Abstract
Background
A greater understanding of the factors that are associated with favourable health may help increase longevity and healthy life expectancy. We examined sociodemographic, psychosocial, lifestyle and environmental exposures associated with multiple health indicators.
Methods
UK Biobank recruited > 500,000 participants, aged 37–73, between 2006 and 2010. Health indicators examined were 81 cancer and 443 non-cancer illnesses used to classify participants' health status; long-standing illness; and self-rated health. Exposures were sociodemographic (age, sex, ethnicity, education, income and deprivation), psychosocial (loneliness and social isolation), lifestyle (smoking, alcohol intake, sleep duration, BMI, physical activity and stair climbing) and environmental (air pollution, noise and residential greenspace) factors. Associations were estimated using logistic and ordinal logistic regression.
Results
In total, 307,378 participants (mean age = 56.1 years [SD = 8.07], 51.9% female) were selected for cross-sectional analyses. Low income, being male, neighbourhood deprivation, loneliness, social isolation, short or long sleep duration, low or high BMI and smoking were associated with poor health. Walking, vigorous-intensity physical activity and more frequent alcohol intake were associated with good health. There was some evidence that airborne pollutants (PM2.5, PM10 and NO2) and noise (Lden) were associated with poor health, though findings were not consistent across all models.
Conclusions
Our findings highlight the multifactorial nature of health, the importance of non-medical factors, such as loneliness, healthy lifestyle behaviours and weight management, and the need to examine efforts to improve the health outcomes of individuals on low incomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-021-02097-z.
Keywords: Health status, Self-rated health, Long-standing illness, Lifestyle, Environmental exposures, Sociodemographic characteristics, Psychosocial factors, UK Biobank, Epidemiology, Public health
Background
Substantial improvements in human health and significant increases in average life expectancy are major achievements of civilization over the past two centuries [1]. Life expectancy at birth in England and Wales has doubled from about 40 years in 1850 to more than 80 years in 2013 [2]. Future life expectancy is projected to increase across 35 industrialised nations, with a median increase in life expectancy at birth of approximately 3 years for women and 4 years for men in the UK between 2010 and 2030 [3].
Many adults, however, spend a substantial portion of their lives with late-life morbidities and decreased sensory, motor and cognitive functioning, which may reduce their quality of life [4, 5]. More than 80% of the population of England and Wales aged 85 and above report having a disability and 50% require care or help with daily activities [6]. Recent data suggest that healthy life expectancy in the UK has decreased between 2008 and 2016 [7]. Nevertheless, some individuals experience very little functional decline in old age and rate their health as good or excellent [8]. A greater understanding of the factors associated with good health may help increase longevity and healthspan, i.e. the length of time that a person lives healthy [9].
The health effects of lifestyle factors such as smoking [10], diet [11], excessive alcohol intake [12] and physical activity [13] are well documented. However, the number and type of covariates that have been adjusted for in previous analyses vary widely [14], making a systematic comparison of risk factor associations across studies difficult. Few studies have jointly examined sociodemographic, psychosocial, lifestyle and environmental factors. A review of multivariable models examining determinants of self-reported health concluded that most factors except for age, sex and education were examined in only one or a few studies [15]. In addition, research has often focused on predicting mortality or disease incidence instead of overall health status, despite its potential to provide insights into the factors associated with increased healthspan.
The UK Biobank study provides an unprecedented data resource to investigate determinants of health and ageing trajectories. One of its strengths is that the data collection was not merely focused on established predictors of health and disease, such as lifestyle, but also included relevant non-medical factors such as social isolation and loneliness, as well as road traffic noise and ambient air pollution [16, 17].
The aim of this study was to explore predictors of health status from a wide range of potential risk factor mechanisms. More specifically, we examined how health status was associated with (i) sociodemographic characteristics and psychosocial factors, (ii) lifestyle factors, and (iii) environmental exposures, both independently and jointly. We also examined, as a secondary aim, whether there was evidence of similar associations between these factors and (i) reporting a long-standing illness and (ii) self-rated health.
Methods
Study population
The UK Biobank is a prospective study of over 500,000 UK residents aged 37–73 at baseline, recruited between 2006 and 2010. Details of the study rationale and design have been reported elsewhere [18]. Briefly, individuals registered with the UK National Health Service (NHS) and living within a 25-mile (~ 40 km) radius of one of 22 assessment centres were invited to participate (9,238,453 postal invitations sent). At the baseline assessment, participants completed questionnaires and were interviewed by nurses to provide data on sociodemographic characteristics, health behaviours and their medical history. A small subset of participants completed repeat measurements: first revisit of participants living within a 35-km radius of the assessment centre at Stockport, England, between 2012 and 2013 (20,344 participants); second revisit as part of the UK Biobank Imaging Study [19] between 2014 and 2019 (43,190 participants at the time of this analysis). Participants consented to use of their de-identified data. See Additional file 1: Table S1 for the UK Biobank data fields used.
Exposures
Multiple explanatory variables were considered based on their likely relevance to public health in terms of modifiability and potential for risk stratification, representing a wide range of potential risk factor mechanisms.
Sociodemographic characteristics
We considered individual-level sociodemographic characteristics including age at baseline assessment, sex, ethnicity (White, Asian, Black, Chinese, Mixed-race or other), highest educational or professional qualification (four categories, reflecting similar years of education [20]: (1) College/University Degree; (2) Education to age 18 or above, but not reaching degree level: General Certificate of Education Advanced Level (A levels) / General Certificate of Education Advanced Subsidiary Level (AS levels) or equivalent, National Vocational Qualification (NVQ) / Higher National Diploma (HND) / Higher National Certificate (HNC) or equivalent, other professional qualifications; (3) Education to age 16 qualifications: General Certificate of Education Ordinary Level (O levels) / General Certificate of Secondary Education (GCSEs) or equivalent, Certificate of Secondary Education (CSEs) or equivalent; (4) No qualifications) and gross annual household income (< £18,000, £18,000–£30,999, £31,000–£51,999, £52,000–£100,000 or > £100,000).
We also included the Index of Multiple Deprivation for England which is a small-area level measure derived by government from data on income, employment, health and disability, education skills and training, barriers to housing and services, living environment and crime [21]. Higher values on the index reflect greater deprivation.
Psychosocial factors
Loneliness was assessed using two questions: “Do you often feel lonely?” (no = 0 / yes = 1) and “How often are you able to confide in someone close to you?” (almost daily to about once a month = 0 / once every few months to never or almost never = 1). Individuals who received a sum score of 2 were classified as lonely [22].
Social isolation was assessed using three questions: “Including yourself, how many people are living together in your household?” (living alone = 1), “How often do you visit friends or family or have them visit you?” (less than once a month = 1) and “Which of the following [leisure/social activities] do you attend once a week or more often?” (none of the above = 1). Individuals who received a sum score of 2 or 3 were classified as socially isolated [22].
Lifestyle factors
Smoking status was assessed using two questions summarising current and past smoking behaviour. Individuals who responded “Yes, on most or all days” or “Only occasionally” to current tobacco smoking were coded as “current”. Individuals who responded “Smoked on most or all days” or “Smoked occasionally” to past tobacco smoking were coded as “former”. Individuals who responded “No” to current tobacco smoking and “Just tried once or twice” or “I have never smoked” to past tobacco smoking were coded as “never”.
Alcohol intake frequency was assessed using one question: “About how often do you drink alcohol?”. Response options included “Daily or almost daily”, “Three or four times a week”, “Once or twice a week”, “One to three times a month”, “Special occasions only” and “Never”.
Sleep duration was assessed using one question: “About how many hours sleep do you get in every 24 hours? (please include naps)”. Values below 1 h or above 23 h were rejected, and UK Biobank asked participants to confirm values below 3 h or above 12 h.
Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Weight measurements were obtained with a Tanita BC-418 MA body composition analyser. Standing height measurements were obtained using a Seca 202 height measure.
Physical activity was assessed using the International Physical Activity Questionnaire (IPAQ) short form [23]. Specifically, we included data on the number of days per week spent walking, engaging in moderate-intensity physical activity (e.g. “carrying light loads, cycling at normal pace”) or engaging in vigorous-intensity physical activity (i.e. “activities that make you sweat or breathe hard such as fast cycling, aerobics, heavy lifting”) for ≥ 10 min continuously.
Daily frequency of stair climbing was assessed using one question: “At home, during the last 4 weeks, about how many times a day do you climb a flight of stairs? (approx. 10 steps)”. These data were only collected from individuals who indicated that they were able to walk. Response options included “None”, “1–5 times a day”, “6–10 times a day”, “11–15 times a day”, “16–20 times a day” and “More than 20 times a day”.
Environmental exposures
Exposure to airborne pollutants was estimated using a Land Use Regression model developed as part of the European Study of Cohorts for Air Pollution Effects (ESCAPE) project [24, 25]. We included annual average concentration of particulate matter with an aerodynamic diameter of < 2.5 μm (PM2.5) and < 10 μm (PM10), as well as nitrogen dioxide (NO2) modelled at participants’ residential addresses for the year 2010.
Residential road traffic noise was modelled for the year 2009 using the Common Noise Assessment Methods (CNOSSOS-EU) algorithm [26, 27]. We used Lden (day-evening-night noise level) which is an annual average 24-h sound pressure level in decibels with a 10-decibel penalty added between 11 pm and 7 am. This penalty has previously been added in epidemiological analyses to account for annoyance / sleep disruption at night [28].
The percentage of the home location classed as greenspace, as a proportion of all land use types, was modelled using 2005 data from the Generalized Land Use Database for England (GLUD) [29] for the 2001 Census Output Areas in England. Each residential address was allocated a circular distance buffer of 1000 m, representing wider-area greenspace.
Health status
Data on 81 cancer and 443 non-cancer illnesses (past and current) were ascertained through touchscreen self-report questionnaire and confirmed during a verbal interview by a trained nurse. In order to provide a single health indicator (“health status”) based on a previously defined algorithm, we used a classification developed by the Reinsurance Group of America (RGA) in which an experienced underwriter classified each illness according to whether it was “likely acceptable for standard life insurance” [30]. Participants were thus classified as healthy or unhealthy based on their reported cancer and non-cancer illnesses (Additional file 1: Table S1; Additional file 2). In developing this algorithm, the main determinant of whether to classify specific illnesses as healthy or unhealthy was their corresponding all-cause mortality risk. This classification does not account for the number of illnesses or temporality of diseases. In a separate analysis, we have shown that UK Biobank participants classified as unhealthy had a twofold increase in their risk of all-cause mortality compared to participants who were classified as healthy [31].
Two secondary health outcomes were assessed. Firstly, whether patients had a long-standing illness, disability or infirmity was assessed using the question “Do you have any long-standing illness, disability or infirmity?” to which individuals could respond “Yes” or “No”. Secondly, participants’ perceived health was assessed using the question “In general how would you rate your overall health?”. Response options included “Poor”, “Fair”, “Good” and “Excellent”. These health outcomes will be termed “long-standing illness” and “self-rated health”.
Exclusion criteria
Women who were pregnant at the time of assessment were excluded from the analysis based on the assumption that lifestyle patterns change during pregnancy [32] (0.0003% of participants were excluded for this reason). Participants for whom their genetic sex, inferred from the relative intensity of biological markers on the Y and X chromosomes, and self-reported sex did not match were also excluded as this may reflect poor data quality (0.0007% of participants were excluded for this reason).
Statistical analyses
Participants with baseline data on all explanatory variables and health indicators were selected for cross-sectional analyses. Participants with missing data or who responded “do not know” or “prefer not to answer” were excluded.
