Abstract
Cancer-associated fibroblasts (CAFs) are continuously activated and are one of the most important cellular components of the tumor matrix. The role of CAFs in the tumor microenvironment has been widely recognized. However, the underlying molecular mechanism by which CAFs promote tumor characteristics in breast cancer (BC) remains poorly understood. The aim of the present study was to investigate the potential mechanisms and the possible pathways of collagen triple helix repeat containing-1 (CTHRC1) in the epithelial-mesenchymal transition (EMT) of BC cells. The level of CTHRC1 in BC tissues was found to be higher than that in adjacent-normal tissues. CAFs isolated from BC tissues secreted significantly greater amounts of CTHRC1 than normal fibroblasts. Furthermore, CAFs promoted the migration, invasiveness and EMT of BC cells by secreting CTHRC1, which activates the Wnt/β-catenin signaling pathway. However, the use of neutralizing antibodies towards CTHRC1, or the specific inhibitor Dickkopf-1, to inhibit the Wnt/β catenin pathway significantly alleviated the CAF-induced malignant phenotypes of BC cells. Collectively, the data indicate that CAFs in the tumor microenvironment promote BC cell malignant behaviors via the CTHRC1/Wnt/β-catenin signaling pathway. Furthermore, weakening CAF-BC cell communication by suppressing CTHRC1 expression may be a novel strategy for treating BC.
Keywords: cancer-associated fibroblasts, collagen triple helix repeat containing-1, epithelial-mesenchymal transition, breast cancer, Wnt/β-catenin signaling pathway
Introduction
Breast cancer (BC) is one of the malignant tumors with the highest morbidity and mortality rates in women worldwide, of which metastasis is the primary cause of poor patient prognosis (1). The tumor microenvironment has been found to play a crucial role in the process of tumor metastasis (2), and is primarily composed of immune cells, vascular cells, fibroblasts, myofibroblasts, mesenchymal stem cells, fat cells and the extracellular matrix (ECM) (3,4). Among these cell types, fibroblasts can be activated to become cancer-associated fibroblasts (CAFs), which are the most abundant cell type in the BC matrix, accounting for 80% of BC tissue (5,6). Via both autocrine and paracrine pathways, these activated CAFs acquire an invasive phenotype and promote tumor cell proliferation, invasiveness and metastasis, angiogenesis, ECM remodeling, inflammatory responses and tumor microenvironment reprogramming, thus promoting the evolution of BC (7). Clinical and epidemiological studies have demonstrated a strong association between CAFs and poor prognosis in several types of cancer, including BC (5,8,9). However, the mechanism by which CAFs promote the development of BC remains undefined.
Collagen triple helix repeat containing-1 (CTHRC1), originally identified as an upregulated gene in injured rat arteries, is involved in vascular remodeling, osteoblast formation and wound repair (10). In injured and diseased arteries, CTHRC1 increases cellular motility to repair the injury by restricting the deposition of collagen matrix and promoting cellular migration (11). In previous years, studies have revealed that the upregulation of CTHRC1 is associated with a variety of solid tumors, to include the invasion and metastasis of tumor tissues such as those of BC (12–14). Immunohistochemical results have indicated significantly higher expression of CTHRC1 in BC than in normal tissues or precursor lesions (15). The results of cDNA microarray and in situ hybridization also suggested high CTHRC1 expression in BC. High CTHRC1 expression was also reported to be of prognostic value in multiple cancer types, including pancreatic (16), gastric (17), non-small cell lung (18) and gastrointestinal stromal cancer (19). Furthermore, CTHRC1 overexpression was recently reported to promote human colorectal cancer cell proliferation and invasiveness by activating the Wnt/planar cell polarity (PCP) signaling pathway (20). As an important component of the tumor matrix, CAFs are a key component for regulating tumor progression; active communication between CAFs and cancer cells is achieved through growth factors or inflammatory cytokines that promote tumorigenesis and progression (21). Although several studies have revealed the involvement of CAFs in solid tumor progression, it remains unclear whether CTHRC1 is also involved in this process, and whether it acts as an intermediary between BC and CAFs.
Wnt, a member of the family of secreted signaling molecules, plays a key role in the regulation of stem cell proliferation and differentiation, embryogenesis and organogenesis, cell carcinogenesis, tumor invasion and other pathological processes (22). At the cellular level, such versatility of Wnt signaling is achieved by activating various intracellular signaling pathways. The canonical Wnt pathway is the best- characterized pathway, which stabilizes β-catenin proteins. An increase in β-catenin level results in its translocation to the nucleus, where it interacts with T cell factor/lymphoid enhancer factor family of transcription factors to trigger the expression of target genes (23). Abnormal activation of the Wnt/β-catenin signaling pathway, associated with epithelial-mesenchymal transformation (EMT), promotes the metastasis and invasion capacities of cancer cells by decreasing the expression of cell-cell contact protein E-cadherin in vitro and in vivo (24,25). The role of CTHRC1 in the biological behaviors of BC (by Wnt/β-catenin signaling pathway activity), especially the invasion and EMT mediated by the tumor microenvironment, remains poorly understood. Therefore, the aim of the present study was to determine how CAFs regulate the migration, invasiveness and EMT of BC cells, and how CAFs are associated with the activation of CTHRC1/Wnt/β-catenin signaling in BC progression.
In the current study, the expression of CTHRC1 was first verified in BC cancer tissues and serum, and their secretion sources were investigated. Then, changes in BC cell malignant behaviors were evaluated by altering the expression of CTHRC1 in the microenvironment. Finally, the Wnt/β-catenin signaling pathway inhibitor Dickkopf-1 (DKK1) was introduced to explore the mechanisms of action of CTHRC1.
Materials and methods
Tissues samples
Preoperative blood samples from 43 patients with BC (provided by Cangzhou Central Hospital between August 2016 and April 2018), as well as tumor and adjacent tissues removed during surgery, were analyzed. The tumor tissue was removed from the deepest infiltration of the tumor, and the adjacent tissue was within 3 cm of the tumor. Each patient was sampled and numbered. Concurrently, blood samples from 27 healthy subjects, collected at the same hospital between July 2017 and February 2018, were selected for comparative analysis. The inclusion criteria were as follows: i) Histopathological sections identified as BC; and ii) complete pathological information. The exclusion criteria were as follows: i) Patients aged <18 years; ii) patients with a history of tumor disease; and iii) patients who had received chemotherapy, radiation therapy or immunotherapy. All tissue samples were identified by a clinical pathologist. Written informed consent was obtained from each study participant, and the research program was reviewed and approved by the Human Subject Research Ethics Committee of Cangzhou Central Hospital, and implemented under the guidelines formulated by the Committee. Detailed clinical characteristics of the patients are summarized in Table I.
Table I.
