Abstract
Purpose
To identify if noninfectious uveitis (NIU) is associated with a greater risk of Coronavirus Disease 2019 (COVID-19) infection, hospitalization, and death.
Design
A retrospective cohort study from January 20, 2020 to December 31, 2020, using a national claims-based database.
Participants
Enrollees who had continuous enrollment with both medical and pharmacy coverage for 3 years before January 20, 2020. Patients with an NIU diagnosis within 3 years of the start of the study were included in the NIU cohort. Those with infectious uveitis codes or new NIU diagnoses during the risk period were excluded.
Methods
Cox proportional hazard models were used to identify unadjusted hazard ratios (HRs) and adjusted HRs for all covariates for each outcome measure. Adjusted models accounted for patient demographics, health status, and immunosuppressive medication use during the risk period.
Main Outcome Measures
Rates of COVID-19 infection, COVID-19-related hospitalization, and COVID-19-related in-hospital death identified with International Classification of Disease 10th revision codes.
Results
This study included 5 806 227 patients, of whom 29 869 (0.5%) had a diagnosis of NIU. On unadjusted analysis, patients with NIU had a higher rate of COVID-19 infection (5.7% vs. 4.5%, P < 0.001), COVID-19-related hospitalization (1.2% vs. 0.6%, P < 0.001), and COVID-19-related death (0.3% vs. 0.1%, P < 0.001). However, in adjusted models, NIU was not associated with a greater risk of COVID-19 infection (HR, 1.05; 95% confidence interval [CI], 1.00–1.10; P = 0.04), hospitalization (HR, 0.98; 95% CI, 0.88–1.09; P = 0.67), or death (HR, 0.90, 95% CI, 0.72–1.13, P = 0.37). Use of systemic corticosteroids was significantly associated with a higher risk of COVID-19 infection, hospitalization, and death.
Conclusions
Patients with NIU were significantly more likely to be infected with COVID-19 and experience severe disease outcomes. However, this association was due to the demographics, comorbidities, and medications of patients with NIU, rather than NIU alone. Patients using systemic corticosteroids were significantly more likely to be infected with COVID-19 and were at greater risk of hospitalization and in-hospital death. Additional investigation is necessary to identify the impact of corticosteroid exposure on COVID-19-related outcomes.
Keywords: Corticosteroids, COVID-19, COVID-19 deaths, COVID-19 hospitalizations, Noninfectious uveitis
Abbreviations and Acronyms: CI, confidence interval; COVID-19, Coronavirus Disease 2019; DMARD, disease-modifying anti-rheumatic drug; HR, hazard ratio; ICD-10, International Classification of Disease 10th Revision; NIU, noninfectious uveitis; OLDW, OptumLabs Data Warehouse; SD, standard deviation; TNF-α, tumor necrosis factor alpha
Since the advent of Coronavirus Disease 2019 (COVID-19), a major public health goal has been to protect individuals at highest risk of infection. In light of pre-existing literature indicating that select inflammatory diseases are associated with greater rates of infection,1, 2, 3 there was concern that patients with inflammatory conditions may constitute a high-risk group for COVID-19. To date, studies have yielded mixed findings even among those examining the same disease. Reports from the initial phase of the pandemic found that patients with rheumatic disease had higher rates of COVID-19–related hospitalization and were more likely to require ventilation,4, 5, 6 whereas more recent long-term studies have indicated no difference in outcomes after controlling for comorbidities.7
Noninfectious uveitis (NIU) comprises a spectrum of ocular inflammatory conditions that can manifest in isolation or in the context of other systemic inflammatory diseases. Thus, during the early phase of the pandemic, concerns arose that uveitis may similarly increase COVID-19 susceptibility or worsen the severity of infection due to underlying dysregulated immune function.3 , 8, 9, 10 Patients with uveitis also frequently require in-person care to assess disease status and modify treatment regimens, increasing the risk of exposure.
Currently, COVID-19–specific evidence-based uveitis management guidelines have not been established because of limited understanding of the relationship between ocular inflammatory conditions and COVID-19–related outcomes. Furthermore, existing studies on this topic have been limited in sample size and have mostly focused on uveitis management during the pandemic. Therefore, the purpose of this study was to determine if NIU confers a greater risk for COVID-19 infection, severe disease course, or death.
Methods
Study Design
A retrospective cohort study was conducted using a de-identified healthcare claims database, OptumLabs Data Warehouse (OLDW, OptumLabs).11 The OLDW contains de-identified, longitudinal health information on enrollees, representing a diverse mixture of ages, ethnicities, and geographical regions across the United States. The claims data in OLDW include medical and pharmacy claims, laboratory results, and enrollment records for Medicare Advantage and commercial insurance enrollees of all ages. The OLDW does not contain data for patients who only have original Medicare (Parts A and B) or Medicaid, or who are Veterans Administration enrollees.
To be eligible for inclusion in the study, enrollees were required to be continuously enrolled with both medical and pharmacy coverage for 3 years (1095 days) before and on January 20, 2020. This date was chosen as the index date and start of the risk period for all patients because this was the date of the first known COVID-19 case in the United States. Patients with NIU were identified by an International Classification of Disease 10th Revision (ICD-10) code appearing at any time during the lookback period of continuous enrollment from January 20, 2017, to January 20, 2020. The NIU diagnosis could have been incidental or prevalent during this lookback period. All codes used to identify NIU are included in the Appendix (eBox 1; available at www.aaojournal.org). Patients without any of these codes in the 3-year lookback period were considered to not have a prior history of NIU. Individuals with an infectious uveitis ICD-10 code (Appendix [eBox 1], available at www.aaojournal.org) at any time during the 3-year lookback period were excluded from the study. Individuals who were newly diagnosed with NIU (no NIU ICD-10 codes in the lookback period but an NIU ICD-10 code during the risk period) were also excluded from the study.
Outcomes related to COVID-19 were assessed from January 20, 2020, to December 31, 2020. Outcomes of interest included COVID-19 infection, COVID-19 hospitalization, and COVID-19–related in-hospital death. Coronavirus Disease 2019 infection was identified using ICD-10 codes B97.29 (other coronavirus as the cause of diseases classified elsewhere; used before 4/1/2020) or U07.1 (COVID-19 infection; used on and after 4/1/2020) in any type of encounter,12 or a positive polymerase chain reaction lab test during the study period. Hospitalization for COVID-19 was identified by ICD-10 codes B97.29 or U07.1 in any position associated with an inpatient encounter during the study period. In-hospital mortality during the study period was determined on the basis of discharge status codes. The mortality data also included additional levels of de-identification such that a death could not be attributed to a specific claim or cause; however, deaths in patients with medical claims including ICD-10 codes B97.29 or U07.1 that appeared within 30 days before the death date were classified as COVID-19 deaths.
