Abstract
Human parasitic infections—including malaria, and many neglected tropical diseases (NTDs)—have long represented a Gordian knot in global public health: ancient, persistent, and exceedingly difficult to control. With the coronavirus disease (Covid-19) pandemic substantially interrupting control programmes worldwide, there are now mounting fears that decades of progress in controlling global parasitic infections will be undone. With Covid-19 moreover exposing deep vulnerabilities in the global health system, the current moment presents a watershed opportunity to plan future efforts to reduce the global morbidity and mortality associated with human parasitic infections. In this chapter, we first provide a brief epidemiologic overview of the progress that has been made towards the control of parasitic diseases between 1990 and 2019, contrasting these fragile gains with the anticipated losses as a result of Covid-19. We then argue that the complementary aspirations of the United Nations Sustainable Development Goals (SDGs) and the World Health Organization (WHO)’s 2030 targets for parasitic disease control may be achieved by aligning programme objectives within the One Health paradigm, recognizing the interdependence between humans, animals, and the environment. In so doing, we note that while the WHO remains the preeminent international institution to address some of these transdisciplinary concerns, its underlying challenges with funding, authority, and capacity are likely to reverberate if left unaddressed. To this end, we conclude by reimagining how models of multisectoral global health governance—combining the WHO's normative and technical leadership with greater support in allied policy-making areas—can help sustain future malaria and NTD elimination efforts.
Keywords: Neglected tropical diseases, Malaria, COVID-19, Global health governance, World Health Organization (WHO), Sustainable Development Goals (SDGs), One Health
1. Introduction
Human parasitic infections—including malaria, and many neglected tropical diseases (NTDs)—have long represented a Gordian knot in global public health: ancient, persistent, and exceedingly difficult to control. With the coronavirus disease (Covid-19) pandemic placing a halt on mass drug administration (MDA) programmes and also interrupting vector control worldwide, there are now mounting fears that decades of painstaking progress in controlling global parasitic infections will soon disappear (Ehrenberg et al., 2020; Hotez et al., 2021). Yet the breathtaking speed at which Covid-19 diagnostic tests, medical therapies, and vaccines have been developed, while deserving of our collective marvel, also raises deeply troubling questions regarding health equity and justice within the global health system. Central to these concerns is the notion that pre-existing infectious diseases—which exact an overwhelmingly disproportionate burden on the world's poor—are apparently not worthy of the same attention nor resources as those that affect higher-income countries. A sobering assessment of this dilemma was recently offered by Peter Sands, executive director of the Global Fund, who lamented the persistence of HIV/AIDS, tuberculosis, vaccine-preventable infections, and indeed parasitic diseases, as “a policy choice and budgetary decision” (Sands, 2021).
With Covid-19 exposing deep vulnerabilities in the global health system, the current moment presents a watershed opportunity to plan future efforts to reduce the global morbidity and mortality associated with human parasitic infections. To this end, our short perspective is divided into three sections. First, we provide a brief epidemiologic overview of the progress that has been made towards the control of malaria and certain NTDs between 1990 and 2019, contrasting these fragile gains with the losses that have occurred and are anticipated as a result of Covid-19. Second, we argue that the complementary aspirations of the United Nations Sustainable Development Goals (SDGs) and the World Health Organization (WHO)’s 2030 targets for malaria (WHO, 2021) and NTD (WHO, 2020b) control may be achieved by aligning programme objectives within the One Health paradigm (Zinsstag et al., 2005), recognizing the interdependence between humans, animals, and the environment. This comprehensive approach addresses not only the clinical manifestations of parasitic disease, but also those that emphasize the mutually reinforcing social, economic, and environmental factors that allow human parasitic diseases to flourish in the first instance. These include, but are not limited to, lack of universal primary healthcare, poverty, food insecurity, unequal access to education, armed conflict, agricultural intensification, rapid urbanization, and gender and/or racial discrimination. Clearly, the WHO is a preeminent international institution to address some of these transdisciplinary concerns, but the Covid-19 pandemic has exposed issues with the WHO's funding, authority, and capacity that are likely to reverberate if left unaddressed. Such considerations will form the bases of our final aim: to reimagine how models of multisectoral global health governance—combining the WHO's normative and technical leadership with greater support in allied policy-making areas—can help sustain future malaria and NTD elimination efforts as part of our ageless bid to “leave no one behind” (Kharas et al., 2019).
2. Global malaria and NTD control 1990–2019: An epidemiological overview
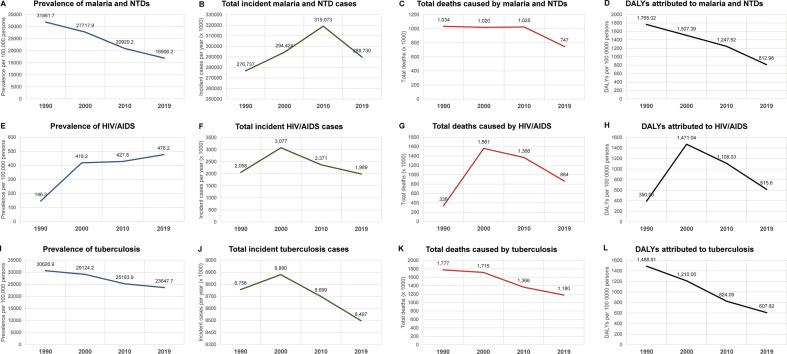
The most comprehensive data on the burden of global parasitic diseases are derived from the Global Burden of Disease (GBD) study. A longitudinal analysis of the GBD from 1990 to 2019 reveals significant advances made towards the global control of parasitic diseases over the last 30 years, reflecting similar declines observed for HIV/AIDS and tuberculosis (Fig. 1 ) (GBD Collaborative Network, 2020). The total number of prevalent cases of malaria and NTDs was estimated to be in the order of 1.7 billion cases in both 1990 and 2000, 1.46 billion in 2010, and 1.30 billion in 2019 (GBD Collaborative Network, 2020; Murray et al., 2012; Vos et al., 2020). Although these reductions appear rather modest, a more accurate assessment of reduced disease burden was observed in terms of prevalence proportion per 100,000 persons, taking into consideration human population growth over the same period. The prevalence per 100,000 persons diagnosed with malaria and/or any NTD almost halved from 1990 through 2019, from almost 32,000 cases to just under 17,000 cases per 100,000 persons (Fig. 1A). Reductions were also observed in total deaths (1.03 million to 747,000) and incidence rate (5200 to 3700 new cases per 100,000 persons, Fig. 1B and C).
Fig. 1.
Prevalence, incident cases, total deaths, and disability-adjusted life years (DALYs) for malaria and the NTDs, HIV/AIDS, and tuberculosis. Note that the total incident cases and deaths attributed to malaria and the NTDs follow similar trends observed for HIV/AIDS and tuberculosis. The total burden of malaria and NTDs, in terms of DALYs, has remained higher than HIV/AIDS and tuberculosis since 1990.
Data from the GBD Collaborative Network, 2020. Global Burden of Disease Study 1990, 2000, 2010, and 2019 Results. Institute for Health Metrics and Evaluation (IHME): Seattle, United States. http://ghdx.healthdata.org/gbd-results-tool.
Perhaps the single-most notable decrease in malaria and NTD disease burden was observed in terms of disability-adjusted life years (DALYs), which represents the loss of a single life-year lived in “full” or optimal health, thereby distilling into a single measure both the mortality of disease (as the years of life lost, or YLL, as a result of premature death), and morbidity (as the years lived with disability, YLD). The introduction of the DALY within the inaugural GBD of 1990 (Murray et al., 1994) was among the first attempts in global health epidemiology to systematically capture the chronic complications of disease, which are particularly pertinent to parasitic infections. The DALYs associated with malaria and NTDs totaled 94, 92, and 87 million in 1990, 2000, and 2010, respectively, followed by more substantial reduction in the last decade to 63 million in 2019 (Fig. 1D) (GBD Collaborative Network, 2020; Murray et al., 2012; Vos et al., 2020). There are several caveats that should be noted, however, including the systematic underestimation of DALYs for some conditions where data are not available. For example, because the African Programme for Onchocerciasis Control MDA initiatives explicitly target high-prevalence regions (> 60% communities infected), data from hypoendemic regions do not feature in GBD estimates (Boatin and Richards, 2006). Furthermore, non-parasitic diseases (dengue, leprosy, and rabies) are incorporated in these reported totals, though their contribution represents only ~ 5% of overall DALYs attributed to malaria and NTDs.
