Abstract
Background:
Sleep disturbance in individuals prescribed medications for opioid use disorder (MOUD) is common, though the nature and progression of such concerns are difficult to discern due to differing terminology and assessment type between studies. Accurately identifying and treating sleep problems in this growing population has the potential to improve comorbidity and other MOUD outcomes.
Objective:
The aim of the present review is to provide an overview of sleep in individuals stabilized on MOUD. Specifically, the following aspects of sleep were reviewed: 1) prevalence of clinically significant sleep disturbance; 2) sleep disturbance compared to findings in those not prescribed MOUD; 3) correlates of sleep disturbance; 4) self-reported sleep compared to objective measures.
Method:
Studies were identified using 6 large databases and included if they contained at least one measure of sleep during MOUD treatment as usual. Studies were excluded if they were case studies, not available in English, or participants were in withdrawal or detoxification.
Results:
Forty-two studies were included and categorized by type of sleep assessment: validated self-report questionnaire; provider-assessed; polysomnography; multi-method. Correlates were included if they were statistically significant (generally p < 0.05).
Conclusions:
This review indicates there is a high prevalence of chronic self-reported sleep disturbance (eg, insomnia symptoms) in this population and suggests quantitative sleep parameters (eg, total sleep time) and respiratory problems during sleep are worse than in the general population. These sleep problems are correlated with psychiatric comorbidity and other substance use. Other correlates (eg, sociodemographic factors) require further study to draw definitive conclusions.
Keywords: Sleep, Addiction, Assessment, Opioid use disorder, Medication assisted treatment
1. Introduction
Opioid use disorder (OUD) is a highly prevalent problem that has a significant global impact on public health and the economy [1]. Opioid agonists for OUD, such as methadone or buprenorphine, assist by activating opioid receptors to prevent opioid withdrawal [2]. Prescribing these Food and Drug Administration-approved medications for OUD (MOUD) is a first-line treatment for OUD, and though access to MOUD is growing, rates of both treatment entry and treatment retention for MOUD have marked room for improvement [3–5]. Sleep problems that occur during the transition from detoxification through initiation of MOUD have been increasingly addressed as an intervention target to assist with successful treatment entry [3,6–8]. However, treating sleep problems that persist in the months and years after initiating MOUD has received far less attention [9]. Understanding the prevalence and nature of sleep complaints during this time is important, as MOUD is a long-term, often lifelong, treatment intervention [10], and sleep disturbance that persists once stabilized on MOUD may be a potential target to assist with successful treatment retention and other important treatment outcomes, such as psychiatric comorbidity and other substance use.
Comprehensively assessing characteristics of sleep disturbance across studies is often difficult due to the variability of assessment types utilized for the multifactorial components that make up the sleep period and overall sleep experience. Questionnaires and clinical interviews ask about subjective aspects of the sleep experience, and multiple approaches are aimed at assessing quantitative sleep parameters (eg, total sleep time [TST]), such as daily sleep diaries and polysomnography (PSG). PSG is also able to assess sleep staging and sleep-related respiratory events. Terminology also makes this difficult as one variable or concept can be referred to by multiple different abbreviations or reported in differing metrics (see Table 1 for a basic overview of such variables pertinent to this review). As such, sleep disturbance will be the general term used throughout this review to refer to any negative aspect of the sleep experience. This varies based on assessment type, and the type of sleep disturbance is thus explicitly described within each section. The purpose of the present study was to integrate studies with various sleep assessment types in adults stabilized on MOUD to examine: 1) the prevalence of clinically significant sleep disturbance; 2) sleep disturbance compared to findings in those not prescribed MOUD; 3) correlates of sleep disturbance; 4) self-reported sleep compared to objective measures of sleep when provided within a single sample.
Table 1.
Sleep variable names, abbreviations, and descriptions.
| SD | PSC | Names/Abbreviation | Definition | Reported as: | |
|---|---|---|---|---|---|
| Quantitative parameters assessed | X | X | Time in bed/TIB | Time between getting in bed and out of bed for the major sleep period | Minutes or hours |
| X | X | Sleep onset latency/SOL | Time between trying to go to sleep and falling asleep | Minutes or hours | |
| X | X | Total sleep time/TST | Time spent asleep during the major sleep period | Minutes or hours | |
| X | X | Wake after sleep onset/WASO | Time awake during the major sleep period after initial sleep onset | Minutes or hours | |
| X | X | Sleep efficiency/SE | Time asleep of the time spent in bed, calculated as(TST/TIB)*100 | Percentage ([TST/TIB]*100) | |
| Sleep stages | X | Non-rapid eye movement/Non-REM | N1, N2, N3 each represent a deeper stage. typically passed through sequentially before REM | Total minutes in each stage or percentage ([REM/TST]*100) | |
| Stage 1/N1 | |||||
| Stage 2/N2 | |||||
| Stage 3/N3 or Slow wave sleep/SWS | |||||
| X | Rapid eye movement/REM | Lightest stage, where dreams often occur | Total minutes of REM or percentage ([REM/TST]*100) | ||
| Respiratory events during sleep | X | Apnea hypopnea index/AHI | Index indicating number of apnea (complete air blockage) and hypopnea (partial blockage) events | Number of events/hour; generally AHI > 5 indicates apnea diagnosis | |
| X | Obstructive sleep apnea hypopnea index/OSAHI or OAHI | AHI of events caused by obstruction | Number of events/hour | ||
| X | Central apnea index/CAI | AHI of events with no identifiable obstruction | Number of events/hour | ||
| Sleep-related breathing disorders | X | Obstructive sleep apnea/OSA | Clinically significant disruption in breathing caused by obstruction | Yes/No or Mild/Moderate/Severe | |
| X | Central sleep apnea/CSA | Clinically significant disruption in breathing with no identifiable cause | Yes/No or Mild/Moderate/Severe | ||
| X | Sleep apnea syndrome/SAS | Referring to both OSA and CSA | Yes/No or Mild/Moderate/Severe |
Note: SD = sleep diary, a subjective/self-report measure; PSG = polysomnography/overnight sleep study in a sleep laboratory or at home, an objective measure.
2. Method
2.1. Search strategy and selection criteria
The electronic databases PubMed, PsychlNFO, CINAHL, Scopus, Cochrane Central Register of Controlled Trials, and clinicaltrials.gov (studies with posted results only) were searched from inception through July 2020. The search strategy included the following key words and their variants: opioid use disorder (eg, dependence, addiction, opium-dependent), medication assisted treatment (eg, medication assisted therapy, methadone, buprenorphine, opioid agonist), and sleep (eg, sleep disturbance, sleep quality, sleep disorder). No attempts were made to find unpublished studies.
2.2. Inclusion and exclusion criteria
Only original full-text research studies were included. Studies were eligible for inclusion if they met the following criteria: 1) included human subjects at least 18 years of age; 2) at least part of the sample was stabilized in MOUD treatment as usual (clearly documented as stabilized or consistently in treatment for at least 3 weeks); 3) at least one sleep measure was administered. Studies were excluded if any of the following were true: 1) not available in English; 2) included only case studies; 3) included only subjects in withdrawal or engaged in detoxification; 4) measured sleep as part of an experimental paradigm (eg, performance following sleep deprivation in a laboratory); 5) assessed sleep with fewer than 3 self-report items; 6) measured sleep as part of an assessment in a randomized MOUD clinical trial. This final criterion was included as the primary purpose of this review was to assess the typical, natural course of sleep and prevalence of sleep problems in individuals stabilized in MOUD treatment. Well controlled randomized clinical trials (eg, comparing 2 types of MOUD) often have strict exclusion criteria related to sleep, such as past or current illicit drug use and comorbid psychiatric diagnoses. Thus, publications coming from such trials may not provide an accurate representation of sleep in this population and were subsequently not included in this review. As such, any reference to comparison groups is referring to naturalistic groups occurring in the clinic or community, rather than a group randomized to a control condition. Papers were reviewed for major findings and categorized by sleep assessment type.
3. Results
3.1. Search results
The initial search identified 858 articles, with 738 remaining after removal of duplicates (see Fig. 1). Of these, titles, abstracts, and key words were reviewed for eligibility criteria and 241 were chosen for full text review. Forty-two articles met all eligibility criteria and were included. Upon review by the authors four main categories of sleep assessment emerged: 1) self-report with validated sleep questionnaire; 2) provider-assessed sleep disorder (ie, sleep disorder documented in medical record by provider or diagnosed via provider interview); 3) PSG; 4) multi-method (ie, self-report and objective measure of sleep). It should be noted there may be multiple publications that analyzed data from the same sample of participants. Though each publication has a different focus and producing multiple publications from the same parent study is common practice (eg, Nguyen et al. [11], Teichtahl et al. [12], and Wang et al. [13,14]), it is important to be mindful of this when reviewing and interpreting all 42 studies together.
Fig. 1.
