Abstract
Background:
Nasal allergen challenge (NAC) could be a means to assess indication and/or an outcome of allergen-specific therapies, particularly for perennial allergens. NACs are not commonly conducted in children with asthma and cockroach NACs are not well established.
This study’s objective was to identify a range of German cockroach extract doses that induce nasal symptoms and to assess the safety of cockroach NAC in children with asthma.
Methods:
Ten adults (18–37 years) followed by 25 children (8–14 years) with well-controlled, persistent asthma and cockroach sensitization underwent NAC with diluent followed by up to 8 escalating doses of cockroach extract (0.00381 −11.9 mcg/mL Bla g 1). NAC outcome was determined by Total Nasal Symptom Score (TNSS) and/or sneeze score. Cockroach allergen-induced T cell activation and IL-5 production were measured in peripheral blood mononuclear cells.
Results:
67% (6/9) of adults and 68% (17/25) of children had a positive NAC at a median response dose of 0.120 mcg/mL [IQR 0.0380–0.379 mcg/mL] of Bla g 1. Additionally, three children responded to diluent alone, and did not receive any cockroach extract. Overall, 32% (11/34) were positive with sneezes alone, 15% (5/34) with TNSS alone, and 21% (7/34) with both criteria. At baseline, NAC responders had higher cockroach-specific IgE (p=0.03), lower cockroach-specific IgG/IgE ratios (children, p=0.002), and increased cockroach-specific IL-5-producing T lymphocytes (p=0.045). The NAC was well tolerated.
Conclusion:
We report the methodology of NAC development for children with persistent asthma and cockroach sensitization. This NAC could be considered a tool to confirm clinically relevant sensitization and to assess responses in therapeutic studies.
Keywords: nasal allergen challenge, children, asthma, inner-city, cockroach allergy, allergic rhinitis
INTRODUCTION
Confirming clinical allergy to a perennial allergen or the efficacy of immunotherapy can be difficult because exposure varies greatly, no clear seasonality of exposure exists, and other allergens can confound the relationship between exposure and clinical manifestations. Nasal allergen challenge (NAC), an established test in the research setting, may offer an objective assessment of nasal airway allergen responsiveness.1,2 NAC can also be used to evaluate the pathophysiology of allergic reactions in a readily accessible part of the respiratory tract.1,2
Cockroach sensitization and exposure have been established as a leading risk factor for morbidity in children with asthma living in low-income, US urban communities, and many other parts of the world.3–8 Environmental approaches, eradication and avoidance, have been the focus for ameliorating the effects of cockroach exposure. However, these approaches may be unrealistic in the environment of a lower socioeconomic urban population.9 Allergen immunotherapy with German cockroach extract is a potential alternative for treating cockroach allergy.
Three studies examining NAC with cockroach allergen have been reported, but did not provide clear data on measures of responsiveness, information on standardization of the procedure, or adequate safety and dosing information in children with asthma.10–12 We conducted a study first in adults, then children, to develop a cockroach NAC protocol and assess its safety in children with asthma. Additionally, we conducted an initial evaluation of the T-cell epitope repertoire against cockroach allergens and we tested for NAC-induced changes in T cell responsiveness to cockroach in order to lay the groundwork for the evaluation of immunologic changes seen with this type of NAC.
METHODS
Study Design
The Cockroach Nasal Allergen Challenge (CoNAC) study was a multi-center, open label study to determine the feasibility and safety of German cockroach NAC in cockroach sensitive adults (Stage 1, n=10) and children ages 8–14 (Stage 2, n=25) with persistent, well-controlled asthma (history of doctor-diagnosed asthma ≥ 1 year, daily inhaled corticosteroid equivalent to 100–500 mcg of fluticasone, FEV1 ≥ 80% predicted, Asthma Control Test score ≥ 20), and German cockroach sensitization (skin prick test wheal (SPT) ≥ 3mm and cockroach-specific IgE ≥0.35 kUA/L). The data from Stage 1 were used to identify a dose range that was safe and elicited a clinical response determined by a Total Nasal Symptom Score (TNSS) ≥ 8 or a sneezing score of 3. The sneezing score threshold was implemented after 6 adult subjects completed the NAC to improve sensitivity of the responder criteria.13 Based on Stage 1 results, the TNSS threshold for NAC response in Stage 2 was lowered to 6 to limit the severity of the reaction, shorten the study procedure, and decrease overall participant study burden; the sneezing score threshold was unchanged.
The protocol was reviewed for ethical compliance by a central institutional review board. Written informed consent was obtained from Stage 1 participants and Stage 2 participants’ legal guardians. Stage 2 participants provided verbal (ages 8–11) or written (ages 12–14) assent.
