Abstract
Cryptosporidiosis has been considered as a serious diarrheal disease, especially in immunodeficient patients, where they failed to clear the infection leading to several consequences of infection (i.e death). The role of cell mediated immunity in clearing the infection was demonstrated by the increased susceptibility of HIV/AIDS patients to infection. To date, no specific treatment has been proven for cryptosporidiosis in immunodeficient patients. The study aimed to evaluate the efficacy of Aloe vera gel for the treatment of cryptosporidiosis in immunocompetent and dexamethasone immunosuppressed mice in comparison to that of nitazoxanide. Mice were orally administrated with Aloe vera gel, in a daily dose of 250 mg/L in drinking water, for 14 consecutive days post infection. Parasitological, molecular and immunological measurements were recorded on the 7th, 14th, 21st and 32nd days post infection. Our in vitro results showed that 250 mg/L of prepared gel achieved the highest parasitic reduction. The body weights of Aloe vera treated mice on the 21st and 32nd day post infection, either in immunocompetent or immunosuppressed groups, were nearly the same as those of their corresponding control groups. Aloe vera gel succeeded in clearing cryptosporidiosis with a percent reduction of 100% in immunocompetent mice and 99.67% in immunosuppressed mice. The anti-inflammatory effect of Aloe vera reduced the levels of IFN-γ, IL-4, -6 and -17. The success of Aloe vera gel, in clearing cryptosporidiosis in immunosuppressed mice, was obvious either from the reduction of Cryptosporidium DNA or the oocysts in stool samples; and from the improvement of histopathological sections.
Keywords: Cryptosporidium parvum, Oocyst, Dexamethasone, IFN-γ, IL-10
Graphical abstract
1. Introduction
More than forty Cryptosporidium (C.) spp. have been described, but C. hominis and C. parvum account for the most cases of cryptosporidiosis in human (Feng et al., 2018). Moreover, twenty other species/genotypes have been reported in humans, such as C. meleagridis, C. cuniculus, C. ubiquitum, C. canis, C. felis and C. viatorum (Feng et al., 2018; Zahedi and Ryan, 2020; Lebbad et al., 2021). C. parvum is an apicomplexan protozoan parasite (Chen et al., 2002) that leads to gastrointestinal problems such as severe diarrhea in both immunocompromised and immunocompetent patients. It is responsible for acute self-limiting diarrhea in immunocompetent patients and fatal diarrhea in immunocompromised ones; especially in transplant recipients, persons receiving immunosuppressive drugs and HIV/AIDS patients (Chen et al., 2002).
The immune condition of the patients plays an important role in determining the degree and severity of the infection. C. parvum mainly infects the distal small intestine; but in immunodeficient patients it may infect extraintestinal places like the lungs, biliary tract and pancreas (Borad and Ward, 2010). Cryptosporidiosis was reported in 24% of HIV/AIDS patients especially before the use of highly active antiretroviral therapy (HAART). The severity of the disease was linked to the degree of immunosuppression in these patients (Huang and White, 2006). In developing countries, patients can't afford HAART resulting in the re-emergence of cryptosporidiosis (Nannini and Okhuysen, 2002). Although more than 100 anti-cryptosporidial drugs have been identified, nitazoxanide (NTZ) is the only FDA approved drug in immunocompetent patients (Abubakar et al., 2007). In HIV/AIDS patients, the immune reconstitution with HARRT results in abrogation or clearance of the infection (Borad and Ward, 2010).
NTZ is a thiazolide compound that shows broad spectrum activities against many helminths and protozoa (Hemphill et al., 2006). However, the treatment efficacy of NTZ can be determined by the patient's immune system (Sparks et al., 2015). NTZ achieved a poor result in treatment of immunocompromised patients and a temporary effect with relapses in immunocompetent patients (Cabada and White, 2010). These facts necessitate the search for other alternatives. Plants possess many potential effective agents against protozoans. World Health Organization has considered medicinal plants as the best source for obtaining a variety of drugs. Several plants have powerful anti-inflammatory, antioxidant and anti-protozoal effects (Farid et al., 2020b&c and 2021a). Aloe vera is a succulent plant that grows in tropical climates. The parenchymal cells of the fresh leaves produce a colorless substance, known as Aloe gel, which contains several polysaccharides such as pectins, hemicelluloses, glucomannan, acemannan and other mannose derivatives. Nowadays, the gel is used in treatment of many inflammatory diseases such as arthritis, wound healing, radiation and thermal burns (Bałan et al., 2014). Ancient Egyptian papyrus described the use of Aloe vera in treatment of infections, skin problems and as a laxative (Shelton, 1991).
The plant leaf has two layers; the latex lining and the inner gel. The potentially active chemical constituents of Aloe vera are polysaccharides (glucomannan and acemannan), carboxypeptidase, magnesium, zinc, calcium, glucose, cholesterol, salicylic acid, prostaglandin precursors, vitamins (A, C, E), lignins, saponins, plant sterols and amino acids (Atherton, 1998) besides anthraquinone glycosides (aloin, aloe-emodin and barbaloin) from the latex lining. The gel, obtained from the leaf flesh, has quite different compounds from the bitter latex extracted from the leaf lining (Klein and Penneys, 1988). Aloe gel is 99% water (pH of 4.5) and contains an emollient polysaccharide, known as glucomannan. Glucomannan has a good moisturizing effect that accounts for its usage in many cosmetics. Acemannan, a carbohydrate fraction, is a water soluble long chain mannose polymer that stimulates wound healing, modulates immune function (particularly macrophage activation and cytokines production) and demonstrates antineoplastic and antiviral effects (Peng et al., 1991). The gel also contains bradykininase (an anti-inflammatory agent) (Yagi et al., 1982), magnesium lactate (which can prevent itching), salicylic acid and other antiprostaglandin compounds that relieve inflammation. Pathak and Sharma (2017) reported the anti-inflammatory, anti-arthritic, antibacterial and hypoglycaemic effects of Aloe vera total leaf extract in in vitro studies and animal models. Abdulrahman et al. (2019) examined the synergistic effect of Aloe vera and Hyptis suaveolens against Giardia lamblia and Salmonella species infections among children in Nigeria. They showed that Aloe vera have better activities than Hyptis suaveolens extracts; and added that the combined extracts showed better activities against giardiasis. Previously, Farid et al. (2020a) reported that Aloe vera could be used as a therapy for G. intestinalis in infected rats with no side effects, as it significantly decreased the count of both cysts and trophozoites. Moreover, the anti-inflammatory effect of Aloe vera was obvious from the reduction of inflammatory cytokines (IFN-γ, IL-4 and IL-6). Therefore, the study aimed to examine the use of Aloe vera gel against cryptosporidiosis in immunocompetent and immunosuppressed infected mice. Moreover, its anti-inflammatory effect was examined by measuring T helper (Th)-1, Th-2 and Th-17 cytokines; in addition to the histopathological studies.
2. Materials and methods
2.1. Drugs
NTZ (Alinia, Romark laboratories, USA): 100 mg/kg/day were orally administrated starting ten days post infection for 14 consecutive days (Li et al., 2003). Dexamethazone (Dexazone): 0.5 mg tablets were provided by Kahira Pharmaceuticals and Chemical Industries Company, Cairo, Egypt.
2.2. Preparation of Aloe vera gel powder
Aloe vera leaves were collected from 2-year old plant with approximately 50 cm length from the garden of Faculty of Science, Cairo University, Egypt. Plant was identified by a specialized botanist in the department of Botany, Faculty of Science, Cairo University, Egypt. The voucher specimens were collected, identified and deposited at the herbarium of the department of Botany, Faculty of Science; Cairo University, Egypt. The plant name has been checked with http://www.theplantlist.org.
Leaves were washed with distilled water then by 0.5% chlorine. Aloe vera gel was collected carefully using a sharp knife and kept for 1 h at −18 °C. Frozen gel was reduced to powder by lyophilization under vacuum. Moisture, acidity, fiber content, ash content and pH were determined according to AOAC (AOAC, 2005). Protein content was determined spectrophotometrically according to Bradford (1976). Polysaccharide, acemannan, was quantified using the Congo red colorimetric assay (Eberendu et al., 2005). Analysis was performed in three samples and results were expressed as mean ± SD.
2.3. C. parvum oocysts identification
Oocysts were obtained from calves from the Faculty of Veterinary Medicine, Cairo University. The oocysts were identified as C. parvum oocysts at the molecular level by using nested PCR and PCRRFLP.
2.3.1. Collection of fecal samples
Oocysts were obtained by the collection of scrapings and cecal content of ileal mucous membrane according to Anderson (1985). Samples were examined by modified Ziehl–Neelsen staining method according to Henriksen and Pohlenz (1981) to confirm the presence of oocysts.
2.3.2. Immunofluorescence method
Immunofluorescence method was, also, used to prove the presence of Cryptosporidium oocysts prior to molecular identification. Samples were concentrated using formalin-ether method (FECT); briefly, 2 g of stool were mixed well with 10 ml of 10% formalin then filtered with a mesh screen (No. 10). Three ml of ether were added to the fecal suspension followed by strong shaking and centrifugation for 15 min at 500g. Supernatants were discarded and the sediments were placed on the slide and fixed with methanol and allowed to dry at room temperature. Fifty μl of diamino4’,6-diamidino-2-phenylindole dihydrochloride hydrate(DAPI, Sigma Chemical Co., USA) were added to slides and left for 2 min. Fifty μl of Cryptosporidium-specific IgG1 monoclonal antibody (EasyStain™, Biotechfrontiers, Australia) were added to the well followed by incubation at 37 °C for 15 min in a humidified chamber. Slides were washed and kept for 5 min in PBS (pH = 7.5). Slides were allowed to dry at room temperature, mounted and covered with coverslips. The slides were examined by a fluorescent microscope at 400x, where oocysts have ovoid or spherical brilliant apple/green structure with thick walls.