Logistic regression analyses were used to estimate associations between sociodemographic characteristics, psychosocial factors, lifestyle factors and environmental exposures with health status and long-standing illness. Ordinal logistic regression analyses were used to estimate associations between these factors and self-rated health (four categories). Across these outcomes, poor health was the reference group. In determining the reference groups for categorical explanatory variables, we focused on interpretability of the results for middle-aged and older UK residents.
For each explanatory variable, we fitted incrementally adjusted models using the baseline UK Biobank data: Model 1 included only individual explanatory variables; Model 2 included individual explanatory variables plus age and sex; Model 3 included all explanatory variables within a given domain (sociodemographic, psychosocial, lifestyle or environmental) plus age and sex; Model 4 was a full multivariable model that included all explanatory variables. All models were fitted in the analytical sample without missing data to ensure that any differences between models were not due to inclusion of different participants. We calculated odds ratios and Bonferroni-adjusted (~ 99.9%) confidence intervals. Adjusted p values were calculated to account for multiple testing within each model (i.e. typically for 39 tests). Two methods were used: (1) Bonferroni and (2) Benjamini & Hochberg [33], all two-tailed with α = .05, and false discovery rate of 5%, respectively. We report Bonferroni-adjusted p values for most results. For the interaction analyses (see below), we report in-text the most conservative correction at which statistical significance was reached (i.e. either Bonferroni or Benjamini & Hochberg) and present both sets of adjusted p values in Additional File 1. Multicollinearity was assessed using generalised variance inflation factors [34].
To compare the magnitudes of association between the explanatory variables and health indicators, we calculated standardised regression coefficients by rescaling all variables included in Model 4 to have a mean of zero and by dividing the coefficients of numeric variables with more than two values by twice their standard deviation [35].
To assess whether any associations between explanatory variables and health indicators were modified by sex or age, we stratified analyses by sex and by age at baseline assessment (< 65 years and ≥ 65 years) and assessed potential age and sex interactions by adding cross-product terms to Model 4. The choice of age strata was based on the current UK retirement age.
To assess whether the explanatory variables assessed at baseline predicted self-rated health at follow-up, we applied the same modelling strategy (ordinal logistic regression analyses) to the subsets of participants with repeat assessment data. We additionally adjusted for the number of days between the baseline and follow-up assessments in these analyses. This additional adjustment was included to account for the possibility that individuals who had their first or second revisit (t1 and t2, respectively) closer to the baseline assessment would be less likely to have had changes in their self-rated health.
As variable selection and classification might impact results, we conducted multiple additional analyses to examine related variables, categorised continuous variables according to health guidelines or previously determined cut-offs and tested the robustness of our findings to additional exclusion criteria. We repeated the main analysis (i) with the Townsend deprivation index instead of the Index of Multiple Deprivation for England as measure of neighbourhood deprivation; (ii) examining each individual component of the Index of Multiple Deprivation for England separately; (iii) with sleep duration as categorical variable (< 7, 7–8 [reference] and ≥ 9 h of sleep/day) [36]; (iv) with BMI as categorical variable (< 18.5, 18.5–24.9 [reference] and > 24.9 kg/m2); (v) with body fat percentage (estimated by electrical bio-impedance measurement) instead of BMI as measure of body composition; (vi) excluding individuals who reported that they stopped drinking alcohol because of “Illness or ill health” or “Doctor’s advice”; (vii) sub-dividing individuals who reported that they never drink alcohol into current and lifetime abstainers; (viii) with current tobacco smoking only (three categories: “Yes, on most or all days”, “Only occasionally” and “No”); (ix) with Metabolic Equivalent Task (MET) minutes per week for walking, moderate physical activity and vigorous physical activity [37] instead of the number of days per week spent engaging in these activities for ≥ 10 min; (x) reducing the greenspace percentage circular distance buffer from 1000 to 300 m to examine nearby greenspace; (xi) with air pollution and noise exposure estimates dichotomised according to WHO recommendation thresholds [38, 39]: PM2.5 ≤ 10 μg/m3, PM10 ≤ 20 μg/m3, NO2 ≤ 40 μg/m3 and Lden ≤ 53 decibels; (xii) restricting analyses to participants assessed after 31 December 2008 (regarding noise estimates) and restricting analyses to participants assessed after 31 December 2009 (regarding air pollution estimates); (xiii) restricting analyses to those individuals who had lived at their current address for at least 10 years; (xiv) truncating continuous explanatory variables at the 1st and 99th percentile of the distribution.
Statistical analyses were conducted using R (version 3.6.0).
Results
Study population
Of 502,521 UK Biobank participants, 5.32% (n = 26,757) had missing health data and 33.51% (n = 168,386) had missing data on explanatory variables or did not meet our inclusion criteria. Hence our analytical sample included n = 307,378 adults. Missing data for each explanatory variable are described in Additional file 1: Fig. S1.
Descriptive statistics are presented in Additional file 1: Tables S2–S5. There were few differences between the full and analytical sample, with the analytical sample slightly healthier and more educated. Of the participants in the analytical sample, 8.18% (n = 25,142) reported at least one cancer and 73.30% (n = 225,312) at least one non-cancer illness (Additional file 1: Fig. S2 and S3). Approximately two thirds of participants (69.04%, n = 212,201) were classified as healthy, while 30.96% (n = 95,177) were unhealthy. A similar percentage of participants (30.5%, n = 93,757) reported having a long-standing illness (72.9% agreement with health status). Finally, 3.60% (n = 11,066) rated their health as poor, while 19.25% (n = 59,169), 59.44% (n = 182,699) and 17.71% (n = 54,444) rated their health as fair, good and excellent, respectively.
Regression analyses
We found weak correlations between most continuous explanatory variables and moderate to strong correlations between the environmental exposures (Additional file 1: Fig. S4). Generalised variance inflation factor (VIF) values for Model 4 were between 1.02 and 2.62 for 19/21 explanatory variables. The VIFs for NO2 and PM2.5 were 6.08 and 4.27, respectively. Excluding NO2 from the model reduced the highest VIF to 2.42 (for PM2.5). Fitting Model 4 without NO2 or with each air pollutant separately had little impact on their associations with health status. As such, we report the results for Model 4 that included all explanatory variables. Unless indicated otherwise, results presented below correspond to multivariable-adjusted odds ratios and Bonferroni-adjusted (~ 99.9%) confidence intervals from Model 4. A simplified overview of our findings is presented in Additional file 1: Tables S6–S9.
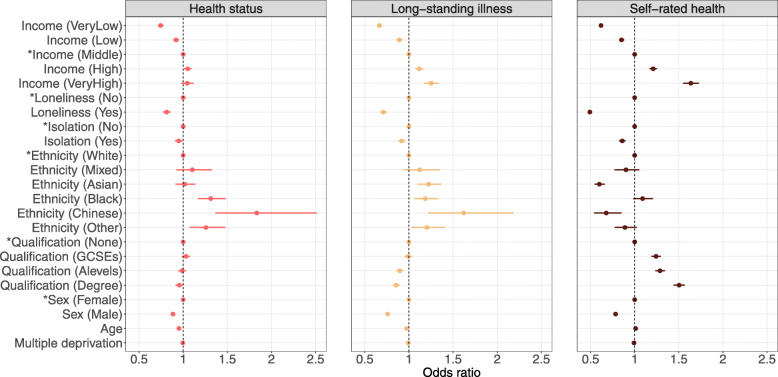
Increased age was associated with lower odds of favourable health status (OR = 0.953, 99.9% CI 0.951–0.955, pBonf. < 0.001). Men had lower odds of being healthy (OR = 0.88, 99.9% CI 0.86–0.91, pBonf. < 0.001). Individuals with a high income had higher odds of being healthy (OR = 1.05, 99.9% CI 1.01–1.09, pBonf. = 0.003 [£52,000–£100,000 vs £31,000–£51,999]), while those with lower levels of income had lower odds of being healthy (e.g. OR = 0.74, 99.9% CI 0.71–0.78, pBonf. < 0.001 [< £18,000 vs £31,000–£51,999]). Increased neighbourhood deprivation was associated with lower odds of being healthy (OR = 0.995, 99.9% CI 0.994–0.996, pBonf. < 0.001). Compared to Whites, individuals of Black, Chinese, Mixed-race, and “other” ethnic background had higher odds of being healthy in Model 1, but only Chinese ethnicity was associated with higher odds of being healthy across all models (Model 4: OR = 1.83, 99.9% CI 1.36–2.51, pBonf. < 0.001). Compared to individuals without educational or professional qualification, participants with any qualification had higher odds of being healthy in Model 1, after adjustment for age and sex (Model 2) and, except for A levels or equivalent education level, in Model 3 that included all sociodemographic characteristics. However, there was only limited evidence of associations between participants’ qualifications and health in Model 4 (Table 1).
Table 1.