Study Assessments
During the NAC, diluent control and up to 8 escalating intranasal doses (0.00381–11.9 mcg/mL Bla g 1) of German cockroach extract were administered as a nasal spray with a nasal drug delivery system (LMA/Teleflex, San Diego, CA), which delivers 0.1 mL per spray. For each dose, one spray was delivered in each nostril at the end of inspiration. Nasal symptoms, PEF, and peak nasal inspiratory flow (PNIF) were assessed at the beginning of the NAC and 10 minutes after each dose. The TNSS score was determined by tallying the results of four symptom categories, -- sneezing, runny nose, stuffy nose, and itchy nose; the participant graded each symptom on a scale of 0 to 3 (Appendix 1). 14 The sneeze score noted was derived from the total number of sneezes (Appendix 1). Participants could not undergo a NAC if their baseline TNSS was >3 or an individual symptom score was >1. Antihistamines, anticholinergic agents, cromolyn, systemic glucocorticosteroids, nasal decongestants, and nasal steroids were withheld prior undergoing to the NAC (Appendix 2). Blood was drawn before the NAC, 6–10 days post-NAC, and 30 days post-NAC. In a subset, additional blood was obtained 24–72 hours post-NAC. Dust from the participant’s bedroom was collected and analyzed by ELISA for concentration of Bla g 1, 2 and 5.15
Adverse events were graded according to the National Cancer Institue (NCI) Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03 (published June 14, 2010).16 For local adverse events related to the NAC and to skin prick testing, for reduction in lung function (PEF), and for anaphylaxis study specific grading scales were utilized (Appendix 3).
German cockroach extract, peptide synthesis and T cell in vitro culture expansion
German cockroach extract was obtained from Greer (cat#XPB46D3A4, Lenoir, NC). For in vitro studies, German cockroach allergen-derived peptides were synthesized by A & A (San Diego, CA) as crude material on a 1-mg scale. Peptides were re-suspended in DMSO, and equal amounts of each peptide were pooled. T cell in vitro expansions were performed as previously described.17 See Appendix 4 for details.
T cell response and antigen presenting cell activation assays
ELISPOT assays, T cell activation as measured by flow cytometry, as well as activation status and frequency of circulating antigen presenting cells were determined as previously described (see Appendix 4).17–19
Statistical methods
The probability of positive NAC response at each dose was estimated as the cumulative proportion of participants meeting at least one response threshold. Participants were assumed to have a NAC response for all doses beyond the dose at which threshold criteria were met. Exact 95% confidence intervals were estimated using the Clopper-Pearson method. SPT for cockroach and other allergens, cockroach-specific immunoglobulins, IL-5-producing T lymphocytes, and T cell activation were summarized by geometric mean and were compared between responders and non-responders with a Mann-Whitney U Test. IL-5-producing T lymphocytes and T cell activation before, 1 week after, and 1 month post-NAC were compared between responders and non-responders with a Wilcoxon test. Dust allergen levels were summarized as being below or above the lower limit of detection (yes/no) and compared between responders and non-responders with a Fisher’s exact test. Activation and mobilization of antigen-presenting cells were compared pre- and post-NAC with a Wilcoxon matched-pairs signed rank test. Monocyte frequency and T cell activation were correlated separately pre- and post-NAC.
RESULTS
Clinical Findings
The 10 adult participants had a median age of 22.5 years (range 18.0–37.0) and were predominantly female (70%); the 25 pediatric participants had a median age of 12.0 years (range 8.0–14.0) and were predominantly male (64%) (Table 1). Most of the participants were Black (adult 100%; pediatric 72%). The median baseline TNSS was 0 for adults and 1 for children. Baseline characteristics, including asthma medication use, are shown in Table 1.
Table 1.
Demographics
| Adult | Pediatric | ||
| N=10 | N=25 | ||
| Site – n (%) | Cincinnati | 3 (30) | 6 (24) |
| Dallas | 2 (20) | 5 (20) | |
| Denver | 2 (20) | 7 (28) | |
| New York | 0 (0) | 3 (12) | |
| Washington, DC | 3 (30) | 4 (16) | |
| Age in Years | Mean (SD) | 26.0 ( 7.5) | 11.2 ( 1.9) |
| Median (Min – Max) | 22.5 (18 – 37) | 12 (8 – 14) | |
| Gender – n (%) | Female | 7 (70) | 9 (36) |
| Male | 3 (30) | 16 (64) | |
| Ethnicity – n (%) | Hispanic or Latino | 0 (0) | 6 (24) |
| Not Hispanic or Latino | 10 (100) | 19 (76) | |
| Primary Race – n (%) | Black | 10 (100) | 18 (72) |
| White | 0 (0) | 6 (24) | |
| Unknown | 0 (0) | 1 (4) | |
| Baseline Total Nasal Symptom | Mean (SD) | 0.43 (0.65) | 1.04 ( 1.06) |
| Score | Median (Min – Max) | 0 (0 – 2) | 1 (0 – 3) |
| Inhaled Corticosteroid Medication | 100 mcg Fluticasone daily | 0 (0) | 1 (4) |
| Equivalent– n (%) | 200 mcg Fluticasone daily | 1 (10) | 9 (36) |
| 500 mcg Fluticasone daily | 5 (50) | 9 (36) | |
| 500 mcg Fluticasone daily + LABA | 4 (40) | 6 (24) | |
| Allergic Rhinitis – n (%) | Has History of Rhinitis | 4 (40) | 12 (48) |
| Has Ongoing Rhinitis | 3 (30) | 9 (36) | |
Sixty-seven percent of adults had a positive NAC based on meeting the TNSS threshold or sneeze score (Appendix 5; Appendix 6). The median response dose in the adult portion of the trial was 0.120 mcg/mL [IQR 0.0380–0.379] Bla g 1 (Appendix 5).
