Abstract
Spatial memory in vertebrates requires brain regions homologous to the mammalian hippocampus. Between vertebrate clades, however, these regions are anatomically distinct and appear to produce different spatial patterns of neural activity. We asked whether hippocampal activity is fundamentally different even between distant vertebrates that share a strong dependence on spatial memory. We studied tufted titmice – food-caching birds capable of remembering many concealed food locations. We found mammalian-like neural activity in the titmouse hippocampus, including sharp-wave ripples and anatomically organized place cells. In a non-food-caching bird species, spatial firing was less informative and was exhibited by fewer neurons. These findings suggest that hippocampal circuit mechanisms are similar between birds and mammals, but that the resulting patterns of activity may vary quantitatively with species-specific ethological needs.
One Sentence Summary:
Avian hippocampus shows mammalian-like neural activity patterns, with enhancement of spatial coding in a food-caching species
Vertebrates differ greatly in their forebrain anatomy but are capable of remarkably similar cognitive functions. The extent to which these functions share neural mechanisms across species is unclear. One example is spatial memory, which depends on hippocampal regions in fish, reptiles, birds, and mammals (1–4). In spite of shared embryological origin (5, 6), these regions differ in anatomy and cytoarchitecture (7–9). Non-mammals also appear to lack hippocampal activity patterns that are central to models of spatial memory: place cells, whose firing represents location during movement through space (10, 11), and sharp-wave ripples (SWRs), which replay activity during immobility and sleep (12, 13). Unlike place cells observed in mammals, hippocampal activity reported in non-mammals is neither confined in space nor stable over time (14–18). In addition, non-mammalian SWRs have only been found outside the hippocampus (19–22).
The prevailing explanation of these results is that non-mammalian spatial memory operates via mechanisms that are fundamentally distinct from those in mammals and do not require place cells or SWRs (14, 22). However, another possibility is that these firing patterns exist across vertebrates, but are quantitatively different or less prevalent in non-mammals, and thus difficult to detect. We also considered the possibility that differences in hippocampal activity are related to species-specific ethological demands. In fact, mammals with well-documented hippocampal activity (rodents, primates, and bats) are all renowned for their spatial abilities (10, 23, 24). Therefore, it may be informative to determine whether classic hippocampal activity patterns exist in a non-mammal that also has exceptional spatial memory.
We chose to record in a food-caching bird, the tufted titmouse. Food-caching birds are memory specialists, capable of remembering many scattered, concealed food locations (25). Accurate cache retrieval requires the hippocampus, which is enlarged in food-caching birds (2, 3, 26). We designed miniature microdrives that allowed these small birds to move freely in a two-dimensional arena. We recorded in the hippocampus (Fig. S1) while titmice foraged for randomly dispensed sunflower seed fragments (Fig. 1A–C, Fig. S2, Movie S1). These experiments mimicked classic rodent studies that probed neural representations of space without explicitly requiring memory use (27).
Fig. 1. Place cells in the hippocampus of tufted titmice.