Sociodemographic characteristics and psychosocial factors associated with health status
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Term | OR | Bonferroni-corrected CI | OR | Bonferroni-corrected CI | OR | Bonferroni-corrected CI | OR | Bonferroni-corrected CI | ||||
| Sociodemographic characteristics | ||||||||||||
| Household income1 | ||||||||||||
| Very low | 0.5161 | 0.4978 | 0.5351 | 0.6298 | 0.6066 | 0.6538 | 0.6825 | 0.6558 | 0.7102 | 0.7447 | 0.7148 | 0.7759 |
| Low | 0.7548 | 0.7288 | 0.7818 | 0.8728 | 0.8419 | 0.9048 | 0.8963 | 0.8643 | 0.9295 | 0.9196 | 0.8864 | 0.9541 |
| Middle | Ref | – | – | Ref | – | – | Ref | – | – | Ref | – | – |
| High | 1.2084 | 1.1626 | 1.2561 | 1.1016 | 1.0591 | 1.1458 | 1.0774 | 1.0354 | 1.1212 | 1.0510 | 1.0096 | 1.0941 |
| Very high | 1.2867 | 1.2088 | 1.3703 | 1.1483 | 1.0778 | 1.2239 | 1.1151 | 1.0457 | 1.1897 | 1.0472 | 0.9812 | 1.1181 |
| Sex | ||||||||||||
| Female | Ref | – | – | Ref | – | – | Ref | – | – | Ref | – | – |
| Male | 0.8642 | 0.8428 | 0.8862 | NA | NA | NA | 0.8810 | 0.8584 | 0.9043 | 0.8847 | 0.8610 | 0.9090 |
| Age | 0.9462 | 0.9446 | 0.9478 | NA | NA | NA | 0.9527 | 0.9510 | 0.9545 | 0.9528 | 0.9510 | 0.9546 |
| Multiple deprivation | 0.9912 | 0.9903 | 0.9921 | 0.9885 | 0.9876 | 0.9894 | 0.9923 | 0.9913 | 0.9933 | 0.9952 | 0.9941 | 0.9963 |
| Ethnicity | ||||||||||||
| White | Ref | – | – | Ref | – | – | Ref | – | – | Ref | – | – |
| Mixed-race | 1.2991 | 1.0925 | 1.5524 | 0.9743 | 0.8163 | 1.1684 | 1.0443 | 0.8742 | 1.2533 | 1.1037 | 0.9221 | 1.3273 |
| Asian | 1.0196 | 0.9213 | 1.1298 | 0.8560 | 0.7714 | 0.9511 | 0.9252 | 0.8333 | 1.0285 | 1.0188 | 0.9149 | 1.1359 |
| Black | 1.2994 | 1.1612 | 1.4571 | 0.9831 | 0.8764 | 1.1050 | 1.1929 | 1.0619 | 1.3426 | 1.3125 | 1.1657 | 1.4805 |
| Chinese | 2.1528 | 1.6132 | 2.9286 | 1.7413 | 1.2994 | 2.3777 | 1.7988 | 1.3413 | 2.4580 | 1.8339 | 1.3646 | 2.5106 |
| Other | 1.2077 | 1.0369 | 1.4115 | 0.9952 | 0.8519 | 1.1667 | 1.1096 | 0.9491 | 1.3018 | 1.2570 | 1.0727 | 1.4779 |
| Highest qualification | ||||||||||||
| None | Ref | – | – | Ref | – | – | Ref | – | – | Ref | – | – |
| O levels/GCSEs/CSEs | 1.6519 | 1.5859 | 1.7207 | 1.2266 | 1.1757 | 1.2796 | 1.0692 | 1.0236 | 1.1168 | 1.0315 | 0.9870 | 1.0779 |
| A levels/NVQ/HND/HNC2 | 1.5125 | 1.4508 | 1.5769 | 1.2102 | 1.1595 | 1.2631 | 1.0270 | 0.9825 | 1.0735 | 0.9879 | 0.9445 | 1.0332 |
| Degree | 1.7949 | 1.7257 | 1.8667 | 1.3373 | 1.2838 | 1.3929 | 1.0469 | 1.0018 | 1.0940 | 0.9558 | 0.9137 | 0.9997 |
| Psychosocial factors | ||||||||||||
| Loneliness | ||||||||||||
| Not lonely | Ref | – | – | Ref | – | – | Ref | – | – | Ref | – | – |
| Lonely | 0.7283 | 0.6915 | 0.7672 | 0.6947 | 0.6588 | 0.7326 | 0.7207 | 0.6832 | 0.7604 | 0.8129 | 0.7697 | 0.8587 |
| Social isolation | ||||||||||||
| Not isolated | Ref | – | – | Ref | – | – | Ref | – | – | Ref | – | – |
| Isolated | 0.7544 | 0.7226 | 0.7877 | 0.7542 | 0.7218 | 0.7882 | 0.7795 | 0.7457 | 0.8149 | 0.9496 | 0.9067 | 0.9947 |
Note: Bonferroni-adjusted (~ 99.9%) confidence intervals. OR odds ratio, CI confidence interval, GCSEs general certificate of secondary education, CSE certificate of secondary education, NVQ national vocational qualification, HND higher national diploma, HNC higher national certificate, NA not applicable. 1Annual household income groups: very low (< £18,000), low (£18,000–£30,999), middle (£31,000–£51,999), high (£52,000–£100,000) and very high (> £100,000). 2also includes “other professional qualifications”
Model 1—only individual explanatory variables
Model 2—adjusted for age and sex
Model 3—age, sex and all sociodemographic characteristics / age, sex and all psychosocial factors
Model 4—all explanatory variables
Loneliness was associated with lower odds of favourable health status (OR = 0.81, 99.9% CI 0.77–0.86, pBonf. < 0.001). Socially isolated individuals also had lower odds of being healthy, but the strength of association was weaker than for loneliness in Model 4 (OR = 0.95, 99.9% CI 0.91–0.99, pBonf. = 0.01) (Table 1).
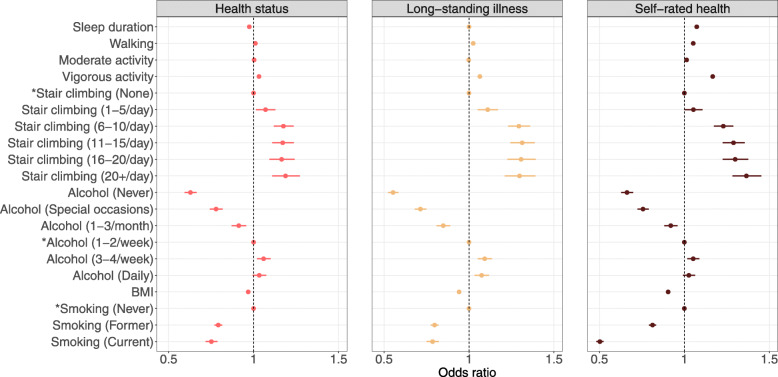
Longer sleep duration was associated with lower odds of favourable health status (OR = 0.97, 99.9% CI 0.96–0.99, pBonf. < 0.001). Walking frequently and engaging in frequent vigorous physical activity was associated with higher odds of being healthy across most analyses (OR = 1.010, 99.9% CI 1.003–1.018, pBonf. < 0.001 and OR = 1.03, 99.9% CI 1.02–1.04, pBonf. < 0.001, respectively). Although moderate physical activity was associated with higher odds of being healthy in Models 1 and 2, we did not find evidence of an association in Models 3 and 4 (OR = 1.003, 99.9% CI 0.996–1.010, pBonf > 0.99, Model 4). Frequent daily stair climbing was associated with higher odds of being healthy (ranging from OR = 1.07, 99.9% CI 1.01–1.13, pBonf. = 0.002 to OR = 1.19, 99.9% CI 1.11–1.27, pBonf. < 0.001 [1–5 times/day and > 20 times/day vs none, respectively]). We found some evidence that frequent alcohol intake was associated with higher odds of being healthy (e.g. OR = 1.06, 99.9% CI 1.02–1.10, pBonf. < 0.001 [3–4 vs 1–2 times/week]), although for daily/almost daily alcohol drinking only in Models 2 and 3 (OR = 1.06, 99.9% CI 1.02–1.10, pBonf. < 0.001, Model 2), while infrequent alcohol intake was associated with lower odds of being healthy (ranging from OR = 0.91, 99.9% CI 0.87–0.96, pBonf. < 0.001 to OR = 0.63, 99.9% CI 0.59–0.66, pBonf. < 0.001 [1–3 times/month and never vs 1–2 times/week, respectively]). Higher BMI was associated with lower odds of being healthy (OR = 0.968, 99.9% CI 0.966–0.971, pBonf. < 0.001). Past and current tobacco smoking was associated with lower odds of being healthy (OR = 0.79, 99.9% CI 0.77–0.82, pBonf. < 0.001 and OR = 0.75, 99.9% CI 0.72–0.79, pBonf. < 0.001, respectively) (Table 2).
Table 2.
Lifestyle factors associated with health status
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Term | OR | Bonferroni-corrected CI | OR | Bonferroni-corrected CI | OR | Bonferroni-corrected CI | OR | Bonferroni-corrected CI | ||||
| Sleep duration (hours/day) | 0.9574 | 0.9460 | 0.9688 | 0.9817 | 0.9699 | 0.9937 | 0.9743 | 0.9626 | 0.9862 | 0.9748 | 0.9631 | 0.9868 |
| Physical activity (days/week)1 | ||||||||||||
| Walking | 1.0099 | 1.0034 | 1.0164 | 1.0258 | 1.0190 | 1.0326 | 1.0059 | 0.9987 | 1.0131 | 1.0105 | 1.0032 | 1.0178 |
| Moderate activity | 1.0095 | 1.0040 | 1.0149 | 1.0253 | 1.0197 | 1.0310 | 0.9995 | 0.9928 | 1.0062 | 1.0032 | 0.9964 | 1.0100 |
| Vigorous activity | 1.0571 | 1.0502 | 1.0642 | 1.0502 | 1.0431 | 1.0573 | 1.0347 | 1.0267 | 1.0429 | 1.0319 | 1.0238 | 1.0400 |
| Stair climbing frequency | ||||||||||||
| None | Ref | – | – | Ref | – | – | Ref | – | – | Ref | – | – |
| 1–5/day | 1.3009 | 1.2362 | 1.3688 | 1.0980 | 1.0422 | 1.1568 | 1.1045 | 1.0477 | 1.1643 | 1.0704 | 1.0148 | 1.1289 |
| 6–10/day | 1.5529 | 1.4813 | 1.6278 | 1.3354 | 1.2725 | 1.4013 | 1.2467 | 1.1872 | 1.3091 | 1.1756 | 1.1188 | 1.2352 |
| 11–15/day | 1.6271 | 1.5457 | 1.7128 | 1.3923 | 1.3211 | 1.4672 | 1.2503 | 1.1855 | 1.3186 | 1.1714 | 1.1098 | 1.2363 |
| 16–20/day | 1.6494 | 1.5530 | 1.7520 | 1.4123 | 1.3280 | 1.5020 | 1.2433 | 1.1681 | 1.3235 | 1.1650 | 1.0937 | 1.2410 |
| 20+/day | 1.7494 | 1.6390 | 1.8675 | 1.4402 | 1.3474 | 1.5396 | 1.2632 | 1.1806 | 1.3518 | 1.1881 | 1.1097 | 1.2723 |
| Alcohol intake frequency | ||||||||||||
| Never | 0.5651 | 0.5364 | 0.5954 | 0.5849 | 0.5545 | 0.6171 | 0.5944 | 0.5632 | 0.6275 | 0.6285 | 0.5946 | 0.6644 |
| Special occasions | 0.7015 | 0.6705 | 0.7341 | 0.7109 | 0.6785 | 0.7448 | 0.7450 | 0.7107 | 0.7809 | 0.7792 | 0.7429 | 0.8174 |
| 1–3/month | 0.9082 | 0.8678 | 0.9505 | 0.8730 | 0.8333 | 0.9146 | 0.8947 | 0.8537 | 0.9378 | 0.9129 | 0.8708 | 0.9570 |
| 1–2/week | Ref | – | – | Ref | – | – | Ref | – | – | Ref | – | – |
| 3–4/week | 1.0652 | 1.0271 | 1.1047 | 1.1059 | 1.0656 | 1.1478 | 1.0882 | 1.0481 | 1.1298 | 1.0590 | 1.0198 | 1.0998 |
| Daily/almost daily | 0.9293 | 0.8957 | 0.9643 | 1.0627 | 1.0232 | 1.1037 | 1.0744 | 1.0339 | 1.1166 | 1.0334 | 0.9939 | 1.0745 |
| BMI (kg/m2) | 0.9561 | 0.9536 | 0.9587 | 0.9580 | 0.9554 | 0.9606 | 0.9659 | 0.9632 | 0.9687 | 0.9683 | 0.9656 | 0.9711 |
| Smoking status | ||||||||||||
| Never | Ref | – | – | Ref | – | – | Ref | – | – | Ref | – | – |
| Former | 0.6977 | 0.6792 | 0.7168 | 0.7879 | 0.7663 | 0.8100 | 0.7765 | 0.7548 | 0.7989 | 0.7922 | 0.7698 | 0.8152 |
| Current | 0.7309 | 0.7003 | 0.7629 | 0.6850 | 0.6556 | 0.7158 | 0.6882 | 0.6583 | 0.7196 | 0.7518 | 0.7183 | 0.7869 |
Note: Bonferroni-adjusted (~ 99.9%) confidence intervals. OR odds ratio, CI confidence interval, BMI body mass index. 1number of days per week engaging in these activities for 10+ min continuously
Model 1—only individual explanatory variables
Model 2—adjusted for age and sex
Model 3—age, sex and all lifestyle factors
Model 4—all explanatory variables
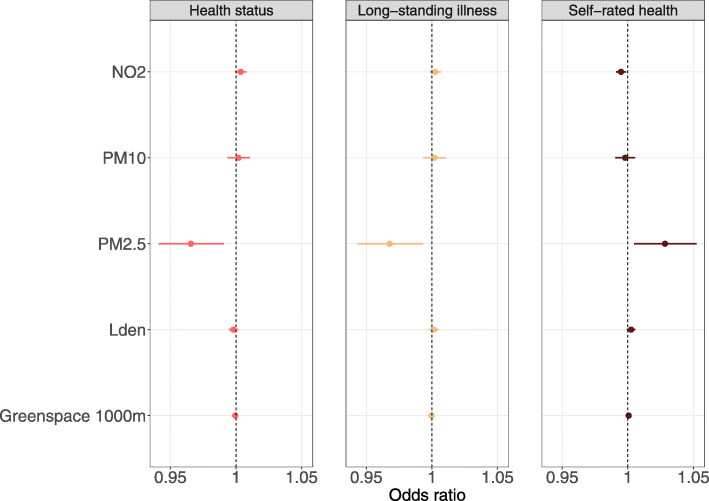
In our analyses of environmental exposures, higher PM2.5 concentration was associated with lower odds of favourable health status (OR = 0.97, 99.9% CI 0.94–0.99, pBonf. < 0.001). PM10 was also associated with lower odds of being healthy in Models 1 and 2, but there was no evidence of an association between PM10 and health status in Models 3 and 4 (OR = 1.00, 99.9% CI 0.99–1.01, pBonf. > 0.99, Model 4). NO2 was associated with lower odds of being healthy in Models 1 and 2. However, in Models 3 and 4, there was no evidence that NO2 was associated with health status after Bonferroni correction (OR = 1.004, 99.9% CI 0.999–1.008, pBonf. = 0.27, Model 4). Ambient sound level was associated with lower odds of being healthy in Models 1 and 2, but there was no evidence of an association with health status in Models 3 and 4 (OR = 0.998, 99.9% CI 0.995–1.002, pBonf. > 0.99, Model 4). Finally, we did not find consistent evidence of an association between percentage greenspace within a 1000 m circular distance buffer and health status (Table 3).
