Abstract
Objective.
The electrode–tissue interface surrounding a deep brain stimulation (DBS) lead is known to be highly dynamic following implantation, which may have implications on the interpretation of intraoperatively recorded local field potentials (LFPs). We characterized beta-band LFP dynamics following implantation of a directional DBS lead in the sensorimotor subthalamic nucleus (STN), which is a primary target for treating Parkinson’s disease.
Approach.
Directional STN-DBS leads were implanted in four healthy, non-human primates. LFPs were recorded over two weeks and again 1–4 months after implantation. Impedance was measured for two weeks post-implant without stimulation to compare the reactive tissue response to changes in LFP oscillations. Beta-band (12–30 Hz) peak power was calculated from the LFP power spectra using both common average referencing (CAR) and intra-row bipolar referencing (IRBR).
Results.
Resting-state LFPs in two of four subjects revealed a steady increase of beta power over the initial two weeks post-implant whereas the other two subjects showed variable changes over time. Beta power variance across days was significantly larger in the first two weeks compared to 1–4 months post-implant in all three long-term subjects. Further, spatial maps of beta power several hours after implantation did not correlate with those measured two weeks or 1–4 months post-implant. CAR and IRBR beta power correlated across short- and long-term time points. However, depending on the time period, subjects showed a significant bias towards larger beta power using one referencing scheme over the other. Lastly, electrode–tissue impedance increased over the two weeks post-implant but showed no significant correlation to beta power.
Significance.
These results suggest that beta power in the STN may undergo significant changes following DBS lead implantation. DBS lead diameter and electrode recording configurations can affect the post-implant interpretation of oscillatory features. Such insights will be important for extrapolating results from intraoperative and externalized LFP recordings.
Keywords: deep brain stimulation, local field potentials, impedance spectroscopy, electrode tissue interface, reactive tissue response, recording configuration, directional leads
1. Introduction
Similar to other intracranial electrode arrays, deep brain stimulation (DBS) leads with electrodes around and along the lead body provide opportunities for bidirectional interfacing, including the ability to record oscillatory activity in the form of local field potentials (LFPs) from the surrounding tissue. Recent translational efforts that leverage LFPs to collect and interpret data for evaluation of closed-loop DBS technologies [1–3], post-operative programming techniques [4–8], and new lead designs [9] depend on understanding how the reactive tissue response influences LFP signatures over the duration of the implant. While the reactive tissue response to cortical microelectrode implantation has been well studied in rodents [10–15], primates [16, 17], and humans [18, 19], less is known about how the time course of the tissue response to DBS lead implantation [20] affects the ability to record neural activity from DBS leads.
Electrochemical impedance spectroscopy (EIS) is one approach to characterize the reactive tissue response of implantable neural devices and gain insight about the surrounding tissue encapsulation [14, 21]. Impedance of implanted cortical microelectrodes typically increases during the first two weeks post-implant before stabilizing thereafter [10, 14, 21]. Larger cross-sectional area microelectrodes also cause more tissue damage and, hence, larger increases in impedance [21]. Not surprisingly, preclinical and clinical studies have shown that impedance of DBS lead electrodes, with lead diameters much greater than that of microelectrodes, increases substantially in the initial weeks following implantation [14, 20, 22, 23].
A common LFP feature recorded from DBS leads in the subthalamic nucleus (STN) are beta-band oscillations [7, 24–26]. Beta oscillations have long been used intraoperatively to guide DBS lead placement, as they are known to be robust in the sensorimotor region of the STN in drug-naïve [27, 28] and parkinsonian [29–32] brains. Beta oscillatory activity recorded from conventional DBS leads (4 rows × 1 column) in the STN has been shown to change between intraoperative and 30 d post-implant time periods [33]. Due to clinical constraints, there is a lack of studies detailing the dynamics of LFP oscillatory activity over the initial two weeks following STN-DBS lead implant and how these compare with known increases in electrode–tissue impedance at later time points. Such knowledge is critical for interpreting results from intra-operative studies that evaluate the use of beta-band activity as a feature for post-operative programming and intraoperative evaluation of closed-loop DBS systems.
In this study, we examined post-implant spatial and temporal dynamics of beta-band oscillatory power in the STN through directional DBS leads in four non-human primates. Recordings were performed over a two-week post-implant period without stimulation and then tracked again 1–4 months later. The non-parkinsonian state was chosen over a parkinsonian state in order to control for any day-to-day fluctuations in beta-band oscillatory power stemming from changes in parkinsonian behaviors. LFP recordings were analyzed using common average referencing (CAR) and intra-row bipolar referencing (IRBR) schemes and tracked alongside electrode–tissue impedance measurements.