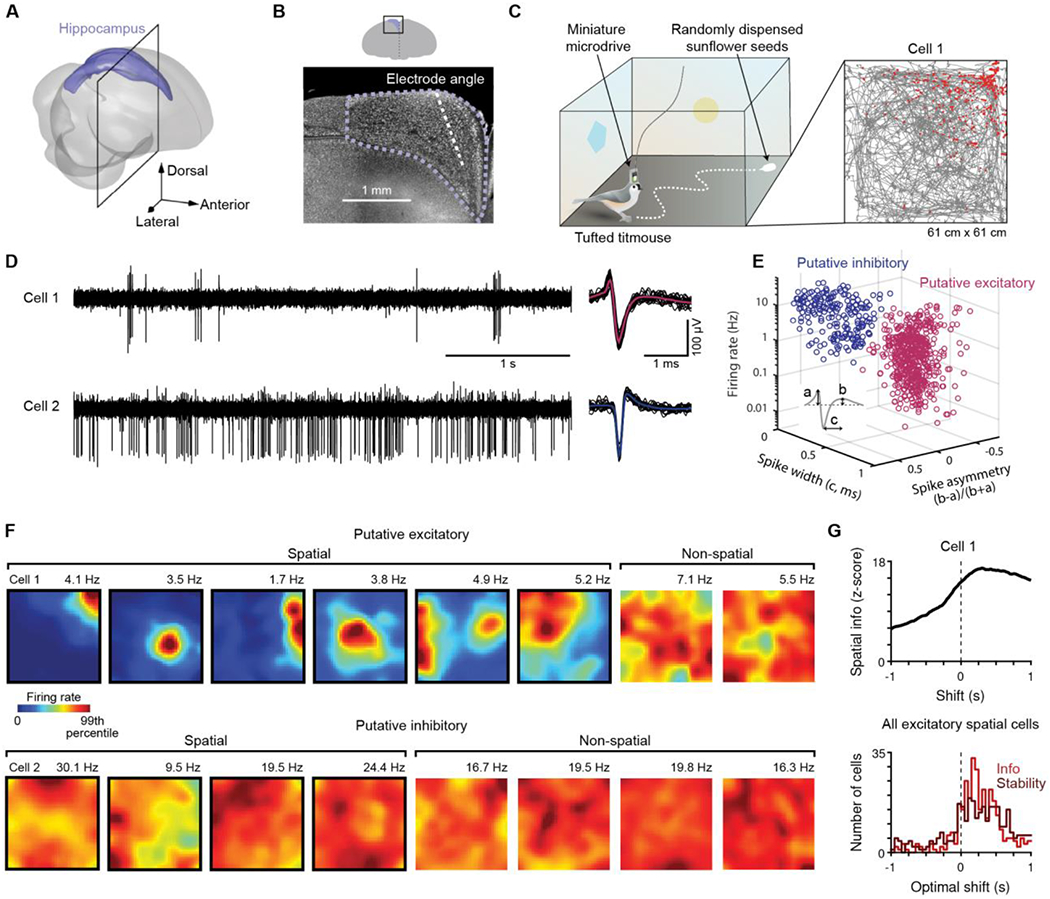
(A) Reconstruction of the titmouse hippocampus. (B) Fluorescent Nissl-stained coronal section at the location indicated by the black box in (A). Dashed purple: hippocampal boundary. Dashed white: electrode approach angle. (C) Left, schematic of the random foraging arena. Right, bird’s trajectory (grey line) and locations of spikes (red dots) for an example hippocampal cell. Cell 1 refers to the same neuron in all panels. (D) Voltage traces and 20 example spike waveforms for two example cells (black: examples; pink or blue: mean). (E) Electrophysiological characteristics for all cells recorded during the random foraging task, classified as excitatory (n = 538) and inhibitory cells (n = 217). (F) Example spatial rate maps for excitatory and inhibitory neurons. Numbers above plot indicate maximum of color scale. (G) Top, spatial information as the time shift between spikes and behavior was varied for an example cell. The peak at a positive shift (“optimal shift”) means that spikes were most informative about the bird’s future position. Bottom, histogram of optimal shifts for spatial information and spatial stability.
Two clusters of recorded units were revealed by analysis of electrophysiological properties (n = 538 and 217 cells). Cells in the first cluster had lower firing rates, wider spikes, a larger first peak of the spike waveform (Fig. 1D,E), and were more bursty (CV2 1.1 ± 0.2 and 0.9 ± 0.1, respectively, p = 10−88, t-test) than cells in the second cluster. These properties match those of excitatory and inhibitory neurons in the mammalian hippocampus, respectively (28, 29). Spike time cross-correlograms for pairs of simultaneously recorded neurons confirmed this categorization (Fig. S3). Thus, similar criteria can distinguish putative excitatory and inhibitory neurons in birds and mammals.
We observed spatially localized neural activity in the titmouse hippocampus (Fig. 1F). We used conventional criteria (Materials and Methods) to quantify spatial tuning (“spatial information”) and the stability of this tuning within a session (“spatial stability”). Neurons for which both measures were larger than expected by chance (p < 0.01) were considered significantly spatial (321/538 excitatory and 144/217 inhibitory cells). The firing fields of such excitatory neurons fully tiled the environment (Fig. S4), reminiscent of rodent place cells. We will therefore refer to significantly spatial excitatory neurons as “place cells”.
In rats, place cell firing is most strongly tuned to position 100–200 ms in the future (27). Despite different methods of locomotion in titmice and rats (discrete hops vs. continuous walking), titmouse place cells were also tuned to future position (median delay 225 ms and 250 ms for spatial information and stability, respectively, n = 321 place cells; both >0, p < 10−14, Wilcoxon signed-rank test; Fig. 1G, Fig. S5). Some neurons also displayed head direction and speed tuning (254/522 and 224/538 excitatory cells, respectively; Fig. S6). Note that many place cells (107/318) were not modulated by head direction, implying that their spatial tuning could not be explained entirely by visual inputs (30). Place cells were also found in separate experiments on a linear track (77/105 excitatory cells), and displayed directional tuning (54/77 place cells; Fig. S7, as in (18)). The titmouse hippocampus therefore displayed multiple features of spatial activity observed in mammals, suggesting that mechanisms of hippocampal coding in birds are not fundamentally distinct from those in mammals.