Clinical characteristics of patients with breast cancer (n=43).
| Clinical characteristic | n (%) |
|---|---|
| Sex | |
| Male | 0 (0.00) |
| Female | 43 (100.00) |
| Age, years | |
| ≤45 | 18 (41.86) |
| >45 | 25 (58.14) |
| TNM stage | |
| I+II | 16 (37.21) |
| III+IV | 27 (62.79) |
| Lymph node metastasis | |
| No | 19 (44.19) |
| Yes | 24 (55.81) |
| Tumor sidedness | |
| Left-sided | 28 (65.11) |
| Right-sided | 15 (34.89) |
Cell lines and reagents
CAFs and their matched normal fibroblasts (NFs) were isolated from primary BC and adjacent tissues, respectively. The fresh tissues were sliced and digested with 150 mg/ml collagenase A (Sigma-Aldrich; Merck KGaA) and 25 mg/ml hyaluronidase (Sigma-Aldrich; Merck KGaA) at 37°C temperature for 4 h. Thereafter, the cells were collected and cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) until a confluent monolayer was obtained. After 2~3 passages, a unique homogeneity of stromal fibroblasts was formed. All stromal fibroblasts (CAFs and NFs) were passaged <8 times. Normal human mammary epithelial cells MCF10A (cat. no. SCSP-575) and BC cell lines HCC1937 (cat. no. TCHu148), Hs578T (cat. no. TCHu127), MCF7 (cat. no. TCHu74) and MDA-MB- 468 (cat. no. TCHu136), were obtained from the Chinese Academy of Sciences Cell Bank, and cultured in DMEM containing 10% FBS, 100 U/ml penicillin and 100 g/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at 37°C (5% CO2) in humidified air. The neutralizing CTHRC1 antibody (Ab-CTHRC1) and IgG isotype control antibody (IgG) were supplied by Sigma-Aldrich; Merck KGaA. DKK1, an inhibitor of Wnt/β-catenin signaling, was purchased from StemRD, Inc.
Preparation of conditioned medium (CM) and CTHRC1 expression detection
The CAFs and their matching NFs were seeded into 6-well plates (2×106/well) containing serum-free DMEM. After culture at 37°C for 48 h, the medium was collected and centrifuged at 2,000 × g, 4°C for 5 min, and the supernatant (CM) was subjected to further experimentation. Prior to in vitro experiments, the CTHRC1 protein expression level in the CAF-CM and NF-CM was determined by western blotting. Each experiment was performed in triplicate.
ELISA
The CTHRC1 protein levels in serum, tissue homogenates and cell culture media were quantified using a Human CTHRC1 ELISA kit (cat. no. EH135RB; Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The absorbance (450 nm) of each sample was detected using a standard automatic microplate reader (BioTek Instruments, Inc.). Each sample of blood, cancer tissue and adjacent tissue was divided into three for ELISA, and the CTHRC1 protein level is presented as an average value according to the experimental results. Finally, independent or paired samples were tested and analyzed according to grouping.
Immunohistochemical staining
BC and adjacent tissues from the same patient were fixed with 4% paraformaldehyde at room temperature for 24 h, embedded in paraffin and cut into 5–6 µm-thick slices for immunohistochemical staining. Briefly, the tissue slices were placed onto a slide and heated at 60°C for 20 min, and then exposed to xylene and graded alcohol (100, 95, 90, 80 and 70%) in turn, each at room temperature for 10 min. After rinsing with water, the sections were immersed in 3% H2O2 for 10 min to remove endogenous catalases, and 10% serum (Gibco; Thermo Fisher Scientific, Inc.) was used for blocking at 37°C for 30 min. Rabbit anti-human CTHRC1 primary antibodies were applied at 4°C for 12 h (1:500; cat. no. MABT889; MilliporeSigma), followed by horseradish peroxidase- conjugated goat anti-rabbit IgG secondary antibodies (1:1,000; cat. no. ab6721; Abcam) at 37°C for 30 min. The sections were then stained using a DAB kit (Nanjing Jiancheng Bioengineering Institute) at 37°C for 5 min, and images were captured under an optical microscope. This experiment was repeated twice, and the most representative images are displayed.
Invasion and migration assays
To evaluate cellular invasiveness, an 8-µm pore polycarbonate membrane filter was inserted into each Transwell chamber and coated with 50 µl Matrigel at a final concentration of 4 mg/ml (37°C for 30 min). Then, 5×103 MDA-MB-468 cells were seeded into the upper chamber with 100 µl serum-free medium, and 1 ml DMEM containing 20% FBS was added to the bottom chamber (Control group). For the experimental groups, 1 ml CAF-CM, 1 ml CAF-CM containing 100 µg lgG, 1 ml CAF-CM containing 100 µg Ab-CTHRC1, 1 ml CAF-CM containing 100 ng DKK1, and 1 ml CAF-CM containing 100 µg Ab-CTHRC1 and 100 ng DKK1 were added to the respective lower chambers. The cells were incubated at 37°C for 24 h, and the non-invasive cells were removed from the upper surface of the filter membrane. The invaded cells on the lower surface were stained with crystal violet at room temperature for 1 h, rinsed with water, and dried. Images of cells from three random visual fields per filter were captured using an optical microscope (Olympus Corporation) at ×200 magnification, and then counted manually. A cellular migration assay was performed in the same manner, but without Matrigel. The experiment was performed in triplicate.
MTT Assay
A total of 5×103 cells per well were seeded into 96-well plates. The absorbance value was determined after incubation with CM at different concentrations of DKK1 (0, 50, 100 and 200 ng/ml) for 24 h (each concentration was repeated three times). After 24 h, the absorbance of each group of cells was measured according to the instructions of the MTT cell proliferation and cytotoxicity assay kit (Nanjing Jiancheng Bioengineering Institute), and the proliferation rate was calculated based on the absorbance at 0 h. Western blotting was subsequently used to verify the effects of DKK1, and to determine the optimal concentration.