2. Materials and methods
2.1. Preclinical animals
Four healthy, adult female rhesus macaques (Macaca mulatta) were used in this study (subject 1, 17 years old, 9.5 kg; subject 2, 17 years old, 8.9 kg; subject 3, 15 years old, 11 kg; subject 4, 17 years old, 10.0 kg). All experimental procedures and care were performed in compliance with the institutional animal care and use committee at the University of Minnesota and with the United States Public Health Service policy on the humane care and use of laboratory animals. The subjects were pair-housed, provided with water ad libitum, and fed a standard diet supplemented daily with a variety of fresh fruits and vegetables. Subjects were housed in a humidity-controlled room maintained on a 12 h/12 h light/dark cycle. All efforts were made to provide quality care and alleviate medical conditions for the animals through regular veterinary support and consultation. The animals were trained with a cooperative behavioral training paradigm using positive reinforcement methods [34, 35].
2.2. Imaging and surgical procedures
Pre-operative CT (Siemens Biograph) and 7T MRI (Magnex Scientific-passively shielded) data were acquired in each subject at the Center for Magnetic Resonance Research at the University of Minnesota. The T1-, T2- and susceptibility-weighted images were used to construct subject-specific surgical plans through a combination of Amira (Thermo Fischer Scientific) and the Monkey Cicerone surgical navigation software [36] (contact the corresponding author for an updated copy of this software platform). All surgeries were performed under deep isoflurane anesthesia and aseptic conditions as described previously [6, 37, 38]. All subjects were instrumented with a chamber over the right (S1 and S2) or left (S3 and S4) hemisphere and oriented in an oblique, anterior–posterior/lateral–medial trajectory to target the STN in a manner consistent with the trajectory of STN-DBS lead implantation in humans. The chamber material was PEEK in subject 4 and titanium in all other subjects (Gray Matter Research).
2.3. Microelectrode mapping and DBS lead implant
Electrophysiological mapping techniques utilized glass tipped tungsten microelectrodes (250 μM diameter) to record neuronal spike responses to passive and active joint manipulation to locate the sensorimotor region of the STN. Additionally, microstimulation (<50 μA) which evoked motor contractions was used to define the location and functional representation of the corticospinal tract of the internal capsule. The mapping track which yielded the longest run of spike activity and high thresholds for evoked motor movements in the sensorimotor STN was chosen for DBS lead implantation. Along this trajectory, a scaled-down, 12-electrode, directionally segmented DBS lead (four rows by three columns, manufactured by Heraeus Medical Components) was implanted in subjects 1 and 2 (figure 1(A)). The 4 × 3 lead diameter was 0.8 mm, and each electrode had 0.5 mm height (SA = 0.36 mm2) with a 0.5 mm spacing between rows. A scaled-down, six-electrode, directionally segmented DBS lead (two rows by three columns, manufactured by Abbott Neuromodulation) was implanted in subjects 3 and 4 (figure 1(B)). The 2 × 3 lead had 0.51 mm diameter, and each