2.3.3. Nested PCR-RFLP
2.3.3.1. DNA extraction
QIAamp Fast DNA Stool Mini Kit (cat. no. 51604; Qiagen- Germany) was used for DNA extraction from stool samples of different experimental groups according to the manufacturer's instructions. Five minute freeze/thaw cycle was enabled for 5 times to ensure the rupture of oocyst wall. The kit isolated DNA from stool by a fast spin-column procedure; and the InhibitEX Buffer removed the PCR inhibitors from DNA efficiently.
2.3.3.2. DNA amplification by nested PCR
All primer specific to the C. parvum target genes used in this study have been described previously by several studies (Spano et al., 1997a&b; Yu et al., 2009). The primary PCR primers were BCOWPF (5′-ACCGCTTCTCAACAACCATCTTGTCCTC-3′) and BCOWPR (5′-CGCACCTGTTCCCACTCAATGTAAACCC-3′); while the nested PCR primers were Cry-15 (5′-GTAGATAATGGAAGAGATTGTG-3′) and Cry-9 (5′-GGACTGAAATACAGGCATTATCTTG-3′). The expected product sizes were 769-bp and 553-bp, respectively. Extracted DNA (2.5 μl) in 1x PCR buffer was mixed with MgCl2 (1.5 mM), dNTP (250 μM), primers (10 pmoles) and Taq DNA polymerase (1.25 units) to reach a volume of 25 μl. Samples were exposed to 30 cycles of denaturation at 94 °C for 1 min, annealing at 65 °C for 1 min and extension at 72 °C for 10 min. The previous reaction is known as extended COWP reaction. Nested COWP reaction used 2.5 μl of the extended COWP product to amplify 553-bp gene fragment (Spano et al., 1997b). All PCR procedures included negative and positive controls.
2.3.3.3. Restriction fragment length polymorphism (RFLP)
RsaI enzyme kit (ER1121 – Fermentas UAB, V. Graiciuno 8,LT-02241Vilnius, Lithuania) was used for digestion of nested PCR products. Where, 10 μl nested PCR product were mixed with 17 μl Nuclease-free water, 2 μl Green buffer and 1 μl RsaI Enzyme followed by gentle mixing and spinning for few seconds. Samples were incubated at 37 °C for 5 min. Digested fragments were visualized by agarose gel electrophoresis and UV light trans-illumination.
2.3.4. Animal infection
Infective oocysts were pooled, sieved and preserved with an equal volume of 2.5% potassium dichromate (Current et al., 1983) at 4 °C. For mice inoculation, oocysts were washed three times with PBS and counted with a haemocytometer; then diluted in distilled water to obtain 105oocysts/ml to be used for mice infection. Where mice were prevented from drinking water overnight before infection; then intraesophageally inoculated with the prepared inoculums using a tuberculin syringe connected to a polythene tube.
2.4. In vitro anti-C. parvum activity of Aloe vera gel
2.4.1. Culture media and cell maintenance
Human ileocecal adenocarcinoma cells (HCT-8, sigma-Aldrich Co., St. Louis, Missouri, USA) were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal calf serum, 1% L-glutamine, 20 mM HEPES, 100 U/mL penicillin G, 100 mg/L streptomycin and 0.5 mg/L amphotericin B. The cells (5 × 105) were maintained in well chamber slides (5% CO2 atmosphere, 37 °C and 100% humidity). Trypsinization, with phosphate buffered saline-EDTA/0.25% trypsin (by the ratio 1:1), was used for cells' passage.
2.4.2. C. parvum oocysts preparation and in vitro cell infection
C. parvum oocysts were suspended in a 10.5% bleach solution, possessing 9:1 sterile deionized water and 5.25% sodium hypochlorite, for 10 min. Oocysts were washed in sterile water, centrifuged and resuspended in DMEM (37 °C). Oocysts were added to the cells and incubated to facilitate excystation (5% CO2 atmosphere at 37 °C for 90 min). Unexcysted oocysts and oocyst debris were removed from the cells through washing with sterile PBS and the addition of DMEM. Cells were incubated at 37 °C under 5% CO2 for an additional 4 h. The culture medium was supplemented with DMEM containing Aloe vera gel at a concentration of 50, 100, 150, 200, 250 or 300 mg/L. Experiments were performed in triplicate in antibiotic-free plates. Forty eight hours after infection, the culture medium was aspirated and the cells were washed with sterile PBS; and then fixed with 100% methanol for 10 min. The cells were rehydrated with blocking buffer for 30 min. The slides were stained by the indirect antibody method according to Slifko et al. (1997); briefly, cells were labeled with a primary polyclonal antibody [prepared in Theodor Bilharz Research Institute (TBRI)], which reacts with all reproductive stages (sporozoites and merozoites) of C. parvum. Slides were washed three times with PBS followed by the addition of fluorescein isothiocyanate conjugated secondary goat antibody (Sigma). Ten ml of 2% 1,4-diazabicyclo[2.2.2]octane (DABCO)/glycerol (Sigma) was added to each well; and then the cells were viewed under epifluorescence microscopy. Within a 175-m-diameter area, a cluster of infection was defined as an average three intracellular C. parvum developmental stages according to Sifuentes et al. (2007).
2.5. Immunosuppression
After the determination of the daily water consumption of mouse (≈4 ml), 0.25 mg/kg/day dexamethasone was orally administrated in drinking water (at a concentration of 62.5 μg/ml) for 14 consecutive days prior to infection with C. parvum oocysts. The immunosuppressed mice continued to receive dexamethasone at the same dose throughout the experiment (Rehg et al., 1988).
2.6. Experimental animals
Six-week-old male BALB/c mice weighing 20–25 g were purchased from TBRI. All animals were bred and maintained under specific pathogen free conditions with standard diet (24% protein, 4% fat and about 4–5% fiber) and water provided ad libitum in the animal house, Faculty of Science, Cairo University. Animals were administrated with Aloe vera gel, in daily doses of 250 mg/L, for 14 successive days starting 10 days post infection. Animals were distributed into two divisions that contained six groups each (10 mice/group). Division one involved group Ic: Immunocompetent control mice, group IIc: NTZ treated immunocompetent non-infected mice, group IIIc: Aloe vera treated immunocompetent non-infected mice, group IVc: Non-treated immunocompetent infected mice, group Vc: NTZ treated immunocompetent infected mice and group VIc: Aloe vera treated immunocompetent infected mice. Division two involved group Is: Immunosuppressed control mice, group IIs: NTZ treated immunosuppressed non-infected mice, group IIIs: Aloe vera treated immunosuppressed non-infected mice, group IVs: Non-treated immunosuppressed infected mice, group Vs: NTZ treated immunosuppressed infected mice and group VIs: Aloe vera treated immunosuppressed infected mice.
Animals were maintained under controlled temperature (21 ± 2 °C) and on 12/12 h dark/light cycle. All the experimental procedures were accomplished according to the international care and use of laboratory animals’ guidelines. The study was approved by the ethics committee of Cairo University. Mice were weighed on day 0, 1st, 7th, 14th, 21st and 32nd days post infection. At the end of the experiment, animals were euthanised with pentobarbital (80 mg/kg). Blood was collected by direct cardiac puncture with heparin syringe. Coagulated blood was centrifuged, for 10 min at 2500 rpm, to separate the serum. Serum aliquots were stored at −80 °C till immunological measurement.
2.7. Parasitological examination
Fresh fecal samples from each animal of different groups were collected for examination on the 7th, 14th, 21st and 32nd days post infection. The number of C. parvum oocysts per gram stool of each animal was determined; and then the means of oocysts count for each group was calculated. Stool samples were concentrated by formalin-ether concentration method (FECT); briefly, each stool sample was mixed well with 10 ml of 10% formalin then filtered with a mesh screen (No. 10). Three ml of ether were added to the fecal suspension followed by strong shaking and centrifugation for 15 min at 500g. Supernatants were discarded and 1 g of the sediment was prepared as a fecal smear. Fecal smears were stained by modified Ziehl Neelsen method according to Tahvildar-Biderouni and Salehi (2014). Smears were air-dried and fixed by ethanol. Slides were flooded with alkaline fushin then heated for 5 min followed by washing with water, decolorization by 2.5% sulfuric acid and finally counter stained with 1% methylene blue for 1 min. Slides were air-dried and examined with 100X objective. Each slide was carefully examined for 5 min in a systematic manner. Where the objective was focused on the top left corner of the slide; and then the slide was moved systematically up and down or forwards and downwards to ensure careful examination. Percent reduction (PR) that expressed the decrease in oocysts' number of treated group in comparison to that of infected untreated group was calculated by the formula.
2.8. Molecular examination
2.8.1. DNA extraction from stool samples
DNA was extracted from mice stool samples by FavorPrep™ Stool DNA Isolation Mini Kit (FASTI 001-1). Briefly, two hundred mg of each stool sample was added to a bead tube placed on ice. Three hundred μl of SDE1 buffer and 20 μl of 10 mg/ml proteinase K were added to the sample, followed by incubation at 60 °C for 20 min with vortexing every 5 min during this incubation. One hundred μl of SDE2 buffer was added to the mixture followed by 5 min incubation on ice. After incubation, the mixture was centrifuged at maximum speed (18000×g) for 5 min. The supernatant was mixed with 200 μl of SDE3 buffer with vortexing; the resultant mixture was incubated at room temperature for 2 min followed by centrifugation for 2 min. Two hundred and fifty μl of SDE4 buffer and 250 μl of ethanol were added to the supernatant with mixing thoroughly by pulse-vortexing. All of the sample mixture was transferred to SDE column, centrifuged for 1 min at full speed and the flow through was discarded and washed by 750 μl of washing buffer two times. Two hundred μl of preheated elution buffer was added to the membrane center of SDE column followed by centrifugation for 1 min to elute DNA.
