Abstract
Study Objectives
Disrupted daily rhythms are associated with mild cognitive impairment (MCI) and dementia. The specific nature of how rhythms and cognition are related, however, is unknown. We hypothesized characteristics from a nonparametric estimate of circadian rest-activity rhythm patterns would be associated to the development of MCI or dementia.
Methods
Wrist actigraphy from 1232 cognitively healthy, community-dwelling women (mean age 82.6 years) from the Study of Osteoporotic Fractures was used to estimate rest-activity patterns, including intradaily variability (IV), interdaily stability (IS), most active 10-hour period (M10), least active 5-hour period (L5), and relative amplitude (RA). Logistic regression examined associations of these predictors with 5-year incidence of MCI or dementia. Models were adjusted for potential confounders.
Results
Women with earlier sleep/wake times had higher risk of dementia, but not MCI, (early vs. average L5 midpoint: OR, 1.66; 95% CI, 1.08–2.55) as did women with smaller day/night activity differentials (low vs. high RA: OR, 1.96; 95% CI, 1.14–3.35). IV, IS, and M10 were not associated with MCI or dementia.
Conclusion
The timing and difference in day/night amplitude, but not variability of activity, may be useful as predictors of dementia.
Keywords: dementia, mild cognitive impairment, circadian rest-activity rhythms, aging, prospective cohort, nonparametric, relative amplitude, circadian timing, Study of Osteoporotic Fractures
Statement of Significance.
Characteristics describing circadian rest-activity rhythm patterns that were derived using a nonparametric approach were related to 5-year odds of developing dementia in a prospective cohort of cognitively healthy, community-dwelling older women. No associations were found between these characteristics and the development of mild cognitive impairment. This approach does not assume predictable activity patterns, which may not apply to those with impairments associated with aging. These results add to the literature that predominately focus on associations of parametrically derived parameters of circadian rest-activity rhythm patterns with the development of dementia. Future work is needed to confirm these findings in other cohorts. These findings may support the development of interventions to adjust activity patterns that may delay the onset of dementia in the elderly.
Introduction
Alzheimer’s disease, the most common cause of dementia, is known to affect nearly six million Americans as of 2019 [1–3]. Despite the high prevalence of dementia among the elderly, only 16% of older adults receive regular cognitive assessments at their routine doctor visits [1], partly due to the intensive nature of cognitive adjudication, which involves physician review of a patient’s demographics, lifestyle, family, and medical history as well as employment of a “battery” of cognitive tests, which ascertains deficiencies across four neuropsychological domains: global cognition, working memory, verbal memory, and executive function. Neuroimaging examinations are also often required [4, 5]. Identifying potential risk factors may help to detect early-stage cognitive decline in older adults.
Concerted, multidisciplinary research in recent years has refined our understanding of the relationship between brain pathophysiology and age-related changes in patterns of circadian biology. Chronobiology and circadian medicine—fields focused on the study of biological and clinical features that follow roughly 24-hour periods [6]—have helped identify preclinical biomarkers of cognitive decline in older adults. With age, circadian rhythms phase advance, resulting in earlier onset of sleepiness at night and earlier mean morning wake time on average [7]. Rhythm amplitude (the maximum daily activity level) also decreases over time [8]. Disturbances of the sleep-wake cycle are prevalent among those with Alzheimer’s disease [9] and frequently precede the transition from community dwelling to institutional residence [10, 11]. Furthermore, nighttime restlessness, poor sleep, and sleep fragmentation are associated with cognitive decline [12–14], dementia [15], and mortality [16] in the elderly.
We previously examined circadian rest-activity rhythm (RAR) patterns derived from activity data captured with wrist actigraphy in relation to cognitive decline, including the development of mild cognitive impairment (MCI) and dementia [17], in older women. Those with weaker, less robust, or delayed rhythms had significantly higher 5-year odds of MCI or dementia. However, the parametric approach we used to estimate RAR patterns invokes the extended cosine model, which presumes a modified sinusoidal “shape” to the 24-hour activity rhythm, or that resembling a squared-off cosine wave [18–20]. This assumption of a predictable pattern is useful for those with robust activity patterns, but this shape may not apply to those with physical impairments, such as those associated with aging. Here, we utilized a nonparametric, “shape-naïve” approach [21] to estimate circadian rest-activity rhythm patterns from activity data gathered with wrist actigraphy, and examined characteristics describing these patterns in relation to 5-year odds of developing MCI or dementia in a prospective cohort of cognitively healthy, community-dwelling older women.
Methods
Participants
Women were participants in the Study of Osteoporotic Fractures (SOF), a longitudinal study of 10,366 community-dwelling women age 65 and older, recruited from four clinic sites in Baltimore, MD; Minneapolis, MN; Portland, OR; and the Monongahela Valley near Pittsburgh, PA. Participant recruitment is described elsewhere [22]. Women were excluded if they had a bilateral hip replacement or were unable to walk without assistance. The SOF baseline visit was conducted from 1986 to 1988, when 9704 white women were recruited [23]. The initial study population consisted of white women because of their high prevalence of hip fractures, but from 1997 to 1998, 662 African American women were separately recruited [24]. Participants were re-contacted every 2–5 years for in-person visits. The institutional review board on human research at each institution approved the study, and all participating women provided written informed consent.
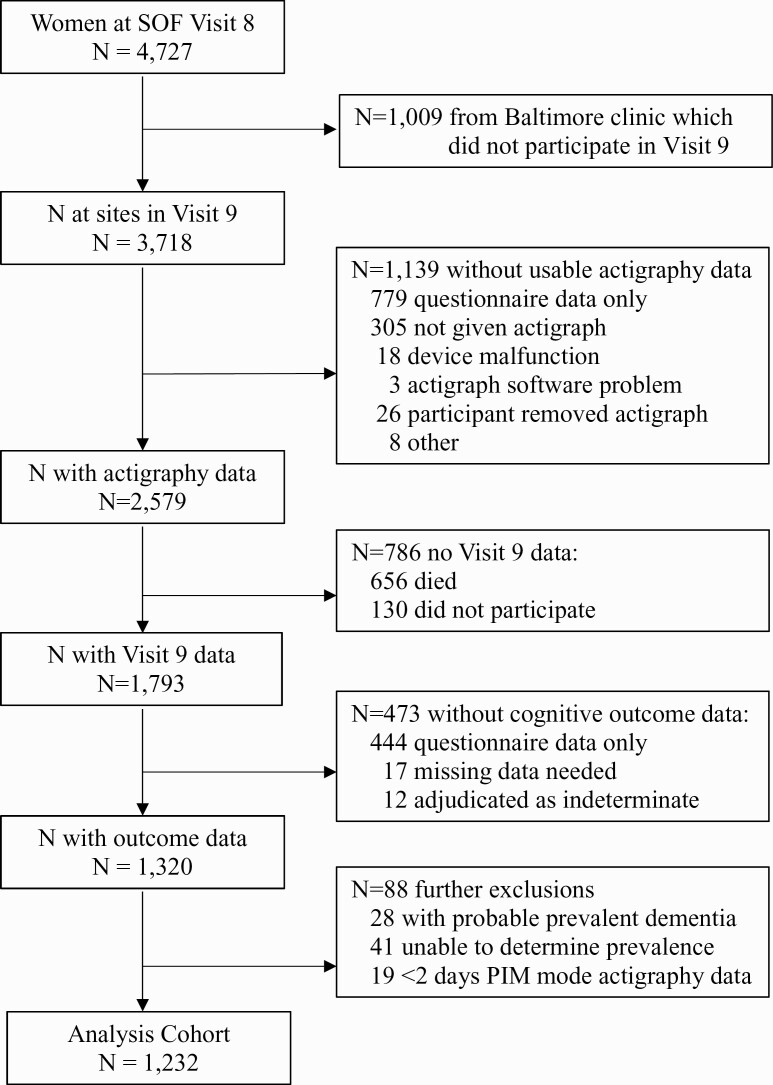
For this analysis, we defined the baseline as the eighth SOF visit that occurred from 2002 to 2004 (Visit 8) (Figure 1). MCI and dementia outcomes were identified at the ninth SOF visit that occurred approximately 5 years later from 2006 to 2008 (Visit 9) in three of the four clinic sites (The Baltimore, MD was not included in Visit 9). Participants included had activity data collected via wrist actigraphy at Visit 8 and clinical cognitive status adjudicated by expert panel at Visit 9. Three thousand and seven hundred eighteen women were still participating in the longitudinal study at Visit 8 across the three clinic sites with data at Visit 9. Across these three sites, 2579 women had actigraphy data collected at Visit 8, and 1793 participated in Visit 9. 1320 women completed the expanded neuropsychological cognitive assessment at Visit 9. We excluded 28 women with probable cognitive impairment at Visit 8 identified from self-reported dementia diagnosis, dementia medication use, and/or a Mini-Mental State Examination (MMSE) score < 24 [25] and 41 women who had missing data to determine probable cognitive impairment. Of the remaining 1245, we also excluded 19 women with fewer than two days of actigraphy data from the Proportional Integration Mode of activity collection. As such, our analysis cohort comprised 1232 women who had at least 2 days of activity data successfully collected at Visit 8, cognitive status adjudicated at Visit 9, and no probable cognitive impairment at baseline.