Protein extraction and western blotting
The total cellular protein was extracted using a total protein extraction kit (Nanjing Jiancheng Bioengineering Institute) and quantified using the BCA method. Then, 100 µg protein per lane was then electrophoresed using 12% SDS-PAGE, and transferred to polyvinylidene difluoride membranes. Nonspecific binding was blocked with 5% skimmed milk for 2 h at 37°C, after which the membranes were incubated with the following primary antibodies at 4°C overnight: Rabbit anti-human E-cadherin (cat. no. ab15148), rabbit anti-human N-cadherin (cat. no. ab18203), rabbit anti-human vimentin (cat. no. ab92547), rabbit anti-human β-catenin (cat. no. ab16051), rabbit anti-human cyclin D1 (cat. no. ab134175) and rabbit anti-human c-myc (cat. no. ab32072); all at 1:1,000, all purchased from Abcam. A GAPDH antibody (1:1,000; cat. no. ab37168; Abcam) was used as the internal reference control. Then, the membranes were incubated with goat anti-rabbit secondary antibody IgG (HRP) (1:5,000; cat. no. ab6721; Abcam) for 1 h at room temperature. Finally, images were obtained using the multifunctional Gel Imaging System (Gel Doc XR; Bio-Rad Laboratories, Inc.), and the gray value of each band was quantified and analyzed using Quantity One software (v4.6.6; Bio-Rad Laboratories, Inc.). Furthermore, the detection and evaluation of CTHRC1 protein expression levels in the CAF-CM and NF-CM were assessed in the same manner using Rabbit anti-human CTHRC1 (1:2,000; cat. no. MABT889; MilliporeSigma) as primary antibodies and GAPDH as the internal control. This experiment was performed in triplicate.
Statistical analysis
All values are presented as the mean ± standard deviation. Statistical analysis was performed using SPSS version 19.0 (IBM Corp). Differences between two groups were analyzed using an unpaired t-test. However, the differences in CTHRC1 expression level between BC and adjacent tissues were examined using a paired t-test. One-way ANOVA was performed to compare differences between multiple groups (>2), followed by Tukey's post hoc test or Bonferroni's correction. P<0.05 was considered to indicate a statistically significant difference.
Results
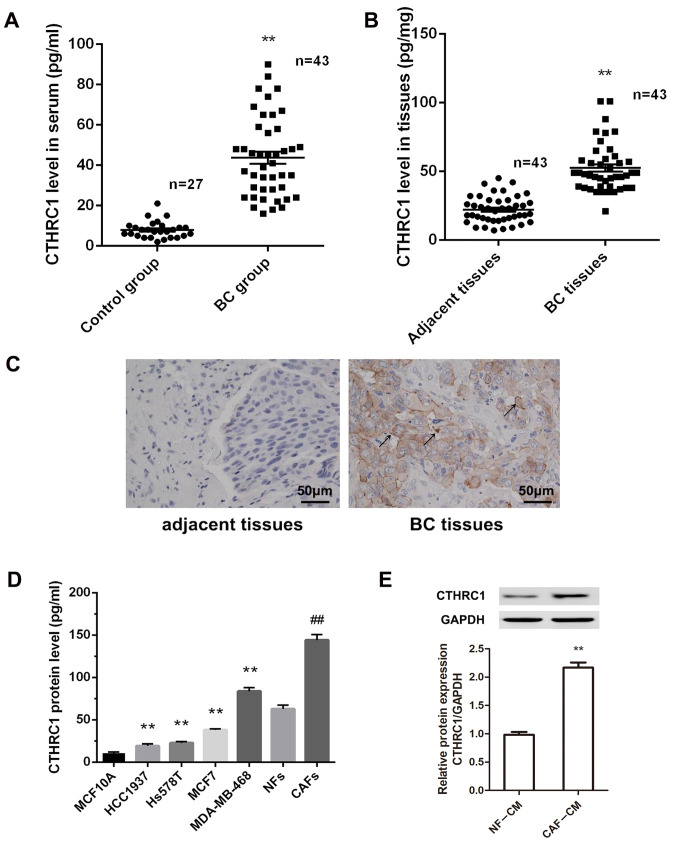
CTHRC1 is highly expressed in CAFs in the BC tumor microenvironment
Previous studies have shown the relevance of CTHRC1 to specific epithelial tumors, including BC (12,26). To determine the protein level and cellular source of CTHRC1 in BC, an ELISA kit was used to detect the level of CTHRC1 in the serum and tumor tissues of patients with BC. Fig. 1A and B show significant elevation of CTHRC1 protein levels in the serum and BC tissues of patients compared with healthy volunteers (P<0.01). To confirm which BC cellular component was associated with the high expression of CTHRC1, the expression pattern in BC tissues was detected by immunohistochemistry. Fig. 1C suggests that CTHRC1-positive cells were primarily located at the boundary between tumor and stroma tissues, in stroma between tumor nests, and rarely within tumor tissues. However, CTHRC1-positive cells could not be detected in adjacent non-tumor tissues. Thus, we hypothesize that CAFs are the source of CTHRC1, and are the most abundant cell type in the breast tumor stroma. Therefore, the expression levels of CTHRC1 in CAFs, NFs, BC cell lines (HCC1937, Hs578T, MCF7 and MDA-MB-468) and normal human mammary epithelial cells MCF10A were evaluated by ELISA. CTHRC1 levels were significantly higher in BC cell lines compared with MCF10A cells (Fig. 1D; all P<0.01). Furthermore, the CTHRC1 level in CAFs was significantly higher than that in NFs (P<0.01). To confirm these results, the CTHRC1 protein expression level was evaluated in CAF-CM and NF-CM by western blotting, and revealed that the relative expression of CTHRC1 was significantly higher in CAF-CM than in NF-CM (Fig. 1E; P<0.01). These results suggest that CTHRC1 is significantly upregulated in BC, and that CAFs are a primary cell source of CTHRC1 in the BC microenvironment.
Figure 1.
CTHRC1 is highly expressed in CAFs in the BC tumor microenvironment. Expression of CTHRC1 in (A) serum and (B) cancer tissues of patients with BC was detected by ELISA. **P<0.01. (C) Expression of CTHRC1 in adjacent and BC tissues was detected by immunohistochemical staining; arrows indicate CTHRC1-positive cells; magnification, ×200. (D) CTHRC1 protein expression level in MCF10A normal human mammary epithelial cells, BC cell lines (HCC1937, Hs578T, MCF7 and MDA-MB-468), CAFs and NFs was determined by ELISA. **P<0.01 vs. MCF10A cells; ##P<0.01 vs. the NFs. (E) CTHRC1 protein expression level in CAF-CM and NF-CM was determined by western blotting. **P<0.01. CTHRC1, collagen triple helix repeat containing-1; CAF, cancer-associated fibroblast; NF, normal fibroblast; BC, breast cancer; CM, conditioned media.