2.8.2. Quantitative PCR
This quantification assay was composed of two real time PCR that based on the amplification of a DNA sequence located in the 18S rRNA gene (GenBank accession no. EU675853.1, positions 33 to 211); and was performed according to Mary et al. (2013). The assay used the direct (CATGGATAACCGTGGTAAT) and reverse (TACCCTACCGTCTAAAGCTG) primers described by Mary et al. (2013); in addition to a TaqMan probe homologous to a conserved region of the sequence (Pan-crypto, FAM-CTAGAGCTAATACATGCGAAAAAA-MGB-BHQ) to detect Cryptosporidium. The second PCR used a hybridization probe 6-carboxyrhodamine (VIC)-ATCACATTAAATGT-MGB-BHQ (C. parvum). The final concentration of primers and probes were 0.2 and 0.1 μM, respectively. Extracted DNA (1 μl) was added to the reaction tubes to reach a final volume of 25 μl. The amplification was composed of Taq DNA polymerase activation at 94 °C for 10 min. To ensure probe hybridization, Taq activation was followed by forty five cycles: 1- for 10 s at 94 °C, 2- for 30 s at 54 °C and 3- for 10 s at 72 °C. The assays included positive control (C. parvum) and negative control.
2.9. Immunological measurements
The concentrations of serum interleukin (IL)-4, −6, −10, −17 and interferon (IFN)-γ were measured on day 0, 7th, 14th, 21st and 32nd days post infection by using mouse ELISA kit (# BMS613, # BMS603-2, RAB0245, # BMS6001 and # KMC4021, respectively).
2.10. Histopathological study
At the end of experiment, 32nd day, small parts of the intestine from each mouse was separated, fixed in 10% formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin stains for histopathological study.
2.11. Statistical analysis
Statistical analyses were carried out using SPSS v.15 software. One-way analysis of variance (ANOVA) was used to analyse the effect of treatment on the studied parameters. Duncan's multiple range test was used to test the similarities and differences between the experimental groups. P values less than 0.05 were considered statistically significant.
3. Results
3.1. Characterization of Aloe vera gel
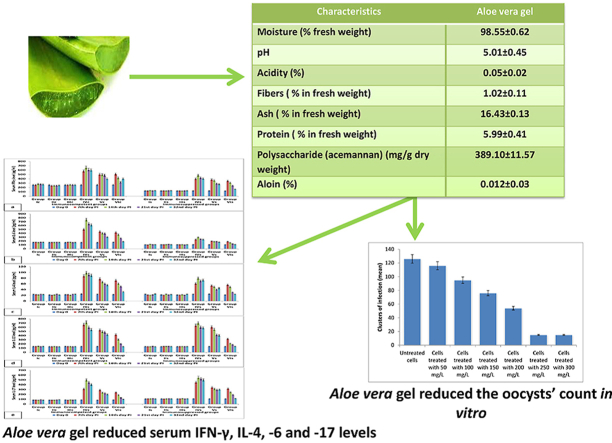
According to Table 1, Aloe vera gel has a moisture content of 98.55% and pH equal to 5. The gel contained 389.1 mg/g acemannan and 5.99% protein. No aloin was detected in Aloe vera gel.
Table 1.
Characteristics of Aloe vesra gel.
| Characteristics | Aloe veragel |
|---|---|
| Moisture (% fresh weight) | 98.55 ± 0.62 |
| pH | 5.01 ± 0.45 |
| Acidity (%) | 0.05 ± 0.02 |
| Fibers (% in fresh weight) | 1.02 ± 0.11 |
| Ash (% in fresh weight) | 16.43 ± 0.13 |
| Protein (% in fresh weight) | 5.99 ± 0.41 |
| Polysaccharide (acemannan) (mg/g dry weight) | 389.10 ± 11.57 |
| Aloin (%) | 0.012 ± 0.03 |
3.2. C. parvum oocysts preparation
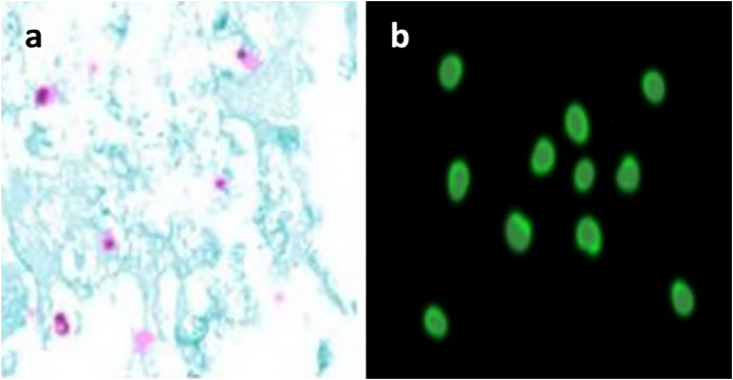
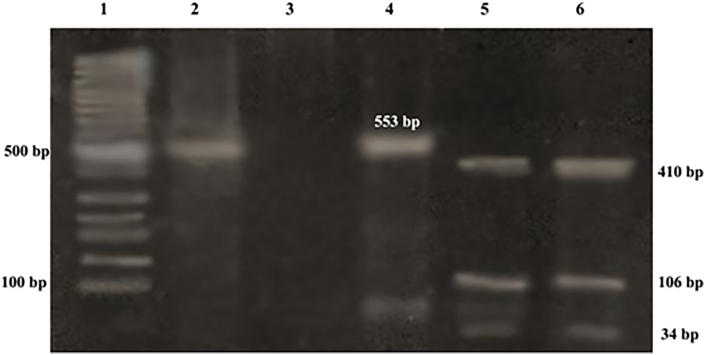
Fig. 1 showed Cryptosporidium oocysts in fecal smears stained with modified Ziehl–Neelsen staining method; where it had a pink- purple color. The oocysts have ovoid or spherical brilliant apple/green structure with thick walls in the immunofluorescence detection method. The restriction enzyme Rsa I digestion of nPCR product targeting COWP gene revealed the presence of Cryptosporidium parvum genotype. The genotype digestion products at 34, 106 and 410 bp were shown in Fig. 2.
Fig. 1.
Cryptosporidium oocyst (stained pink with modified Ziehl–Neelsen method), b] immunofluorescence staining of Cryptosporidium oocyst. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Agarose gel electrophoresis showing: Lane 1: 100 bp DNA molecular weight marker, Lane 2: nested PCR products of the sample targeting COWP gene of Cryptosporidium at 553 bp, Lane 3: Negative control, Lane 4: positive control. Lane 5: RFLP products of sample after digestion with RsaI endonuclease (C. parvum genotype 2 digestion products at 34, 106 and 410 bp), and Lane 6: RFLP products of positive control (C. parvum).
3.3. In vitro studies
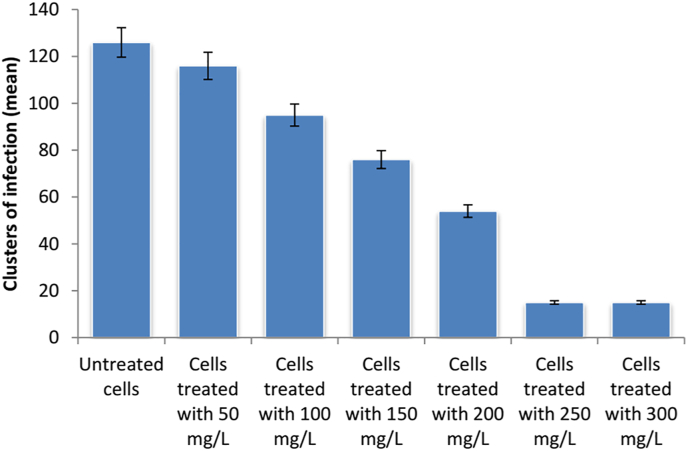
It was noticed that both of 250 and 300 mg/L of Aloe vera gel achieved the same result in the reduction of clusters of infection (Fig. 3). Therefore, 250 mg/L was the selected dose for the in vivo study.
Fig. 3.
Clusters of C. parvum infection against different concentration of Aloe vera gel (In vitro anti-C. parvum activity of Aloe vera gel).
3.4. Body weight
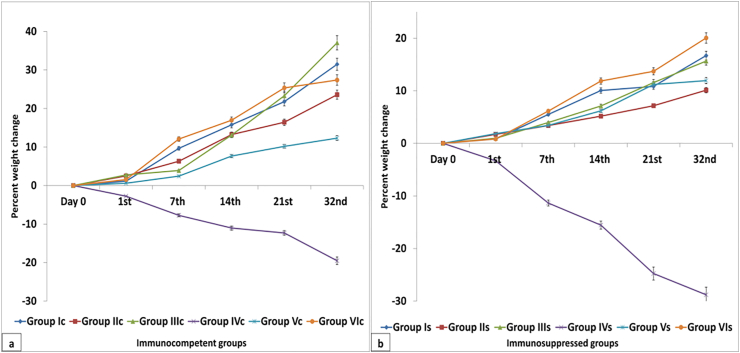
Body weight of healthy immunocompetent group Ic showed a significant increase along all experimental periods. On day 0 and 1st day PI, no significant change was observed between different experimental groups. A significant reduction in body weight was observed in non-treated C. parvum infected either immunocompetent group IVc or immunosuppressed group IVs. However, this reduction along all experimental periods was significantly higher in group IVs than in group IVc. An enhancement in the reduction of body weight was noticed upon treatment with NTZ, group Vc and Vs, along all experimental periods. The body weights of Aloe vera treated groups at 21st and 32nd day PI, either immunocompetent group VIc or immunosuppressed group VIs, were nearly the same as those of their corresponding control groups Ic and Is. Fig. 4 showed the percent weight change in different immunocompetent and immunosuppressed groups. Tables for body weight measurements and percent weight change were submitted as supplementary materials.
