Abstract
Background:
Osteoarthritis (OA) is a common inflammatory disorder with no disease modifying therapies. Whether inhibition of interleukin-1β (IL-1β) can reduce the consequences of large joint OA is unclear.
Objective:
To determine whether IL-1β inhibition with canakinumab reduces incident total hip or knee replacement (THR/TKR).
Design:
Exploratory analysis of a randomized trial.
Setting:
1091 clinical sites in 39 countries.
Participants:
10,061 participants in the Canakinumab Anti-inflammatory Thrombosis Outcomes Study.
Intervention:
Random allocation to placebo or canakinumab (50mg, 150mg, or 300mg) subcutaneously once every 3 months.
Measurements:
The primary and secondary outcomes were time to first incident THR/TKR and time to first occurrence of an OA related adverse event. Data were obtained through blinded ascertainment of trial clinical and safety databases.
Results:
The median follow-up period was 3.7 years. For the individual canakinumab dose groups, compared to placebo, hazard ratios [HR] for incident THR/TKR during follow-up were 0.60 [95% CI 0.38–0.95] for the 50 mg group; 0.53 [95%CI 0.33–0.84] for the 150 mg group, and 0.60 [95%CI 0.38–0.93] for the 300 mg group. Thus, in the pooled canakinumab groups compared to the placebo group, incidence rates for THR/TKR were 0.31 and 0.54 events per 100-person years (HR 0.58, [95%CI 0.42–0.80], p=0.001). The HR for the secondary endpoint of OA related AEs was 0.73 (95% CI 0.61–0.87). Similar findings were observed in analyses restricted to those with a prior history of OA.
Limitations:
As the parent trial was not designed to examine the efficacy of IL-1β inhibitors in OA, information on structural joint outcomes was not collected.
Conclusion:
Findings from this exploratory analysis of a randomized-controlled trial support further investigation of IL-1β inhibition for treatment of large joint OA.
Introduction
Osteoarthritis (OA), a slowly progressive disease with a multifactorial pathophysiology, is a common chronic health condition and a leading cause of pain and disability among adults (1). Few effective and tolerated symptomatic therapies for OA exist other than joint replacement surgery, and no structure-modifying drugs are available (2). Due to demographic changes, the prevalence of OA is steadily increasing, posing a substantial disease burden to global healthcare systems (1). Chronic joint inflammation is common in OA, with a range of inflammatory mediators implicated in pain and structural progression (3–7). Interleukin (IL)-1β is a critical cytokine involved in the OA inflammatory process. However, whether IL-1β inhibition has clinical efficacy in OA is uncertain (8–11).
We addressed this issue in an exploratory analysis of the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) in which 10,061 men and women with elevated high sensitivity C-reactive protein (hsCRP) and a previous history of myocardial infarction were randomly allocated to placebo or to canakinumab, a human therapeutic monoclonal antibody targeting IL-1β, in doses of 50, 150, or 300 mg given subcutaneously every three months for up to 5 years. As previously described, cardiovascular event rates fell among those allocated to either the 150 mg or 300 mg doses of canakinumab with the greatest magnitude of effect accruing among those with the most robust reductions in hsCRP and IL-6 (12–14). CANTOS therefore provided a unique opportunity to explore the effects of therapy targeting IL-1β as compared to placebo on incidence rates of total hip and total knee replacement (THR/TKR) surgeries in a large middle-aged population with long-term follow-up. We also evaluated a secondary sensitivity endpoint of worsening or new OA symptoms reported during trial follow-up.
Methods
Design Overview and Study Sample
CANTOS was a multi-national, randomized, double-blind, placebo-controlled trial in which 10,061 stable post-myocardial infarction patients with hsCRP>=2 mg/L were allocated to receive canakinumab (50 mg, 100 mg, or 300 mg) or matching placebo given subcutaneously every three months. Conducted between 2011 and 2017 at 1091 clinical sites in 39 countries, CANTOS excluded patients with a history of chronic or recurrent infections, previous malignancy other than basal cell skin carcinoma, a suspected or known immunocompromised state, a history of (or at high risk for) tuberculosis or HIV-related disease, and those using systemic anti-inflammatory treatments. All trial participants provided written informed consent to participate in the trial, which was overseen by an independent data and safety monitoring board. The results of the main trial, the effects of canakinumab as compared to placebo on incident major adverse cardiovascular events, have been previously published (12).
Outcomes and Follow-up
For the purpose of this exploratory analysis, the primary endpoint of time to first occurrence of THR or TKR was evaluated over a mean follow-up time of 3.7 years (maximum 5 years). The trial clinical and the safety databases were searched for incidences of THR and TKR in the investigator reported surgery listings and in the serious adverse event (AE) narratives. Any AE reported by the investigator as serious is accompanied by a narrative with a description of actions taken (including arthroplasty); surgical procedures were considered per protocol to require AE reporting. The AE narratives were reviewed by two physicians to identify patients in whom the THR or TKR were ascribed to OA. To improve specificity, on an a priori basis events of THR or TKR ascribed in the AE reports as being due to acute fracture or that occurred in a joint with a previous arthroplasty were not counted as incident OA.
While the “hard” endpoint of surgical joint replacement was our principle analysis of interest and had robust reporting, we also sought consistency of effects by evaluating a secondary sensitivity endpoint of time to a first or recurrent OA event reported as an AE during the trial. OA in the medical history and AE reports were classified using the Medical Dictionary for Regulatory Activities (MedDRA) System Organ Class “Musculoskeletal and connective tissue disorders” which contains the high-level term “osteoarthropathies” (OAP), encompassing both peripheral and spinal OA. MedDRA terms used by the investigators to describe the nature of the AEs are listed in Supplementary Table 1.