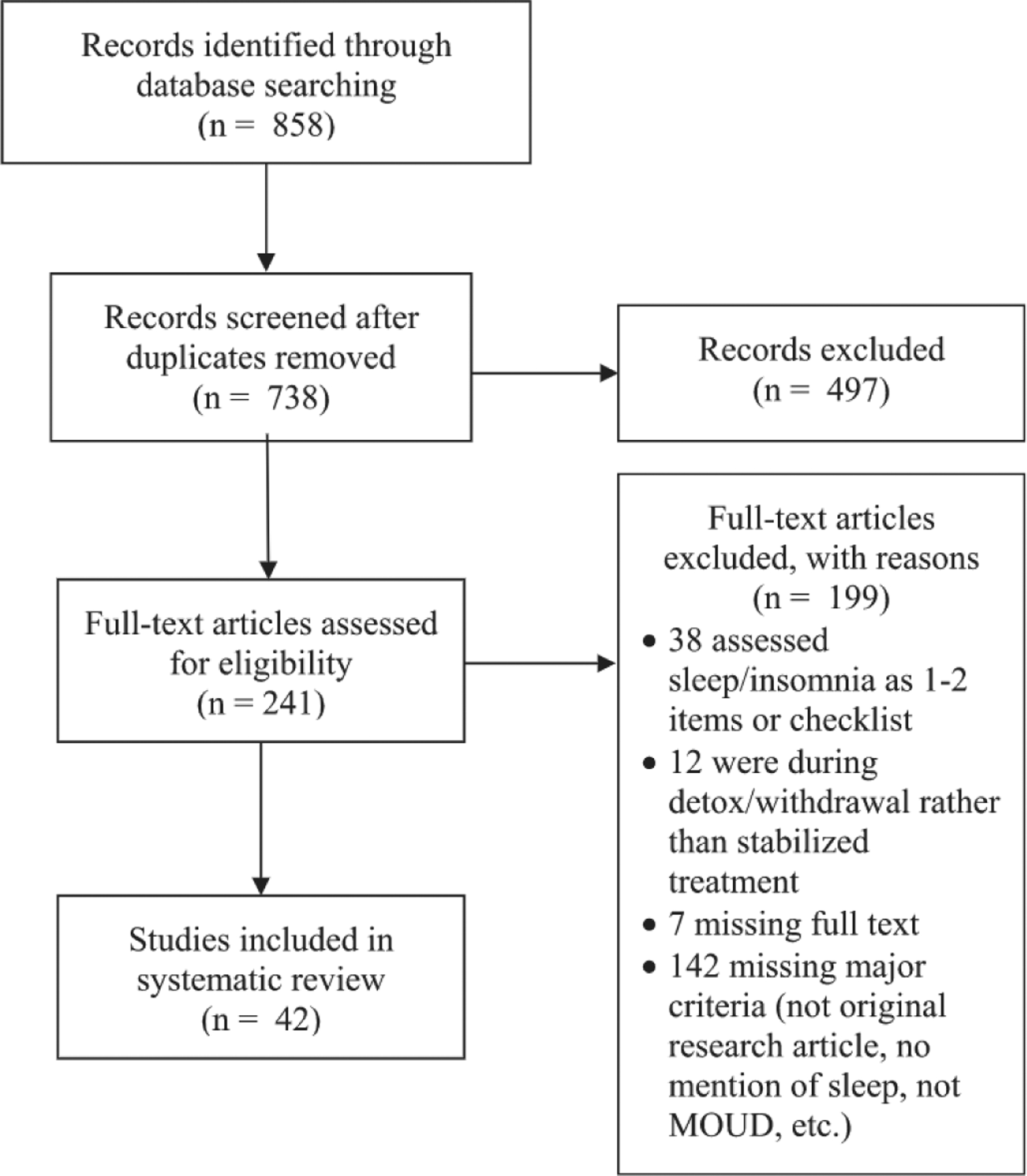
Selection of articles for inclusion.
3.2. Self-report sleep questionnaires
Twenty-four studies utilized questionnaires designed specifically to measure various aspects of sleep disturbance, with sample sizes ranging from 54 to 489 (see Table 2). Sleep disturbance in these studies was typically identified by a cut-off point on the total score or pulling items from these questionnaires that ask for an estimation of recent quantitative sleep parameters, such as TST or sleep onset latency (SOL).
Table 2.
Self-report sleep questionnaires.
| Authors | N(men) | Groups | Age M(SD) yrs (unless noted) | MOUD dose M(SD) mg (unless noted) | MOUD duration M(SD) (unless noted) | Sleep Questionnaire(s) | Other sleep-related findings |
|---|---|---|---|---|---|---|---|
| Albonaim et al. [45] | 82(82) | MMT | 44.6(14.6) | 93.1(36.0) | ≥3 mos | Mean ISI scores in 3 gene variant groups ranged from 11 to 16 | Of 3 gene variants studied 1 (rs997917) was associated with worse ISI score (p = 0.0001) |
| Baykara & Alban [16] | 107(107) | B/NMT(4:1 b:n ratio) | 24.6(4.3) | 3.79(2.8) | 0, 4 mos |
PSQI M(SD) 0 mos: 8.9(3.6) 4 mos: 6.9(3.3) |
SOL, sleep disturbance, and daytime dysfunction improved from 0 to 4 mos (ps < 0.001); TST, SE, and sleep medication use did not |
| Chan et al. [17] | 54(54) | (1) 34 MMT (2) 20 No opioid |
(1) 40.1(1.2) (2) 37.9(2.1) |
59.1(5.6) | 23.6(5.6) mos |
PSQI M(SD) (1) 10.8 (1.2) (2) NR |
PSQI score not correlated with IL-1β, IL-6, IL-8, IL-10, or TNF-α |
| Chrobok et al. [18] | 199(147) | (1) 84 MMT (2) 65 DMT (3) 23 BMT (4) 27 DTX |
(1) 42(8) (2) 41 (9) (3) 41(7) (4) 39(8) |
(1) 46.3(21.7) (2) 417.3(161.6) (3) 11.1(4.8) |
≥12 mos |
PSQI M (Range) (1) 10.4(2–18) (2) 9(3–18) (3) 8.8(2–14) (4) 5.3(1–13) RIS M (Range) (1) 17.3(6–36) (2) 15.1(4–29) (3) 16.1(5–31) (4) 10.4(3–23) |
MMT dose related to PSQI and RIS (ps < 0.05) but DMT and BMT dose were not; women had higher PSQI (ps ≤ 0.0083) but similar RIS; employment was related to RIS score (ps ≤ 0.0083); age was not related to score on either measure |
| Dunn et al. [38] | 185(89) | (1) 185 MMT (2) 125 BMT |
46.1 (10.1) | NR | 4.8(5.3) yrs |
MOS Sleep Scale TST M(SD) 336(132); 53% SOL > 30; 6 of 7 subscales worse than normative sample |
No significant differences in severe impairment between (1) and (2); higher scores on Sleep Problems subscale associated with more medical and psychiatric diagnoses (ps < 0.05) |
| Fudalej et al. [39] | 240(169) | (1) 94 MMT hx suicide attempt (2) 146 MMT no attempt hx |
Med = 45 (IQR 31–36) | Med = 80 (IQR 60–90) | Med = 34 (IQR 23–55) mos |
SDQ-7 Med(IQR) (1) 11(8–17) (2) 8(4–14) |
|
| Hallinan et al. [46] | 489(264) | (1) 113 BMT (2) 184 MMT (3) 87 OUD (4) 105 No opioid |
(1) 37.8(8.7) (2) 38.9(8.5) (3) 37.6(8.8) (4) 36.9(10.9) |
(1) Med = 12 (IQR 7.2–20) (2) Med = 85 (IQR 55–120) |
(1) Med = 2.0 (IQR 1.0–4.5) yrs (2) Med = 4.0 (IQR 1.5–8.0) yrs |
AIS M(SD) (1) 9.5(6.1) (2) 10.1(6.4) (3) 11.3(5.8) (4) 4.9(4.2) ESS All Ms < 10 |
No significant AIS or ESS differences between sexes in groups 1–3; anxiety and depressive symptoms and cannabis use significantly increased odds of insomnia (ps < 0.05) |
| Hsu et al. [19] | 121(105) | MMT | 39.6(8.6) | 51.4(24.9) | 15.7(11.1) mos |
PSQI M(SD) 9.1(5.4) |
PSQI score positively correlated with methadone dose (p = 0.047) but not age, duration of opiate exposure, or duration or attendance in MMT; group differences in PSQI score in education level and hypnotic use (ps < 0.05) but not gender, employment, marital status; 22.3% use sleep aids; 23.1% alcohol to sleep; 38.0% saw clinician for sleep; 29.8% might use heroin again due to sleep problem |
| Khazaie et al. [30] | 126(126) | (1) 65 MMT (2) 61 active OUD |
(1) 38.9(11.2) (2) 34.0(9.3) | NR | ≥2 mos |
PSQI> 5 (1) 78.5% (2) 86.9% GSAQ: (2) > (1) in SOL, fatigue, sleepiness, and PLMs |
(1) reported more daytime dysfunction related to sleep than (2), p = 0.001 |
| Le et al. [31] | 395(395) | MMT | 25.9(7.8) | 69.2(37.0) | 2.8(2.2) yrs | PSQI > 5 in 26.6% | PSQI > 5 was associated with unemployment, being single, concurrent tobacco or drug use, higher depression, anxiety, and stress scores, and longer duration of MMT (ps < 0.05) |
| Peles et al. [20] | 54(0) | (1) 26 MMT hx of sexual abuse (2) 15 No MMT hx sexual abuse (3) 13 MMT no hx sexual abuse |
(1) 42.2(7.6) (2) 34.9(9.5) (3) 46.9(9.5) |
NR | (1) 7.6(5.8) yrs (3) 8.9(6.6) yrs |
PSQI M(SD) (1) 10.4(4.2) (2) 7.9(4.8) (3) 6.3(4.8) ESS All Ms < 8 |
Significant differences in PSQI score between groups (p = 0.03) but not in ESS scores |
| Peles et al. [21] | 154(116) | (1) 55 MMT ≥ 10 yrs (2) 99 MMT-free ≥ 10 yrs |
(1) 51.5(7.7) (2) 53.5(7.9) |
NR | 14.5 (3.1) yrs |
PSQI M(SD) (1) 7.2(4.0) (2) 5.1(3.4) ESS M(SD) (1) 7.4(4.0) (2) 8.7(3.6) |
No significant correlation between PSQI and 2 measures of cognitive functioning |
| Peles et al. [22] | 101(79) | MMT | 40.4(9.6) | 157.0(52.9) | 5.0 (3.3) yrs | PSQI M(SD) 9(4.8) | Higher PSQI correlated with BZD use, pain, psychiatric disorders, years of opiate abuse before admission, and dose (ps < 0.05) but not with duration in MMT. gender, age, abuse of opiates, cannabis. or cocaine |
| Peles et al. [23] | 123(89) | MMT THDP (1) 31 Never (2) 12 Not current (3) 5 < 1 wk (4) 31 1 wk (5) 25 2 wks |