Fig. 4.
Percent weight change of different immunocompetent (a) and immunosuppressed (b) groups.
3.5. Parasitological results
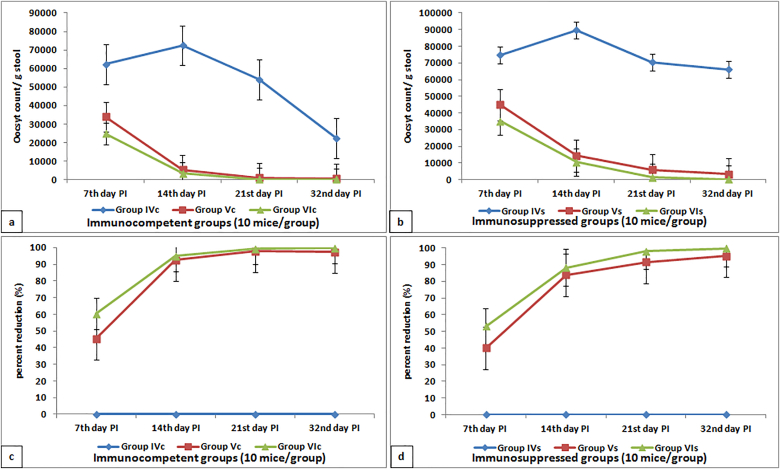
According to the parasitological results, the oocyst shedding in stool samples of non-treated immunosuppressed infected group IVs was significantly higher than that of non-treated immunocompetent infected group IVc along all experimental periods (Fig. 5). NTZ treated infected groups, either immunocompetent group Vc or immunosuppressed group Vs, showed a significant gradual decrease in oocyst count starting from 7th day PI to achieve a PR of 97.56% and 95.09%, respectively. On the 32nd day PI, Aloe vera gel treatment succeeded in reducing the oocyst count to zero and achieving 100% PR in immunocompetent infected group VIc. On the other hand, immunosuppressed Aloe vera treated infected group VIs showed a significant reduction in oocyst count (215.40 and 99.67%) more than that of NTZ immunosuppressed infected treated group Vs (3241.76 and 95.09%). Tables for oocyst count and PR were submitted as supplementary materials.
Fig. 5.
Percent reduction and oocysts count in stool samples of different infected immunocompetent (a and c) and immunosuppressed (b and d) groups.
3.6. Molecular studies
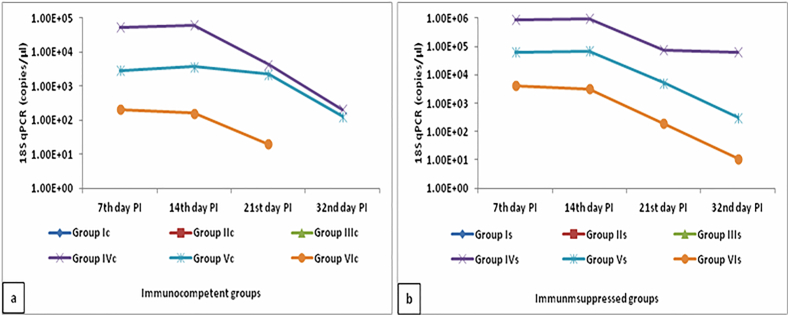
The real time PCR results indicated the complete treatment of infected immunocompetent group VIc with Aloe vera gel, where C. parvum DNA was not detected in mice stool samples (Fig. 6). Detected DNA copies in stool samples of Aloe vera treated infected immunosuppressed group VIs were lower than that of NTZ infected immunosuppressed group Vs. Table for molecular quantification was submitted as supplementary materials.
Fig. 6.
Real time PCR results in stool samples of different infected immunocompetent (a) and immunosuppressed (b) groups.
3.7. Cytokines measurements
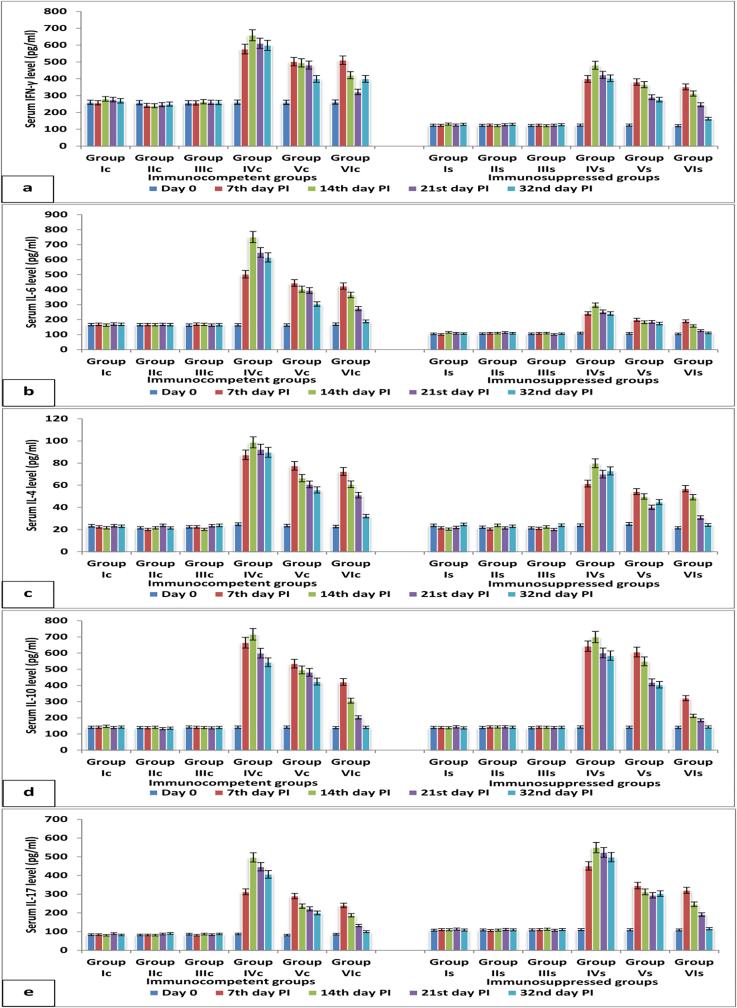
Cytokines secretion (IFN-γ, IL-4, -6, -10 and −17) among all experimental periods in group Ic or Is showed no significant difference. However, levels of IFN-γ and IL-6 in dexamethasone immunosuppressed group Is were significantly lower than those of immunocompetent group Ic along all experimental periods. On the other hand, IL-17 levels in group Is was higher than those of group Ic; IL-4 and IL-10 levels showed no significant change between group Ic and Is on the 7th, 14th, 21st and 32nd days post infection. C. parvum infection led to a significant elevation in all measured cytokines secretions either in non-treated immunocompetent group IVc or immunosuppressed group IVs. This elevation started on the 7th day post infection and continued to reach the highest level on the 14th day post infection; after that a slight non-significant decrease was observed on the 21st and 32nd days post infection. The levels of different measured cytokines on the 14th day post infection, in NTZ treated infected immunocompetent group Vc and immunosuppressed group Vs, were lower than their corresponding on the 7th day post infection. This reduction continued till the 32nd day post infection. But the cytokines levels on the 32nd day post infection, in group Vc and Vs, remained significantly higher than their corresponding in groups Ic and Is. Aloe vera, in groups VIc and VIs, succeeded in decreasing all cytokines levels on the 32nd day post infection to be similar to their corresponding control groups Ic and Is (Fig. 7). Tables for cytokines’ results were submitted as supplementary materials.
Fig. 7.
Cytokines' level (a] IFN-γ, b] IL-6, c] IL-4, d] IL-10 and e] IL-17) in serum samples of different immunocompetent and immunosuppressed groups.