Figure 1.
Progression of participants.
Actigraphy
Activity data were collected using wrist actigraphs (Sleep-Watch-O, Ambulatory Monitoring, Inc., Ardsley, NY) worn on participants’ nondominant wrists. Participants were instructed to wear the actigraph for a minimum of three consecutive 24-hour periods (i.e., ≥72 hours), although 110 (8.9%) had fewer (mean 85.8 hours). Movement is measured by a piezoelectric linear accelerometer, which generates a voltage each instance the actigraph is moved [26]. These voltages are gathered continuously and summarized over 1-minute intervals, providing 1440 distinct time points per day. The accelerometer is optimized for highly sensitive sleep-wake inference from wrist activity, which has been previously validated [27, 28]. Circadian imputations derived from wrist actigraphy (e.g., sleep/wake predictions and acrophase) show strong concordance with measures derived from reference standards like polysomnography, urinary melatonin, and core body temperature [29–33]. Actigraphy has also been shown to effectively assess activity levels [34–36]. Actigraphy data were analyzed using ActionW-2 software (Ambulatory Monitoring, Inc., Ardsley, NY), which derived estimates of total sleep time and sleep efficiency (the proportion of time in bed that was spent asleep; a measure of sleep fragmentation) [37]. Additional measures obtained from actigraphy were parameters related to sleep timing (time to bed, sleep onset, sleep offset), duration of the main in-bed interval, time spent napping, number of long (>5 min) sleep episodes outside of the main in-bed interval and average activity level outside the main in-bed interval.
An approach developed by van Someren and colleagues [21] was utilized to estimate the following nonparametric variables from activity data: intradaily variability (IV), a measure of the fragmentation of the 24-hour activity rhythm, reflecting the frequency and extent of transitions between periods of rest and activity on an hourly basis (range 0–2: IV≈0 for a perfect sine wave, IV≈2 for Gaussian noise; higher IV indicating more fragmented rhythms); interdaily stability (IS), a measure reflecting how closely the 24-hour activity rhythm synchronizes to the 24-hour light-dark cycle and other environmental cues that regulate the biological clock (range 0–1: IS≈0 for Gaussian noise, IS≈1 for perfect stability; higher IS indicating more stable rhythms); the average activity level of the most active 10-hour period (M10), a measure reflecting how active the wake periods are (measured in arbitrary units of activity [counts/min]), and the M10 midpoint, which indicates whether a person is more active earlier or later in the day; the average activity level of the least active 5-hour period (L5), a measure reflecting activity during periods of rest (measured in arbitrary units of activity [counts/min]), as well as the L5 midpoint, indicating whether inactivity (likely sleep) is earlier or later in the day; and relative amplitude (RA), the difference in activity between M10 and L5 in the average 24-hour pattern, normalized by their sum (; higher RA reflecting relatively lower activity during the night and greater activity when awake).
IV, IS, RA, and average activity in M10 and L5 were examined based on quartile distributions. Previous studies have reported U-shaped associations between acrophase and health conditions such as dementia [17]. Therefore, we examined predictors describing sleep timing, the midpoints of M10 and L5, in three categories based on midpoints more than one standard deviation (SD) above and below the respective means for the study population.
Adjudication of cognitive status
Cognitive status was determined in a two-step process that is described elsewhere [38]. First, women were screened for five criteria (based on cognitive test scores, self-report of dementia diagnosis, or nursing home residence) that could indicate possible cognitive impairment. Those who did not meet any of the criteria for possible cognitive impairment were considered to have normal cognitive status. Those who did meet one or more criteria had an assessment of cognitive status performed by a panel of clinical experts, which included a neurologist, two neuropsychologists, and a geropsychologist. Information used for assessment included the Visit 9 neuropsychological battery scores, prior cognitive test scores, demographics, medical history, medications, depression symptoms, and functional status. Dementia diagnoses were based on criteria from the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), and likely dementia etiology (vascular dementia, Alzheimer’s disease, dementia due to multiple etiologies, or other) was also determined. MCI was diagnosed using a modified Petersen Criteria, which requires generally intact functional status and cognitive impairment that is insufficient to be dementia. Those who initially screened positive for possible impairment at Visit 9 but did not meet diagnostic criteria for MCI or dementia were classified as having normal cognitive status.
Other measurements
Additional data were collected at participants’ clinic or home visits at Visit 8. For this analysis, data on covariates from the following three categories were collected, measured, or estimated: demographics; medical history, including comorbid conditions and current medications; and lifestyle.
Demographic variables included age in years; race (white or African American); and years of education. Body mass index (BMI) in kg/m2 was calculated based on measurement of participants’ body weight and height by clinic site staff. Medical history variables were all binary and included depression; antidepressant use; benzodiazepine use; sleep medication use; self-reported health status (good or fair/poor); self-reported history of hypertension; and a composite medical history variable indicating self-report of prior physician diagnosis of one or more of the following: chronic obstructive pulmonary disease (COPD); diabetes; stroke; cardiovascular disease (including myocardial infarction, angina, congestive heart failure, or other heart disease); any cancer; and Parkinson’s disease. The Geriatric Depression Scale-Short Form (GDS) was used by clinic site staff to assess depressive symptoms, with a score of six or more symptoms (out of 15 possible) used to define depression [39]. Medication use was ascertained by asking participants to bring all medications (prescription and nonprescription) used in the past 30 days to their clinic visits, and a computerized medication coding dictionary was used to categorize all medications (antidepressant, benzodiazepine, prescription sleep medication, and others) [40].
Lifestyle variables included smoking status (whether the participant currently smokes cigarettes), number of alcoholic drinks consumed per week in the past 30 days, caffeine intake in mg/day, actigraphic sleep efficiency and total sleep time, whether participants walk for exercise, and functional status. Functional status was assessed by collecting information on six instrumental activities of daily living (IADL), which included walking two to three blocks on level ground, climbing up to 10 steps, walking down 10 steps, preparing meals, doing heavy housework, and shopping for groceries or clothing [41, 42].