Statistical Analyses
Cox proportional hazards models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the primary analysis of time to first incident THR or TKR for each canakinumab dose compared to placebo and for all canakinumab doses combined. All analyses were performed on an intention-to-treat basis. Individual participant follow-up time was censored at the time of death, loss to follow-up, or withdrawal of consent; participants who had THR or TKR due to acute fracture or in a joint with a previous arthroplasty were not censored as other joints were still at risk. Similar analyses were performed for the secondary endpoint of time to new diagnosis of OA or worsening of existing OA symptoms. In addition to the primary analysis inclusive of the full trial cohort, additional analyses were performed excluding participants with a history of gout, gouty arthritis, or rheumatoid arthritis at baseline, and among the subgroup with a prior history of peripheral OA. Analyses were conducted using SAS Version 9.4 © SAS Institute Inc, Cary, NC, USA.
Role of the funding source
The investigator-initiated CANTOS trial was financially supported by Novartis Pharmaceuticals who were responsible for site management and data collection. All authors were involved in the design, execution, and analysis of the current manuscript. The first and corresponding authors had full access to all study data and were responsible for the decision to submit for publication.
Results
Of 10,061 CANTOS participants, 3344, 2170, 2284, and 2263 were randomly allocated to placebo or to canakinumab at doses of 50mg, 150mg, and 300mg, respectively (approximate allocation ratio 1.5:1:1:1). As previously reported and as shown in Supplemental Table 2, baseline clinical characteristics were equally distributed across randomized study groups (12). Regarding visit adherence after randomization among participants who remained alive and had not reached the end of the trial, 99.7% had a 12-month visit, 99.5% had a 24-month visit, 88.0% had a 36-month visit, 82.5% had a 48-month visit, and 60.2% had a 60-month visit.
Among CANTOS participants, 1569 (15.6%) had a reported medical history of OA at baseline. Of these, 1369 were reported as peripheral OA while the remainder had a medical history consistent with various forms of spinal OA. As anticipated, baseline clinical characteristics differed between those with or without medical history of OA (Table 1) in that patients with prevalent OA were older, more likely to be female, and had higher body mass indices and waist circumference. Baseline hsCRP levels were comparable between patients with or without OA at baseline.
Table 1.
Baseline clinical characteristics of the CANTOS population according to randomized treatment assignment
| Placebo | Canakinumab | ||||
|---|---|---|---|---|---|
| 50 mg | 150 mg | 300 mg | All doses | ||
| Participants, (N) | 3344 | 2170 | 2284 | 2263 | 6717 |
| Age, years, mean (SD) | 61.1±10.0 | 61.1±10.1 | 61.2±10.0 | 61.1±10.1 | 61.1±10.1 |
| Female sex, no. (%) | 865 (25.9) | 541 (24.9) | 575 (25.2) | 606 (26.8) | 1722 (25.6) |
| Body-mass index, kg/m2, median (IQR) | 29.7 (26.6–33.8) | 29.9 (26.6–33.9) | 29.8 (26.5–33.7) | 29.8 (26.5–33.8) | 29.9 (26.6–33.8) |
| Current smoking, no. (%) | 765 (22.9) | 531 (24.5) | 534 (23.4) | 536 (23.7) | 1601 (23.8) |
| Hypertension, no. (%) | 2644 (79.1) | 1751 (80.7) | 1814 (79.4) | 1799 (79.5) | 5364 (79.9) |
| Diabetes, no. (%) | 1333 (39.9) | 854 (39.4) | 954 (41.8) | 888 (39.2) | 2696 (40.1) |
| Osteoarthritis, peripheral, no. (%) | 434 (13.0%) | 261 (12.0%) | 331 (14.5%) | 343 (15.2%) | 935 (13.9%) |
| hs-CRP, mg/L, median (IQR) | 4.10 (2.75–6.85) | 4.25 (2.80–7.15) | 4.25 (2.85–7.05) | 4.15 (2.85–7.15) | 4.20 (2.80–7.10) |
In the full trial population, incidence rates for any THR/TKR over the median 3.7 years of follow-up were significantly lower among those allocated to canakinumab as compared to placebo. For the individual dose groups, compared to placebo, HRs for incident THR/TKR were 0.60 [95% CI 0.38–0.95] for the 50 mg group; 0.53 [95%CI 0.33–0.84] for the 150 mg group, and 0.60 [95%CI 0.38–0.93] for the 300 mg group. Thus, in the pooled canakinumab groups compared to the placebo group, incidence rates for THR/TKR were 0.31 and 0.54 events per 100-person years (HR=0.58, [95%CI 0.42–0.80], p=0.001) (Table 2 and Figure 1). We observed similar overall effects of canakinumab on arthroplasty outcomes in analyses stratified by gender; in comparisons of event rates in the placebo group to event rates in the combined canakinumab groups, the hazard ratios for THR/TKR were 0.54 (95%CI 0.36–0.81) among men (92 incident THR/TKR events) and 0.66 (95%CI 0.38–1.12) among women (55 incident THR/TKR events).