3.8. Histopathological results
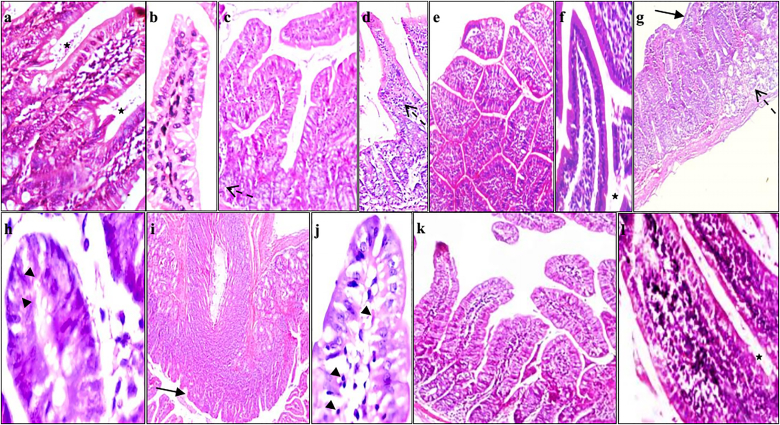
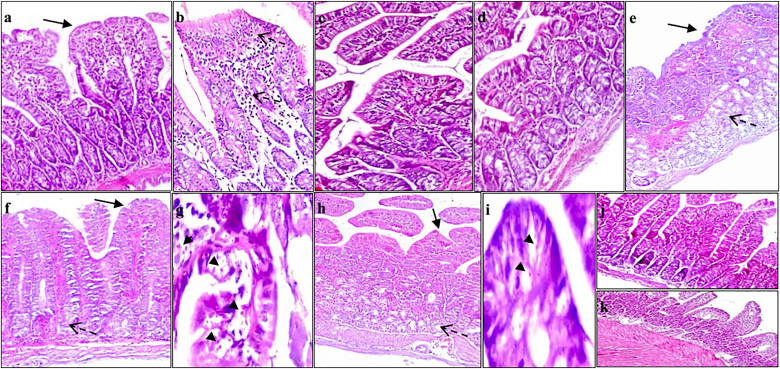
Histopathological examination of sections of small intestine of non-infected immunocompetent group Ic showed normal villous architecture with average length and width of villi (crypt villous ratio was 1:3 to 1:5). Goblet cells were moderate in number with a well-defined brush border (Fig. 8a and b). The same observations were noticed in NTZ and Aloe vera administrated control groups IIc and IIIc. Immunosuppressed non-infected group Is showed short broad villous architecture with mild inflammation (Fig. 9a and b). C. parvum infected immunocompetent group IVc showed alterations in the intestinal mucosa as a result of the infection. These alterations involved shortening and broading of villi, loss of villous architecture with decreased ratio of villous height to crypt length. Goblet cell depletion, mucosal ulceration and inflammatory cells infiltration in the lamina propria with diffuse loss of brush border microvillous surface area were observed (Fig. 8g and h). These alterations were more severe in immunosuppressed infected non-treated group IVs. Intestinal sections of group IVs showed mild intra-villous inflammatory infiltrate with many superficial and deep mucosal C. parvum oocysts; and submucosa with marked inflammatory infiltrate extending to musculosa with dilated congested blood vessels (Fig. 9e,f,g). Intestinal sections of NTZ treated immunocompetent and immunosuppressed infected groups Vc and Vs showed no improvement in the histopathological changes following C. parvum infection in the form of persistent moderate to severe villous atrophy, broad villi, infiltration of the lamina propria with inflammatory cells and many superficial and deep mucosal oocysts. Aloe vera treated infected immunocompetent group VIc revealed several improvements in the histopathological alterations following cryptosporidiosis infection. Evidences of improvement were the returning of the villous like pattern with focal flattening of surface enterocytes, mild depletion of goblet cells, mild decrease in the ratio between villous height to crypt length and no observed oocysts (Fig. 8k,l). Group VIs, Aloe vera treated immunosuppressed infected mice, showed average villi, mild intra-villous inflammatory infiltrate with very few deep mucosal oocysts (Fig. 9j and k).
Fig. 8.
Hematoxylin and eosin intestinal section of a] and b] group Ic showing intestinal wall with average mucosal thickness, average villi, average crypts (star) and average submucosa (x200); c] and d] group IIc showing normal villous architecture with mild inflammation (dashed arrows) and no villus atrophy(X100 and X200, respectively; e] and f] group IIIc showing normal villous architecture with crypt villous ratio of 1:3 to 1:5 with normal brush border (x100 and X200, respectively); g] and h] group IVc showing short broad villi with partially ulcerated covering mucosa (arrow), mild intra-villous inflammatory infiltrate (dashed arrow) and Cryptosporidium oocysts (arrow heads) (X100 and X400, respectively); i] and j] group Vc showing short broad villi (arrow), Cryptosporidium oocysts (arrow heads), marked intra-mucosal inflammatory infiltrate, and marked submucosal edema (x100 and X400, respectively); k] and l] group VI showing average villi, average crypts (star), no intra-villous inflammatory infiltrate, average submucosa and absence of Cryptosporidium oocysts (X200 and x400, respectively). Group Ic: Immunocompetent control mice, group IIc: NTZ treated immunocompetent non-infected mice, group IIIc: Aloe vera treated immunocompetent non-infected mice, group IVc: Non-treated immunocompetent infected mice, group Vc: NTZ treated immunocompetent infected mice and group VIc: Aloe vera treated immunocompetent infected mice.
Fig. 9.
Hematoxylin and eosin intestinal section of a] and b] group Is showing short broad villi with partially ulcerated covering mucosa (arrow), sever intra-villous inflammatory infiltrate (dashed arrow), submucosa with moderate edema, and moderate inflammatory infiltrate (dashed arrow) (X200); c] group IIs showing short broad villi with mild inflammatory infiltrate (x200); d] group IIIs showing short broad villi (x200); e], f] and g] group IVs showing severe to complete villous atrophy with sever inflammatory infiltrate (dashed arrow), numerous superficial and deep oocysts (arrow heads), and submucosa showing marked edema with inflammatory infiltrate extending to musculosa (dashed arrow) (X100, x100 and x400, respectively); h] and i] group Vs showing short blunted villi (arrow) and mild inflammatory infiltrate (dashed arrow) with superficial and deep mucosal oocysts (arrow heads) (x100 and x400, respectively); j] and k] group VIs showing average villi with average crypts and mild intra-villous inflammatory infiltrate (X200). Group Is: Immunosuppressed control mice, group IIs: NTZ treated immunosuppressed non-infected mice, group IIIs: Aloe vera treated immunosuppressed non-infected mice, group IVs: Non-treated immunosuppressed infected mice, group Vs: NTZ treated immunosuppressed infected mice and group VIs: Aloe vera treated immunosuppressed infected mice.
4. Discussion
Immunosuppression can occur during cancer therapy, in infected HIV patients or after organ transplantation. Moreover, infants, preschool children and pregnant woman are susceptible to infection with C. parvum. The parasite can spread in genetically immunosuppressed animals and, also, in dexamethasone treated ones (Miller et al., 2007). Therefore, the present study aimed to evaluate the efficacy of Aloe vera gel in treatment of cryptosporidiosis especially in immunosuppressed mice. Dexamethasone was used for the induction of immunosuppressed mice model. Dexamethasone has been used as an immunosuppressive drug in many studies. Baishanbo et al. (2006) used dexamethasone to induce immunosuppressed gerbil model in order to evaluate the therapeutic efficacy of paromomycin, in comparison to that of NTZ, for the treatment of cryptosporidiosis. Gargala et al. (2013) showed the activity of halogeno-thiazolides against cryptosporidiosis in experimentally infected dexamethasone immunosuppressed gerbils. Abdou et al. (2013) evaluated the role of C. parvum infection in inducing intestinal dysplasia in immunocompromised mice. This was achieved by the oral administration of dexamethasone (0.25 μg/g/day) for 14 successive days prior to inoculation with C. parvum oocysts. Asadpour et al. (2018) used the dexamethasone immunosuppressed mice to examine curcumin in treatment of cryptosporidiosis. In many studies, dexamethasone dose was selected according to Rehg et al. (1988) who reported that dexamethasone immunosuppressed animals were susceptible to C. parvum infection. They examined several doses of dexamethasone (1, 0.5, 0.25, 0.125 and 0.0625 mg); then reported that 0.25 μg/g/day of dexamethasone was the selected dose and recommended its administration by fourteen days before inoculation. Where, the higher doses produced unacceptable toxicity level and the lower doses were ineffective.
According to Pantenburg et al. (2008), CD4+ T lymphocytes have a crucial role in the immune response against cryptosporidiosis in human and murine models. It is known that CD4+ T lymphocytes are an important component in the intestinal lamina propria (Ullrich et al., 1991); and were the first T-cell subsets to decrease during HIV infection (Brenchley et al., 2004). Dexamethasone, mainly, induce immunosuppression by the inhibition of cytokines transcription and sequestration of CD4+ T lymphocytes in the reticuloendothelial system (Barshes et al., 2004). CD4+ T lymphocytes need two signals for an effective immune response: 1- The binding of T cell receptor to peptide-MHC complex that results in CD3 intracellular signaling cascade; 2- The co-stimulatory signal that is received when CD28 on T cells interact with CD80 or CD86 on the antigen presenting cells (Lanier et al., 1995). Dexamethasone induces its action by blocking naïve T cell proliferation and differentiation through attenuating CD28 co-stimulation (Giles et al., 2018). Therefore, it can be concluded that dexamethasone immunosuppressed mice model can be appropriate for this study. This model can be related to immune suppression in human and SCID mice (deficient in functional B and T lymphocytes) (Bosma and Carroll, 1991).
Dexamethasone is a type of corticosteroid that inhibits cytokines' secretion of Th1 cells more than that of Th2 cells (Franchimont et al., 1998). Th1 cells are known to secret IFN-γ and IL-6; while IL-4 and -10 are secreted from Th2 cells (Farid et al., 2020d&e and 2021b). This was obvious in uninfected immunosuppressed group Is, where the levels of IFN-γ and IL-6 were significantly lower than those of healthy group Ic along all experimental periods. On the other hand, no significant changes were observed in IL-4 and IL-10 levels between groups Ic and Is on day 0, 7th, 14th, 21st and 32nd days post infection. Also, a significant elevation in IL-17 levels (secreted from Th17) in group Is was observed in comparison to group Ic. This meant a reduction in pro-inflammatory cytokines more than anti-inflammatory cytokines leading to a shift from Th1 to Th2 immune response. However, C. parvum infection led to a significant increase in all measured cytokines (IFN-γ, IL-4, -6, -10 and −17) that began on the 7th day post infection and continued to reach the highest level on the 14th day post infection. Histopathological intestinal sections showed the loss of villous architecture, goblet cell depletion, mucosal ulceration and infiltration of lamina propria with inflammatory cells. These symptoms were more severe in immunosuppressed infected untreated group that showed many superficial and deep mucosal C. parvum oocysts.
The immunostimulant and anti-inflammatory effects of Aloe vera gel have been proved in many researches. 800 mg/day of acemannan, extracted from Aloe vera gel, improved the clinical conditions of fourteen HIV-1+ patients by the elevation in the number of white blood cells, circulating monocytes and macrophages (McDaniel et al., 1990a&b). Also, Shida (1985) showed the increased phagocytosis in asthmatic patients upon treatment with Aloe vera extract. Several antiseptic agents have been extracted from Aloe vera such as salicylic acid, urea nitrogen, cinnamonic acid, phenols and sulfur (Moghaddasi and Verma, 2011). Kahlon et al. (1991) reported that acemannan blocked the reproduction of Herpes and AIDS virus. Kawai et al. (1998) treated guinea pig feet infected with Trichophyton mentagrophytes by Aloe vera extract and reported 70% inhibition in the fungal growth. Farid et al. (2020a) showed that Aloe vera gel successfully cleared giardiasis with no side effects in rats and acted as an anti-inflammatory agent to reduce IFN-γ, IL-4 and -6.
