Abstract
Study Objectives
Studies have demonstrated a daily, bidirectional relationship between sleep and physical activity. However, little is known about how other health behaviors, such as alcohol consumption affect this relationship. This study examined how daily and average alcohol consumption affects the relationships between sleep and physical activity.
Methods
Participants included 70 men and women, ages 18–50 with sleep duration >6.5 hours. Participants wore an actigraph, physical activity monitor and recorded number of alcoholic drinks by daily food logs for 7 days. Results were analyzed using multi-level models to evaluate the 7-day average (i.e. between-person effects) and daily effects (i.e. within-person effects) simultaneously.
Results
Those with more average (7 day) minutes of vigorous physical activity had less wake after sleep onset (WASO). Furthermore, a higher number of alcoholic drinks was associated with longer sleep duration and higher WASO over 7 days. Days with a higher number of alcoholic drinks were associated with higher WASO and sleep fragmentation that night. Alcohol intake moderated the average (7 days) and daily relationships between sleep and physical activity such that high average (7 days) WASO was associated with shorter average total physical activity duration, but only for those with higher alcohol intake. In addition, longer physical activity duration during the day was associated with lower sleep fragmentation that night, but only for those with lower alcohol intake.
Conclusions
These data demonstrate that in a naturalistic setting, alcohol intake negatively impacts sleep and diminishes the benefits of physical activity on sleep.
Keywords: alcohol, physical activity, actigraphy, sleep
Statement of Significance.
Although physical activity is known to promote sleep health, little is known about how other health behaviors impact these relationships. This study evaluated how alcohol affects the links between physical activity and sleep both on average (over 1 week) and day-to-day. Results showed that alcohol reduces the benefits of physical activity on sleep.
Introduction
Along with proper nutrition, sleep and physical activity are two of the three pillars of modifiable health-protective behaviors [1]. Sleep serves an important role in maintaining and promoting health such as brain function, metabolism, immune system health, cardiovascular function, and endocrine system function [2–5]. Regular physical activity also plays a vital role in overall health, fitness, and emotional well-being and in decreasing the risk for chronic diseases [6]. As such, lack of adequate good quality sleep and physical activity are associated with poor health outcomes [6, 7]. According to a 24-hours health behavior framework, it is important to understand and examine both sleep health behavior and waking health behaviors (WHB) such as physical activity and alcohol consumption simultaneously [8]. Several prior studies have reported associations between sleep and physical activity both overall and in daily analyses [9–14]. For example, midlife women who are more physically active are likely to have better sleep [9]. However, few studies have examined how other WHB such as alcohol consumption play a role in the relationship between sleep and physical activity.
Alcohol consumption, a prevalent, modifiable, health risk behavior among adults, can affect both sleep and physical activity [15, 16]. Even though alcohol is commonly used as a sleep aid in the general population, alcohol can negatively affect sleep health [17]. Specifically, acute alcohol consumption can increase sleep fragmentation and cause a decrease in rapid eye movement (REM) sleep [18, 19]. Alcohol consumption can also impact daytime functioning, including daytime alertness, which might hamper an individual’s ability to engage in physical activity. However, little is known about the role of other health behaviors such as alcohol consumption in relationships between sleep and physical activity. Identifying whether the relationship between sleep and physical activity and are influenced by alcohol use can inform strategies aimed at promoting and sustaining health-protective behaviors and inhibiting health risk behaviors.
Sleep quality and quantity, physical activity level, and quantity of alcohol consumption can each fluctuate from day-to-day and the interactions among these health behaviors can also vary day-to-day. These health behaviors are time-varying constructs that include both between-person and within-person variability.[20, 21]. The use of daily diary data can inform researchers about how health behaviors fluctuate and influence each other, day-to-day [8, 22], by examining both between-person and within-person effects [20, 21]. For example, some people tend to engage in more physical activity or alcohol use than others (i.e. between-person variation). In addition, an individual may experience better sleep some days more than others (i.e. within-person variation). Recently, the daily relationships between sleep and physical activity has been studied in several samples [11–14]. For example, a study reported the relationship between sleep and physical activity among young adults and found that longer total sleep duration and greater wake after sleep onset (WASO) predicted less activity the following day [11]. In addition, a recent study of daily associations between alcohol consumption and sleep among in a sample of African Americans found that alcohol consumption was related to lower sleep efficiency that night [23]. However, little is known about the role of alcohol consumption in the daily relationships between sleep and physical activity. Therefore, the goals of this study are to examine both the average (7-day or between-person) and day-to-day (within-person) associations between sleep, physical activity, and alcohol consumption in a sample of healthy adults.
First, we examined daily, bidirectional relationships between sleep and physical activity both in terms of daily relationships (within-person variability, or deviation from one’s average across the 7 days) and the weekly average (between-person variability, averaged across the 7 days [20, 21]) We hypothesized that there would be a bidirectional association between sleep and physical activity in both daily (nights with lower sleep fragmentation and WASO would be followed by higher physical activity duration and vice versa) and average levels (individuals with lower average sleep fragmentation and WASO would have higher physical activity over the 7 days period) (Hypothesis 1).
Second, we examined the association between alcohol consumption with sleep and physical activity respectively for both the daily (within-person) and weekly average level (between-person levels). We hypothesized that alcohol would be associated with poorer sleep at the daily and average level (i.e. on days when participants consumed a higher number of alcoholic drinks than usual, they would demonstrate poorer sleep on the subsequent night and participants with a higher number of alcoholic drinks across 7 days would report poorer sleep across 7 days compared to their counterparts). Regarding the role of alcohol consumption on physical activity, we hypothesized that higher alcohol intake would be associated with lower physical activity both in daily and weekly associations.
Finally, we examined whether alcohol consumption moderated the relationship between sleep and physical activity at both within-person and between-person levels. We hypothesized that higher number of alcoholic drinks would moderate the relationships between physical activity duration at both within-person and between-person levels, respectively.