We asked whether place cells were anatomically organized within the hippocampus by systematically varying recording locations. We constructed a three-dimensional model of the titmouse hippocampus (Fig. S1) and registered recording locations to this template. Spatial information and stability were correlated to location along the anterior-posterior axis (p < 10−3 for both; Materials and Methods; Fig. 2), but not along the other stereotaxic axes (p > 0.27; Fig. S8) or between published subdivisions of the avian hippocampus (31) (p > 0.18). Place cells were concentrated in the anterior two-thirds of the hippocampus, with incidence increasing from <10% to >70% of excitatory cells from the posterior to the anterior pole. In rodents, place cells follow a similar gradient along the dorso-ventral (“long”) axis (32), which is in fact hypothesized to be homologous to the avian anterior-posterior axis (6, 33).
Fig. 2. Spatial representations are organized along the long axis of the hippocampus.
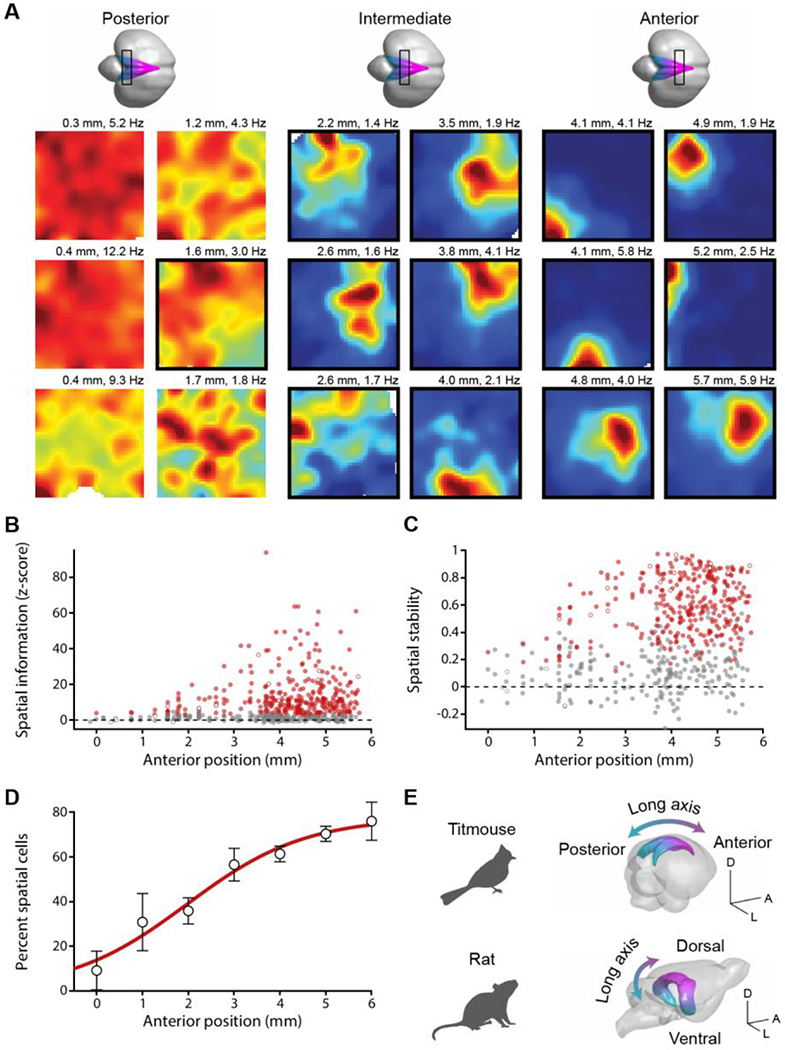
(A) Example spatial rate maps for excitatory neurons from posterior, intermediate, or anterior hippocampus, plotted as in Fig. 1. Place cells are outlined in black. The location on the anterior-posterior axis (distance from lambda) is indicated above each map. (B) Spatial information, normalized by taking the z-score of the actual value relative to a shuffled dataset, plotted for all 538 excitatory cells. Anterior position is relative to lambda. Red: place cells; grey: non-place cells; open markers: example cells in (A). (C) Spatial stability plotted as in (B). (D) Fraction of excitatory cells that passed place cell criteria binned across anterior position. Error bars: mean ± SEM; red line: logistic sigmoid function fit. (E) Schematic of the spatial gradient along the hippocampal long axis in tufted titmice and in rats (3D model generated using published data (48)). Scale bars 5 mm.
Why did previous recordings in birds not reveal similar spatial representations (15, 18)? If spatial coding is related to ethological demands or experiences, place cells may be less common, less spatially informative, or more anatomically restricted in other species. To explore these possibilities while ruling out effects of experimental technique, we repeated our experiments in the zebra finch — a species that, like those previously studied, does not cache food.