47.2(10.5) | (1) 126.8(39) (2) 115.3(42) (3) 119.2(46) (4) 117.3(42) (5) 106.9(48) |
7.6 (5.5) yrs |
PSQI M(SD) 8.2(4.1) ESS M(SD) 7,6(3.8) |
No significant differences between THDP groups on PSQI or ESS |
| Sharafshah et al. [47] | 82(82) | MMT | 44.6(14.6) | 93.1(35.9) | ≥3 mos | Mean ISI in 4 gene variant groups ranged from 11 to 16 | None of the four variants were significantly associated with ISI |
| Stein et al. [24] | 225(122) | MMT | 40.7(7.7) | 39.7% > 100 mg | ≥3 mos |
PSQI M(SD) 10.7(4.9) |
Worse sleep associated with: employment, depression, anxiety, methadone dose > 100 mg, more severe nicotine dependence, and pain (ps < 0.01) |
| Torrens et al. [40] | 80(52) | MMT | 30.3(6.3) | 1 mo: 60(18) 3 mos: 73(30) 6 mos: 83(39) 12 mos: 91(54) |
0, 1, 3,6, 12 mos | NHP sleep items M(SD) scores decreased from 75(30) to 40(35) | Most significant drop in scores between 0 and 1 month; at month 12 still clinically significant range |
| Tripathi et al. [25] | 106(106) | BMT | 41.1(14.3) | 10.2(3.8) | Med = 60 mos (IQR 17–120) |
PSQI M(SD) 6.6(3.4) ESS “normal” |
|
| Zahari, Ibrahim, et al. [26] | 319(319) | (1) 160 MMT (2) 159 opioid naïve |
(1) 37.2(6.19) (2) 24.8(5.31) |
NR | ≥1 mo |
PSQI M(SD) (1) 5.4(2.8) (2) 4.8(2.3) |
PSQI scores significantly different between groups (P = 0.03) |
| Zahari, Lee, Ibrahim, et al. [27] | 165(165) | MMT | 37.3(6.2) | 76.6(37.6) | 2.9(2.1) yrs |
PSQI M(SD) 5.5(2.7) |
Those with one combined genotype (118AA and IVS2 + 691 GC)of the OPRM1 gene had lower PSQI than those without this diplotype (p = 0.018) |
| Zahari, Lee, Mohamad, et al. [28] | 119(119) | MMT | 37.5(6.8) | 77.7(39.5) | 2.8(2.1) yrs |
PSQI M(SD) 5.6(2.8) |
Risk factors for higher PSQI score: first illicit drug use age ≤20, opioid abuse age ≤ 20, opioid exposure duration >10 years, opioid addiction duration >10 years. MMT duration ≥5 years, and lower monthly income (ps < 0.05) |
| Zahari, Siong, Musa, et al. [29] | 119(119) | MMT | 34.7(6.9) | 77.8(39.5) | 2.8(2.1) yrs |
PSQI M(SD) 5.6(2.8) |
39% TST less than 7 h |
| Zhang et al. [32] | 480(331) | MMT | 38.3(7.0) | 69.6(30.0) | 25.6(10.8) mos | Chinese PSQI 35.2% ≥ 8 |
Higher PSQI score correlated with unmarried, unemployed, injecting heroin before MMT. longer duration of heroin use and MMT, depression, and lower sexual satisfaction (ps < 0.05) |
| Zheng et al. [9] | 40(22) | B/NMT | 35.3(11.6) | Increased during study from 0 to 11.9(2.3) | 0, 30, 60, 90 days | MOS-Sleep SLPD4. SLPD6, SLPD9 improved over time (ps < 0.04) | 50% participants reported that their sleep change was “some” to “completely” related to MOUD; even after some scores improved they were still in clinical range |
Note: M = mean; SD = standard deviation; yrs = years; MOUD = medication for opioid use disorder; mg = milligrams; MMT = methadone maintenance treatment; mos = months; ISI = Insomnia Severity Index; B/NMT = buprenorphine/naloxone maintenance treatment; PSQI = Pittsburgh Sleep Quality Index; SOL = sleep onset latency, TST = total sleep time; SE = sleep efficiency; NR = not reported; IL-1β = Interleukin 1 beta; IL-6 = Interleukin 6; IL-8 = Interleukin 8; IL-10 = Interleukin 10; TNF-α = tumor necrosis factor alpha; DMT = diacetylmorphine maintenance treatment; BMT = buprenorphine maintenance treatment; DTX = recent detoxification; RIS = Regensburger Insomnia Scale; MOS = Medical Outcome Study Sleep Scale; Med = median; IQR = interquartile range; SDQ-7 = Sleep Disorders Questionnaire; OUD = opioid use disorder; AIS = Athens Insomnia Scale; ESS = Epworth Sleepiness Scale; GSAQ = Global Sleep Assessment Questionnaire; PLM = periodic limb movements; BZD = benzodiazepine; THDP = take home dose privileges; wk = week; NHP = Nottingham Health Profile; SLPD4/SLPD6/SLPD9 = overlapping MOS subscales that include SOL, restlessness, sufficient sleep, awakenings, drowsiness, trouble falling or staying asleep, and perceived sleep need.
3.2.1. Prevalence of sleep disturbance
Eighteen of these studies administered the Pittsburgh Sleep Quality Index (PSQI) [15], 15 of which reported the mean PSQI score exceeded the clinical cut-off of 5 [16–29], and the other three studies reported percentages exceeding the cut-off ranged from 26.6% to 78.5% [30–32]. Other questionnaires aimed at getting an overview of sleep were the Medical Outcomes Study Sleep Scale (MOS) [33] the Sleep Disorders Questionnaire (SDQ) [34], the Global Sleep Assessment Questionnaire (GSAQ) [35], and the Nottingham Health Profile (NHP) [36,37]. The studies that used these measures also consistently found elevated reports of sleep disturbance during MOUD, which included problems with an insufficient amount of sleep, restless sleep, excessive time to fall asleep, snoring, and shortness of breath [9,30,38–40]. A handful of studies specifically targeted insomnia symptoms [41], including the Insomnia Severity Index (ISI) [42], the Athens Insomnia Scale (AIS) [43], and the Regensburg Insomnia Scale (RIS) [44], and found the mean scores indicated at least mild insomnia symptoms [18,45–47]. Interestingly, daytime sleepiness, an issue often associated with sleep problems, seemed to be prevalent in some patients, but the majority of individuals in every sample were experiencing “normal” sleepiness, as defined by an Epworth Sleepiness Scale (ESS) [48] score less than 10 [9,20,21,23,25,46].