Methods
This is a secondary analysis of a larger study of circadian timing, alignment, and obesity-related behaviors [24]. Participants were recruited from the community using flyers, web advertisements, and participant registries with advertisements for healthy volunteers with “normal” or “late” sleep timing. Inclusionary criteria included: age 18–50, habitual sleep duration ≥6.5 hours and ≤8.5 hours, and ability to read and write English. Exclusionary criteria included: comorbid sleep, medical or psychiatric disorders, current use of psychiatric medications and medications that could affect sleep, melatonin production, or metabolism (e.g. antidepressants, decongestants, anti-inflammatory medications, cardiac medications), use of illicit drugs or alcohol intake >2 drinks per day, shift work or travel over two time zones in the past six months, caffeine >300 mg per day, current smoking, pregnancy or the desire to become pregnant during the study period. Our analytic sample was limited to persons who completed actigraphy, daily sleep, and food diary (n = 70).
Procedure
A full description of the procedures is reported elsewhere [24]. Briefly, participants completed preliminary screening for study criteria via questionnaires, 7 days of actigraphy, and one-night home apnea monitoring. After screening was completed, participants were scheduled for a one-night overnight in the clinical research unit (CRU). Participants were discharged in the morning after breakfast. Prior to the CRU session, participants completed 7 days of actigraphy, as well as daily sleep and food diaries. Participants were instructed to complete the daily sleep diaries upon waking. On the food diaries, participants recorded time, location, type of food, amount of food they consumed and a description of each component, including brands and restaurant names. Participants were instructed to complete the food diary as they consume and drink and to email, fax, or call in their diary each day. All procedures were approved by Northwestern’s Institutional Review Board and participants provided written informed consent.
Measures
Sleep
Sleep/wake patterns were estimated using 7 days of wrist actigraphy using the Actiwatch Spectrum (Philips/Respironics, Inc, Bend, OR). Actiwatches were worn on the non-dominant wrist and set with 30 seconds epoch length and medium sensitivity. Actigraphic sleep parameters were calculated using Actiware-Sleep 6.0 software with default settings. Rest intervals were manually entered using a standardized algorithm [25]. Sleep variables utilized in this analysis included sleep duration (total minutes of sleep from sleep onset to sleep offset), WASO (minutes of wake between sleep onset and sleep offset), and fragmentation index (an index of restlessness calculated as the sum of two percentages: (1) The percentage of the sleep period spent moving and (2) The percentage of the number of immobile phases that are only 1 minute long. Actigraphic sleep parameters were calculated using Actiware-Sleep 6.0 software with default settings.
Physical activity
Physical activity was assessed using the Sensewear Pro armband monitor (Bodymedia Inc., Pittsburgh, PA). The Sensewear monitor estimates physical activity and energy expenditure using several sensors, including a three-axis accelerometer, heat flux sensor, skin temperature sensor, near body ambient temperature sensor, and galvanic skin response sensor. The Sensewear monitor has demonstrated accuracy and reliability for physical activity assessment [26–28]. Physical activity values were calculated using Sensewear Professional software (Version 8.1). The Sensewear recordings were considered valid if participants had at least 3 days of recording with ≥18 hours of wear time each day. Physical activity variables included in our analyses included (1) total physical activity duration (minutes of PA per day >3 METs) and (2) vigorous activity duration (minutes of PA per day >6 METs).
Number of alcoholic drinks
The number of alcoholic drinks were collected in the 7-day food logs and reviewed with study staff using a multiple pass interviewing procedure that included review of each entry to query missing items, brand names and confirm estimated portion size in order to improve the calculation of alcohol intake. Participants were asked to report the amount and contents of each drink and based on the logs, the servings of alcohol per day were calculated (1 drink = 1.5 oz of hard liquor, 6 oz of wine and 12 oz of beer).
Analytic strategy
We first ran a descriptive analysis for demographic data and main research variables of the participants included in our analyses. Second, we conducted analyses to determine whether there was sufficient variability in our variables of interest (i.e. if there was insufficient daily variability in sleep, alcohol or physical activity, then analyses should not be conducted on change). Thus we ran preliminary unconditional models to calculate intraclass correlation coefficients (ICCs) and examine within- and between-person variability in sleep, physical activity, and alcohol consumption across the 7 diary days to make sure that multilevel modeling is appropriate for this study. There was both within-person (72.25%) and between-person variability (27.75%) in sleep duration, sleep fragmentation (60.82% within; 39.18% between), WASO (60.64% within; 39.36% between), total physical activity duration (42.75% within; 57.25% between), vigorous physical duration (73.62% within; 26.38% between), and alcohol consumption (85.71% within;14.29% between). Therefore, we proceeded with conducting multilevel models [29] in IBM SPSS Mixed (version 25 [30]) to account for the data structure, wherein diary days were nested within individuals.
We employed a two-level model to address the study’s central questions. Level 1 represented variability due to within-person repeated measures for participants (i.e. day-to-day within an individual), and Level 2 represented between-person variability for participants (on average, differences between individuals) [21, 22]. Day (1–7 days) of the daily diary was centered and then included in all models. Random effects were allowed on the intercept.
We separated the within- and between-person variability by group (person) mean (i.e. subtracting the individual’s group mean (average across 7 days) from the individual’s score measured everyday) centering at Level 1 and grand mean centering at Level 2 [22]. Modeling within- and between-person variability decomposes the effect of individuals’ day-to-day fluctuations in the constructs of interest while accounting for between-person (individual) differences in these constructs. For instance, the models that examined the effect of daily sleep on daily physical activity allowed for the separation of the effect of individuals’ day-to-day fluctuations in sleep while accounting for between-person differences in sleep (i.e. average levels of a participant’s sleep across the 7 days) on daily physical activity. We used maximum likelihood (ML) estimation to handle missing data in multilevel model.[31] Age and gender were included as covariates in every model. A p value of <.05 was considered significant.
For the main research questions 1 and 2, our multilevel model includes two levels. As an example, the equations below were based on the role of sleep on physical activity.