Baseline covariates considered as potential confounders included sex (female, male, unknown), age in 2020, years of continuous enrollment, race/ethnicity (Asian, Black, Hispanic, White, other/multiple races/unknown), homeownership (probable homeowner, probable renter, unknown), region (Midwest, Northeast, South, West, other/unknown), smoking status (never smoker, current/former smoker, unknown), and presence or absence of comorbidities in the 1 year before the index date based on ICD-10 codes. Comorbidities were chosen on the basis of the risk factors for severe COVID-19 illness reported by the Centers for Disease Control and Prevention as of February 2021 (Appendix, eBox 2; available at www.aaojournal.org).13 Use of systemic immunosuppressive medication was identified during the risk period and categorized into the following groups: systemic corticosteroids, disease-modifying anti-rheumatic drugs (DMARDs), tumor necrosis factor alpha (TNF-α) inhibitors, interleukin-6 (IL-6) inhibitors, other biologic immunosuppressive therapies, and other nonbiologic immunosuppressive drugs that do not fit into previous categories. Hydroxychloroquine was not included in the analysis to avoid introducing confounding by indication. Medication use was determined by text search of drug names in pharmacy claims, with 1 or more fills during the risk period considered active use of that medication. Generic names of medications and routes used to search for prescriptions and their categorizations are listed in the Appendix (eBox 3, available at www.aaojournal.org).
Statistical Analysis
Baseline covariates, characteristics of NIU patients, and immunosuppressive medication use during the risk period were summarized using descriptive statistics. Unadjusted hazard ratios (HRs) were estimated for each variable and COVID-19 outcome (infection, hospitalization, in-hospital death) using Cox proportional hazards models. Adjusted HRs for each outcome were also estimated using Cox proportional hazards models to compare patients with NIU to those without NIU. Each adjusted model was adjusted for baseline demographics, comorbidities, and immunosuppressive medication use during the risk period. In a sensitivity analysis, immunosuppressive medications were excluded from models to identify if they might mediate, and thus potentially mask, the effect of NIU on COVID-19 outcomes. A subgroup adjusted analysis was performed by age group (<50 vs. ≥50 years) for each COVID-19 outcome to understand if the effect of NIU may differ in younger individuals versus older individuals. These models were similarly adjusted for demographics, comorbidities, and immunosuppressive medications.
Coronavirus Disease 2019 infection and hospitalization dates were recorded as the first date when the previously outlined outcome criteria were met. For example, if a patient tested positive for COVID-19 more than once, the date of the first positive test was recorded as the event date. Likewise, if a patient was hospitalized more than once with COVID-19, the date of the first hospitalization was recorded as the event date. In each model, patients could be censored at disenrollment from the medical plan, death unrelated to COVID-19, or the end of the risk period (12/31/2020). A patient was considered to have experienced the event if their event date occurred before any of the other censoring events. Time to event or censoring was calculated as days from January 20, 2020. Proportional hazards assumptions were checked using plots of survival curves versus time and Schoenfeld residual tests and plots.
Statistical analyses were performed in R (Version 4.0.2, R Foundation for Statistical Computing, https://www.R-project.org/). P values less than 0.005 were considered statistically significant to be conservative. This study was approved by the Institutional Review Board of the University of California, San Francisco, and was conducted in adherence with the tenets of the Declaration of Helsinki. The requirement for informed consent was waived because of the retrospective nature of the study.
Results
Characteristics of Study Population
A total of 5 806 227 patients were included in the analysis, and 29 869 of those had NIU (0.5%). Table 1 summarizes the baseline characteristics of the cohort by NIU status. The mean age of patients in the overall cohort in the year 2020 was 50.3 years (standard deviation [SD] = 23.5), and the mean age of patients with NIU was 65.2 years (SD = 17.4). Women comprised a larger proportion of patients with NIU compared with those without NIU (60.7% vs. 51.4%). Patients with NIU were also more likely to be Black, probable homeowners, and current or former smokers. Approximately 20% of patients with NIU had another autoimmune disease, and almost all other comorbidities were more prevalent in the NIU group at baseline (Table 1).
Table 1.
Baseline Characteristics of Cohort by Uveitis Status (N = 5 806 227)
| Characteristic | No NIU N = 5 776 358 (99.5%) |
NIU N = 29 869 (0.5%) |
All N = 5 806 227 (100.0%) |
|---|---|---|---|
| Age (yrs) | |||
| Mean (SD) | 50.2 (23.5) | 65.2 (17.4) | 50.3 (23.5) |
| Median [Q1, Q3] | 53.0 [32.0, 71.0] | 70.0 [56.0, 77.0] | 53.0 [32.0, 71.0] |