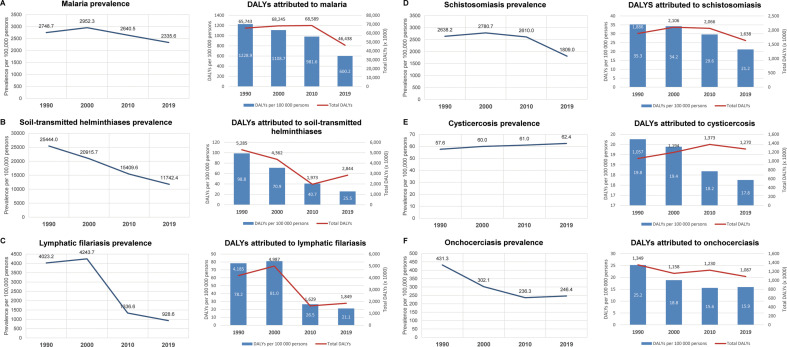
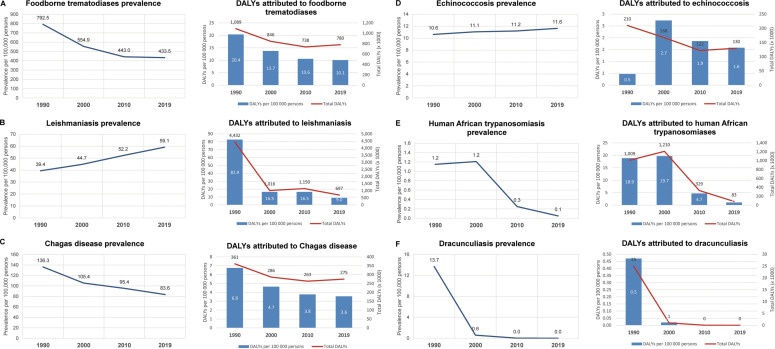
Data disaggregated by disease show that the reduced burden of parasitic infections since 1990 can be attributed mostly to a fall in DALYs lost to malaria, which reached a peak of nearly 69 million in 2010, falling to 46 million by 2019 (1229 to 600 DALYs per 100,000) (Fig. 2 ) (GBD Collaborative Network, 2020; Murray et al., 2012; Vos et al., 2020). This reduction in DALYs was most likely due to decreases in the yearly malaria deaths over the last decade, which hovered in the range of 800,000–900,000 until 2010, falling to approximately 640,000 by 2019. This progress has been in large part due to intensified campaigns to distribute long-lasting insecticide-treated bed nets (LLINs), complemented with indoor residual insecticide spraying (IRS), provision of topical repellents, and the increased availability of artemisinin-based combination therapy (ACTs) (Bhatt et al., 2015). However, reductions in malaria-associated DALYs may mask signs that progress for many other parasitic diseases and NTDs had reached an impasse even before Covid-19. It is true that human African trypanosomiasis and dracunculiasis (Guinea worm disease) now appear feasible candidates for eradication, and impressive reductions in DALYs per 100,000 persons have been observed for leishmaniasis (82.9 in 1990 to 9.0 in 2019), soil-transmitted helminthiases (98.8 to 25.5), lymphatic filariasis (78.2 to 21.1), and schistosomiasis (35.3 to 21.2) from 1990 to 2019 (Fig. 3 ). Notably, these latter three diseases are all predominantly controlled through preventive chemotherapy with cheap and readily available medication, administered via mass drug distribution campaigns, with substantial efforts made over the last decade by Ministries of Health, donors, and implementing partners to integrate delivery for more complete and effective coverage (Standley et al., 2018).
Fig. 2.
Prevalence and disability-adjusted life years (DALYs) for malaria and selected NTDs. Malaria (A), soil-transmitted helminthiases (B), lymphatic filariasis (C), schistosomiasis (D), cysticercosis (E), and onchocerciasis (F) represent the top six contributors to total DALYs attributed to global parasitic diseases in 2019.
Data from the GBD Collaborative Network, 2020. Global Burden of Disease Study 1990, 2000, 2010, and 2019 Results. Institute for Health Metrics and Evaluation (IHME): Seattle, United States. http://ghdx.healthdata.org/gbd-results-tool.
Fig. 3.
Prevalence and disability-adjusted life years (DALYs) for selected NTDs, including foodborne trematodiases (A), leishmaniasis (B), Chagas disease (C), echinococcosis (D), human African trypanosomiasis (E), and dracunculiasis (F). These conditions have been ranked according to total DALYs in 2019. Note that human African trypanosomiasis and dracunculiasis now appear feasible targets of eradication.
Data from the GBD Collaborative Network, 2020. Global Burden of Disease Study 1990, 2000, 2010, and 2019 Results. Institute for Health Metrics and Evaluation (IHME): Seattle, United States. http://ghdx.healthdata.org/gbd-results-tool.
In contrast, despite earlier gains, data from the last decade (2010 to 2019) showed that progress has now reached a plateau for lymphatic filariasis (26.5 to 21.1 DALYs per 100,000 persons), onchocerciasis (15.6 to 15.9), food-borne trematodiases (10.6 to 10.1), echinococcosis (11.2 to 11.6), and Chagas disease (3.8 to 3.6). In some cases, the prevalence of disease had appeared to be rising, as in cysticercosis (61.0 to 62.4 per 100,000 persons) and leishmaniasis (52.2 to 59.1) (Fig. 2, Fig. 3). Others experienced increases in total attributable DALYs (in thousands) despite stable per-capita estimates, as in soil-transmitted helminthiases (1973 to 2844), lymphatic filariasis (1629 to 1849), foodborne trematodiases (738 to 780), Chagas disease (263 to 275), and echinococcosis (122 to 130), as population numbers grow in endemic areas, placing more people at risk of these diseases. Overall, analysis of these data suggest that reductions in the global burden of parasitic diseases since 1990 have been dominated by gains made in the control of malaria, soil-transmitted helminthiases, lymphatic filariasis, leishmaniasis, and schistosomiasis, though trends may be starting to stagnate overall. Indeed, figures from the last decade had already foreshadowed a resurgence for a number of parasitic diseases over coming years, well before the start of the Covid-19 pandemic.
3. The impact of Covid-19 on global control of human parasitic infections
While it is not yet possible to assess the full impact of Covid-19 on the global control of human parasitic infections, the negative consequences will almost certainly be profound. Anticipated setbacks include, but are certainly not limited to: (a) excess morbidity and mortality caused by interruptions to transnational, national, and subnational efforts for malaria and NTD control, including MDA programmes (Hogan et al., 2020); (b) already-fragile health systems that have diverted precious resources—including frontline health workers, healthcare administrators, and medical supplies—to the Covid-19 effort (Abdela et al., 2020; Chaumont et al., 2020); (c) delayed presentation to care for the evaluation of febrile illnesses, including malaria (Brooke et al., 2020); (d) freezing of global supply chains for antimalarials and antihelmintics, LLINs, insecticides, and diagnostic tests, due to government lockdown measures, international trade restrictions, and/or lost funding streams, e.g., the retrenchment of UK Official Development Assistance (de Souza et al., 2020; Ehrenberg et al., 2021; Royal Society of Tropical Medicine and Hygiene, 2021); (e) further impoverishment and health inequity within populations most vulnerable to parasitic diseases; (f) loss of research productivity, including for urgently needed medical countermeasures (e.g., vaccines), in addition to point-of-care diagnostic tests for both clinical use and epidemiologic surveillance within low-resource settings (Adepoju, 2020; Tchuem Tchuenté et al., 2018); and (g) postponing the introduction of other neglected diseases into the official WHO NTD portfolio. By mid-2020, interim advice from the WHO recommended for core services (i.e. LLIN distribution, seasonal antimalarial chemoprevention, and ACT) to continue within malaria endemic countries (WHO, 2020c), but recommended the cessation of all MDA programmes, case-finding missions, and epidemiologic surveys for NTDs (WHO, 2020a). While many national malaria and NTD control services in sub-Saharan Africa (e.g. in Guinea, Guinea-Bissau, and Nigeria) remain operational (Malaria Consortium, 2021; United Nations Development Programme, 2021), it is not known when such campaigns will return to pre-pandemic capacities. Ongoing outbreaks caused by SARS-CoV-2 variants and the devastating collapse of health systems, as seen in Brazil, India, Iran, and South Africa (Silva and Pena, 2021; Slater et al., 2021), together with low Covid-19 vaccine coverage across the global South for the foreseeable future, do not bode well for the early resumption and enduring provision of disease and vector control programmes.
The extensive multisectoral effects of Covid-19 have raised serious questions regarding whether key disease and vector control targets will be met over the next decade. In the WHO Global Technical Strategy for Malaria 2016–2030, the main objectives listed include the reduction of malaria-related mortality and case incidence by ≥ 90% compared to 2015, elimination of malaria in 35 countries, and preventing the re-emergence of malaria in countries that have previously achieved elimination (WHO, 2021). Prior to Covid-19, there had already been increasing alarm that global progress had stalled over the last 5 years, with data from the World Malaria Programme showing that only 31 of 92 malaria-endemic countries were on track to achieve the 2020 target of ≥ 40% reduction in incidence rates compared to 2015 (WHO Global Malaria Programme, 2020). However, there are now signs that even partial service interruptions may result in catastrophic setbacks. A recent cross-sectional analysis conducted by the Global Fund, surveying 502 health facilities in 24 sub-Saharan and 7 Asian countries, showed that the number of malaria diagnoses and malaria treatment services from April to September 2020 both fell by at least 15% and 56%, respectively, compared to the same period in 2019 (Global Fund, 2021). These findings were presumably due to diminished diagnostic testing capacity and medication shortfalls. Indeed, over 20% of surveyed healthcare services in Africa had run out of paediatric doses of antimalarial treatment, portending chronic medication shortages for children for whom the risk of death is greatest.