Level 1 was represented by the following equation:
Level 2 was represented by these equations:
In Level 1, the measure of “sleep” was group (person) mean-centered, whereas “mean health” at Level 2 represents each person’s average sleep score across the 7 days, which then was grand-mean centered. The slope (β20) represents the within-person sleep effect on physical activity and β01 represents the between-person sleep effect on physical activity. These are the coefficients used for hypothesis testing. Day (β10) is level 1 covariates because they are time-varying. Age (β02) and Gender (β03) are level 2 covariates because they are time invarying.
For the moderating role of alcohol consumption (main question 3), independent variables are sleep (both at the daily level and on average), alcohol consumption (both at the daily level and on average), and the daily-level (daily sleep by daily alcohol consumption), average-level (average sleep by average alcohol consumption), and cross-level (daily sleep by average alcohol consumption) interactions between alcohol consumption and sleep. We tested all three interactions at the same time (daily, average, and cross-level interaction). Significant interactions decomposed one standard deviation above and below the mean number of alcoholic drinks and simple slopes were tested.
As an example, the equations below were based on moderating role of alcohol consumption between sleep and physical activity.
Level 1 for the research question 3 represented by the following equation:
and Level 2 for the research question 3 represented by these equations:
The slope β40 represents the daily level interaction, β03 represents the average level interaction, and β21 represents cross-level interaction. These are the coefficients used for hypothesis testing.
Results
Participant characteristics
Table 1 describes our analytic sample, which consisted of men (38.6%) and women (61.4%), ranging in age from 18 to 50 (M = 26.71, SD = 7.30). The sample was largely non-Hispanic (92.9%) and white (64.3%). Descriptive statistics for main research variables were calculated as averages across all 7 days. Average sleep duration was 435.53 minutes (7 hours 15 minutes; range 164–729; SD = 82.47) WASO for 32.67 minutes (range 5–134; SD = 16.66) and sleep fragmentation index was 15.49 percent (range 3–38; SD = 6.78) and. In addition, total physical activity duration was 151.57 minutes (2 hours 32 minutes; range 0–663; SD = 107.89) and vigorous physical activity for 6.05 minutes (range 0–88; SD = 12.12). In addition, weekly alcohol consumption ranged from 0 to 16.25 drinks per week with an average of 3.96 (SD = 4.15) drinks. A substantial number of participants (22.1%) did not report any alcoholic drinks across the 7 days, 57.3% of participants reported at least 1 and up to 7 drinks across the 7 days. A total of 17.7% of participants reported >7 to 14 drinks across the 7 days. A small number (2.9%) of participants reported >14 drinks across the 7 days.
Table 1.
Demographic information and descriptive statistics of sleep, physical activity, and number of alcoholic drinks across 7 days for participants
| M/% (SD) | Range | |
|---|---|---|
| Age | 26.71 (7.30) | 18.28–50.12 |
| Gender (% Women) | 61.4% | |
| Race (%White) | 64.3% | |
| Ethnicity (% Hispanic) | 7.1% | |
| Sleep duration (minute) | 435.53 (82.47) | 164–729 |
| Sleep fragmentation | 15.49 (6.78) | 3–38 |
| WASO (minute) | 32.67 (16.66) | 5–134 |
| Total Physical Activity | 151.47 (107.87) | 0–663 |
| Vigorous Physical Activity | 6.05 (12.12) | 0–88 |
| Weekly number of alcohol consumption | 3.96 (4.15) | 0–16.25 |
| 0 drink per week (% Participants) | 22.1% | |
| 0 < drink per week ≤ (% Participants) | 57.3% | |
| 7 < drink per week ≤ 14 (% Participants) | 17.7% | |
| 14< drink per week (% Participants) | 2.9% |
WASO, wake after sleep onset.
Is sleep associated with physical activity? (Research question 1)
There were no daily (i.e. within-person) effects between daily sleep and daily physical activity for either direction (Tables 2 and 3), in that daily sleep did not impact sleep that night, and sleep did not impact next day activity. We, however, found some effects between sleep and physical activity on average (7 days or between-person). For example, persons who, on average, reported longer sleep duration reported shorter total physical activity duration for the 7-day period (Table 2). In addition, people who, on average, reported longer vigorous physical activity duration reported lower WASO, respectively (see Table 3).
Table 2.
Effect of sleep on physical activity
| Sleep > Physical activity | ||
|---|---|---|
| Total physical activity duration | Vigorous physical activity duration | |
| b (SE) | b (SE) | |
| Intercept | 146.52 (14.38)*** | 7.34 (1.47)*** |
| Day | 1.72 (2.86) | 0.54 (0.39) |
| Age | −0.08 (1.41) | −0.14 (0.14) |
| Gender | 18.85 (23.60) | −1.33(2.37) |
| Sleep duration (WP) | −0.09 (0.06) | −0.005(0.01) |
| Sleep duration (BP) | −0.66 (0.22)** | −0.04 (0.02) |
| b (SE) | b (SE) | |
| Intercept | 135.28 (14.39)*** | 6.65 (1.43)*** |
| Day | 1.74 (2.87) | 0.56 (0.39) |
| Age | −0.53 (1.48) | −0.12 (0.14) |
| Gender | 44.30 (23.09) | 0.50(2.24) |
| Sleep fragmentation (WP) | 0.62 (0.90) | −0.07 (0.12) |
| Sleep fragmentation (BP) | −0.19 (2.34) | −0.33 (0.23) |
| b (SE) | b (SE) | |
| Intercept | 136.63 (14.36)*** | 7.03 (1.40)*** |
| Day | 1.85 (2.87) | 0.55 (0.39) |
| Age | −0.22 (1.49) | −0.08 (0.14) |
| Gender | 41.38 (22.90) | −0.58 (2.19) |
| WASO (WP) | 0.04 (0.35) | −0.01 (0.05) |
| WASO (BP) | −0.81 (0.95) | −0.19 (0.09)* |
wp = within-person. bp = between-person. Table reports unstandardized coefficients (b) with standard errors (SE) in parentheses.
