to regimen
| Regimen | EDSS: pre-HSCT baseline | EDSS: 6 months | EDSS: 1 year | EDSS: 2 years | EDSS: 3 years | EDSS: 4 years | EDSS: 5 years |
|---|---|---|---|---|---|---|---|
| All regimens | |||||||
|
Mean, median, (range), STD, 1st Q, 3rd Q (number) Δ EDSS P value |
4.09, 4, (1–8), 1.48, 3, 5.5 (n = 507) |
3.04, 2.5, (0–7.5), 1.66, 2, 4 (n = 314) P < 0.001 |
2.95, 2.5, (0–7), 1.73, 2, 4 (n = 366) P < 0.001 |
2.91, 2.5, (0–7.5), 1.90, 2, 4 (n = 272) P < 0.001 |
2.86, 2.5, (0–7.5), 1.90, 2, 4 (n = 192) P < 0.001 |
2.78, 2, (0–7.5), 2.02, 1.5, 4 (n = 117) P < 0.001 |
2.54, 2, (0–6.5), 1.9, 1.5, 4 (n = 95) P < 0.001 |
| Cy / ATG | |||||||
|
Mean, median, (range), STD, 1st Q, 3rd Q (number) Δ EDSS P value |
4.11, 4, (1–8), 1.48, 3, 5.5 (n = 376) |
3.02, 3, (0–7.5), 1.62, 2, 4 (n = 239) P < 0.001 |
2.98, 2.5, (0–7), 1.73, 2, 4 (n = 283) P < 0.001 |
2.97, 2.5, (0–7.5), 1.84, 2, 4 (n = 218) P < 0.001 |
2.94, 2.5, (0–7.5), 1.93, 1.5, 4 (n = 171) P < 0.001 |
2.82, 2, (0–7.5), 2.05. 1.5, 4 (n = 100) P < 0.001 |
2.5, 2, (0–6.5), 1.91, 1.5, 3.5 (n = 77) P < 0.001 |
| Cy / alemtuzumab | |||||||
|
Mean, median, (range), STD, 1st Q, 3rd Q (number) Δ EDSS P value |
3.98, 3.5 (2–8), 1.56, 3, 4.5, (n = 26) |
3.02, 2, (1–6.5), 1.77, 1.6, 4 (n = 22) P = 0.006 |
2.71, 2, (0–6) 1.77, 1.5, 3.5 (n = 21) P = 0.003 |
2.25, 2 (1–6) 1.44, 1.5, 3, (n = 22) P = 0.0003 |
2.21, 2, (1–6), 1.62, 1, 3 (n = 21) P = 0.006 |
2.56, 2 (0–6), 1.86, 1.5, 2.5 (n = 17) P = 0.004 |
2.72, 2.5 (0–6), 1.9. 1.5, 3.75, (n = 18) P = 0.015 |
| Cy / ATG/ RIX | |||||||
|
Mean, median, (range), STD, 1st Q, 3rd Q (number) Δ EDSS P value |
4.37, 4 (1.5–8) 1.68, 3, 6, (n = 63) |
3.41, 3 (0–7), 1.81, 2, 5.5 (n =37) P < 0.001 |
3.15, 3 (0–6.5), 1.73, 2, 4, (n =44) P < 0.001 |
2.96, 2.5 (0–7), 1.87, 2, 4 (n =33) P < 0.001 |
|||
| Cy / ATG / IVIG | |||||||
|
Mean, median, (range), STD, 1st Q, 3rd Q (number) Δ EDSS P value |
3.69, 3.5 (2–6.5), 0.97, 3, 4 (n = 46) |
2.4, 2.25, (0–6.5), 1.3, 1.5, 3 (n = 36) P < 0.001 |
2.22, 2, (0–6) 1.39, 1.25, 2.75 (n = 27) P < 0.001 |
||||
Δ Change in mean EDSS from mean at baseline, 1st Q first quartile, 3rd Q third quartile, ATG rabbit anti-thymocyte globulin (Thymoglobulin®), Cy cyclophosphamide, EDSS expanded disability status scale, HSCT hematopoietic stem cell transplantation, IVIG intravenous immunoglobulin, RIX rituximab, STD standard deviation
Table 4.