The MMSE, a test of global cognition [43], and a modified version of Trails B, a test of executive function [44], were administered at both clinic visits (Visits 8 and 9).
Statistical analysis
Participant characteristics are summarized for SOF participants who were included and excluded from our analysis cohort. Similar comparisons were made across clinical cognitive status categories and across quartiles and categories of circadian RAR predictor patterns. Sleep timing variables were compared across category of L5 midpoint. We compared group-specific summary statistics using a t-test or analysis of variance (ANOVA) for normally distributed continuous covariates, a Wilcoxon rank-sum test or Kruskal–Wallis test for skewed continuous covariates, and a chi-squared (χ2) test for categorical covariates.
To determine the relationship between circadian activity rhythms and incident MCI and dementia, we used logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs). For dementia, this model compared participants with dementia to those without dementia (i.e., with either MCI or normal cognitive status); for models with MCI as the outcome, participants with incident dementia at Visit 9 were excluded, and participants with MCI were compared to those with normal status. The minimally adjusted models included clinic site, age, race, and education. To determine covariates for the multivariable models, we identified potential confounders by first considering a list of characteristics thought to be associated with circadian activity rhythms or cognitive function based on biological plausibility or previous studies. The final multivariable model included the following covariates: clinic site, age, race, education, BMI, history of one or more select medical conditions, hypertension, depression, antidepressant use, benzodiazepine use, sleep medication use, current smoking, alcohol consumption, caffeine intake, self-reported health status, and walking for exercise.
We also examined associations using a binomial logistic model adapted from Tranah et al. [17] that further adjusted for participants’ total IADL impairments.
Because associations have been found with sleep fragmentation and cognitive impairment in this cohort [45], sensitivity analyses were performed further adjusting the multivariable model by actigraphic sleep efficiency to examine if associations between activity rhythms and incident MCI or dementia were driven by underlying sleep fragmentation.
In secondary analyses, additional multivariable adjusted models were performed examining the associations of incident cognitive impairment with sleep timing variables (time to bed, sleep onset time, sleep offset time, midpoint of the main in-bed interval, midpoint of sleep interval) to determine if associations seen with L5 or M10 midpoint were also observed with these parameters.
Lastly, those parameters with statistically significant associations to an outcome were included in the same multivariable adjusted model to determine if the associations were independent. Statistical analysis was performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Characteristics of the study population
The average age was 82.6 (±3.3) years. 89.1% of participants were white and 10.9% were African American. The average MMSE score at baseline was 28.5 (±1.4), and the average total sleep time was 6.8 (±1.2) hours.
Compared to the remaining SOF cohort excluded from this analysis (n = 2486), on average, women in the analysis cohort were younger, had more years of education, had higher BMI, had fewer medical conditions overall, were less likely to have concurrent depression or use antidepressants, less likely to take benzodiazepines, consumed more alcohol, were less likely to smoke, were more likely to report walking for exercise, had fewer functional impairments and better self-reported health status, had higher MMSE scores and shorter Trails B completion times, and had greater actigraphic sleep efficiency (Table 1). Similar comparisons across other circadian RAR predictor patterns can be found in Supplemental Tables 1–4. Compared to those participants in our analysis cohort with normal cognitive status, on average, those with incident dementia at Visit 9 were older, had fewer years of education, were more likely to have concurrent depression and use antidepressants, consumed less alcohol, had more functional impairments, and had lower MMSE scores and higher Trails B completion times (Table 2). The later midpoint of L5 was associated in a linear fashion with later sleep onset time, sleep offset time, and the start and midpoint of the main sleep interval (p < 0.001). Both earlier and later times for midpoint of L5 were associated with longer time in bed (p < 0.001), while the time spent napping and average physical activity level were similar (p ≥ 0.10) (Supplemental Table 3).
Table 1.
Characteristics of SOF participants according to analysis cohort inclusion.
| Characteristic | Analysis Cohort* N = 1232 |
Excluded N = 2486 |
P-value |
|---|---|---|---|
| Age, years | 82.6 ± 3.3 | 84.9 ± 4.4 | <0.0001 |
| Median (range) | 82 (70–96) | 84 (70–100) | |
| African American | 134 (10.9) | 229 (9.2) | 0.11 |
| Education, years | 12.9 ± 2.6 | 12.4 ± 2.8 | <0.0001 |
| Median (range) | 12 (1–19) | 12 (0–19) | |
| BMI, kg/m2 | 27.5 ± 4.8 | 26.6 ± 5.2 | <0.0001 |
| Median (range) | 26.9 (17.2–51.7) | 25.9 (14.4–51.4) | |
| Any medical history† | 700 (56.9) | 1543 (65.8) | <0.0001 |
| COPD | 148 (12.0) | 299 (12.7) | 0.53 |
| Diabetes | 118 (9.6) | 304 (13.0) | 0.003 |
| Stroke | 120 (9.7) | 423 (18.0) | <0.0001 |
| Cardiovascular disease‡ | 274 (22.2) | 625 (26.6) | 0.004 |
| Cancer | 279 (22.7) | 482 (20.5) | 0.13 |
| Parkinson’s disease | 3 (0.2) | 48 (2.0) | <0.0001 |
| History of hypertension | 732 (59.4) | 1416 (60.4) | 0.58 |
| Depression (GDS score ≥ 6) | 99 (8.0) | 459 (20.2) | <0.0001 |
| Current antidepressant user | 130 (10.6) | 325 (19.2) | <0.0001 |
| Current benzodiazepine user | 83 (6.7) | 153 (9.0) | 0.02 |
| Current sleep medication user | 14 (1.1) | 12 (0.7) | 0.22 |
| Current smoker | 27 (2.2) | 80 (3.4) | 0.04 |
| Alcoholic consumption, drinks/week | 1.1 ± 2.9 | 0.8 ± 22.2 | <0.0001 |
| Median (range) | 0 (0–42) | 0 (0–24.5) | |
| Caffeine intake, mg/day | 156.2 ± 155.0 | 149.6 ± 153.0 | 0.15 |
| Median (range) | 110 (0–950) | 110 (0–960) | |
| Walks for exercise | 510 (41.8) | 729 (31.4) | <0.0001 |
| IADL impairments (of 6) | 1.1 ± 1.6 | 2.2 ± 2.1 | <0.0001 |
| Median (range) | 0 (0–6) | 2 (0–6) | |
| Self-reported health status, fair/poor/very poor | 238 (19.3) | 875 (35.5) | <0.0001 |
| MMSE score (0–30) | 28.5 ± 1.4 | 27.2 ± 2.46 | <0.0001 |
| Median (range) | 29 (24–30) | 28 (9–30) | |
| Trails B time to complete, seconds | 137.0 ± 63.6 | 189.2 ± 90.7 | <0.0001 |
| Median (range) | 120 (46–421) | 166 (54–421) | |
| Actigraphic sleep efficiency, % | 79.1 ± 10.8 | 75.8 ± 12.7 | <0.0001 |
| Median (range) | 81.9 (9.8–97.1) | 78.4 (5.7–96.0) | |
| Actigraphic total sleep time, minutes | 407.9 ± 69.4 | 408.7 ± 84.9 | 0.80 |
| Median (range) | 414.8 (59.8–618.8) | 413.1 (29.0–721.0) |
SOF, Study of Osteoporotic Fractures; BMI, body mass index; COPD, chronic obstructive pulmonary disease; GDS, Geriatric Depression Scale; IADL, instrumental activities of daily living; MMSE, Mini-Mental State Examination; SD, standard deviation.