Despite inherent uncertainties when attempting to quantify the excess morbidity and mortality that could result from such disruptions, several independent modelling studies have reached the stark conclusion that the total malaria burden in sub-Saharan Africa may have doubled in 2020 when compared to 2019, with up to an additional 400,000 annual deaths forecast under the most pessimistic scenarios (Sherrard-Smith et al., 2020; Weiss et al., 2021). It is critically important to note that the modelling assumptions used in such studies only take into account interruptions in malaria-specific interventions, such as suspension of LLIN distribution or reduced case treatment, and do not consider the rise in comorbid conditions that may increase malaria-specific mortality. For instance, it is anticipated that up to an additional 1.5 million children in sub-Saharan Africa will experience acute malnutrition and wasting due to Covid-19-related unemployment, food insecurity, and decreased access to nutrition and social assistance programmes (Headey et al., 2020). Though difficult to comprehend, it is quite possible that the actual scale of devastation may be even more dire than current projections.
The global aspirations set by the WHO NTD Roadmap 2021–2030 also face formidable challenges. Key objectives of the roadmap include a ≥ 90% reduction in persons requiring intervention against NTDs, ≥ 75% reduction in the DALYs attributable to NTDs, eradication of endemic treponematosis (yaws) and Guinea worm disease, and for the remaining NTDs to be either eliminated as a public health problem (EPHP) or for transmission to be disrupted (WHO, 2020b). Achieving these highly ambitious but necessary goals would require at least 1–1.5 billion of the world's poorest people to no longer require prevention and/or treatment for NTDs by the end of 2030. The extent to which Covid-19 will derail these ambitions will depend on a range of factors, including the transmission dynamics of the particular NTD, disease endemicity, the nature of public health interventions, length of service disruption, resilience of local healthcare systems, school closures, and funding, as well, of course, as the continued trajectory of the Covid-19 pandemic (Hollingsworth et al., 2021). For communities that rely on preventive MDA, setbacks are likely to be greater for those infections with higher effective reproductive numbers and therefore higher growth rates. Expected resurgences in schistosomiasis (Kura et al., 2021; Toor et al., 2020), soil-transmitted helminthiases (Malizia et al., 2021) and trachoma (Borlase et al., 2021) may mean that these conditions will likely not achieve EPHP status until after 2030, particularly in high-prevalence settings (NTD Modelling Consortium, 2020). On the other hand, delays in achieving 2030 WHO NTD targets may not be as pronounced for lymphatic filariasis (Prada et al., 2021) and onchocerciasis (Hamley et al., 2021), which do not transmit as quickly and may be more responsive to early implementation of catch-up strategies such as biannual MDAs and/or expanded coverage. For diseases that rely on active case searching and epidemiologic surveys, including human African trypanosomiasis, leishmaniasis, Guinea worm disease, and Chagas disease, setbacks will depend heavily on when local health systems and community-based programmes are ready to resume elimination activities. Of course, this is contingent on the end of the Covid-19 pandemic, which will remain a distant hope without efforts to ensure global vaccine equity and uptake in underserved communities (Katz et al., 2021).
Unfortunately, the broad range of health, social, and economic benefits that come with effective malaria and NTD control may also be lost over the coming years. For example, MDA-administered ivermectin for lymphatic filariasis and onchocerciasis has non-target effects on scabies, other ectoparasitoses, and strongyloidiasis; praziquantel used for schistosomiasis also treats foodborne trematodiases and cysticercosis (Hotez et al., 2019); and azithromycin used for the control of blinding trachoma, which could be considered a disease caused by an obligate intracellular parasite, may be associated with a reduction in all-cause childhood mortality (Keenan et al., 2020). Beyond health outcomes, effective control of malaria and NTDs has been associated with the preservation of international peace and security (Fürst et al., 2009; Jacobson and Bush, 2018), economic and work productivity (Conteh et al., 2010; Lenk et al., 2016), educational attainment (Houweling et al., 2016; Lucas, 2010), decreased stigma and social isolation (Litt et al., 2012; Weiss, 2008), and alleviation of gender inequities (Manandhar et al., 2018; Sun and Amon, 2018). These interwoven benefits—while not a feature of traditional measures of disease burden, nor explicitly mentioned as part of programme objectives—interrupt cycles of poverty, and thereby create the conditions in which communities can thrive. Finally, it would be remiss not to mention that delays in controlling currently-designated NTDs will invariably undermine advocacy for other conditions that have been proposed as also deserving of NTD status, including infectious corneal ulceration (in part caused by Acanthamoeba spp.) (Ung et al., 2019), giardiasis (Coelho et al., 2017), loiasis (Zouré et al., 2011), and toxocariasis (Hotez and Wilkins, 2009). Compelling arguments could certainly be made for infections that often co-exist with existing NTDs, such as waterborne giardia and schistosome infections (Archer et al., 2020), and the major filarial nematode infections loiasis, lymphatic filariasis, and onchocerciasis (Cano et al., 2018). The costs of suspended global malaria and NTD programmes will therefore have consequences that stretch far beyond the control of parasitic infections themselves, with significant implications for where, when, and how future elimination efforts might be conducted.
4. Controlling global parasitic diseases through the “One Health” paradigm
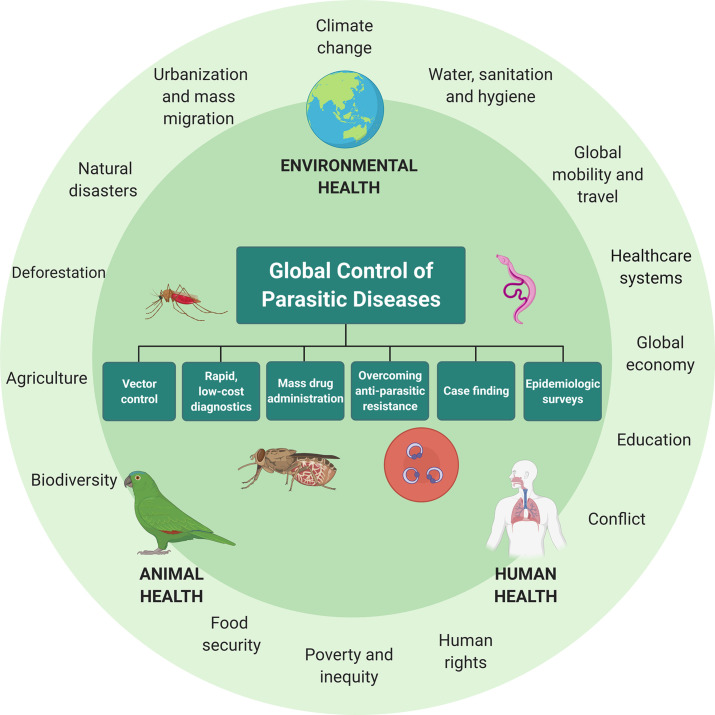
The growing burden of global parasitic diseases in the Covid-19 era will require renewed efforts that address not only the clinical manifestations of disease—for which field-deployable rapid diagnostics (Solomon et al., 2012), new treatments (Keller et al., 2021), vaccines (Datoo et al., 2021), and surveillance programmes (Galatas et al., 2020) are essential—but also the social, economic, and environmental substrate that characterize the world's poorest communities. A cross-disciplinary approach to disease prevention and control that has gained increasing traction is One Health, which recognizes the embedded contexts and interactions of human, animal, and environmental health (Fig. 4 ) (Standley et al., 2019; Zinsstag et al., 2005). Global parasitic infections today flourish owing to interconnected issues such as population growth, urbanization, globalization, environmental and climate change, conflict, and natural and humanitarian disasters (Bedford et al., 2019). The consequences of malaria and NTDs spill freely into areas such as the global economy, trade, food security, human rights, poverty, gender inequity, education, and global peace and security (Frenk and Moon, 2013). These issues are in part currently recognized by the UN SDGs—including the global alleviation of poverty (SDG 1) and hunger (SDG 2), universalized healthcare (SDG 3), access to quality education (SDG 4), gender equity (SDG 5), clean water and sanitation (SDG 6), infrastructure (SDG 9), access to decent work and economic growth (SDG 8), reduced inequality (SDG 10), and peace, justice, and strong institutions (SDG 16). The essence of One Health is also found in the NTD Roadmap for 2021–2030 and, albeit to a less detailed extent, within the WHO Global Technical Strategy for Malaria 2016–2030, which emphasize the need for biomedical interventions to be grounded in broader efforts to address the underlying socioeconomic determinants of disease, and also the animal reservoirs that allow parasites and their vectors to persist (WHO, 2020b, WHO, 2021). WHO has provided firm operational commitment to One Health, for example, via its participation in the Tripartite Alliance together with the Food and Agriculture Organization of the United Nations (FAO) and World Organization for Animal Health (OIE) (Frieden et al., 2014). For global parasitic diseases, the question is how One Health approaches are best operationalized to reduce community transmission, conduct case-finding in both human and animal populations, and prevent the re-emergence of disease owing to unaddressed animal reservoirs and/or disease vectors.