Table 2:
Incidence rates (per 100 person years) and hazard ratios for the primary endpoint of THR/TKR according to randomized treatment allocation for the full trial cohort; for those without a prior history of gout, gouty arthritis, or rheumatoid arthritis; and for those with a prior history of OA.
| Placebo | Canakinumab | ||||
|---|---|---|---|---|---|
| 50 mg | 150 mg | 300 mg | All doses | ||
| THR/TKR event rate per 100 person-years | 0.51 | 0.32 | 0.30 | 0.30 | 0.31 |
| Hazard Ratio (95% CI) | 1.00 | 0.64 (0.40;1.03) | 0.59 (0.36;0.94) | 0.59 (0.37;0.95) | 0.61 (0.43;0.85) |
| Participants with rheumatoid arthritis at baseline excluded | 3296 | 2139 | 2264 | 2240 | 6643 |
| THR/TKR event (n) | 67 | 25 | 25 | 28 | 78 |
| THR/TKR event rate per 100 person-years | 0.54 | 0.32 | 0.29 | 0.33 | 0.31 |
| Hazard Ratio (95% CI) | 1.00 | 0.59 (0.37–0.94) | 0.53 (0.34–0.84) | 0.60 (0.39–0.94) | 0.58 (0.41–0.80) |
| Participants with gout, gouty arthritis or rheumatoid arthritis at baseline excluded | 3049 | 1987 | 2089 | 2061 | 6137 |
| THR/TKR events (n) | 59 | 23 | 24 | 24 | 71 |
| THR/TKR event rate per 100 person-years | 0.52 | 0.31 | 0.30 | 0.31 | 0.31 |
| Hazard Ratio (95% CI) | 1.00 | 0.61 (0.39–1.00) | 0.58 (0.36–0.94) | 0.59 (0.37–0.95) | 0.60 (0.42–0.84) |
| Patients with a history of OA at baseline | 434 | 261 | 331 | 343 | 935 |
| All THR/TKR events (n) | 47 | 18 | 18 | 22 | 58 |
| THR/TKR events rate per 100 person-years | 2.93 | 1.94 | 1.41 | 1.70 | 1.65 |
