Abstract
Acral melanoma (AM) is a malignant cutaneous melanocytic tumour specifically located on the palms, soles, and nail apparatus, which are areas of glabrous (hairless) skin. Acral lentiginous melanoma, a subtype of AM, represents a histopathological subtype diagnosis of cutaneous melanoma with unique morphological and structural features. Despite clear definitions, the misuse of these terms and the inconsistency in reporting the histopathological features of AM cases have become a major obstacle to the study of the disease. In this review, we discuss the epidemiology, histopathological features, prognosis, and genetic profile of AM, highlighting the differences observed when histopathological subtypes are considered. The increasing global effort to characterise AM cases from ethnically diverse populations would benefit greatly from a more consistent classification of the disease.
Keywords: acral melanoma, epidemiology, histopathology, prognosis, genetics
Introduction
The terms used to denote melanomas from volar surfaces of hands and feet or the nail apparatus, ‘acral melanoma’ and ‘acral lentiginous melanoma’, embody distinct diagnoses. According to the World Health Organization (WHO), acral melanoma (AM) is an anatomical term that refers to melanoma located on glabrous (hairless) skin of the extremities. On the other hand, acral lentiginous melanoma (ALM) represents a histopathological subtype of cutaneous melanoma with characteristic morphological and structural features, recognised alongside others such as lentigo maligna melanoma (LMM), superficial spreading melanoma (SSM), and nodular melanoma (NM) [1].
Almost two decades ago, attention was drawn to the various controversial aspects and unanswered questions about AM, including the misuse of the terms AM and ALM [2]. Apparently, the lack of consensus on how to refer to melanoma in acral skin continues until today: from all original studies retrieved from a search of PubMed using ‘acral melanoma’ as the search term, only 38% specified the histopathological subtype of the lesions, 78% reported the anatomical sites, and 37% reported information on both (Table 1). In 21% of the studies, no specification of the histopathological subtype and anatomical site was reported. The search does not retrieve all studies on AM, but it offers us a picture of what is available in the literature. These studies involve different populations and illustrate the lack of reporting rigour and consensus.
Table 1.
Articles focusing on AM according to the histopathological subtype and anatomical site.
| Publication year | Histopathological subtypes | Anatomical site | Reference |
|---|---|---|---|
| 2020 | ALM | Sole, palm, nail apparatus | [3] |
| 2020 | Not specified | Acral non‐nail, nail apparatus* | [4] |
| 2020 | Not specified | Sole, toe, finger, nail apparatus* | [5] |
| 2020 | Not specified | Hand, foot, finger, toe, sole, nail apparatus* | [6] |
| 2020 | Not specified | Sole, palm, nail apparatus | [7] |
| 2020 | Not specified | Not specified | [8] |
| 2020 | ALM, NM | Sole, palm, nail apparatus | [9] |
| 2020 | ALM, NM, SSM | Hand, foot, nail apparatus* | [10] |
| 2020 | ALM, NM, SSM, LMM | Hand, foot* | [11] |
| 2020 | Not specified | Not specified | [12] |
| 2020 | Not specified | Not specified | [13] |
| 2020 | Not specified | Not specified | [14] |
| 2019 | ALM | Sole, palm, nail apparatus | [15] |
| 2019 | ALM, NM, SSM, LMM | Not specified | [16] |
| 2019 | Not specified | Hand, foot* | [17] |
| 2019 | Not specified | Not specified | [18] |
| 2019 | ALM, NM, SSM | Sole, palm, dorsum of hand and foot, nail apparatus | [19] |
| 2019 | Not specified | Not specified | [20] |
| 2019 | ALM | Sole, lateral great toe | [21] |
| 2019 | ALM, NM, SSM | Sole, palm, nail apparatus | [22] |
| 2019 | Not specified | Not specified | [23] |
| 2019 | Not specified | Sole | [24] |
| 2019 | Not specified | Sole, palm, nail apparatus | [25] |
| 2018 | Not specified | Sole, palm, nail apparatus, digit | [26] |
| 2018 | Not specified | Hand, foot* | [27] |
| 2018 | ALM, NM, SSM | Sole, palm, nail apparatus | [28] |
| 2018 | Not specified | Nail apparatus, non‐nail apparatus* | [29] |
| 2018 | Not specified | Sole, palm | [30] |
| 2018 | Not specified | Not specified | [31] |
| 2018 | ALM, SSM, NM | Sole, palm, nail apparatus | [32] |
| 2018 | Not specified | Not specified | [33] |
| 2018 | Not specified | Hand, foot* | [34] |
| 2018 | Not specified | Hand, finger, foot and toe* | [35] |
| 2018 | Not specified | Hand, foot, sole, finger* | [36] |
| 2018 | ALM, NM, SSM | Palm, sole, nail apparatus | [37] |
| 2018 | ALM, NM, SSM, LMM | Sole, palm, dorsum of hand and foot, nail apparatus, interdigital | [38] |
| 2017 | Not specified | Not specified | [39] |
| 2017 | Not specified | Not specified | [40] |
| 2017 | Not specified | Foot, palm, nail apparatus* | [41] |
| 2017 | ALM | Sole, nail apparatus | [42] |
| 2017 | Not specified | Foot, hand, nail apparatus* | [43] |
| 2017 | ALM | Sole, palm, nail apparatus | [44] |
| 2016 | Not specified | Hand, foot, nail apparatus* | [45] |
| 2016 | Not specified | Sole, toe, top of the foot, palm, finger, near the fingernail* | [46] |
| 2016 | ALM, NM | Sole, palm, dorsum of hand and foot, nail apparatus | [47] |
| 2016 | ALM, NM, SSM | Sole, toe, dorsum of the foot, finger, nail apparatus | [48] |
| 2016 | ALM, non‐ALM | Sole, palm, nail apparatus | [49] |
| 2016 | ALM, NM, SSM | Sole, palm, nail apparatus | [50] |
| 2016 | ALM, non‐ALM | Sole, palm, nail apparatus | [51] |
| 2015 | Not specified | Sole, palm | [52] |
| 2015 | Not specified | Not specified | [53] |
| 2015 | Not specified | Sole, palm, nail apparatus | [54] |
| 2015 | Not specified | Hand, foot* | [55] |
| 2014 | Not specified | Foot, sole, toe* | [56] |
| 2013 | Not specified | Not specified | [57] |
| 2013 | ALM, NM, SSM | Sole, palm, nail apparatus | [58] |
| 2013 | ALM, NM | Sole, palm, nail apparatus | [59] |
| 2013 | Not specified | Sole, palm, nail apparatus | [60] |
| 2013 | ALM, NM, desmoplastic | Sole, palm, nail apparatus | [61] |
| 2013 | Not specified | Sole, palm, dorsum of hand and foot | [62] |
| 2013 | ALM, NM | Nail apparatus | [63] |
| 2012 | Not specified | Sole, nail apparatus, web space | [64] |
| 2012 | Not specified | Sole, toe, web space | [65] |
| 2012 | Not specified | Hand, foot* | [66] |
| 2011 | Not specified | Not specified | [67] |
| 2011 | Not specified | Not specified | [68] |
| 2011 | Not specified | Not specified | [69] |
| 2009 | Not specified | Not specified | [70] |
| 2005 | ALM | Sole, palm, finger, toe, nail apparatus* | [71] |
| 2004 | Not specified | Sole, palm, nail apparatus | [72] |
| 2004 | ALM | Sole, palm | [73] |
| 2000 | ALM, NM, SSM | Sole, palm, nail apparatus, dorsum of hand, foot | [74] |
| 2000 | ALM, SSM | Sole, toe, foot* | [75] |
| 1998 | ALM, NM, SSM | Sole, palm, nail apparatus | [76] |
| 1993 | Not specified | Sole, palm, nail apparatus, dorsum of hand and foot | [77] |
| 1990 | ALM, NM, SSM | Sole, palm, nail apparatus | [78] |
| 1990 | Not specified | Sole, palm, nail apparatus | [79] |
| 1988 | Not specified | Sole, palm, nail apparatus | [80] |
| 1985 | ALM, NM, SSM | Sole, palm, nail apparatus | [81] |
| 1983 | Not specified | Sole, palm, nail apparatus, toe, finger, dorsum of foot | [82] |
| 1982 | Not specified | Sole, palm, dorsum of hand and foot | [83] |
A search using the term ‘acral melanoma’ on PubMed in June 2020 (English language and journal article type) retrieved 239 articles (published and ahead of print), of which 154 were original studies. Of those, 94 used the words ‘acral’ and ‘melanoma’ in the title. Four were excluded for using the word ‘lentiginous’ in the title and nine were excluded for other reasons (file not available, only in situ AM, naevi, or cell lines), resulting in 81 articles. Among these articles, 38% reported the histopathological subtypes studied, 78% reported the anatomical sites, and 37% reported information on both. In 21% of the studies, there was no specification of the histopathological subtype and anatomical site.