Fig. 4.
A One Health approach to the global control of parasitic diseases, emphasizing the interconnectedness of human, environmental, and animal health. One Health approaches favour programme interventions that are both disease-specific (i.e. vector control, rapid and deployable point of care diagnostics, mass drug administration, overcoming antiparasitic resistance, case finding, and epidemiologic surveys), and that target the underlying conditions that allow parasitic infections to persist, including but not limited to intractable poverty, urbanization and mass migration, lack of universalized healthcare, and armed conflict. Created under a standard academic licence using BioRender.com.
A One Health approach to malaria and the NTDs presents both extensive opportunities and daunting challenges. Perhaps the most urgent cross-cutting need is to address intractable poverty and economic stagnation in low-to-middle income countries (LMICs) (Smith et al., 2019). The World Bank recently estimated that over 500 million of the world's 711 million extreme poor (earnings of < US $1.90 per day) lived in Africa and the Middle East (Mahler et al., 2021). Though initial predictions suggested that LMICs would be spared the worst effects of Covid-19 (Cash and Patel, 2020), these latest data also reflect up to an additional 100 million people who will return to extreme poverty in 2021, a large proportion of whom reside within transitioning economies such as India and Nigeria. The International Monetary Fund has projected that sub-Saharan Africa's per capita gross domestic product (GDP) growth would likely range in the order of 3–4% over the next 5 years, compared to nearly 15% for all remaining countries (Selassie and Hakobyan, 2021). Furthermore, public debt in this region now sits at nearly 60% of GDP, a level not seen for nearly 20 years. Economic forecasts are also ominous across Latin America and the Caribbean, where a regionwide economic contraction of 7% of GDP in 2020—the highest in the world—will be met with a projected growth of only 4–5% over the coming year (Werner et al., 2021). The effect of increased poverty and economic shortfalls will likely be felt in the years to come, with dire consequences for SDG attainment, and also creating the conditions in which conflict and mass migrations are more likely to arise. For example, outbreaks of cutaneous and visceral leishmaniasis among refugees and internally displaced persons in conflict-ridden Somalia (Raguenaud et al., 2007), South Sudan (Al-Salem et al., 2016), and Syria (Youssef et al., 2019) highlight the intimate connection between economic prosperity and peace and security, two preconditions required for the successful implementation of global health programmes writ large.
The One Health approach also emphasizes the management of livestock and agriculture, particularly as the boundaries between human and animal habitats erode due to factors such as population growth and human migration. Historically, efforts to manage both definitive and intermediate animal reservoirs has been somewhat overlooked in the control of zoonotic NTDs, including Chagas disease, cysticercosis, echinococcosis, foodborne trematodiases, human African trypanosomiasis, leishmaniasis and schistosomiasis (Webster et al., 2016). A particularly recent example is Guinea worm disease, where eradication targets have been stymied by the growing recognition of widespread infection in dogs, accounting now for thousands of annual cases, and prompting calls for a re-think of control and surveillance strategies to target both human and canine infections (Boyce et al., 2020; Guagliardo et al., 2021; Standley and Schermerhorn, 2021). MDAs alone are rarely sufficient to interrupt disease transmission over the long-term; the use of praziquantel for the treatment of schistosomiasis in school-aged children, for example, is repeated every 6- to 12 months owing to reinfections arising from contact with freshwater sources contaminated with schistosome-infected snails. One Health coordination would supplement such efforts with the financing of appropriate sewage systems in underserved communities, establishing and maintaining water, sanitation, and hygiene (WASH) campaigns (Grimes et al., 2015), and targeted vector or intermediate host interventions (Rollinson et al., 2013), for instance, the implementation of targeted mollusciding (King et al., 2015). One Health approaches would also prioritize the research and development of novel prevention strategies and therapeutics (Pink et al., 2005), which will be important in light of growing concerns regarding selection-driven resistance against common antiparasitic and insecticide treatments (Crellen et al., 2016; Hemingway et al., 2016; Kelly-Hope et al., 2008). These considerations are particularly complex, of course, because treating animal reservoirs with antiparasitic treatment, and at veterinary dosages, may diminish the future utility of medications currently used to treat human infections (Gower et al., 2017).
Another major strength of One Health approaches is that they are inherently context-specific, allowing for interventions to be readily tailored to the biology, epidemiology, and the socioeconomic determinants that govern the transmission dynamics of each disease. For example, treating trypanosome-harbouring cattle with insecticides is seen as an effective method of tsetse fly control in Uganda, but such an approach has been argued to be less effective in other countries such as Kenya, Zimbabwe, and Tanzania, where tsetse flies are more likely to seek blood meals from wild mammals (Hargrove et al., 2003; Muturi et al., 2011). On the other hand, oral transmission of Chagas disease—historically associated with impoverished rural communities with poor housing and domiciliary infestations of triatomine (kissing) bugs—has become increasingly common across Latin America, driven by mass urbanization, conflict, and migration of populations from endemic to non-endemic areas, and therefore requiring measures beyond vector control alone (Bastos et al., 2010; Dias et al., 2008; Rassi et al., 2010). In other contexts, One Health considerations might lead to the implementation of programme synergies, for instance, within communities where polyparasitism is common, and where interventions could feasibly overlap. Prime examples would include the integration of malaria and lymphatic filariasis vector control initiatives (Kelly-Hope et al., 2013; van den Berg et al., 2013), and concurrent MDA and source control measures for onchocerciasis, soil-transmitted helminthiases and schistosomiasis (Mwinzi et al., 2012; Ndyomugyenyi and Kabatereine, 2003). Perhaps most importantly, the holistic outlook of a One Health approach to parasitic and neglected disease control has the potential to address development objectives beyond control or elimination of disease in humans, including food security (Wielinga and Schlundt, 2014), wildlife conservation (Lu et al., 2017), and tourism (Kasozi et al., 2021).
5. The role of the World Health Organization and multisectoral global health governance on control of parasitic diseases
Despite courting unprecedented controversy since the start of the pandemic (Buranyi, 2020; Gostin et al., 2020a, Gostin et al., 2020b), the WHO has shown that its unique combination of legal, normative, and technical authority remains unmatched by any other international health agency. Covid-19 has demonstrated, however, that the WHO may not be adequately equipped with the mandate or resources to mount optimal responses to the contemporary multisectoral challenges of infectious diseases. The far reaching consequences of Covid-19 on the global control of parasitic infections will require a broad range of global health bodies—including intergovernmental, national, and local health agencies, in addition to the private sector—to harmonize their activities towards common objectives, to meet pre-specified timelines, and to do so in a way that promotes global consistency, accountability, and solidarity (Bennett et al., 2018). Historically, interagency and private–public partnerships have formed the backbone of global efforts to control parasitic infections. For over 20 years, malaria elimination efforts have been coordinated primarily through collaborations between the WHO, the US President's Malaria Initiative, Global Fund, Unitaid, and the Bill and Melinda Gates Foundation (Haakenstad et al., 2019). On the other hand, private–public partnerships between the WHO and multinational pharmaceutical companies have resulted in the free supply of MDA treatments for decades, notably ivermectin for onchocerciasis and lymphatic filariasis (Merck & Co.), albendazole for lymphatic filariasis (GlaxoKlineSmith), mebendazole for soil-transmitted helminthiasis (Johnson & Johnson), and praziquantel for schistosomiasis (Merck KGaA) (Bradley et al., 2021; Molyneux et al., 2021). However, most of these partnerships are still heavily geared towards vertical programme implementation, which are critically important but may not be sufficient in themselves to address the socioeconomic and environmental dimensions that allow parasitic diseases to endure. Acknowledging that the WHO requires further support in cross-sector policy venues should not be taken as a criticism, but rather as a natural evolution in the character of modern infectious diseases that warrant global multisectoral responses.