Zebra finches exhibited similar behavior to titmice in the random foraging task (Fig. S2). As in titmice, zebra finch hippocampal neurons had electrophysiological characteristics matching those of putative excitatory and inhibitory cells (Fig. S9A). A fraction of these neurons had spatially modulated firing (48/179 excitatory cells and 13/59 inhibitory cells significantly spatial). As in titmice, place cells were predictive of future location (Fig. S9B), were found mainly in the anterior hippocampus (Fig. S9C–E), and their firing tiled the environment (Fig. S4). However, despite these similarities, there appeared to be differences in spatial coding between species. To quantify these differences, we sought to account for the larger size of the titmouse hippocampus and for uneven sampling of the long axis. We therefore compared activity across species in two ways, using landmarks defined functionally or anatomically.
First, we defined an anterior segment of the hippocampus in each species as the region with a high density of place cells (Materials and Methods). This segment was proportionately larger in titmice than in zebra finches (60% vs. 49% of the anterior-posterior extent of the hippocampus). Further, even within this anterior segment place cells were more prevalent in titmice (64% vs. 47% of cells at the anterior pole; Fig. 3A). To illustrate this difference, we sorted cells in the anterior segment by spatial information and compared neurons with corresponding rank. For all ranks, spatial information was higher in titmice than in zebra finches (Fig. 3B).
Fig. 3. Spatial representations differ across avian species.
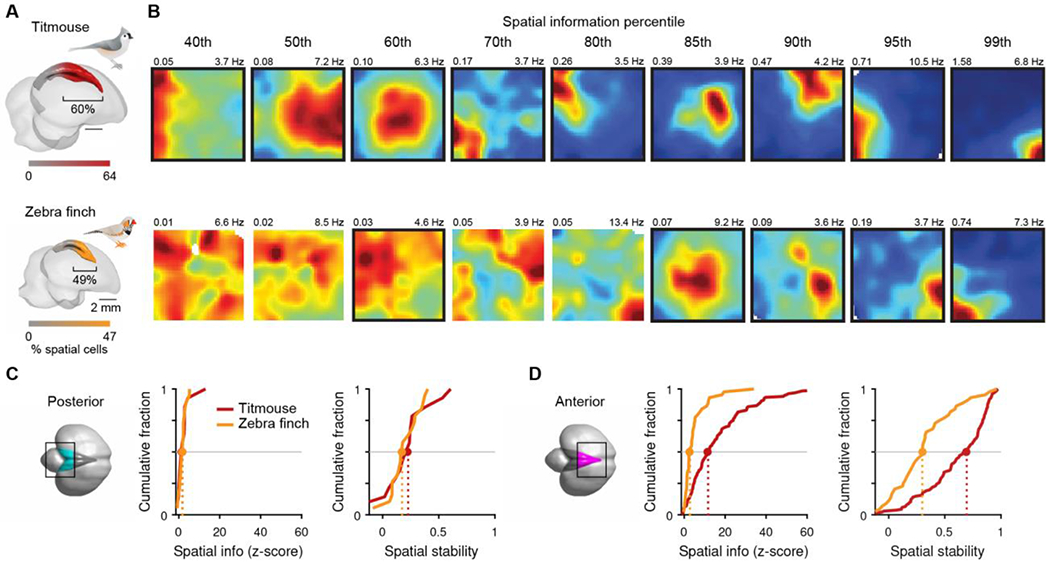
(A) Titmouse (top) and zebra finch (bottom) hippocampus colored according to a logistic sigmoid fit to the percent of place cells at each anterior position. The bracket indicates the percent of hippocampal length anterior to the inflection point of this fit. (B) Example spatial rate maps. All excitatory cells within the bracketed region in (A) with peak rates >3 Hz were ranked by spatial information, and rate maps for the cells corresponding to the given percentiles are shown. Place cells are outlined in black. Raw spatial information (left) and maximum of color scale (right) are given above each plot. (C) Cumulative distributions of normalized spatial information and spatial stability for excitatory cells with peak rates >3 Hz from the posterior hippocampus, defined anatomically (black box; circles: median values). (D) Same as (C) but for anterior hippocampus.
Second, we identified a reliable anatomical landmark that divided the hippocampus roughly in half volumetrically (Materials and Methods). We compared spatial information and stability between species on the anterior and posterior sides of this landmark. Both measures were larger in titmice than in zebra finches in the anterior hippocampus (n = 136 and 44 excitatory cells with peak rates >3 Hz, respectively, p < 0.005; Fig 3C), but not in the posterior hippocampus (n = 14 and 19 cells, p > 0.5; species difference was larger in anterior vs. posterior hippocampus, p < 0.01; Fig 3D). These analyses reveal a difference between species: place cells were more abundant, and activity was more spatially informative and stable in titmice than in zebra finches.