| Hazard Ratio (95% CI) | 1.00 | 0.66 (0.38; 1.14) | 0.48 (0.28;0.83) | 0.58 (0.35;0.97) | 0.57 (0.39;0.83) |
CI, confidence interval; THR/TKR, total hip or knee replacement
Figure 1:
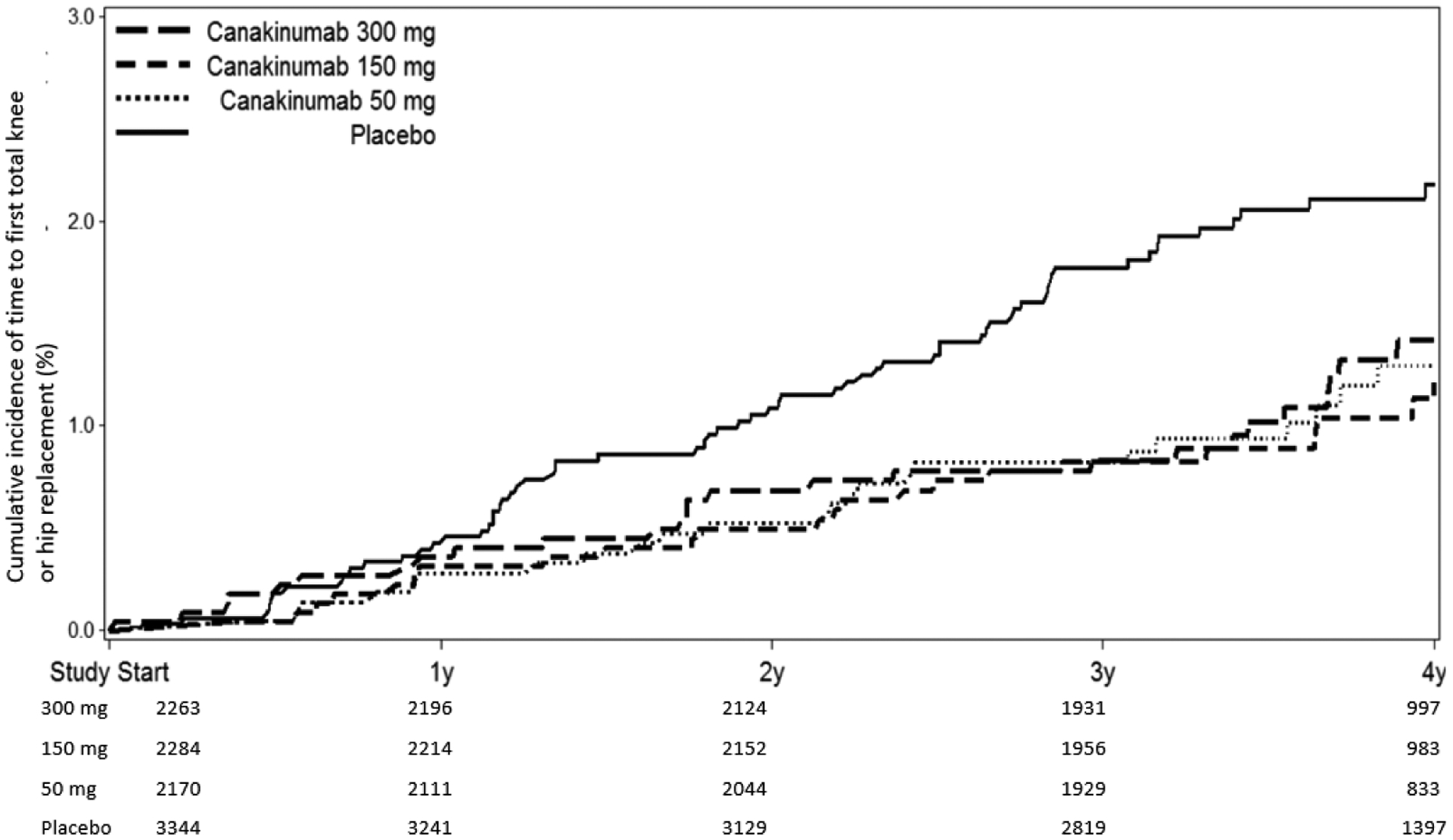
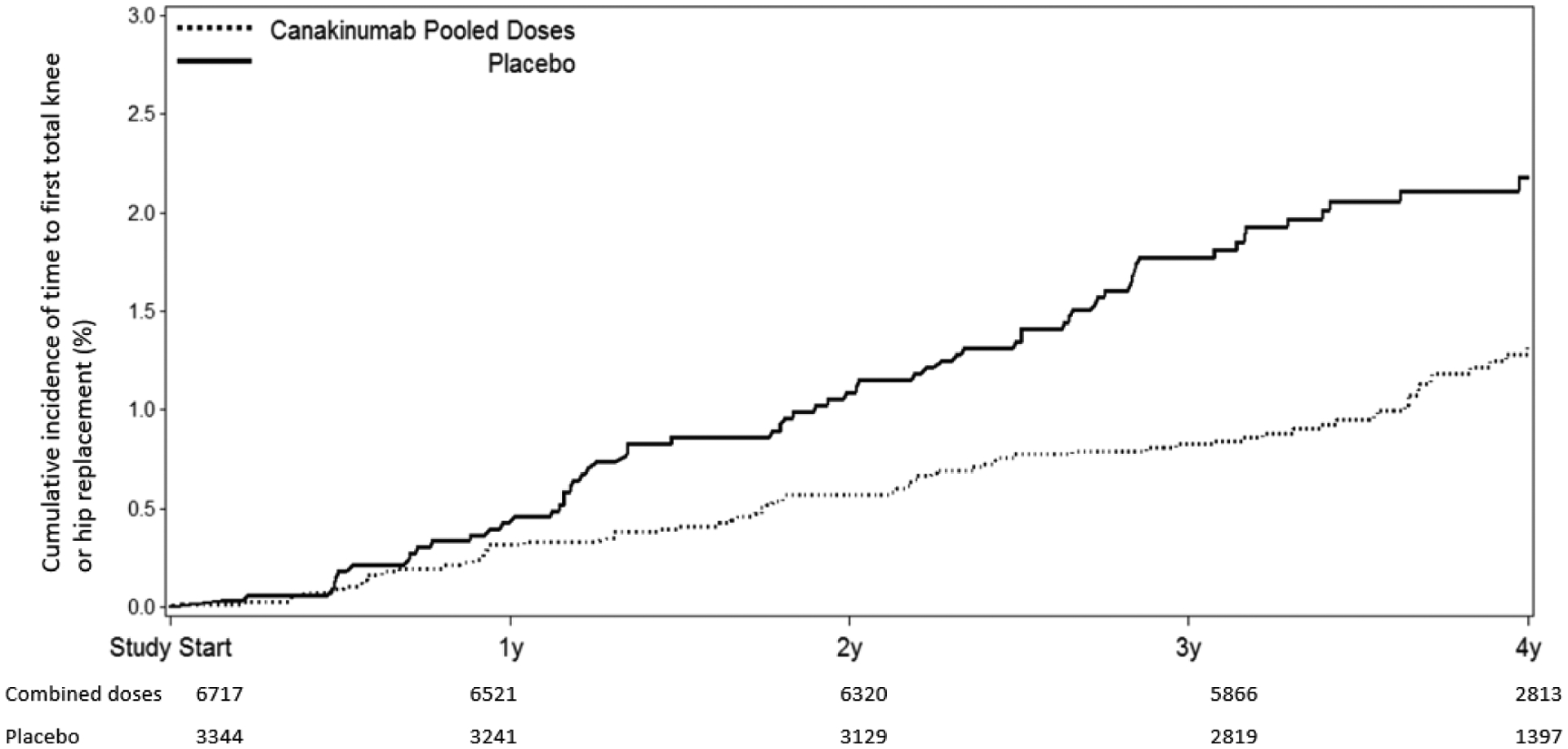
Cumulative incidence of THR or TKR in participants treated with placebo compared to canakinumab at 50 mg, 150 mg, or 300 mg administered once every three months (A, top); and placebo compared to all participants treated with canakinumab regardless of dose (B, bottom). Data are shown on an intention-to-treat basis for the full trial population.
Table 2 also presents incident rate data for THR/TKR according to randomized treatment assignment in subgroup analyses that eliminated trial participants with a history of gout, gouty arthritis, or rheumatoid arthritis at trial entry. In all of these subgroup analyses, statistically significant findings consistent with the overall effect of canakinumab as compared to placebo on incident THR or TKR were observed. For example, among CANTOS participants with no prior history of any of these inflammatory conditions, incidence rates for THR/TKR were 0.31 and 0.52 events per 100-person years in the pooled canakinumab and placebo groups, respectively (HR=0.60, 95%CI 0.42–0.84)(Supplemental Figure 1). Importantly, similar benefits of canakinumab as compared to placebo was observed in the subgroup with a history of peripheral OA at baseline (HR 0.57, 95%CI 0.39–0.83)(Table 2, Figure 2).