One potential strategy for greater multisectoral collaboration would be to operationalize malaria and NTD elimination efforts within the United Nations (UN) system, which in recent years has increasingly viewed healthcare as a matter of international peace and security. This was demonstrated with the establishment of UNAIDS in 1994 (UN, 2000), and the UN Mission for Ebola Emergency Response in 2014 (UN, 2014). Largely unexplored synergies exist between the global control of parasitic diseases and the relief of international debt (through the IMF), poverty and hunger relief (UN Development Programme, World Food Programme, and the Food and Agriculture Organization), responsible environmental practices (UN Environment Programme), gender equality (UN Women), and child health (UN Children's Fund). The health-related focus of these agencies has been historically limited to HIV/AIDS, and tuberculosis to a lesser extent, with far less attention paid to the burden of malaria and NTDs. Furthermore, the control of parasitic infections could be explicitly identified as a requirement to achieving universal healthcare and poverty alleviation under the SDGs, and implementation supported by the Sendai Framework's all-hazards approach to strengthen healthcare systems and build community resilience against disasters (Aitsi-Selmi and Murray, 2016). A similar case could be made to establish programmes to monitor emerging zoonoses at the animal–human interface, and invest in rapidly scalable research and development platforms for diagnostics and therapeutics. Deeper collaborations between WHO efforts and those of sister UN agencies may also allow for the “boots on ground” operational and logistical capacity that the WHO was not designed to maintain nor oversee. Indeed, an added benefit of the WHO coordinating multisectoral action would be the explicit return of the WHO to its primary role as the world's executive medical scientific body, emphasizing its historic strengths in issuing technical advice, information-sharing, research and development, and advocacy for the equitable distribution of global public health goods.
Equally, however, the WHO simply cannot function to its potential without renewed support from its member states. Perhaps the greatest organizational challenge faced by the WHO in recent decades has been the dire state of its financing, reflecting decades of state neglect and divestiture. Prior to Covid-19, the WHO budget for the 2020–21 biennium was US $4.84 billion (US $2.42 billion per year) (WHO, 2019). For comparison, the US Centres for Disease Control and Prevention's operational budget for 2019 was over US $7.3 billion (US CDC, 2019). The funding required by the Global Malaria Programme to achieve targets is estimated to be in the order of US $6.6 billion, with another US $720 million required for malaria research and development efforts, amounting to a total that is over three times the annual WHO budget (WHO, 2020d). Even worse, over 80% of WHO's paltry budget is spoon-fed through earmarked voluntary streams, more than 90% of which are earmarked for specific programmes (WHO, 2019). The remaining assessed contributions from member states are subject to a zero nominal growth policy that prevents inflationary adjustment (Frenk, 2016). Future WHO financing must involve mechanisms to dramatically increase and incentivize contributions. Strategies could include returning the ratio of assessed to voluntary contributions to at least parity, designating a fixed proportion of non-earmarked voluntary funds, broadening the donor base by highlighting clear links between core WHO activities and UN SDGs, and releasing the WHO from operational responsibilities that could be more successfully met through other global health bodies. Above all, funding investments should be commensurate to the WHO's many essential functions, including the control of major infectious diseases.
6. Conclusions
Global parasitic diseases represent some of the most distressing conditions known to humanity, often consigning the world's poorest people to premature death, lifelong financial hardship, physical debilitation and disfigurement, social stigma, and mental distress. Although some progress has been made towards control and elimination of malaria and the NTDs in the last 30 years, these tenuous gains have come under imminent threat owing to worldwide interruptions to disease and vector control programmes during the Covid-19 pandemic. Averting calamitous increases in the burden of global parasitic diseases over coming decades will require the prompt resumption of disease elimination campaigns, and embedding such efforts within a broader One Health framework that explicitly addresses the social, economic, and environmental conditions that allow parasitic infections to persist. Ultimately, whether target aspirations are met will depend largely on the role of the WHO, still the world's public health agency, and the degree to which its activities can be supported by multisectoral action in health-adjacent policy areas. With renewed political will, social advocacy, and sustained financial resources, it is both possible and necessary to make decisive strides towards the elimination of global parasitic diseases over years to come.
Acknowledgments
Disclosures
The authors have no disclosures or financial conflicts of interest to declare.
References
- Abdela S.G., van Griensven J., Seife F., Enbiale W. Neglecting the effect of COVID-19 on neglected tropical diseases: the Ethiopian perspective. Trans. R. Soc. Trop. Med. Hyg. 2020;114(10):730–732. doi: 10.1093/trstmh/traa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adepoju P. COVID-19 puts health research to the test in Africa. Nat. Med. 2020;26(9):1312–1314. doi: 10.1038/s41591-020-1055-5. [DOI] [PubMed] [Google Scholar]
- Aitsi-Selmi A., Murray V. Protecting the health and well-being of populations from disasters: health and health Care in the Sendai Framework for disaster risk reduction 2015–2030. Prehosp. Disaster Med. 2016;31(1):74–78. doi: 10.1017/S1049023X15005531. [DOI] [PubMed] [Google Scholar]
- Al-Salem W., Herricks J.R., Hotez P.J. A review of visceral leishmaniasis during the conflict in South Sudan and the consequences for east African countries. Parasit. Vectors. 2016;9(1):1–11. doi: 10.1186/s13071-016-1743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer J., O'Halloran L., Al-Shehri H., Summers S., Bhattacharyya T., Kabaterine N.B., et al. Intestinal schistosomiasis and giardiasis co-infection in sub-Saharan Africa: can a one health approach improve control of each waterborne parasite simultaneously? Trop. Med. Infect. Dis. 2020;5(3):137. doi: 10.3390/tropicalmed5030137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos C.J.C., Aras R., Mota G., Reis F., Dias J.P., de Jesus R.S., et al. Clinical outcomes of thirteen patients with acute chagas disease acquired through oral transmission from two urban outbreaks in northeastern Brazil. PLoS Negl. Trop. Dis. 2010;4(6):e711. doi: 10.1371/journal.pntd.0000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford J., Farrar J., Ihekweazu C., Kang G., Koopmans M., Nkengasong J. A new twenty-first century science for effective epidemic response. Nature. 2019;575(7781):130–136. doi: 10.1038/s41586-019-1717-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett S., Glandon D., Rasanathan K. Governing multisectoral action for health in low-income and middle-income countries: unpacking the problem and rising to the challenge. BMJ Glob. Health. 2018;3(Suppl 4) doi: 10.1136/bmjgh-2018-000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S., Weiss D.J., Cameron E., Bisanzio D., Mappin B., Dalrymple U., et al. The effect of malaria control on plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526(7572):207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatin B.A., Richards F.O., Jr. Control of onchocerciasis. Adv. Parasitol. 2006;61:349–394. doi: 10.1016/S0065-308X(05)61009-3. [DOI] [PubMed] [Google Scholar]
- Borlase A., Blumberg S., Callahan E.K., Deiner M.S., Nash S.D., Porco T.C., et al. Modelling trachoma post-2020: opportunities for mitigating the impact of COVID-19 and accelerating progress towards elimination. Trans. R. Soc. Trop. Med. Hyg. 2021;115(3):213–221. doi: 10.1093/trstmh/traa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M.R., Carlin E.P., Schermerhorn J., Standley C.J. A one health approach for Guinea worm disease control: scope and opportunities. Trop. Med. Infect. Dis. 2020;5(4):159. doi: 10.3390/tropicalmed5040159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M., Taylor R., Jacobson J., Guex M., Hopkins A., Jensen J., et al. Medicine donation programmes supporting the global drive to end the burden of neglected tropical diseases. Trans. R. Soc. Trop. Med. Hyg. 2021;115(2):136–144. doi: 10.1093/trstmh/traa167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke B.D., Raman J., Frean J., Rundle J., Maartens F., Misiani E., et al. Implementing malaria control in South Africa, Eswatini and southern Mozambique during the COVID-19 pandemic. S. Afr. Med. J. 2020;110(11):1072–1076. [PubMed] [Google Scholar]