Change in EDSS for all patients with RRMS segregated by pre-HSCT EDSS and for SPMS segregated by MRI enhancement within the prior year
| Patients | EDSS: pre-HSCT baseline | EDSS: 6 months | EDSS: 1 year | EDSS: 2 years | EDSS: 3 years | EDSS: 4 years | EDSS: 5 years |
|---|---|---|---|---|---|---|---|
| RRMS | |||||||
|
RRMS ALL@ Mean, median, (range), STD, 1st Q, 3rd Q (number) Δ EDSS P value |
3.87, 3.5 (1–8) @ 1.43, 3, 4 (n = 414) |
2.70, 2.5 (0–7) 1.48, 2, 3.25 (n = 259) P < 0.001 |
2.51, 2 (0–7) 1.48, 1.5, 3 (n = 299) P < 0.001 |
2.50, 2 (0–7.5) 1.60, 1.5, 3 (n = 224) P < 0.001 |
2.41, 2 (0–7.5) 1.61, 1.5, 3 (n = 158) P < 0.001 |
2.33, 2 (0–7.5) 1.75, 1, 3 (n = 96) P < 0.001 |
2.19, 2 (0–6.5) 1.70, 1, 3 (n = 80) P < 0.001 |
| RRMS Pre-HSCT EDSS 2–4 @ | |||||||
|
Mean, median, (range), STD, 1st Q, 3rd Q (number) Δ EDSS P value |
3.14, 3 (1–4) @ 0.66, 2.5, 3.5 (n = 302) |
2.17, 2 (0–4) 0.99, 2.5, 3.5 (n = 179) P < 0.001 |
2.03, 2 (0–6) 1.06, 1.5–2.5 (n = 14) P < 0.001 |
1.99, 2 (0–5.5) 1.15, 1.37, 2.5 (n = 160) P < 0.001 |
1.94, 2 (0–6) 1.15, 1, 2.5 (n = 114) P < 0.001 |
1.78, 1.5 (0–6.5) 1.18, 1, 2 (n = 63) P < 0.001 |
1.95, 1,5 (0–6.5) 1.65, 1, 2.5 (n = 58) P < 0.001 |
| RRMS Pre-HSCT EDSS 4.5–6 | |||||||
|
Mean, median, (range), STD, 1st Q, 3rd Q (number) Δ EDSS P value |
5.5, 5.75 (4.5–6) 0.58, 5, 6 (n = 74) |
3.38, 3 (0–6.5) 1.52, 2.4, 4.5 (n = 56) P < 0.001 |
3,21, 3 (0–6.5) 1.54, 2.5, 4 (n = 57) P < 0.001 |
2.81, 2.5 (0–6.5) 1.34, 2, 3,4 (n = 38) P < 0.001 |
2.85, 2.5 (0–6.5) 1.59, 1.5, 3.75 (n = 27) P < 0.001 |
2.5, 2.25 (0–6) 1.84, 1.37, 4 (n = 20) P < 0.001 |
2.38, 2 (0–6) 1.73, 1, 3 (n = 13) P < 0.001 |
| RRMS Pre-HSCT EDSS 6.5 | |||||||
|
Mean, median, (range), STD, 1st Q, 3rd Q (number) Δ EDSS P value |
6.5, 6.5 6.5 0, 6.5, 6.5 (n = 30) |
5.13, 6 (2.5–7) 1.43, 4, 6,5 (n = 19) P < 0.001 |
4.88, 5 (2.5–7) 1,41, 3.5, 6 (n = 22) P < 0.001 |
5.21, 6 (2.5–7.5) 1.54, 4, 6.5 (n = 21) P = 0.001 |
5.1, 6 (1–7.5) 1.91, 4.1, 6.3 (n-14) P = 0.02 |
4.88, 5.5 (0–5.5) 2.02, 4, 6 (n = 12) P = 0.01 |
3.37, 3.75 (0–6) 1.7, 2.8, 4 (n = 8) P < 0.001 |
| RRMS Pre-HSCT EDSS 7–8 | |||||||
|
Mean, median, (range), STD, 1st Q, 3rd Q (number) Δ EDSS P value |
7.13, 7 (7–8) 0.45, 7, 7.6 (n = 8) |
5.5, 6 (4–6) 1.0, 5.5, 6 (n = 4) P = 0.05 |
4.6, 5 (3–6) 1.51, 3, 6 (n = 4) P = 0.05 |
||||
| SPMS | |||||||
| All SPMS | |||||||
|
Mean, median, (range), STD, 1st Q, 3rd Q (number) Δ EDSS P value |
5.09, 5.5 (3–8) 1.32, 3, 6 n = 93 |
4.71, 4.25 (1–8) 1.56, 3.5, 6 n = 54 P < 0.001 |
4.85, 5.25 (2–6.5) 1.39, 3.5, 6 n = 64 P = 0.04 |
4.88, 5.5 (1.5–7) 1.54, 3.5, 6.5 n = 45 P = 0.20 |
4.92, 6 (0–7) 1.77, 3, 6.5 n = 33 p = 0.27 |
4.72, 6 (1.5–7.5) 1.95, 3, 6.4 n = 22 p = 0.07 |
4.21, 4 (1.5–6.5) 1.95, 2.5, 6 N = 16 P = 0.21 |
| naSPMS MRI gadolinium negative in prior year | |||||||
|