Not specified: information on histopathological subtype or anatomical location of AM cases was not provided. The volar surfaces of the fingers and toes are included as palms and soles, respectively. Specific locations of the soles, such as the heels, are included as soles.
Articles in which it is unclear whether the classification was performed according to the 2018 WHO guidelines.
These inconsistencies regarding the reporting of histopathological subtypes have a major impact in the interpretation of data derived from studies aimed at understanding the epidemiological, clinical, and biological characteristics of AM. In the following sections, we discuss different aspects of AM, highlighting the differences observed when the histopathological subtypes are considered, and in doing so we aim to draw attention to the importance of an accurate anatomical and histopathological classification. As an important note, we clarify that we only reviewed articles that followed the WHO guidelines for diagnosing AM [1].
AM epidemiology
According to several population‐based epidemiology studies, AM represents only about 3% of melanomas that occur in European‐descent individuals [84, 85, 86], whereas it represents a higher proportion of cases in Asian, Hispanic, and African populations [1]. For example, AM accounts for more than 40% of cutaneous melanoma in Asia [87, 88] and 20.1% in Latin American countries [89]. Furthermore, the relative frequency of AM is higher in genetically heterogeneous or Amerindian‐descent populations (indigenous Latin Americans) than in European‐descent individuals from Latin American countries [90, 91].
In the case of ALM, its absolute global incidence is similar across populations and is much lower than that of SSM or LMM. However, it also accounts for a higher percentage of total melanoma cases in non‐European descent populations compared to populations of European descent [92].
These contrasts in the relative incidence of AM and ALM may be ascribed to marked differences in sun‐induced melanoma incidence among these populations; for example, the incidence of cutaneous melanoma is 49 per 100,000 individuals in Australia, which has a predominantly European‐descent population [93], but is typically estimated to be less than 5 per 100,000 inhabitants in several countries in Latin America [91, 94, 95]. As AM and ALM have similar incidences around the globe and are not thought to be ultraviolet (UV)‐related, they constitute a higher proportion of cases in countries with a lower incidence of melanoma.
Among studies that have reported the histopathological subtypes of AM, a high relative frequency of ALM is consistently reported, but the frequencies of AM histopathological subtypes vary considerably. ALM has been reported to represent 44–90% of AM, while acral NM (2.5–41%) and acral SSM (0.7–28%) are relatively less frequent subtypes of AM [16, 22, 28, 38, 50, 58, 76, 96]. When studying specific populations, these variations are noteworthy. In European‐descent individuals, ALM represents ~72% of AM [16, 38, 76], followed by acral SSM (~18%) [16, 38, 76]. In Asians, ~88% of AM patients were reported as ALM, whereas the second most frequent subtype was NM, with ~11% of cases [50, 58]. In African individuals, ALM corresponds to ~78% of AM [96]. In Brazil, which consists of a highly genetically heterogeneous population with African, European, and indigenous heritage, ALM (56%) and acral NM (22%) represent the majority of cases, whereas acral SSM represents only 13% of AM cases [22, 28].
While this variation is interesting, it requires special attention because, although it may indicate real differences among populations, it may also indicate a lack of consensus in the classification of these types of tumours. It also shows how important it is to perform studies considering the diversity of the world population in order to obtain a more accurate picture of the characteristics of this disease.
The distinct clinicopathology of AM
According to the 2018 WHO classification of melanoma, AM is grouped together with melanomas not consistently associated with cumulative solar damage. In fact, because of the anatomical location and the thick stratum corneum of glabrous skin, UV radiation is not believed to play a significant role in the pathogenesis of AM [97]. Since 1935, trauma has been suspected to influence the development of melanoma on the soles of the feet (Hewer, as cited in Ref. [98]). A strong association has yet to be proven, but several studies have shown a higher incidence of plantar melanoma in areas of greater physical stress such as the heels [58, 99, 100].
Clinically, AM is usually characterised by an asymmetric pigmented macule with highly irregular notched borders and various colour shades (ranging from tan to dark brown to black) in the centre of which a nodule may develop. In subungual locations, melanoma presents as longitudinal or total (damage of the entire nail plate) melanonychia. Periungual pigmentation (Hutchinson sign) and nail plate dystrophy are also clues to a diagnosis of nail apparatus melanoma (or subungual melanoma) [101].
Histopathologically, ALM is characterised by a lentiginous growth pattern, i.e. a continuous proliferation of atypical melanocytes with enlarged hyperchromatic nuclei and prominent dendrites along the dermoepidermal junction. The confluence of the cells increases with the stage of the lesion varying from scattered cells along the basal layer of an epidermal hyperplasia at early stage to pagetoid spread and junctional nests at late stage, the latter usually at the tips of the epidermal ridges or haphazardly oriented. Established lesions show acanthosis with marked elongation of the rete ridges (Figure 1). Cells are usually spindle (Figure 2A) and pigmented. However, an epithelioid component is often present in invasive lesions and can be predominant. Giant cells can also be seen. Extension along sweat glands is commonly observed (syringotropism) (Figure 2B). Invasive tumours can display a desmoplastic reaction (Figure 2C) and neurotropism. A band‐like chronic inflammatory cell infiltrate can surround the tumours (Figure 2D) [1, 101, 102].