In addition to the similarities in “online” activity during locomotion, are there also similarities in “offline” activity? In the mammalian hippocampus, periods of quiescence contain SWRs, defined by 1) a fast “ripple” oscillation in the local field potential (LFP), 2) a slower “sharp wave” deflection, 3) synchronization of spikes to the ripple, and 4) propagation across the hippocampus (12, 34). We examined activity during sleep (Materials and Methods) in the avian hippocampus and found events with these characteristics (in titmice: Fig. 4A,B and in zebra finches: Fig. S9; 100–200 Hz “ripple” frequency band). SWRs were frequent (0.3–1.1 events/s, n = 5 titmice). Both excitatory and inhibitory cells increased firing during SWRs, but preferred different phases of the ripple oscillation (Fig. S10). In contrast to ripple-frequency oscillations, we did not observe oscillations at lower frequencies, including in the theta band (similar to bats (35); Fig. S11).
Fig. 4. SWRs in the avian hippocampus.
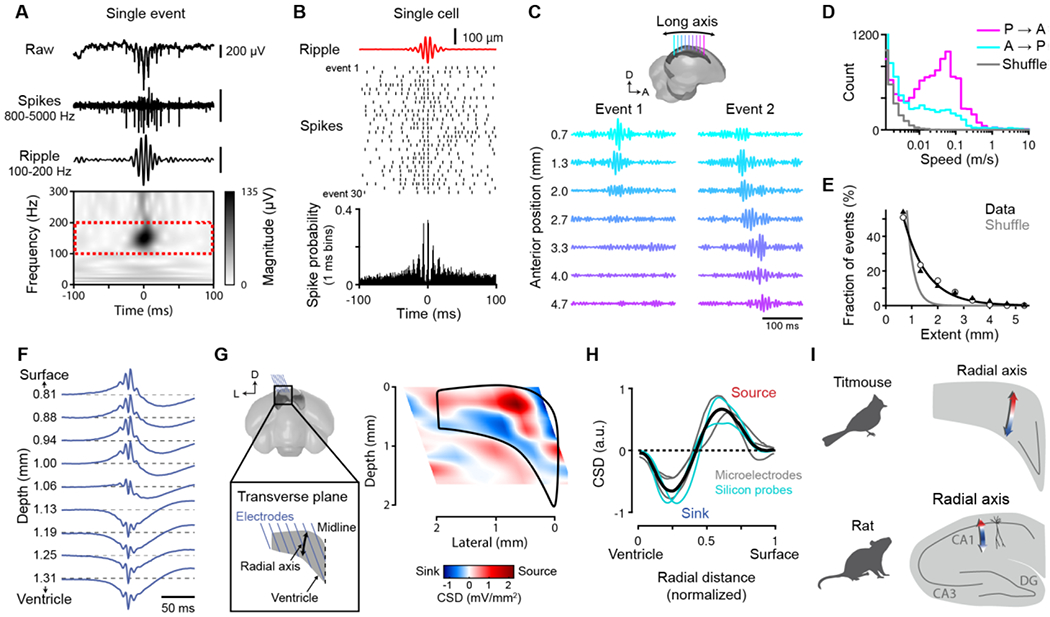
(A) Single SWR in the titmouse hippocampus across frequency bands. (B) Spike raster (top, 30 consecutive SWRs) and spike histogram (bottom, all SWRs) aligned to SWR times (defined in Materials and Methods) for a single cell. (C) Top: electrode placement along the hippocampal long axis. Bottom: example SWRs detected on multiple electrodes. Event 1 is more locally restricted, whereas Event 2 propagates through the entire recorded length of the hippocampus. (D) Speed of SWRs propagating in the posterior-to-anterior (P→A) and anterior-to-posterior (A→P) directions, compared to shuffled data. (E) Distribution of SWR extent across the long axis. Markers: individual titmice; black line: exponential fit to all points; grey line: exponential fit to shuffled data. (F) LFP averaged across SWRs recorded sequentially at different depths in the hippocampus. (G) Left: electrode placement within the transverse plane of the hippocampus. Right: 2D CSD map within the transverse plane of one bird. Black: hippocampus outline. (H) One-dimensional CSD across the radial axis. Grey and cyan lines: individual birds; black: average. (I) Layered CSD organization during SWRs across species. In rat, the primary current sources (red) and sinks (blue) correspond to the pyramidal cell layer and the stratum radiatum, respectively (36).