3.2.2. Comparison to non-MOUD groups and the general population
Questionnaires administered across multiple time points and/or with groups who were not prescribed MOUD suggest that sleep is slightly better for those receiving MOUD than for those with OUD not receiving medication [30,46] and indicate that while sleep disturbance may improve over the course of treatment it is often still worse than comparison groups with no opioid use and reports found in the general population [9,16,17,26,30,40,46]. Studies that reported items estimating recent quantitative sleep parameters found the majority of those using MOUD reported SOL greater than 30 min with a TST less than 7 h per night, which are both indicators of poor sleep quality [25,29,38,49].
3.2.3. Correlates of sleep disturbance
Many past and present psychosocial factors were found to be correlated with worse self-reported sleep. Historical factors include less education, past experience of sexual abuse, one or more suicide attempt(s), and a diagnosis of cancer, endocrine disorder, hepatitis, or significant injury; current factors include psychiatric comorbidity, pain, decreased sexual satisfaction, nicotine dependence, and use of cannabis, hypnotics, or benzodiazepines [19,20,22,24,32,38,39,46]. There were mixed findings related to employment, with four studies concluding unemployment and disability status were related to worse sleep quality [18,24,31,32] though one reported no relationship [19]. Similarly, two studies found a relationship between being single and having worse sleep [31,32] while another did not [19]. Finally, one study found women reported worse sleep quality than men [18], though three other studies found that sleep was not different between men and women in this population [19,22,46].
Several studies examined the relationship between sleep and various MOUD factors. Four found higher PSQI scores were related to a higher MOUD dose, all of which were samples of methadone only [19,22,24,28]; interestingly, in comparing three types of MOUD, one study found this to be true for methadone but not for diacetylmorphine or buprenorphine [18]. Other attempts to clarify the nature of the relationship of sleep with opioid use and OUD treatment were markedly inconsistent. For instance, two studies found higher PSQI scores were associated with longer opioid exposure prior to MOUD treatment [22,28] while another found no such relationship [19]. Additionally, three studies found higher PSQI scores were associated with longer MOUD duration [28,31,32] while two did not [19,22] did not.
Four studies attempted to explore the relationship of sleep with genetic variants and inflammatory response markers. One study found a relationship between PSQI score and one diplotype they were studying (118AA and IVS2 + 691 GC) [27], and another found a relationship between ISI score and one gene variant (rs997917) [45]. No other sleep correlates were found in these studies [17,47].
3.3. Provider-assessed sleep disorder
Four studies examined provider-assessed sleep disorders via provider assessment, with sample size of these studies ranging from 152 to 2414 (see Table 3). Two studies were single time-point assessments [50,51] and two collected data at two time points, separated by at least one year [52,53]. Sleep disturbance in these studies was identified as documented treatment for a sleep disorder by a provider in a medical record review [50,52] or documentation of a sleep disorder diagnosed by a provider via interview [51,53].
Table 3.
Provider-assessed sleep disorder (chart review or provider interview).
| Authors | N(men) | Groups | Age M(SD) yrs (unless noted) | MOUD dose M(SD) mg (unless noted) | MOUD duration M(SD) (unless noted) | Sleep assessment | Sleep-related findings |
|---|---|---|---|---|---|---|---|
| Li et al. [50] | 1290 (1100) | MMT | 43.7(7.9) | 61.2 | ≥3 mos | Single time-point retrospective chart review for documented psychoeducation or psychotropic medication for sleep problem | 469 with documented sleep problem also had higher dose of MMT than those without, even after controlling for demographic and MMT characteristics (p < 0.001) |
| Li et al. [52] | 152(127) | MMT | 42.1(7.8) | Increased during study from 0 to 69.7(30.0) | 0, 2 yrs | 2 time-point retrospective chart review (upon entering MMT, 2 years later) for documented psychoeducation or psychotropic medication for sleep problem | 29 developed sleep problem during 2-year period: predictors were earlier age of onset heroin exposure, low attendance rate, shorter time to maximum methadone dose (ps < 0.05) |
| Roncero et al. [51] | 621(525) | MOUD Phases: (1) 29 Induction (2) 504 Maintenance (3) 86 Reduction (94% MMT) |
38.9(NR) | Of those on MMT: (1) 50.5(18.1) (2) 61.5(49.1) (3) 29.2(29.8) |
Phase reported rather than time | Physician interview assessing for sleep disorder based on DSM-IV-TR criteria | 42% of men and 40% of women met criteria for a sleep disorder; those with sleep disorders were older, worse overall working status, not driving, and currently abusing heroin or benzodiazepines (ps < 0.05); prevalence did not vary by methadone dose |
| Schäfer et al. [53] | 2414 (1653) | (1) 1261 MMT HCV+ (2) 536 MMT HCV− (3) 353 BMT HCT+ (4) 264 BMT HCV− |
34.8(8.1) | Time point 1: (1) 79.5(46.9) (2) 68.4(43.5) (3) 6.9(4.9) (4) 6.8(5.6) |
Time point 1: (1) 6.6(5.4) yrs (2) 4.2(4.1) yrs (3) 5.4(5.0) yrs (4) 3.6(3.5) yrs |
2 time-point structured interview (1 year apart) assessing for 1CD 10 sleep disorder diagnosis | In HCV+ patients rates of new sleep disorder doubled in one-year time period (12%–24%); significantly more than HCV− patients (p < 0.05) |
Note: M = mean; SD = standard deviation; yrs = years; MOUD = medication for opioid use disorder; mg = milligrams; MMT = Methadone Maintenance Treatment; mos = months; NR = not reported; DSM-IV-TR = Diagnostic and Statistical Manual of Mental Disorders, fourth edition, Text Revision; HCV+ = hepatitis C virus; HCV− = No hepatitis C virus; BMT = buprenorphine assisted treatment ICD 10 = International Statistical Classification of Diseases and Related Health Problems 10th Revision.
3.3.1. Prevalence of sleep disturbance
Similar to self-report measures, this approach revealed a high number of sleep issues. The two single time-point studies found a documented sleep disorder in 36% of a sample of 1290 individuals receiving MOUD [50] and 41% of a sample of 621 [51]. The studies that administered assessments twice across multiple years allowed for prospective analysis to determine incidence of new sleep issues during the course of MOUD treatment. One found 19% of 152 individuals had an incidence of a new sleep problem requiring treatment from the time they entered MOUD treatment until the second time point two years later [52]. The other found an increased rate of sleep disorders in those with hepatitis C (12% at the first time point, 24% one year later) but no increase in new sleep disorders in those without hepatitis C.
3.3.2. Comparison to non-MOUD groups and the general population
These studies did not have a comparison group nor did they discuss results in the context of the general population.
3.3.3. Correlates of sleep disturbance
Correlates of sleep disturbance found in these studies were older current age, younger age of heroin exposure, being unemployed or on disability, not driving, abusing benzodiazepines, and a diagnosis of hepatitis C [51–53].
MOUD dose returned mix results, as one found the presence of a sleep disturbance was correlated with a higher MOUD dose [50] and the other did not [51] (both samples were methadone only). Regarding other MOUD factors, low MOUD attendance and shorter time to maximum MOUD dose were associated with provider-assessed sleep disorder [52].
3.4. Sleep assessed with polysomnography (PSG)
Four studies assessed sleep using PSG in sample sizes ranging from 30 to 88 (see Table 4 for details). In three studies sleep disturbance was examined in the context of sleep parameters, sleep staging, or apnea indexes [12,13,54]. The fourth study did not examine sleep disturbance, but rather assessed the potential of conducting in-home PSG in this population, concluding this is a feasible method of sleep measurement of objective sleep data in this population, with 82% of the sample returning with at least one acceptable night of data [55].
Table 4.