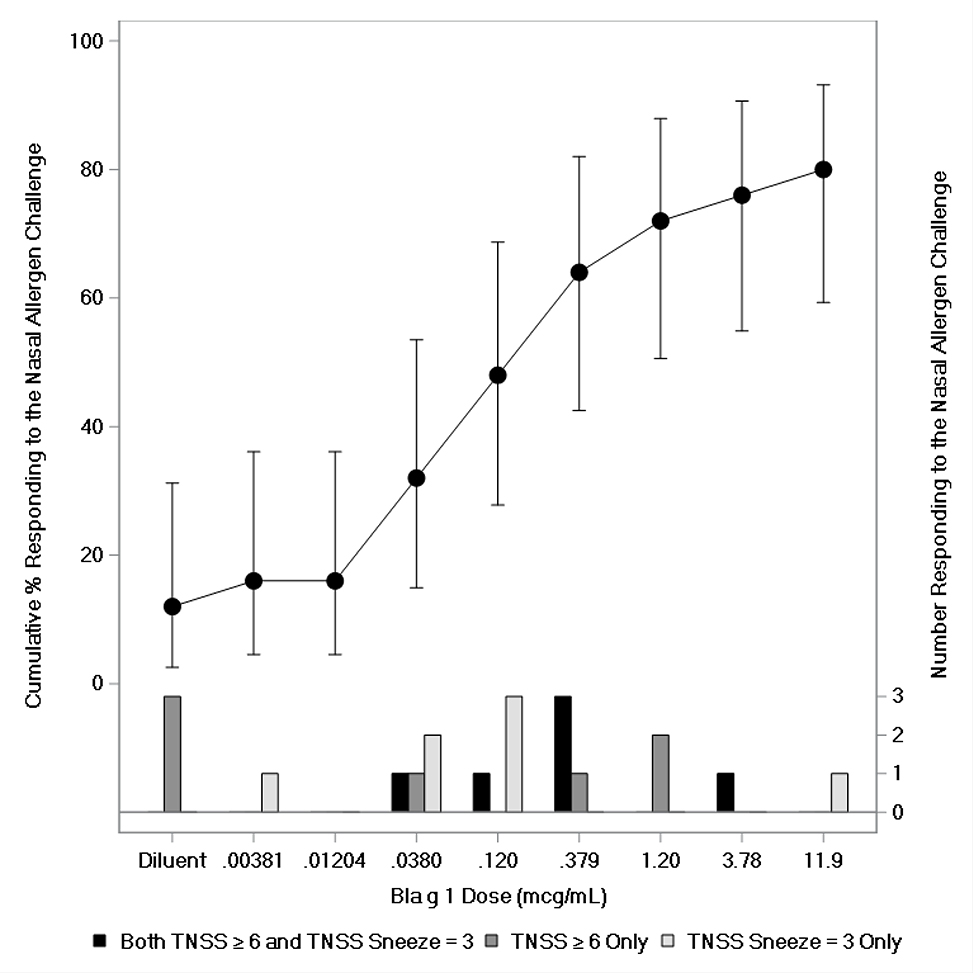
Eighty percent (20/25) of the children responded to the NAC, including 28% (7/25) based on sneeze count alone, 28% (7/25) on TNSS alone, and 24% (6/25) meeting both criteria (Figure 1; Appendix 5; Appendix 7). Three children responded to the diluent on TNSS alone and did not receive any of the cockroach allergenic extract. The median response dose in the pediatric portion of the trial was the same as in adults (Figure 1; Appendix 5). None of the Stage 1 and Stage 2 participants with a negative NAC had a TNSS higher than a 4 (Appendix 6 and 7).
Figure 1 – Pediatric Response to Nasal Allergen Challenge with German Cockroach Extract.
Error bars represent 95% confidence limits.
In general, the PNIF results tended to reflect the symptoms experienced by the participant in both the adult and pediatric participants (Appendix 6 and 7).
Overall there were twelve adverse events related to the NAC; eleven were grade 1. Headache was most frequently reported with an incidence of 20% in adults and 8% in children (Table 2). One adult had a PEF decline of 20.5% from baseline during the NAC that resolved without treatment after the challenge was stopped (Appendix 6, participant 9). There were no reports of asthma symptoms worsening during the NAC or 24-hours post-NAC.
Table 2.