To analyze SWR propagation, we implanted electrode arrays spanning >5 mm of the hippocampal long axis. About half of the events occurred on more than one electrode, and some spanned most of the recorded extent of the hippocampus (length constant 0.90 mm; Fig. 4C–E). Propagation speed was 0.12 ± 0.07 m/s (median ± median absolute deviation, n = 15790 SWRs), with a bias for propagation in the posterior-to-anterior direction (70% of SWRs). Avian SWRs are therefore global, propagating events in the hippocampus.
During mammalian SWRs, current sinks and sources (net electrical current flowing into or out of cells, respectively) occur within specific layers of the hippocampus (36). Does a similar laminar organization exist in birds? We examined SWRs across the hippocampal transverse plane in titmice, either by incrementally advancing electrodes or by recording synchronously across depths with silicon probes. We found that the sharp-wave component often inverted from positive to negative polarity between dorsal and ventral locations (Fig. 4F). To relate these changes in waveform to electrical currents, we calculated the current source density (CSD) either across the entire transverse plane or collapsed along the radial axis (Fig. 4G,H). The CSD was organized along the radial axis, with a current source dorsal to a sink. Thus, SWRs display laminar organization in the titmouse hippocampus (Fig. 4I).
There have been relatively few studies of neural activity in the non-mammalian hippocampus, and these studies have not reported neurons resembling classic place cells. Rather, these studies have found other types of spatial neurons, including head direction cells, border cells, and broadly tuned cells (14–18). In contrast, we found place cells that fired in restricted regions of space and, as a population, tiled the environment. As in mammals, these cells were anatomically organized along the long axis of the hippocampus. Our findings provide evidence for shared neural processes underlying spatial representation across hippocampal circuits separated by 320 million years of evolution (5).
Mechanisms that produce place cells are debated, but are hypothesized to depend on specialized internal connections within the hippocampus and external inputs (37). Furthermore, patterns of external inputs are thought to explain differences in spatial coding along the long axis (38). Our results suggest that similar features of hippocampal circuitry may give rise to the observed place cells in birds.
We also report SWRs in the avian hippocampus. It is unknown whether these events originate in the hippocampus itself. In fact, SWRs have been reported in other brain regions of birds and reptiles (19–21). Regardless of their origin, it is unclear why hippocampal SWRs are experimentally detectable in birds. In mammals, hippocampal SWRs are thought to be detectable due to the crystalline cytoarchitecture: a dense pyramidal cell layer and parallel dendrites that allow summation of small currents into large LFP fluctuations (12). In the avian hippocampus (unlike in non-avian reptiles and mammals (9)), cell clustering is modest and limited to a medial V-shaped region, and dendrites are not strictly aligned (7, 31, 39) (Fig. 1B). It is possible that detectable SWRs result from a more subtle arrangement of cells in birds. It is also possible that they result from other patterns of hippocampal organization, such as differences in synaptic input (36, 40), morphology (41), or intrinsic cell properties (7) along the radial axis. Note that the organization of current flow in birds is inverted along this axis compared to mammals (source is superficial to sink; Fig. 4I). This is reminiscent of the inverted cerebral cortex in mammals compared to other amniotes (5). Regardless of the mechanisms, our results suggest that as-of-yet unidentified patterns of radial axis organization may exist in the avian hippocampus.
Despite these similarities between clades, there were also significant differences across bird species. We found weaker spatial coding in zebra finches than in titmice. Previous studies reported even weaker place coding in other non-food-caching birds (pigeons and quails): a near absence of place cells (18) and low reliability of spatial patterns across time (15). Apparent differences between zebra finches and these species could potentially be due to the relatively sparse sampling of the anterior hippocampus in previous recordings. However, because we densely sampled the entire anterior-posterior extent of the hippocampus, stronger place coding in titmice likely reflects a true species difference.
There are many innate and experience-related differences between titmice and other recorded birds, but it is tempting to speculate that enhanced spatial coding in titmice is related to the demands of food caching. Place cell activity is sparse (42) — that is, firing occurs in a small fraction of the environment. Although sparse coding requires more neurons, it may allow new memories to form quickly without interfering with old memories (42, 43). Increased sparsity may thus confer an adaptive advantage to food-caching birds. Our results demonstrate functional and anatomical similarity in a higher brain region of distant vertebrates. At the same time, these findings contribute to the growing evidence that hippocampal coding may vary according to the ethological demands of different species (23, 24, 44–47).