Table 3.
Environmental exposures associated with health status
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Term | OR | Bonferroni-corrected CI | OR | Bonferroni-corrected CI | OR | Bonferroni-corrected CI | OR | Bonferroni-corrected CI | ||||
| PM2.5 | 0.9640 | 0.9525 | 0.9757 | 0.9357 | 0.9243 | 0.9473 | 0.9159 | 0.8933 | 0.9392 | 0.9656 | 0.9411 | 0.9908 |
| PM10 | 0.9917 | 0.9851 | 0.9983 | 0.9842 | 0.9775 | 0.9909 | 1.0048 | 0.9965 | 1.0132 | 1.0019 | 0.9935 | 1.0104 |
| NO2 | 0.9972 | 0.9955 | 0.9988 | 0.9929 | 0.9912 | 0.9945 | 1.0000 | 0.9959 | 1.0041 | 1.0036 | 0.9993 | 1.0078 |
| Lden | 0.9967 | 0.9938 | 0.9996 | 0.9945 | 0.9916 | 0.9975 | 1.0002 | 0.9967 | 1.0038 | 0.9981 | 0.9945 | 1.0017 |
| Greenspace 1000 m | 1.0001 | 0.9995 | 1.0006 | 1.0013 | 1.0007 | 1.0019 | 0.9988 | 0.9978 | 0.9997 | 0.9995 | 0.9985 | 1.0004 |
Note: Bonferroni-adjusted (~ 99.9%) confidence intervals. OR odds ratio, CI confidence interval, PM particulate matter, NO2 nitrogen dioxide, Lden day-evening-night noise level
Model 1—only individual explanatory variables
Model 2—adjusted for age and sex
Model 3—age, sex and all environmental exposures
Model 4—all explanatory variables
Full results for long-standing illness and self-rated health are presented in Additional file 1: Tables S10–S21. Model 4 results across health indicators are presented in Figs. 1, 2 and 3.
Fig. 1.
Sociodemographic characteristics and psychosocial factors associated with health indicators. Confidence interval plot (odds ratio ± Bonferroni-adjusted (~ 99.9%) confidence intervals) for Model 4 (i.e. including all explanatory variables). GCSEs = general certificate of secondary education. *Indicates reference group for categorical explanatory variables. For categorical explanatory variables the odds ratios for self-rated health indicate the changes in odds of reporting better self-rated health associated with the explanatory variable group relative to the reference group. Odds ratios for continuous explanatory variables for self-rated health indicate proportional odds ratios for a 1-unit increase in the explanatory variable on level of self-rated health. Annual household income groups: very low (< £18,000), low (£18,000–£30,999), middle (£31,000–£51,999), high (£52,000–£100,000) and very high (> £100 000). “GCSEs” also includes O levels and certificate of secondary education (CSE). “A levels” also includes national vocational qualification (NVQ), higher national diploma (HND), higher national certificate (HNC) and “other professional qualifications”
Fig. 2.
Lifestyle factors associated with health indicators. Confidence interval plot (odds ratio ± Bonferroni-adjusted (~ 99.9%) confidence intervals) for Model 4 (i.e. including all explanatory variables). BMI = body mass index. *Indicates reference group for categorical explanatory variables. For categorical explanatory variables the odds ratios for self-rated health indicate the changes in odds of reporting better self-rated health associated with the explanatory variable group relative to the reference group. Odds ratios for continuous explanatory variables for self-rated health indicate proportional odds ratios for a 1-unit increase in the explanatory variable on level of self-rated health
Fig. 3.
Environmental exposures associated with health indicators. Confidence interval plot (odds ratio ± Bonferroni-adjusted (~ 99.9%) confidence intervals) for Model 4 (i.e. including all explanatory variables). PM = particulate matter; NO2 = nitrogen dioxide; Lden = day-evening-night noise level. Odds ratios for self-rated health indicate proportional odds ratios for a 1-unit increase in the explanatory variable on level of self-rated health
Findings regarding income, sex and neighbourhood deprivation were mostly consistent across health indicators, although with some variation in the magnitude of associations. Compared to Whites, participants of Chinese ethnicity had lower odds of favourable self-rated health (OR = 0.68, 99.9% CI 0.54–0.85, pBonf. < 0.001) but had higher odds of being classified healthy (OR = 1.83, 99.9% CI 1.36–2.51, pBonf. < 0.001) and being free from long-standing illness (OR = 1.62, 99.9% CI 1.22–2.18, pBonf. < 0.001). Individuals of non-White ethnic backgrounds tended to rate their health less favourable, but there was some evidence that they had higher odds of being free from long-standing illness, especially in the full multivariable model. Individuals with any qualification had higher odds of rating their health more favourable (e.g. OR = 1.50, 99.9% CI 1.44–1.57, pBonf. < 0.001 [university/college degree vs no qualification]), while results were less consistent for health status and long-standing illness, especially in Models 3 and 4. Finally, older individuals tended to rate their health more favourable (OR = 1.011, 99.9% CI 1.010–1.013, pBonf. < 0.001).
Findings regarding psychosocial factors were consistent across health indicators, but the association was strongest for loneliness and self-rated health (OR = 0.49, 99.9% CI 0.47–0.52, pBonf. < 0.001).
Findings regarding lifestyle factors were consistent across health indicators, except that there were some inconsistencies in the associations between daily/almost daily alcohol intake and health. More frequent moderate physical activity was associated with better self-rated health also in Model 4 (OR = 1.01, 99.9% CI 1.01–1.02, pBonf. < 0.001). Longer sleep duration was associated with better self-rated health (OR = 1.07, 99.9% CI 1.06–1.09, pBonf. < 0.001), but there was little evidence of an association with having a long-standing illness.
Higher levels of PM2.5 were also associated with lower odds of being free from long-standing illness (OR = 0.97, 99.9% CI 0.94–0.99, pBonf. = 0.002). While we found similar associations with self-rated health in Models 1–3, PM2.5 was associated with better self-rated health in Model 4 (OR = 1.028, 99.9% CI 1.005–1.052, pBonf. = 0.004). There was no evidence of an association between PM10 and any health indicator in Models 3 and 4, although PM10 was associated with poor health across outcomes in Models 1 and 2. Higher NO2 concentration was associated with less favourable self-rated health (OR = 0.995, 99.9% CI 0.991–0.999, pBonf. < 0.001), while there was no evidence of an association with health status and having a long-standing illness after adjusting for covariates in Models 3 and 4. Higher Lden was associated with poor health in Models 1 and 2, but we found no evidence of an association in Model 4. For long-standing illness and self-rated health, there was some evidence that higher Lden was associated with better health in Model 3. Percentage greenspace was associated with better self-rated health and higher odds of being free from long-standing illness in Models 1 and 2. Results from Model 3 suggested that greenspace was associated with less favourable health across all health indicators, but we found no evidence of any associations in Model 4 after Bonferroni correction.
Magnitude of associations
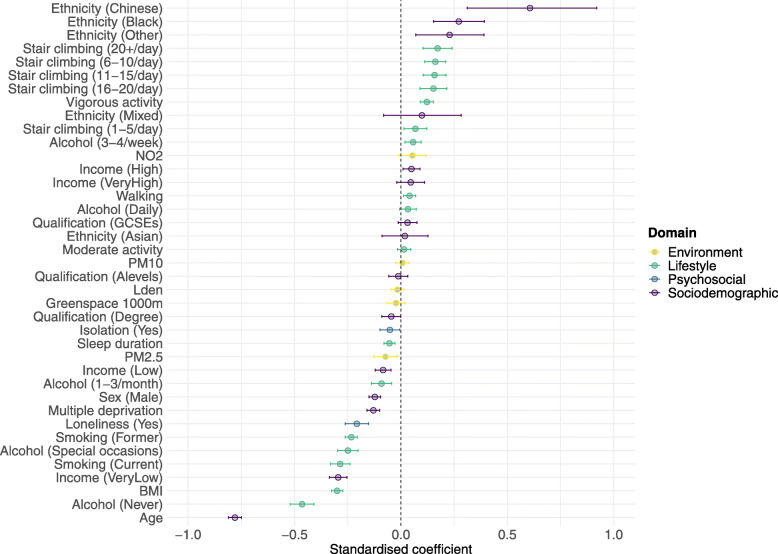
Standardised regression coefficients for health status are presented in Fig. 4. Most notably, the magnitude of association between BMI (β = −0.30, 99.9% CI −0.33 to −0.27) or being on a very low income (β = −0.29, 99.9% CI −0.34 to −0.25) and health status were comparable to that of current smoking (β = −0.29, 99.9% CI −0.33 to −0.24). There was also a substantial difference in the magnitude of association with health status between the very low and low income groups (β = −0.29, 99.9% CI −0.34 to −0.25, and β = −0.08, 99.9% CI −0.12 to −0.05, respectively). The magnitude of association between loneliness and health status was substantially larger than that of social isolation (β = −0.21, 99.9% CI −0.26 to −0.15, and β = −0.05, 99.9% CI −0.10 to −0.01, respectively). Finally, associations between the environmental exposures and health status were relatively small compared to those observed for the sociodemographic, psychosocial and lifestyle factors (Additional file 1: Tables S22).
Fig. 4.
Confidence interval plot for health status. Standardised β estimates and Bonferroni-adjusted (~ 99.9%) confidence intervals. Model 4 regression coefficients were rescaled to have a mean equal to zero and, for numeric variables with more than two values, divided by two standard deviations
Associations with long-standing illness and self-rated health compared to health status were stronger for household income, sex, neighbourhood deprivation, loneliness, social isolation, walking frequency, vigorous physical activity, BMI and stair climbing frequency (Additional file 1: Tables S23 and S24, Fig. S5 and S6).