Mean, median, (range), STD, 1st Q, 3rd Q (number) Δ EDSS P value |
4.92, 5 (3–7) 1.26, 3.6, 6 n = 58 |
4.56, 4 (1–8), 1.57, 3.5, 6 n = 33 p = 0.04 |
4.88, 5.25 (2–6.5) 1.37, 3.5, 6 n = 38 p = 0.54 |
5.13, 5.5 (3–7) 1.34, 4, 6.4 n = 26 p = 1.0 |
4.89, 6 (0–6.5) 1.79, 3.5, 6.26 n = 19 p = 0.74 |
4.54, 5 (1.5–7.5) 2.15, 2.5, 6 n = 11 p = 0.27 |
4.71, 6 (1.5–6.5) 2.13, 3.25, 6.25 n = 7 p = 0.75 |
| aSPMS, MRI gadolinium positive in prior year | |||||||
|
Mean, median, (range), STD, 1st Q, 3rd Q (number) Δ EDSS P value |
5.37, 6 (3–8) 1.40, 4, 6.5 n = 35 |
4.95, 5.5 (2–7.5) 1.56. 3.5, 6.5 n = 21 P = 0.002 |
4.82, 5 (2–6.5) 1.44, 3.5, 6 n = 26 P = 0.001 |
4.55, 4 (1.5–6.5) 1.76, 3, 6.5 n = 19 P = 0.003 |
4.93, 6 (2.5–7) 1.8, 3.1, 6.5 n = 14 P = 0.02 |
4.9, 5.5 (2.5–7) 1.81, 3, 6.5 n = 14 p = 0.1 |
3.88, 3 (2.5–6.5) 1.83, 2.5, 6 n = 9 p = 0.03 |
@ while awaiting HSCT a single patient improved to EDSS 1.0. Δ Change in mean EDSS from mean at baseline, 1st aSPMS active secondary progressive multiple sclerosis, Q first quartile, 3rd Q third quartile EDSS = expanded disability status scale, HSCT hematopoietic stem cell transplantation, naSPMS non-active secondary progressive multiple sclerosis, RRMS relapsing remitting multiple sclerosis, SPMS secondary progressive multiple sclerosis, STD standard deviation
Fig. 2.
Pre- and post-EDSS for entire cohort by non-myeloablative regimen. ATG anti-thymocyte globulin, Cy cyclophosphamide, EDSS expanded disability status scale, gad gadolinium, HSCT Hematopoietic stem cell transplantation, IVIG intravenous immunoglobulin, mo month, RFS relapse-free survival, RRMS relapsing remitting multiple sclerosis, RT rituximab, SPMS secondary progressive multiple sclerosis
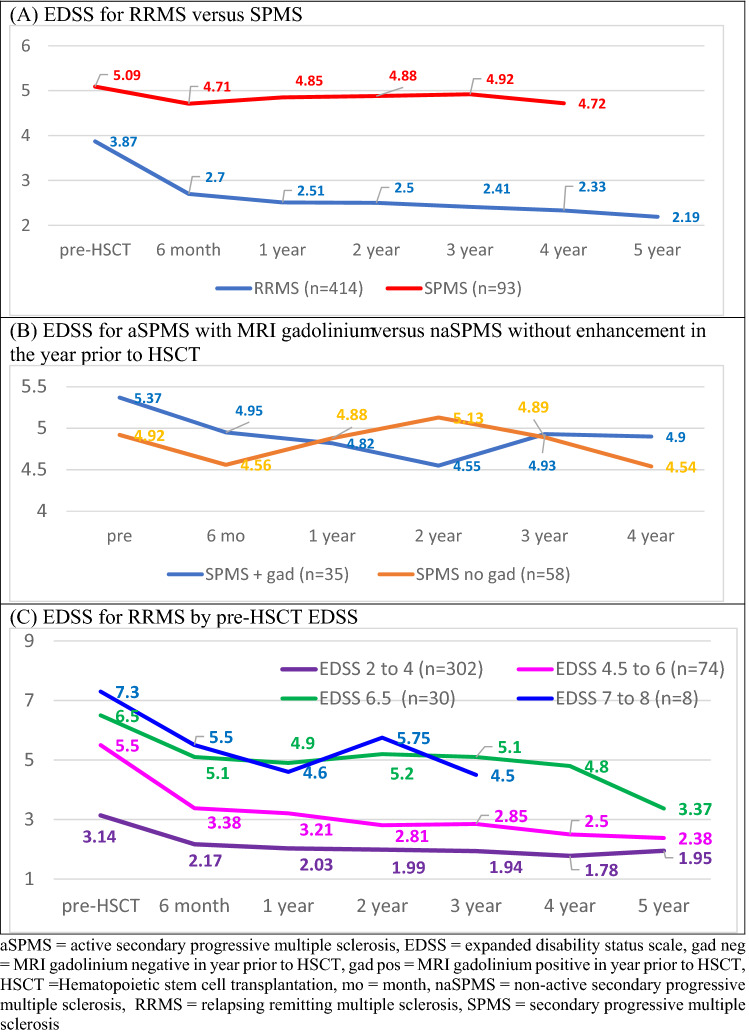
The 414 patients with RRMS had a pre-HSCT mean baseline EDSS of 3.87 that improved to 2.70 (P < 0.0001) at 6 months, 2.51 (P < 0.0001) at 1 year, 2.50 (P < 0.0001) at 2 years, 2.41 (P < 0.0001) at 3 years, 2.33 (P < 0.0001) at 4 years, and 2.19 (P < 0.0001) at 5 years (Table 4; Fig. 3A). When RRMS patients are separated by pre-HSCT EDSS, significant improvement in EDSS occurred in all categories (Table 4; Fig. 3C), For pre-HSCT EDSS between 2 and 4, mean EDSS improved by p < 0.001 at each follow-up from a mean baseline of 3.14 to post-HSCT at 1, 2, 3, 4, and 5 years of 2.03. 1.99, 1.94, 1.78, 1.95, respectively. For pre-HSCT EDSS score between 4.5 and 6.0, the mean improved from 5.5 to 3.21 at 1 year, 2.81 at 2 years, 2.85 at 3 years, 2.5 at 4 years, and 2.38 at 5 years. Baseline EDSS of 6.5 significantly improved (p < 0.001) at each follow-up to 4.88 at 1 year, 5.2 at 2 years, 5.1 at 3 years, 4.88 at 4 years, and 3.37 at 5 years. Only eight RRMS patient had a pre-HSCT of between 7 and 8 who improved (p = 0.05) from a mean of 7.13 to 4.6 at 1 year.