Adverse Events
| Adult | Pediatric | |
| N=10 | N=25 | |
| Number of Participants with at Least One Event | 4 (40%) | 8 (32%) |
| Most Frequent Adverse Event- Headache | 2 (20%) | 2 (8%) |
| Number of Adverse Events | 9 | 12 |
| Adverse Events Related to the Nasal Allergen Challenge | 6 (67%) | 6 (50%) |
| Serious Adverse Events Related to the Nasal Allergen Challenge | 1 | 0 |
There was one serious adverse event (SAE) (Table 2). The SAE was a reactivation of infranasal Herpes Simplex Virus in an adult that occurred three days post-NAC, which resolved without sequelae. There were no instances of anaphylaxis related to the NAC in the study.
In Vitro Parameters
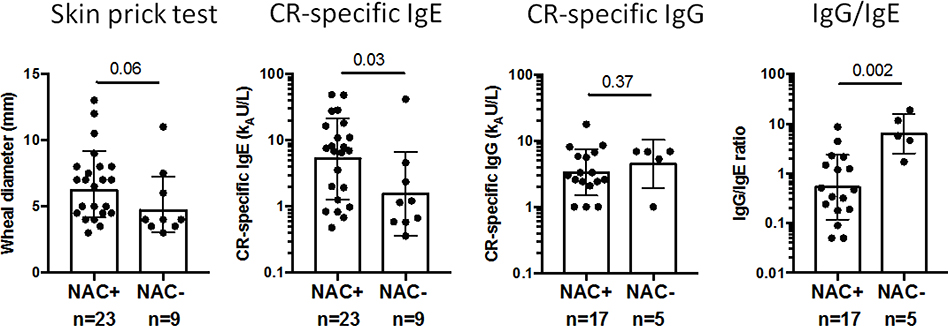
NAC responders (to a cockroach-containing dose) had significantly higher levels of cockroach-specific IgE (median 7.0 kU/L vs. 1.2 kU/L; p=0.03) and lower cockroach-specific IgG/IgE ratios (0.5 vs. 5.7; p=0.002) compared to non-responders (Figure 2). Cockroach SPT, specific-IgG, and house dust cockroach allergen levels were not significantly different between responders and nonresponders, although SPT tended to be larger in responders (p=0.06) (Figure 2; Appendix 8). When comparing other allergen sensitizations, SPT to cat (p=0.008), dog (p<0.001) and Alternaria (p=0.056) were smaller in responders (Appendix 9).
Figure 2 – German CR Skin Prick Test Wheal, CR-specific IgE, and Pediatric CR-specific IgG and IgG/IgE in NAC-positive versus NAC-negative Participants.
Bars show the geometric mean with 95% CI.
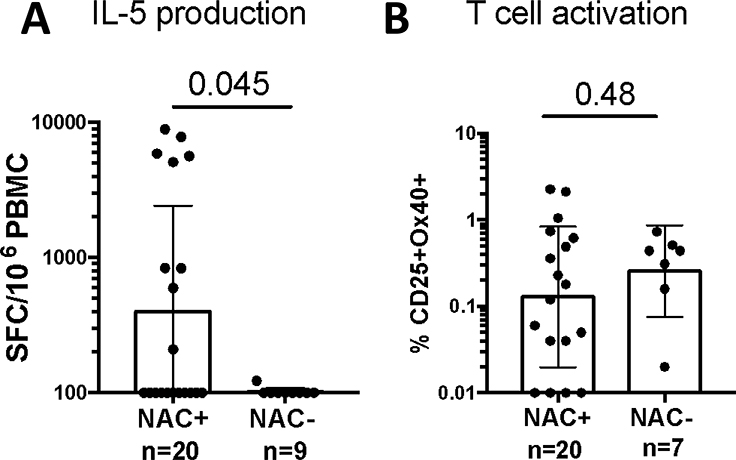
NAC-positive participants had significantly more IL-5 producing cells than NAC-negative participants (Figure 3A). However, there were no significant differences in overall activated T cells between groups (Figure 3B). Cockroach-specific IL-5 production and T cell activation were assessed both one week and one month post-NAC and showed no consistent pattern of CR-specific T cell response modulation at either time point (Appendix 10).
Figure 3 – German Cockroach-Specific IL-5 (A) and T Cell (B) Activation for Combined Adult and Pediatric Populations.
Bars shown the geometric mean ± 95% confidence intervals.
Monocytes, plasmacytoid dendritic cells (pDCs) and basophils were identified by flow cytometry in blood acquired pre- and 48 hours post-NAC in a subset of participants (n=6). Significant increases in surface FcεRIα expression (monocytes and basophils), cell frequency (monocytes and pDCs), and expression of surface MHC-Class II (HLA-DR; monocytes and pDCs) were observed post-NAC. CD14 expression was also increased on monocytes post-NAC. These findings were also observed in samples from two participants who reacted to challenge with the diluent alone (uncolored symbols in Appendix 11).
A significant correlation was observed between antigen-specific ex vivo T cell activation and monocyte frequency both pre- and post-NAC (R2= 0.67, p=0.04; R2= 0.8, p=0.015, respectively) (Appendix 12). No correlations were observed for IL-5 production (data not shown).