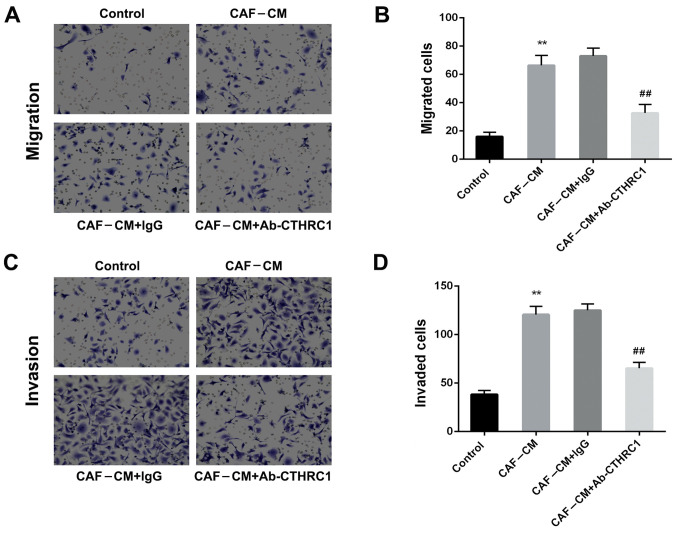
CAFs enhance the migration and invasiveness of BC cells by secreting CTHRC1
As BC cells are the key determinants of metastasis in tumor progression, the migration and invasion capacities of MDA-MB-468 cells (induced by CAFs) were determined. The CAF-CM-induced migration ability of MDA-MB-468 cells was significantly increased compared with that of the control group (Fig. 2A and B; P<0.01). However, the addition of a neutralizing CTHRC1 antibody (Ab-CTHRC1) significantly decreased migration compared with that of the isotype control (CAF-CM+lgG) (P<0.01). Furthermore Fig. 2C and D demonstrate that CAF-CM induction enhanced the invasive ability of MDA-MB-468 cells (P<0.01). Ab-CTHRC1 also reduced the invasiveness of MDA-MB-468 cells compared with the isotype control antibody (P<0.01). Hence, these findings indicate that CAFs enhanced the migration and invasion abilities of BC cells by secreting CTHRC1.
Figure 2.
CAFs enhance the migration and invasion abilities of breast cancer cells via the secretion of CTHRC1. Effect of CAF-CM induction on MDA-MB-468 cells (A and B) migration and (C and D) invasiveness was determined following addition of a CTHRC1 neutralizing antibody or IgG isotype control antibody for 24 h. **P<0.01 vs. the control group; ##P<0.01 vs. the CAF-CM+lgG group. CTHRC1, collagen triple helix repeat containing-1; CAF, cancer-associated fibroblast; CM, conditioned media.
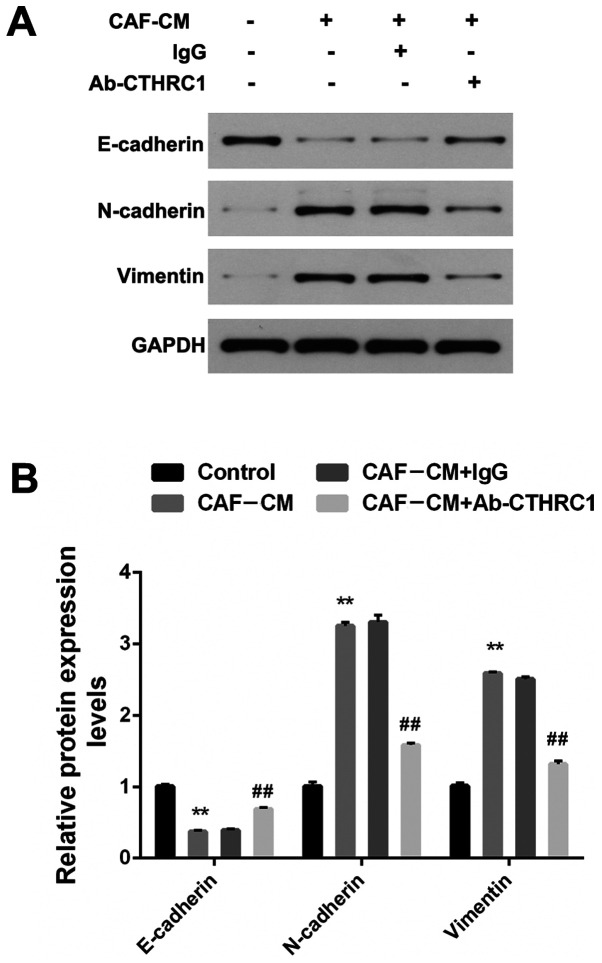
CAFs promote EMT alterations in BC cells by secreting CTHRC1
EMT is a recognized biological process that plays a key role in tumor metastasis, and is characterized by the loss of epithelial markers (such as E-cadherin) and the acquisition of stromal markers (such as N-cadherin and vimentin) (27). To ascertain the role of CAFs in the EMT regulation of BC cells, MDA-MB-468 cells that crossed the filter membrane (under the premise of repeating the migration assay) were collected. Fig. 3A and B show that the expression of E-cadherin induced by CAF-CM was significantly decreased, while the expression of N-cadherin and vimentin was increased (all P<0.01). To determine whether CTHRC1 contributes to the EMT effect of CAFs on BC cells, Ab-CTHRC1 was added into the CAF-CM induction system. EMT was induced by CAF-CM, but was impaired by Ab-CTHRC1, revealing a reduction in E-cadherin and an increase in N-cadherin and vimentin (all P<0.01). Therefore, these findings indicate that CAFs promoted EMT alterations in BC cells by secreting CTHRC1.
Figure 3.
CAFs promote EMT changes of BC cells via the secretion of CTHRC1. (A) Protein expression of E-cadherin, N-cadherin and vimentin in MDA-MB-468 BC cells induced by CAF-CM in the presence of a CTHRC1 neutralizing or IgG isotype control antibody was analyzed by western blotting. (B) Quantitative analysis of E-cadherin, N-cadherin and vimentin expression level. **P<0.01 vs. the control group; ##P<0.01 vs. the CAF-CM+lgG group. CTHRC1, collagen triple helix repeat containing-1; CAF, cancer-associated fibroblast; CM, conditioned media.