Figure 1.
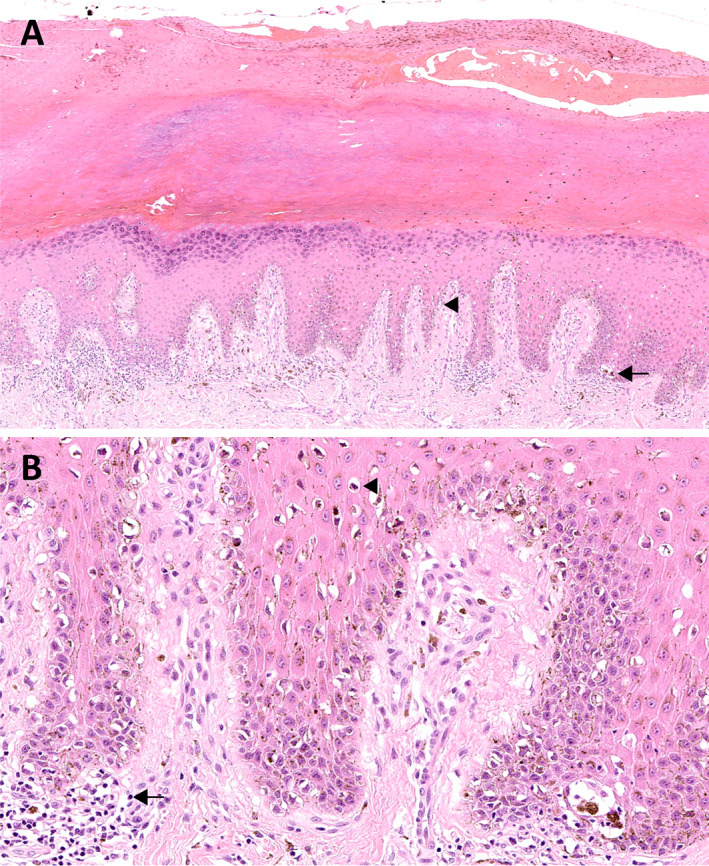
Acral melanoma. (A) In situ acral lentiginous melanoma with a lentiginous proliferation of atypical melanocytes along the dermoepidermal junction (arrowhead), with pagetoid spread and scattered nests at the tips of the rete ridges (arrow). There is also acanthosis, hypergranulosis, and hyperkeratosis (H&E, ×100). (B) Tumour cells are hyperchromatic and have a cytoplasmic fixation retraction artefact (arrowhead). The dermis contains a chronic inflammatory cell infiltrate associated with macrophages (arrow) (H&E, ×400).
Figure 2.
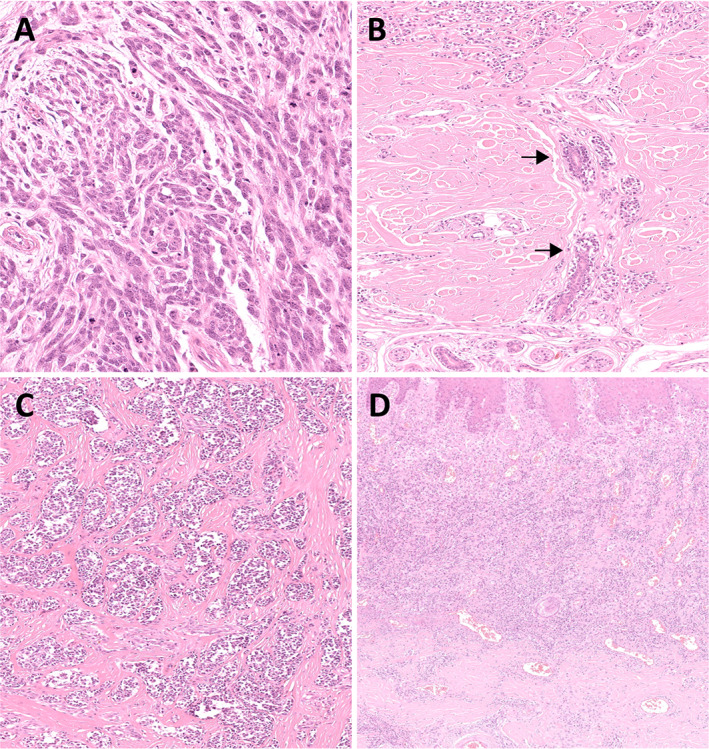
Acral lentiginous melanoma. (A) Tumour cells are usually spindle. (B) Deep extension along sweat gland (arrows) is frequent. Desmoplastic reaction (C) and band‐like chronic inflammatory cell infiltrate (D) are associated features (H&E, ×400, ×200, ×200, and ×100).
Distinguishing ALM from SSM can sometimes be challenging. Compared to ALM, SSM is mainly characterised by a nested and pagetoid proliferation of epithelioid melanocytes along the dermoepidermal junction (Figure 3). However, it is not entirely specific as both of these features can also be observed in advanced ALM. Changes at the periphery of the lesions may be more relevant for classification. Importantly, SSM is mainly encountered on the dorsa of hands and feet, which, according to the WHO, are not considered acral sites [97]. Although the prevalence of KIT mutations is low in ALM (~15%) [103], the presence of KIT expression can be useful in complex diagnostic cases. By immunohistochemistry, in general (subject to exception [104]), KIT expression is in favour of ALM [103] as KIT mutations appear to be related to the lentiginous growth pattern [105], while the expression of BRAFV600E argues for non‐ALM/SSM subtype [37, 106]. These two histopathological subtypes can present with a prominent vertical growth phase, which can be misdiagnosed for an NM. They are distinguished from NM by the presence of an adjacent radial growth phase [1, 102, 107, 108] (Figure 4).
Figure 3.
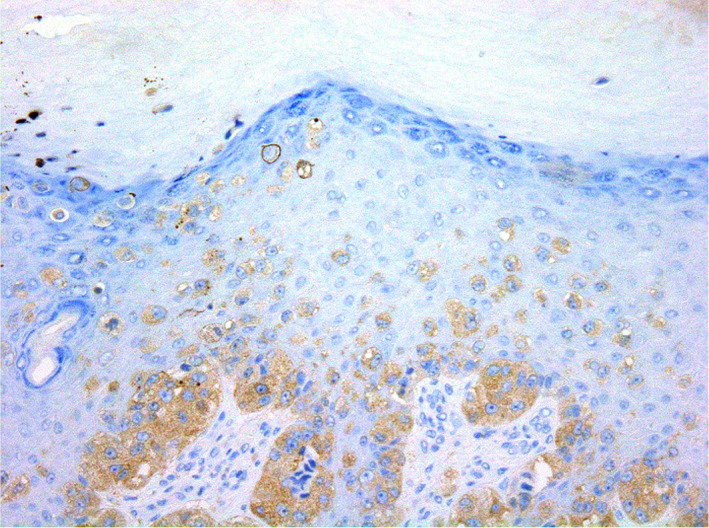
Superficial spreading melanoma in acral skin. Because of the upward spread of abnormal melanocytic cells in the epidermis, it may also be referred to as acral pagetoid melanoma. Lesion stained positively for the BRAFV600E protein (BRAFV600E/DAB, ×400).