*p < .05, **p < .01, ***p < .001.
Table 3.
Effect of physical activity on sleep
| Sleep duration | Sleep fragmentation | WASO | |
|---|---|---|---|
| Total physical activity duration | b (SE) | b (SE) | b (SE) |
| Intercept | 447.49 (8.34)*** | 15.59 (0.83)*** | 33.59 (2.03)*** |
| Day | −2.06 (2.60) | 0.15 (0.19) | −0.60 (0.49) |
| Age | 0.58(0.77) | 0.12 (0.08) | 0.47 (0.20)* |
| Gender | −29.99 (12.35)* | 1.50 (1.30) | −2.37 (3.15) |
| Physical activity (WP) | −0.02 (0.06) | −0.003 (0.005) | 0.004 (0.01) |
| Physical activity (BP) | −0.20 (0.07)** | −0.001 (0.01) | −0.01 (0.02) |
| Vigorous physical activity duration | b (SE) | b (SE) | b (SE) |
| Intercept | 450.61 (8.69)*** | 15.65 (0.81)*** | 33.91 (1.96)*** |
| Day | −2.11 (2.60) | 0.15 (0.19) | −0.61 (0.49) |
| Age | 0.53 (0.82) | 0.10 (0.08) | 0.41 (0.19)* |
| Gender | −38.35 (12.74)** | 1.41 (1.23) | −3.09 (2.96) |
| Physical activity (WP) | −0.12 (0.41) | 0.01 (0.03) | 0.05 (0.08) |
| Physical activity (BP) | −0.94 (0.81) | −0.15 (0.08) | −0.42 (0.19)* |
wp = within-person. bp = between-person. Table reports unstandardized coefficients (b) with standard errors (SE) in parentheses.
*p < .05, **p < .01, ***p < .001.
Is alcohol consumption associated with sleep and physical activity, respectively? (Research question 2)
We found some daily (i.e. within-person) effects between daily alcohol consumption and subsequent daily sleep. That is, we found that on days when individuals reported higher number of alcoholic drinks (more than their own average), they demonstrated higher sleep fragmentation and WASO, respectively during the subsequent night. We also found some average (between-persons) effects between alcohol consumption and sleep. That is, people who, on average, reported higher number of alcoholic drinks demonstrated longer sleep duration and higher WASO, respectively (Table 4).
Table 4.
Effects of number of alcoholic drinks on sleep and physical activity
| Number of alcoholic drinks > Sleep | |||
|---|---|---|---|
| Sleep duration | Sleep fragmentation | WASO | |
| b (SE) | b (SE) | b (SE) | |
| Intercept | 439.20 (7.55)*** | 14.71 (0.72)*** | 32.43 (1.75)*** |
| Day | −3.85 (1.98) | −0.04 (0.15) | −0.88 (0.37)* |
| Age | 0.97 (0.79) | 0.09 (0.08) | 0.35 (0.19) |
| Gender | −25.90 (11.84)* | 2.06 (1.15) | −1.28 (2.78) |
| Alcohol consumption (WP) | 3.08 (3.23) | 0.64 (0.25)** | 2.03 (0.60)** |
| Alcohol consumption (BP) | 24.58 (9.14)* | 1.06 (.88) | 4.27 (2.13)* |
| Number of alcoholic drinks > Physical activity | |||
| Total physical activity duration | Vigorous physical activity duration | ||
| b (SE) | b (SE) | ||
| Intercept | 137.52 (15.68)*** | 6.67 (1.46)*** | |
| Day | −0.18 (2.45) | 0.39 (0.34) | |
| Age | −0.64 (1.54) | −0.15 (0.14) | |
| Gender | 36.76 (24.39) | −0.15 (2.19) | |
| Alcohol consumption (WP) | −4.59 (3.65) | −0.16 (0.50) | |
| Alcohol consumption (BP) | −12.46 (17.83) | −0.83 (1.61) | |
wp = within-person. bp = between-person. Table reports unstandardized coefficients (b) with standard errors (SE) in parentheses.
*p < .05, **p < .01, ***p < .001.
We found no daily or average effects between daily alcohol consumption and daily physical activity the following day (Table 4).
Does alcohol consumption moderate the bidirectional relationships between daily sleep and daily physical activity? (Research question 3)
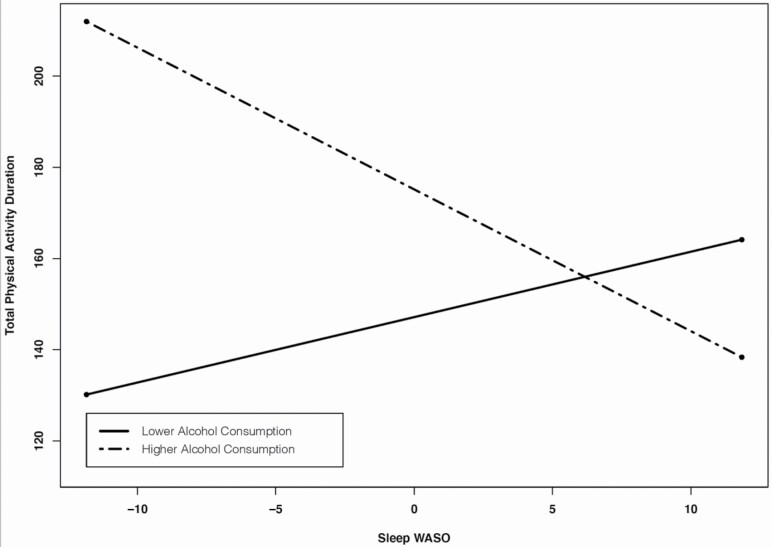
Regarding the moderating role of alcohol consumption in the effect of sleep on physical activity, we found that average number of alcoholic drinks moderated the between-person effects between WASO and total physical activity duration (level 2 interactions). That is, greater average WASO over 7 days was associated with shorter average total physical activity duration for those with higher average number of alcoholic drinks (1 SD above the mean) (b = −2.63, z = −2.06, p = .04). However, average WASO was not associated with average total physical activity duration for those with lower average number of alcoholic drinks (1 SD below the mean) (b = 1.49, z = .10, p = .33) (Table 5; Figure 1)
Table 5.