Supplementary Material
Acknowledgments:
We thank D. Scheck, S. Hale, T. Tabachnik, R. Hormigo, and K. Gutnichenko for technical assistance; M. Fee for the contribution to microdrive design; the Black Rock Forest Consortium, J. Scribner and Hickory Hill Farm, and T. Green for help with field work; L. Abbott and members of the Aronov lab for comments on the manuscript. The illustration of the arena in Fig. 1C and birds in Fig. 3A are by J. Kuhl.
Funding:
This work was supported by the Helen Hay Whitney Foundation Fellowship (H.L.P.), New York Stem Cell Foundation – Robertson Neuroscience Investigator Award, the Beckman Young Investigator Award, and the NIH New Innovator Award (DP2 AG071918-01).
Footnotes
Competing interests: The authors declare no competing interests.
Data and materials availability:
Data and code are available at: https://doi.org/10.5061/dryad.pg4f4qrp7
References and Notes:
- 1.Morris RGM, Garrud P, Rawlins JNP, O’Keefe J, Place navigation impaired in rats with hippocampal lesions. Nature. 297, 681–683 (1982). [DOI] [PubMed] [Google Scholar]
- 2.Krushinskaya N, Some complex forms of feeding behavior of nutcracker nucifraga caryocatactes, after removal of old cortex. Z. Evouz. Biochem. Fisiol II, 563–568 (1966). [Google Scholar]
- 3.Sherry DF, Vaccarino AL, Hippocampus and memory for food caches in black-capped chickadees. Behav. Neurosci 103, 308–318 (1989). [Google Scholar]
- 4.Rodríguez F, López JC, Vargas JP, Broglio C, Gómez Y, Salas C, Spatial memory and hippocampal pallium through vertebrate evolution: Insights from reptiles and teleost fish. Brain Res. Bull 57, 499–503 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Tosches MA, Yamawaki TM, Naumann RK, Jacobi AA, Tushev G, Laurent G, Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science. 360, 881–888 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Abellán A, Desfilis E, Medina L, Combinatorial expression of Lef1, Lhx2, Lhx5, Lhx9, Lmo3, Lmo4, and Prox1 helps to identify comparable subdivisions in the developing hippocampal formation of mouse and chicken. Front. Neuroanat 8, 59 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montagnese CM, Krebs JR, Meyer G, The dorsomedial and dorsolateral forebrain of the zebra finch, Taeniopygia guttata: A Golgi study. Cell Tissue Res. 283, 263–282 (1996). [DOI] [PubMed] [Google Scholar]
- 8.Naumann RK, Ondracek JM, Reiter S, Shein-Idelson M, Tosches MA, Yamawaki TM, Laurent G, The reptilian brain. Curr. Biol 25, R317–R321 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Striedter GF, Evolution of the hippocampus in reptiles and birds. J. Comp. Neurol 524, 496–517 (2016). [DOI] [PubMed] [Google Scholar]
- 10.O’Keefe J, Dostrovsky J, The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175 (1971). [DOI] [PubMed] [Google Scholar]
- 11.O’Keefe J, Nadel L, The hippocampus as a cognitive map (Oxford University Press, Oxford, 1978). [Google Scholar]
- 12.Buzsáki G, Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus. 25, 1073–1188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buzsáki G, Moser EI, Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci 16, 130–138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherry DF, Grella SL, Guigueno MF, White DJ, Marrone DF, Are there place cells in the avian hippocampus? Brain. Behav. Evol 90, 73–80 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Kahn MC, Siegel JJ, Jechura TJ, Bingman VP, Response properties of avian hippocampal formation cells in an environment with unstable goal locations. Behav. Brain Res 191, 153–163 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Vinepinsky E, Cohen L, Perchik S, Ben-Shahar O, Donchin O, Segev R, Representation of edges, head direction, and swimming kinematics in the brain of freely-navigating fish. Sci. Rep 10, 1–16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fotowat H, Lee C, Jun JJ, Maler L, Neural activity in a hippocampus-like region of the teleost pallium are associated with navigation and active sensing. Elife. 8, 1–25 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben-Yishay E, Krivoruchko K, Ron S, Ulanovsky N, Derdikman D, Gutfreund Y, Directional tuning in the hippocampal formation of birds. Curr. Biol, 1–11 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Shein-Idelson M, Ondracek JM, Liaw HP, Reiter S, Laurent G, Slow waves, sharp waves, ripples, and REM in sleeping dragons. Science. 352, 590–595 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Norimoto H, Fenk LA, Li HH, Tosches MA, Gallego-Flores T, Hain D, Reiter S, Kobayashi R, Macias A, Arends A, Klinkmann M, Laurent G, A claustrum in reptiles and its role in slow-wave sleep. Nature. 578, 413–418 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Yeganegi H, Luksch H, Ondracek JM, Hippocampal-like network dynamics underlie avian sharp wave-ripples. bioRxiv (2019), doi: 10.1101/825075. [DOI] [Google Scholar]