Figure 2:
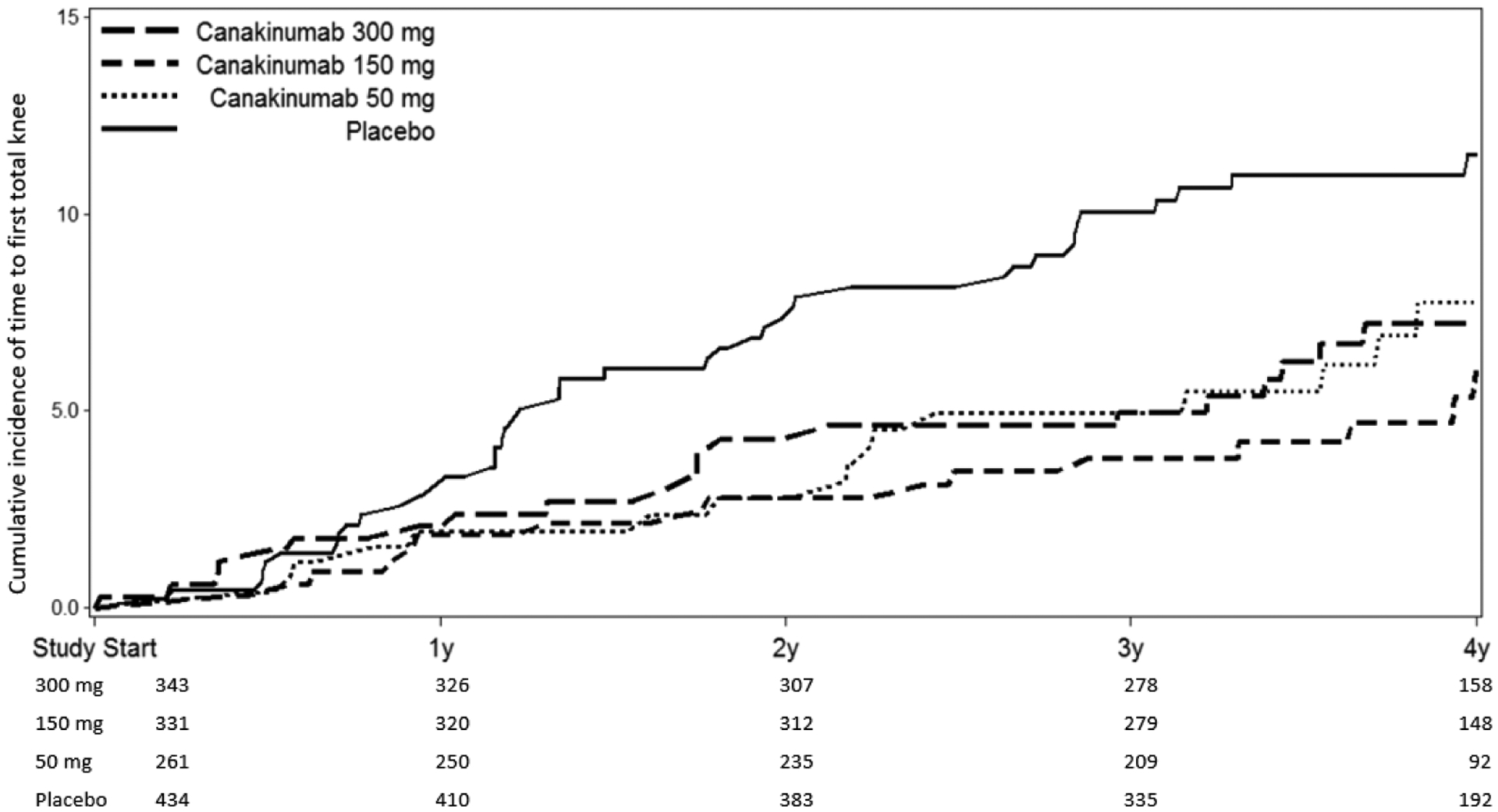
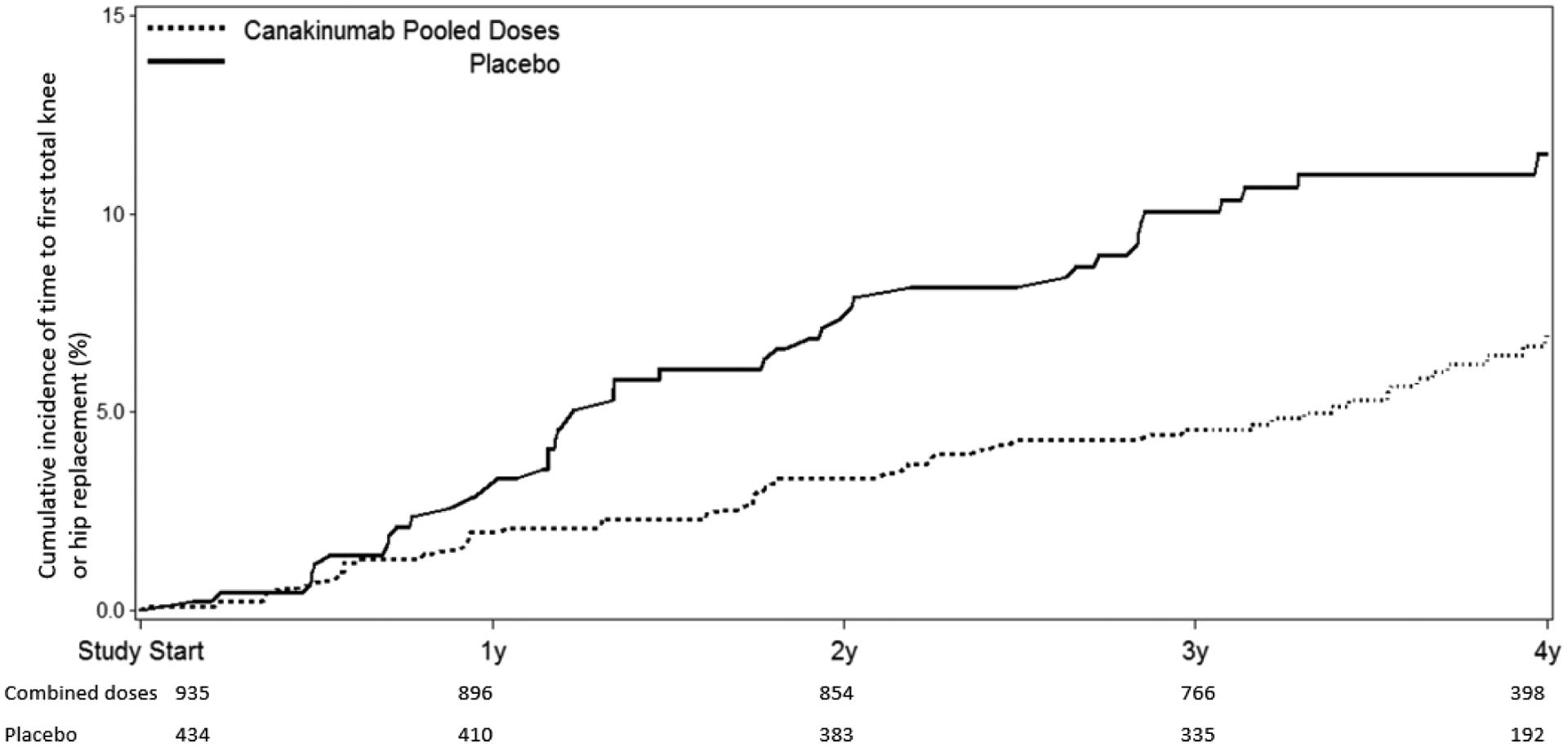
Cumulative incidence of THR or TKR in participants treated with placebo compared to canakinumab at 50 mg, 150 mg, or 300 mg administered once every three months (A, top); and placebo compared to all participants treated with canakinumab regardless of dose (B, bottom). Data are shown on an intention-to-treat basis for the subgroup of participants with a baseline history of peripheral osteoarthritis.
Table 3 presents data for the secondary supportive sensitivity endpoint of worsening OA symptoms or new OA adverse events. For the full trial cohort, incidence rates for the secondary endpoint were 1.17 and 1.63 events per 100-person years in the pooled canakinumab and placebo groups, respectively (HR=0.73, 95%CI 0.61–0.87). Comparable effects for the secondary endpoint were also present in the subgroup with a history of peripheral OA at baseline (HR 0.66, 95%CI 0.51–0.87)(Table 3, Supplemental Figure 2).
Table 3:
Incidence rates (per 100 person years) and hazard ratios for the secondary sensitivity endpoint of incident OA adverse events according to randomized treatment allocation for the full trial cohort and for participants with a prior history of OA.
| Placebo | Canakinumab | ||||
|---|---|---|---|---|---|
| 50 mg | 150 mg | 300 mg | All doses | ||
| Full Trial Cohort | 3344 | 2170 | 2284 | 2263 | 6717 |
| OA adverse events (n) | 203 | 95 | 95 | 109 | 299 |
| OA adverse event rate per 100 person-yrs | 1.63 | 1.18 | 1.08 | 1.26 | 1.17 |
| Hazard Ratio (95% CI) | 1.00 | 0.72 [0.56,0.92] | 0.68 [0.53,0.86] | 0.79 [0.62,0.99] | 0.73 [0.61,0.87] |
| Patients with a history of OA at baseline | 434 | 261 | 331 | 343 | 935 |