Moderating role of number of alcoholic drinks on the relationships between sleep and physical activity
| Total physical activity duration | Vigorous physical activity duration | |
|---|---|---|
| Total sleep duration | b (SE) | b (SE) |
| Intercept | 151.44 (15.18)*** | 7.66 (1.64)*** |
| Day | 1.58 (2.94) | 0.59 (0.42) |
| Age | 0.05 (1.43) | −0.14 (0.15) |
| Gender | 14.97 (24.60) | −1.37 (2.59) |
| Sleep (WP) | −0.07 (0.07) | −0.01 (0.01) |
| Sleep (BP) | −0.66 (0.25)* | −0.03 (0.03) |
| Alcohol use (WP) | −5.24 (4.36) | −0.33 (0.62) |
| Alcohol use (BP) | 10.65 (18.43) | −0.14 (1.99) |
| Sleep (WP)*Alcohol use (WP) | −0.07 (0.05) | −0.002 (0.01) |
| Sleep (BP)*Alcohol use (BP) | −0.39 (0.41) | −0.02 (0.04) |
| Sleep (WP)*Alcohol use (BP) | −0.17 (0.11) | 0.01 (0.02) |
| Sleep fragmentation | b (SE) | b (SE) |
| Intercept | 141.64 (15.38)*** | 6.76 (1.58)*** |
| Day | 1.46 (2.98) | 0.56 (0.42) |
| Age | −0.45 (1.51) | −0.13 (0.15) |
| Gender | 41.23 (24.06) | 0.43 (2.42) |
| Sleep (WP) | 0.71 (0.94) | −0.09 (0.13) |
| Sleep (BP) | 0.83 (2.48) | −0.33 (0.24) |
| Alcohol use (WP) | −6.63 (4.45) | −.22 (0.63) |
| Alcohol use (BP) | 6.71 (20.77) | −0.99 (2.10) |
| Sleep (WP)*Alcohol use (WP) | 0.13 (0.99) | 0.10 (0.13) |
| Sleep (BP)*Alcohol use (BP) | −6.19 (3.46) | 0.09 (0.35) |
| Sleep (WP)*Alcohol use (BP) | −0.76 (1.32) | −0.004 (0.19) |
| WASO | b (SE) | b (SE) |
| Intercept | 147.12 (15.58)*** | 7.24 (1.58)*** |
| Day | 1.70 (2.98) | 0.57 (0.42) |
| Age | −0.04 (1.51) | −0.10 (0.15) |
| Gender | 33.46 (24.15) | −0.73 (2.39) |
| Sleep (WP) | 0.06 (0.36) | −0.02 (0.05) |
| Sleep (BP) | −0.57 (1.00) | −0.21 (0.10)* |
| Alcohol use (WP) | −7.90 (4.41) | −0.32 (0.62) |
| Alcohol use (BP) | 13.27 (20.22) | −0.33 (2.03) |
| Sleep (WP)*Alcohol use (WP) | −0.41 (0.35) | −0.01 (0.05) |
| Sleep (BP)*Alcohol use (BP) | −3.16 (1.54)* | 0.06 (0.15) |
| Sleep (WP)*Alcohol use (BP) | 0.10 (0.48) | 0.01 (0.07) |
wp = within-person. bp = between-person. Table reports unstandardized coefficients (b) with standard errors (SE) in parentheses.
*p < .05, **p < .01, ***p < .001.
Figure 1.
Between-person effects of WASO on total physical activity duration moderated by average number of alcoholic drinks.
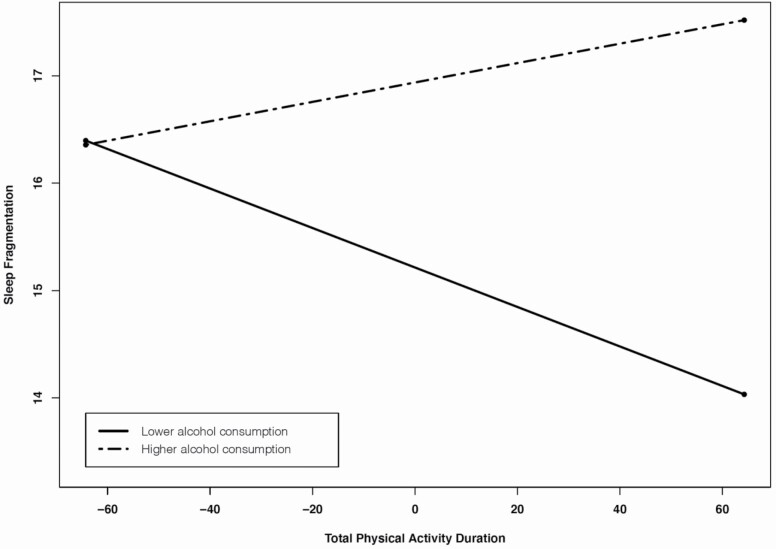
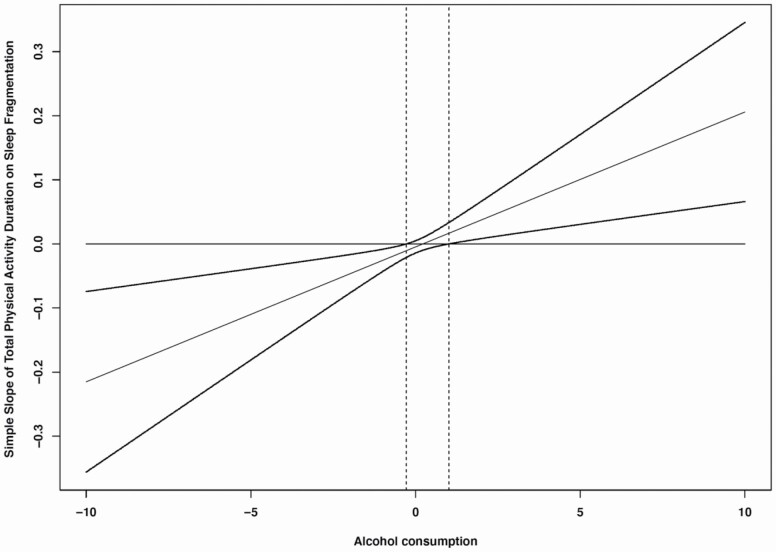
Regarding the moderating role of alcohol consumption in the effect of physical activity on sleep, we found that average number of alcoholic drinks moderated the relationships between daily total physical activity duration and daily sleep fragmentation. That is, longer daily total physical activity duration was associated with lower sleep fragmentation that same night for those with lower average number of alcoholic drinks (1 SD below the mean) (cross-level interaction; b= −.02, z = −2.65, p = .01), whereas daily total physical activity duration was not associated with daily sleep fragmentation for those with higher average number of alcoholic drinks (1 SD above the mean) (cross-level interaction; b = .01, z =1.35, p = .18) (see Table 6; Figure 2). We further used the Johnson-Neyman technique to probe for interaction and to identify ranges of values of the moderator (alcohol consumption) for which the interaction effect is significant. Figure 3 is the J-N plot of the simple slope of total physical activity duration on sleep fragmentation when total physical activity duration is held at its mean and alcohol consumption is allowed to vary. It indicates that when alcohol consumption is low (specifically < .28), an increase in total physical activity duration is expected to result in significantly lower levels of sleep fragmentation. The simple slope of total physical activity duration does not differ significantly from zero from −.28 to 1.10 units of alcohol consumption, but when alcohol consumption is >1.10 units, one would expect that an increase in total physical activity duration would result in a significant, increase in sleep fragmentation.