- 22.Rattenborg NC, Martinez-Gonzalez D, Roth TC, Pravosudov VV, Hippocampal memory consolidation during sleep: A comparison of mammals and birds. Biol. Rev 86, 658–691 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Killian NJ, Jutras MJ, Buffalo EA, A map of visual space in the primate entorhinal cortex. Nature. 491, 761–764 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yartsev MM, Ulanovsky N, Representation of three-dimensional space in the hippocampus of flying bats. Science. 340, 367–372 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Sherry DF, Food storage by black-capped chickadees: Memory for the location and contents of caches. Anim. Behav 32, 451–464 (1984). [Google Scholar]
- 26.Sherry DF, Vaccarino AL, Buckenham K, Herz R, The hippocampal complex of food-storing birds. Brain Behav Evol (1989). [DOI] [PubMed] [Google Scholar]
- 27.Muller RU, Kubie JL, The firing of hippocampal place cells predicts the future position of freely moving rats. J. Neurosci 9, 4101–4110 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Csicsvari J, Hirase H, Czurkó A, Mamiya A, Buzsáki G, Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J. Neurosci 19, 274–287 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizuseki K, Sirota A, Pastalkova E, Buzsáki G, Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron. 64, 267–280 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller RU, Bostock E, Taube JS, Kubie JL, On the directional firing properties of hippocampal place cells. J. Neurosci 14, 7235–51 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atoji Y, Wild JM, Anatomy of the avian hippocampal formation. Rev. Neurosci 17, 3–15 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Jung MW, Wiener SI, McNaughton BL, Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J. Neurosci 14, 7347–7356 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smulders TV, The avian hippocampal formation and the stress response. Brain. Behav. Evol 90, 81–91 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Patel J, Schomburg EW, Berényi A, Fujisawa S, Buzsáki G, Local generation and propagation of ripples along the septotemporal axis of the hippocampus. J. Neurosci 33, 17029–17041 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yartsev MM, Witter MP, Ulanovsky N, Grid cells without theta oscillations in the entorhinal cortex of bats. Nature. 479, 103–107 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, Buzsáki G, Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: Network and intracellular mechanisms. J. Neurosci 15, 30–46 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moser EI, Kropff E, Moser M-B, Place cells, grid cells, and the brain’s spatial representation system. Annu. Rev. Neurosci 31, 69–89 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Strange BA, Witter MP, Lein ES, Moser EI, Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci 15, 655–669 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Tömböl T, Davies DC, Németh A, Sebestény T, Alpár A, A comparative Golgi study of chicken (Gallus domesticus) and homing pigeon (Columba livia) hippocampus. Anat. Embryol. (Berl). 201, 85–101 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Valero M, Cid E, Averkin RG, Aguilar J, Sanchez-Aguilera A, Viney TJ, Gomez-Dominguez D, Bellistri E, De La Prida LM, Determinants of different deep and superficial CA1 pyramidal cell dynamics during sharp-wave ripples. Nat. Neurosci 18, 1281–1290 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montagnese CM, Krebs JR, Székely AD, Csillag A, A subpopulation of large calbindin-like immunopositive neurones is present in the hippocampal formation in food-storing but not in non-storing species of bird. Brain Res. 614, 291–300 (1993). [DOI] [PubMed] [Google Scholar]
- 42.Skaggs WE, McNaughton BL, Computational approaches to hippocampal function. Curr. Biol 2, 198 (1992). [DOI] [PubMed] [Google Scholar]
- 43.Fiete IR, Hahnloser RHR, Fee MS, Seung HS, Temporal sparseness of the premotor drive is important for rapid learning in a neural network model of birdsong. J. Neurophysiol 92, 2274–2282 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Omer DB, Maimon SR, Las L, Ulanovsky N, Social place-cells in the bat hippocampus. Science. 359, 218–224 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Danjo T, Toyoizumi T, Fujisawa S, Spatial representations of self and other in the hippocampus. Science. 359, 213–218 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Radvansky BA, Dombeck DA, An olfactory virtual reality system for mice. Nat. Commun 9, 1–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tavares RM, Mendelsohn A, Grossman Y, Williams CH, Shapiro M, Trope Y, Schiller D, A map for social navigation in the human brain. Neuron. 87, 231–243 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calabrese E, Badea A, Watson C, Johnson GA, A quantitative magnetic resonance histology atlas of postnatal rat brain development with regional estimates of growth and variability. Neuroimage. 71, 196–206 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code are available at: https://doi.org/10.5061/dryad.pg4f4qrp7