Figure 4.
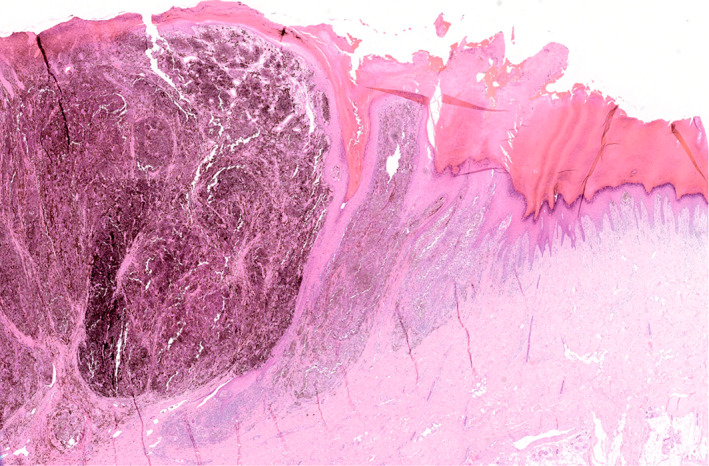
Acral lentiginous melanoma with a ‘nodular‐like’ vertical growth phase (on the left). There also is an adjacent radial growth phase including a lentiginous proliferation of atypical melanocytes along the dermoepidermal junction (on the right) (H&E, ×20).
Similar to non‐acral cutaneous melanomas, histopathological features such as Breslow depth, ulceration, and sentinel lymph node (SLN) status have been identified as prognostic factors in AM [22, 28, 60, 109, 110]. In AM, Breslow depth is usually high and is considered an independent prognostic factor and positively correlates with ulceration [28]. Consistently, in amelanotic AM, in which acral NM was the most common histopathological subtype (63.6%), ulceration was observed in 83% of cases, and more than 50% of cases had Breslow depth over 4 mm [59]. Also, it has been observed that ulceration is an important predictive factor for SLN involvement in ALM [111]. Thus, although the importance of these histopathological features is well established in AM, studies that look at the different subtypes of AM in order to better understand the impact of these characteristics on patient prognosis are still needed.
The molecular profile of AM
The consensus that AMs are molecularly different from cutaneous melanomas of non‐glabrous skin is well accepted in the literature. AM displays a lower frequency of BRAF V600E mutations than non‐acral cutaneous melanoma but achieves activation of the MAPK pathway through alterations in NRAS, NF1, KIT, and other genes such as SPRED1, a negative regulator of RAS [25, 112, 113, 114, 115]. Genomic aberrations in KIT, CCND1, CDK4, GAB2, and TERT are common [25, 114, 116, 117], but what best describes the genomic landscape of AM is a lower number of point somatic mutations and an increased frequency of genomic aberrations [112, 113, 114, 115].
Most studies, however, were based on heterogeneous cohorts that pooled together several distinct AM subtypes and, in most cases, without discriminating tumours according to the WHO histopathological classification (Table 1). Although these studies mostly agree on the general molecular features of AM, the lack of information regarding histopathological parameters prevents the investigation of potential differences among AM subtypes.
Unanswered questions and perspectives
Herein, we have highlighted different aspects of AM drawing attention to the lack of information on the histopathological classification of the tumours.
Our review of the literature revealed that only 38% of the studies that included AM cases reported histopathological subtype information. Because most studies of AM do not classify samples by their histopathological subtype, it is difficult to evaluate whether the different aspects of the disease – e.g. incidence, prognosis, and molecular landscape – apply to all patients with AM or just to patients with a specific histopathological subtype such as ALM.
There are other concerns not discussed here in great depth that deserve major attention. One is related to the inconsistency in diagnosing AM according to the type of skin in which it occurs. Several studies consider melanomas arising at the dorsal surface of the hands and feet as AMs, disregarding the WHO guidelines that recommend that AMs always occur on glabrous acral skin [1]. Not surprisingly, whereas 78% of the studies retrieved in our literature search on AM reported anatomical localisation of the lesions, only half of these confirmed their location on glabrous acral skin (Table 1). This is worrisome because studies that compared melanomas involving dorsal surfaces of hands and feet, which are more frequently classified as SSM, to melanomas on glabrous acral skin reported significant differences, including in the genetics of these tumours and in patient prognosis [38, 89, 91].
Another relevant point of discussion is how similar subungual melanomas are to AMs occurring on the palms and soles. Recent evidence shows significant clinical and molecular differences that may impact diagnosis and treatment [118]. Among them, reported trauma and KIT mutations have been more frequently associated with subungual melanomas than with AMs occurring in other locations. Whether these differences are sufficient to categorise subungual melanomas as a separate entity still needs to be discussed, but findings like these demonstrate both the advantage of considering different groupings when studying AM and how this can help arrive at optimal definitions.
In conclusion, AM is a cutaneous melanoma subtype which exhibits its own clinical and genetic features. Nevertheless, the inconsistency regarding the definition used for AM and the lack of histopathological information observed in many scientific articles limits our knowledge of its biology. In the future, greater attention should be given to the definition and classification of AMs to clarify the extent to which different histopathological subtypes do occur on acral sites across different ethnic populations. This information will be key to helping characterise the biological, clinical, and molecular aspects of AM and its histopathological subtypes. All these studies will hopefully contribute to decreasing the gaps in knowledge of this disease and advance its therapeutic possibilities.
Author contributions statement
SSB, CDR‐E, DJA and PAP conceived and designed the review. SSB performed the bibliographical search. IF, DEE and SSB designed the figures. All authors participated in writing and approved the final version of the manuscript.
Acknowledgements
The authors wish to thank Jair S. García‐Sotelo, Alejandro de León, Carlos S. Flores, and Luis A. Aguilar of the Laboratorio Nacional de Visualización Científica Avanzada from the National Autonomous University of Mexico, and Alejandra Castillo, Carina Díaz, Abigayl Hernández, and Eglee Lomelin of the International Laboratory for Human Genome Research, UNAM. This work was supported by the Medical Research Council grants (MR/S01473X/1 and MR/V000292/1) to CDR‐E, PAP, and DJA; Royal Society Newton Advanced Fellowship (NAF\R2\202163) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (E‐26/010.002427/2019) to PAP; Melanoma Research Alliance Pilot Award (825924) and Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT UNAM) (IA202020) to CDR‐E; and Cancer Research UK and Wellcome Trust to DJA. PAP is also supported by the International Centre for Genetic Engineering and Biotechnology (CRP/BRA17‐05_EC). CDR‐E is also supported by CONACyT (A3‐S‐31603), the Academy of Medical Sciences through a Newton Advanced Fellowship (NAF\R2\180782), and a Wellcome Sanger Institute International Fellowship. SSB was supported by an INCA/Ministry of Health scholarship. IF is supported by Wallonia‐Brussels International, Belgium.