Stratified and interaction analyses
Descriptive statistics of the analytical sample stratified by sex and age group are presented in Additional file 1: Tables S25 and S26. Findings presented below correspond to results for which the stratified and interaction analyses provided consistent conclusions. Full results and effect sizes are presented in Additional file 1: Tables S27–S50, Fig. S7–S24.
The odds of being healthy were lower for men than for women in the lowest income group (pBonf.(interaction) < 0.001). The association between increased age and lower odds of being healthy was stronger in men (pBonf.(interaction) < 0.001). Asian women had higher odds of being healthy (pBH(interaction) = 0.007). The association between neighbourhood deprivation and lower odds of being healthy was stronger in men (pBonf.(interaction) < 0.001). Men who were lonely had lower odds of being healthy (pBonf.(interaction) = 0.008) and social isolation was associated with lower odds of being healthy only in men (pBH(interaction) = 0.006). Longer sleep duration was associated with lower odds of being healthy only in men (pBonf.(interaction) < 0.001). Reporting frequent alcohol intake was associated with higher odds of being healthy only in men (pBH(interaction) = 0.023 for 3–4 times/week and pBH(interaction) = 0.039 for daily/almost daily). The association between higher BMI and lower odds of being healthy was stronger in men (pBonf.(interaction) < 0.001). The association between former smoking and health status was stronger in men (pBonf.(interaction) < 0.001), while the association between current smoking and health status was stronger in women (pBH(interaction) = 0.005). PM2.5 was associated with lower odds of being healthy only in men (pBH(interaction) = 0.011). Finally, we found that NO2 was associated with higher odds of favourable health status only in women (pBH(interaction) = 0.024) (Additional file 1: Tables S27–S30, Fig. S7–S9).
The association between being on a very low income and lower odds of being healthy was stronger in participants below the age of 65 than in participants aged 65 and above (pBonf.(interaction) < 0.001). Reporting an income of £52,000–£100,000 was associated with higher odds of being healthy only in participants younger than 65 (pBH(interaction) = 0.016). Men aged 65 and above had lower odds of being healthy (pBonf.(interaction) < 0.001). Loneliness and social isolation were associated with lower odds of being healthy only in individuals younger than 65 (pBH(interaction) = 0.024 and pBonf.(interaction) = 0.011, respectively). The association between walking frequency and higher odds of being healthy was stronger in individuals aged 65 and above (pBonf.(interaction) < 0.001). Climbing stairs 1–5 times/day was associated with favourable health status only in individuals younger than 65 (pBonf.(interaction) < 0.001). There was some evidence that the association between never drinking alcohol and poor health status was stronger in individuals younger than 65 (pBonf.(interaction) = 0.002). The association between higher BMI and lower odds of being healthy was stronger in individuals aged 65 and above (pBH(interaction) < 0.001). Finally, the association between current smoking and poor health status was stronger in individuals younger than 65 (pBH(interaction) = 0.038) (Additional file 1: Tables S31–S34, Fig. S10–S12).
Although there were some differences, stratified and interaction analyses of long-standing illness and self-rated health indicated a large degree of consistency with the results observed for health status (Additional file 1: Tables S35–S50, Fig. S13–S24).
Longitudinal analyses
We repeated our analyses of self-rated health in participants with follow-up data collected between 2012 and 2013 (n = 16,058; mean follow-up = 4.26 years, SD = 0.87) and between 2014 and 2019 (n = 32,617; mean follow-up = 8.57 years, SD = 1.64). Descriptive statistics and full results are presented in Additional file 1: Tables S51–S59. The findings were fully consistent with cross-sectional analyses for very low, high and very high levels of income, sex, loneliness, sleep duration, walking frequency, vigorous physical activity, infrequent alcohol intake, BMI and smoking status. Although many results were consistent across timepoints, we found some differences between cross-sectional and longitudinal analyses for low income, age, neighbourhood deprivation, ethnicity, highest qualification, social isolation, moderate physical activity, stair climbing frequency, regular alcohol intake and the environmental exposures.
Additional analyses
Additional descriptive statistics are presented in Additional file 1: Table S60.
Repeating the main analysis with the Townsend deprivation index as measure of neighbourhood deprivation that does not include a health dimension led to similar conclusions as for the Index of Multiple Deprivation for England (OR = 0.974, 99.9% CI 0.969–0.980, pBonf. < 0.001). We also found that higher scores on each individual component of the Index of Multiple Deprivation for England, except for the housing dimension, were associated with lower odds of favourable health status (results not shown).
For sleep duration and BMI, we found evidence of non-linearity in the association with health status (Additional file 1: Fig. S25). Hence, we also examined these explanatory variables as categorical variables. Compared to individuals with optimal sleep duration (7–8 h/day), those who slept < 7 or ≥ 9 h/day had lower odds of being healthy (OR = 0.90, 99.9% CI 0.87–0.92, pBonf. < 0.001 and OR = 0.70, 99.9% CI 0.67–0.74, pBonf. < 0.001, respectively). We also found that low (< 18.5 kg/m2) and high (> 24.9 kg/m2) BMI was associated with lower odds of being healthy, compared to a BMI of 18.5–24.9 kg/m2 (OR = 0.70, 99.9% CI 0.58–0.85, pBonf. < 0.001 and OR = 0.85, 99.9% CI 0.83–0.88, pBonf. < 0.001, respectively). There was no evidence of substantial departure from a linear association with health status for all other continuous variables (Additional file 1: Fig. S25). Repeating the main analysis with body fat percentage as measure of body composition led to similar conclusions as for BMI: higher fat percentage was associated with lower odds of being healthy (n = 307,202; OR = 0.978, 99.9% CI 0.975–0.980, pBonf. < 0.001). Excluding individuals who reported that they had stopped drinking alcohol due to illness or their doctor’s advice (n = 2790) attenuated the association between never drinking alcohol and health status (n = 304,588; OR = 0.74, 99.9% CI 0.70–0.79, pBonf. < 0.001). When sub-dividing individuals who reported that they never drink alcohol into current and lifetime abstainers, the association with health status was stronger in current abstainers (n = 307,346; OR = 0.53, 99.9% CI 0.49–0.57, pBonf. < 0.001 and OR = 0.75, 99.9% CI 0.70–0.81, pBonf. < 0.001, respectively). Restricting analyses to current smoking, we found that regular smoking and smoking only occasionally was associated with lower odds of being healthy, although the magnitude of association was stronger for regular smoking (n = 307,372; OR = 0.81, 99.9% CI 0.77–0.85, pBonf. < 0.001 and OR = 0.90, 99.9% CI 0.83–0.98, pBonf. = 0.002, respectively). Examining MET minutes instead of the number of days per week spent walking, engaging in moderate or vigorous physical activity led to similar results (n = 268,674; data not shown).
When we examined air pollution and noise exposure estimates dichotomised according to WHO recommendation thresholds, we found no evidence that being exposed to ≤ 10 μg/m3 PM2.5 on average annually was associated with higher odds of being healthy after Bonferroni correction (OR = 1.029, 99.9% CI 0.997–1.062, pBonf. = 0.15). There was also no evidence of an association with health status for exposures to ≤ 20 μg/m3 PM10, ≤ 40 μg/m3 NO2 or ≤ 53 decibels Lden in Model 4. We also did not find evidence of a threshold in the association between PM10 or NO2 and health status when including quintiles of these exposures in Model 4 (data not shown). Restricting analyses to participants assessed after 2008 (regarding noise estimates; n = 182,342) or 2009 (regarding air pollution estimates; n = 60,521) or to those who had lived at their current address for at least 10 years (n = 209,239) did not lead to different conclusions regarding the associations between environmental exposures and health status. There was no evidence that percentage greenspace within a 300-m circular distance buffer was associated with health status after Bonferroni correction (n = 307,378; OR = 0.9993, 99.9% CI 0.9985–1.0001, pBonf. = 0.29). Truncating continuous explanatory variables at the 1st and 99th percentile did not materially change our results (n = 278,065; data not shown).
Discussion
Increased age was strongly associated with unfavourable health status and having a long-standing illness. However, older participants generally rated their health positively, which is consistent with several smaller studies [40, 41], though not all [42, 43]. Ageing might be the single most important factor underlying disease [44], with an almost universally accepted expectation of declining health as people get older. As attainable health states shift with age [45], older participants might evaluate their health more favourable, despite higher rates of illness and disability.
Although women, on average, report more illnesses, disabilities and limitations in daily life [46–48], one of the most robust findings in human biology is that they live longer than men [49]. Our findings are consistent with results from the Newcastle 85+ cohort study in which women rated their health more favourably than men [46]. However, other studies reported that women rated their health less favourably than men [50–52], or did not find evidence of sex differences in self-rated health [53, 54]. Sex differences in health status could result from differences in the frequency of specific illnesses, sex-specific reporting patterns, or biological and social factors [52]. Discrepancies in findings between studies might reflect differences in age group or socio-cultural factors.
High income and low levels of neighbourhood deprivation were associated with better health, which is broadly consistent with previous studies [55–57]. Notably, we found only a small difference in the strength of association with health status and having a long-standing illness between the high-income groups. The difference between the low-income groups, however, was substantial, supporting previous findings, which suggested a non-linear association between family income and mortality [58]. For self-rated health, we also found evidence of substantial differences between the high-income groups. A possible explanation for the observation that there was less evidence of associations between household income and health in individuals aged 65 and over than in the younger age group is that more individuals in this age group received a pension income. Future studies could examine associations between health and related socioeconomic variables such as family income or household income per capita.
Our study provides limited evidence that education was independently associated with favourable health status after accounting for other factors, although higher levels of qualification remained associated with better self-rated health, consistent with previous research [42]. A recent UK Biobank analysis showed that remaining longer in school causally reduced participants’ risk of diabetes and mortality [59]. A potential explanation for why we did not find a consistent pattern in the full model is that most differences in health status result from educated individuals engaging in healthier lifestyle behaviours [60] that we had accounted for, or they could be due to socioeconomic or genomic factors.
Social isolation and loneliness were associated with poor health. The strength of association was greater for loneliness, particularly in men and in individuals below the age of 65. Social isolation and loneliness were not always correlated [61, 62] and represent different aspects of social relations (scarcity of contact with others and discrepancies between the need for, and the fulfilment of, social interaction, respectively). In a meta-analysis of 70 studies, social isolation, loneliness and living alone were associated with a 26–32% increased mortality risk [16]. There was no evidence of differences in mortality between these measures, although the strength of association was greater in individuals below the age of 65, consistent with our findings. A recent UK Biobank analysis found that socially isolated and lonely individuals had an increased risk of death, but only social isolation predicted all-cause mortality in a joint model [22]. The discrepancy with our finding (loneliness was more strongly associated with poor health) might reflect differences in outcome measures (general health in the present study vs mortality in previous investigations).
Long sleep duration, high BMI and past and current smoking were associated with poor health. Sleeping less than 7 h/day was also associated with poor health, consistent with a meta-analysis that provided evidence of a U-shaped association between sleep duration and all-cause mortality [36]. A BMI outside the optimal range of 18.5–24.9 kg/m2 was also associated with poor health, consistent with previous research that examined all-cause mortality [60].
Physical activity is a key lifestyle factor recommended for primary and secondary prevention of chronic health conditions [63] and is associated with lower mortality risk [64]. In this study, walking frequency, especially in individuals aged 65 and above, stair climbing, and engaging in vigorous physical activity was associated with good health. Moderate physical activity was associated with better self-rated health, especially in men. A study in middle-aged British men found evidence of an association between vigorous, but not moderate, physical activity and reduced mortality [65]. Reviews of the literature report mixed findings on the relative contributions of moderate and vigorous physical activity [66, 67], with some evidence suggesting stronger associations for vigorous activity [68].