Table 6.
Moderating role of number of alcoholic drinks on the relationships between physical activity and sleep
| Sleep duration | Sleep fragmentation | WASO | |
|---|---|---|---|
| Total physical activity duration | b (SE) | b (SE) | b (SE) |
| Intercept | 445.45 (8.17)*** | 15.62 (0.84)*** | 34.07 (2.00)*** |
| Day | −2.72 (2.69) | 0.16 (0.19) | −0.62 (0.51) |
| Age | 0.41 (0.73) | 0.12 (0.08) | 0.40 (0.19)* |
| Gender | −31.31 (11.81)* | 1.05 (1.30) | −3.82 (3.06) |
| Physical activity (WP) | 0.01 (0.07) | −0.005 (0.005) | 0.002 (0.01) |
| Physical activity (BP) | −0.19 (0.07) | 0.003 (0.01) | −0.01 (0.02) |
| Alcohol use (WP) | 1.01 (4.07) | 0.83 (0.29)** | 2.29 (0.77)** |
| Alcohol use (BP) | 21.61 (8.91) | 1.03 (.95) | 4.40 (2.26) |
| Physical activity (WP)*Alcohol use (WP) | −0.01 (0.05) | −0.003 (0.004) | −0.004 (0.01) |
| Physical activity (BP)*Alcohol use (BP) | −0.10 (0.09) | −0.02 (0.01) | −0.04 (0.02) |
| Physical activity (WP)*Alcohol use (BP) | −0.08 (0.10) | 0.02 (0.01)** | 0.05 (0.02)* |
| Vigorous physical activity duration | b (SE) | b (SE) | b (SE) |
| Intercept | 447.72 (8.62)*** | 15.52 (0.84)*** | 34.56 (2.01)*** |
| Day | −3.39 (2.69) | 0.14 (0.20) | −0.78 (0.52) |
| Age | 0.51 (0.81) | 0.12 (0.08) | 0.38 (0.20) |
| Gender | −39.92 (12.31)** | 1.15 (1.26) | −4.32 (2.98) |
| Physical activity (WP) | −0.06 (0.46) | 0.02 (0.03) | 0.05 (0.09) |
| Physical activity (BP) | −0.40 (0.88) | −0.19 (0.09)* | −0.42 (0.21)* |
| Alcohol use (WP) | 1.32 (3.90) | 0.74 (0.28)** | 1.92 (0.75)* |
| Alcohol use (BP) | 25.40 (10.52) | 0.26 (1.07) | 3.96 (2.53) |
| Physical activity (WP)*Alcohol use (WP) | 0.99 (0.40)* | −0.06 (0.03)* | −0.02 (0.08) |
| Physical activity (BP)*Alcohol use (BP) | 0.05 (1.95) | −0.34 (0.20) | −0.38 (0.47) |
| Physical activity (WP)*Alcohol use (BP) | −.84 (0.94) | −.005 (0.07) | −0.18 (0.18) |
wp = within-person. bp = between-person. Table reports unstandardized coefficients (b) with standard errors (SE) in parentheses.
*p < .05, **p < .01, ***p < .001.
Figure 2.
Within-person effects of total physical activity duration on sleep fragmentation duration moderated by average number of alcoholic drinks.
Figure 3.
Johnson-Neyman plot of the simple slope of total physical activity duration on sleep fragmentation at the average value (0) of total physical activity across the range of alcohol consumption. Dotted lines represent the 95% of confidence interval.
We also found that daily number of alcoholic drinks moderated the relationships between daily vigorous physical activity duration and daily sleep duration; however, simple slope test showed that neither slopes are significant (within-person interactions; b = −1.09, z = −1.71, p = .09; b = .97, z = 1.64, p = .10). We also found that daily number of alcoholic drinks moderated the relationships between daily vigorous physical activity and daily sleep fragmentation, but simple slope test showed that neither slopes are significant (within-person interactions; b = .08, z = 1.70, p = .09; b = −.05, z = −1.10, p = .27). Finally, we found that average number of alcoholic drinks moderated the relationships between daily total physical activity duration and daily WASO, but simple slope test showed that neither slopes are significant (cross-level interactions; b = −.03, z = −1.56, p = .12; b = .03, z =1.82, p = .07; Table 6).