| Continuous enrollment (yrs) | |||
| Mean (SD) | 6.6 (3.8) | 6.5 (3.7) | 6.6 (3.8) |
| Median [Q1, Q3] | 5.1 [4.0, 8.1] | 5.1 [4.1, 8.1] | 5.1 [4.0, 8.1] |
| Gender | |||
| Female | 2 968 650 (51.4%) | 18 128 (60.7%) | 2 986 778 (51.4%) |
| Male | 2 804 701 (48.6%) | 11 705 (39.2%) | 2 816 406 (48.5%) |
| Unknown | 3007 (0.1%) | 36 (0.1%) | 3043 (0.1%) |
| Race | |||
| Asian | 298 833 (5.2%) | 1228 (4.1%) | 300 061 (5.2%) |
| Black | 581 630 (10.1%) | 5667 (19.0%) | 587 297 (10.1%) |
| Hispanic | 673 319 (11.7%) | 2927 (9.8%) | 676 246 (11.6%) |
| White | 3 719 784 (64.4%) | 17 912 (60.0%) | 3 737 696 (64.4%) |
| Other/Unknown | 502 792 (8.7%) | 2135 (7.1%) | 504 927 (8.7%) |
| Region | |||
| Midwest | 1 528 179 (26.5%) | 7053 (23.6%) | 1 535 232 (26.4%) |
| Northeast | 635 989 (11.0%) | 4387 (14.7%) | 640 376 (11.0%) |
| South | 2 488 756 (43.1%) | 14 750 (49.4%) | 2 503 506 (43.1%) |
| West | 944 533 (16.4%) | 3620 (12.1%) | 948 153 (16.3%) |
| Other/Unknown | 178 901 (3.1%) | 59 (0.2%) | 178 960 (3.1%) |
| Homeownership | |||
| Probable homeowner | 3 959 324 (68.5%) | 22 037 (73.8%) | 3 981 361 (68.6%) |
| Probable renter | 440 749 (7.6%) | 2119 (7.1%) | 442 868 (7.6%) |
| Unknown | 1 376 285 (23.8%) | 5713 (19.1%) | 1 381 998 (23.8%) |
| Smoking status (baseline or risk period) | |||
| Never | 844 880 (14.6%) | 6183 (20.7%) | 851 063 (14.7%) |
| Current or former | 931 095 (16.1%) | 7382 (24.7%) | 938 477 (16.2%) |
| Unknown | 4 000 383 (69.3%) | 16 304 (54.6%) | 4 016 687 (69.2%) |
| Asthma | 324 875 (5.6%) | 2745 (9.2%) | 327 620 (5.6%) |
| Autoimmune disease | 419 741 (7.3%) | 6013 (20.1%) | 425 754 (7.3%) |
| Cancer | 311 186 (5.4%) | 3131 (10.5%) | 314 317 (5.4%) |
| Cardiovascular disease | 605 002 (10.5%) | 6003 (20.1%) | 611 005 (10.5%) |
| Cerebrovascular disease | 272 104 (4.7%) | 3102 (10.4%) | 275 206 (4.7%) |
| Chronic kidney disease | 440 518 (7.6%) | 4893 (16.4%) | 445 411 (7.7%) |
| Chronic lung disease | 437 757 (7.6%) | 4315 (14.4%) | 442 072 (7.6%) |
| Diabetes (any type) | 841 908 (14.6%) | 8652 (29.0%) | 850 560 (14.6%) |
| Hemoglobin disease | 8476 (0.1%) | 121 (0.4%) | 8597 (0.1%) |
| HIV/AIDS | 11 916 (0.2%) | 107 (0.4%) | 12 023 (0.2%) |
| Hypertension | 1 998 776 (34.6%) | 18 145 (60.7%) | 2 016 921 (34.7%) |
| Liver disease | 11 030 (0.2%) | 102 (0.3%) | 11 132 (0.2%) |
| Neurologic disease | 230 073 (4.0%) | 2100 (7.0%) | 232 173 (4.0%) |
| Obesity | 623 910 (10.8%) | 4970 (16.6%) | 628 880 (10.8%) |
| Solid organ transplantation | 15 753 (0.3%) | 355 (1.2%) | 16 108 (0.3%) |
| Pregnancy (risk period) | 62 129 (1.1%) | 180 (0.6%) | 62 309 (1.1%) |
HIV = human immunodeficiency virus; AIDS = acquired immunodeficiency syndrome; NIU = noninfectious uveitis; Q1 = first quartile; Q3 = third quartile; SD = standard deviation.
Less than 11 patients were missing age.
Overall, 676 927 patients (11.7%) were prescribed at least 1 of the 6 immunosuppressive drug categories during the risk period up to COVID-19 infection or censoring. Table 2 summarizes the immunosuppressive medications taken during the risk period up to infection date or censoring by NIU status. Systemic corticosteroids were the most prescribed immunosuppressive treatments during this period, with 18.0% of NIU patients and 10.7% of patients without NIU taking these drugs. Patients with NIU were also prescribed the other 5 drug categories more frequently than patients without NIU (Table 2).
Table 2.
Immunosuppressive Drug Prescriptions during the Risk Period up to COVID-19 Infection Outcome by Uveitis Status (N = 5 806 227)
| Immunosuppressive Drug Category | No NIU (N = 5 776 358) | NIU (N = 29 869) | All (N = 5 806 227) |
|---|---|---|---|
| Systemic corticosteroids | 617 953 (10.7%) | 5369 (18.0%) | 623 322 (10.7%) |
| DMARDs | 51 208 (0.9%) | 1347 (4.5%) | 52 555 (0.9%) |
| TNF-α inhibitors | 15 891 (0.3%) | 758 (2.5%) | 16 649 (0.3%) |
| IL-6 inhibitors | 734 (0.01%) | 26 (0.1%) | 760 (0.01%) |
| Other biologic therapies | 13 488 (0.2%) | 192 (0.6%) | 13 680 (0.2%) |
| Other immunosuppressive drugs | 20 346 (0.4%) | 360 (1.2%) | 20 706 (0.4%) |
DMARD = disease-modifying anti-rheumatic drug; IL-6 = interleukin 6; NIU = noninfectious uveitis; TNF-α = tumor necrosis factor alpha.
Frequencies and percentages reflect the proportion of patients who filled 1 or more prescriptions during risk period up to COVID-19 infection outcome date (results differ slightly for hospitalization and death outcomes).
Factors Associated with COVID-19 Infection
There were 1708 cases of COVID-19 infection in patients with NIU compared with 258 388 in patients without NIU, corresponding to a cumulative incidence of 5.7% and 4.5%, respectively. The unadjusted HR for COVID-19 infection comparing patients with NIU to patients without NIU was 1.25 (95% confidence interval [CI], 1.19–1.31; P < 0.001) (Table 3 ). Age, Black race, Hispanic ethnicity, current or former smoking, and all other comorbidities were also associated with increased hazard of infection in the unadjusted analyses (Table 3). By medication category, use of corticosteroids, DMARDs, TNF-α inhibitors, other biologics, and other immunosuppressive drugs conferred a higher unadjusted hazard of COVID-19 infection.
Table 3.