All continuous data are mean ± SD; P-values are from a t-test if normally distributed or Wilcoxon rank-sum test if skewed. All categorical data are N (%); P-values are from a χ2 test.
*Participants in the analysis cohort were from three of four SOF clinic sites, had no probable cognitive impairment at Visit 8, had at least 2 days of actigraphy data collected at Visit 8, and had clinical cognitive status successfully adjudicated at Visit 9.
Includes all members of SOF cohort at Visit 8 from three of four clinic sites (OR, MN, PA).
†Any medical history indicates prior physician diagnosis of one or more of the following: COPD, diabetes, stroke, cardiovascular disease, cancer, or Parkinson’s disease.
‡Cardiovascular disease includes myocardial infarction, angina, congestive heart failure, or other heart disease.
Table 2.
Characteristics of the SOF cohort among categories of incident clinical cognitive status.
| Characteristic | Normal N = 767 |
MCI N = 287 |
Dementia N = 178 |
P-value |
|---|---|---|---|---|
| Age, years | 82.3 ± 3.1 | 82.8 ± 3.3 | 83.7 ± 4.0 | <0.0001 |
| Median (range) | 82 (70–92) | 83 (73–91) | 83.5 (73–96) | |
| African American | 74 (9.7) | 36 (12.5) | 24 (13.5) | 0.20 |
| Education, years | 13.1 ± 2.6 | 12.4 ± 2.5 | 12.7 ± 2.6 | 0.0001 |
| Median (range) | 12 (1–19) | 12 (7–19) | 12 (5–19) | |
| BMI, kg/m2 | 27.5 ± 4.8 | 27.6 ± 4.6 | 27.2 ± 5.3 | 0.74 |
| Median (range) | 26.8 (17.4–51.7) | 27.5 (18.8–46.0) | 26.8 (17.2–45.8) | |
| Any medical history* | 436 (56.9) | 158 (55.1) | 106 (59.6) | 0.63 |
| COPD | 89 (11.6) | 36 (12.5) | 23 (12.9) | 0.84 |
| Diabetes | 69 (9.0) | 29 (10.1) | 20 (11.2) | 0.62 |
| Stroke | 66 (8.6) | 30 (10.5) | 24 (13.5) | 0.13 |
| Cardiovascular disease† | 172 (22.4) | 60 (20.9) | 42 (23.6) | 0.78 |
| Cancer | 187 (24.4) | 54 (18.8) | 38 (21.3) | 0.14 |
| Parkinson’s disease | 1 (0.1) | 1 (0.3) | 1 (0.6) | 0.53 |
| History of hypertension | 455 (59.3) | 163 (56.8) | 114 (64.0) | 0.30 |
| Depression (GDS score ≥ 6) | 46 (6.0) | 28 (9.8) | 25 (14.0) | 0.0009 |
| Current antidepressant user | 58 (7.6) | 37 (12.9) | 35 (19.7) | <0.0001 |
| Current benzodiazepine user | 51 (6.6) | 17 (5.9) | 15 (8.4) | 0.57 |
| Current sleep medication user | 9 (1.2) | 1 (0.3) | 4 (2.2) | 0.17 |
| Current smoker | 14 (1.8) | 10 (3.5) | 3 (1.7) | 0.23 |
| Alcohol consumption, drinks/week | 1.2 ± 3.1 | 1.2 ± 2.8 | 0.5 ± 1.4 | <0.0001 |
| Median (range) | 0 (0–42) | 0 (0–17.5) | 0 (0–7) | |
| Caffeine intake, mg/day | 159.9 ± 156.5 | 158.0 ± 157.6 | 137.6 ± 143.5 | 0.23 |
| Median (range) | 140 (0–950) | 110 (0–815) | 95 (0–760) | |
| Walks for exercise | 330 (43.5) | 119 (41.8) | 61 (34.9) | 0.11 |
| IADL impairments (of 6) | 1.0 ± 1.5 | 1.2 ± 1.6 | 1.6 ± 1.8 | <0.0001 |
| Median (range) | 0 (0–6) | 0 (0–6) | 1 (0–6) | |
| Self-reported health status, fair/poor/very poor | 136 (17.7) | 61 (21.3) | 41 (23.0) | 0.17 |
| MMSE score (0–30) | 28.7 ± 1.2 | 28.3 ± 1.4 | 27.7 ± 1.5 | <0.0001 |
| Median (range) | 29 (24–30) | 29 (24–30) | 28 (24–30) | |
| Trails B time to complete, seconds | 123.9 ± 53.9 | 157.2 ± 69.6 | 164.0 ± 76.9 | <0.0001 |
| Median (range) | 111 (46–421) | 138 (55–421) | 145.5 (66 – 421) | |
| Actigraphic sleep efficiency, % | 79.6 ± 10.6 | 78.5 ± 11.3 | 78.0 ± 10.5 | 0.10 |
| Median (range) | 82.2 (30.5–96.8) | 81.7 (9.8–95.2) | 80.4 (34.8–97.1) | |
| Actigraphic total sleep time, minutes | 407.7 ± 69.4 | 407.0 ± 70.7 | 410.0 ± 67.2 | 0.90 |
| Median (range) | 414.5 (142.7–618.8) | 415 (59.8–564.8) | 415 (199–576.5) | |
| Time wearing actigraph, days | 3.6 ± 0.6 | 3.6 ± 0.6 | 3.5 ± 0.7 | 0.17 |
| Median (range) | 3.5 (2.3–7.3) | 3.5 (2.3–6.6) | 3.4 (2.3–6.4) |
SOF, Study of Osteoporotic Fractures; MCI, mild cognitive impairment; BMI, body mass index; COPD, chronic obstructive pulmonary disease; GDS, Geriatric Depression Scale; IADL, instrumental activities of daily living; MMSE, Mini-Mental State Examination; SD, standard deviation.
All continuous data are mean ± SD; P-values are from an ANOVA if normally distributed or Kruskal-Wallis test if skewed. All categorical data are N (%); P-values are from a χ2 test for homogeneity.
*Any medical history indicates prior physician diagnosis of one or more of the following: COPD, diabetes, stroke, cardiovascular disease, cancer, or Parkinson’s disease.
†Cardiovascular disease includes myocardial infarction, angina, congestive heart failure, or other heart disease.