DISCUSSION
This study assessed whether NAC with German cockroach extract can induce nasal responses in adults and children with cockroach sensitization and well-controlled, persistent asthma, to identify the dosing range and to assess the safety of this procedure. The reason for involving children was because cockroach sensitization has been strongly associated with asthma in this age group. Furthermore, establishing the cockroach NAC in children was a pre-requisite for us to proceed with a clinical trial where we would test the effectiveness of cockroach allergen immunotherapy (https://clinicaltrials.gov/ct2/show/NCT03541187?term=cockroach&recrs=ab&cond=Asthma+in+Children&cntry=US&draw=2&rank=1) in this population. 3, 20
As with some other perennial allergens, it is difficult for cockroach-allergic patients to link symptoms to exposure and consequently the clinical significance of allergic sentitization demonstrated by the presence of serum specific IgE or positive skin prick testing is not clear. With acute delivery of allergen to the nasal passages, induction of symptomatology may be a stronger indicator that an individual is allergic and a candidate for allergen-specific immunotherapy. Also, reduction in the NAC response may be a good clinical outcome surrogate, but this requires investigation.
We designed this study to identify a narrow range of doses, refine the threshold definition, and assess safety in adults before engaging children. The NAC reached a threshold of nasal symptoms in 76% (26/34) of participants; 9% (3/34) responded to the administration of diluent alone, leaving 68% (23/34) who responded to cockroach extract. Additionally, 26% (9/34) did not respond to the challenge suggesting that, in the case of German cockroach, allergic sensitization alone may not be reliable enough for the selection of individuals who should participate in an allergen immunotherapy study. NAC responsiveness did not differ between adults and children, but only children had diluent responses possibly reflecting a higher degree of nonspecific nasal reactivity or increased sensitivity to the relatively unpleasant aspects of the challenge.
In both adults and children, the PNIF results tended to be concordant with the direction of the symptoms, but, in several instances, the reductions in PNIF were either too small or absent when the symptomatic responses were clear (Appendix 6 and 7). In addition, we encountered technical difficulties conducting PNIF in the pediatric population leading to increased burden and longer time periods to complete a NAC, indicating that the pros and cons of adding PNIF as part of a NAC in a pediatric population should be carefully considered.
Data from the adults did not help reduce the number of allergen doses, but they did allow us to adjust the threshold TNSS score from 8 to 6 to reduce the burden of the procedure in children and to include sneezing as another indicator of positive response.21 Although recent NAC studies use the TNSS as the primary outcome of the challenge,22, 23 it became obvious in adults that several participants responded to the NAC primarily with sneezing and little or no other symptoms. A threshold based on sneezing alone has been used previously, when NAC was used to elucidate the mechanisms of allergic rhinitis.13 In our study, the NAC response based on sneezing score threshold was similar in adult and pediatric participants.
Overall, no participants with a negative NAC had a TNSS higher than a 4. In both study stages, individuals with negative NAC, would either have a flat dose-response or would experience mild symptom elevation which would flatten thereafter showing no dose-response (Appendix 6 and 7). Given this pattern, we do not believe that false negative results were observed. Additionally, while three participants (two adults and one child) had a maximum TNSS of 5 after receiving all 8 doses, these three participants met the criteria for a positive response based on the sneeze score, highlighting the importance of adding this cut-off for a positive NAC as the sneezing score converted the NAC to “positive” and prevented false negative results (Appendix 6 and 7).
Despite the large body of evidence supporting the safety of NAC, safety was of utmost consideration in this study. One SAE related to the NAC occurred in the adult group with a participant experiencing an infranasal reactivation of herpes simplex virus (HSV). Because of the unusual presentation and the originally unknown nature of the reaction, this participant was hospitalized as a precaution, which led to the characterization of the event as a SAE. The study clinicians felt the impetus for the reactivation may have been nasal discharge and manipulation of the nasal and infranasal area as there was no theoretical reason for the cockroach extract to have triggered the reactivation of HSV. PCR testing of the allergen extract used for the NAC was negative for HSV. Future studies may wish to consider HSV as an exclusion criterion for NAC.
The most common adverse event was mild headache. Concerns that the NAC could trigger bronchospasm in participants with persistent asthma were largely unrealized. No asthma symptoms related to the NAC were reported during the NAC or within 24-hours post-NAC. This supports the general experience with other allergens that NAC is low risk in children and specifically in children with asthma,24–28 although caution is still advised. In particular, NAC in adults or children with severe or uncontrolled asthma has not been tested.
NAC responders had significantly elevated indicators of allergen-specific immunity. Although it is important to emphasize that the NAC response can not be reliably predicted with these parameters, the elevated cockroach sIgE, and the low sIgG/sIgE ratio in the pediatric participants support the relationship between a strong systemic, allergen-specific immunologic process and the airway response to allergens and are consistent with prior studies demonstrating that asthma in children is associated with low allergen-specific IgG/IgE ratios to cat and dust mite.29, 30
Similarly, T cell IL-5 production in response to a cockroach allergen-derived peptide pool measured at baseline after in vitro culture was significantly higher in responders compared to non-responders. However, cockroach allergen-induced ex vivo T cell activation pre-NAC was not predictive of NAC response, suggesting that an in vitro culture step coupled with measurement of functional T cells may be a better indicator of clinical reactivity compared to direct ex vivo antigen-specific T cell activation.