Fig. 3.
EDSS change after HSCT. aSPMS active secondary progressive multiple sclerosis, EDSS expanded disability status scale, gad neg MRI gadolinium negative in year prior to HSCT, gad pos MRI gadolinium positive in year prior to HSCT, HSCT Hematopoietic stem cell transplantation, mo month, naSPMS non-active secondary progressive multiple sclerosis, RRMS relapsing remitting multiple sclerosis, SPMS secondary progressive multiple sclerosis
For the 93 patients with newly diagnosed SPMS, the EDSS improved for the first year after transplant but not thereafter with a pre-HSCT EDSS mean of 5.09 and post-HSCT EDSS of 4.85 (P = 0.04) at 1 year, 4.88 (p = 0.2) at 2 years, 4.92 (p = 0.27) at 3 years, and 4.72 (0.07) at 4 years (Table 4; Fig. 3A).The outcome of patients with newly diagnosed SPMS was separated into those with aSPMS who had at least one gadolinium enhancing lesion (n = 35) within one year of HSCT versus those with naSPMS who did not (n = 58) (Table 4; Fig. 3B). For those newly diagnosed naSPMS patients without enhancement, the EDSS, except at 6 months, did not significantly improve. The mean EDSS pre-HSCT was 4.92, and mean post-transplant EDSS was 4.88 (P = 0.54), 5.13 (p = 1), 4.89 (P = 0.73), and 4.54 (P = 0.27) at 1, 2, 3, and 4 years post-HSCT, respectively. Newly diagnosed aSPMS patients with gadolinium enhancement during the prior year improved their EDSS for 3 years after HSCT from 5.37 pre-HSCT to 4.82 (P = 0.001), 4.55 (p = 0.003), 4.93 (p = 0.2) at 1, 2, and 3 years post-HSCT (Table 4; Fig. 3B), respectively.
Discussion
Despite regimen-induced neutropenia, only one of 507 patients developed bacteremia. The low incidence of bacteremia may be related to the use of non-myeloablative regimens that do not cause mucositis, i.e., the gut endothelium barrier remains closed, placement and removal of the large bore apheresis catheter on the same day and subsequent insertion of a PICC line in the upper inner arm on day of admission resulting in less risk of cutaneous barrier breaches, and the use of pre-emptive antibiotics when neutropenic. Equally important, no patient receiving prophylactic antibiotics developed a drug-resistant organism. During the first 100 days, URTIs and UTIs predominate which diminish between 100 days and 1 year after which the incidence of VZV dermatomal reactivation predominates. While one group has reported EBV reactivation and post-transplant lymphoproliferative disease (PTLD) using a non-myeloablative regimen [31], the risk of opportunistic viral infection is related to the degree of regimen immune suppression. Neither symptomatic EBV nor PTLD occurred herein when utilizing a less intense non-myeloablative regimen with ATG ≤ 6.0 mg/kg, and mobilization with 2.0 g/m2 cyclophosphamide without CD34 selection (lymphocyte depletion) of the graft.
One patient died due to hospital-acquired legionella pneumonia. Several organisms can be spread via hospital water including legionella and gram-negative bacteria such as pseudomonas and klebsiella [34, 35]. Monitoring and routinely documenting hospital water sterility / contamination is of major importance since legionella contamination may occur even despite copper–silver ionization of the water as hyperchlorination alone is not effective in clearing legionella [36].
ITP was highest with alemtuzumab (11.5%) and 0–2.8% for the non-alemtuzumab regimens. Characterizing B and T cell phenotype recovery after autologous HSCT may allow better selection of the most appropriate conditioning regimen to prevent 2ndADs that have been hypothesized to arise from rapid naïve and immature B cell recovery without T cell regulation due to delayed post-transplant regulatory CD4+CD25+FoxP3+ T cell recovery [30, 37].
Unlike DMT trials, in which subjects had never received a prior DMT or had only received a single prior first-generation interferon or glatiramer acetate, patients in this analysis had previously received a mean / median of 4 prior DMTs including a majority who had received second- or third-generation DMTs (Table 1). While the data demonstrate that the EDSS reversed by > 1.0 point in the RRMS cohort, RRMS patients were selected for persistently active inflammatory disease. EDSS improvement by > 1.0 point could, therefore, represent post-treatment regression to the mean.