A more frequent drinking pattern was associated with better health in this study. Alcohol drinking often occurs in a social context and might therefore constitute a proxy for social wellness, supported by the finding that non-drinkers tend to be characterised by poor psychosocial health and low socioeconomic status [69]. Moderate drinkers also perform more sports than lifelong abstainers [70]. Drinking less frequent than 1–2 times/week was associated with poor health and could not be fully accounted for by excluding individuals who had discontinued alcohol intake for health reasons or because of their doctor’s advice. However, the association with health status was stronger for current abstainers, suggesting that some individuals are non-drinkers in later life due to illness [71]. Current abstainers who quit drinking for health reasons could exaggerate poor health outcomes associated with not drinking alcohol [72]. Those who never drink might also differ from current drinkers in other characteristics [69].
Road traffic noise and ambient air pollution are environmental risk factors for health. While we found some evidence that higher levels of all airborne pollutants were associated with poor health, only PM2.5 was associated with unfavourable health status and having a long-standing illness after adjustment for other factors. Higher levels of NO2 were associated with less favourable self-rated health. A joint analysis of UK Biobank, HUNT, and EPIC-Oxford data did not find evidence of an association between NO2 and incident cardiovascular disease, although both PM2.5 and PM10 were associated with higher incident cardiovascular disease [73]. Adjustment for physical activity and neighbourhood deprivation in the present study could have contributed to differences in findings between studies. Few individuals in our study were exposed to levels of PM10 and NO2 above recommended guidelines, which might explain why we did not find strong associations between these pollutants and health. The only exception was PM2.5, for which 45% of our sample were exposed to levels above the WHO recommendation threshold of ≤ 10 μg/m3. Positive associations between PM2.5 and self-rated health might represent spurious associations or statistical artefacts; we are not aware of any mechanisms linking higher levels of pollution to good health. While higher levels of residential noise were associated with poor health in Models 1 and 2, there was no evidence of an association after adjustment for other factors. Our findings are consistent with a previous analysis that did not find evidence of an independent association between Lden and cardiovascular disease, ischemic heart disease or cerebrovascular disease [73].
We found some evidence that greenspace was associated with better self-rated health, consistent with a recent meta-analysis [74], although not after adjustment for other factors. Several previous studies examining associations between greenspace and health did not adjust for physical activity and most did not include other environmental exposures such as air pollution or noise [74]. These differences could partially explain why we did not find consistent associations in this study.
Strengths and limitations
The large sample size enabled high precision in the estimation of associations, and it allowed us to explore a wide range of explanatory variables, sex and age-specific associations, interactions and additional analyses with further classification of explanatory variables. Many of the findings from previous studies that we replicated in our analyses were conducted in smaller populations and with one exposure at a time, often providing limited insight into the multifactorial nature of health. One of the contributions of this study is that it allows for systematic comparisons of a broad range of factors associated with health. We examined three health indicators with slightly different ascertainment. While our findings were fairly consistent across these outcomes, we also identified differences between objective and subjective health. Overall health is arguably what matters most to people, and exploring factors associated with health indicators that transcend traditional disease-boundaries could represent an effective strategy to increase longevity and improve healthy life expectancy. Previous studies have often used composite scores of healthy lifestyles [14] in which individual behaviours were weighted equally, even though the strength of their respective associations with health might differ. In the present study, we estimated associations separately for each lifestyle factor.
Nevertheless, our study has limitations. Cross-sectional analyses prevent examination of temporal sequences. Reverse causality such as poor health and disability leading to an unhealthy lifestyle, less favourable socioeconomic outcomes, fewer social interactions or living in more deprived and polluted areas needs to be considered, except for fixed characteristics such as sex and ethnicity. Associations reported here cannot be interpreted as causal effects and there are model-specific limitations that need to be considered when interpreting these results. Residual confounding needs to be considered when examining associations from Model 1 and Model 2, while associations from Model 4 might be over-adjusted and there is the possibility that some variables included in Model 4 could lie on the causal pathway of other variables. The intermediate Model 3 in which we examined only variables from each broad group of explanatory variables plus age and sex partially addresses this concern and provided mostly consistent results. Although we have focussed on associations that were consistent across Models 1–4 in the main body of the text and highlighted inconsistencies, there was a large degree of consistency in direction of association across models and health indicators. As noted above, the notable exception to this pattern was education, for which we did not find consistent associations with favourable health in the full multivariable model. This is likely due to other explanatory variables (e.g. lifestyle) being on the causal pathway from education, which is determined fairly early in life, to health in middle to old age. Indeed, a potential limitation of using a standard set of covariates across models is that this approach does not fully consider the specific role each covariate may have for a given exposure-outcome pair.
As many explanatory variables were self-reported, we cannot exclude the possibility that social desirability might have influenced responses. For example, previous research has shown that up to half of participants who claim to never have had an alcoholic drink reported alcohol intake during previous surveys [75]. For most participants, explanatory variables were measured at a single time point, which could increase the potential for measurement error and did not allow us to model changes across the life course. However, there is evidence that lifestyle is fairly stable in this age group, with few people newly adopting a healthy lifestyle in middle age [76]. Future research should explore predictors of changes in health status over time using longitudinal data, for example using linked patient records. We did not include a measure of diet quality or quantity in our analyses, despite it being a key health-related behaviour, as detailed information on diet at the baseline assessment was only available for about 13% of participants (prior to applying exclusion criteria) [77]; examining the frequency of specific food consumption available for most of the UK Biobank sample was beyond the scope of this study. There might be some misclassification in the reporting of medical illnesses. However, participants were asked to report illnesses that had been diagnosed by a doctor and these were confirmed during the nurse-led interview.
Generalisability
The overall response rate of the UK Biobank was low (5.5%) and compared with non-responders, participants were older, more likely to be female and lived in less deprived neighbourhoods. Participants were also less likely to be obese, to smoke, to never drink or to drink alcohol daily/almost daily and they reported fewer health conditions compared with data from a nationally representative survey [78]. While the UK Biobank states that “valid assessment of exposure-disease relationships are nonetheless widely generalizable and do not require participants to be representative of the population at large” [79], concordant with the finding that there is little evidence of considerable bias due to non-participation in epidemiological research [80], the magnitude of exposure-disease associations may depend on the prevalence of effect modifiers [81]. A recent empirical investigation comparing the UK Biobank with data from 18 prospective cohort studies with conventional response rates showed that the direction of risk factor associations were similar, although with differences in magnitude [82].
Conclusion
Our findings shed light on some of the behavioural, psychosocial and environmental pathways to health and are thus relevant to public health policies aimed at promoting health in later life. Public health and medicine could put greater focus on non-medical factors such as loneliness, further encourage healthy lifestyle behaviours and weight management and examine efforts to improve the health outcomes of individuals on a low income. Our findings support the view that promoting individual responsibility for one’s health (e.g. engaging in healthy lifestyle behaviours) and policy-level commitments (e.g. reducing long-term exposure to environmental pollutants [83] or improving neighbourhoods) both need to be considered in population health. While associations between lifestyle and health might reflect a lifelong commitment to healthy behaviours, it is not too late to newly adopt healthy behaviours later in life [76]. Prevention, in addition to drug discovery and disease treatment, should be the top priority for health policy.
Supplementary Information
Additional file 1: Table S1. Data fields. Figure S1. Study flowchart. Table S2. Baseline characteristics. Figures S2-S3. Self-reported illnesses. Figure S4. Correlation matrix of continuous explanatory variables. Tables S3-S5. Descriptive statistics stratified by health indicators. Tables S6-S9. Visual summary of findings. Tables S10-S13. Regression tables long-standing illness. Tables S14-S17. Regression tables self-rated health. Tables S18-S21. Regression tables health indicators. Tables S22-S24. Standardised regression coefficients tables. Figures S5-S6. Standardised regression coefficients plots. Table S25. Baseline characteristics stratified by sex. Table S26. Baseline characteristics stratified by age. Table S27-S30. Regression tables health status stratified by sex. Figures S7-S9. Confidence interval plots health status stratified by sex. Tables S31-S34. Regression tables health status stratified by age. Figures S10-S12. Confidence interval plots health status stratified by age. Tables S35-S38. Regression tables long-standing illness stratified by sex. Figures S13-S15. Confidence interval plots long-standing illness stratified by sex. Tables S39-S42. Regression tables long-standing illness stratified by age. Figures S16-S18. Confidence interval plots long-standing illness stratified by age. Tables S43-S46. Regression tables self-rated health stratified by sex. Figures S19-S21. Confidence interval plots self-rated health stratified by sex. Tables S47-S50. Regression tables self-rated health stratified by age. Figures S22-S24. Confidence interval plots self-rated health stratified by age. Table S51. Baseline characteristics longitudinal samples. Tables S52-S55. Regression tables self-rated health t1. Tables S56-S59. Regression tables self-rated health t2. Table S60. Descriptive statistics additional analyses. Figure S25. Generalised additive models.
Additional file 2. Health status classification.
Acknowledgements
This project made use of time on Rosalind HPC, funded by Guy’s & St Thomas’ Hospital NHS Trust Biomedical Research Centre (GSTT-BRC), South London & Maudsley NHS Trust Biomedical Research Centre (SLAM-BRC), and Faculty of Natural Mathematics & Science (NMS) at King’s College London.
Authors’ contributions
CML acquired the studentship funding. JM and CML conceived the idea of the study; JM acquired the data; JM carried out the statistical analysis; JM and CML interpreted the findings; JM wrote the manuscript. CJR and CML critically reviewed the manuscript and JM revised the manuscript for final submission. All authors read and approved the final manuscript. JM had full access to all the data used in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
JM receives studentship funding from the Biotechnology and Biological Sciences Research Council (BBSRC) (ref: 2050702) and Eli Lilly and Company Limited. CML is part-funded by the National Institute for Health Research (NIHR) Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Availability of data and materials
The data used in the present study are available to all bona fide researchers for health-related research that is in the public interest, subject to an application process and approval criteria. Study materials are publicly available online at http://www.ukbiobank.ac.uk.
Declarations
Ethics approval and consent to participate
Ethical approval for the UK Biobank study has been granted by the National Information Governance Board for Health and Social Care and the NHS North West Multicentre Research Ethics Committee (11/NW/0382). No project-specific ethical approval is needed. Data access permission has been granted under UK Biobank application 45514. Participants consented to use of their de-identified data.
Consent for publication
Not applicable.
Competing interests
JM receives studentship funding from the Biotechnology and Biological Sciences Research Council (BBSRC) and Eli Lilly and Company Limited. CML is a member of the Scientific Advisory Board of Myriad Neuroscience. CJR does not declare any potential conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature. 2018;561(7721):45–56. doi: 10.1038/s41586-018-0457-8. [DOI] [PubMed] [Google Scholar]
- 2.Roser M. Life expectancy. Our World in Data. 2013. https://ourworldindata.org/life-expectancy.