Unadjusted Analyses of Associations with COVID-19 Infection (N = 5 806 227)
| Variable | No COVID-19 Infection N = 5 546 131 |
COVID-19 Infection N = 260 096 |
Unadjusted Analysis |
|
|---|---|---|---|---|
| N (%) or Mean (SD) | N (%) or Mean (SD) | Hazard Ratio (95% CI) | P Value | |
| Noninfectious uveitis | 28 161 (0.5) | 1708 (0.7) | 1.25 (1.19, 1.31) | <0.001 |
| Age (yrs) | 50.1 (23.5) | 53.8 (22.2) | 1.006 (1.005, 1.006) | <0.001 |
| Continuous enrollment (yrs) | 6.5 (3.8) | 6.6 (3.8) | 1.001 (1.000, 1.002) | 0.30 |
| Gender | ||||
| Female | 2 848 089 (51.4) | 138 689 (53.3) | Reference | |
| Male | 2 695 085 (48.6) | 121 321 (46.6) | 0.94 (0.93, 0.94) | <0.001 |
| Unknown | 2957 (0.05) | 86 (0.03) | 0.56 (0.45, 0.69) | <0.001 |
| Race/ethnicity | ||||
| White | 3 578 712 (64.5) | 158 984 (61.1) | Reference | |
| Asian | 292 676 (5.3) | 7385 (2.8) | 0.60 (0.59, 0.62) | <0.001 |
| Black | 557 820 (10.1) | 29 477 (11.3) | 1.20 (1.18, 1.21) | <0.001 |
| Hispanic | 636 600 (11.5) | 39 646 (15.2) | 1.45 (1.43, 1.46) | <0.001 |
| Other/Unknown | 480 323 (8.7) | 24 604 (9.5) | 1.11 (1.10, 1.13) | <0.001 |
| Region | ||||
| South | 2 384 178 (43.0) | 119,328 (45.9) | Reference | |
| Midwest | 1 465 322 (26.4) | 69,910 (26.9) | 0.94 (0.94, 0.95) | <0.001 |
| Northeast | 603 354 (10.9) | 37,022 (14.2) | 1.22 (1.20, 1.23) | <0.001 |
| West | 914 564 (16.5) | 33 589 (12.9) | 0.77 (0.76, 0.78) | <0.001 |
| Other/Unknown | 178 713 (3.2) | 247 (0.1) | 0.03 (0.03, 0.04) | <0.001 |
| Homeownership | ||||
| Probable homeowner | 3 806 910 (68.6) | 174 451 (67.1) | Reference | |
| Probable renter | 421 650 (7.6) | 21 218 (8.2) | 1.15 (1.13, 1.17) | <0.001 |
| Unknown | 1 317 571 (23.8) | 64 427 (24.8) | 1.11 (1.10, 1.12) | <0.001 |
| Smoking status | ||||
| Never smoker | 805 248 (14.5) | 45 815 (17.6) | Reference | |
| Current/former smoker | 887 685 (16.0) | 50 792 (19.5) | 1.02 (1.01, 1.04) | <0.001 |
| Unknown | 3 853 198 (69.5) | 163 489 (62.9) | 0.79 (0.78, 0.79) | <0.001 |
| Asthma | 308 506 (5.6) | 19 114 (7.4) | 1.33 (1.31, 1.35) | <0.001 |
| Autoimmune disease | 400 567 (7.2) | 25 187 (9.7) | 1.34 (1.32, 1.36) | <0.001 |
| Cancer | 297 991 (5.4) | 16 326 (6.3) | 1.16 (1.14, 1.18) | <0.001 |
| Cardiovascular disease | 568 500 (10.3) | 42 505 (16.3) | 1.67 (1.65, 1.69) | <0.001 |
| Cerebrovascular | 255 328 (4.6) | 19 878 (7.6) | 1.69 (1.66, 1.71) | <0.001 |
| Chronic kidney disease | 413 847 (7.5) | 31 564 (12.1) | 1.67 (1.65, 1.69) | <0.001 |
| Chronic lung disease | 413 014 (7.5) | 29 058 (11.2) | 1.54 (1.53, 1.56) | <0.001 |
| Diabetes | 793 551 (14.3) | 57 009 (21.9) | 1.62 (1.61, 1.64) | <0.001 |
| Hemoglobin disorder | 8087 (0.2) | 510 (0.2) | 1.32 (1.21, 1.44) | <0.001 |
| HIV/AIDS | 11 157 (0.2) | 866 (0.3) | 1.67 (1.56, 1.79) | <0.001 |
| Hypertension | 1 902 370 (34.3) | 114 551 (44.0) | 1.43 (1.42, 1.44) | <0.001 |
| Liver disease | 10 363 (0.2) | 769 (0.3) | 1.72 (1.60, 1.85) | <0.001 |
| Neurologic disease | 207 005 (3.7) | 25 168 (9.7) | 2.84 (2.81, 2.88) | <0.001 |
| Obesity | 588 969 (10.6) | 39 911 (15.3) | 1.48 (1.47, 1.50) | <0.001 |
| Solid organ transplant | 15 075 (0.3) | 1033 (0.4) | 1.44 (1.35, 1.53) | <0.001 |
| Pregnancy | 58 723 (1.1) | 3586 (1.4) | 1.30 (1.26, 1.34) | <0.001 |
| Systemic corticosteroids | 585 400 (10.6) | 37 922 (14.6) | 1.34 (1.33, 1.36) | <0.001 |
| DMARDs | 49 539 (0.9) | 3016 (1.2) | 1.22 (1.18, 1.27) | <0.001 |
| TNF-α inhibitors | 15 731 (0.3) | 918 (0.4) | 1.20 (1.13, 1.28) | <0.001 |
| IL-6 inhibitors | 718 (0.01) | 42 (0.02) | 1.18 (0.87, 1.60) | 0.28 |
| Other biologics | 12 896 (0.2) | 784 (0.3) | 1.24 (1.16, 1.33) | <0.001 |
| Other immunosuppressive drugs | 19 496 (0.4) | 1210 (0.5) | 1.25 (1.18, 1.33) | <0.001 |
AIDS = acquired immunodeficiency syndrome; CI = confidence interval; COVID-19 = Coronavirus Disease 2019; DMARD = disease-modifying anti-rheumatic drug; HIV = human immunodeficiency virus; IL-6 = interleukin 6; SD = standard deviation; TNF-α = tumor necrosis factor alpha.
P values calculated from Cox proportional hazards models. Reference groups for comorbidities and medications are the group of patients without the given disease or without a prescription for the given medication.
However, after adjusting for demographic, comorbid, and treatment covariates, NIU was not significantly associated with COVID-19 infection (HR, 1.05; 95% CI, 1.00–1.10; P = 0.04) (Table 4 ). Systemic corticosteroid use remained significantly associated with COVID-19 infection after adjustment (HR, 1.19; 95% CI, 1.18–1.20; P < 0.001). Tumor necrosis factor-α inhibitors were also significantly associated with COVID-19 infection after adjustment, and DMARDs and other immunosuppressive drugs were associated with decreased hazard of infection in the full model. In the sensitivity analysis that removed medication covariates to assess mediation, the estimated hazard of infection was similar to that in the full model (Table 4).
Table 4.