Rest-activity rhythms and incident dementia
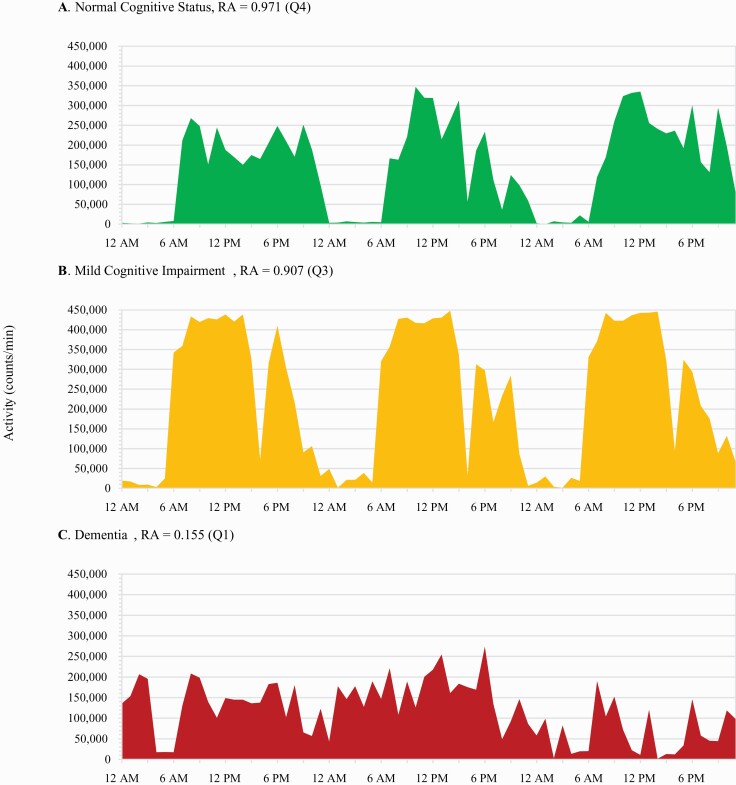
After a mean 4.9 (±0.6) years of follow-up, there were 178 cases of dementia (14%) identified. Women in the lowest quartile of relative amplitude (n = 296) had nearly twice the odds of developing dementia compared to those (n = 297) in the highest quartile (Multivariable adjusted OR, 1.96; 95% CI, 1.14–3.35; Table 3). A significant linear trend across quartiles was also observed, such that a linear increase in odds of developing dementia was found with corresponding decreases across quartiles of RA (P value for trend = 0.03). Results were largely unchanged after further adjusting for actigraphic sleep efficiency in sensitivity analyses, and in all three lower quartiles of RA, estimated odds of dementia increased slightly (Table 3). RA and rest-activity levels for three SOF participants are illustrated in Figure 2.
Table 3.
Associations between rest-activity rhythm patterns and incident dementia across logistic models.
| Pattern | N Events | Minimal* | Multivariable† | Sleep‡ | Prior§ |
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | |||||
| Intradaily variability, 0–2 | 177 | ||||
| Q1: 0.26 to <0.51 | 42 | Ref | Ref | Ref | Ref |
| Q2: 0.51 to <0.63 | 40 | 0.92 (0.58, 1.49) | 0.96 (0.59, 1.56) | 0.95 (0.58, 1.55) | 0.94 (0.57, 1.53) |
| Q3: 0.63 to <0.77 | 35 | 0.76 (0.47, 1.24) | 0.79 (0.48, 1.31) | 0.78 (0.47, 1.30) | 0.75 (0.45, 1.26) |
| Q4: 0.77 to 1.89 | 60 | 1.35 (0.87, 2.10) | 1.17 (0.72, 1.89) | 1.15 (0.71, 1.89) | 1.12 (0.69, 1.82) |
| P-value for linear trend | 0.25 | 0.65 | 0.72 | 0.80 | |
| Interdaily stability, 0–1 | 177 | ||||
| Q1: 0.20 to <0.73 | 50 | 0.99 (0.64, 1.55) | 0.95 (0.59, 1.53) | 0.92 (0.57, 1.48) | 0.93 (0.57, 1.49) |
| Q2: 0.73 to <0.80 | 37 | 0.71 (0.44, 1.13) | 0.72 (0.44, 1.18) | 0.71 (0.43, 1.16) | 0.68 (0.42, 1.13) |
| Q3: 0.80 to <0.85 | 43 | 0.99 (0.67, 1.45) | 0.92 (0.57, 1.48) | 0.91 (0.56, 1.46) | 0.90 (0.56, 1.44) |
| Q4: 0.85 to 0.98 | 47 | Ref | Ref | Ref | Ref |
| P-value for linear trend | 0.76 | 0.64 | 0.53 | 0.55 | |
| L5, activity counts/min | 178 | ||||
| Q1: 41 to <185 | 32 | Ref | Ref | Ref | Ref |
| Q2: 185 to <263 | 41 | 1.38 (0.84, 2.27) | 1.52 (0.91, 2.53) | 1.50 (0.89, 2.53) | 1.48 (0.89, 2.48) |
| Q3: 263 to <384 | 55 | 1.86 (1.16, 3.00) | 1.88 (1.15, 3.08) | 1.84 (1.09, 3.12) | 1.82 (1.11, 2.98) |
| Q4: 384 to 2027 | 50 | 1.58 (0.97, 2.57) | 1.50 (0.90, 2.51) | 1.43 (0.72, 2.84) | 1.43 (0.85, 2.39) |
| P-value for linear trend | 0.03 | 0.08 | 0.13 | 0.13 | |
| L5 midpoint | 178 | ||||
| < 1:42 AM | 38 | 1.67 (1.10, 2.53) | 1.66 (1.08, 2.55) | 1.66 (1.08, 2.55) | 1.63 (1.06, 2.51) |
| 1:42 AM to 4:39 AM | 106 | Ref | Ref | Ref | Ref |
| > 4:39 AM | 34 | 1.36 (0.88, 2.10) | 1.32 (0.84, 2.09) | 1.31 (0.83, 2.06) | 1.29 (0.82, 2.04) |
| M10, activity counts/min | 175 | ||||
| Q1: 1633 to <3534 | 58 | 1.28 (0.82, 1.99) | 1.10 (0.67, 1.79) | 1.11 (0.68, 1.81) | 1.05 (0.64, 1.71) |
| Q2: 3534 to <4077 | 45 | 0.99 (0.62, 1.58) | 0.91 (0.56, 1.49) | 0.92 (0.56, 1.50) | 0.87 (0.53, 1.43) |
| Q3: 4077 to <4612 | 30 | 0.66 (0.40, 1.09) | 0.66 (0.39, 1.12) | 0.67 (0.40, 1.12) | 0.66 (0.39, 1.11) |
| Q4: 4612 to 7578 | 42 | Ref | Ref | Ref | Ref |
| P-value for linear trend | 0.11 | 0.45 | 0.43 | 0.61 | |
| M10 midpoint | 175 | ||||
| < 11:59 AM | 19 | 0.83 (0.49, 1.40) | 0.88 (0.51, 1.50) | 0.89 (0.52, 1.52) | 0.88 (0.51, 1.51) |
| 11:59 AM to 3:17 PM | 131 | Ref | Ref | Ref | Ref |
| > 3:17 PM | 25 | 0.93 (0.58, 1.50) | 0.95 (0.58, 1.55) | 0.93 (0.57, 1.53) | 0.94 (0.57, 1.53) |
| Relative amplitude, 0–1 | 175 | ||||
| Q1: 0.16 to <0.82 | 57 | 2.06 (1.25, 3.40) | 1.96 (1.14, 3.35) | 2.15 (1.11, 4.15) | 1.84 (1.07, 3.16) |
| Q2: 0.82 to <0.88 | 47 | 1.82 (1.09, 3.02) | 1.78 (1.05, 3.03) | 1.86 (1.06, 3.24) | 1.71 (1.00, 2.93) |
| Q3: 0.88 to <0.92 | 44 | 1.69 (1.01, 2.83) | 1.82 (1.07, 3.10) | 1.86 (1.09, 3.18) | 1.79 (1.05, 3.04) |
| Q4: 0.92 to 0.98 | 27 | Ref | Ref | Ref | Ref |
| P-value for linear trend | <.01 | 0.03 | 0.03 | 0.06 |
CI, confidence interval; L5, least active 5-hour period; M10, most active 10-hour period; BMI, body mass index; IADL, instrumental activities of daily living.
*Minimal: Adjusted for clinic site, age, race, and education.
†Multivariable: Adjusted for Minimal + BMI, history of any of six select medical conditions, history of hypertension, depression, antidepressant use, benzodiazepine use, sleep medication use, smoking, alcohol use, caffeine intake, walking for exercise, and self-reported health status.