No changes were detected in cockroach-specific T cell responses between pre-NAC and 7 or 30 days post-NAC. It is possible that the effects of a local allergen provocation on circulating lymphocytes are too small to detect, or the kinetics of changes did not match the timepoints we chose.
The activation and mobilization of pDCs, monocytes and basophils observed post-NAC, although not specific to NAC responders, indicates a link between environmental airway mucosal stimuli and systemic innate immune responses. This is consistent with results of previous studies, where both allergen challenge and administration of intranasal live viral vaccines have been shown to similarly activate systemic innate immune responses.1, 31, 32. However, activation and mobilization of monocytes and pDCs was observed in two participants who reacted to challenge with the diluent alone, suggesting these changes may not be allergen-specific, but could be induced by mucosal irritation or some other stimulus associated with the NAC.
There are two limitations of this pilot trial. First, we did not test non-cockroach sensitized individuals. We reasoned that, provocation of cockroach non-allergic controls may only be necessary if every cockroach sensitive participant was to have a positive response to the NAC. However, 8 adults and children did not show any evidence of reactivity after receiving all 8 consecutive doses of the cockroach allergen (Appendices 6 and 7). Additional support for our decision not to challenge cockroach non-allergic controls comes from the fact that we found a significant relationship between cockroach-specific IgE and NAC responsiveness suggesting that IgE sensitization to cockroach allergen played an important role in defining the NAC response and that the NAC response was not secondary to non-specific reactivity.
Second, while the dosing range was based on only one allergen, Bla g 1, cockroach extracts are not standardized and their content in other allergens, together with the study participants’ pattern of specific cockroach allergen sensitization, may play a role in determining in vivo responsiveness.33 Although matching the sensitization pattern of a study participant with the allergen content of the provocation extract will be very difficult, knowledge of these parameters may help better predict NAC responsiveness in future studies.
In conclusion, we established a NAC model utilizing German cockroach extract that can be safely performed in children with well-controlled, persistent asthma. We have shown that not all sensitized individuals respond to the NAC and, although NAC responders appear to have higher sensitization based on IgE and skin prick testing, these evaluations are not adequate to identify who will develop nasal symptoms upon exposure. Next steps should be to test whether German cockroach NAC may serve as a tool in selecting appropriate candidates for and assessing responses to allergen-specific immunotherapy.
Supplementary Material
Key Message:
Nasal allergen challenge with German cockroach extract is feasible and safe in children with well-controlled, persistent asthma.
ACKNOWLEDGEMENTS
We are grateful to the CoNAC study participants and their families who gave of themselves to be our investigational partners, as well as to the entirety of the Inner City Asthma Consortium (ICAC), who are dedicated to our inner-city asthma mission and clinical research excellence. In particular, we would like to thank the following ICAC institutions and investigators (principal investigators are indicated by asterisks):
Cincinnati Children’s Hospital – G Hershey*, C Kercsmar*, J Brewington, S Zak, S Austin, A Blust, K Connolly, Z Ehsan, L Henkes, A Jagpal, G McPhail, M Rechtin, J Torres-Garcia, A Witt, K Curtsinger, P Groh; UT Southwestern Medical Center – M Gill*, R Gruchalla*, D Gonzales, B Lewis, D Santoyo, B Shao, H Zhao, K DeBacco, C Ordinario; Children’s Hospital Colorado – A Liu*, A Anderson, W Anderson, C Dutmer, K Freeman, M Gleason, H Hainey, H Hoch, V Lopez, N Miyazawa, P Pinedo-Estrada, A Schiltz, D Searing, B Tippin, C Campos, M Christie, D Liptzin; Columbia University Medical Center – M Kattan*, Y Fernandez-Pau, C Lamm, S Lovinsky-Desir, E Arteaga-Solis, S Tsang, N Whitney, M Pierce, P Yaniv; Children’s National Hospital – S Teach*, E Cowin, A Mathis, D Pillai, D Quint, D Hall, M Savitz, M Sullivan, E Gorin, A Sullivan.
Financial Support:
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract numbers HHSN272200900052C and HHSN272201000052I, and cooperative agreement numbers 1UM1AI114271-01 and UM2AI117870. Additional support was provided by the National Center for Research Resources, and National Center for Advancing Translational Sciences, National Institutes of Health, under grants NCRR/NIH UL1TR000451, UL1RR025780, UL1TR000075 and NCATS/NIH UL1TR000154, UL1TR001082, UL1TR000077-04, UL1TR000040, UL1TR000150, and UL1TR001105.