In vitro studies of the Cryptosporidium life cycle and metabolic development requirements have previously been conducted using cell culture (Slifko et al., 1997). In a comparison between many cell lines, HCT-8 cells were shown to support the life phases of C. parvum infection in cultures. Usually, an enzyme-linked immunosorbent test using a polyclonal antibody produced against C. parvum sporozoites was used to detect the developmental stages in the cell culture. Also, the antibody responds with epitopes on other stages of reproduction. According to Yu et al. (2000), the in vitro system has many advantages like low cost, more effective in studying the parasite's biology and doses of anti-cryptosporidial tested drugs. In this study, the in vitro assay was performed to determine the most effective anti-cryptosporidial dose of Aloe vera gel. According to our results, a dose of 250 mg/L of Aloe vera gel was effective in the reduction of infection.
In this study, the dexamethasone immunosuppressed infected untreated group IVs continued to shed oocysts in large numbers till the 32nd day. This was in agreement with Lacroix et al. (2001 and 2002) who reported that the duration of oocyst shedding was approximately 4 weeks in neonatal mice. Mahmood et al. (2016) found that the maximum shedding of oocysts in feces were observed on day 20 post-treatment in infected immunocompromised mice. Also, Benamrouz et al. (2012) who showed that in Dex-SCID mice inoculated with low doses of oocysts, the excretion of oocysts increased, reaching a mean of oocysts shedding of more than 10,000 oocysts per gram of feces at the forty five days post infection. Gaafar (2012) reported that the infected immunosuppressed mice showed a significant increase in the number of cryptosporidial oocysts in stool and ileal sections, when compared to the corresponding immunocompetent subgroups. They attributed this result to the immunodeficient situation that flared up the infection. Chai et al. (1999) found that oocyst excretion started on the 4th day PI in both immunocompetent and immunosuppressed mice. Sonzogni-Desautels et al. (2015) reported that the oocyst shedding started between the 5th and 7th days PI and continued to the 30th day PI in immunocompromised mice.
Our study showed that although the oocyst shedding in immunocompetent infected untreated group IVc was much lower than that of its corresponding immunosuppressed group, the duration of oocyst shedding in immunocompetent infected untreated group IVc extended to the 32 days PI. Our results were in agreement with El Shafei et al. (2018); who reported that oocyst sedding in immunocompetent untreated female albino mice was 3.7 × 103 in mg of stool samples on the 30th day PI. Furthermore, they reported the presence of endogenous developmental stages of C. parvum/villous unit in the same group. Matsui et al. (2001) reported that the natural shedding period of C. muris infection in mice was nearly 24 days. Sadek et al. (2020) showed that immunocompetent mice continued to shed Cryptosporidium oocysts till the 19 day post infection. Furthermore, the maximum oocyst shedding in immunocompetent and immunosuppressed groups, in the study of Abdou et al. (2013), was observed between the 13th and 15th days PI. The mean oocyst count in mg of stool samples was 3.2 and 4.8 for infected untreated immunocompetent and immunosuppressed groups, respectively. Our results were in agreement with Abdou et al. (2013) where the maximum oocyst shedding was recorded on the 14th day PI. On the other hand, immunocompetent infected untreated group IVc continued the oocyst shedding (22471.96 in g stool ≈ 22.4 in mg stool) till the 32nd day PI. This result was slightly different from that of Abdou et al. (2013) who reported oocyst count (1 ± 1 in mg stool) on the 30th day PI. This difference was attributed to the methods for oocysts preparation, the number of oocysts to be inoculated, the difference in mice species and sex. Where, Abdou et al. (2013) used a Swiss albino female mouse that was completely different from our mouse model (Six-week-old male BALB/c mice). Also, the study of Aboelsoued et al. (2019) showed the anticryptosporidial effect of Punica granatum nano-form red and white peel extracts in male albino mice. They reported that a gradual reduction in oocyst shedding, in the infected non-treated group, was noticed from the 9th day PI and continued till the 24th day PI. They, also, added that the interval which covered the natural C. parvum oocyst shedding period in mice was about 24 days or about 3–4 weeks. Metawae et al. (2021) studied the treatment of C. parvum infected immunocompetent mice by nitazoxanide alone or loaded with silica nanoparticles. They extended the experiment for 30 days to evaluate the infection course and to cover the period of natural shedding of Cryptosporidium infection in mice, which is stated to be about 24 days. Their results showed that the oocyst count per gram stool in infected untreated mice was 34437.1 and 13304.9 after two and three weeks, respectively.
In this study, the molecular results indicated the undetectable level of C. parvum in Aloe vera treated infected immunocompetent group VIc, where C. parvum DNA was not detected in mice stool samples. Detected DNA copies in stool samples of Aloe vera treated infected immunosuppressed group VIs were lower than that of NTZ infected immunosuppressed group Vs. This was accompanied by a decrease in all cytokines levels on the 32nd day post infection to be similar to those of uninfected corresponding control group; and an improvement in the histopathological changes and body weight. On the other hand, NTZ treatment neither succeeded in clearing the infection nor reducing the elevated cytokines. Our results were in agreement with Yim et al. (2011) and Akhtar et al. (2012) who showed that Aloe vera-based diet could be used as an alternative treatment for controlling coccidiosis in chickens. This can be explained by the immunostimulant and anti-inflammatory effects of Aloe vera. Bałan et al. (2014) reported that oral administration of Aloe vera gel to mice stimulated the cellular and humoral immune response. An elevation in number of CD4 cells and immunoglobulin level was described in rabbits fed with Aloe vera (Vahedi et al., 2011). Aloe vera was involved in the differentiation of CD4 lymphocytes by regulating cytokines secretion in Th1 and Th2 (Yu et al., 2002). Aloe vera gel contains several polysaccharides like acemannan that was responsible for its immunomodulatory effects (Choi and Chung, 2003). Acemannan stimulated the differentiation of immature dendritic cells (Lee et al., 2001). Aloe vera gel improved the anti-coxsackievirus antibody level in mice (Gauntt et al., 2000), survival rate of cats infected with oncornavirus (Sheets et al., 1991) and stimulated the secretion of TNF-α, INF-γ, IL-1β, −2 and −6 from splenic lymphocytes and peritoneal macrophages (Leung et al., 2004).
In conclusion, oral administration of Aloe vera gel cleared cryptosporidiosis with a PR of 100% in immunocompetent mice and 99.67% in immunosuppressed mice. Where, it acted as an anti-inflammatory agent to reduce IFN-γ, IL-4, -6 and -17 levels.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2021.09.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abdou A.G., Harba N.M., Afifi A.F., Elnaidany N.F. Assessment of Cryptosporidium parvum infection in immunocompetent and immunocompromised mice and its role in triggering intestinal dysplasia. Int. J. Infect. Dis. 2013;17(8):e593–e600. doi: 10.1016/j.ijid.2012.11.023. [DOI] [PubMed] [Google Scholar]
- Abdulrahman O., Samaila A.B., Panda S.M., Aliyu A., Sahal M.R. Antidiarrhoeagenic potentials of synergistic activities of water extracts of Aloe vera and hyptis suaveolens against Giardia lamblia and salmonella species infections among children 0-5 years in Bauchi State, Nigeria. Asian J. Biotechnol. Genet. Eng. 2019;2(2):1–7. [Google Scholar]
- Aboelsoued D., Abo-Aziza F.A.M., Mahmoud M.H., Abdel Megeed K.N., Abu El Ezz N.M.T., Abu-Salem F.M. Anticryptosporidial effect of pomegranate peels water extract in experimentally infected mice with special reference to some biochemical parameters and antioxidant activity. J. Parasit. Dis. 2019;43(2):215–228. doi: 10.1007/s12639-018-01078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abubakar I., Aliyu S.H., Arumugam C., Usman N.K., Hunter P.R. Treatment of cryptosporidiosis in immunocompromised individuals: systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2007;63(4):387–393. doi: 10.1111/j.1365-2125.2007.02873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar M., Hai A., Awais M.M., Iqbal Z., Muhammad F., ul Haq A., Anwar M.I. Immunostimulatory and protective effects of Aloe vera against coccidiosis in industrial broiler chickens. Vet. Parasitol. 2012;186:170–177. doi: 10.1016/j.vetpar.2011.11.059. [DOI] [PubMed] [Google Scholar]