- Buranyi S. The Guardian; London, UK: 2020. The WHO V Coronavirus: Why it can't Handle the Pandemic.http://www.theguardian.com/news/2020/apr/10/world-health-organization-who-v-coronavirus-why-it-cant-handle-pandemic Retrieved August 3, 2021. [Google Scholar]
- Cano J., Basáñez M.-G., O'Hanlon S.J., Tekle A.H., Wanji S., Zouré H.G., et al. Identifying co-endemic areas for major filarial infections in sub-Saharan Africa: seeking synergies and preventing severe adverse events during mass drug administration campaigns. Parasit. Vectors. 2018;11(1):70. doi: 10.1186/s13071-018-2655-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash R., Patel V. Has COVID-19 subverted global health? Lancet. 2020;395(10238):1687–1688. doi: 10.1016/S0140-6736(20)31089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont C., Kamara K., Baring E., Palacio K., Power A., Lancaster W. The SARS-CoV-2 crisis and its impact on neglected tropical diseases: threat or opportunity? PLoS Negl. Trop. Dis. 2020;14(9) doi: 10.1371/journal.pntd.0008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho C.H., Durigan M., Leal D.A.G., Schneider A.d.B., Franco R.M.B., Singer S.M. Giardiasis as a neglected disease in Brazil: systematic review of 20 years of publications. PLoS Negl. Trop. Dis. 2017;11(10) doi: 10.1371/journal.pntd.0006005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conteh L., Engels T., Molyneux D.H. Socioeconomic aspects of neglected tropical diseases. The Lancet. 2010;375(9710):239–247. doi: 10.1016/S0140-6736(09)61422-7. [DOI] [PubMed] [Google Scholar]
- Crellen T., Walker M., Lamberton P.H.L., Kabatereine N.B., Tukahebwa E.M., Cotton J.A., et al. Reduced efficacy of Praziquantel against Schistosoma mansoni is associated with multiple rounds of mass drug administration. Clin. Infect. Dis. 2016;63(9):1151–1159. doi: 10.1093/cid/ciw506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datoo M.S., Natama M.H., Somé A., Traoré O., Rouamba T., Bellamy D., et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant matrix-M, with seasonal administration to children in Burkina Faso: a randomised controlled trial. Lancet. 2021;397(10287):1809–1818. doi: 10.1016/S0140-6736(21)00943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza D.K., Picado A., Biéler S., Nogaro S., Ndung'u J.M. Diagnosis of neglected tropical diseases during and after the COVID-19 pandemic. PLoS Negl. Trop. Dis. 2020;14(8) doi: 10.1371/journal.pntd.0008587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias J.P., Bastos C., Araújo E., Mascarenhas A.V., Martins Netto E., Grassi F., et al. Acute Chagas disease outbreak associated with oral transmission. Rev. Soc. Bras. Med. Trop. 2008;41(3):296–300. doi: 10.1590/s0037-86822008000300014. [DOI] [PubMed] [Google Scholar]
- Ehrenberg J.P., Zhou X.N., Fontes G., Rocha E.M.M., Tanner M., Utzinger J. Strategies supporting the prevention and control of neglected tropical diseases during and beyond the COVID-19 pandemic. Infect. Dis. Poverty. 2020;9(1):86. doi: 10.1186/s40249-020-00701-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg J.P., Utzinger J., Fontes G., da Rocha E.M.M., Ehrenberg N., Zhou X.-N., et al. Efforts to mitigate the economic impact of the COVID-19 pandemic: potential entry points for neglected tropical diseases. Infect. Dis. Poverty. 2021;10(1):2. doi: 10.1186/s40249-020-00790-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenk J. Finance and governance: critical challenges for the next WHO director-general. Am. J. Public Health. 2016;106(11):1906–1907. doi: 10.2105/AJPH.2016.303399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenk J., Moon S. Governance challenges in global health. N. Engl. J. Med. 2013;368(10):936–942. doi: 10.1056/NEJMra1109339. [DOI] [PubMed] [Google Scholar]
- Frieden T.R., Tappero J.W., Dowell S.F., Hien N.T., Guillaume F.D., Aceng J.R. Safer countries through global health security. Lancet. 2014;383(9919):764–766. doi: 10.1016/S0140-6736(14)60189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst T., Raso G., Acka C.A., Tschannen A.B., N'Goran E.K., Utzinger J. Dynamics of socioeconomic risk factors for neglected tropical diseases and malaria in an armed conflict. PLoS Negl. Trop. Dis. 2009;3(9):e513. doi: 10.1371/journal.pntd.0000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatas B., Saúte F., Martí-Soler H., Guinovart C., Nhamussua L., Simone W., et al. A multiphase program for malaria elimination in southern Mozambique (the Magude project): a before-after study. PLoS Med. 2020;17(8) doi: 10.1371/journal.pmed.1003227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD Collaborative Network . Institute for Health Metrics and Evaluation (IHME); Seattle, United States: 2020. Global Burden of Disease Study 1990, 2000, 2010, and 2019 Results.http://ghdx.healthdata.org/gbd-results-tool [Google Scholar]
- Global Fund . Global Fund; Geneva, Switzerland: 2021. The Impact of Covid-19 on HIV, TB, and Malaria Services and Systems for Health: A Snapshot from 502 Health Facilities across Africa and Asia.http://www.theglobalfund.org/media/10776/covid-19_2020-disruption-impact_report_en.pdf Retrieved July 26, 2021. [Google Scholar]
- Gostin L.O., Koh H.H., Kavanagh M.M., Benjamin G.C., Bradley E.H., Williams M.A., et al. Georgetown University O'Neill Institute for National and Global Health Law; Washington, DC: 2020. Letter to Congress on WHO Withdrawal from Public Health, Law and International Relations Leaders.http://oneill.law.georgetown.edu/letter-to-congress-on-who-withdrawal-from-public-health-law-and-international-relations-leaders/ Retrieved July 21, 2021. [Google Scholar]
- Gostin L.O., Koh H.H., Williams M., Hamburg M.A., Benjamin G., Foege W.H., et al. US withdrawal from WHO is unlawful and threatens global and US health and security. Lancet. 2020;396(10247):293–295. doi: 10.1016/S0140-6736(20)31527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower C.M., Vince L., Webster J.P. Should we be treating animal schistosomiasis in Africa? The need for a one health economic evaluation of schistosomiasis control in people and their livestock. Trans. R. Soc. Trop. Med. Hyg. 2017;111(6):244–247. doi: 10.1093/trstmh/trx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes J.E.T., Croll D., Harrison W.E., Utzinger J., Freeman M.C., Templeton M.R. The roles of water, sanitation and hygiene in reducing schistosomiasis: a review. Parasit. Vectors. 2015;8(1):156. doi: 10.1186/s13071-015-0766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guagliardo S.A.J., Ruiz-Tiben E., Hopkins D.R., Weiss A.J., Ouakou P.T., Zirimwabagabo H., et al. Surveillance of human Guinea worm in Chad, 2010–2018. Am. J. Trop. Med. Hyg. 2021;105(1):188–195. doi: 10.4269/ajtmh.20-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haakenstad A., Harle A.C., Tsakalos G., Micah A.E., Tao T., Anjomshoa M., et al. Tracking spending on malaria by source in 106 countries, 2000-16: an economic modelling study. Lancet Infect. Dis. 2019;19(7):703–716. doi: 10.1016/S1473-3099(19)30165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamley J.I., Blok D.J., Walker M., Milton P., Hopkins A.D., Hamill L.C., et al. What does the COVID-19 pandemic mean for the next decade of onchocerciasis control and elimination? Trans. R. Soc. Trop. Med. Hyg. 2021;115(3):269–280. doi: 10.1093/trstmh/traa193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove J., Torr S., Kindness H. Insecticide-treated cattle against tsetse (Diptera: Glossinidae): what governs success? Bull. Entomol. Res. 2003;93(3):203–217. doi: 10.1079/BER2003234. [DOI] [PubMed] [Google Scholar]
- Headey D., Heidkamp R., Osendarp S., Ruel M., Scott N., Black R., et al. Impacts of COVID-19 on childhood malnutrition and nutrition-related mortality. The Lancet. 2020;396(10250):519–521. doi: 10.1016/S0140-6736(20)31647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway J., Ranson H., Magill A., Kolaczinski J., Fornadel C., Gimnig J., et al. Averting a malaria disaster: will insecticide resistance derail malaria control? Lancet. 2016;387(10029):1785–1788. doi: 10.1016/S0140-6736(15)00417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan A.B., Jewell B.L., Sherrard-Smith E., Vesga J.F., Watson O.J., Whittaker C., et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob. Health. 2020;8(9):e1132–e1141. doi: 10.1016/S2214-109X(20)30288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth T.D., Mwinzi P., Vasconcelos A., de Vlas S.J. Evaluating the potential impact of interruptions to neglected tropical disease programmes due to COVID-19. Trans. R. Soc. Trop. Med. Hyg. 2021;115(3):201–204. doi: 10.1093/trstmh/trab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Wilkins P.P. Toxocariasis: America's Most common neglected infection of poverty and a helminthiasis of global importance? PLoS Negl. Trop. Dis. 2009;3(3) doi: 10.1371/journal.pntd.0000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Fenwick A., Molyneux D.H. Collateral benefits of preventive chemotherapy — expanding the war on neglected tropical diseases. N. Engl. J. Med. 2019;380(25):2389–2391. doi: 10.1056/NEJMp1900400. [DOI] [PubMed] [Google Scholar]