Discussion
Guided by the 24-health behavior framework [8], the current study sought to examine how alcohol consumption influences the bidirectional, within-person, and between-person relationship between sleep and physical activity. Consistent with our hypotheses we found individuals with higher WASO engaged in less vigorous physical activity. We also found that a higher number of alcoholic drinks was associated with poorer sleep at both the daily and weekly average levels.
Somewhat unexpectedly, we also found that sleep and activity were unrelated in the daily analyses and that on average, across the 7-day period, longer sleep duration was associated with shorter physical activity duration. Previous research has also reported contradictory findings in this area but our result does appear to be in line with results from younger, healthier samples [11, 12]. For example, a study conducted by Master and colleagues among adolescents found that shorter sleep duration was associated with more next-day sedentary behavior and moderate and vigorous physical activity was associated with longer sleep duration [12]. Furthermore, a recent study conducted by Mead and colleagues in a sample of undergraduate students found that longer sleep duration was associated with shorter physical activity the following day, which is consistent with our findings [11]. Our study, combined with these prior studies, suggests that among healthy, young adults without sleep disorders, there may be a trade-off between physical activity and sleep, in that additional sleep may limit time for physical activity and vice versa.
We also found that although alcohol affected sleep, both in daily (within-person) and on average (between-person), there was not a relationship between alcohol use on physical activity the following day. There are several potential explanations for this lack of effect. First, our participants tended to have low alcohol consumption (97% of participants reported consuming <14 drinks in the week). Furthermore, as alcohol consumption in this study was measured by the number of alcoholic drinks only, the severity of hangovers after drinks was not directly captured. Therefore, at this level of alcohol intake, perhaps the effects on next day functioning were not noticed by participants on the following day. Previous studies found that severity of hangover is associated with sleep and physical activity experiences respectively [32–35]. Therefore, future research is needed to examine severity of hangover symptoms along with the number of alcoholic drinks, in particular in clinical samples with participants who are heavy drinkers in daily life.
As anticipated, alcohol moderated the associations between sleep and physical activity both at the daily and average (7-day) level. We found that longer daily total physical activity duration was associated lower sleep fragmentation only among those with lower alcohol consumption. In other words, individuals with higher alcohol consumption did not receive the benefits of physical activity on their objective sleep quality. Thus, alcohol consumption, even in lower levels, may cancel out the positive effect of physical activity on sleep. Furthermore, we found that greater mean WASO was associated with less total physical activity duration only among those with higher alcohol consumption. All in all, these findings imply that a higher number of alcoholic drinks can play a role in diminishing the positive effect between longer physical activity duration and better sleep and enhancing the adverse relationship between poorer sleep and shorter physical activity duration.
Furthermore, we found between-person main effects between sleep and physical activity, but we did not find within-person main effects between sleep and physical activity. Several potential explanations exist for why fluctuations of sleep did not affect physical activity and those of physical activity did not affect sleep. First, other factors may affect the relationship between sleep and physical activity at within-person levels. For example, we found that average alcohol consumption moderated the relationship between sleep and physical activity at within-person levels, even we did not find the significant main effects. These results can be found when cross-over interaction occurs. That is, the relationship between daily sleep and physical activity may have the opposite direction depending on alcohol consumption. Second, as mentioned above, our sample consisted of healthy individuals with at least 6.5 hours of habitual sleep duration, so they may be less sensitive to fluctuation of sleep or physical activity in daily life.
Strengths of this study include the collection of seven consecutive days of sleep, physical activity, and alcohol consumption data, which allow us to examine both within-person variability on a daily basis and between-person variability [21]. Furthermore, this study also used objective measures of sleep and physical activity, and a rigorous multiple-pass method of collecting alcohol intake over 7 days. Despite these strengthens this study is not without limitations which include the generalizability of the findings to groups other than young and middle-aged adults who had healthy sleep and were largely non-Hispanic white. This sample, overall, contained highly active participants with low to moderate alcohol intake, which may not generalize to less healthy or samples with higher alcohol intake (e.g. binge drinkers). In addition, our study did not examine the underlying mechanisms of this associations between sleep, physical activity and alcohol, such as EEG changes sleep or exercise capacity. We also did not measure self-reported sleep quality, which may be an important factor in the sleep and physical activity relationships. For example, in our prior study in older adults, self-reported fatigue was strongly correlated with exercise minutes.[36] Future research is needed to identify potential physiological, psychological, and relational mechanisms of this association. For example, a previous study found that alcohol interfered with the body’s ability to regulate sleep homeostasis.[37] This study also did not evaluate other important determinants of physical activity, such as fatigue, self-efficacy and reward.
In conclusion, the findings of this study demonstrate that alcohol intake negatively affected objective sleep quality and diminished the benefits of physical activity on sleep. Our results also demonstrate the complex interactions between sleep and physical activity and may be a foundation to explore the role of other WHBs to understand the relationships between sleep and physical activity. Finally, given that exercise is often used as a technique to improve sleep among individuals with poor sleep quality, further research should investigate whether alcohol impairs the benefits for this population as well.
Acknowledgments
The authors thank Phyllis Zee, PhD, Lisa Wolfe, MD and Hrayr Attarian, MD for their mentorship and support in completing this project. We also thank Ivy Cheung Mason, PhD, Leah Hecht, PhD, David Clough, Leland Bardsley, Lori Koch, PhD and Tiffany Kim MD for their assistance in data collection and processing.
Funding
Research reported in this publication was supported in part by the National Institutes of Health’s National Heart Lung and Blood Institute 1K23HL109110-01 (KGB), 1R01HL141706-01A1 (KGB) and the National Center for Advancing Translational Sciences, Grant Number UL1TR001422. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Individual support for ELT was provided by Clinical and Translational Research Training in Geriatric Mental Health (MH019986) and Translational Research Training in Sleep Medicine (T32HL082610).
Disclosure Statement
None declared.
Data Availability
Data will be made available upon request.