- 3.Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017;389(10076):1323–1335. doi: 10.1016/S0140-6736(16)32381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Netuveli G, Wiggins RD, Hildon Z, Montgomery SM, Blane D. Quality of life at older ages: evidence from the English Longitudinal Study of Aging (wave 1). J Epidemiol Community Health. 2006;60(4):357–63. 10.1136/jech.2005.040071. [DOI] [PMC free article] [PubMed]
- 5.Van Hecke O, Torrance N, Smith B. Chronic pain epidemiology and its clinical relevance. Brit J Anaesthesia 2013;111(1):13–8. 10.1093/bja/aet123. [DOI] [PubMed]
- 6.Brown GC. Living too long. EMBO Rep. 2015;16(2):137–141. doi: 10.15252/embr.201439518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welsh CE, Matthews FE, Jagger C. Trends in life expectancy and healthy life years at birth and age 65 in the UK, 2008–2016, and other countries of the EU28: An observational cross-sectional study. Lancet Regional Health-Eur. 2021;2:100023. doi: 10.1016/j.lanepe.2020.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nybo H, Gaist D, Jeune B, McGue M, Vaupel JW, Christensen K. Functional status and self-rated health in 2,262 nonagenarians: the Danish 1905 Cohort Survey. J Am Geriatr Soc. 2001;49(5):601–609. doi: 10.1046/j.1532-5415.2001.49121.x. [DOI] [PubMed] [Google Scholar]
- 9.Olshansky SJ. From Lifespan to Healthspan. JAMA. 2018;320(13):1323–1324. doi: 10.1001/jama.2018.12621. [DOI] [PubMed] [Google Scholar]
- 10.Carter BD, Abnet CC, Feskanich D, Freedman ND, Hartge P, Lewis CE, Ockene JK, Prentice RL, Speizer FE, Thun MJ, Jacobs EJ. Smoking and mortality—beyond established causes. N Engl J Med. 2015;372(7):631–640. doi: 10.1056/NEJMsa1407211. [DOI] [PubMed] [Google Scholar]
- 11.Lai HT, de Oliveira Otto MC, Lemaitre RN, McKnight B, Song X, King IB, et al. Serial circulating omega 3 polyunsaturated fatty acids and healthy ageing among older adults in the Cardiovascular Health Study: prospective cohort study. BMJ. 2018;363:k4067. 10.1136/bmj.k4067. [DOI] [PMC free article] [PubMed]
- 12.Room R, Babor T, Rehm J. Alcohol and public health. Lancet. 2005;365(9458):519–530. doi: 10.1016/S0140-6736(05)17870-2. [DOI] [PubMed] [Google Scholar]
- 13.Cooper R, Kuh D, Hardy R. Objectively measured physical capability levels and mortality: systematic review and metaanalysis. BMJ. 2010;341:c4467. 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed]
- 14.Loef M, Walach H. The combined effects of healthy lifestyle behaviors on all cause mortality: a systematic review and meta-analysis. Prev Med. 2012;55(3):163–170. doi: 10.1016/j.ypmed.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Mantzavinis GD, Pappas N, Dimoliatis ID, Ioannidis JP. Multivariate models of self-reported health often neglected essential candidate determinants and methodological issues. J Clin Epidemiol. 2005;58(5):436–443. doi: 10.1016/j.jclinepi.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspectives Psychol Sci. 2015;10(2):227–237. doi: 10.1177/1745691614568352. [DOI] [PubMed] [Google Scholar]
- 17.Smith RB, Fecht D, Gulliver J, Beevers SD, Dajnak D, Blangiardo M, et al. Impact of London's road traffic air and noise pollution on birth weight: retrospective population based cohort study. BMJ. 2017;359:j5299. 10.1136/bmj.j5299. [DOI] [PMC free article] [PubMed]
- 18.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O’Connell J, Cortes A, Welsh S, Young A, Effingham M, McVean G, Leslie S, Allen N, Donnelly P, Marchini J. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Littlejohns TJ, Holliday J, Gibson LM, Garratt S, Oesingmann N, Alfaro-Almagro F, et al. The UK Biobank imaging enhancement of 100,000 participants: rationale, data collection, management and future directions. Nat Commun. 2020;11:2624. 10.1038/s41467-020-15948-9. [DOI] [PMC free article] [PubMed]
- 20.Guggenheim JA, Williams C. Childhood febrile illness and the risk of myopia in UK Biobank participants. Eye. 2016;30(4):608–614. doi: 10.1038/eye.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Department for Communities and Local Government 2011. The English indices of deprivation 2010. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2010.
- 22.Elovainio M, Hakulinen C, Pulkki-Råback L, Virtanen M, Josefsson K, Jokela M, Vahtera J, Kivimäki M. Contribution of risk factors to excess mortality in isolated and lonely individuals: an analysis of data from the UK Biobank cohort study. Lancet Public Health. 2017;2(6):e260–e2e6. doi: 10.1016/S2468-2667(17)30075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.IPAQ-Group. IPAQ scoring protocol - International Physical Activity Questionnaire. https://sites.google.com/site/theipaq/scoring-protocol.
- 24.Beelen R, Hoek G, Vienneau D, Eeftens M, Dimakopoulou K, Pedeli X, Tsai MY, Künzli N, Schikowski T, Marcon A, Eriksen KT, Raaschou-Nielsen O, Stephanou E, Patelarou E, Lanki T, Yli-Tuomi T, Declercq C, Falq G, Stempfelet M, Birk M, Cyrys J, von Klot S, Nádor G, Varró MJ, Dėdelė A, Gražulevičienė R, Mölter A, Lindley S, Madsen C, Cesaroni G, Ranzi A, Badaloni C, Hoffmann B, Nonnemacher M, Krämer U, Kuhlbusch T, Cirach M, de Nazelle A, Nieuwenhuijsen M, Bellander T, Korek M, Olsson D, Strömgren M, Dons E, Jerrett M, Fischer P, Wang M, Brunekreef B, de Hoogh K. Development of NO2 and NOx land use regression models for estimating air pollution exposure in 36 study areas in Europe–The ESCAPE project. Atmos Environ. 2013;72:10–23. doi: 10.1016/j.atmosenv.2013.02.037. [DOI] [Google Scholar]
- 25.Eeftens M, Beelen R, de Hoogh K, Bellander T, Cesaroni G, Cirach M, Declercq C, Dėdelė A, Dons E, de Nazelle A, Dimakopoulou K, Eriksen K, Falq G, Fischer P, Galassi C, Gražulevičienė R, Heinrich J, Hoffmann B, Jerrett M, Keidel D, Korek M, Lanki T, Lindley S, Madsen C, Mölter A, Nádor G, Nieuwenhuijsen M, Nonnemacher M, Pedeli X, Raaschou-Nielsen O, Patelarou E, Quass U, Ranzi A, Schindler C, Stempfelet M, Stephanou E, Sugiri D, Tsai MY, Yli-Tuomi T, Varró MJ, Vienneau D, Klot S, Wolf K, Brunekreef B, Hoek G. Development of land use regression models for PM2. 5, PM2. 5 absorbance, PM10 and PMcoarse in 20 European study areas; results of the ESCAPE project. Environ Sci Technol. 2012;46(20):11195–11205. doi: 10.1021/es301948k. [DOI] [PubMed] [Google Scholar]
- 26.Kephalopoulos S, Paviotti M, Anfosso-Lédée F, Van Maercke D, Shilton S, Jones N. Advances in the development of common noise assessment methods in Europe: the CNOSSOS-EU framework for strategic environmental noise mapping. Sci Total Environ. 2014;482:400–10. 10.1016/j.scitotenv.2014.02.031. [DOI] [PubMed]
- 27.Morley D, De Hoogh K, Fecht D, Fabbri F, Bell M, Goodman P, et al. International scale implementation of the CNOSSOS-EU road traffic noise prediction model for epidemiological studies. Environ Pollut. 2015;206:332–341. doi: 10.1016/j.envpol.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 28.Vienneau D, Schindler C, Perez L, Probst-Hensch N, Röösli M. The relationship between transportation noise exposure and ischemic heart disease: a meta-analysis. Environ Res. 2015;138:372–380. doi: 10.1016/j.envres.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 29.Department for Communities and Local Government. Generalised Land Use Database Statistics for England 2005 (Enhanced Basemap). https://data.gov.uk/dataset/land_use_statistics_generalised_land_use_database2007.
- 30.Maxwell JM, Russell RA, Wu HM, Sharapova N, Banthorpe P, O’Reilly PF, et al. Multifactorial disorders and polygenic risk scores: predicting common diseases and the possibility of adverse selection in life and protection insurance. Ann Actuarial Sci. 2020:1–16. 10.1017/S1748499520000226.
- 31.Mutz J, Lewis CM. Cross-classification between self-rated health and health status: longitudinal analyses of all-cause mortality and leading causes of death in the UK. medRxiv. 2021. 10.1101/2021.04.23.21255982. [DOI] [PMC free article] [PubMed]
- 32.Fell DB, Joseph K, Armson BA, Dodds L. The impact of pregnancy on physical activity level. Matern Child Health J. 2009;13(5):597–603. doi: 10.1007/s10995-008-0404-7. [DOI] [PubMed] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Methodol. 1995;57(1):289–300. 10.1111/j.2517-6161.1995.tb02031.x.
- 34.Fox J, Monette G. Generalized collinearity diagnostics. J Am Stat Assoc. 1992;87(417):178–183. doi: 10.1080/01621459.1992.10475190. [DOI] [Google Scholar]
- 35.Gelman A. Scaling regression inputs by dividing by two standard deviations. Stat Med. 2008;27(15):2865–2873. doi: 10.1002/sim.3107. [DOI] [PubMed] [Google Scholar]
- 36.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cassidy S, Chau JY, Catt M, Bauman A, Trenell MI. Cross-sectional study of diet, physical activity, television viewing and sleep duration in 233 110 adults from the UK Biobank; the behavioural phenotype of cardiovascular disease and type 2 diabetes. BMJ Open. 2016;6(3):e010038. doi: 10.1136/bmjopen-2015-010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization. Air quality guidelines: global update 2005: particulate matter, ozone, nitrogen dioxide, and sulfur dioxide. 2006. https://www.who.int/publications/i/item/WHO-SDE-PHE-OEH-06.02. [PubMed]
- 39.Mayor S. Noise pollution: WHO sets limits on exposure to minimise adverse health effects. BMJ. 2018;363:k4264. 10.1136/bmj.k4264. [DOI] [PubMed]
- 40.Cockerham WC, Sharp K, Wilcox JA. Aging and perceived health status. J Gerontol. 1983;38(3):349–355. doi: 10.1093/geronj/38.3.349. [DOI] [PubMed] [Google Scholar]
- 41.Ferraro KF. Self-ratings of health among the old and the old-old. J Health Soc Behav. 1980;21(4):377–383. doi: 10.2307/2136414. [DOI] [PubMed] [Google Scholar]
- 42.Wu S, Wang R, Zhao Y, Ma X, Wu M, Yan X, et al. The relationship between self-rated health and objective health status: a population-based study. BMC Public Health. 2013;13(1):320. 10.1186/1471-2458-13-320. [DOI] [PMC free article] [PubMed]
- 43.Kelleher C, Friel S, Gabhainn SN, Tay JB. Socio-demographic predictors of self-rated health in the Republic of Ireland: findings from the National Survey on Lifestyle, Attitudes and Nutrition, SLAN. Soc Sci Med. 2003;57(3):477–486. doi: 10.1016/S0277-9536(02)00371-4. [DOI] [PubMed] [Google Scholar]
- 44.Sinclair DA, LaPlante MD. Lifespan: why we age—and why we don’t have to: Atria Books. 2019. [Google Scholar]
- 45.Brouwer WB, van Exel NJA, Stolk EA. Acceptability of less than perfect health states. Soc Sci Med. 2005;60(2):237–246. doi: 10.1016/j.socscimed.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 46.Collerton J, Davies K, Jagger C, Kingston A, Bond J, Eccles MP, et al. Health and disease in 85 year olds: baseline findings from the Newcastle 85+ cohort study. BMJ. 2009;339:b4904. 10.1136/bmj.b4904. [DOI] [PMC free article] [PubMed]
- 47.Gorman BK, Read JG. Gender disparities in adult health: an examination of three measures of morbidity. J Health Soc Behav. 2006;47(2):95–110. doi: 10.1177/002214650604700201. [DOI] [PubMed] [Google Scholar]
- 48.McDonough P, Walters V. Gender and health: reassessing patterns and explanations. Soc Sci Med. 2001;52(4):547–559. doi: 10.1016/S0277-9536(00)00159-3. [DOI] [PubMed] [Google Scholar]
- 49.Austad SN, Bartke A. Sex differences in longevity and in responses to anti-aging interventions: a mini-review. Gerontology. 2016;62(1):40–6. 10.1159/000381472. [DOI] [PubMed]
- 50.Phaswana-Mafuya N, Peltzer K, Chirinda W, Kose Z, Hoosain E, Ramlagan S, et al. Self-rated health and associated factors among older South Africans: evidence from the Study on Global Ageing and Adult Health. Glob Health Action. 2013;6(1):19880. 10.3402/gha.v6i0.19880. [DOI] [PMC free article] [PubMed]
- 51.Benyamini Y, Blumstein T, Lusky A, Modan B. Gender differences in the self-rated health–mortality association: is it poor self-rated health that predicts mortality or excellent self-rated health that predicts survival? Gerontologist. 2003;43(3):396–405. 10.1093/geront/43.3.396. [DOI] [PubMed]
- 52.Singh L, Arokiasamy P, Singh PK, Rai RK. Determinants of gender differences in self-rated health among older population: evidence from India. SAGE Open. 2013;3(2). 10.1177/2158244013487914.