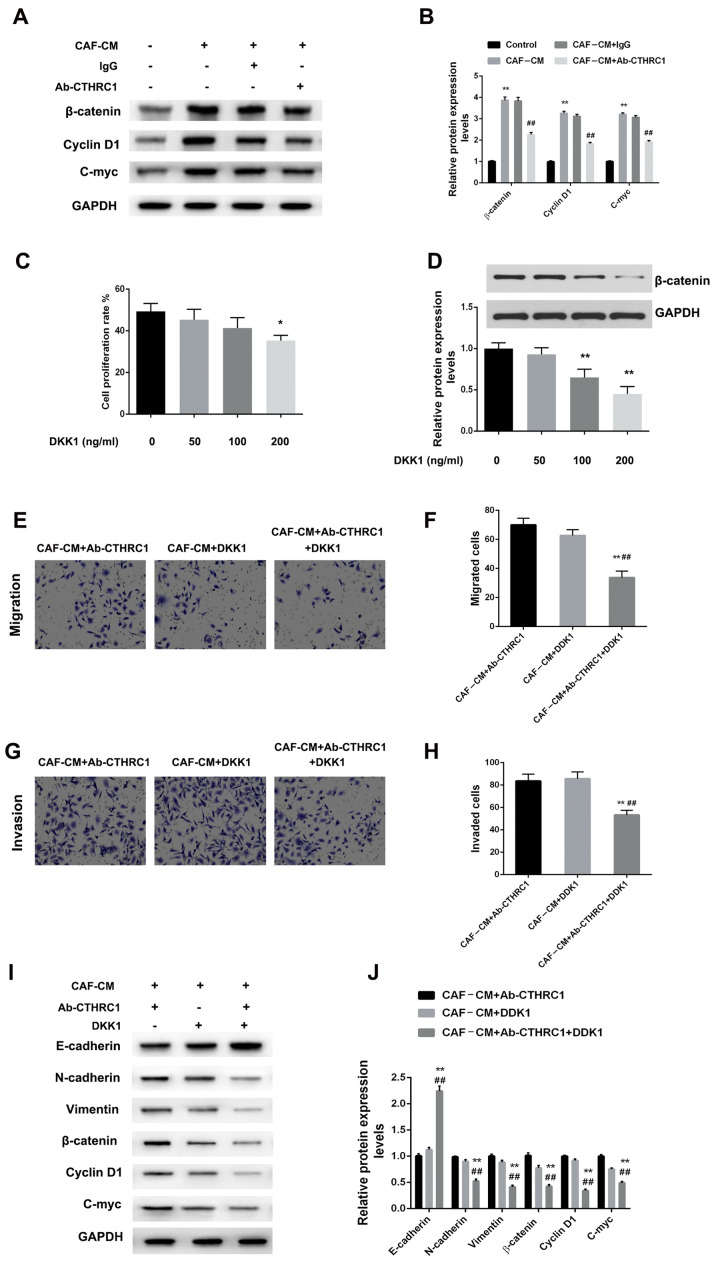
CAF-secreted CTHRC1 mediates the migration, invasiveness and EMT of BC cells by activating the Wnt/β-catenin signaling pathway
Studies have reported that CTHRC1 drives human oral cancer cell migration by activating the Wnt/β-catenin signaling pathway (28), and promotes human colorectal cancer cell proliferation and invasiveness by activating Wnt/PCP signaling (20). However, to the best of our knowledge, their reports identifying whether CTHRC1 enhances the migration, invasiveness and EMT of BC cells via Wnt/β-catenin signaling pathway activation. Therefore, the effects of different induction systems on the Wnt/β-catenin signaling pathway were determined using MDA-MB-468 cells. Fig. 4A and B suggest that CAF-CM induction markedly increased the expression of β-catenin, cyclin D1 and c-Myc in MDA-MB-468 cells (all P<0.01). However, Wnt/β-catenin signaling induced by CAF-CM and lgG was disrupted when CTHRC1 was inhibited by Ab-CTHRC1, which showed a degree of β-catenin, cyclin D1 and c-Myc expression inhibition (all P<0.01).
Figure 4.
CAF-secreted CTHRC1 mediates the migration, invasiveness and EMT of BC cells via activation of the Wnt/β-catenin signaling pathway. (A) Protein expression of β-catenin, cyclin D1 and c-Myc in MDA-MB-468 BC cells induced by CAF-CM in the presence of CTHRC1 neutralizing or IgG isotype control antibody was analyzed by western blotting. (B) Quantitative analysis of β-catenin, cyclin D1 and c-Myc expression level. **P<0.01 vs. the control group; ##P<0.01 vs. the CAF-CM+lgG group. (C) proliferation and (D) β-catenin expression was concentration-dependent. *P<0.05 and **P<0.01 vs. the control group (0 ng/ml DKK1). Effect of CAF-CM induction on MDA-MB-468 cell (E and F) migration and (G and H) invasiveness was evaluated 24 h after Wnt/β-catenin pathway inhibition via neutralizing antibody or DKK1 administration. **P<0.01 vs. the CAF-CM+Ab-CTHRC1 group; ##P<0.01 vs. the CAF-CM+DKK1 group. (I and J) Protein expression of E-cadherin, N-cadherin, vimentin, w-catenin, cyclin D1 and c-Myc in MDA-MB-468 BC cells induced by CAF-CM in the presence of CTHRC1 neutralizing antibody or DKK1. **P<0.01 vs. the CAF-CM+Ab-CTHRC1 group; ##P<0.01 vs. the CAF-CM+DKK1 group. CTHRC1, collagen triple helix repeat containing-1; CAF, cancer-associated fibroblast; CM, conditioned media; DKK1, Dickkopf-1.
The blocking effect of DKK1 on the Wnt/β-catenin pathway was also assessed for its ability to inhibit the tumorigenic effects on BC cells. Fig. 4C and D indicate that the effect of DKK1 was enhanced in a concentration-dependent manner, with a significant anti-proliferative effect at high concentrations. Therefore, an optimal concentration of 100 ng/ml was selected. In this instance, Fig. 4E-H indicate that compared with CAF-CM/Ab-CTHRC1 or CAF-CM/DKK1, CAF-CM/Ab-CTHRC1/DKK1 significantly reduced cellular migration and invasion ability (all P<0.01). For EMT changes, CAF-CM/Ab-CTHRC1/DKK1 further decreased the EMT of MDA-MB-468 cells compared with CAF-CM/Ab-CTHRC1 and CAF-CM/DKK1 (P<0.01). Finally, to confirm the involvement of CAF-secreted CTHRC1 in malignant phenotypes via Wnt/β-catenin signaling pathway activation, the expression changes of pathway-related proteins were assessed. Fig. 4I and J show that compared with CAF-CM/Ab-CTHRC1 or CAF-CM/DKK1, the CAF-CM/Ab-CTHRC1/DKK1 combined induction system significantly inhibited Wnt/β-catenin signaling pathway activation (all P<0.01). The results suggest that the CTHRC1/Wnt/β-catenin pathway is essential for the CAF-induced migration, invasiveness and EMT of BC cells.
Discussion
CAFs have been reported to promote tumor growth and provide an appropriate microenvironment for the proliferation, angiogenesis, migration and invasion of a variety of cancers, by secreting different pro-oncogenic factors (29,30). Therefore, CAFs may serve as an effective tool for cancer treatment in the future. Consequently, a deeper understanding of the molecular mechanism by which CAFs stimulate tumor growth is of great significance for understanding BC progression and potential treatment strategies. The current study concluded that CAF-secreted CTHRC1 plays a vital role in the progression of BC. We hypothesize that CAFs promote the migration, invasiveness and EMT of BC cells by secreting CTHRC1, which activates the Wnt/β-catenin signaling pathway. Suppressing the Wnt/β-catenin pathway with a neutralizing antibody to deplete CTHRC1, or a specific inhibitor (DKK1), significantly attenuated these CAF-induced malignant phenotypes. Therefore, activation of the Wnt/β-catenin pathway by CTHRC1 may play a useful role in the interaction between CAFs and BC cells.