‡Sleep: Adjusted for Multivariable + actigraphic sleep efficiency.
§Prior: Adjusted for Multivariable + number of IADL impairments. Adapted from Tranah et al. [17]
P-values are from tests for linear trends across quartiles. The associations between L5 and M10 midpoint and the outcome is hypothesized to be nonlinear, so the test for linear trend was not performed.; Bold values indicate significance at P < 0.05.
Figure 2.
Relative amplitude and cognitive status. Footnote: Activity levels and average relative amplitude (RA) over 72 hours for three individuals across clinical cognitive status categories.
Additionally, women with an earlier than average L5 midpoint (< 1:42 AM; n = 200) experienced a 66% increase in odds of developing dementia compared to those (n = 833) in the mean midpoint range of 1:42–4:39 AM (OR, 1.66; 95% CI, 1.08–2.55). This significant finding was consistent across all models.
In the minimally adjusted model, evidence for a linear increase in odds of developing dementia was found with associated increases in average activity in L5 (p value for trend = 0.03), although evidence for this trend attenuated slightly upon multivariable adjustment (p value for trend = 0.08; Table 3).
Across models, IV, IS, average activity in M10, and M10 midpoint were not associated with elevated 5-year odds of developing dementia in our cohort.
Results were largely similar when including both quartile or RA and category of L5 midpoint in the same multivariable adjusted model. (L5 midpoint < 1:42 AM vs. mean midpoint range of 1:42–4:39 AM: OR, 1.57; 95% CI, 1.01–2.45; Quartile 1 vs. Quartile 4 of RA: OR, 1.92; 95% CI, 1.12–3.29).
Rest-activity rhythms and incident MCI
After a mean 4.9 years (±0.6) of follow-up, there were 287 cases of MCI identified (23%). There were no significant associations between rest-activity rhythm patterns and 5-year odds of MCI. The only RAR predictor that was suggestive of elevated odds of developing MCI or differences across quartiles was average activity in M10. Women in the lower two quartiles of average activity in M10 experienced an increase in odds of developing MCI of approximately 50% compared to those in the highest quartile (Multivariable adjusted OR: Q1 vs. Q4: 1.49; 95% CI, 0.95–2.32; Table 4); similarly, increases in average activity in M10 were suggestive of decreasing odds of developing MCI (p value for trend = 0.05). Across models, IV, IS, RA, average activity in L5, L5 midpoint, and M10 midpoint were not associated with elevated 5-year odds of developing MCI in our cohort.
Table 4.
Associations between rest-activity rhythm patterns and incident MCI across logistic models.
| Pattern | N Events | Minimal* | Multivariable† | Sleep‡ | Prior§ |
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | |||||
| Intradaily variability, 0–2 | 285 | ||||
| Q1: 0.26 to <0.51 | 68 | Ref | Ref | Ref | Ref |
| Q2: 0.51 to <0.63 | 67 | 1.00 (0.67, 1.48) | 1.00 (0.67, 1.51) | 1.00 (0.67, 1.51) | 1.00 (0.66, 1.50) |
| Q3: 0.63 to <0.77 | 72 | 1.05 (0.71, 1.56) | 1.07 (0.71, 1.62) | 1.07 (0.71, 1.62) | 1.06 (0.70, 1.61) |
| Q4: 0.77 to 1.89 | 78 | 1.39 (0.93, 2.06) | 1.36 (0.89, 2.07) | 1.34 (0.88, 2.05) | 1.35 (0.89, 2.06) |
| P-value for linear trend | 0.11 | 0.15 | 0.17 | 0.16 | |
| Interdaily stability, 0–1 | 285 | ||||
| Q1: 0.20 to <0.73 | 73 | 1.30 (0.86, 1.94) | 1.27 (0.83, 1.94) | 1.25 (0.81, 1.91) | 1.27 (0.83, 1.94) |
| Q2: 0.73 to <0.80 | 76 | 1.24 (0.83, 1.85) | 1.27 (0.84, 1.91) | 1.25 (0.83, 1.89) | 1.26 (0.84, 1.90) |
| Q3: 0.80 to <0.85 | 75 | 1.29 (0.86, 1.92) | 1.39 (0.80, 2.05) | 1.36 (0.90, 2.04) | 1.36 (0.91, 2.05) |
| Q4: 0.85 to 0.98 | 61 | Ref | Ref | Ref | Ref |
| P-value for linear trend | 0.26 | 0.34 | 0.40 | 0.35 | |
| L5, activity counts/min | 287 | ||||
| Q1: 41 to <185 | 72 | Ref | Ref | Ref | Ref |
| Q2: 185 to <263 | 79 | 1.25 (0.85, 1.83) | 1.27 (0.86, 1.87) | 1.20 (0.81, 1.79) | 1.26 (0.85, 1.86) |
| Q3: 263 to <384 | 63 | 0.94 (0.63, 1.40) | 0.93 (0.62, 1.40) | 0.84 (0.54, 1.30) | 0.92 (0.61, 1.39) |
| Q4: 384 to 2027 | 73 | 1.08 (0.73, 1.60) | 1.07 (0.71, 1.61) | 0.83 (0.47, 1.46) | 1.05 (0.70, 1.58) |
| P-value for linear trend | 0.96 | 0.9 | 0.32 | 0.84 | |
| L5 midpoint | 287 | ||||
| < 1:42 AM | 43 | 0.94 (0.64, 1.39) | 0.92 (0.62, 1.37) | 0.92 (0.62, 1.37) | 0.92 (0.62, 1.37) |
| 1:42 AM to 4:39 AM | 203 | Ref | Ref | Ref | Ref |
| > 4:39 AM | 41 | 0.90 (0.60, 1.34) | 0.88 (0.58, 1.34) | 0.87 (0.57, 1.31) | 0.88 (0.58, 1.33) |
| M10, activity counts/min | 275 | ||||
| Q1: 1633 to <3534 | 73 | 1.41 (0.93, 2.13) | 1.49 (0.95, 2.32) | 1.50 (0.96, 2.35) | 1.48 (0.94, 2.31) |
| Q2: 3534 to <4077 | 74 | 1.42 (0.94, 2.14) | 1.48 (0.96, 2.26) | 1.49 (0.97, 2.29) | 1.47 (0.95, 2.26) |
| Q3: 4077 to <4612 | 70 | 1.15 (0.76, 1.73) | 1.20 (0.79, 1.82) | 1.20 (0.79, 1.83) | 1.20 (0.79, 1.82) |
| Q4: 4612 to 7578 | 58 | Ref | Ref | Ref | Ref |
| P-value for linear trend | 0.06 | 0.05 | 0.05 | 0.06 | |
| M10 midpoint | 275 | ||||
| < 11:59 AM | 41 | 1.24 (0.82, 1.88) | 1.29 (0.85, 1.97) | 1.31 (0.86, 1.99) | 1.30 (0.85, 1.98) |
| 11:59 AM to 3:17 PM | 193 | Ref | Ref | Ref | Ref |
| > 3:17 PM | 41 | 1.11 (0.74, 1.68) | 1.00 (0.65, 1.52) | 0.98 (0.64, 1.49) | 1.00 (0.65, 1.52) |
| Relative amplitude, 0–1 | 275 | ||||
| Q1: 0.16 to <0.82 | 70 | 1.11 (0.74, 1.66) | 1.12 (0.73, 1.72) | 0.92 (0.52, 1.62) | 1.11 (0.72, 1.70) |
| Q2: 0.82 to <0.88 | 63 | 0.98 (0.65, 1.47) | 0.96 (0.63, 1.46) | 0.88 (0.57, 1.38) | 0.95 (0.63, 1.45) |
| Q3: 0.88 to <0.92 | 73 | 1.23 (0.83, 1.82) | 1.24 (0.83, 1.86) | 1.19 (0.79, 1.79) | 1.24 (0.83, 1.85) |
| Q4: 0.92 to 0.98 | 69 | Ref | Ref | Ref | Ref |
| P-value for linear trend | 0.88 | 0.88 | 0.55 | 0.95 |
CI, confidence interval; L5, least active 5-hour period; M10, most active 10-hour period; MCI, mild cognitive impairment; BMI, body mass index; IADL, instrumental activities of daily living.