- 53.Jylhä M, Guralnik JM, Ferrucci L, Jokela J, Heikkinen E. Is self-rated health comparable across cultures and genders? J Gerontol B Psychol Sci Soc Sci. 1998;53(3):S144–SS52. doi: 10.1093/geronb/53B.3.S144. [DOI] [PubMed] [Google Scholar]
- 54.Gold CH, Malmberg B, McClearn GE, Pedersen NL, Berg S. Gender and health: a study of older unlike-sex twins. J Gerontol B Psychol Sci Soc Sci. 2002;57(3):S168–SS76. doi: 10.1093/geronb/57.3.S168. [DOI] [PubMed] [Google Scholar]
- 55.Shibuya K, Hashimoto H, Yano E. Individual income, income distribution, and self rated health in Japan: cross sectional analysis of nationally representative sample. BMJ. 2002;324(7328):16. 10.1136/bmj.324.7328.16. [DOI] [PMC free article] [PubMed]
- 56.Der G, Macintyre S, Ford G, Hunt K, West P. The relationship of household income to a range of health measures in three age cohorts from the West of Scotland. Eur J Public Health. 1999;9(4):271–277. doi: 10.1093/eurpub/9.4.271. [DOI] [Google Scholar]
- 57.Godhwani S, Jivraj S, Marshall A, Bécares L. Comparing subjective and objective neighbourhood deprivation and their association with health over time among older adults in England. Health Place. 2019;55:51–58. doi: 10.1016/j.healthplace.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 58.Rehkopf DH, Berkman LF, Coull B, Krieger N. The non-linear risk of mortality by income level in a healthy population: US National Health and Nutrition Examination Survey mortality follow-up cohort, 1988–2001. BMC Public Health. 2008;8(1):383. doi: 10.1186/1471-2458-8-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davies NM, Dickson M, Smith GD, Van Den Berg GJ, Windmeijer F. The causal effects of education on health outcomes in the UK Biobank. Nat Hum Behav. 2018;2(2):117–125. doi: 10.1038/s41562-017-0279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ding D, Rogers K, van der Ploeg H, Stamatakis E, Bauman AE. Traditional and emerging lifestyle risk behaviors and allcause mortality in middle-aged and older adults: evidence from a large population-based Australian cohort. PLoS Medicine. 2015;12(12). 10.1371/journal.pmed.1001917. [DOI] [PMC free article] [PubMed]
- 61.Coyle CE, Dugan E. Social isolation, loneliness and health among older adults. J Aging Health. 2012;24(8):1346–1363. doi: 10.1177/0898264312460275. [DOI] [PubMed] [Google Scholar]
- 62.Perissinotto CM, Cenzer IS, Covinsky KE. Loneliness in older persons: a predictor of functional decline and death. Arch Intern Med. 2012;172(14):1078–1084. doi: 10.1001/archinternmed.2012.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174(6):801–9. 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed]
- 64.Arem H, Moore SC, Patel A, Hartge P, De Gonzalez AB, Visvanathan K, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med. 2015;175(6):959–967. doi: 10.1001/jamainternmed.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu S, Yarnell J, Sweetnam P, Murray L. What level of physical activity protects against premature cardiovascular death? The Caerphilly study. Heart. 2003;89(5):502–6. 10.1136/heart.89.5.502. [DOI] [PMC free article] [PubMed]
- 66.Lee I, Paffenbarger R, Hennekens C. Physical activity, physical fitness and longevity. Aging Clin Exp Res. 1997;9(1-2):2–11. doi: 10.1007/BF03340123. [DOI] [PubMed] [Google Scholar]
- 67.Oguma Y, Sesso H, Paffenbarger R, Lee I. Physical activity and all cause mortality in women: a review of the evidence. Br J Sports Med. 2002;36(3):162–172. doi: 10.1136/bjsm.36.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee I-M, Paffenbarger RS., Jr Associations of light, moderate, and vigorous intensity physical activity with longevity: the Harvard Alumni Health Study. Am J Epidemiol. 2000;151(3):293–299. doi: 10.1093/oxfordjournals.aje.a010205. [DOI] [PubMed] [Google Scholar]
- 69.Fat LN, Cable N, Marmot M, Shelton N. Persistent long-standing illness and non-drinking over time, implications for the use of lifetime abstainers as a control group. J Epidemiol Community Health. 2014;68(1):71–77. doi: 10.1136/jech-2013-202576. [DOI] [PubMed] [Google Scholar]
- 70.Piazza-Gardner AK, Barry AE. Examining physical activity levels and alcohol consumption: are people who drink more active? Am J Health Promot. 2012;26(3):e95–e104. doi: 10.4278/ajhp.100929-LIT-328. [DOI] [PubMed] [Google Scholar]
- 71.Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: explaining the U-shaped curve. Lancet. 1988;332(8623):1267–1273. doi: 10.1016/S0140-6736(88)92890-5. [DOI] [PubMed] [Google Scholar]
- 72.Stockwell T, Zhao J, Panwar S, Roemer A, Naimi T, Chikritzhs T. Do “moderate” drinkers have reduced mortality risk? A systematic review and meta-analysis of alcohol consumption and all-cause mortality. J Stud Alcohol Drugs. 2016;77(2):185–198. doi: 10.15288/jsad.2016.77.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai Y, Hodgson S, Blangiardo M, Gulliver J, Morley D, Fecht D, Vienneau D, de Hoogh K, Key T, Hveem K, Elliott P, Hansell AL. Road traffic noise, air pollution and incident cardiovascular disease: a joint analysis of the HUNT, EPIC-Oxford and UK Biobank cohorts. Environ Int. 2018;114:191–201. doi: 10.1016/j.envint.2018.02.048. [DOI] [PubMed] [Google Scholar]
- 74.Twohig-Bennett C, Jones A. The health benefits of the great outdoors: a systematic review and meta-analysis of greenspace exposure and health outcomes. Environ Res. 2018;166:628–637. doi: 10.1016/j.envres.2018.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rehm J, Irving H, Ye Y, Kerr WC, Bond J, Greenfield TK. Are lifetime abstainers the best control group in alcohol epidemiology? On the stability and validity of reported lifetime abstention. Am J Epidemiol. 2008;168(8):866–871. doi: 10.1093/aje/kwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.King DE, Mainous AG, III, Geesey ME. Turning back the clock: adopting a healthy lifestyle in middle age. Am J Med. 2007;120(7):598–603. doi: 10.1016/j.amjmed.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 77.Galante J, Adamska L, Young A, Young H, Littlejohns TJ, Gallacher J, Allen N. The acceptability of repeat Internet-based hybrid diet assessment of previous 24-h dietary intake: administration of the Oxford WebQ in UK Biobank. Br J Nutr. 2016;115(4):681–686. doi: 10.1017/S0007114515004821. [DOI] [PubMed] [Google Scholar]
- 78.Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, Collins R, Allen NE. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.UK Biobank. Access matter: representativeness of the UK Biobank resource. 2017. http://www.ukbiobank.ac.uk/wpcontent/uploads/2017/03/access-matters-representativeness-1.pdf.
- 80.Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17(9):643–653. doi: 10.1016/j.annepidem.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 81.Keyes KM, Westreich D. UK Biobank, big data, and the consequences of non-representativeness. Lancet. 2019;393(10178):1297. doi: 10.1016/S0140-6736(18)33067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Batty GD, Gale CR, Kivimäki M, Deary IJ, Bell S. Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta-analysis. BMJ 2020;368:m131. 10.1136/bmj.m131. [DOI] [PMC free article] [PubMed]
- 83.Holgate ST. ‘Every breath we take: the lifelong impact of air pollution’–a call for action. Clin Med. 2017;17(1):8–12. doi: 10.7861/clinmedicine.17-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Data fields. Figure S1. Study flowchart. Table S2. Baseline characteristics. Figures S2-S3. Self-reported illnesses. Figure S4. Correlation matrix of continuous explanatory variables. Tables S3-S5. Descriptive statistics stratified by health indicators. Tables S6-S9. Visual summary of findings. Tables S10-S13. Regression tables long-standing illness. Tables S14-S17. Regression tables self-rated health. Tables S18-S21. Regression tables health indicators. Tables S22-S24. Standardised regression coefficients tables. Figures S5-S6. Standardised regression coefficients plots. Table S25. Baseline characteristics stratified by sex. Table S26. Baseline characteristics stratified by age. Table S27-S30. Regression tables health status stratified by sex. Figures S7-S9. Confidence interval plots health status stratified by sex. Tables S31-S34. Regression tables health status stratified by age. Figures S10-S12. Confidence interval plots health status stratified by age. Tables S35-S38. Regression tables long-standing illness stratified by sex. Figures S13-S15. Confidence interval plots long-standing illness stratified by sex. Tables S39-S42. Regression tables long-standing illness stratified by age. Figures S16-S18. Confidence interval plots long-standing illness stratified by age. Tables S43-S46. Regression tables self-rated health stratified by sex. Figures S19-S21. Confidence interval plots self-rated health stratified by sex. Tables S47-S50. Regression tables self-rated health stratified by age. Figures S22-S24. Confidence interval plots self-rated health stratified by age. Table S51. Baseline characteristics longitudinal samples. Tables S52-S55. Regression tables self-rated health t1. Tables S56-S59. Regression tables self-rated health t2. Table S60. Descriptive statistics additional analyses. Figure S25. Generalised additive models.
Additional file 2. Health status classification.
Data Availability Statement
The data used in the present study are available to all bona fide researchers for health-related research that is in the public interest, subject to an application process and approval criteria. Study materials are publicly available online at http://www.ukbiobank.ac.uk.