CTHRC1 is a secretary protein that is reportedly upregulated in various solid tumors, including melanoma (31), epithelial ovarian cancer (EOC) (32), hepatocellular carcinoma (33) and BC (12). Hou et al (32) reported that the overexpression of CTHRC1 promoted the EMT of EOC, and was associated with poor prognosis. Ip et al (31) revealed that CTHRC1 protein expression was weak or undetectable in non-invasive melanoma tumors, but highly expressed in invasive melanoma. In melanoma cell line-related studies, overexpression of CTHRC1 enhanced cellular migration and adhesion, and protected melanoma cells from serum deprivation-induced apoptosis (31). Furthermore, Jin et al (34) reported that CTHRC- knockdown inhibited the proliferation of renal cell carcinoma (RCC) cells. In BC patients with bone metastasis, the stromal expression of CTHRC1 is markedly enhanced (15). In the current study, CTHRC1 protein levels in the serum and tumor tissues of patients with BC were significantly elevated compared with those of healthy volunteers. Furthermore, CTHRC1-positive cells were primarily located at the boundary between tumor and stroma tissues, and in stroma between tumor nests. Similar clinical results for CTHRC1 expression level have also been demonstrated in colorectal and endometrial cancer, and in others solid human tumors (26,35,36). In a comprehensive review, Jiang et al (37) also noted that the multidimensional function of CTHRC1 was closely associated with its abnormal expression. Collectively, these data suggest that CTHRC1 is an important regulator of the tumor microenvironment. However, there are few studies on the specific microenvironmental origin of CTHRC1. Using cellular isolation and validation experiments, the results of the present study further demonstrated that the cells were derived from CAFs, which was the basis of subsequent experimentation. Considering previous reports, CTHRC1 was found to be a migration- and invasion-promoting protein in the tumor microenvironment. Secretion of CTHRC1, mainly by CAFs, strongly suggests that CAF-generated CTHRC1 promotes BC cell metastasis through several autocrine and paracrine processes.
EMT is an important embryonic process, and a vital reversible process by which fully differentiated cells lose their epithelial features and gain a migratory mesenchymal phenotype (38). EMT contributes to the metastatic potential of several types of solid tumor via activating invasion-metastasis cascade responses, including in BC. A previous study reported that knockdown of CTHRC1 expression in EOC cells resulted in the downregulation of both N-cadherin and vimentin (32). Similarly, downregulation of CTHRC1 markedly accelerated the expression of E-cadherin, suppressed the expression of N-cadherin and vimentin, and inhibited the migration and invasiveness of RCC cells (34). E-cadherin is an epithelial calcium-binding transmembrane glycoprotein, whose downregulation is a representative biomarker of EMT, enabling tumor cells to develop an invasive phenotype, while N-cadherin exhibits the opposite function (39). Migration and invasiveness are prominent biological characteristics of cancer cells, and related experiments can be used as a means to study their EMT ability. In epithelial ovarian cancer, SKOV3 and HO8910 cell migration and invasion activities were significantly increased by the CM of recombinant CTHRC1-treated macrophages (40). In the current study, EMT induced by CAF-CM was retarded following CTHRC1 depletion by specific neutralizing antibodies, showing a reduction in E-cadherin, an increase in N-cadherin and vimentin, and the attenuation of migration and invasion ability. These results indicate that CAF-secreted CTHRC1 significantly induced EMT. In summary, the two studies have something in common: altering the microenvironment necessary for the proliferation of cancer cells to study malignant biological changes, and achieving the desired results (that decreasing CTHRC1 expression in the tumor microenvironment significantly inhibited the migration, invasion and EMT abilities of BC cells). Thus we hypothesize that the success of the experiment lies in an understanding of the primary source of CTHRC1 and its secretion characteristics, which is of great significance for subsequent in-depth research.
A growing body of evidence suggests that the Wnt/β-catenin signaling pathway plays a key role in the carcinogenesis and progression of several types of solid tumor, including BC (41,42). Therefore, in the medical community, influencing the Wnt/β-catenin pathway has been considered an effective way to treat BC. Wnt/β-catenin pathway activation leads to the stabilization and accumulation of β-catenin protein in the cytoplasm, which translocates into the nucleus and induces the transcription of downstream target genes, such as cyclin D1 and c-Myc (43). Several studies have reported that activation of these genes increases cellular proliferation, reduces cell-cell adhesion, and promotes EMT, migration and invasiveness (44,45). In the present study, CTHRC1 from CAFs promoted the migration, invasiveness and EMT of BC cells by activating the Wnt/β-catenin signaling pathway, which primarily manifests in significantly upregulated levels of β-catenin, cyclin D1 and c-Myc. However, depleting CTHRC1 partially blocked CAF-induced activation of the Wnt/β-catenin pathway. In addition, the combination of neutralizing antibodies and the specific inhibitor (DKK1) of the Wnt/β-catenin pathway further weakened CAF-induced pathway activation. These data demonstrate that CTHRC1 promotes the malignant biological properties of BC cells by activating the Wnt/β-catenin signaling pathway.