Sleep assessed with polysomnography (PSG).
| Authors | N(mcn) | Groups | Age M(SD) yrs (unless noted) | MOUD dose M(SD) mg | MOUD duration M(SD) (unless noted) | PSC Description | Sleep-related findings |
|---|---|---|---|---|---|---|---|
| Kurth et al. [55] | 88(41) | MMT PSQI > 5 | 37.6 | 104.9 | 71.8 wks | 1 or 2 nights of in-home PSC | 81.7% completed at least one night of acceptable PSG data; self-report indicated 77.4% slept the same or better than usual |
| Staedt et al. [54] | 30(27) | (1) 10 MMT (2) 10 NMT (3) 10 No opioids |
(1) 36.6(4.4) (2) 30.0(5.0) (3) 26.0(4.0) |
(1) 47 (2) 50 |
(1) 33.0(13.4) mos (2) 10.0(9.8) mos |
1 night of in-lab PSG | TST M(SD) (1) 363(56) (2) 409(62) (3) 457(63) SOL M(SD) (1) 20(12) (2) 15(7) (3) 13(10) WASO M(SD) (1) 45(6) (2) 17(16) (3) 13(11) REM M(SD) (1) 76(20) (2) 98(42) (3) 97(16) SWS M(SD) (1) 36(35) (2) 44(22) (3) 87(22) |
| Teichtahl et al. [12] | 70(35) | (1) 50 MMT (2) 20 No opioids |
35(9) | NR | ≥2 mos | 2 in-lab PSGs (before and after HCVR and HVR) | OSAHI correlated with HCVR in MMT patients (p = 0.00001) |
| Wang et al. [13] | 70(35) | (1) 50 MMT (2) 20 No opioids |
35(9) | NR | ≥2 mos | 2 nights of in-lab PSG | TST M(SD) (1) 380(56) (2) 382(56) SOL M(SD) (1) 10(9) (2) 14(17) N1 M(SD) (1) 27(17) (2) 37(16) N2 M(SD) (1) 243(63) (2) 209(34) SWS M(SD) (1) 55(40) (2) 65(38) REM M(SD) 55(29) (2) 71(23) CAI M(SD) (1) 7(14) (2) 0(0) OSAHI M(SD) (1) 11 (10) (2) 9(9) methadone blood concentration associated with CAI (p = 0.025) |
Note: M = mean; SD = standard deviation; yrs = years; MOUD = medication for opioid use disorder; mg = milligrams PSG = polysomnography MMT = methadone maintenance treatment; PSQI = Pittsburgh Sleep Quality Index; wks = weeks; NMT = naltrexone maintenance treatment; mos = months; TST = total sleep time; SOL = sleep onset latency; WASO = wake after sleep onset; REM = rapid eye movement; SWS = slow wave sleep; NR = not reported; HCVR = hypercapnic ventilatory response; HVR = hypoxic ventilatory response; CAI = central apnea index; OSAHI = obstructive sleep apnea hypopnea index; N1 = stage 1 sleep; N2 = stage 2 sleep.
3.4.1. Prevalence of sleep disturbance
Two studies looked at PSG-assessed TST and SOL. Similar to self-reported sleep, mean TST in each sample was less than 7 h, though mean SOL in each sample was less than 30 min [13,54]. One study found the mean apnea indexes (central apnea index [CAI] and obstructive apnea hypopnea index OAHI) were both greater than 5, the cut-off for indicating sleep apnea [56].
3.4.2. Comparison to non-MOUD groups and the general population
One study found that those receiving MOUD generally had worse sleep than a group with no opioid use, with those on methadone experiencing less TST, rapid eye movement stage (REM), and slow wave sleep (SWS) with more SOL and wake after sleep onset (WASO) than those on naltrexone, while those on naltrexone were still worse than the non-opioid group in most areas [54]. Another study did not find significant differences between those receiving MOUD and those with no opioid use in PSG-measured SOL or TST, but did find significant differences in sleep architecture, with MOUD patients experiencing significantly less REM, stage 1 (N1), and stage 2 (N2) sleep [13]. Regarding respiratory events, one study reported increased respiratory events in those receiving MOUD when compared to controls, which was primarily driven by the central apnea index CAI, rather than the OAHI [12].
3.5. Multi-method sleep assessment
Ten studies were found that utilized at least one self-report sleep measure and at least one objective measure of sleep; sample sizes in these studies ranged from 19 to 71 (see Table 5) [11,14,57–64]. The most common combination was utilization of a sleep questionnaire or sleep diary with PSG.
Table 5.
Multi-method sleep assessment.
| Authors | N (men) | Groups | Age M(SD) yrs (unless noted) | MOUD dose M(SD) mg (unless noted) | MOUD duration M(SD) (unless noted) | Sleep Assessment M(SD) (unless noted) | Sleep-related Findings |
|---|---|---|---|---|---|---|---|
| Charpentier et al. [57] | 25(21) | MMT | 33.7(7.3) | 99.6(39.3) | >2 mos |
PSQI 10.4(4.7) ESS 5.0(4.0) 1 night in-home pulse oxiraetry % with SAS 20 |
No correlation between dose and ODI or ESS; PSQI and ESS correlated (p = 0.028) |
| Finan et al. [58] | 55(41) | (1) 26MMT (2) 29 BMT |
(1) 49.9(7.9) (2) 48.2(10.4) |
(1) 81.7(31.1) (2) 14.2(6.0) |
≥3 wks |
Daily Sleep Diary for 54(25) days SOL (1) 22(12) (2) 25(18) WASO (1) 23(18) (2) 26(23) TST (1) 418(96) (2) 405(101) SE% (1) 82(9) (2) 80(12) Daily in-home EEG for 5(1) days SOL (1) 21(18) (2) 19(17) WASO (1) 119(66) (2) 95(57) TST (1) 241(61) (2) 257(83) SE% (1) 65(17) (2) 69(16) N1% (1) 19(8) (2) 19(9) N2% (1) 46(14) (2) 53(13) SWS%(1) 14(8) (2) 13(13) REM% (1)14(8) (2) 15(7) |
(1) and (2) did not differ on any measures of sleep. Women had longer EEG TST, lower % N2, and greater % SWS than men (ps ≤ 0.03); Sleep diaries differed from EEG on WASO, TST, and SE (ps < 0.001) |
| Nguyen et al. [11] | 70(35) | (1) 50 MMT (2) 20 No opioids | 35(9) | NK | ≥2 mos |
FOSQ (1) 15.5(3.2) (2) 19.4(0.5) 1 night of in-lab PSG CAI (1) 7(14) (2) 0(0) AHI (l) 18(17) (2) 10(10) |
(1) had higher CAI and AHI and worse IBI variability and FOSQ than (2) (ps < 0.01); IBI variability related to blood methadone concentration (p < 0.01); CAI/AHI not correlated with FOSQ |
| Peles et al. [59] | 44(36) | MMT: (1)25 High dose (2) 19 Low dose |
(1) 49.2(9.0) (2) 41.8(8.7) |
(1) >150 (2) < 80 | (1) 6.8(3.1) yrs (2) 6.1(4.0) yrs |
PSQI (1) 12.9(3.8) (2) 10.9(3.6) ESS (l) 12.0(4.9) (2) 13.1(4.9) 1 night of in-lab PSG: AHI (1) 18(17) (2) 10(10) TST (1) 288(102) (2) 306(114) N1% (1) 0(0) (2) 0(0) N2% (1) 66(17) (2) 59(15) SWS% (1) 5(6) (2) 10(9) REM% (1)10(8) (214(10) SE% (1)72(23) 74(19) |
Higher PSQI and worse PSG variables those with chronic pain and/or BZD abuse, regardless of dose (ps < 0.05) |
| Peles et al. [60] | 23(19) | MMT | 23.6(9.0) | 6 mos 133.5(31.1); 12 mos 141.1(33.5) | 0, 6, 12 mos |
Time 0 mo. 6 mo, 12 mo PSQI 11.2(4.9), 11.0(5.6), 10.9(4.0) ESS 11.4(5.6), 12.5(10.8), 11.5(10.3) 1 night in-lab PSG SOL 26(24), 34(20), 32(53) TST 330(66). 312(114), 330(66) SE% 81(11), 74(22), 80(12) N1% 0(0). 0(0). 0(2) N2% 66(10), 60(17), 61(10) SWS% 7(9), 8(9), 10(8) REM% 14(8), 16(9), 17(8) % with OSA 26, 39. 44% with CSA 0, 0, 0 |
No significant changes in any variables at 6 mos or 12 mos |
| Sharkey et al. [61] | 71(29) | MMT PSQI > 5 | 37.7(8.1) | 108.3 (53.9) | 22.3(24.2) mos |
1 night of in-home PSG % with OSA 35 % with CSA 14 |
OSA related to higher BMI. longer MMT duration, and non-Caucasian race (ps < 0.05); CAI not related to close; PSQI not related to OSA/CSA |
| Sharkey et al. [62] | 62(24) | MMT PSQI > 5 | 39.2(8.3) | 107.9(51.7) | Med = 13 mos |
Pre-PSG 1-week sleep diary TST 340 (129) 1-night in-home-PSG TST 332 (131) Post-PSG morning questionnaire TST 323 (129) |
TST not related to gender, MMT duration, dose, or other drug use; questionnaire TST related to BZD use (p < 0.05); PSQI correlated with diary TST, feeling rested, and PSG SE (ps < 0.05); PSQI not related to AH1 or arousals |
| Sharkey et al. [63] | 50(23) | MMT PSQI > 5 (1) 14 1 PSG (2) 36 2 PSGs |
36.8 (range 22–52) | Med = 100 (range 25–310) |
Med = 286 days |
PSQI 12.6(2.9) 1 or 2 nights of in-home PSG WASO (1) 65(40) (2) 55(36) TST (l) 369(81) (2) 271(101) SE% (1) 85(9) (2) 83(10) N1% (1) 2.2(1.0) (2) 2.2(1.3) N2% (1) 68.4(9.4) (2) 65.2(10.0) SWS%(1) 16.4(8.1) (2) 15.8(8.0) REM%(1) 13.0(5.8) (2) 16.8(7.2) |
(2) had no significant differences between nights 1 and 2; (1) had worse sleep than (2) on TST, N2, REM (ps < 0.05) |
| Teichtahl et al. [64] | 19(12) | (1) 10 MMT (2) 9 No opioids |
(1) 33(6) (2) 32(6) |
50(19.7) | Range = 2–60 mos |
ESS (1) 11(6) (2) 3(4) 1-night in lab PSG TST (1) 326(90) (2) 392(62) SOL (1) 8(12) (2) 7(6) WASO (1) 59(32) (2) 29(8) N1 (1) 22(28) (2) 4(2) N2 (1) 222(67) (2) 225(45) SWS (1) 26(31) (2) 61(18) REM (1) 57(39) (2) 89(23) % with OSA (1) 60 (2) NR % with CSA (1) 60 (2) NR |
|
| Wang et al. [14] | 70(35) | (1) 50 MMT (2) 20 No opioids |
35(9) | NR | ≥2 mos |
ESS (1) 7.1(5.0) (2) 2.05(1.8) FOSQ (1) 15.5(3.2) (2) 19.4(0.5) 2 nights in-lab PSG TST (l) 380(56) (2) 382(53) SOL (1) 10(9) (2) 14(17) N1 (1) 27(17) (2) 38(16) N2 (1) 243(63) (2) 209(34) SWS (1) 55(40) (2) 65(38) REM (1) 55(29) (2) 71(23) OSAHI (1) 10.8(10.3) (2) 9.4(9.1) CAI (1) 6.7(14.2) (2) 0.25(0.33) |
CSA, blood methadone concentration, and sleep architecture did not predict ESS or FOSQ |
Note: M = mean; SD = standard deviation; yrs = years; MOUD = Medication for opioid use disorder; mg = milligrams; MMT = methadone maintenance treatment; mos = months; PSQI = Pittsburgh Sleep Quality Index; ESS = Epworth Sleepiness Scale; SAS = sleep apnea syndrome; ODI = oxygen desaturation index; BMT = buprenorphine maintenance treatment; wks = weeks; SOL = sleep onset latency; WASO = wake after sleep onset; TST = total sleep time; SE = sleep efficiency; EEG = electroencephalogram; N1 = stage 1 sleep; N2 = stage 2 sleep; SWS = slow wave sleep; REM = rapid eye movement; NR = not reported; FOSQ = Functional Outcomes of Sleep Questionnaire; PSG = polysomnography; CAI = central apnea index; AHI = apnea-hypopnea index; IBI = inter-breath intervals; BZD = benzodiazepine; mo = months; OSA = obstructive sleep apnea; CSA = central sleep apnea; BMI = body mass index; Med = median; OSAHI = obstructive sleep apnea-hypopnea index.