Fully Adjusted and Reduced (No Immunosuppressives) Model Results Showing Associations between Demographic and Clinical Characteristics and COVID-19 Infection
|
Variable |
Fully Adjusted Model Immunosuppressives Included |
Reduced Model Immunosuppressives Removed |
||
|---|---|---|---|---|
| Adjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | |
| Noninfectious uveitis | 1.05 (1.00, 1.10) | 0.04 | 1.06 (1.01, 1.12) | 0.03 |
| Age (yrs) | 0.998 (0.998, 0.999) | <0.001 | 0.998 (0.998, 0.999) | <0.001 |
| Continuous enrollment (yrs) | 1.004 (1.003, 1.005) | <0.001 | 1.004 (1.003, 1.005) | <0.001 |
| Male vs. Female | 0.98 (0.98, 0.99) | <0.001 | 0.98 (0.97, 0.99) | <0.001 |
| Unknown vs. Female | 0.55 (0.44, 0.68) | <0.001 | 0.55 (0.44, 0.68) | <0.001 |
| Asian vs. White | 0.70 (0.68, 0.71) | <0.001 | 0.69 (0.68, 0.71) | <0.001 |
| Black vs. White | 1.12 (1.11, 1.14) | <0.001 | 1.12 (1.11, 1.13) | <0.001 |
| Hispanic vs. White | 1.57 (1.55, 1.59) | <0.001 | 1.57 (1.55, 1.58) | <0.001 |
| Other/Unknown vs. White | 1.10 (1.08, 1.11) | <0.001 | 1.10 (1.08, 1.11) | <0.001 |
| Midwest vs. South | 1.05 (1.04, 1.06) | <0.001 | 1.05 (1.04, 1.06) | <0.001 |
| Northeast vs. South | 1.28 (1.27, 1.30) | <0.001 | 1.27 (1.26, 1.29) | <0.001 |
| West vs. South | 0.87 (0.86, 0.88) | <0.001 | 0.86 (0.85, 0.87) | <0.001 |
| Other/Unknown vs. South | 0.03 (0.03, 0.04) | <0.001 | 0.03 (0.03, 0.04) | <0.001 |
| Probable renter vs. Probable homeowner |
1.12 (1.11, 1.14) | <0.001 | 1.12 (1.11, 1.14) | <0.001 |
| Unknown vs. Probable homeowner | 1.24 (1.23, 1.25) | <0.001 | 1.24 (1.23, 1.25) | <0.001 |
| Current/former smoker vs. Never smoker |
0.91 (0.90, 0.93) | <0.001 | 0.92 (0.91, 0.93) | <0.001 |
| Unknown smoking status vs. Never smoker |
0.88 (0.88, 0.89) | <0.001 | 0.88 (0.87, 0.89) | <0.001 |
| Asthma | 1.10 (1.09, 1.12) | <0.001 | 1.13 (1.11, 1.15) | <0.001 |
| Autoimmune disease | 1.05 (1.03, 1.06) | <0.001 | 1.06 (1.04, 1.07) | <0.001 |
| Cancer | 0.95 (0.93, 0.96) | <0.001 | 0.95 (0.94, 0.97) | <0.001 |
| Cardiovascular disease | 1.20 (1.19, 1.22) | <0.001 | 1.20 (1.19, 1.22) | <0.001 |
| Cerebrovascular | 1.05 (1.03, 1.06) | <0.001 | 1.05 (1.03, 1.06) | <0.001 |
| Chronic kidney disease | 1.14 (1.13, 1.16) | <0.001 | 1.14 (1.13, 1.16) | <0.001 |
| Chronic lung disease | 1.12 (1.11, 1.14) | <0.001 | 1.14 (1.13, 1.16) | <0.001 |
| Diabetes | 1.24 (1.23, 1.25) | <0.001 | 1.24 (1.22, 1.25) | <0.001 |
| Hemoglobin disorder | 1.10 (1.01, 1.20) | 0.03 | 1.10 (1.01, 1.20) | 0.03 |
| HIV/AIDS | 1.41 (1.32, 1.51) | <0.001 | 1.41 (1.32, 1.51) | <0.001 |
| Hypertension | 1.04 (1.03, 1.05) | <0.001 | 1.05 (1.03, 1.06) | <0.001 |
| Liver disease | 1.16 (1.08, 1.25) | <0.001 | 1.16 (1.08, 1.24) | <0.001 |
| Neurologic disease | 2.33 (2.29, 2.36) | <0.001 | 2.31 (2.28, 2.34) | <0.001 |
| Obesity | 1.21 (1.20, 1.22) | <0.001 | 1.22 (1.20, 1.23) | <0.001 |
| Solid organ transplant | 1.11 (1.04, 1.18) | 0.001 | 1.11 (1.04, 1.18) | <0.001 |
| Pregnancy | 1.36 (1.31, 1.40) | <0.001 | 1.36 (1.31, 1.40) | <0.001 |
| Systemic corticosteroids | 1.19 (1.18, 1.20) | <0.001 | ||
| DMARDs | 0.91 (0.88, 0.95) | <0.001 | ||
| TNF-α inhibitors | 1.11 (1.04, 1.19) | 0.002 | ||
| IL-6 inhibitors | 0.96 (0.71, 1.30) | 0.79 | ||
| Other biologics | 1.00 (0.93, 1.07) | 0.94 | ||
| Other immunosuppressive drugs | 0.87 (0.83, 0.93) | <0.001 | ||
AIDS = acquired immunodeficiency syndrome; CI = confidence interval; DMARD = disease-modifying anti-rheumatic drug; HIV = human immunodeficiency virus; HR = hazard ratio; IL-6 = interleukin 6; TNF-α = tumor necrosis factor alpha.
P values calculated from Cox proportional hazards models. Reference groups for comorbidities and medications are the group of patients without the given disease or without a prescription for the given medication.
Factors Associated with COVID-19–Related Hospitalization
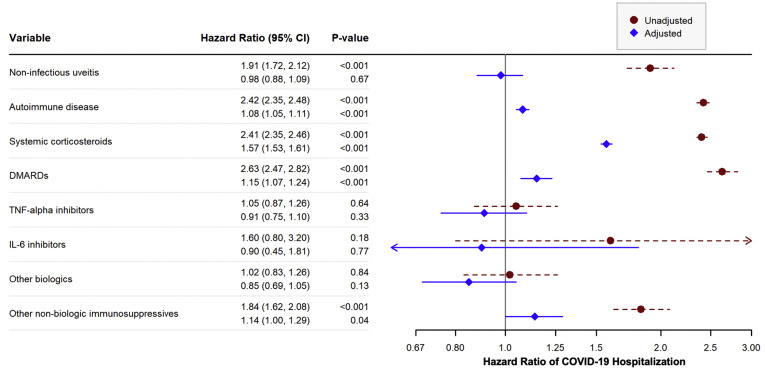
There were 363 NIU patients hospitalized with COVID-19 during the risk period, compared with 35 958 patients without NIU, corresponding to a cumulative incidence of 1.2% and 0.6%, respectively. In the unadjusted analysis, the HR of COVID-19 hospitalization comparing patients with NIU to those without NIU was 1.91 (95% CI, 1.72–2.12; P < 0.001) (Fig 1 and Table S5, available at www.aaojournal.org). Many factors associated with greater risk of COVID-19 infection, including most comorbidities, age, Black race or Hispanic ethnicity, current or former smoking, and use of any immunosuppressive medication, were also associated with increased hazard of hospitalization in the unadjusted analyses. In addition, male gender was also associated with COVID-19 hospitalization (Fig 1 and Table S5, available at www.aaojournal.org).
Figure 1.