| OA adverse events (n) | 90 | 43 | 42 | 49 | 134 |
| OA adverse event rate per 100 person-yrs | 6.02 | 4.84 | 3.35 | 3.85 | 3.92 |
| Hazard Ratio (95% CI) | 1.00 | 0.80 [0.56,1.15] | 0.57 [0.40, 0.82] | 0.65 [0.46,0.93] | 0.66 [0.51,0.87] |
CI, confidence interval; pOA, peripheral osteoarthritis; AE, adverse events
Discussion
These exploratory data derived from a randomized, double-blind, placebo-controlled trial suggest that inhibition of IL-1β with canakinumab may significantly reduce rates of total hip and knee replacement as well as OA related symptoms during a median follow-up period of 3.7 years. These data thus provide support for the hypothesis that inhibition of IL-1β could represent a novel pathway for future therapies targeting OA.
Various cytokines contribute to OA pathogenesis through mechanisms including downregulation of anabolic and upregulation of catabolic and inflammatory pathways (15). IL-1β is produced by chondrocytes, mononuclear cells, osteoblasts and synovial cells; it stimulates chondrocytes to release proteolytic enzymes that drive cartilage destruction (16,17). Moreover, IL-1β up-regulates pro-nociceptive mediators (e.g., the neurotrophin nerve growth factor (NGF)) resulting in increased pain (18). IL-1β has been identified as one prominent factor that induces catabolic changes in cartilage and bone homeostasis by direct induction of degradative enzymes such as metalloproteinases and collagenases in chondrocytes and differentiation and activation of osteoclasts, respectively. Further, IL-1β induced catabolic reprogramming in bone and cartilage is at least in part facilitated by epigenetic regulation involving bromodomain proteins and micro RNAs (19–21).