3.5.1. Prevalence of sleep disturbance
PSQI scores were reported in six studies, three of which required PSQI >5 for inclusion in the study [61–63]. For the three studies that did not require this, the average score was still well above the cut-off of 5, ranging from 10 to 13 [57,59,60]. Five studies administered the ESS. Again using the cut-off score of 10, two studies found “normal” sleepiness in those receiving MOUD [14,57] and three found averages just above cut-off, ranging from 11 to 13 [59,60,64].
Mean TST measured by sleep diaries and PSG was under 7 h in multiple studies [14,55,58], and under 6 h in several others [59,60,62,64], which was noted to be enough deprivation to cause daytime impairment. Of note, a multi-week daily diary study found no differences in TST, SOL, WASO, or sleep efficiency (SE) in those on methadone and those on buprenorphine [58].
Studies that reported percentage of sample with sleep apnea were variable, with central sleep apnea (CSA) ranging from 0 to 60% and obstructive sleep apnea (OSA) ranging from 26% to 60%, with one study combining CSA and OSA and finding prevalence of 20% [57,60,61,64]. All studies that documented apnea indexes reported means greater than the cut-off of 5 [11,14,59].
3.5.2. Comparison to non-MOUD groups and the general population
Two studies utilized the Functional Outcomes of Sleep (FOSQ) [65], a self-report questionnaire, and found control subjects reported fewer sleep-related daytime functioning problems than those receiving MOUD [11,14]. In examining respiratory variables two studies found the mean CAI to be worse than controls while mean OAHI was comparable to those with no opioid use [11,14], however, these and two others all found that overall prevalence and severity of OSA was worse than CSA in the MOUD samples [60,61], with the exception of one that found an equal rate (60%) of CSA and OSA in those engaged in MOUD [64].
3.5.3. Correlates of sleep disturbance
Notable factors presented in these multi-method studies are those of gender and use of benzodiazepines. One study found women tended to have longer TST and more SWS than men [58], while another found no relationship between gender and TST [62]. Regarding benzodiazepine use, two studies found worse self-reported and objectively measured sleep variables in those who using benzodiazepines, regardless of reason for use [59,62].
Several studies attempted to examine the relationship between sleep and MOUD dose. Using self-report sleep measures, one study found no relationship between blood methadone concentration and the FOSQ or the ESS [14]. Another study found no relationship between methadone dose or duration and TST [62]. Two studies also examined the relationship between MOUD dose and respiratory variables, with one finding no correlation between dose and the amount of oxygen desaturation [57] while another found increased MOUD dose was related to increased variability between breaths [11].
3.5.4. Comparison of self-report measure(s) to objective measure(s)
When comparing self-report measures to PSG or EEG (the aspect of PSG that allows for assessment of sleep staging) results were mixed. One study found significant differences between subjective and objective TST, WASO, and SE [58] but another study found TST as measured by PSQI, sleep diaries, and PSG were all consistent with each other [62].
The three that examined respiratory measurements and self-reported sleep experience found no relationship between self-reported measures of sleep and sleepiness and various apnea indices [11,14,61].
4. Discussion
In attempting to clarify the prevalence of sleep disturbance in the population, how sleep compares to non-MOUD groups, correlates of sleep disturbance, and the relationship between self-report and objective measures of sleep, several consistent findings emerged across studies. First, there was a high prevalence of persistent perceived sleep disturbance, with self-report scores across all studies reflecting numerous subjective sleep issues, including difficulty falling and/or staying asleep, poor sleep quality, short sleep duration, and perceived daytime impairment (eg, difficulty concentrating, irritability, etc.) as a result of sleep disturbance. Second, unsurprisingly, there were multiple psychiatric comorbidities and substance use factors linked to sleep disturbance, including past or present depression and anxiety, earlier age of first opiate use, and concurrent benzodiazepine use. Third, subjective and objective measures of quantitative sleep parameters were generally worse than that found in the general population. Most notably, the mean TST of every study was less than seven hours, which is less than the recommended seven to nine hours per night [66], and many reported a mean less than 6 h, which has been linked to increased mortality hazard [67], Fourth, studies that reported apnea indices found that apnea was worse in those receiving MOUD than in those with no opioid use, though self-reported sleep found no relationship between severity of apnea and self-reported sleep problems. Fifth, daytime sleepiness was not a problem despite the self-reported sleep disturbance and objectively measured short sleep duration.
All aspects of sleep disturbance, however, were not this straightforward. First, the relationship between sleep problems and some sociodemographic factors were inconsistent, with unemployment, relationship status, and gender being the most commonly assessed of these types of variables. Unemployment and relationship status were likely due to great differences in measurement of these variable across studies (eg, some giving 2 options for relationship status while some gave 5), making it difficult to compare studies and clearly interpret how these factors impact sleep. Gender is perhaps the most complicated of these factors, as there were discrepancies in both self-reported and objective measures across studies. It is difficult to say what this means or propose any interpretations, in part because women are largely underrepresented in these studies overall. Eleven of the studies that met criteria for the current review had only men as participants and 16 other studies had fewer than 30% women. This represents a critical gap in the literature as the most recent evidence suggests the number of women seeking treatment for OUD is equal to or greater than the number of men [68].
Second, and perhaps most important in the context of this review, findings related to the relationship between sleep disturbance and various MOUD factors returned mixed results. Broadly speaking, a higher dose was related to worse self-reported sleep but was not related to having a sleep disorder or worse PSG measures. Importantly, all of the studies that examined this relationship heavily consisted of individuals receiving methadone. One study examined methadone, diacetylmorphine, and buprenorphine and found worse self-reported sleep related to dose only in those engaged in methadone treatment, suggesting perhaps this dose/sleep disturbance relationship is a characteristic of methadone but not the other two MOUD approaches [18]. Regarding MOUD duration, the majority, though not all, found longer treatment duration associated with increased self-reported sleep. Given the purpose of this review was simply to follow sleep of those “stabilized” on MOUD, the resulting mean MOUD duration of the studies included ranged from 3 weeks to 7 years. There may be countless nuances to this relationship that are lost in that lengthy timeframe, and future studies are needed to further clarify the relationship between sleep disturbance and time course of MOUD.