Unadjusted and adjusted hazard ratios (HRs) of Coronavirus Disease 2019 (COVID-19) hospitalization for selected variables. P values calculated from Cox proportional hazards models. The HRs compare the hazards of COVID-19 hospitalization for patients with the given disease or on the given medication to patients without the disease or not on the medication. The first HR given in each row corresponds to the unadjusted HR for that variable, whereas the second HR corresponds to the adjusted HR for that variable. Adjusted models were adjusted for all variables in the figure and all variables given in Table 1 including demographics and other comorbidities. CI = confidence interval; DMARD = disease-modifying anti-rheumatic drug; IL-6 = interleukin 6; TNF-α = tumor necrosis factor alpha. See Appendix (eBox 3, available at www.aaojournal.org) for the specific medications included in each category.
In the adjusted analysis, NIU was not significantly associated with an increased hazard of COVID-19 hospitalization (HR, 0.98; 95% CI, 0.88–1.09; P = 0.67) (Fig 1 and Table S6, available at www.aaojournal.org). Other comorbidities, including autoimmune disease, cardiovascular disease, diabetes, age, male gender, and Black or Hispanic race/ethnicity, remained significantly associated with increased hazard of hospitalization after adjustment (Table S6, available at www.aaojournal.org). Patients taking systemic corticosteroids had a 57.1% increase in their hazard of hospitalization after adjustment (95% CI, 53.1–61.1; P < 0.001), and those taking DMARDs had a 14.7% increase (95% CI, 6.6–23.5, P = P < 0.001). The estimated HR for NIU did not change substantially when the immunosuppressive medications were removed from the model (Table S6, available at www.aaojournal.org).
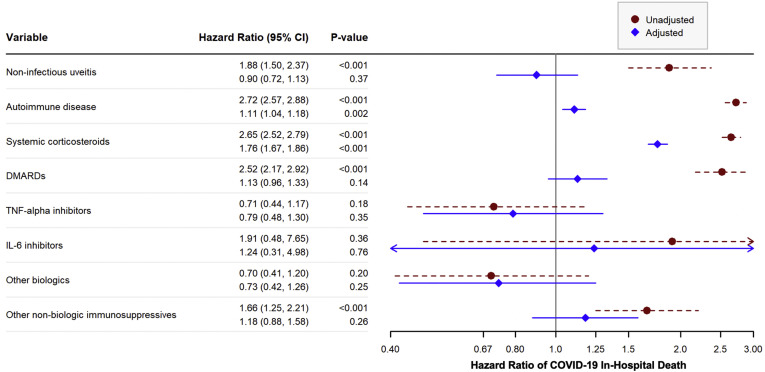
Factors Associated with COVID-19–Related In-Hospital Death
Among patients with NIU, 75 had a COVID-19–related death in an inpatient facility compared with 7518 in the group without NIU, corresponding to a cumulative incidence of 0.3% and 0.1%, respectively. The unadjusted HR for in-hospital COVID-19 death among patients with NIU compared with those without NIU was 1.88 (95% CI, 1.50–2.37; P < 0.001) (Fig 2 and Table S7, available at www.aaojournal.org). In other unadjusted analyses, age, male gender, Black race, Hispanic ethnicity, current or former smoking, and nearly all comorbid conditions also increased the hazard of in-hospital death (Table S7, available at www.aaojournal.org). Systemic corticosteroids, DMARDs, and other immunosuppressive drugs were all associated with in-hospital death in the unadjusted analyses (Fig 2 and Table S7, available at www.aaojournal.org).
Figure 2.
Unadjusted and adjusted hazard ratios (HRs) of COVID-19 in-hospital death for selected variables. P values calculated from Cox proportional hazards models. The HRs compare the hazards of COVID-19 in-hospital death for patients with the given disease or on the given medication to patients without the disease or not on the medication. The first HR given in each row corresponds to the unadjusted HR for that variable, whereas the second HR corresponds to the adjusted HR for that variable. Adjusted models were adjusted for all variables in the figure and all variables given in Table 1 including demographics and other comorbidities. CI = confidence interval; DMARD = disease-modifying anti-rheumatic drug; IL-6 = interleukin 6; TNF-α = tumor necrosis factor alpha. See Appendix (eBox 3,available at www.aaojournal.org) for the specific medications included in each category.
After adjusting for all other covariates, NIU was no longer associated with in-hospital COVID-19 death (HR, 0.90, 95% CI, 0.72–1.13, P = 0.37) (Fig 2 and Table S8, available at www.aaojournal.org). Age, male gender, Black race, and Hispanic ethnicity remained significantly associated with increased hazard of in-hospital death after adjustment (Table S8, available at www.aaojournal.org). Most comorbidities, including autoimmune disease, cardiovascular disease, hypertension, and chronic kidney disease, also remained significant predictors of death. Use of systemic corticosteroids was associated with a 75.9% increase in hazard of death (95% CI, 66.7–85.6; P < 0.001) after adjusting for demographics and comorbidities, but no other immunosuppressive drugs had significant effects on the hazard of COVID-19 death after adjustment (Fig 2 and Table S8, available at www.aaojournal.org). In the sensitivity analysis, the estimated hazard of in-hospital death for NIU patients was similar when the immunosuppressive drug categories were removed. However, for other conditions that often require immunosuppressive treatments, including autoimmune diseases, cancer, and solid organ transplantation, the estimated hazards of in-hospital death increased substantially when the medications were removed from the model (Table S8, available at www.aaojournal.org).
Subgroup Analysis by Age
There were 2 641 914 patients aged less than 50 years, 5488 of whom had NIU (0.2%). There were 3 164 310 patients aged ≥50 years, 24 381 of whom had NIU (0.8%). In the subgroup of patients aged less than 50 years, rates of COVID-19 infection and hospitalization were slightly higher in patients with NIU compared with patients without NIU. There were no COVID-19 in-hospital deaths in patients with NIU in the subgroup aged less than 50 years. After adjusting for demographics, comorbidities, and immunosuppressive medication use, NIU was not associated with COVID-19 infection, hospitalization, or in-hospital death in the age <50 years subgroup or the ≥50 years and subgroup (Table S9, available at www.aaojournal.org).
Discussion
Patients with NIU had higher unadjusted hazards of COVID-19 infection, hospitalization, and in-hospital death. However, after adjusting for demographics and comorbidities, there was no significant difference in the risk of COVID-19 infection, hospitalization, or in-hospital death between patients with and without NIU. These results indicate that the higher rates of COVID-19 infection and severe disease in patients with uveitis may be explained by the characteristics of patients with NIU, rather than a history of NIU alone. Age, gender, race/ethnicity, and comorbidities such as autoimmune disease, cardiovascular disease, diabetes, and chronic kidney disease were all strongly associated with COVID-19 hospitalization and in-hospital death in this analysis. These characteristics are known risk factors for severe COVID-19 outcomes.14 , 15
The removal of immunosuppressive medications from regression models in the sensitivity analysis did not impact the estimates of hazards of the 3 COVID-19 outcomes for patients with NIU, suggesting that the use of immunosuppressive medications among patients with NIU did not mediate the association between NIU and COVID-19 outcomes. However, for patients with autoimmune disease, cancer, or solid organ transplants, immunosuppressive medications may act as partial mediators in the relationship between the disease and COVID-19 in-hospital death.