No conflicts of interest were declared.
References
- 1. Yun SJ, Bastian BC, Dunca LM, et al. Chapter 2: Melanocytic tumours in acral skin. In: WHO Classification of Skin Tumours (4th edn), Elder DE, Massi D, Scoyler RA, et al. (Eds). IARC: Lyon, 2018; 116–120. [Google Scholar]
- 2. Stalkup JR, Orengo IF, Katta R. Controversies in acral lentiginous melanoma. Dermatol Surg 2002; 28: 1051–1059. [DOI] [PubMed] [Google Scholar]
- 3. Lee AY, Friedman EB, Sun J, et al. The devil's in the details: discrepancy between biopsy thickness and final pathology in acral melanoma. Ann Surg Oncol 2020; 27: 5259–5266. [DOI] [PubMed] [Google Scholar]
- 4. Jo G, Cho SI, Cho Y, et al. Tumor growth rate as a prognostic factor of acral melanoma in a Korean population. Medicine (Baltimore) 2020; 99: e19936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gong HZ, Zheng HY, Li J. Clinicopathological characteristics and prognosis of amelanotic acral melanoma: a comparative study with pigmented acral melanoma. Australas J Dermatol 2020; 61: 358–361. [DOI] [PubMed] [Google Scholar]
- 6. Lim Y, Yoon D, Lee DY. Novel mutations identified by whole‐exome sequencing in acral melanoma. J Am Acad Dermatol 2020; 83: 1792–1794. [DOI] [PubMed] [Google Scholar]
- 7. Wei X, Wu D, Li H, et al. The clinicopathological and survival profiles comparison across primary sites in acral melanoma. Ann Surg Oncol 2020; 27: 3478–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dai J, Yang L, Xu T, et al. A functional synonymous variant in PDGFRA is associated with better survival in acral melanoma. J Cancer 2020; 11: 2945–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ito T, Kaku‐Ito Y, Murata M, et al. Immunohistochemical BRAF V600E expression and intratumor BRAF V600E heterogeneity in acral melanoma: implication in melanoma‐specific survival. J Clin Med 2020; 9: 690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dika E, Veronesi G, Altimari A, et al. BRAF, KIT, and NRAS mutations of acral melanoma in white patients. Am J Clin Pathol 2020; 153: 664–671. [DOI] [PubMed] [Google Scholar]
- 11. Zou Z, Ou Q, Ren Y, et al. Distinct genomic traits of acral and mucosal melanomas revealed by targeted mutational profiling. Pigment Cell Melanoma Res 2020; 33: 601–611. [DOI] [PubMed] [Google Scholar]
- 12. Forschner A, Hilke FJ, Bonzheim I, et al. MDM2, MDM4 and EGFR amplifications and hyperprogression in metastatic acral and mucosal melanoma. Cancers (Basel) 2020; 12: 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mikoshiba A, Ashida A, Sakaizawa K, et al. Detecting copy number alterations of oncogenes in cell‐free DNA to monitor treatment response in acral and mucosal melanoma. J Dermatol Sci 2020; 97: 172–178. [DOI] [PubMed] [Google Scholar]
- 14. Lim Y, Lee J, Lee DY. Is the survival rate for acral melanoma actually worse than other cutaneous melanomas? J Dermatol 2020; 47: 251–256. [DOI] [PubMed] [Google Scholar]
- 15. Magdaleno‐Tapial J, Valenzuela‐Oñate C, Ortiz‐Salvador JM, et al. Acral melanoma with eccrine involvement: comments and controversies. J Am Acad Dermatol 2020; 83: 600–602. [DOI] [PubMed] [Google Scholar]
- 16. Pradhan D, Jour G, Milton D, et al. Aberrant DNA methylation predicts melanoma‐specific survival in patients with acral melanoma. Cancers (Basel) 2019; 11: 2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ito T, Kaku‐Ito Y, Murata M, et al. Intra‐ and inter‐tumor BRAF heterogeneity in acral melanoma: an immunohistochemical analysis. Int J Mol Sci 2019; 20: 6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu H, Wang W, Zhao J, et al. Aberrant hTERT promoter methylation predicts prognosis in Chinese patients with acral and mucosal melanoma: a CONSORT‐compliant article. Medicine (Baltimore) 2019; 98: e17578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zaremba A, Murali R, Jansen P, et al. Clinical and genetic analysis of melanomas arising in acral sites. Eur J Cancer 2019; 119: 66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang L, Dai J, Ma M, et al. Identification of a functional polymorphism within the 3'‐untranslated region of denticleless E3 ubiquitin protein ligase homolog associated with survival in acral melanoma. Eur J Cancer 2019; 118: 70–81. [DOI] [PubMed] [Google Scholar]
- 21. Love NR, Lang UE, Cheung C, et al. Depletion of primary cilium in acral melanoma. J Cutan Pathol 2019; 46: 665–671. [DOI] [PubMed] [Google Scholar]
- 22. Nunes LF, Mendes GLQ, Koifman RJ. Sentinel lymph node biopsy in patients with acral melanoma: analysis of 201 cases from the Brazilian National Cancer Institute. Dermatol Surg 2019; 45: 1026–1034. [DOI] [PubMed] [Google Scholar]
- 23. Kato J, Hida T, Someya M, et al. Efficacy of combined radiotherapy and anti‐programmed death 1 therapy in acral and mucosal melanoma. J Dermatol 2019; 46: 328–333. [DOI] [PubMed] [Google Scholar]
- 24. Zhang X, Peng Y, Li C, et al. Genomic heterogeneity and branched evolution of early stage primary acral melanoma shown by multiregional microdissection sequencing. J Invest Dermatol 2019; 139: 1526–1534. [DOI] [PubMed] [Google Scholar]
- 25. Yeh I, Jorgenson E, Shen L, et al. Targeted genomic profiling of acral melanoma. J Natl Cancer Inst 2019; 111: 1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Wet J, Tod B, Visser WI, et al. Clinical and pathological features of acral melanoma in a South African population: a retrospective study. S Afr Med J 2018; 108: 777–781. [DOI] [PubMed] [Google Scholar]
- 27. Yu S, Xu T, Dai J, et al. TERT copy gain predicts the outcome of high‐dose interferon α‐2b therapy in acral melanoma. Onco Targets Ther 2018; 11: 4097–4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nunes LF, Quintella Mendes GL, Koifman RJ. Acral melanoma: a retrospective cohort from the Brazilian National Cancer Institute (INCA). Melanoma Res 2018; 28: 458–464. [DOI] [PubMed] [Google Scholar]
- 29. Lee M, Yoon J, Chung YJ, et al. Whole‐exome sequencing reveals differences between nail apparatus melanoma and acral melanoma. J Am Acad Dermatol 2018; 79: 559–561. [DOI] [PubMed] [Google Scholar]