- Anderson B.C. Moist heat inactivation of Cryptosporidium sp. Am. J. Publ. Health. 1985;75:1433–1434. doi: 10.2105/ajph.75.12.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . fifteenth ed. 2005. Official Methods of Analysis. Association of Official Analytical Chemist. 14th Edition 1984. eighteenth ed. [Google Scholar]
- Asadpour M., Namazi F., Razavi S.M., Nazifi S. Curcumin: a promising treatment for Cryptosporidium parvum infection in immunosuppressed BALB/c mice. Exp. Parasitol. 2018;195:59–65. doi: 10.1016/j.exppara.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Atherton P. Aloe vera: magic or medicine? Nurs. Stand. 1998;12:49–52. doi: 10.7748/ns.12.41.49.s40. [DOI] [PubMed] [Google Scholar]
- Baishanbo A., Gargala G., Duclos C., François A., Rossignol J.F., Ballet J.J., Favennec L. Efficacy of nitazoxanide and paromomycin in biliary tract cryptosporidiosis in an immunosuppressed gerbil model. J. Antimicrob. Chemother. 2006;57(2):353–355. doi: 10.1093/jac/dki456. [DOI] [PubMed] [Google Scholar]
- Bałan B.J., Niemcewicz M., Kocik J., Jung L., Skopińska-Różewska E., Skopiński P. Oral administration of Aloe vera gel, anti-microbial and anti-inflammatory herbal remedy, stimulates cell-mediated immunity and antibody production in a mouse model. Cent. Eur. J. Immunol. 2014;39(2):125–130. doi: 10.5114/ceji.2014.43711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barshes N.R., Goodpastor S.E., Goss J.A. Pharmacologic immunosuppression. Front. Biosci. 2004;9:411–420. doi: 10.2741/1249. [DOI] [PubMed] [Google Scholar]
- Benamrouz S., Guyot K., Gazzola S., Mouray A., Chassat T., Delaire B., Chabé M., Gosset P., Viscogliosi E., Dei-Cas E., Creusy C., Conseil V., Certad G. Cryptosporidium parvum infection in SCID mice infected with only one oocyst: qPCR assessment of parasite replication in tissues and development of digestive cancer. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0051232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borad A., Ward H. Human immune responses in cryptosporidiosis. Future Microbiol. 2010;5:507–519. doi: 10.2217/fmb.09.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma M.J., Carroll A.M. The SCID mouse mutant: definition, characterization, and potential uses. Annu. Rev. Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brenchley J.M., Schacker T.W., Ruff L.E., Price D.A., Taylor J.H., Beilman G.J., Nguyen P.L., Khoruts A., Larson M., Haase A.T., Douek D.C. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabada M.M., White A.C., Jr. Treatment of cryptosporidiosis: do we know what we think we know? Curr. Opin. Infect. Dis. 2010;23(5):494–499. doi: 10.1097/QCO.0b013e32833de052. [DOI] [PubMed] [Google Scholar]
- Chai J.Y., Guk S.M., Han H.K., Yun C.K. Role of intraepithelial lymphocytes in mucosal immune responses of mice experimentally infected with Cryptosporidium parvum. J. Parasitol. 1999;85:234–239. [PubMed] [Google Scholar]
- Chen X.M., Keithly J.S., Paya C.V., LaRusso N.F. Cryptosporidiosis. N. Engl. J. Med. 2002;346(22):1723–1731. doi: 10.1056/NEJMra013170. [DOI] [PubMed] [Google Scholar]
- Choi S., Chung M.H. A review on the relationship between Aloe vera components and their biologic effects. Semin. Integr. Med. 2003;1:53–62. [Google Scholar]
- Current W.L., Reese N.C., Ernst J.V., Bailey W.S., Heyman M.B., Weinstein W.M. Human cryptosporidiosis in immunocompetent and immunodeficient persons. Studies of an outbreak and experimental transmission. N. Engl. J. Med. 1983;308:1252–1257. doi: 10.1056/NEJM198305263082102. [DOI] [PubMed] [Google Scholar]
- Eberendu A., Luta G., Edwards J., McAnalley B., Davis B. Quantitative colorimetric analysis of aloe polysaccharides as a measure of aloe vera. Quality in commercial products. J. AOAC Int. 2005;88:684–691. [PubMed] [Google Scholar]
- El Shafei O.K., Saad A.G.E., Harba N.M., Sharaf O.F., Samaka R.M., Farag S.A. Therapeutic effect of phenyl vinyl sulfone and nitazoxanide on experimentally infected mice with cryptosporidiosis. Menoufia Med. J. 2018;31:786–794. [Google Scholar]
- Farid A., Amadou M., Safwat G. Treatment potential of Aloe Vera gel in Gairdia Intestinalis infected albino rats. J. Egypt. Soc. Parasitol. 2020;50(1):79–85. [Google Scholar]
- Farid A., Haytham M., Essam A., Safwat G. Efficacy of the aqueous extract of Siwa dates in protection against the whole body γ irradiation induced damages in mice. J.Radiat. Res.Appl. Sci. 2021;14(1):322–335. [Google Scholar]
- Farid A., El-Dewak M., Safwat G., Diab A. Anti-apoptotic and antioxidant effects of melatonin protect spleen of whole body γ-irradiated male Sprague-dawley rats. Int. J. Radiat. Res. 2021 IJRR–21–3042. [Google Scholar]
- Farid A., Kamel D., Montaser S.A., Ahmed M.M., El Amir M., El Amir A. Assessment of antioxidant, immune enhancement, and antimutagenic efficacy of fennel seed extracts in irradiated human blood cultures. J.Radiat. Res.Appl. Sci. 2020;13(1):260–266. [Google Scholar]
- Farid A., Kamel D., Montaser S.A., Ahmed M.M., El Amir M., El Amir A. Synergetic role of senna and fennel extracts as antioxidant, anti–inflammatory and anti–mutagenic agents in irradiated human blood lymphocyte cultures. J.Radiat. Res.Appl. Sci. 2020;13(1):191–199. [Google Scholar]
- Farid A., Tawfik A., Elsioufy B., Safwat G. Narrow band ultraviolet B therapy deactivates Th1/Th17 pathway and activates Th2 cytokines secretion in Egyptian psoriatic arthritis patients. J.Radiat. Res.Appl. Sci. 2020;13(1):356–361. [Google Scholar]
- Farid A., Hesham M., El-Dewak M., Amin A. The hidden hazardous effects of stevia and sucralose consumption in male and female albino mice in comparison to sucrose. Saudi Pharmaceut. J. 2020;28(10):1290–1300. doi: 10.1016/j.jsps.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Ryan U.M., Xiao L. Genetic diversity and population structure of Cryptosporidium. Trends Parasitol. 2018;34:997–1011. doi: 10.1016/j.pt.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Franchimont D., Louis E., Dewe W., Martens H., Vrindts-Gevaert Y., De Groote D., Belaiche J., Geenen V. Effects of dexamethasone on the profile of cytokine secretion in human whole blood cell cultures. Regul. Pept. 1998;73(1):59–65. doi: 10.1016/s0167-0115(97)01063-x. [DOI] [PubMed] [Google Scholar]
- Gaafar M.R. Efficacy of Allium sativum (garlic) against experimental cryptosporidiosis. Alexandria.J. Med. 2012;48(1):59–66. [Google Scholar]
- Gargala G., François A., Favennec L., Rossignol J.F. Activity of halogeno-thiazolides against Cryptosporidium parvum in experimentally infected immunosuppressed gerbils (Meriones unguiculatus) Antimicrob. Agents Chemother. 2013;57(6):2821–2823. doi: 10.1128/AAC.01538-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauntt C.J., Wood H.J., McDaniel H.R., McAnalley B.H. Aloe polymannose enhances anti-coxsackievirus antibody titres in mice. Phytother Res. 2000;14:261–266. doi: 10.1002/1099-1573(200006)14:4<261::aid-ptr579>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Giles A.J., Hutchinson M.N.D., Sonnemann H.M., Jung J., Fecci P.E., Ratnam N.M., Zhang W., Song H., Bailey R., Davis D., Reid C.M., Park D.M., Gilbert M.R. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J. Immunother. Cancer. 2018;6(1):51. doi: 10.1186/s40425-018-0371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemphill A., Mueller J., Esposito M. Nitazoxanide, a broad spectrum thiazolide antiinfective agent for the treatment of gastrointestinal infections. Expet Opin. Pharmacother. 2006;7:953–964. doi: 10.1517/14656566.7.7.953. [DOI] [PubMed] [Google Scholar]
- Henriksen S.A., Pohlenz J.F. Staining of cryptosporidia by a modified Ziehl–neelsen technique. Acta Vet. Scand. 1981;22:594–596. doi: 10.1186/BF03548684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D.B., White A.C. An updated review on Cryptosporidium and Giardia. Gastroenterol. Clin. N. Am. 2006;35(2):291–314. doi: 10.1016/j.gtc.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Kahlon J.B., Kemp M.C., Carpenter R.H., McAnalley B.H., McDaniel H.R., Shannon W.M. Inhibition of AIDS virus replication by acemannan In vitro. Mol. Biother. 1991;3:127–135. [PubMed] [Google Scholar]
- Kawai K., Beppu H., Shimpo K., Chihara T., Yamamoto N., Nagatsu T., Ueda H., Yamada Y. In vivo effects of Aloe arborescens Miller var. natalensis Berger on experimental tinea pedis in Guinea pig feet. Phytother Res. 1998;12:178–182. [Google Scholar]
- Klein A.D., Penneys N.S. Aloe vera. J. Am. Acad. Dermatol. 1988;18:714–720. doi: 10.1016/s0190-9622(88)70095-x. [DOI] [PubMed] [Google Scholar]
- Lacroix S., Mancassola R., Naciri M., Laurent F. Cryptosporidium parvum-specific mucosal immune response in C57BL/6 neonatal and gamma interferon-deficient mice: role of tumor necrosis factor alpha in protection. Infect. Immun. 2001;69:1635–1642. doi: 10.1128/IAI.69.3.1635-1642.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix-Lamandé S., Mancassola R., Naciri M., Laurent F. Role of gamma interferon in chemokine expression in the ileum of mice and in a murine intestinal epithelial cell line after Cryptosporidium parvum infection. Infect. Immun. 2002;70:2090–2099. doi: 10.1128/IAI.70.4.2090-2099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L.L., O'Fallon S., Somoza C., Phillips J.H., Linsley P.S., Okumura K., Ito D., Azuma M. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J. Immunol. 1995;154(1):97–105. [PubMed] [Google Scholar]