In our analysis of a national-level claims database, the cumulative incidence of COVID-19 among patients with NIU was 5.7%, which is in the range of reported incidence among previous international studies with more limited cohorts. In a report of 59 patients with uveitis in Saudi Arabia, 15.3% tested positive for COVID-19, but none developed any symptoms during follow-up or required hospitalization.16 In Spain, Fanlo et al17 surveyed patients with uveitis associated with a systemic autoimmune disease in April and May of 2020. Of 28 patients with uveitis, half reported symptoms of possible COVID-19 infection, but only 2 were tested and just 1 tested positive. A study in Italy monitored 125 children with juvenile idiopathic arthritis–associated uveitis during the initial lockdown in March 2020, and there were no known cases of infection.18 As in all estimates of COVID-19 incidence, our analysis likely underestimates the true incidence of COVID-19 infection among patients with NIU due to lack of diagnostic testing. Additionally, patients with NIU enrolled in commercial and Medicare Advantage plans may have different rates of COVID-19 infection than a population with different insurance coverage or lack of insurance coverage.
Independent of uveitis or other autoimmune disease, this study found that use of systemic corticosteroids during the risk period was associated with significantly higher hazard of infection, hospitalization, and death with COVID-19. Use of DMARDs during the risk period was associated with a significantly lower hazard of infection but a higher hazard of hospitalization after adjustment. Although TNF-α inhibitors and other nonbiologic treatments were associated with higher hazard of infection, no other immunosuppressive treatment categories had a significant impact on the hazard of hospitalization or death. Other studies have identified systemic corticosteroids as a risk factor for COVID-19 hospitalization and death in the general population19 and among cohorts with existing autoimmune disease,20 , 21 although conflicting studies have found no effect of corticosteroids or other immunosuppressive treatments on the risk of severe COVID-19.22 , 23 Among studies that looked at TNF-α inhibitors or DMARDs specifically, several found no impact on COVID-19 hospitalization or severe outcomes,19 , 20 whereas others found a protective effect of TNF-α inhibitors against hospitalization for COVID-19.21 , 22
Some studies investigating autoimmune disease and immunosuppressive therapy as risk factors for COVID-19 infection and severe outcomes included patients with uveitis, but the number of patients with uveitis was too small to draw conclusions about uveitis as a risk factor itself.22 , 24 , 25 To the best of our knowledge, our study provides the first estimates of the hazard of COVID-19 infection, hospitalization, and death in patients with NIU, as well as estimates of the cumulative incidence of these outcomes in NIU patients during 2020. In addition, this study provides novel information on whether patients with NIU carry a higher risk of severe COVID-19 outcomes compared with the general population. Our large sample size allowed observation of rare events such as hospitalization and death, and allowed for adjustment of many potential confounders, giving clearer insight into the effects of uveitis independent of other COVID-19 risk factors.
The results of this study have several implications for patients with NIU as well as for those receiving systemic corticosteroid therapy. Immunosuppressed patients, including those with NIU, may not be as protected by COVID-19 vaccination given their immune status,26 so mitigating their risk of infection remains an important issue. We found that patients on systemic corticosteroid therapy experience COVID-19 hospitalization and death at higher rates than the general population, raising concerns about the use of systemic corticosteroid therapy to treat active inflammation during the pandemic. It is unknown if the association we observed is dose or duration dependent, and further research is necessary to elucidate the impact of systemic corticosteroid use on COVID-19 infection susceptibility and adverse outcomes. Given our findings, future uveitis treatment guidelines may want to encourage physicians caring for patients with NIU, particularly those on systemic corticosteroids, to provide counseling about possible increased risk and to encourage infection mitigation efforts. Local corticosteroid therapies may be considered as an alternative, although this carries some risks of ocular adverse events.
There are several limitations to this study. First, there is limited granularity inherent to healthcare claims data that could contribute to misclassification of NIU patients versus non-NIU patients and COVID-19 outcomes, particularly infection, if testing was incompletely reported in the claims database. However, the cumulative incidence of infection, hospitalization, and death up to December 31, 2020, reported by both the Centers for Disease Control and Prevention and Johns Hopkins University is similar to the rates reported in this study.27 , 28 Second, we could not obtain data on deaths occurring outside of a healthcare facility, so it is possible that not all deaths related to COVID-19 (or other causes) were captured. Knowledge of COVID-19 pathophysiology and treatment significantly improved over the study’s risk period. It is possible that over the course of the study, trends in COVID-19 management differentially affected outcomes among NIU and non-NIU patients. Additionally, OLDW does not include individuals who are enrolled in basic Medicare plans and Medicaid, or are uninsured, so the cohort analyzed in this study may represent a more economically advantaged population in the United States. We made efforts to adjust for socioeconomic factors in the analysis; however, we did not have access to socioeconomic variables other than homeownership and race/ethnicity in the database. Other measures of socioeconomic status such as education and occupation, which could impact risk for COVID-19, were not available in the database. Last, the inclusion of Medicare Advantage enrollees and the strict 3-year continuous enrollment requirement used for this study may skew the study population to be older than the overall US population. However, the age distribution of the NIU group is comparable to that reported in other US claims-based studies on NIU incidence and prevalence.29 Although the older age of the cohort could increase crude rates of COVID-19 cases and severe illness, this should not greatly impact the ascertainment of risk factors in the adjusted analyses, which adjusted for age and known comorbidity risk factors for COVID-19. We also conducted a subgroup analysis of patients aged less than 50 years versus ≥50 years to understand if a younger group of NIU patients may have a higher risk of severe COVID-19 outcomes and found similar results in the younger and older groups. Overall, the sheer size of the population included in OLDW, as well as the diversity in geography, ethnicity, and age, makes this a more generalizable sample than other alternatives.
In conclusion, in the United States and abroad, many patients remain unvaccinated and susceptible to COVID-19, and potential for resurgence of the virus remains. In this study, patients with NIU experienced COVID-19 infection, hospitalization, and death at significantly greater rates than patients without NIU. Increased risk is likely due to the demographic characteristics and medical conditions of patients with NIU, rather than NIU itself. Furthermore, patients treated with systemic corticosteroid therapy may be at increased risk of infection and severe COVID-19 outcomes. Future studies are needed to evaluate the impact of the level of corticosteroid exposure on COVID-19 risk.