- Hotez P.J., Fenwick A., Molyneux D. The new COVID-19 poor and the neglected tropical diseases resurgence. Infect. Dis. Poverty. 2021;10(1):10. doi: 10.1186/s40249-020-00784-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houweling T.A.J., Karim-Kos H.E., Kulik M.C., Stolk W.A., Haagsma J.A., Lenk E.J., et al. Socioeconomic inequalities in neglected tropical diseases: a systematic review. PLoS Negl. Trop. Dis. 2016;10(5) doi: 10.1371/journal.pntd.0004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson J., Bush S. Neglected tropical diseases, neglected communities, and conflict: how do we leave no one behind? Trends Parasitol. 2018;34(3):175–177. doi: 10.1016/j.pt.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasozi K.I., Zirintunda G., Ssempijja F., Buyinza B., Alzahrani K.J., Matama K., et al. Epidemiology of trypanosomiasis in wildlife—implications for humans at the wildlife Interface in Africa. Front. Vet. Sci. 2021;8(565) doi: 10.3389/fvets.2021.621699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz I.T., Weintraub R., Bekker L.-G., Brandt A.M. From vaccine nationalism to vaccine equity—finding a path forward. N. Engl. J. Med. 2021;384(14):1281–1283. doi: 10.1056/NEJMp2103614. [DOI] [PubMed] [Google Scholar]
- Keenan J.D., Arzika A.M., Maliki R., Elh Adamou S., Ibrahim F., Kiemago M., et al. Cause-specific mortality of children younger than 5 years in communities receiving biannual mass azithromycin treatment in Niger: verbal autopsy results from a cluster-randomised controlled trial. Lancet Glob. Health. 2020;8(2):e288–e295. doi: 10.1016/S2214-109X(19)30540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller L., Welsche S., Patel C., Sayasone S., Ali S.M., Ame S.M., et al. Long-term outcomes of ivermectin-albendazole versus albendazole alone against soil-transmitted helminths: results from randomized controlled trials in Lao PDR and Pemba Island, Tanzania. PLoS Negl. Trop. Dis. 2021;15(6) doi: 10.1371/journal.pntd.0009561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Hope L., Ranson H., Hemingway J. Lessons from the past: managing insecticide resistance in malaria control and eradication programmes. Lancet Infect. Dis. 2008;8(6):387–389. doi: 10.1016/S1473-3099(08)70045-8. [DOI] [PubMed] [Google Scholar]
- Kelly-Hope L.A., Molyneux D.H., Bockarie M.J. Can malaria vector control accelerate the interruption of lymphatic filariasis transmission in Africa; capturing a window of opportunity? Parasit. Vectors. 2013;6(1):1–12. doi: 10.1186/1756-3305-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharas H., McArthur J.W., Ohno I. Brookings Institution Press; Washington, D.C., USA: 2019. Leave no One behind: Time for Specifics on the Sustainable Development Goals. [Google Scholar]
- King C.H., Sutherland L.J., Bertsch D. Systematic review and meta-analysis of the impact of chemical-based mollusciciding for control of Schistosoma mansoni and S. haematobium transmission. PLoS Negl. Trop. Dis. 2015;9(12) doi: 10.1371/journal.pntd.0004290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kura K., Ayabina D., Toor J., Hollingsworth T.D., Anderson R.M. Disruptions to schistosomiasis programmes due to COVID-19: an analysis of potential impact and mitigation strategies. Trans. R. Soc. Trop. Med. Hyg. 2021;115(3):236–244. doi: 10.1093/trstmh/traa202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenk E.J., Redekop W.K., Luyendijk M., Rijnsburger A.J., Severens J.L. Productivity loss related to neglected tropical diseases eligible for preventive chemotherapy: a systematic literature review. PLoS Negl. Trop. Dis. 2016;10(2) doi: 10.1371/journal.pntd.0004397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt E., Baker M.C., Molyneux D. Neglected tropical diseases and mental health: a perspective on comorbidity. Trends Parasitol. 2012;28(5):195–201. doi: 10.1016/j.pt.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Lu H., McComas K.A., Buttke D.E., Roh S., Wild M.A., Decker D.J. One health messaging about bats and rabies: how framing of risks, benefits and attributions can support public health and wildlife conservation goals. Wildl. Res. 2017;44(3):200–206. [Google Scholar]
- Lucas A.M. Malaria eradication and educational attainment: evidence from Paraguay and Sri Lanka. Am. Econ. J. Appl. Econ. 2010;2(2):46–71. doi: 10.1257/app.2.2.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler D.G., Yonzan N., Lakner C., Aguilar R.A.C., Wu H. Washington, D.C., USA; The World Bank: 2021. Updated Estimates of the Impact of COVID-19 on Global Poverty: Turning the Corner on the Pandemic in 2021?http://blogs.worldbank.org/opendata/updated-estimates-impact-covid-19-global-poverty-turning-corner-pandemic-2021 Retrieved July 28, 2021. [Google Scholar]
- Malaria Consortium . London, UK; Malaria Consorium: 2021. Malaria and COVID-19—What Can we Learn from Nigeria?http://www.malariaconsortium.org/news-centre/malaria-and-covid-19-andndash-what-can-we-learn-from-nigeria.htm Retrieved July 28, 2021. [Google Scholar]
- Malizia V., Giardina F., Vegvari C., Bajaj S., McRae-McKee K., Anderson R.M., et al. Modelling the impact of COVID-19-related control programme interruptions on progress towards the WHO 2030 target for soil-transmitted helminths. Trans. R. Soc. Trop. Med. Hyg. 2021;115(3):253–260. doi: 10.1093/trstmh/traa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manandhar M., Hawkes S., Buse K., Nosrati E., Magar V. Gender, health and the 2030 agenda for sustainable development. Bull. World Health Organ. 2018;96(9):644–653. doi: 10.2471/BLT.18.211607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux D.H., Asamoa-Bah A., Fenwick A., Savioli L., Hotez P. The history of the neglected tropical disease movement. Trans. R. Soc. Trop. Med. Hyg. 2021;115(2):169–175. doi: 10.1093/trstmh/trab015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C.J., Lopez A.D., Jamison D.T. The global burden of disease in 1990: summary results, sensitivity analysis and future directions. Bull. World Health Organ. 1994;72(3):495–509. [PMC free article] [PubMed] [Google Scholar]
- Murray C.J.L., Vos T., Lozano R., Naghavi M., Flaxman A.D., Michaud C., et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Muturi C.N., Ouma J.O., Malele I.I., Ngure R.M., Rutto J.J., Mithöfer K.M., et al. Tracking the feeding patterns of tsetse flies (Glossina genus) by analysis of Bloodmeals using mitochondrial cytochromes genes. PLoS One. 2011;6(2) doi: 10.1371/journal.pone.0017284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwinzi P.N.M., Montgomery S.P., Owaga C.O., Mwanje M., Muok E.M., Ayisi J.G., et al. Integrated community-directed intervention for schistosomiasis and soil transmitted helminths in western Kenya—a pilot study. Parasit. Vectors. 2012;5(1):182. doi: 10.1186/1756-3305-5-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndyomugyenyi R., Kabatereine N. Integrated community-directed treatment for the control of onchocerciasis, schistosomiasis and intestinal helminths infections in Uganda: advantages and disadvantages. Trop. Med. Int. Health. 2003;8(11):997–1004. doi: 10.1046/j.1360-2276.2003.01124.x. [DOI] [PubMed] [Google Scholar]
- NTD Modelling Consortium . Big Data Institute, University of Oxford; Oxford, UK: 2020. Impact of COVID-19 on NTD Programmes Progress.http://www.who.int/neglected_diseases/news/NTDs-mitigation-and-recovery-from-COVID-19/en/ Retrieved July 26, 2021. [Google Scholar]
- Pink R., Hudson A., Mouriès M.-A., Bendig M. Opportunities and challenges in Antiparasitic drug discovery. Nat. Rev. Drug Discov. 2005;4(9):727–740. doi: 10.1038/nrd1824. [DOI] [PubMed] [Google Scholar]
- Prada J.M., Stolk W.A., Davis E.L., Touloupou P., Sharma S., Muñoz J., et al. Delays in lymphatic filariasis elimination programmes due to COVID-19, and possible mitigation strategies. Trans. R. Soc. Trop. Med. Hyg. 2021;115(3):261–268. doi: 10.1093/trstmh/trab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raguenaud M.-E., Jansson A., Vanlerberghe V., Van der Auwera G., Deborggraeve S., Dujardin J.-C., et al. Epidemiology and clinical features of patients with visceral Leishmaniasis treated by an MSF Clinic in Bakool region, Somalia, 2004–2006. PLoS Negl. Trop. Dis. 2007;1(1) doi: 10.1371/journal.pntd.0000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassi A., Rassi A., Marin-Neto J.A. Chagas disease. The Lancet. 2010;375(9723):1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- Rollinson D., Knopp S., Levitz S., Stothard J.R., Tchuenté L.-A.T., Garba A., et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013;128(2):423–440. doi: 10.1016/j.actatropica.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Royal Society of Tropical Medicine and Hygiene . RSTMH; London, UK: 2021. RSTMH Responds to Cuts to UK Official Development Aid.http://rstmh.org/news-blog/news/rstmh-responds-to-cuts-to-uk-official-development-aid Retrieved August 1, 2021. [Google Scholar]