- Lebbad M., Winiecka-Krusnell J., Stensvold C.R., Beser J. High diversity of Cryptosporidium species and subtypes identified in cryptosporidiosis acquired in Sweden and abroad. Pathogens. 2021;10(5):523. doi: 10.3390/pathogens10050523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.K., Lee M.K., Yun Y.P., Kim Y., Kim J.S., Kim Y.S., Kim K., Han S.S., Lee C.K. Acemannan purified from Aloe vera induces phenotypic and functional maturation of immature dendritic cells. Int. Immunopharm. 2001;1:1275–1284. doi: 10.1016/s1567-5769(01)00052-2. [DOI] [PubMed] [Google Scholar]
- Leung M.Y., Liu C., Zhu L.F., Hui Y.Z., Yu B., Fung K.P. Chemical and biological characterization of a polysaccharide biological response modifier from Aloe vera L. var. Chinensis (Haw.) Berg. Glycobiology. 2004;14:501–510. doi: 10.1093/glycob/cwh050. [DOI] [PubMed] [Google Scholar]
- Li X., Brasseur P., Agnamey P., Lemeteil D., Favennec L., Ballet J.J., Rossignol J.F. Long lasting anticryptosporidial activity of nitazoxanide in an immunosuppressed rat model. Folia Parasitol. 2003;50:19–22. doi: 10.14411/fp.2003.003. [DOI] [PubMed] [Google Scholar]
- Mahmood M.N., Ramadan F.N., Hassan M.S., Sabry H.Y., Magdy M.M. Introducing miltefosine as an anti-cryptosporidial agent in immunocompromised mice. J. Plant Pathol. Microbiol. 2016;7:354. [Google Scholar]
- Mary C., Chapey E., Dutoit E., Guyot K., Hasseine L., Jeddi F., Menotti J., Paraud C., Pomares C., Rabodonirina M., Rieux A., Derouin F. ANOFEL Cryptosporidium National Network. Multicentric evaluation of a new real-time PCR assay for quantification of Cryptosporidium spp. and identification of Cryptosporidium parvum and Cryptosporidium hominis. J. Clin. Microbiol. 2013;51(8):2556–2563. doi: 10.1128/JCM.03458-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T., Fujino T., Kajima J., Tsuji M. Infectivity and oocyst excretion patterns of Cryptosporidium muris in slightly infected mice. J. Vet. Med. Sci. 2001;63:319–320. doi: 10.1292/jvms.63.319. [DOI] [PubMed] [Google Scholar]
- McDaniel H., Carpenter R., Kemp M., Kahlon J., McAnalley B. Extended survival and prognostic criteria for Acemannan (ACE-M) treated HIV Patients. Antivir. Res. 1990;1:117. [Google Scholar]
- McDaniel H., Combs C., HR M., Carpenter R., Kemp M., McAnalley B. An increase in circulating monocyte/macrophages (M/M) is induced by oral acemannan in HIV-1 patients. Am. J. Clin. Pathol. 1990;94:516–517. [Google Scholar]
- Metawae A.G., Bayoumy A.M., Ali I.R., Hammam O.A., Temsah K.A. Efficacy of nitazoxanide alone or loaded with silica nanoparticle for treatment of cryptosporidiosis in immunocompetent hosts. IJMA. 2021;3(2):1229–1239. [Google Scholar]
- Miller T.A., Ware M.W., Wymer L.J., Schaefer F.W. Chemically and genetically immunocompromised mice are not more susceptible than immunocompetent mice to infection with Cryptosporidium muris. Vet. Parasitol. 2007;143:99–105. doi: 10.1016/j.vetpar.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Moghaddasi M.S., Verma S.K. Aloe vera their chemicals composition and applications: a review. Int. J. Biol. Med. Res. 2011;2(1):466–471. [Google Scholar]
- Nannini E.C., Okhuysen P.C. HIV1 and the gut in the era of highly active antiretroviral therapy. Curr. Gastroenterol. Rep. 2002;4(5):392–398. doi: 10.1007/s11894-002-0009-z. [DOI] [PubMed] [Google Scholar]
- Pantenburg B., Dann S.M., Wang H.C., Robinson P., Castellanos-Gonzalez A., Lewis D.E., White A.C. Intestinal immune response to human Cryptosporidium sp. infection. Infect. Immun. 2008;76(1):23–29. doi: 10.1128/IAI.00960-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak D., Sharma R. Review on "Aloe vera- medicinal plant. IJARIIE. 2017;3:661–671. [Google Scholar]
- Peng S.Y., Norman J., Curtin G., Corrier D., McDaniel H.R., Busbee D. Decreased mortality of Norman murine sarcoma in mice treated with the immunomodulator. Acemannan. Mol. Biother. 1991;3:79–87. [PubMed] [Google Scholar]
- Rehg J.E., Hancock M.L., Woodmansee D.B. Characterization of a dexamethasone treated rat model of Cryptosporidium infection. J. Infect. Dis. 1988;158:1406–1407. doi: 10.1093/infdis/158.6.1406. [DOI] [PubMed] [Google Scholar]
- Sadek H., Abdel-Rahman S., Bakir H., Arafa M., Ahmed A., Gaber M. The potential convention of garlic and black seed different extracts as an effective treatment of Cryptosporidium spp.: an experimental study. J. Egypt. Soc. Parasitol. 2020;50(3):613–621. [Google Scholar]
- Sheets M.A., Unger B.A., Giggleman G.F., Jr., Tizard I.R. Studies of the effect of acemannan on retrovirus infections: clinical stabilization of feline leukemia virus-infected cats. Mol. Biother. 1991;3:41–45. [PubMed] [Google Scholar]
- Shelton R.M. Aloe vera: its chemical and therapeutic properties. Int. J. Dermatol. 1991;30:679–683. doi: 10.1111/j.1365-4362.1991.tb02607.x. [DOI] [PubMed] [Google Scholar]
- Shida T. Effect of Aloe extract on peripheral phagocytosis in adult bronchial asthma. Planta Med. 1985;51:273–275. doi: 10.1055/s-2007-969480. [DOI] [PubMed] [Google Scholar]
- Sifuentes L.Y., Di Giovanni G.D. Aged HCT-8 cell monolayers support Cryptosporidium parvum infection. Appl. Environ. Microbiol. 2007;73(23):7548–7551. doi: 10.1128/AEM.01579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifko T.R., Friedman D., Rose J.B., Jakubowski W. An in vitro method for detecting infectious Cryptosporidium oocysts with cell culture. Appl. Environ. Microbiol. 1997;63(9):3669–3675. doi: 10.1128/aem.63.9.3669-3675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonzogni-Desautels K., Renteria A.E., Camargo F.V., Di Lenardo T.Z., Mikhail A., Arrowood M.J., Fortin A., Ndao M. Oleylphosphocholine (OlPC) arrests Cryptosporidium parvum growth in vitro and prevents lethal infection in interferon gamma receptor knock-out mice. Front. Microbiol. 2015;6:973. doi: 10.3389/fmicb.2015.00973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano F., Puri C., Ranucci L., Putignani L., Crisanti A. Cloning of the entire COWP gene of Cryptosporidium parvum and ultrastructural localization of the protein during sexual parasite development. Parasitology. 1997;114:427–437. doi: 10.1017/s0031182096008761. [DOI] [PubMed] [Google Scholar]
- Spano F., Putignani L., McLauchlin J., Casemore D.P., Crisanti A. PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene discriminates between C. wrairi and C. parvum isolates of human and animal origin. FEMS Microbiol. Lett. 1997;150:209–217. doi: 10.1016/s0378-1097(97)00115-8. [DOI] [PubMed] [Google Scholar]
- Sparks H., Nair G., Castellanos-Gonzalez A., White A.C. Treatment of Cryptosporidium: what we know, gaps, and the way forward. Curr. Trop. Med. Rep. 2015;2(3):181–187. doi: 10.1007/s40475-015-0056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahvildar-Biderouni F., Salehi N. Detection of Cryptosporidium infection by modified zeihl-neelsen and PCR methods in children with diarrheal samples in pediatric hospitals in Tehran. Gastroenterol. Hepatol. Bed. Bench. 2014;7(2):125–130. [PMC free article] [PubMed] [Google Scholar]
- Ullrich R., Riecken E.O., Zeitz M. Human immunodeficiency virus-induced enteropathy. Immunol. Res. 1991;10:456–464. doi: 10.1007/BF02919742. [DOI] [PubMed] [Google Scholar]
- Vahedi G., Taghavi M., Maleki A.K., Habibian R. The effect of Aloe vera extract on humoral and cellular immune response in rabbit. Afr. J. Biotechnol. 2011;10:5225–5228. [Google Scholar]
- Yagi A., Harada N., Yamada H., Iwadare S., Nishioka I. Antibradykinin active material in Aloe saponaria. J. Pharmaceut. Sci. 1982;71:1172–1174. doi: 10.1002/jps.2600711024. [DOI] [PubMed] [Google Scholar]
- Yim D., Kang S.S., Kim D.W., Kim S.H., Lillehoj H.S., Min W. Protective effects of Aloe-vera based diets in Eimeria maxima-infected broiler chickens. Exp. Parasitol. 2011;127:322–325. doi: 10.1016/j.exppara.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Yu H., Dong Z., Yang Z. Molecular biological study of Aloe vera in the treatment of experimental allergic rhinitis in rat. Lin Chuang Er Bi Yan Kou He Za Zhi. 2002;16 229–23. [PubMed] [Google Scholar]
- Yu J.R., Choi S.D., Kim Y.W. In vitro infection of Cryptosporidium parvum to four different cell lines. Kor. J. Parasitol. 2000;38(2):59–64. doi: 10.3347/kjp.2000.38.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.R., Lee S.U., Park W.Y. Comparative sensitivity of PCR primer sets for detection of Cryptosporidium parvum. Kor. J. Parasitol. 2009;47(3):293–297. doi: 10.3347/kjp.2009.47.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi A., Ryan U. Cryptosporidium-An update with an emphasis on foodborne and waterborne transmission. Res. Vet. Sci. 2020;132:500–512. doi: 10.1016/j.rvsc.2020.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.