The present review was limited by multiple factors. It is possible that there have been other studies that did not meet criteria for this review that can add to a comprehensive picture of sleep disturbance during MOUD, such as well controlled clinical trials that were intentionally excluded from this review. Additionally, the majority of studies that met criteria for this review examined methadone and thus may reflect primarily the sleep problems specific to methadone maintenance therapy. However, as can be seen in the tables, multiple studies have shown other types of MOUD also have a high prevalence of self-reported sleep disturbance and poor sleep parameters, staging, and respiratory variables. Several of the studies that included other types of MOUD have been done in recent years. In fact, there has been a marked increase in the study of sleep in this population. Of the 34 studies that used a validated self-report measure of sleep (Tables 2 and 5), 21 (61.7%) were published in 2015 or later, perhaps indicating the field is beginning to find this type of information useful in studying treatment process and outcomes in MOUD.
These results show strong evidence for persistence of insomnia and sleep related breathing problems even after OUD is managed with MOUD, and they stress the importance of comprehensive assessment and treatment of sleep concerns in this population. Assessment may include a clinical interview that screens for insomnia and other sleep disorders, such as the Structured Clinical Interview for Sleep Disorders [69], and an assessment of the bedroom and sleeping environment to understand external factors that may be impacting sleep. Of particular importance is screening for signs of sleep related breathing problems (eg, sleep apnea), including snoring, gasping during sleep, and daytime sleepiness, which warrant referral for a sleep study. Evidence-based treatments, such as cognitive behavioral therapy for insomnia and continuous positive airway pressure for sleep apnea, have the potential to markedly improve sleep and subsequent daytime dysfunction. Additionally, given the high comorbidity of depression, anxiety, and substance use in many of these studies, it is possible improvement in sleep will positively impact these symptoms [70]. Conversely, due to the bidirectional nature of sleep and psychiatric comorbidity, it is likely targeting these symptoms with evidence-based treatment, such as cognitive behavioral therapy for depression or acceptance and commitment therapy, could improve insomnia and other self-reported sleep complaints.
5. Conclusion
Accurately assessing and interpreting findings related to sleep in any population can be difficult due to the various assessment types utilized to study the numerous and diverse aspects of the sleep experience. As more evidence emerges that addressing sleep problems in those with OUD may help improve rates of MOUD treatment entry and retention [8], it is increasingly important to identify and understand the nature and course of sleep disturbance in this population. This review provides strong evidence there is a high prevalence of self-reported and objectively measured persistent sleep disturbance in individuals receiving MOUD and highlights the importance of assessment and treatment of sleep problems in this population. Similar to other substance use disorders, it is possible poor sleep could be targeted as a potential intervention for improving comorbidity as well as MOUD treatment entry and retention, though there is a critical need for further research to fully understand this relationship. To fill some of the gaps in the literature found here, futures studies would benefit from more clearly defined demographic information, greater inclusion of percentage of women in the samples, longitudinal, multi-method data collection across multiple days or even weeks to more accurately assess the nuances of the relationship of sleep with MOUD dose, duration, and treatment outcomes.
Funding
This work was supported by NIDA Grant NI K 12 DA031794.
Footnotes
Conflict of interest
None declared.
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: https://doi.org/10.1016/j.sleep.2021.05.021.
References
- [1].Reinhart M, Scarpati LM, Kirson NY, et al. The economic burden of abuse of prescription opioids: a systematic literature review from 2012 to 2017. Appl Health Econ Health Policy 2018;16(5):609–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bell J, Strang J. Medication treatment of opioid use disorder. Biol Psychiatry 2020;87(1):82–8. [DOI] [PubMed] [Google Scholar]
- [3].Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet 2019;393(10182):1760–72. [DOI] [PubMed] [Google Scholar]
- [4].McCarty D, Priest KC, Korthuis PT. Treatment and prevention of opioid use disorder: challenges and opportunities. Annu Rev Public Health 2018;39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].National Academies of Sciences E, Medicine. Medications for opioid use disorder save lives. National Academies Press; 2019. [PubMed] [Google Scholar]
- [6].Examining the role of the orexin system in sleep and stress in persons with opioid use disorder. NHLBI; 2020. Clinicaltrials.gov [Internet] Identifier NCT04287062, https://clinicaltrials.gov/ct2/show/NCT04287062. [Accessed 1 January 2021]. [Google Scholar]
- [7].Carroll KM, Nich C, Frankforter TL, et al. Accounting for the uncounted: physical and affective distress in individuals dropping out of oral naltrexone treatment for opioid use disorder. Drug Alcohol Depend 2018;192:264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tripathi R, Rao R, Dhawan A, et al. Opioids and sleep — a review of literature. Sleep Med 2020;67:269–75. [DOI] [PubMed] [Google Scholar]
- [9].Zheng W, Wakim R, Geary R, et al. Self-reported sleep improvement in buprenorphine MAT (medication assisted treatment) population. Austin J Drug Abuse Addict 2016;3(1). [PMC free article] [PubMed] [Google Scholar]
- [10].Saloner B, Daubresse M, Alexander GC. Patterns of buprenorphine-naloxone treatment for opioid use disorder in a multi-state population. Med Care 2017;55(7):669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nguyen CD, Kim JW, Grunstein RR et al. Respiratory variability during sleep in methadone maintenance treatment patients. J Clin Sleep Med 2016; 12(4): 607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Teichtahl H, Wang D, Cunnington D, et al. Ventilatory responses to hypoxia and hypercapnia in stable methadone maintenance treatment patients. Chest 2005; 128(3): 1339–47. [DOI] [PubMed] [Google Scholar]
- [13].Wang D, Teichtahl H, Drummer O, et al. Central sleep apnea in stable methadone maintenance treatment patients. Chest 2005;128(3):1348–56. [DOI] [PubMed] [Google Scholar]
- [14].Wang D, Teichtahl H, Goodman C, et al. Subjective daytime sleepiness and daytime function in patients on stable methadone maintenance treatment: possible mechanisms. J Clin Sleep Med 2008;4(6):557–62. [PMC free article] [PubMed] [Google Scholar]
- [15].Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28: 193–213. [DOI] [PubMed] [Google Scholar]
- [16].Baykara S, Alban K. The effects of buprenorphine/naloxone maintenance treatment on sexual dysfunction, sleep and weight in opioid use disorder patients. Psychiatry Res 2019;272:450–3. [DOI] [PubMed] [Google Scholar]
- [17].Chan Y-Y, Yang S-N, Lin J-C, et al. Inflammatory response in heroin addicts undergoing methadone maintenance treatment. Psychiatry Res 2015;226(1): 230–4. [DOI] [PubMed] [Google Scholar]
- [18].Al Chrobok, Krause D, Winter C, et al. Sleeping patterns in patients with opioid use disorder: effects of opioid maintenance treatment and detoxification. J Psychoact Drugs 2020:1–8. [DOI] [PubMed] [Google Scholar]
- [19].Hsu W-Y, Chiu N-Y, Liu J-T, et al. Sleep quality in heroin addicts under methadone maintenance treatment. Acta Neuropsychiatr 2012;24(6): 356–60. [DOI] [PubMed] [Google Scholar]
- [20].Peles E, Hacohen S, Sason A, et al. Is a history of sexual abuse related to poor sleep among former opioid-addicted women with and without methadone maintenance treatment? Subst Use Misuse 2017;52(11):1478–85. [DOI] [PubMed] [Google Scholar]
- [21].Peles E, Sason A, Tene O, et al. Ten years of abstinence in former opiate addicts: medication-free non-patients compared to methadone maintenance patients. J Addict Dis 2015;34(4):284–95. [DOI] [PubMed] [Google Scholar]
- [22].Peles E, Schreiber S, Adelson M. Variables associated with perceived sleep disorders in methadone maintenance treatment (MMT) patients. Drug Alcohol Depend 2006;82(2):103–10. [DOI] [PubMed] [Google Scholar]
- [23].Peles E, Schreiber S, Domany Y, et al. Achievement of take-home dose privileges is associated with better-perceived sleep and with cognitive status among methadone maintenance treatment patients. World J Biol Psychiatry 2014;15(8):620–8. [DOI] [PubMed] [Google Scholar]
- [24].Stein MD, Herman DS, Bishop S, et al. Sleep disturbances among methadone maintained patients. J Subst Abuse Treat 2004;26(3): 175–80. [DOI] [PubMed] [Google Scholar]
- [25].Tripathi R Dhawan A, Rao R, et al. Assessment of subjective sleep problems in men with opioid dependence maintained on buprenorphine. J Addict Med 2020;142:132–8. [DOI] [PubMed] [Google Scholar]