- 30. Mun JH, Jo G, Darmawan CC, et al. Association between Breslow thickness and dermoscopic findings in acral melanoma. J Am Acad Dermatol 2018; 79: 831–835. [DOI] [PubMed] [Google Scholar]
- 31. Yan J, Yu J, Wu X, et al. Increased AURKA gene copy number correlates with poor prognosis and predicts the efficacy of high‐dose interferon therapy in acral melanoma. J Cancer 2018; 9: 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Usman HA, Hernowo BS, Tobing MDL, et al. The major role of NF‐κB in the depth of invasion on acral melanoma by decreasing CD8+ T cells. J Pathol Transl Med 2018; 52: 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu T, Ma M, Chi Z, et al. High G2 and S‐phase expressed 1 expression promotes acral melanoma progression and correlates with poor clinical prognosis. Cancer Sci 2018; 109: 1787–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ren M, Dai B, Kong YY, et al. PD‐L1 expression in tumour‐infiltrating lymphocytes is a poor prognostic factor for primary acral melanoma patients. Histopathology 2018; 73: 386–396. [DOI] [PubMed] [Google Scholar]
- 35. Yu C, Yang S, Kim W, et al. Acral melanoma detection using a convolutional neural network for dermoscopy images. PLoS One 2018; 13: e0193321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu T, Ma M, Dai J, et al. Gene expression screening identifies CDCA5 as a potential therapeutic target in acral melanoma. Hum Pathol 2018; 75: 137–145. [DOI] [PubMed] [Google Scholar]
- 37. Moon KR, Choi YD, Kim JM, et al. Genetic alterations in primary acral melanoma and acral melanocytic nevus in Korea: common mutated genes show distinct cytomorphological features. J Invest Dermatol 2018; 138: 933–945. [DOI] [PubMed] [Google Scholar]
- 38. Haugh AM, Zhang B, Quan VL, et al. Distinct patterns of acral melanoma based on site and relative sun exposure. J Invest Dermatol 2018; 138: 384–393. [DOI] [PubMed] [Google Scholar]
- 39. Kong Y, Sheng X, Wu X, et al. Frequent genetic aberrations in the CDK4 pathway in acral melanoma indicate the potential for CDK4/6 inhibitors in targeted therapy. Clin Cancer Res 2017; 23: 6946–6957. [DOI] [PubMed] [Google Scholar]
- 40. Yu J, Wu X, Yu H, et al. Systemic immune‐inflammation index and circulating T‐cell immune index predict outcomes in high‐risk acral melanoma patients treated with high‐dose interferon. Transl Oncol 2017; 10: 719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Y, Wen XZ, Ba HJ, et al. A prognostic model for resectable acral melanoma patients on the basis of preoperative inflammatory markers. Melanoma Res 2017; 27: 469–476. [DOI] [PubMed] [Google Scholar]
- 42. Liang WS, Hendricks W, Kiefer J, et al. Integrated genomic analyses reveal frequent TERT aberrations in acral melanoma. Genome Res 2017; 27: 524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shim JH, Shin HT, Park J, et al. Mutational profiling of acral melanomas in Korean populations. Exp Dermatol 2017; 26: 883–888. [DOI] [PubMed] [Google Scholar]
- 44. Rawson RV, Johansson PA, Hayward NK, et al. Unexpected UVR and non‐UVR mutation burden in some acral and cutaneous melanomas. Lab Invest 2017; 97: 130–145. [DOI] [PubMed] [Google Scholar]
- 45. Shoushtari AN, Munhoz RR, Kuk D, et al. The efficacy of anti‐PD‐1 agents in acral and mucosal melanoma. Cancer 2016; 122: 3354–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lyu SM, Wu JY, Byun JY, et al. Expression of phosphatase and tensin homologue, phospho‐Akt, and p53 in acral benign and malignant melanocytic neoplasms (benign nevi, dysplastic nevi, and acral melanomas). Ann Dermatol 2016; 28: 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee KT, Kim EJ, Lee DY, et al. Surgical excision margin for primary acral melanoma. J Surg Oncol 2016; 114: 933–939. [DOI] [PubMed] [Google Scholar]
- 48. Lv J, Dai B, Kong Y, et al. Acral melanoma in Chinese: a clinicopathological and prognostic study of 142 cases. Sci Rep 2016; 6: 31432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Paolino G, Bekkenk MW, Didona D, et al. Is the prognosis and course of acral melanoma related to site‐specific clinicopathological features? Eur Rev Med Pharmacol Sci 2016; 20: 842–848. [PubMed] [Google Scholar]
- 50. Bae SH, Seon HJ, Choi YD, et al. Other primary systemic cancers in patients with melanoma: analysis of balanced acral and nonacral melanomas. J Am Acad Dermatol 2016; 74: 333–340. [DOI] [PubMed] [Google Scholar]
- 51. Li H, Kim SM, Savkovic V, et al. Expression of soluble adenylyl cyclase in acral melanomas. Clin Exp Dermatol 2016; 41: 425–429. [DOI] [PubMed] [Google Scholar]
- 52. Lallas A, Kyrgidis A, Koga H, et al. The BRAAFF checklist: a new dermoscopic algorithm for diagnosing acral melanoma. Br J Dermatol 2015; 173: 1041–1049. [DOI] [PubMed] [Google Scholar]
- 53. Buchbinder EI, Sosman JA, Lawrence DP, et al. Phase 2 study of sunitinib in patients with metastatic mucosal or acral melanoma. Cancer 2015; 121: 4007–4015. [DOI] [PubMed] [Google Scholar]
- 54. Johnson DB, Peng C, Abramson RG, et al. Clinical activity of ipilimumab in acral melanoma: a retrospective review. Oncologist 2015; 20: 648–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xu S, Yang Z, Zhang J, et al. Increased levels of β‐catenin, LEF‐1, and HPA‐1 correlate with poor prognosis for acral melanoma with negative BRAF and NRAS mutation in BRAF exons 11 and 15 and NRAS exons 1 and 2. DNA Cell Biol 2015; 34: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Furney SJ, Turajlic S, Stamp G, et al. The mutational burden of acral melanoma revealed by whole‐genome sequencing and comparative analysis. Pigment Cell Melanoma Res 2014; 27: 835–838. [DOI] [PubMed] [Google Scholar]
- 57. Braun RP, Thomas L, Dusza SW, et al. Dermoscopy of acral melanoma: a multicenter study on behalf of the international dermoscopy society. Dermatology 2013; 227: 373–380. [DOI] [PubMed] [Google Scholar]
- 58. Jung HJ, Kweon SS, Lee JB, et al. A clinicopathologic analysis of 177 acral melanomas in Koreans: relevance of spreading pattern and physical stress. JAMA Dermatol 2013; 149: 1281–1288. [DOI] [PubMed] [Google Scholar]