*Minimal: Adjusted for clinic site, age, race, and education.
†Multivariable: Adjusted for Minimal + BMI, history of any of six select medical conditions, history of hypertension, depression, antidepressant use, benzodiazepine use, sleep medication use, smoking, alcohol use, caffeine intake, walking for exercise, and self-reported health status.
‡Sleep: Adjusted for Multivariable + actigraphic sleep efficiency.
§Prior: Adjusted for Multivariable + number of IADL impairments. Adapted from Tranah et al. [17].
NOTE. P-values are from tests for trends across quartiles. The associations between L5 and M10 midpoint and the outcome is hypothesized to be nonlinear, so the test for linear trend was not performed.; Bold values indicate significance at P < 0.05.
Secondary analyses of sleep timing and incident MCI or dementia
None of the multivariable adjusted models examining the associations of the sleep timing parameters with incident MCI or dementia were significant (results not shown). Parameters included time to bed, time of sleep onset, time of sleep offset, midpoint of the main in-bed interval and midpoint of the sleep interval (onset to offset). Sleep timing parameters were examined as continuous variables to allow for potential linear relationships with the outcomes and also as categorical to allow for a u-shaped relationship with the outcomes (one SD above and below the mean value, compared to the middle category).
Discussion
We found evidence that among 1232 cognitively healthy older women, variations in nonparametric indices of rest-activity timing and relative amplitude were associated with an elevated 5-year risk of developing dementia but not MCI. Earlier timing of low activity (L5 midpoint) was found to be strongly associated with increased odds of dementia 5 years later, independent of numerous potential confounders including demographic characteristics, self-report of walking for exercise, medical history, smoking status, alcohol use, caffeine intake, and actigraphic sleep efficiency. While neither the amount of activity during the night (L5) nor the amount of activity during the day (M10) were associated with dementia, lower RA was associated with greater risk of dementia, indicating that the ratio of activity in the day and night appears to be more prognostic for dementia than the absolute amount of activity during either time point. IV, IS, and M10 midpoint were not associated with increased risk of dementia, and none of the nonparametric variables was associated with increased risk of MCI.
In previous work in this same SOF cohort, later timing of parametrically derived acrophase was related to the development of dementia [17], yet here earlier timing of L5 was associated with this outcome. Additionally, both the lower category of acrophase and the higher category of L5 midpoint had an elevated risk of development of dementia, however, results did not reach statistical significance. While acrophase and L5 timing are correlated (rho = 0.55) they are not aspects of the same measure. Acrophase is primarily driven by the distribution of the wake (active) data and is dependent on the amplitude of activity data. L5 is mainly driven by when the inactive period occurs irrespective (mostly) of the amplitude of the movement data. These current results add nuance to the past parametric analyses, in which deviations in daily amplitude and acrophase (timing of daily peak activity) were found to be associated with increased risk of both MCI and dementia [17]. Thus, the possibility remains that MCI, representing a transitional stage between healthy aging and dementia, is better predicted by parametric analysis, specifically by examining deviations in amplitude and acrophase from the expected sinusoidal pattern, and that nonparametric analysis, while robust enough to predict 5-year odds of substantial cognitive impairment (i.e., dementia), is not sensitive enough to be utilized in predicting the smaller transition from healthy cognition to MCI or early stage cognitive impairment in older, community-dwelling women more generally. Specifically, acrophase appears to be a strong predictor of MCI in this population, and parametrically derived acrophase and L5 midpoint may play important roles in the larger transition to dementia by reflecting overall endurance, or the ability to sustain periods of rest and activity. Compared to the middle category of L5 timing, women in the low and high category on average had more interruptions of active periods by bouts of sleep. They had about 20–40 min shorter length of time outside the main sleep interval, but during that time had more long sleep episodes and a higher amount of time spent napping, while average activity level was similar across category of L5 midpoint. This supports the theory that those in the extremes of L5 timing have less sustained periods of rest and activity.
The recent work of Li and colleagues reported an association of more suppressed (parametrically derived amplitude) and greater fragmented (IV) daily activity rhythms with higher risk of development of dementia, but no associations were observed between the parameters examined and development of MCI [46]. While this work did not examine the association of RA, L5, M10 or their placement, in general the results are in agreement of an association of parameters measuring circadian disruption with development of incident dementia but not incident MCI. A study of the relationship of white matter microarchitecture with these nonparametric rest–activity rhythm parameters found that RA and L5 timing, but not IV or IS, were associated with white matter fiber density and fiber cross-section in a number of major white matter pathways, which is in line with our findings [47].
In contrast, work examining the association of nonparametric rest-activity rhythm parameters (IV, IS, and L5 onset) with incident dementia in the Rotterdam Study did not observe any increased risk with L5 timing [48]. This difference in results may be due to the use of L5 timing as a continuous variable, where our analyses examined a u-shaped association of L5 timing to development of dementia. Also, the Rotterdam study included both men and women with no presentation of presented results stratified by gender, which makes direct comparisons difficult.
Our study had several strengths, including analysis of a large cohort of mostly community-dwelling older women followed prospectively. The SOF cohort had no inclusion criteria regarding daily activity rhythms, sleep disorders, or particular cognitive function. We adjusted for multiple possible confounders, used validated methods to estimate nonparametric rest-activity rhythm patterns from activity data gathered with wrist actigraphy, and utilized an expansive neuropsychological examination to adjudicate cognitive status. By excluding participants with probable cognitive impairment at Visit 8, we were able to prospectively assess the relationship between rest-activity rhythms and 5-year incidence of MCI and dementia (reflecting the transition from normal to impaired cognitive status). This analysis also had limitations; most notably, our cohort only included older women who were mostly white, so results may not be generalizable to other populations such as men, nonwhite, or younger women. As a result, a concern remains that findings from this study may not be valid when assessing a population of community-dwelling older women that is more racially or socioeconomically diverse. However, in the present study, we controlled for both race and years of education. Participants were instructed to wear their actigraphs for a minimum of three consecutive days, though some only wore it for two days in the current analysis cohort. Typically, five days of activity data is recommended for proper estimation of rest-activity patterns like IV and IS and it is therefore possible that these metrics may not be accurate in the current study. While it would be preferable to perform sensitivity analyses restricted to those women with 5 or more days of data, it was not possible due to small sample size of this subset (n = 56). Participants who agreed to wear the actigraph were also younger on average and somewhat healthier than those who did not. However, we expect that this would have biased our findings toward the null. That said, the possibility of residual confounding cannot be eliminated given the observational design of our study.
This study evaluating the relationship between circadian activity rhythms analyzed nonparametrically and subsequent development of MCI and dementia in participants from a community dwelling setting confirms some findings in prior work [46]. If confirmed in other cohorts, such as older men or women with greater socioeconomic, ethnic, and racial diversity, these findings may support the development of interventions to adjust activity patterns that may delay the onset of dementia in the elderly.