Conflict of Interest Statement: All authors, with the exception of A. Togias and A.K. Rudman Spergel, report grants from NIH/NIAID during the conduct of study. S. Lovinsky-Desir, V. Schulten, A. Frazier, A. Sette, B. Shao, A.K. Rudman Spergel, J. Johnson, D. Searing and A. Togias have nothing to disclose outside the submitted work. M.A. Gill reports an honorarium for and support for travel to the 2017 AAAAI meeting during the conduct of study. M. Gill also reports monetary compensation from the American Academy of Pediatrics for her work teaching the biannual Pediatrics board review course, PREP The Course, outside the submitted work. C. Kercsmar reports personal fees from Glaxo SmithKline and royalties from Up to Date outside the submitted work. S.J. Teach reports royalties from Up to Date, as well as grants from NIH/NHLBI, PCORI, and EJF Philanthropies outside the submitted work. J. Gern reports personal fees from PREP Biopharm Inc, Regeneron, and MedImmune, as well as stock options from Meissa Vaccines Inc. outside the submitted work. W. Busse reports personal fees from Boston Scientific, ICON, Novartis, Glaxo SmithKline, Genentech, Boehringer-Ingelheim, Sanofi Genzyme, AstraZeneca, Teva, 3M, PrEPBiopharm, Circassia, Regeneron, Peptinnovate, and Elsevier outside the submitted work. R.A. Wood reports employment at Johns Hopkins University, royalties from Up to Date, and institutional grants from NIH, DBV, Aimmune, Astellas, and HAL-Allergy outside the submitted work. A.H. Liu reports personal fees from Merck Sharp & Dohma as speaker honoraria and from Phadia ThermoFisher as consulting honoraria. A.H. Liu also reports other support from Glaxo SmithKline for serving as a Data Monitoring Committee member for an asthma study.
Drs. Rudman Spergel’s and Togias’ co-authorship of this publication does not necessarily constitute endorsement by the National Institute of Allergy and Infectious Diseases, the National Institutes of Health or any other agency of the United States government
Abbreviations/Acronyms:
- DSMB
Data and Safety Monitoring Board
- HSV
Herpes Simplex Virus
- ICAC
Inner City Asthma Consortium
- NIH
National Institutes of Health
- NIAID
National Institutes of Allergy and Infectious Diseases
- NAC
Nasal Allergen Challenge
- PCR
Polymerase chain reaction
- SCIT
Subcutaneous immunotherapy
- TNSS
Total Nasal Symptom Score
Footnotes
Clinical Trial Registration:
https://clinicaltrials.gov/ct2/show/NCT02710136?term=conac&rank=1
REFERENCES
- 1.Scadding GW, Eifan A, Penagos M, et al. Local and systemic effects of cat allergen nasal provocation. Clin Exp Allergy 2015;45(3): 613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Togias A, Corren J, Wagenmann M. Nasal Provocation Testing. In: Adkinson NF, Busse WW, Bochner BS, Holgate ST, Simons ER, Lemanske RFJ, eds. Middleton’s Textbook of Allergy. 7th ed: Elsevier; 2009:1281–94. [Google Scholar]
- 3.Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med 1997;336(19):1356–63. [DOI] [PubMed] [Google Scholar]
- 4.Medsker BH, Forno E, Han Y, et al. Cockroach allergen exposure and plasma cytokines among children in a tropical environment. Ann Allergy Asthma Immunol 2017; 119(1): 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sastre J, Ibanez MD, Lombardero M, Laso MT, Lehrer S. Allergy to cockroaches in patients with asthma and rhinitis in an urban area (Madrid). Allergy 1996; 51: 582–586. [DOI] [PubMed] [Google Scholar]
- 6.Stelmach I, Jerzynska J, Stelmach W, et al. Cockroach allergy and exposure to cockroach allergen in Polish children with asthma. Allergy 2002; 57(8):701–705. [DOI] [PubMed] [Google Scholar]
- 7.Tsai JJ, Kao MH, Wu CH. Hypersensitivity of bronchial asthmatics to cockroach in Taiwan: comparative study between American and German cockroaches. Int Arch Allergy Immunol 1998; 117(3): 180–186. [DOI] [PubMed] [Google Scholar]
- 8.Thangam Sudha V, Arora N, Sridhara S, Gaur SN, Singh BP. Biopotency and identification of allergenic proteins in Periplaneta americana extract for clinical applications. Biologicals 2007; 35(2):131–137. [DOI] [PubMed] [Google Scholar]
- 9.Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med 2004;351(11):1068–80. [DOI] [PubMed] [Google Scholar]
- 10.Okuda M, Usami A, Itoh H, Ogino S. [Nationwide investigation of insect allergy in patients with allergic rhinitis]. Nihon Jibiinkoka Gakkai Kaiho 2002;105(12):1181–8. [DOI] [PubMed] [Google Scholar]
- 11.Wierzbicki DA, Majmundar AR, Schull DE, Khan DA. Multiallergen nasal challenges in nonallergic rhinitis. Ann Allergy Asthma Immunol 2008;100(6):533–7. [DOI] [PubMed] [Google Scholar]
- 12.Hosen H Insects as a cause of inhalant respiratory allergy. Ann Allergy 1970;28(12):596–9. [PubMed] [Google Scholar]
- 13.Naclerio RM, Meier HL, Kagey-Sobotka A, et al. Mediator release after nasal airway challenge with allergen. Am Rev Respir Dis 1983;128 (4):597–602 [DOI] [PubMed] [Google Scholar]