Overall, we hypothesize that the crosstalk between BC cells and CAFs is conducive to migration, and invasiveness and EMT via the CTHRC1/Wnt/β-catenin signaling pathway. Similar to normal mammary epithelial cells, CAFs are a major source of CTHRC1 protein. Extracellular CTHRC1 produced in the tumor microenvironment promotes the malignant behaviors of BC cells through autocrine and paracrine pathways. Therefore, weakening CAFs-BC cell communication by suppressing CTHRC1 expression may be a novel strategy for BC treatment. Although the present study achieved the expected results, there were some design limitations. For example, the effect on EMT of directly knocking down or increasing the expression of CTHRC1 in cancer cells was not investigated, as was by Wang et al (46) in gastric cancer. In addition, following the administration of DKK1, necessary controls (such as without CAF-CM or lgG control) for migration and invasion were not introduced, thus it remains to be seen whether the observed effects on migration and invasion are specific to CAF-secreted CTHRC1. Future improved experiments will enhance the level of research, and focus on demonstrating the EMT-promoting effects of CTHRC1 secreted by CAFs in vivo.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YW conceived the present study. HL, XZ and WL designed, performed and interpreted the experimental data. HL drafted the initial manuscript. HL, XZ and WL confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was reviewed and approved by the Human Subject Research Ethics Committee of Cangzhou Central Hospital, and an informed consent form was obtained from each study participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Emens LA. Breast Cancer Immunotherapy: Facts and Hopes. Clin Cancer Res. 2018;24:511–520. doi: 10.1158/1078-0432.CCR-16-3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langley RR, Fidler IJ. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr Rev. 2007;28:297–321. doi: 10.1210/er.2006-0027. [DOI] [PubMed] [Google Scholar]
- 3.Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoppmann SF, Berghoff A, Dinhof C, Jakesz R, Gnant M, Dubsky P, Jesch B, Heinzl H, Birner P. Podoplanin-expressing cancer-associated fibroblasts are associated with poor prognosis in invasive breast cancer. Breast Cancer Res Treat. 2012;134:237–244. doi: 10.1007/s10549-012-1984-x. [DOI] [PubMed] [Google Scholar]
- 6.Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ, Feng YM. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br J Cancer. 2014;110:724–732. doi: 10.1038/bjc.2013.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aboussekhra A. Role of cancer-associated fibroblasts in breast cancer development and prognosis. Int J Dev Biol. 2011;55:841–849. doi: 10.1387/ijdb.113362aa. [DOI] [PubMed] [Google Scholar]
- 8.Saigusa S, Toiyama Y, Tanaka K, Yokoe T, Okugawa Y, Fujikawa H, Matsusita K, Kawamura M, Inoue Y, Miki C, et al. Cancer-associated fibroblasts correlate with poor prognosis in rectal cancer after chemoradiotherapy. Int J Oncol. 2011;38:655–663. doi: 10.3892/ijo.2011.906. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Y, Wang K, Ma W, Zhang X, Song Y, Wang J, Wang N, Song Q, Cao F, Tan B, et al. Cancer-associated fibroblasts are associated with poor prognosis in esophageal squamous cell carcinoma after surgery. Int J Clin Exp Med. 2015;8:1896–1903. [PMC free article] [PubMed] [Google Scholar]
- 10.Pyagay P, Heroult M, Wang Q, Lehnert W, Belden J, Liaw L, Friesel RE, Lindner V. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ Res. 2005;96:261–268. doi: 10.1161/01.RES.0000154262.07264.12. [DOI] [PubMed] [Google Scholar]
- 11.LeClair R, Lindner V. The role of collagen triple helix repeat containing 1 in injured arteries, collagen expression, and transforming growth factor beta signaling. Trends Cardiovasc Med. 2007;17:202–205. doi: 10.1016/j.tcm.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Baek TH, Yim HS, Kim KH, Jeong SH, Kang HB, Oh SS, Lee HG, Kim JW, Kim KD. Collagen triple helix repeat containing-1 (CTHRC1) expression in invasive ductal carcinoma of the breast: The impact on prognosis and correlation to clinicopathologic features. Pathol Oncol Res. 2013;19:731–737. doi: 10.1007/s12253-013-9636-y. [DOI] [PubMed] [Google Scholar]
- 13.Lai YH, Chen J, Wang XP, Wu YQ, Peng HT, Lin XH, Wang WJ. Collagen triple helix repeat containing-1 negatively regulated by microRNA-30c promotes cell proliferation and metastasis and indicates poor prognosis in breast cancer. J Exp Clin Cancer Res. 2017;36:92. doi: 10.1186/s13046-017-0564-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CE, Vincent-Chong VK, Ramanathan A, Kallarakkal TG, Karen-Ng LP, Ghani WM, Rahman ZA, Ismail SM, Abraham MT, Tay KK, et al. Collagen triple helix repeat containing-1 (CTHRC1) expression in oral squamous cell carcinoma (OSCC): Prognostic value and clinico-pathological implications. Int J Med Sci. 2015;12:937–945. doi: 10.7150/ijms.11605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kharaishvili G, Cizkova M, Bouchalova K, Mgebrishvili G, Kolar Z, Bouchal J. Collagen triple helix repeat containing 1 protein, periostin and versican in primary and metastatic breast cancer: An immunohistochemical study. J Clin Pathol. 2011;64:977–982. doi: 10.1136/jclinpath-2011-200106. [DOI] [PubMed] [Google Scholar]
- 16.Park EH, Kim S, Jo JY, Kim SJ, Hwang Y, Kim JM, Song SY, Lee DK, Koh SS. Collagen triple helix repeat containing-1 promotes pancreatic cancer progression by regulating migration and adhesion of tumor cells. Carcinogenesis. 2013;34:694–702. doi: 10.1093/carcin/bgs378. [DOI] [PubMed] [Google Scholar]
- 17.Gu L, Liu L, Zhong L, Bai Y, Sui H, Wei X, Zhang W, Huang P, Gao D, Kong Y, et al. Cthrc1 overexpression is an independent prognostic marker in gastric cancer. Hum Pathol. 2014;45:1031–1038. doi: 10.1016/j.humpath.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Chen S, Li P, Yang R, Cheng R, Zhang F, Wang Y, Chen X, Sun Q, Zang W, Du Y, et al. MicroRNA-30b inhibits cell invasion and migration through targeting collagen triple helix repeat containing 1 in non-small cell lung cancer. Cancer Cell Int. 2015;15:85. doi: 10.1186/s12935-015-0236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma MZ, Zhuang C, Yang XM, Zhang ZZ, Ma H, Zhang WM, You H, Qin W, Gu J, Yang S, et al. CTHRC1 acts as a prognostic factor and promotes invasiveness of gastrointestinal stromal tumors by activating Wnt/PCP-Rho signaling. Neoplasia. 2014;16:265–278. doi: 10.1016/j.neo.2014.03.001. 278.e1-e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang XM, You HY, Li Q, Ma H, Wang YH, Zhang YL, Zhu L, Nie HZ, Qin WX, Zhang ZG, et al. CTHRC1 promotes human colorectal cancer cell proliferation and invasiveness by activating Wnt/PCP signaling. Int J Clin Exp Pathol. 2015;8:12793–12801. [PMC free article] [PubMed] [Google Scholar]