Supplementary Material
Work was performed at California Pacific Medical Center Research Institute.
Financial disclosures
The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, R01 AG027576, R01 AG026720, and R21 AG051380.
Non-financial disclosures
Terri Blackwell and Dr. Stone receive partial salary support from a grant from Merck Sharp & Dohme Corp as well as NIH funding during the conduct of the study.
Dr. Ancoli-Israel is a Consultant for Eisai, Biogen, Idorsia, Merck, Sunovion.
Dr. Leng is supported by NIH award R00AG056598.
References
- 1. Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020;16(3):391–460. [Google Scholar]
- 2. Hebert LE, et al. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barker WW, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16(4):203–212. [DOI] [PubMed] [Google Scholar]
- 4. Snowden JS, et al. The clinical diagnosis of early-onset dementias: diagnostic accuracy and clinicopathological relationships. Brain. 2011;134(Pt 9):2478–2492. [DOI] [PubMed] [Google Scholar]
- 5. Tartaglia MC, et al. Neuroimaging in dementia. Neurotherapeutics. 2011;8(1):82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Halberg F. Chronobiology. Annu Rev Physiol. 1969;31:675–725. [DOI] [PubMed] [Google Scholar]
- 7. Czeisler CA, et al. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340(8825):933–936. [DOI] [PubMed] [Google Scholar]
- 8. Kripke DF, et al. Circadian phase in adults of contrasting ages. Chronobiol Int. 2005;22(4):695–709. [DOI] [PubMed] [Google Scholar]
- 9. Satlin A, et al. Circadian locomotor activity and core-body temperature rhythms in Alzheimer’s disease. Neurobiol Aging. 1995;16(5):765–771. [DOI] [PubMed] [Google Scholar]
- 10. Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16(1):40–81. [DOI] [PubMed] [Google Scholar]
- 11. Van Someren EJ. Circadian and sleep disturbances in the elderly. Exp Gerontol. 2000;35(9–10):1229–1237. [DOI] [PubMed] [Google Scholar]
- 12. Blackwell T, et al. ; Study of Osteoporotic Fractures Group. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61(4):405–410. [DOI] [PubMed] [Google Scholar]
- 13. Spira AP, et al. Actigraphic sleep duration and fragmentation in older women: associations with performance across cognitive domains. Sleep. 2017;40(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeitzer JM, et al. ; Osteoporotic Fractures in Men (MrOS) Study Research Group. Daily patterns of accelerometer activity predict changes in sleep, cognition, and mortality in older men. J Gerontol A Biol Sci Med Sci. 2018;73(5):682–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ancoli-Israel S, et al. Dementia in institutionalized elderly: relation to sleep apnea. J Am Geriatr Soc. 1991;39(3):258–263. [DOI] [PubMed] [Google Scholar]
- 16. Gangwisch JE, et al. Sleep duration associated with mortality in elderly, but not middle-aged, adults in a large US sample. Sleep. 2008;31(8):1087–1096. [PMC free article] [PubMed] [Google Scholar]
- 17. Tranah GJ, et al. ; SOF Research Group. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70(5):722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Refinetti R, et al. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res. 2007;38(4):275–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model. 2014;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marler MR, et al. The sigmoidally transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med. 2006;25(22):3893–3904. [DOI] [PubMed] [Google Scholar]
- 21. Van Someren EJ, et al. Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 1999;16(4):505–518. [DOI] [PubMed] [Google Scholar]
- 22. Cummings SR, et al. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA. 1990;263(5):665–668. [PubMed] [Google Scholar]
- 23. Cummings SR, et al. Risk factors for hip fracture in White Women. N Engl J Med. 1995;332(12):767–774. [DOI] [PubMed] [Google Scholar]
- 24. Vogt MT, et al. Lumbar spine listhesis in older African American women. Spine J. 2003;3(4):255–261. [DOI] [PubMed] [Google Scholar]
- 25. Folstein MF, et al. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40(7):812. [DOI] [PubMed] [Google Scholar]
- 26. Blackwell T, et al. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005;28(12):1599–1605. [DOI] [PubMed] [Google Scholar]
- 27. Ancoli-Israel S, et al. Use of wrist activity for monitoring sleep/wake in demented nursing-home patients. Sleep. 1997;20(1):24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cole RJ, et al. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15(5):461–469. [DOI] [PubMed] [Google Scholar]
- 29. Ancoli-Israel S, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. [DOI] [PubMed] [Google Scholar]
- 30. Pollak CP, et al. How accurately does wrist actigraphy identify the states of sleep and wakefulness? Sleep. 2001;24(8):957–965. [DOI] [PubMed] [Google Scholar]
- 31. Youngstedt SD, et al. Circadian abnormalities in older adults. J Pineal Res. 2001;31(3):264–272. [DOI] [PubMed] [Google Scholar]
- 32. Middleton B, et al. Human circadian rhythms in constant dim light (8 lux) with knowledge of clock time. J Sleep Res. 1996;5(2):69–76. [DOI] [PubMed] [Google Scholar]
- 33. Middleton B, et al. Complex effects of melatonin on human circadian rhythms in constant dim light. J Biol Rhythms. 1997;12(5):467–477. [DOI] [PubMed] [Google Scholar]
- 34. Teicher MH. Actigraphy and motion analysis: new tools for psychiatry. Harv Rev Psychiatry. 1995;3(1):18–35. [DOI] [PubMed] [Google Scholar]
- 35. Brown AC, et al. Actigraphy: a means of assessing circadian patterns in human activity. Chronobiol Int. 1990;7(2):125–133. [DOI] [PubMed] [Google Scholar]
- 36. Müller U, et al. Reduced daytime activity in patients with acquired brain damage and apathy: a study with ambulatory actigraphy. Brain Inj. 2006;20(2):157–160. [DOI] [PubMed] [Google Scholar]
- 37. Ambulatory Monitoring I. Action-W User’s Guide, Version 2.0.
- 38. Yaffe K, et al. Mild cognitive impairment, dementia, and their subtypes in oldest old women. Arch Neurol. 2011;68(5):631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yesavage JA, et al. Geriatric depression scale. Clin Gerontol. 1986;5(1–2):165–173. [Google Scholar]
- 40. Pahor M, et al. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405–411. [DOI] [PubMed] [Google Scholar]
- 41. Fitti JE, et al. The supplement on aging to the 1984 National Health Interview Survey. Vital Health Stat. 1987;21:1–115. [PubMed] [Google Scholar]
- 42. Pincus T, et al. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26(11):1346–1353. [DOI] [PubMed] [Google Scholar]
- 43. Folstein MF, et al. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 44. Reitan RM, et al. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 45. Diem SJ, et al. Measures of sleep-wake patterns and risk of mild cognitive impairment or Dementia in Older Women. Am J Geriatr Psychiatry. 2016;24(3):248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peng Li, et al. Circadian disturbances in Alzheimer’s disease progression: a prospective observational cohort study of community-based older adults. Lancet Healthy Longevity. 2020;1(3):e96–e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Palmer JR, et al. Rest–activity functioning is related to white matter microarchitecture and modifiable risk factors in older adults at-risk for dementia. Sleep. 2021;44(7):zsab007. doi: 10.1093/sleep/zsab007. [DOI] [PubMed] [Google Scholar]
- 48. Lysen TS, et al. Actigraphy-estimated sleep and 24-hour activity rhythms and the risk of dementia. Alzheimers Dement. 2020;16(9):1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.