A potential problem with determining progression from baseline EDSS in RRMS is that the EDSS may improve or revert over 24 weeks [38, 39]. To address this issue, the European Medicines Agency (EMA) recommended that EDSS worsening be confirmed by measurements taken 24 weeks apart [40]. We took the first post-treatment EDSS score at 6 months (24 weeks) and mandated that it persist for at least another 6 months, i.e., for 1 year, and then every year for 5 years.
Going beyond the recent EMA recommendations of a 24-week interval between EDSS scores, it has been suggested that regression to the mean or true baseline EDSS may take up to one year [38, 39]. The data herein indicate that after HSCT, the improvement in EDSS occurred over at least 2 years (with no further DMTs) suggesting that once inflammatory CNS activity is effectively halted, improvement or perhaps regression to the mean may actually continue for 2 years or more. While regression to the mean does occur, it is unlikely that the subsequent EDSS mean will stably persist or continue to improve in patients, as reported herein, if active RRMS persisted. The subsequent prolonged stabile improvement of the EDSS for up to 5 years of follow-up supports the efficacy of HSCT for active RRMS.
Whether post-HSCT improvement in the EDSS in highly active MS is regression to the mean or due to treatment-related effects or both is best approached in different DMT and HSCT treatments for MS by comparison to a control arm within a randomized trial. We had previously published a randomized controlled trial using the same non-myeloablative approach in this subset of highly active RRMS [26]. In the randomized trial, the mean EDSS improved (decreased) to an identical degree and in an identical manner and time interval as reported herein in a larger cohort of patients [26]. In comparison, in the control DMT arm, the EDSS did not regress to the mean but rather continued to worsen (increase) at both 6 and 12 months [26].
The distinction in response to HSCT is not dependent on the EDSS score per se but rather on whether the disease is predominately inflammatory (RRMS) or neurodegenerative (SPMS) [41]. For RRMS, the 5-year RFS was 80% (33 of 414 relapsed), while for SPMS, the 5-year RFS was 98% (1 of 93 relapsed). In contrast, the PFS for RRMS and SPMS was 95% (11 of 414) and 66% (21 of 93), respectively. While SPMS is less likely to relapse after HSCT, SPMS is at a greater risk of continued disease progression which may be related to the underlying pathophysiology differences between RRMS (predominately inflammatory) and SPMS (predominately neurodegenerative).
In summary, autologous non-myeloablative HSCT provides subsequent drug-free reversal of neurologic disability of greater than 1.0 EDSS point for greater than 5 years in patients with RRMS. The data herein suggest that non-myeloablative HSCT may not be effective in newly diagnosed naSPMS and less effective in newly diagnosed aSPMS. ITP usually occurs within the first 2 years, and late thyroid dysfunction for up to 10 years after transplantation.
Declaration
Conflicts of interest
None.
Ethical standards
This study involved human sujects. All procedures were done according to ethical standards of the institution that are compliant with FDA, NIH, and Helsinki guidance. All patients signed an Institutional Review Board approved consent.
References
- 1.Burt RK, Burns W, Hess A. Bone marrow transplantation for multiple sclerosis. Bone Marrow Transplant. 1995;16(1):1–6. doi: 10.1038/sj.bmt.1703081. [DOI] [PubMed] [Google Scholar]
- 2.Fassas A, Anagnostopoulos A, Kazis A, Kapinas K, Sakellari I, Kimiskidis V, Tsompanakou A. Peripheral blood stem cell transplantation in the treatment of progressive multiple sclerosis: first results of a pilot study. Bone Marrow Transplant. 1997;20(8):631–638. doi: 10.1038/sj.bmt.1700944. [DOI] [PubMed] [Google Scholar]
- 3.Burt RK, Traynor AE, Cohen B, Karlin KH, Davis FA, Stefoski D, Terry C, Lobeck L, Russell EJ, Goolsby C, Rosen S, Gordon LI, Keever-Taylor C, Brush M, Fishman M, Burns WH. T cell-depleted autologous hematopoietic stem cell transplantation for multiple sclerosis: report on the first three patients. Bone Marrow Transplant. 1998;21(6):537–541. doi: 10.1038/sj.bmt.1701129. [DOI] [PubMed] [Google Scholar]
- 4.Burt RK, Cohen BA, Russell E, Spero K, Joshi A, Oyama Y, Karpus WJ, Luo K, Jovanovic B, Traynor A, Karlin K, Stefoski D, Burns WH. Hematopoietic stem cell transplantation for progressive multiple sclerosis: failure of a total body irradiation-based conditioning regimen to prevent disease progression in patients with high disability scores. Blood. 2003;102(7):2373–2378. doi: 10.1182/blood-2003-03-0877. [DOI] [PubMed] [Google Scholar]