We observed similar and statistically significant 40 to 50 percent reductions in the hazard for incident arthroplasty at all three active canakinumab doses. This absence of apparent dose-response according to randomization group was similar to what was seen for some other outcomes in CANTOS such as gout and anemia (22,23) but differed from the clear dose-response effect seen for incident lung cancer and cancer death (24) and the partial dose-response effect observed for atherothrombotic events (12). In this regard, our arthroplasty findings are consistent with other work in OA which suggest that agents which may slow progression of disease also have no clear dose response effects. Within CANTOS, a small but statistically significant increase in infection was reported (12). Thus, at least for OA, the observation here that the low and middle doses of canakinumab were of similar efficacy as the highest dose provides optimism regarding the potential for future work in this arena to find an acceptable risk to benefit ratio for long-term intervention.
Limitations of our analysis merit consideration. As CANTOS was not designed to examine the effectiveness of canakinumab as a treatment for OA, information on OA such as pain control, structural outcomes, functional status, biomarkers of cartilage degradation, and radiographic progression were not systematically collected. We used outcome data collected as adverse events, rather than employing specific OA trial criteria with formal disease definition and assessment of therapeutic benefits in a target joint. However, the double-blind nature of our trial ensures a lack of bias in this regard. In addition, we do not have assessment of joint structural outcomes other than the hard endpoint THR/TKR itself. Yet, despite these limitations, the magnitude and consistency of effects observed in this randomized trial are striking, particularly for a common disease with no available structure modifying therapies. As with any secondary analysis of a major trial, replication of these findings is needed.
Previous clinical trials of IL-1 inhibition have been disappointing in terms of pain reduction (9–11). Unlike the current trial, these studies included only patients with clinical and radiographic OA, and all used pain primary endpoints. Determining benefits above placebo in OA pain trials has been very difficult for the OA field, with modern recommendations highlighting ways of improving treatment differentiation. The previous studies were powered for a quite moderate analgesic effect size, with approximately 70 to 90 participants per study arm. It is thus possible that they may have missed smaller benefits on pain. All used different agents with different delivery. Chevalier et al used intra-articular injection of recombinant human IL-1 receptor antagonist in a short duration randomized trial with 4 week primary endpoint using a common OA functional outcome measure, the total WOMAC index (summing pain stiffness and function domains) (9). There was no significant difference between two doses of drug and placebo in terms of the primary endpoint. Another trial used repeated dosing of subcutaneous fully humanized immunoglobulin binding IL-1 receptor type 1 (AMG 108) and again had a short-term endpoint, week 6 WOMAC pain (10). This study showed a non-significant difference in pain between groups, though there was numerically greater improvement in the active arm. This systemic delivery trial did demonstrate clear differentiation over placebo in suppression of CRP. The most recent trial with repeated dosing subcutaneous lutikizumab (an anti-IL-1 α/β dual variable domain immunoglobulin) had a 16 week primary WOMAC pain endpoint, with one dose achieving statistical improvement at that time point (11). The lutikizumab trial also included a co-primary MRI synovitis endpoint in the target knee that did not differentiate between placebo and active treatment at 26 weeks. Of note, the inclusion criteria in these studies were typical for OA trials in excluding significant comorbidities, and likely excluded some of the people with cardiovascular co-morbidities that were included in CANTOS.
The current study is unique in terms of its size, patient inclusion, and long-term outcomes. We recognize that joint replacement, our main outcome, is an unusual outcome for an OA trial, though the United States Food and Drug Administration has recently welcomed discussions on its use as an endpoint. Joint replacement represents a complex outcome with potential confounding variables (including socioeconomic factors, comorbidities and patient preference), though symptoms and radiographic severity are the critical drivers of orthopedic decision-making. The randomized nature of CANTOS makes it likely that potential confounding variables are equally distributed between study groups. It is probable that any study with this endpoint would need to enroll large patient numbers and have long duration in order to detect any treatment differences, an advantage that CANTOS provided.
Given the exploratory results presented here from a large-scale, placebo-controlled treatment trial using the endpoint of surgical joint replacement, we believe further investigation of IL-1β inhibition, especially in OA patient populations with chronic systemic inflammation, is warranted. This is particularly relevant as large joint OA is an increasingly common disorder with few effective and tolerated therapies other than joint replacement surgery, and for which no precision-medicine structure-modifying drugs are currently available.
Supplementary Material
Supplemental Figure 1. Cumulative incidence of THR or TKR in participants treated with placebo compared to canakinumab at 50 mg, 150 mg, or 300 mg administered once every three months (A, top); and placebo compared to all participants treated with canakinumab regardless of dose (B, bottom). Data are shown for the subgroup of trial participants without a prior history of gout, gouty arthritis, or rheumatoid arthritis.
Supplemental Figure 2: Cumulative incidence of the secondary supportive endpoint of OA adverse events during follow-up in participants treated with placebo compared to canakinumab at 50 mg, 150 mg, or 300 mg administered once every three months. Data are shown on an intention-to-treat basis for the subgroup of participants with a baseline history of peripheral osteoarthritis.
Funding Source:
Novartis Pharmaceuticals; ClinicalTrials.gov number NCT01327846
Grant Support:
The CANTOS trial was supported by Novartis, Inc.
Disclosures:
CANTOS was funded by Novartis Pharma AG. MS, JP, CS, TT, KD, RR are employees of, and hold stock in, Novartis Pharma AG. MS was Professor at University of Munich (LMU) until 06/2019, has done speakers bureaus or consultancies for Amgen, GSK, Harvest Technologies, Lilly, MSD, Novartis, Olympus Biotech, P&G, Regeneron and Roche, and is co-owner of LivImplant GmbH and Serenitas GmbH. LM is a former employee of, and holds stock in, Novartis Pharma AG and consulted on this project. PGC is supported in part by the National Institute for Health Research (NIHR) infrastructure at Leeds. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. PGC has done speakers bureaus or consultancies for AbbVie, BMS, Flexion Therapeutics, EMD Serono, Galapagos, GlaxoSmithKline, Novartis, Pfizer, Samumed and Stryker. DHS receives royalties for chapters in UpToDate on symptomatic treatments for osteoarthritis; he has no financial relationship with Novartis. PMR serves as the Principle Investigator of CANTOS and received an investigator-initiated research grant to the Brigham and Women’s Hospital to assist in the conduct of the trial. PMR has also served as a consultant to Novartis, Inflazome, Corvidia, Agepha, Flame, and CiviBiopharm, and is listed as a co-inventor on patents held by the Brigham and Women’s Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease and diabetes that have been licensed by Siemens, Inc.