- 59. Choi YD, Chun SM, Jin SA, et al. Amelanotic acral melanomas: clinicopathological, BRAF mutation, and KIT aberration analyses. J Am Acad Dermatol 2013; 69: 700–707. [DOI] [PubMed] [Google Scholar]
- 60. Bello DM, Chou JF, Panageas KS, et al. Prognosis of acral melanoma: a series of 281 patients. Ann Surg Oncol 2013; 20: 3618–3625. [DOI] [PubMed] [Google Scholar]
- 61. Dai B, Cai X, Kong YY, et al. Analysis of KIT expression and gene mutation in human acral melanoma: with a comparison between primary tumors and corresponding metastases/recurrences. Hum Pathol 2013; 44: 1472–1478. [DOI] [PubMed] [Google Scholar]
- 62. Lee SJ, Lim HJ, Choi YH, et al. The clinical significance of tumor‐infiltrating lymphocytes and microscopic satellites in acral melanoma in a Korean population. Ann Dermatol 2013; 25: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fanti PA, Dika E, Misciali C, et al. Nail apparatus melanoma: is trauma a coincidence? Is this peculiar tumor a real acral melanoma? Cutan Ocul Toxicol 2013; 32: 150–153. [DOI] [PubMed] [Google Scholar]
- 64. Ibrahim ZA, Narihan MZ, Ojep DN, et al. Cyclin D1 expression in acral melanoma: a case control study in Sarawak. Malays J Pathol 2012; 34: 89–95. [PubMed] [Google Scholar]
- 65. Zainal AI, Zulkarnaen M, Norlida DK, et al. Acral melanoma of the extremities: a study of 33 cases Sarawakian patients. Med J Malaysia 2012; 67: 60–65. [PubMed] [Google Scholar]
- 66. Abu‐Abed S, Pennell N, Petrella T, et al. KIT gene mutations and patterns of protein expression in mucosal and acral melanoma. J Cutan Med Surg 2012; 16: 135–142. [DOI] [PubMed] [Google Scholar]
- 67. Mao L, Si L, Chi Z, et al. A randomised phase II trial of 1 month versus 1 year of adjuvant high‐dose interferon α‐2b in high‐risk acral melanoma patients. Eur J Cancer 2011; 47: 1498–1503. [DOI] [PubMed] [Google Scholar]
- 68. Yun J, Lee J, Jang J, et al. KIT amplification and gene mutations in acral/mucosal melanoma in Korea. APMIS 2011; 119: 330–335. [DOI] [PubMed] [Google Scholar]
- 69. Godshalk SE, Paranjape T, Nallur S, et al. A variant in a microRNA complementary site in the 3' UTR of the KIT oncogene increases risk of acral melanoma. Oncogene 2011; 30: 1542–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ashida A, Takata M, Murata H, et al. Pathological activation of KIT in metastatic tumors of acral and mucosal melanomas. Int J Cancer 2009; 124: 862–868. [DOI] [PubMed] [Google Scholar]
- 71. Takata M, Goto Y, Ichii N, et al. Constitutive activation of the mitogen‐activated protein kinase signaling pathway in acral melanomas. J Invest Dermatol 2005; 125: 318–322. [DOI] [PubMed] [Google Scholar]
- 72. Saida T, Miyazaki A, Oguchi S, et al. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol 2004; 140: 1233–1238. [DOI] [PubMed] [Google Scholar]
- 73. Rokuhara S, Saida T, Oguchi M, et al. Number of acquired melanocytic nevi in patients with melanoma and control subjects in Japan: nevus count is a significant risk factor for nonacral melanoma but not for acral melanoma. J Am Acad Dermatol 2004; 50: 695–700. [DOI] [PubMed] [Google Scholar]
- 74. Kuchelmeister C, Schaumburg‐Lever G, Garbe C. Acral cutaneous melanoma in Caucasians: clinical features, histopathology and prognosis in 112 patients. Br J Dermatol 2000; 143: 275–280. [DOI] [PubMed] [Google Scholar]
- 75. Bastian BC, Kashani‐Sabet M, Hamm H, et al. Gene amplifications characterize acral melanoma and permit the detection of occult tumor cells in the surrounding skin. Cancer Res 2000; 60: 1968–1973. [PubMed] [Google Scholar]
- 76. Metzger S, Ellwanger U, Stroebel W, et al. Extent and consequences of physician delay in the diagnosis of acral melanoma. Melanoma Res 1998; 8: 181–186. [DOI] [PubMed] [Google Scholar]
- 77. Kato T, Suetake T, Sugiyama Y, et al. Improvement in survival rate of patients with acral melanoma observed in the past 22 years in Sendai, Japan. Clin Exp Dermatol 1993; 18: 107–110. [DOI] [PubMed] [Google Scholar]
- 78. Slingluff CL Jr, Vollmer R, Seigler HF. Acral melanoma: a review of 185 patients with identification of prognostic variables. J Surg Oncol 1990; 45: 91–98. [DOI] [PubMed] [Google Scholar]
- 79. Lin CS, Wang WJ, Wong CK. Acral melanoma. A clinicopathologic study of 28 patients. Int J Dermatol 1990; 29: 107–112. [DOI] [PubMed] [Google Scholar]
- 80. Shaw JH, Koea JB. Acral (volar‐subungual) melanoma in Auckland, New Zealand. Br J Surg 1988; 75: 69–72. [DOI] [PubMed] [Google Scholar]
- 81. Gutman M, Klausner JM, Inbar M, et al. Acral (volar‐subungual) melanoma. Br J Surg 1985; 72: 610–613. [DOI] [PubMed] [Google Scholar]
- 82. Seiji M, Takematsu H, Hosokawa M, et al. Acral melanoma in Japan. J Invest Dermatol 1983; 80 (Suppl): 56s–60s. [PubMed] [Google Scholar]
- 83. Seiji M, Takahashi M. Acral melanoma in Japan. Hum Pathol 1982; 13: 607–609. [DOI] [PubMed] [Google Scholar]
- 84. Black WC, Goldhahn RT, Wiggins C. Melanoma within a southwestern Hispanic population. Arch Dermatol 1987; 123: 1331–1334. [PubMed] [Google Scholar]
- 85. Johnson DS, Yamane S, Morita S, et al. Malignant melanoma in non‐Caucasians: experience from Hawaii. Surg Clin North Am 2003; 83: 275–282. [DOI] [PubMed] [Google Scholar]
- 86. Reintgen DS, McCarty KM, Cox E, et al. Malignant melanoma in black American and white American populations. A comparative review. JAMA 1982; 248: 1856–1859. [PubMed] [Google Scholar]
- 87. Chi Z, Li S, Sheng X, et al. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: a study of 522 consecutive cases. BMC Cancer 2011; 11: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lee HY, Chay WY, Tang MB, et al. Melanoma: differences between Asian and Caucasian patients. Ann Acad Med Singapore 2012; 41: 17–20. [PubMed] [Google Scholar]
- 89. Quintella Mendes GL, Koifman S. Socioeconomic status as a predictor of melanoma survival in a series of 1083 cases from Brazil: just a marker of health services accessibility? Melanoma Res 2013; 23: 199–205. [DOI] [PubMed] [Google Scholar]