- 5.Nash RA, Bowen JD, McSweeney PA, Pavletic SZ, Maravilla KR, Park MS, Storek J, Sullivan KM, Al-Omaishi J, Corboy JR, DiPersio J, Georges GE, Gooley TA, Holmberg LA, LeMaistre CF, Ryan K, Openshaw H, Sunderhaus J, Storb R, Zunt J, Kraft GH. High-dose immunosuppressive therapy and autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Blood. 2003;102(7):2364–2372. doi: 10.1182/blood-2002-12-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samijn JP, te Boekhorst PA, Mondria T, van Doorn PA, Flach HZ, van der Meché FG, Cornelissen J, Hop WC, Löwenberg B, Hintzen RQ. Intense T cell depletion followed by autologous bone marrow transplantation for severe multiple sclerosis. J Neurol Neurosurg Psychiatry. 2006;77(1):46–50. doi: 10.1136/jnnp.2005.063883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Openshaw H, Lund BT, Kashyap A, Atkinson R, Sniecinski I, Weiner LP, Forman S. Peripheral blood stem cell transplantation in multiple sclerosis with busulfan and cyclophosphamide conditioning: report of toxicity and immunological monitoring. Biol Blood Marrow Transplant. 2000;6(5A):563–575. doi: 10.1016/S1083-8791(00)70066-8. [DOI] [PubMed] [Google Scholar]
- 8.Atkins HL, Bowman M, Allan D, Anstee G, Arnold DL, Bar-Or A, Bence-Bruckler I, Birch P, Bredeson C, Chen J, Fergusson D, Halpenny M, Hamelin L, Huebsch L, Hutton B, Laneuville P, Lapierre Y, Lee H, Martin L, McDiarmid S, O'Connor P, Ramsay T, Sabloff M, Walker L, Freedman MS. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicenter single-group phase 2 trial. Lancet (London, England) 2016;388(10044):576–585. doi: 10.1016/S0140-6736(16)30169-6. [DOI] [PubMed] [Google Scholar]
- 9.Ni XS, Ouyang J, Zhu WH, Wang C, Chen B. Autologous hematopoietic stem cell transplantation for progressive multiple sclerosis: report of efficacy and safety at three yr of follow up in 21 patients. Clin Transplant. 2006;20(4):485–489. doi: 10.1111/j.1399-0012.2006.00510.x. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Ji BX, Su L, Dong HQ, Sun XJ, Liu CY. Clinical outcomes after autologous hematopoietic stem cell transplantation in patients with progressive multiple sclerosis. Chin Med J (Engl) 2006;119(22):1851–1855. doi: 10.1097/00029330-200611020-00001. [DOI] [PubMed] [Google Scholar]
- 11.Saiz A, Blanco Y, Carreras E, Berenguer J, Rovira M, Pujol T, Marín P, Arbizu T, Graus F. Clinical and MRI outcome after autologous hematopoietic stem cell transplantation in MS. Neurology. 2004;62(2):282–284. doi: 10.1212/WNL.62.2.282. [DOI] [PubMed] [Google Scholar]
- 12.Fassas A, Anagnostopoulos A, Kazis A, Kapinas K, Sakellari I, Kimiskidis V, Smias C, Eleftheriadis N, Tsimourtou V. Autologous stem cell transplantation in progressive multiple sclerosis–an interim analysis of efficacy. J Clin Immunol. 2000;20(1):24–30. doi: 10.1023/A:1006686426090. [DOI] [PubMed] [Google Scholar]
- 13.Shevchenko YL, Novik AA, Kuznetsov AN, Afanasiev BV, Lisukov IA, Kozlov VA, Rykavicin OA, Ionova TI, Melnichenko VY, Fedorenko DA, Kulagin AD, Shamanski SV, Ivanov RA, Gorodokin G. High-dose immunosuppressive therapy with autologous hematopoietic stem cell transplantation as a treatment option in multiple sclerosis. Exp Hematol. 2008;36(8):922–928. doi: 10.1016/j.exphem.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Mancardi GL, Sormani MP, Di Gioia M, Vuolo L, Gualandi F, Amato MP, Capello E, Currò D, Uccelli A, Bertolotto A, Gasperini C, Lugaresi A, Merelli E, Meucci G, Motti L, Tola MR, Scarpini E, Repice AM, Massacesi L, Italian BMT Study Group Saccardi R Autologous haematopoietic stem cell transplantation with an intermediate intensity conditioning regimen in multiple sclerosis: the Italian multi-centre experience. Mult Scler. 2012;18(6):835–42. doi: 10.1177/1352458511429320. [DOI] [PubMed] [Google Scholar]
- 15.Mariottini A, Filippini S, Innocenti C, Forci B, Mechi C, Barilaro A, Fani A, Carlucci G, Saccardi R, Massacesi L, Repice AM. Impact of autologous haematopoietic stem cell transplantation on disability and brain atrophy in secondary progressive multiple sclerosis. Mult Scler. 2021;27(1):61–70. doi: 10.1177/1352458520902392. [DOI] [PubMed] [Google Scholar]