References
- 1. Castillo M. The 3 pillars of health. AJNR Am J Neuroradiol. 2015;36(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu B, et al. . Effects of sleep restriction on metabolism-related parameters in healthy adults: A comprehensive review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2019;45:18–30. [DOI] [PubMed] [Google Scholar]
- 3. Ingiosi AM, et al. . Sleep and immune function: glial contributions and consequences of aging. Curr Opin Neurobiol. 2013;23(5):806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cash RE, et al. . Association between sleep duration and ideal cardiovascular health among US adults, national health and nutrition examination survey, 2013-2016. Prev Chronic Dis. 2020;17:E43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Konishi M, et al. . Effects of sleep deprivation on autonomic and endocrine functions throughout the day and on exercise tolerance in the evening. J Sports Sci. 2013;31(3):248–255. [DOI] [PubMed] [Google Scholar]
- 6. McGowan L, et al. . Physical activity and health. In: Llewellyn CD, Ayers S, McManus C, et al. , eds. The Cambridge Handbook of Psychology, Health and Medicine., 3rd Ed. Cambridge Handbooks in Psychology. New York, NY: Cambridge University Press; 2019:61–65. [Google Scholar]
- 7. Cappuccio FP, et al. . Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Irish LA, et al. . A 24-hour approach to the study of health behaviors: temporal relationships between waking health behaviors and sleep. Ann Behav Med. 2014;47(2):189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kline CE, et al. . Consistently high sports/exercise activity is associated with better sleep quality, continuity and depth in midlife women: the SWAN sleep study. Sleep. 2013;36(9):1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Youngstedt SD, et al. . Epidemiology of exercise and sleep. Sleep Biol Rhythms. 2006;4(3):215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mead MP, et al. . Daily associations between sleep and physical activity. Int J Behav Med. 2019;26(5):562–568. [DOI] [PubMed] [Google Scholar]
- 12. Master L, et al. . Bidirectional, daily temporal associations between sleep and physical activity in adolescents. Sci Rep. 2019;9(1):7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dzierzewski JM, et al. . Exercise and sleep in community-dwelling older adults: evidence for a reciprocal relationship. J Sleep Res. 2014;23(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang NK, et al. . Better quality sleep promotes daytime physical activity in patients with chronic pain? A multilevel analysis of the within-person relationship. PLoS One. 2014;9(3):e92158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McIntosh C, et al. . Alcohol and the nervous system. J Neurol Neurosurg Psychiatry. 2004;75(Suppl 3):iii16–iii21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thakkar MM, et al. . Alcohol disrupts sleep homeostasis. Alcohol. 2015;49(4):299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schweizer CA, et al. . Use of alcohol as a sleep aid, unhealthy drinking behaviors, and sleeping pill use among women veterans. Sleep Health. 2019;5(5):495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He S, et al. . Alcohol and sleep-related problems. Curr Opin Psychol. 2019;30:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stein MD, et al. . Disturbed sleep and its relationship to alcohol use. Subst Abus. 2005;26(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoffman L. Longitudinal Analysis: Modeling Within-Person Fluctuation and Change. New York, NY: Routledge; 2015. [Google Scholar]
- 21. Hoffman L, et al. . Persons as contexts: evaluating between-person and within-person effects in longitudinal analysis. Research in Human Development. 2009;6(2–3):97–120. [Google Scholar]
- 22. Bolger N, et al. . Intensive Longitudinal Methods: An Introduction to Diary and Experience Sampling Research. New York, NY: Guilford Press; 2013. [Google Scholar]
- 23. Spadola CE, et al. . Evening intake of alcohol, caffeine, and nicotine: night-to-night associations with sleep duration and continuity among African Americans in the Jackson Heart Sleep Study. Sleep. 2019;42(11). doi: 10.1093/sleep/zsz136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baron KG, et al. . Circadian timing and alignment in healthy adults: associations with BMI, body fat, caloric intake and physical activity. Int J Obes (Lond). 2017;41(2):203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patel SR, et al. . Reproducibility of a standardized Actigraphy scoring algorithm for sleep in a US Hispanic/Latino population. Sleep. 2015;38(9):1497–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johannsen DL, et al. . Accuracy of armband monitors for measuring daily energy expenditure in healthy adults. Med Sci Sports Exerc. 2010;42(11):2134–2140. [DOI] [PubMed] [Google Scholar]
- 27. Fruin ML, et al. . Validity of a multi-sensor armband in estimating rest and exercise energy expenditure. Med Sci Sports Exerc. 2004;36(6):1063–1069. [DOI] [PubMed] [Google Scholar]
- 28. Drenowatz C, et al. . Validation of the SenseWear Armband at high intensity exercise. Eur J Appl Physiol. 2011;111(5):883–887. [DOI] [PubMed] [Google Scholar]
- 29. Raudenbush SW, et al. . Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed. Thousand Oaks, CA: Sage Publications, Inc; 2002. [Google Scholar]
- 30. IBM Corp. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp; Released 2017. [Google Scholar]
- 31. Schafer JL, et al. . Missing data: our view of the state of the art. Psychol Methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- 32. Devenney LE, et al. . Sleep after heavy alcohol consumption and physical activity levels during alcohol hangover. J Clin Med. 2019;8(5):752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Schrojenstein Lantman M, et al. . Total sleep time, alcohol consumption, and the duration and severity of alcohol hangover. Nat Sci Sleep. 2017;9:181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van Schrojenstein Lantman M, et al. . Alcohol hangover, sleep quality, and daytime sleepiness. Sleep Vigilance. 2017;1(1):37–41. [Google Scholar]
- 35. Verster JC, et al. . Relationship of alcohol hangover and physical endurance performance: walking the Samaria Gorge. J Clin Med. 2020;9(1):pii:E114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baron KG, et al. . Exercise to improve sleep in insomnia: exploration of the bidirectional effect. J Clin Sleep Med. 2013;9(8):819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thakkar MM, et al. . Alcohol disrupts sleep homeostasis. Alcohol. 2015;49(4):299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon request.