- 21.Giannoni E, Bianchini F, Calorini L, Chiarugi P. Cancer associated fibroblasts exploit reactive oxygen species through a proinflammatory signature leading to epithelial mesenchymal transition and stemness. Antioxid Redox Signal. 2011;14:2361–2371. doi: 10.1089/ars.2010.3727. [DOI] [PubMed] [Google Scholar]
- 22.Willert K, Nusse R. Wnt proteins. Cold Spring Harb Perspect Biol. 2012;4:a007864. doi: 10.1101/cshperspect.a007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akiyama T. Wnt/beta-catenin signaling. Cytokine Growth Factor Rev. 2000;11:273–282. doi: 10.1016/S1359-6101(00)00011-3. [DOI] [PubMed] [Google Scholar]
- 24.Fatima I, El-Ayachi I, Taotao L, Lillo MA, Krutilina RI, Seagroves TN, Radaszkiewicz TW, Hutnan M, Bryja V, Krum SA, et al. The natural compound Jatrophone interferes with Wnt/β-catenin signaling and inhibits proliferation and EMT in human triple-negative breast cancer. PLoS One. 122017:e0189864. doi: 10.1371/journal.pone.0189864. Erratum in: PLoS One 13: e0197796, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang S, Liu Y, Li MY, Ng CSH, Yang SL, Wang S, Zou C, Dong Y, Du J, Long X, et al. FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer. Mol Cancer. 2017;16:124. doi: 10.1186/s12943-017-0700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ni S, Ren F, Xu M, Tan C, Weng W, Huang Z, Sheng W, Huang D. CTHRC1 overexpression predicts poor survival and enhances epithelial-mesenchymal transition in colorectal cancer. Cancer Med. 2018;7:5643–5654. doi: 10.1002/cam4.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 28.Liu G, Sengupta PK, Jamal B, Yang HY, Bouchie MP, Lindner V, Varelas X, Kukuruzinska MA. N-glycosylation induces the CTHRC1 protein and drives oral cancer cell migration. J Biol Chem. 2013;288:20217–20227. doi: 10.1074/jbc.M113.473785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakarla S, Song XT, Gottschalk S. Cancer-associated fibroblasts as targets for immunotherapy. Immunotherapy. 2012;4:1129–1138. doi: 10.2217/imt.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhome R, Mellone M, Emo K, Thomas GJ, Sayan AE, Mirnezami AH. The colorectal cancer microenvironment: Strategies for studying the role of cancer-associated fibroblasts. Methods Mol Biol. 2018;1765:87–98. doi: 10.1007/978-1-4939-7765-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ip W, Wellman-Labadie O, Tang L, Su M, Yu R, Dutz J, Wang Y, Huang S, Zhang X, Huang C, et al. Collagen triple helix repeat containing 1 promotes melanoma cell adhesion and survival. J Cutan Med Surg. 2011;15:103–110. doi: 10.2310/7750.2011.10014. [DOI] [PubMed] [Google Scholar]
- 32.Hou M, Cheng Z, Shen H, He S, Li Y, Pan Y, Feng C, Chen X, Zhang Y, Lin M, et al. High expression of CTHRC1 promotes EMT of epithelial ovarian cancer (EOC) and is associated with poor prognosis. Oncotarget. 2015;6:35813–35829. doi: 10.18632/oncotarget.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou H, Su L, Liu C, Li B, Li H, Xie Y, Sun D. CTHRC1 may serve as a prognostic biomarker for hepatocellular carcinoma. OncoTargets Ther. 2019;12:7823–7831. doi: 10.2147/OTT.S219429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin XF, Li H, Zong S, Li HY. Knockdown of collagen triple helix repeat containing-1 inhibits the proliferation and epithelial-to-mesenchymal transition in renal cell carcinoma cells. Oncol Res. 2016;24:477–485. doi: 10.3727/096504016X14685034103716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li LY, Yin KM, Bai YH, Zhang ZG, Di W, Zhang S. CTHRC1 promotes M2-like macrophage recruitment and myometrial invasion in endometrial carcinoma by integrin-Akt signaling pathway. Clin Exp Metastasis. 2019;36:351–363. doi: 10.1007/s10585-019-09971-4. [DOI] [PubMed] [Google Scholar]
- 36.Tang L, Dai DL, Su M, Martinka M, Li G, Zhou Y. Aberrant expression of collagen triple helix repeat containing 1 in human solid cancers. Clin Cancer Res. 2006;12:3716–3722. doi: 10.1158/1078-0432.CCR-06-0030. [DOI] [PubMed] [Google Scholar]
- 37.Jiang N, Cui Y, Liu J, Zhu X, Wu H, Yang Z, Ke Z. Multidimensional roles of collagen triple helix repeat containing 1 (CTHRC1) in malignant cancers. J Cancer. 2016;7:2213–2220. doi: 10.7150/jca.16539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sánchez-Tilló E, Liu Y, de Barrios O, Siles L, Fanlo L, Cuatrecasas M, Darling DS, Dean DC, Castells A, Postigo A. EMT-activating transcription factors in cancer: Beyond EMT and tumor invasiveness. Cell Mol Life Sci. 2012;69:3429–3456. doi: 10.1007/s00018-012-1122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noh MG, Oh SJ, Ahn EJ, Kim YJ, Jung TY, Jung S, Kim KK, Lee JH, Lee KH, Moon KS. Prognostic significance of E-cadherin and N-cadherin expression in Gliomas. BMC Cancer. 2017;17:583. doi: 10.1186/s12885-017-3591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bai Y, Yin K, Su T, Ji F, Zhang S. CTHRC1 in ovarian cancer promotes M2-like polarization of tumor-associated macrophages via regulation of the STAT6 signaling pathway. OncoTargets Ther. 2020;13:5743–5753. doi: 10.2147/OTT.S250520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luu HH, Zhang R, Haydon RC, Rayburn E, Kang Q, Si W, Park JK, Wang H, Peng Y, Jiang W, et al. Wnt/beta-catenin signaling pathway as a novel cancer drug target. Curr Cancer Drug Targets. 2004;4:653–671. doi: 10.2174/1568009043332709. [DOI] [PubMed] [Google Scholar]
- 42.Cai J, Guan H, Fang L, Yang Y, Zhu X, Yuan J, Wu J, Li M. MicroRNA-374a activates Wnt/β-catenin signaling to promote breast cancer metastasis. J Clin Invest. 2013;123:566–579. doi: 10.1172/JCI65871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanwar SS, Yu Y, Nautiyal J, Patel BB, Majumdar AP. The Wnt/beta-catenin pathway regulates growth and maintenance of colonospheres. Mol Cancer. 2010;9:212. doi: 10.1186/1476-4598-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiao J, Huang L, Ye F, Shi M, Cheng X, Wang X, Hu D, Xie X, Lu W. Cyclin D1 affects epithelial-mesenchymal transition in epithelial ovarian cancer stem cell-like cells. OncoTargets Ther. 2013;6:667–677. doi: 10.2147/OTT.S44177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inamura N, Kimura T, Wang L, Yanagi H, Tsuda M, Tanino M, Nishihara H, Fukuda S, Tanaka S. Notch1 regulates invasion and metastasis of head and neck squamous cell carcinoma by inducing EMT through c-Myc. Auris Nasus Larynx. 2017;44:447–457. doi: 10.1016/j.anl.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Wang P, Wang YC, Chen XY, Shen ZY, Cao H, Zhang YJ, Yu J, Zhu JD, Lu YY, Fang JY. CTHRC1 is upregulated by promoter demethylation and transforming growth factor-β1 and may be associated with metastasis in human gastric cancer. Cancer Sci. 2012;103:1327–1333. doi: 10.1111/j.1349-7006.2012.02292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.