Footnotes
Data Sharing Statement:
The CANTOS protocol is available upon request.
References
- 1.OARSI. Osteoarthritis Research Society International. Osteoarthritis: A Serious Disease, a white paper submitted to the U.S. Food and Drug Administration 2016. https://www.oarsi.org. Accessed 16 Dec 2019.
- 2.Lohmander LS, Roos EM. Disease modification in OA - will we ever get there? Nat Rev Rheumatol 2019;15:133–5. [DOI] [PubMed] [Google Scholar]
- 3.Wood MJ, Leckenby A, Reynolds G, et al. Macrophage proliferation distinguishes 2 subgroups of knee osteoarthritis patients. JCI Insight 2019;4:pii: 125325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martel-Pelletier J, Barr AJ, Cicuttini FM, et al. Osteoarthritis. Nat Rev Dis Primers 2016;2:16072. [DOI] [PubMed] [Google Scholar]
- 5.Robinson WH, Lepus CM, Wang Q, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2016;12:580–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone 2012;51:249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berenbaum F Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage 2013;21:16–21. [DOI] [PubMed] [Google Scholar]
- 8.Chevalier X, Eymard F. Anti-IL-1 for the treatment of OA: dead or alive? Nat Rev Rheumatol 2019;15:191–2. [DOI] [PubMed] [Google Scholar]
- 9.Chevalier X, Goupille P, Beaulieu AD, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 2009;61:344–52. [DOI] [PubMed] [Google Scholar]
- 10.Cohen SB, Proudman S, Kivitz AJ, et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res Ther 2011;13:R125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleischmann RM, Bliddal H, Blanco FJ, et al. A phase II trial of lutikizumab, an anti-interleukin-1α/β dual variable domain immunoglobulin, in knee osteoarthritis patients with synovitis. Arthritis Rheumatol 2019;71:1056–69. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–31. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, MacFadyen JG, Everett BM, et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet 2018;391:319–28. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Libby P, MacFadyen JG, et al. Modulation of the interleukin-6 signaling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J 2018;39:3499–507. [DOI] [PubMed] [Google Scholar]
- 15.Rahmati M, Mobasheri A, Mozafari M. Inflammatory mediators in osteoarthritis: A critical review of the state-of-the-art, current prospects, and future challenges. Bone 2016;85:81–90. [DOI] [PubMed] [Google Scholar]
- 16.Malda J, Boere J, van de Lest CH, van Weeren P, Wauben MH. Extracellular vesicles - new tool for joint repair and regeneration. Nat Rev Rheumatol 2016;12:243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Hunter D, Xu J, Ding C. Metabolic triggered inflammation in osteoarthritis. Osteoarthritis and Cartilage 2015;23:22–30. [DOI] [PubMed] [Google Scholar]
- 18.Eitner L, Ozgul OS, Enax-Krumova EK, Vollert J, Maier C, Hoffken O. Conditioned pain modulation using painful cutaneous electrical stimulation or simply habituation? Eur J Pain 2018;22:1281–90. [DOI] [PubMed] [Google Scholar]
- 19.Park-Min K, Lim E, Lee M et al. Inhibition of osteoclastogenesis and inflammatory bone resorption by targeting BET proteins and epigenetic regulation. Nate Commun 2014;5:5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen J, Abu-Amer Y, O’Keefe RJ, McAlinden A. Inflammation and epigenetic regulation in osteoarthritis. Connective Tissue Research 2017;58:49–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JH, Jin HM, Kim K, Song I, Youn BU, Matsuo K, Kim N. The Mechanism of Osteoclast Differentiation Induced by IL-1. J Immunology 2009;183:1862–1870. [DOI] [PubMed] [Google Scholar]
- 22.Solomon DH, Glynn RJ, MacFadyen JG, Libby P, Thuren T, Everett BM, Ridker PM. Relationship of interleukin-1β blockade with incident gout and serum uric acid levels: Exploratory analysis of a randomized controlled trial. Ann Intern Med 2018;169:535–42. [DOI] [PubMed] [Google Scholar]
- 23.Vallurupalli M, MacFadyen JG, Glynn RJ, Thuren T, Libby P, Berliner N, Ridker PM. Effects of interleukin-1β inhibition on incident anemia: exploratory analyses from a randomized trial. Ann Intern Med 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10105):1833–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Cumulative incidence of THR or TKR in participants treated with placebo compared to canakinumab at 50 mg, 150 mg, or 300 mg administered once every three months (A, top); and placebo compared to all participants treated with canakinumab regardless of dose (B, bottom). Data are shown for the subgroup of trial participants without a prior history of gout, gouty arthritis, or rheumatoid arthritis.
Supplemental Figure 2: Cumulative incidence of the secondary supportive endpoint of OA adverse events during follow-up in participants treated with placebo compared to canakinumab at 50 mg, 150 mg, or 300 mg administered once every three months. Data are shown on an intention-to-treat basis for the subgroup of participants with a baseline history of peripheral osteoarthritis.