- 90. Zemelman VB, Valenzuela CY, Sazunic I, et al. Malignant melanoma in Chile: different site distribution between private and state patients. Biol Res 2014; 47: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. de Vries E, Sierra M, Piñeros M, et al. The burden of cutaneous melanoma and status of preventive measures in Central and South America. Cancer Epidemiol 2016; 44: S100–S109. [DOI] [PubMed] [Google Scholar]
- 92. Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986‐2005. Arch Dermatol 2009; 145: 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Australian Institute of Health and Welfare . Skin cancer in Australia. Cat. no. CAN 96. AIHW: Canberra, 2016. [Accessed 13 December 2020]. Available from: https://www.aihw.gov.au/getmedia/0368fb8b-10ef-4631-aa14-cb6d55043e4b/18197.pdf.aspx?inline=true
- 94. Instituto Nacional de Câncer José Alencar Gomes da Silva . Estimativa 2020: incidência de câncer no Brasil. 2019. [Accessed 13 December 2020]. Available from: https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//estimativa-2020-incidencia-de-cancer-no-brasil.pdf
- 95. Gómez‐Dantés H, Lamadrid‐Figueroa H, Cahuana‐Hurtado L, et al. The burden of cancer in Mexico, 1990‐2013. Salud Publica Mex 2016; 58: 118–131. [DOI] [PubMed] [Google Scholar]
- 96. Mulenga M, Montgomery ND, Chagomerana M, et al. Epidemiological and histopathological profile of malignant melanoma in Malawi. BMC Clin Pathol 2019; 19: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Elder DE, Bastian BC, Cree IA, et al. The 2018 World Health Organization classification of cutaneous, mucosal, and uveal melanoma: detailed analysis of 9 distinct subtypes defined by their evolutionary pathway. Arch Pathol Lab Med 2020; 144: 500–522. [DOI] [PubMed] [Google Scholar]
- 98. Lewis MG. Malignant melanoma in Uganda. (The relationship between pigmentation and malignant melanoma on the soles of the feet). Br J Cancer 1967; 21: 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Minagawa A, Omodaka T, Okuyama R. Melanomas and mechanical stress points on the plantar surface of the foot. N Engl J Med 2016; 374: 2404–2406. [DOI] [PubMed] [Google Scholar]
- 100. Sheen YS, Liao YH, Lin MH, et al. A clinicopathological analysis of 153 acral melanomas and the relevance of mechanical stress. Sci Rep 2017; 7: 5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Andre J, Sass U, Theunis A. Chapter 23: Diseases of the nails. In: McKee's Pathology of the Skin, Volume 2 (5th edn), Calonje E, Brenn T, Lazar A, et al. (Eds). Elsevier: Edinburgh, 2019; 1129–1155. [Google Scholar]
- 102. North JP, Bastian BC, Lazar A. Chapter 26: Melanoma. In: McKee's Pathology of the Skin, Volume 2 (5th edn), Calonje E, Brenn T, Lazar A, et al. (Eds). Elsevier: Edinburgh, 2019; 1310–1362. [Google Scholar]
- 103. Torres‐Cabala CA, Wang WL, Trent J, et al. Correlation between KIT expression and KIT mutation in melanoma: a study of 173 cases with emphasis on the acral‐lentiginous/mucosal type. Mod Pathol 2009; 22: 1446–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zebary A, Omholt K, Vassilaki I, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci 2013; 72: 284–289. [DOI] [PubMed] [Google Scholar]
- 105. Merkel EA, Gerami P. Malignant melanoma of sun‐protected sites: a review of clinical, histological, and molecular features. Lab Invest 2017; 97: 630–635. [DOI] [PubMed] [Google Scholar]
- 106. Liu W, Kelly JW, Trivett M, et al. Distinct clinical and pathological features are associated with the BRAF(T1799A(V600E)) mutation in primary melanoma. J Invest Dermatol 2007; 127: 900–905. [DOI] [PubMed] [Google Scholar]
- 107. Cochran AJ, Bastian BC, Elder DE. Chapter 2: Nodular, naevoid, and metastatic melanomas. In: WHO Classification of Skin Tumours (4th edn), Elder DE, Massi D, Scoyler RA, et al. (Eds). IARC: Lyon, 2018; 145–146. [Google Scholar]
- 108. Duncan LM, Bastian BC, Elder DE, et al. Chapter 2: Low‐CSD melanoma (superficial spreading melanoma). In: WHO Classification of Skin Tumours (4th edn), Elder DE, Massi D, Scoyler RA, et al. (Eds). IARC: Lyon, 2018; 76–77. [Google Scholar]
- 109. Behbahani S, Malerba S, Samie FH. Acral lentiginous melanoma: clinicopathological characteristics and survival outcomes in the US National Cancer Database 2004‐2016. Br J Dermatol 2020; 183: 952–954. [DOI] [PubMed] [Google Scholar]
- 110. Pavri SN, Han G, Khan S, et al. Does sentinel lymph node status have prognostic significance in patients with acral lentiginous melanoma? J Surg Oncol 2019; 119: 1060–1069. [DOI] [PubMed] [Google Scholar]
- 111. Martínez Saíd H, Cuellar M, Salcedo R, et al. The impact of ulceration on sentinel node biopsy in acral lentiginous melanoma (ALM): an ideal population for adjuvant therapy? Abstract Book: Society of Surgical Oncology 65th Annual Cancer Symposium. Ann Surg Oncol 2012; 19: 1–179.21956610 [Google Scholar]
- 112. Hayward NK, Wilmott JS, Waddell N, et al. Whole‐genome landscapes of major melanoma subtypes. Nature 2017; 545: 175–180. [DOI] [PubMed] [Google Scholar]
- 113. Sheen YS, Tan KT, Tse KP, et al. Genetic alterations in primary melanoma in Taiwan. Br J Dermatol 2020; 182: 1205–1213. [DOI] [PubMed] [Google Scholar]
- 114. Newell F, Wilmott JS, Johansson PA, et al. Whole‐genome sequencing of acral melanoma reveals genomic complexity and diversity. Nat Commun 2020; 11: 5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med 2005; 353: 2135–2147. [DOI] [PubMed] [Google Scholar]
- 116. Chernoff KA, Bordone L, Horst B, et al. GAB2 amplifications refine molecular classification of melanoma. Clin Cancer Res 2009; 15: 4288–4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Krauthammer M, Kong Y, Ha BH, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet 2012; 44: 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Holman BN, Van Gulick RJ, Amato CM, et al. Clinical and molecular features of subungual melanomas are site‐specific and distinct from acral melanomas. Melanoma Res 2020; 30: 562–573. [DOI] [PubMed] [Google Scholar]