- 16.Saccardi R, Mancardi GL, Solari A, Bosi A, Bruzzi P, Di Bartolomeo P, Donelli A, Filippi M, Guerrasio A, Gualandi F, La Nasa G, Murialdo A, Pagliai F, Papineschi F, Scappini B, Marmont AM. Autologous HSCT for severe progressive multiple sclerosis in a multicenter trial: impact on disease activity and quality of life. Blood. 2005;105(6):2601–2607. doi: 10.1182/blood-2004-08-3205. [DOI] [PubMed] [Google Scholar]
- 17.Lee H, Narayanan S, Brown RA, Chen JT, Atkins HL, Freedman MS, Arnold DL. Brain atrophy after bone marrow transplantation for treatment of multiple sclerosis. Mult Scler (Houndmills, Basingstoke, England) 2017;23(3):420–431. doi: 10.1177/1352458516650992. [DOI] [PubMed] [Google Scholar]
- 18.Sharrack B, Saccardi R, Alexander T, Badoglio M, Burman J, Farge D, Greco R, Jessop H, Kazmi M, Kirgizov K, Labopin M, Mancardi G, Martin R, Moore J, Muraro PA, Rovira M, Sormani MP, Snowden JA, European Society for Blood and Marrow Transplantation (EBMT) Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of the International Society for Cellular Therapy (ISCT) and EBMT (JACIE) Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE) Bone Marrow Transplant. 2020;55(2):283–306. doi: 10.1038/s41409-019-0684-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snowden JA, Badoglio M, Labopin M, Giebel S, McGrath E, Marjanovic Z, Burman J, Moore J, Rovira M, Wulffraat NM, Kazmi M, Greco R, Snarski E, Kozak T, Kirgizov K, Alexander T, Bader P, Saccardi R, Farge D, European Society for Blood and Marrow Transplantation (EBMT) Autoimmune Diseases Working Party (ADWP); EBMT Paediatric Working Party (PWP); Joint Accreditation Committee of the International Society for Cellular Therapy (ISCT); EBMT (JACIE) Evolution, trends, outcomes, and economics of hematopoietic stem cell transplantation in severe autoimmune diseases. Blood Adv. 2017;1(27):2742–2755. doi: 10.1182/bloodadvances.2017010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, Wolinsky JS, Balcer LJ, Banwell B, Barkhof F, Bebo B, Jr, Calabresi PA, Clanet M, Comi G, Fox RJ, Freedman MS, Goodman AD, Inglese M, Kappos L, Kieseier BC, Lincoln JA, Lubetzki C, Miller AE, Montalban X, O'Connor PW, Petkau J, Pozzilli C, Rudick RA, Sormani MP, Stüve O, Waubant E, Polman CH. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boffa G, Massacesi L, Inglese M, Mariottini A, Capobianco M, Lucia M, Amato MP, Cottone S, Gualandi F, De Gobbi M, Greco R, Scimè R, Frau J, Zimatore GB, Bertolotto A, Comi G, Uccelli A, Signori A, Angelucci E, Innocenti C, Ciceri F, Repice AM, Sormani MP, Saccardi R, Mancardi G, Italian BMT-MS study group Long-term clinical outcomes of hematopoietic stem cell transplantation in multiple sclerosis. Neurology. 2021;10:1212. doi: 10.1212/WNL.0000000000011461. [DOI] [PubMed] [Google Scholar]
- 22.Burt RK, Balabanov R, Han X, Sharrack B, Morgan A, Quigley K, Yaung K, Helenowski IB, Jovanovic B, Spahovic D, Arnautovic I, Lee DC, Benefield BC, Futterer S, Oliveira MC, Burman J. Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis. JAMA. 2015;313(3):275–284. doi: 10.1001/jama.2014.17986. [DOI] [PubMed] [Google Scholar]
- 23.Burman J, Iacobaeus E, Svenningsson A, Lycke J, Gunnarsson M, Nilsson P, Vrethem M, Fredrikson S, Martin C, Sandstedt A, Uggla B, Lenhoff S, Johansson JE, Isaksson C, Hägglund H, Carlson K, Fagius J. Autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: the Swedish experience. J Neurol Neurosurg Psychiatry. 2014;85(10):1116–1121. doi: 10.1136/jnnp-2013-307207. [DOI] [PubMed] [Google Scholar]
- 24.Burt RK, Loh Y, Cohen B, Stefoski D, Balabanov R, Katsamakis G, Oyama Y, Russell EJ, Stern J, Muraro P, Rose J, Testori A, Bucha J, Jovanovic B, Milanetti F, Storek J, Voltarelli JC, Burns WH. Autologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: a phase I/II study. Lancet Neurol. 2009;8(3):244–253. doi: 10.1016/S1474-4422(09)70017-1. [DOI] [PubMed] [Google Scholar]
- 25.Fagius J, Lundgren J. Early highly aggressive MS successfully treated by hematopoietic stem cell transplantation. Mult Scler. 2009;15(2):229–237. doi: 10.1177/1352458508096875. [DOI] [PubMed] [Google Scholar]
- 26.Burt RK, Balabanov R, Burman J, Sharrack B, Snowden JA, Oliveira MC, et al. Effect of nonmyeloablative hematopoietic stem cell transplantation vs continued disease-modifying therapy on disease progression in patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA. 2019;321(2):165–174. doi: 10.1001/jama.2018.18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nash RA, Hutton GJ, Racke MK, Popat U, Devine SM, Griffith LM, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for relapsing-remitting multiple sclerosis (HALT-MS): a 3-year interim report. JAMA Neurol févr. 2015;72(2):159–169. doi: 10.1001/jamaneurol.2014.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loh Y, Oyama Y, Statkute L, et al. Development of a secondary autoimmune disorder after hematopoietic stem cell transplantation for autoimmune diseases: role of conditioning regimen used. Blood. 2007;109:2643–2548. doi: 10.1182/blood-2006-07-035766. [DOI] [PubMed] [Google Scholar]