- 14.Scadding GW, Calderon MA, Bellido V, et al. Optimisation of grass pollen nasal allergen challenge for assessment of clinical and immunological outcomes. J Immunol Methods. 2012;384(1–2):25–32 [DOI] [PubMed] [Google Scholar]
- 15.Arbes SJ Jr, Sever M, Vaughn B, et al. Feasibility of using subject-collected dust samples in epidemiologic and clinical studies of indoor allergens. Environ Health Perspect 2005;113(6):665–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cancer Institute (NCI) (September 21, 2020), Common Terminology Criteria for Adverse Events (CTCAE), Version 4.03, June 14, 2010. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.html. [Google Scholar]
- 17.Oseroff C, Sidney J, Kotturi MF, et al. Molecular determinants of T cell epitope recognition to the common Timothy grass allergen. J Immunol 2010;185(2):943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dan JM, Lindestam Arlehamn CS, Weiskopf D, et al. A cytokine-independent approach to identify antigen-specific human germinal center T follicular helper cells and rare antigen-specific CD4+ T cells in blood. J Immunol. 2016;197(3):983–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill MA, Bajwa G, George TA, et al. , Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010. 184(11): 5999–6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood RA, Togias A, Wildfire J, et al. Development of cockroach immunotherapy by the Inner-City Asthma Consortium. J Allergy Clin Immunol. 2014; 133(3): 846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis AK, Tsitoura DC, Quint D, Powley W, Lee LA. Safety and pharmacodynamics of intranasal GSK2245035, a TLR7 agonist for allergic rhinitis: A randomized trial. Clin Exp Allergy. 2017; 47 (9): 1193–1203. [DOI] [PubMed] [Google Scholar]
- 22.Scadding GW, Calderon MA, Shamji MH, et al. Effect of 2 years of treatment with sublingual grass pollen immunotherapy on nasal response to allergen challenge at 3 years among patients with moderate to severe seasonal allergic rhinitis: the GRASS randomized clinical trial. JAMA. 2017. 14;317(6):615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neighbour H, Soliman M, Steacy LM, et al. The Allergic Rhinitis Clinical Investigator Collaborative (AR-CIC): verification of nasal allergen challenge procedures in a study utilizing an investigational immunotherapy for cat allergy. Clin Transl Allergy. 2018; 8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lönnkvist K, Moshfegh A, Pedroletti C, Hedlin G, Halldén G, Lundahl J. Increased eosinophil transmigration after nasal allergen challenge in children with allergic asthma and rhinitis. Allergy 2002;57(12): 1200–1204. [DOI] [PubMed] [Google Scholar]
- 25.Jean R, Rufin P, Pfister A, Landais P, et al. Diagnostic value of nasal provocation challenge with allergens in children. Allergy 1998; 53 (10): 990–994. [DOI] [PubMed] [Google Scholar]
- 26.Marcucci F, Passalacqua G, Canonica GW, et al. Lower airway inflammation before and after house dust mite nasal challenge: An age and allergen exposure-related phenomenon. Respir Med. 2007; 101(7):1600–8. Epub 2007 May 7 [DOI] [PubMed] [Google Scholar]
- 27.Hervás D, Rodriguez R, Garde J. Role of aeroallergen nasal challenge in asthmatic children. Allergol Immunopathol (Madr) 2011;39(1): 17–22. [DOI] [PubMed] [Google Scholar]
- 28.Duman H, Bostanci I, Ozmen S, Dogru M. The relevance of nasal provocation testing in children with nonallergic rhinitis. Int Arch Allergy Immunol 2016;170(2):115–121 [DOI] [PubMed] [Google Scholar]
- 29.Custovic A, Soderstrom L, Ahlstedt S, Sly PD, Simpson A, Holt PG. Allergen-specific IgG antibody levels modify the relationship between allergen-specific IgE and wheezing in childhood. J Allergy Clin Immunol. 2011;127(6):1480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holt PG, Strickland D, Bosco A, et al. Distinguising benign from pathologic TH2 immunity in atopic children. J Allergy Clin Immunol. 2016; 137(2):379–87. [DOI] [PubMed] [Google Scholar]
- 31.Jahnsen FL, Lund-Johansen F, Dunne JF, Farkas L, Haye R, Brandtzaeg P. Experimentally induced recruitment of plasmacytoid (CD123high) dendritic cells in human nasal allergy. J Immunol 2000;165(7):4062–8. [DOI] [PubMed] [Google Scholar]
- 32.Bajwa G, DeBerardinis RJ, Shao B, Hall B, Farrar HD, Gill MA. Cutting Edge: Critical Role of Glycolysis in Human Plasmacytoid Dendritic Cell Antiviral Responses. J Immunol 2016;196(5):2004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pomés A, Glesner J, Calatroni A, et al. Cockroach allergen component analysis of children with or without asthma and rhinitis in an inner-city birth cohort. J Allergy Clin Immunol. 2019; 144(4):935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.