- Sands P. Boston, USA; Stat: 2021. Why aren't Diseases like HIV and Malaria, which Still Kill Millions of People a Year, Called Pandemics?http://www.statnews.com/2021/07/06/why-arent-diseases-like-hiv-and-malaria-which-still-kill-millions-of-people-a-year-called-pandemics/ Retrieved July 14, 2021. [Google Scholar]
- Selassie A.A., Hakobyan S. International Monetary Fund; Washington, D.C., USA: 2021. Six Charts Show the Challenges Faced by Sub-Saharan Africa.http://www.imf.org/en/News/Articles/2021/04/12/na041521-six-charts-show-the-challenges-faced-by-sub-saharan-africa Retrieved July 23, 2021. [Google Scholar]
- Sherrard-Smith E., Hogan A.B., Hamlet A., Watson O.J., Whittaker C., Winskill P., et al. The potential public health consequences of COVID-19 on malaria in Africa. Nat. Med. 2020;26(9):1411–1416. doi: 10.1038/s41591-020-1025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva S.J.R.d., Pena L. Collapse of the public health system and the emergence of new variants during the second wave of the COVID-19 pandemic in Brazil. One Health. 2021;13:100287. doi: 10.1016/j.onehlt.2021.100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater J., Masih N., Irfan S. Washington Post; Washington, D.C., USA: 2021. Coronavirus has crushed India's health system. Patients are on their own.http://www.washingtonpost.com/world/2021/04/27/india-coronavirus-health-care/ Retrieved July 23, 2021. [Google Scholar]
- Smith K.M., Machalaba C.C., Seifman R., Feferholtz Y., Karesh W.B. Infectious disease and economics: the case for considering multi-sectoral impacts. One Health. 2019;7:100080. doi: 10.1016/j.onehlt.2018.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A.W., Engels D., Bailey R.L., Blake I.M., Brooker S., Chen J.-X., et al. A diagnostics platform for the integrated mapping, monitoring, and surveillance of neglected tropical diseases: rationale and target product profiles. PLoS Negl. Trop. Dis. 2012;6(7) doi: 10.1371/journal.pntd.0001746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley C.J., Schermerhorn J. Reaching the "last mile”: fresh approaches needed for Guinea worm eradication. Am. J. Trop. Med. Hyg. 2021;105(1):1–2. doi: 10.4269/ajtmh.21-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley C., Boyce M.R., Klineberg A., Essix G., Katz R. Organization of oversight for integrated control of neglected tropical diseases within ministries of health. PLoS Negl. Trop. Dis. 2018;12(11) doi: 10.1371/journal.pntd.0006929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley C.J., Carlin E.P., Sorrell E.M., Barry A.M., Bile E., Diakite A.S., et al. Assessing health systems in Guinea for prevention and control of priority zoonotic diseases: a one health approach. One Health. 2019;7:100093. doi: 10.1016/j.onehlt.2019.100093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N., Amon J.J. Addressing inequity: neglected tropical diseases and human rights. Health Hum. Rights. 2018;20(1):11–25. [PMC free article] [PubMed] [Google Scholar]
- Tchuem Tchuenté L.-A., Stothard J.R., Rollinson D., Reinhard-Rupp J. Precision mapping: an innovative tool and way forward to shrink the map, better target interventions, and accelerate toward the elimination of schistosomiasis. PLoS Negl. Trop. Dis. 2018;12(8) doi: 10.1371/journal.pntd.0006563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toor J., Adams E.R., Aliee M., Amoah B., Anderson R.M., Ayabina D., et al. Predicted impact of COVID-19 on neglected tropical disease programs and the opportunity for innovation. Clin. Infect. Dis. 2020;72(8):1463–1466. doi: 10.1093/cid/ciaa933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UN . UN; Geneva: 2000. S/RES/1308: The Responsibility of the Security Council in the Maintenance of International Peace and Security: HIV/AIDS and International Peacekeeping Operations. S/RES/1308.http://unscr.com/en/resolutions/1308 [Google Scholar]
- UN . UN; Geneva: 2014. S/RES/2177: Resolution 2177 (2014) Adopted by the Security Council at its 7268th Meeting, on 18 September 2014. S/RES/2177.https://www.un.org/en/ga/search/view_doc.asp?symbol=S/RES/2177(2014 [Google Scholar]
- Ung L., Acharya N.R., Agarwal T., Alfonso E.C., Bagga B., Bispo P.J., et al. Infectious corneal ulceration: a proposal for neglected tropical disease status. Bull. World Health Organ. 2019;97(12):854–856. doi: 10.2471/BLT.19.232660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Development Programme . UNDP; Geneva, Switzerland: 2021. Continuing Vital Health Services in Guinea-Bissau during COVID-19.http://undp.medium.com/continuing-vital-health-services-in-guinea-bissau-during-covid-19-872b07aac38a Retrieved July 28, 2021. [Google Scholar]
- US CDC . US CDC; Atlanta, USA: 2019. Centers for Disease Control and Prevention Financial Year 2019 Operating Plan.http://www.cdc.gov/budget/documents/fy2019/fy-2019-cdc-operating-plan.pdf Retrieved August 20, 2020. [Google Scholar]
- van den Berg H., Kelly-Hope L.A., Lindsay S.W. Malaria and lymphatic filariasis: the case for integrated vector management. Lancet infect. Dis. 2013;13(1):89–94. doi: 10.1016/S1473-3099(12)70148-2. [DOI] [PubMed] [Google Scholar]
- Vos T., Lim S.S., Abbafati C., Abbas K.M., Abbasi M., Abbasifard M., et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster J.P., Gower C.M., Knowles S.C.L., Molyneux D.H., Fenton A. One health—an ecological and evolutionary framework for tackling neglected zoonotic diseases. Evol. Appl. 2016;9(2):313–333. doi: 10.1111/eva.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M.G. Stigma and the social burden of neglected tropical diseases. PLoS Negl. Trop. Dis. 2008;2(5):e237. doi: 10.1371/journal.pntd.0000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D.J., Bertozzi-Villa A., Rumisha S.F., Amratia P., Arambepola R., Battle K.E., et al. Indirect effects of the COVID-19 pandemic on malaria intervention coverage, morbidity, and mortality in Africa: a geospatial modelling analysis. Lancet infect. Dis. 2021;21(1):59–69. doi: 10.1016/S1473-3099(20)30700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A., Komatsuzaki T., Pizzinelli C. International Monetary Fund; Washington, D.C., USA: 2021. Short and Long-Term Healing for Latin America and the Caribbean.http://blogs.imf.org/2021/04/15/short-term-shot-and-long-term-healing-for-latin-america-and-the-caribbean/ Retrieved July 25, 2021. [Google Scholar]
- WHO . WHO; Geneva, Switzerland: 2019. World Health Organization Programme Budget.http://www.who.int/about/finances-accountability/budget/WHOPB-PRP-19.pdf?ua=1 Retrieved June 5, 2020. [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2020. Considerations for Implementing Mass Treatment, Active Case-Finding and Population-Based Surveys for Neglected Tropical Diseases in the Context of the COVID-19 Pandemic: Interim Guidance, 27 July 2020.http://apps.who.int/iris/handle/10665/333499 Retrieved June 17, 2021. [Google Scholar]
- WHO . WHO; Geneva, Switzerland: 2020. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030.http://www.who.int/publications/i/item/9789240010352 Retrieved June 30, 2021. [Google Scholar]
- WHO . WHO; Geneva, Switzerland: 2020. Tailoring Malaria Interventions in the COVID-19 Response.http://www.who.int/publications/m/item/tailoring-malaria-interventions-in-the-covid-19-response Retrieved July 21, 2021. [Google Scholar]
- WHO . WHO; Geneva, Switzerland: 2020. World Malaria Report 2020: 20 Years of Global Progress & Challenges.http://www.who.int/publications/i/item/9789240015791 Retrieved July 21, 2021. [Google Scholar]
- WHO . WHO; Geneva, Switzerland: 2021. Global Technical Strategy for Malaria 2015–2030, 2021 Update.http://www.who.int/docs/default-source/documents/global-technical-strategy-for-malaria-2016-2030.pdf?sfvrsn=c82afcc_0 Retrieved June 30, 2021. [Google Scholar]
- WHO Global Malaria Programme . World Health Organization; Geneva, Switzerland: 2020. The Potential Impact of Health Service Disruptions on the Burden of Malaria.http://www.who.int/publications-detail/the-potential-impact-of-health-service-disruptions-on-the-burden-of-malaria Retrieved July 14, 2021. [Google Scholar]
- Wielinga P.R., Schlundt J. In: Confronting Emerging Zoonoses: The One Health Paradigm. Yamada A., Kahn L.H., Kaplan B., Monath T.P., Woodall J., Conti L., editors. Springer Japan; Tokyo: 2014. One health and food safety; pp. 213–232. [Google Scholar]
- Youssef A., Harfouch R., El Zein S., Alshehabi Z., Shaaban R., Kanj S.S. Visceral and cutaneous Leishmaniases in a City in Syria and the effects of the Syrian conflict. Am. J. Trop. Med. Hyg. 2019;101(1):108–112. doi: 10.4269/ajtmh.18-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinsstag J., Schelling E., Wyss K., Mahamat M.B. Potential of cooperation between human and animal health to strengthen health systems. Lancet. 2005;366(9503):2142–2145. doi: 10.1016/S0140-6736(05)67731-8. [DOI] [PubMed] [Google Scholar]
- Zouré H.G.M., Wanji S., Noma M., Amazigo U.V., Diggle P.J., Tekle A.H., et al. The geographic distribution of Loa loa in Africa: results of large-scale implementation of the rapid assessment procedure for Loiasis (RAPLOA) PLoS Negl. Trop. Dis. 2011;5(6) doi: 10.1371/journal.pntd.0001210. [DOI] [PMC free article] [PubMed] [Google Scholar]