Manuscript no. D-21-01499.
Footnotes
Supplemental material available atwww.aaojournal.org.
Disclosure(s): All authors have completed and submitted the ICMJE disclosures form.
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Supported by the National Eye Institute and Office of Research on Women’s Health at the National Institutes of Health (grant no. R01 EY028739, to Principal Investigator N.R.A.) and an OptumLabs Data Warehouse research credit.
HUMAN SUBJECTS: Human subjects were not included in this study. The human ethics committees at the University of California, San Francisco approved the study. All research adhered to the tenets of the Declaration of Helsinki. The requirement for informed consent was waived because of the retrospective nature of the study.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Miller, Sun, Acharya
Data collection: Miller, Sun, Chen, Acharya
Analysis and interpretation: Miller, Sun, Arnold, Acharya
Obtained funding: Acharya
Overall responsibility: Miller, Sun, Chen, Arnold, Acharya
Supplementary Data
References
- 1.Furman D., Campisi J., Verdin E., et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maddur M.S., Vani J., Lacroix-Desmazes S., et al. Autoimmunity as a predisposition for infectious diseases. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Au K., Reed G., Curtis J.R., et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:785–791. doi: 10.1136/ard.2010.128637. [DOI] [PubMed] [Google Scholar]
- 4.D’Silva K.M., Serling-Boyd N., Wallwork R., et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US hot spot. Ann Rheum Dis. 2020;79:1156–1162. doi: 10.1136/annrheumdis-2020-217888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cordtz R., Lindhardsen J., Soussi B.G., et al. Incidence and severeness of COVID-19 hospitalization in patients with inflammatory rheumatic disease: a nationwide cohort study from Denmark. Rheumatology (Oxford) 2021;60(SI):SI59–SI67. doi: 10.1093/rheumatology/keaa897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Silva K.M., Jorge A., Cohen A., et al. COVID-19 outcomes in patients with systemic autoimmune rheumatic diseases compared to the general population: a US multicenter, comparative cohort study. Arthritis Rheumatol. 2020;0:1–7. doi: 10.1002/art.41619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serling-Boyd N., D’Silva K.M., Hsu T.Y.T., et al. Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic. Ann Rheum Dis. 2021;80:660–666. doi: 10.1136/annrheumdis-2020-219279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung J.C.H., Li K.K.W. Implications of COVID-19 for uveitis patients: perspectives from Hong Kong. Eye. 2020;34:1163–1164. doi: 10.1038/s41433-020-0905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seah I., Agrawal R. Can the Coronavirus Disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020;28:391–395. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith J.R., Lai T.Y.Y. Managing uveitis during the COVID-19 Pandemic. Ophthalmology. 2020;127:e65–e67. doi: 10.1016/j.ophtha.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.OptumLabs. OptumLabs Data Warehouse (OLDW) Descriptions and Citation. Eden Prairie, MN: n.p. July 2020. PDF. Reproduced with permission from OptumLabs. Available at: https://www.optumlabs.com/. Accessed July 15, 2021.
- 12.Bohl A., Roozeboom-Baker M. A COVID-19 Primer: Analyzing Health Care Claims, Administrative Data, and Public Use Files. 2020. https://mathematica.org/publications/a-covid-19-primer-analyzing-health-care-claims-administrative-data-and-public-use-files
- 13.Certain Medical Conditions and Risk for Severe COVID-19 Illness | CDC. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed May 27, 2021.
- 14.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albloushi A.F., Alfawaz A.M., Abu El Asrar A.M. Implications of COVID-19 infection on patients with uveitis under biologic treatment. Br J Ophthalmol. 2021;0:1–4. doi: 10.1136/bjophthalmol-2020-318577. [DOI] [PubMed] [Google Scholar]
- 17.Fanlo P., Espinosa G., Adán A., et al. Impact of novel coronavirus infection in patients with uveitis associated with an autoimmune disease: result of the COVID-19-GEAS patient survey. Arch la Soc Española Oftalmol (Engl Ed) 2021;96:347–352. doi: 10.1016/j.oftale.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miserocchi E., Giuffrè C., Modorati G.M., Cimaz R. Management of juvenile idiopathic arthritis-associated uveitis during the COVID-19 pandemic in a pediatric referral center in Lombardy. Ocul Immunol Inflamm. 2020;28:1305–1307. doi: 10.1080/09273948.2020.1800752. [DOI] [PubMed] [Google Scholar]
- 19.Nørgård B.M., Nielsen J., Knudsen T., et al. Hospitalization for COVID-19 in patients treated with selected immunosuppressant and immunomodulating agents, compared to the general population: a Danish cohort study. Br J Clin Pharmacol. 2021;87:2111–2120. doi: 10.1111/bcp.14622. [DOI] [PubMed] [Google Scholar]
- 20.Brenner E.J., Ungaro R.C., Gearry R.B., et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an International Registry. Gastroenterology. 2020;159:481–491.e3. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gianfrancesco M., Hyrich K.L., Hyrich K.L., et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veenstra J., Buechler C.R., Robinson G., et al. Antecedent immunosuppressive therapy for immune-mediated inflammatory diseases in the setting of a COVID-19 outbreak. J Am Acad Dermatol. 2020;83:1696–1703. doi: 10.1016/j.jaad.2020.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen K.M., Mehta H.B., Palamuttam N., et al. Association between chronic use of immunosuppresive drugs and clinical outcomes from Coronavirus Disease 2019 (COVID-19) hospitalization: a retrospective cohort study in a large US Health System. Clin Infect Dis. 2021;73(11):e4124–e4130. doi: 10.1093/cid/ciaa1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Florence A., Nassim A.A., Jean-David A., et al. Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID-19 cohort of 694 patients. Ann Rheum Dis. 2021;80:527–538. doi: 10.1136/annrheumdis-2020-218310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freites Nuñez D.D., Leon L., Mucientes A., et al. Risk factors for hospital admissions related to COVID-19 in patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;79:1393–1399. doi: 10.1136/annrheumdis-2020-217984. [DOI] [PubMed] [Google Scholar]
- 26.Interim Clinical Considerations for Use of COVID-19 Vaccines | CDC. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html. Accessed June 16, 2021.
- 27.CDC COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#datatracker-home. Accessed May 27, 2021.
- 28.Cumulative Cases - Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/data/cumulative-cases. Accessed May 27, 2021.
- 29.Thorne J.E., Suhler E., Skup M., et al. Prevalence of noninfectious uveitis in the United States: a claims-based analysis. JAMA Ophthalmol. 2016;134:1237–1245. doi: 10.1001/jamaophthalmol.2016.3229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.