- [26].Zahari Z, Ibrahim MA, Tan SC, et al. Sleep quality in opioid-naive and opioid-dependent patients on methadone maintenance therapy in Malaysia. Turk J Med Sci 2016;46(6):1743–8. [DOI] [PubMed] [Google Scholar]
- [27].Zahari Z, Lee CS, Ibrahim MA, et al. The AC/AG diplotype for the 118A> G and IVS2+ 691G> C polymorphisms of OPRM1 gene is associated with sleep quality among opioid-dependent patients on methadone maintenance therapy. Pain Ther 2016;5(l):43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zahari Z, Lee CS, Mohamad N, et al. Association between perceived sleep disorders and sleep related factors among patients on methadone maintenance therapy (MMT) in Malaysia. Int Med J 2016;23(2):134–7. [Google Scholar]
- [29].Zahari Z, Siong LC, Musa N, et al. Demographic profiles and sleep quality among patients on methadone maintenance therapy (MMT) in Malaysia. Pak J Pharm Sci 2016;29(1). [PubMed] [Google Scholar]
- [30].Khazaie H, Najafi F, Ghadami MR, et al. Sleep disorders in methadone maintenance treatment volunteers and opium-dependent patients. Addict Health 2016;8(2):84. [PMC free article] [PubMed] [Google Scholar]
- [31].Le TA, Dang AD, Tran AHT, et al. Factors associated with sleep disorders among methadone-maintained drug users in Vietnam. Int J Environ Res Public Health 2019;16(22):4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang H-S, Xu Y-M, Zhu J-H, et al. Poor sleep quality is significantly associated with low sexual satisfaction in Chinese methadone-maintained patients. Medicine 2017;96(39). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stewart A, Ware J. In: The Medical outcomes study approach. Durham, NC: Duke University Press; 1992. [Google Scholar]
- [34].Douglass AB, Bomstein R, Nino-Murcia G, et al. The sleep disorders questionnaire I: creation and multivariate structure of SDQ. Sleep 1994; 17(2): 160–7. [DOI] [PubMed] [Google Scholar]
- [35].Roth T, Zammit G, Kushida C, et al. A new questionnaire to detect sleep disorders. Sleep Med 2002;3(2):99–108. [DOI] [PubMed] [Google Scholar]
- [36].Hunt SM, McKenna S, McEwen J, et al. The Nottingham Health Profile: subjective health status and medical consultations. Soc Sci Med A 1981;15(3): 221–9. [DOI] [PubMed] [Google Scholar]
- [37].Alonso J, Anto JM, Moreno C. Spanish version of the Nottingham Health Profile: translation and preliminary validity. Am J Public Health 1990;80(6): 704–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dunn KE, Finan PH, Tompkins DA, et al. Frequency and correlates of sleep disturbance in methadone and buprenorphine-maintained patients. Addict Behav 2018;76:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fudalej S, Ilgen M, Kolodziejczyk I, et al. Somatic comorbidity and other factors related to suicide attempt among Polish methadone maintenance patients. J Addict Med 2015;9(6):433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Torrens M, San L, Martinez A, et al. Use of the Nottingham Health Profile for measuring health status of patients in methadone maintenance treatment. Addiction 1997;92(6):707–16. [PubMed] [Google Scholar]
- [41].American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. 2013. Washington, DC. [Google Scholar]
- [42].Morin CM, Espie CA. Insomnia: a clinical guide to assessment and treatment. New York: Kluwer Academic/Plenum Publishers; 2003. [Google Scholar]
- [43].Soldatos CR, Dikeos DG, Paparrigopoulos TJ. The diagnostic validity of the Athens insomnia scale. J Psychosom Res 2003;55(3):263–7. [DOI] [PubMed] [Google Scholar]
- [44].Crönlein T, Langguth B, Popp R, et al. Regensburg Insomnia Scale (RIS): a new short rating scale for the assessment of psychological symptoms and sleep in insomnia; study design: development and validation of a new short self-rating scale in a sample of 218 patients suffering from insomnia and 94 healthy controls. Health Qual Life Outcome 2013;11(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Albonaim A, Fazel H, Sharafshah A, et al. Association of OPRK1 gene polymorphisms with opioid dependence in addicted men undergoing methadone treatment in an Iranian population. J Addict Dis 2017;36(4):227–35. [DOI] [PubMed] [Google Scholar]
- [46].Hallinan R, Elsayed M, Espinoza D, et al. Insomnia and excessive daytime sleepiness in women and men receiving methadone and buprenorphine maintenance treatment. Subst Use Misuse 2019:1–10. [DOI] [PubMed] [Google Scholar]
- [47].Sharafshah A, Fazel H, Albonaim A, et al. Association of OPRD1 gene variants with opioid dependence in addicted male individuals undergoing methadone treatment in the north of Iran. J Psychoact Drugs 2017;49(3):242–51. [DOI] [PubMed] [Google Scholar]
- [48].Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14(6):540–5. [DOI] [PubMed] [Google Scholar]
- [49].Ohayon M, Wickwire EM, Hirshkowitz M, et al. National Sleep Foundation’s sleep quality recommendations: first report. Sleep Health 2017;3(1):6–19. [DOI] [PubMed] [Google Scholar]
- [50].Li DJ, Chung KS, Wu HC, et al. Factors affecting the dose of methadone among patients receiving methadone maintenance therapy in Taiwan. Am J Addict 2018;27(3):225–30. [DOI] [PubMed] [Google Scholar]
- [51].Roncero C, Barral C, Rodríguez-Cintas L, et al. Psychiatric comorbidities in opioid-dependent patients undergoing a replacement therapy programme in Spain: the PROTEUS study. Psychiatry Res 2016;243:174–81. [DOI] [PubMed] [Google Scholar]
- [52].Li D-J, Chung K-S, Wu H-C, et al. Predictors of sleep disturbance in heroin users receiving methadone maintenance therapy: a naturalistic study in Taiwan. Neuropsychiatr Dis Treat 2018; 14:2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schäfer A, Wittchen HU, Backmund M, et al. Psychopathological changes and quality of life in hepatitis C virus-infected, opioid-dependent patients during maintenance therapy. Addiction 2009;104(4):630–40. [DOI] [PubMed] [Google Scholar]
- [54].Staedt J, Wassmuth F, Stoppe G, et al. Effects of chronic treatment with methadone and naltrexone on sleep in addicts. Eur Arch Psychiatry Clin Neurosci 1996;246(6):305–9. [DOI] [PubMed] [Google Scholar]
- [55].Kurth ME, Sharkey KM, Millman RP, et al. Insomnia among methadone-maintained individuals: the feasibility of collecting home polysomnographic recordings. J Addict Dis 2009;28(3):219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017;13(3): 479–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Charpentier A, Bisac S, Poirot I, et al. Sleep quality and apnea in stable methadone maintenance treatment. Subst Use Misuse 2010;45(9):1431–4. [DOI] [PubMed] [Google Scholar]
- [58].Finan PH, Mun CJ, Epstein DH, et al. Multimodal assessment of sleep in men and women during treatment for opioid use disorder. Drug Alcohol Depend 2020:207:107698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Peles E, Schreiber S, Adelson M. Documented poor sleep among methadone-maintained patients is associated with chronic pain and benzodiazepine abuse, but not with methadone dose. Eur Neuropsychopharmacol 2009; 19(8): 581–8. [DOI] [PubMed] [Google Scholar]
- [60].Peles E, Schreiber S, Hamburger RB, et al. No change of sleep after 6 and 12 months of methadone maintenance treatment. J Addict Med 2011;5(2):141–7. [DOI] [PubMed] [Google Scholar]
- [61].Sharkey KM, Kurth ME, Anderson BJ, et al. Obstructive sleep apnea is more common than central sleep apnea in methadone maintenance patients with subjective sleep complaints. Drug Alcohol Depend 2010;108(1–2): 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sharkey KM, Kurth ME, Anderson BJ, et al. Assessing sleep in opioid dependence: a comparison of subjective ratings, sleep diaries, and home polysomnography in methadone maintenance patients. Drug Alcohol Depend 2011; 113(2):245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sharkey KM, Kurth ME, Corso RP, et al. Home polysomnography in methadone maintenance patients with subjective sleep complaints. Am J Drug Alcohol Abuse 2009;35(3): 178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Teichtahl H, Prodromidis A, Miller B, et al. Sleep-disordered breathing in stable methadone programme patients: a pilot study. Addiction 2001; 96(3): 395–403. [DOI] [PubMed] [Google Scholar]
- [65].Weaver T, Laizner A, Evans L, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep 1997;20:835–43. [PubMed] [Google Scholar]
- [66].Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health 2015;1(1):40–3. [DOI] [PubMed] [Google Scholar]
- [67].Kripke DF, Garfinkel L, Wingard DL, et al. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry 2002;59(2):131–6. [DOI] [PubMed] [Google Scholar]
- [68].Lyden J, Binswanger IA. The United States opioid epidemic. In: Paper presented at: Seminars in perinatology; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Taylor DJ, Wilkerson AK, Pruiksma KE, et al. Reliability of the structured clinical interview for DSM-5 sleep disorders module. J Clin Sleep Med 2018; 14(3):459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Alvaro PK, Roberts RM, Harris JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep 2013;36(7): 1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]