- 29.Daikeler T, Labopin M, Di Gioia M, et al. Secondary autoimmune diseases occurring after HSCT for an autoimmune disease: a retrospective study of the EBMT Autoimmune Disease Working Party. Blood. 2011;118:1693–1698. doi: 10.1182/blood-2011-02-336156. [DOI] [PubMed] [Google Scholar]
- 30.Burt RK, Muraro PA, Farge D, Oliveria MC, Snowden JA, Saccardi R, Han X, Quigley K, Bueno V, Frasca D, Fedorenko D, Burman J. New autoimmune diseases after autologous hematopoietic stem cell transplantation for multiple sclerosis. Bone Marrow Transplant. 2021;56(7):1509–1517. doi: 10.1038/s41409-021-01277-y. [DOI] [PubMed] [Google Scholar]
- 31.Nicholas RS, Rhone EE, Mariottini A, Silber E, Malik O, Singh-Curry V, Turner B, Scalfari A, Ciccarelli O, Sormani MP, Olavarria E, Mehra V, Gabriel I, Kazmi MA, Muraro P, London Group on Autologous Hematopoietic Stem Cell Transplantation for Multiple Sclerosis Autologous haematopoietic stem cell transplantation in active multiple sclerosis: a real-world case series. Neurology. 2021;10:1212. doi: 10.1212/WNL.0000000000012449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Healy BC, Engler D, Glanz B, Musallam A, Chitnis T. Assessment of definitions of sustained disease progression in relapsing-remitting multiple sclerosis. Mult Scler Int. 2013;2013:189624. doi: 10.1155/2013/189624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodkin DE. EDSS reliability. Neurology. 1991;41(2):332. doi: 10.1212/WNL.41.2_Part_1.332. [DOI] [PubMed] [Google Scholar]
- 34.Kanamori H, Weber DJ, Rutala WA. Healthcare outbreaks associated with a water reservoir and infection prevention strategies. Clin Infect Dis. 2016;62(11):1423–2143. doi: 10.1093/cid/ciw122. [DOI] [PubMed] [Google Scholar]
- 35.Kizny Gordon AE, Mathers AJ, Cheong EYL, Gottleib T, Kotay S, Walker AS, Peto TEA, Crook DW, Stoesser N. Hospital water environment as a reservoir for carbapenem-resistant organisms causing hospital-acquired infections-a systematic review of the literature. Clin Infect Dis. 2017;64(10):1435–1444. doi: 10.1093/cid/cix132. [DOI] [PubMed] [Google Scholar]
- 36.Kessler MA, Osman F, Marx J, Jr, Pop-Vicas A, Safdar N. Hospital-acquired Legionella pneumonia outbreak at an academic medical center: Lessons learned. Am J Infect Control. 2021;S0196–6553(21):00091–92. doi: 10.1016/j.ajic.2021.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Baker D, Herrod SS, Alvarez-Gonzalez C, Giovannoni G, Schmierer K. Interpreting lymphocyte reconstitution data from the pivotal phase 3 trials of alemtuzumab. JAMA Neurol. 2017;74(8):961–969. doi: 10.1001/jamaneurol.2017.0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kappos L, Butzkueven H, Wiendl H, Spelman T, Pellegrini F, Chen Y, Dong Q, Koendgen H, Belachew S, Tysabri® Observational Program (TOP) Investigators Trojano M Greater sensitivity to multiple sclerosis disability worsening and progression events using a roving versus a fixed reference value in a prospective cohort study. Mult Scler. 2018;24(7):963–973. doi: 10.1177/1352458517709619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lepore V, Bosetti C, Santucci C, Iaffaldano P, Trojano M, Mosconi P, Italian Multiple Sclerosis Register Centers Group, the Scientific Committee of Italian SM Register Detection of disability worsening in relapsing-remitting multiple sclerosis patients: a real-world roving Expanded Disability Status Scale reference analysis from the Italian Multiple Sclerosis Register. Eur J Neurol. 2021;28(2):567–578. doi: 10.1111/ene.14589. [DOI] [PubMed] [Google Scholar]
- 40.European Medicines Agency. Guideline on clinical investigation of medicinal products for the treatment of multiple sclerosis, 2015, http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500185161.pdf
- 41.Burt RK, Balabanov R, Voltarelli J, Barreira A, Burman J. Autologous hematopoietic stem cell transplantation for multiple sclerosis–if confused or hesitant, remember: 'treat with standard immune suppressive drugs and if no inflammation, no response. Mult Scler. 2012;18(6):772–775. doi: 10.1177/1352458512442993. [DOI] [PubMed] [Google Scholar]