electrode was 0.76 mm in height (SA = 0.41 mm2) with a 0.51 mm spacing between rows. X-ray imaging (JPI Healthcare) was used during the microelectrode mapping and lead implantation procedures to ensure trajectory and depth were consistent. Post-operative CT scans were registered to each subject’s pre-operational surgical plan to verify DBS lead placement within the STN. The radial orientation of DBS lead electrodes in subjects 1 and 4 was verified with post-mortem histology (figure 1(C)). For more information on obtaining these preclinical DBS lead designs from the above manufacturers, please contact the corresponding author.
Figure 1.
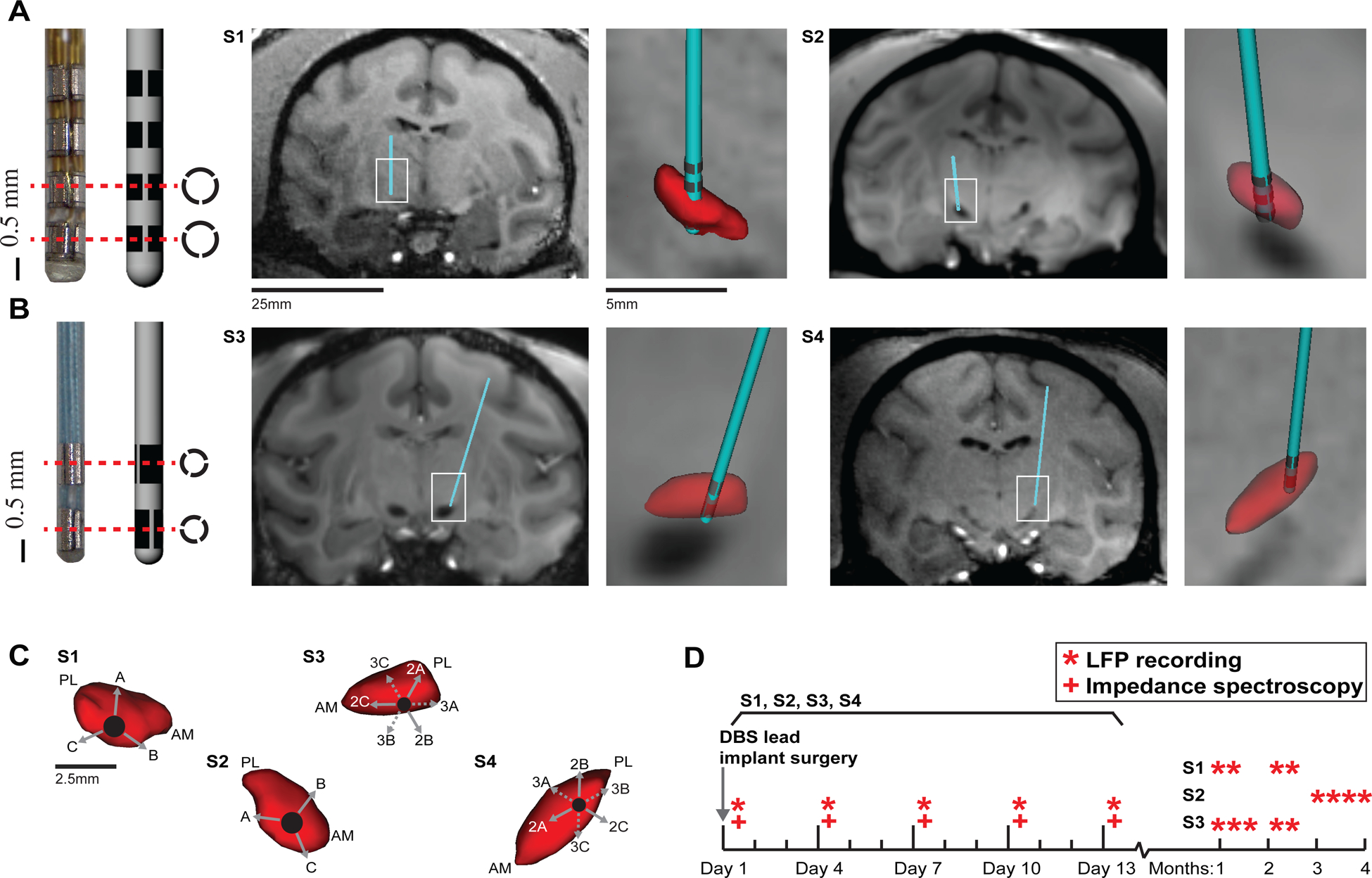
Directional DBS lead implants and experimental timeline. (A) DBS lead with 12 segmented electrodes in a 4 × 3 configuration implanted in the STN of subjects 1 and 2. MRI-T1 images are shown for each subject with segmented reconstructions of the DBS lead (cyan) and STN (red) overlaid on the images. (B) DBS lead with six segmented electrodes in a 2 × 3 configuration was implanted in the STN of subjects 3 and 4. (C) Radial orientation of electrodes relative to the STN (red) for each subject. View is in a plane perpendicular to the oblique DBS lead trajectory. AM-anteromedial; PL-posterolateral. (D) Experimental timeline marks the day of implant and subsequent LFP recordings and impedance spectroscopy measurements.
2.4. LFP recording and analysis
Resting-state LFPs were recorded at 24.4 kHz from each of the electrodes over a two-week period for 30 consecutive movement-free seconds (Tucker-Davis Technologies, Synapse Software) (figure 1(D)). Perioperative (day 1) LFP recordings were taken several hours following DBS lead implantation. Resting-state periods were determined by visual inspection of the animal during the recordings. LFPs were collected in reference to the metallic head post anchored to the cranium with multiple titanium bone screws (subjects 1–3) or a silver chloride wire inserted into the DBS chamber filled with saline (subject 4) [20]. LFPs were analyzed offline using the Chronux toolbox [39] and modified MATLAB scripts (v2017a, Mathworks). Initial post-processing included a 1 Hz high-pass filter and a 300 Hz low-pass filter applied to the raw time series data (figure 2(A)). Two LFP referencing schemes were calculated: common average reference (CAR) and IRBR. CAR is useful for interpretation of the spatial location of beta-band activity, and IRBR is more relevant in currently implemented clinical devices [8, 24]. CAR LFPs were calculated by subtracting the average signal across all electrodes from each individual electrode (figure 2(B)). IRBR LFPs were calculated by subtracting signals from adjacent electrodes within the same row (figure 2(C)). Power spectra from the recording schemes were calculated using the multi-taper method with three tapers (Chronux). The resulting power spectra were smoothed with a moving Gaussian window of length 100 (standard deviation = 20) and normalized by aligning the high frequency baseline power of each spectrum to zero to reduce contribution of any day-to-day wide-band spectral power changes. From the full power spectrum, the beta peak power was calculated from spectral data between 12 and 30 Hz.
Figure 2.
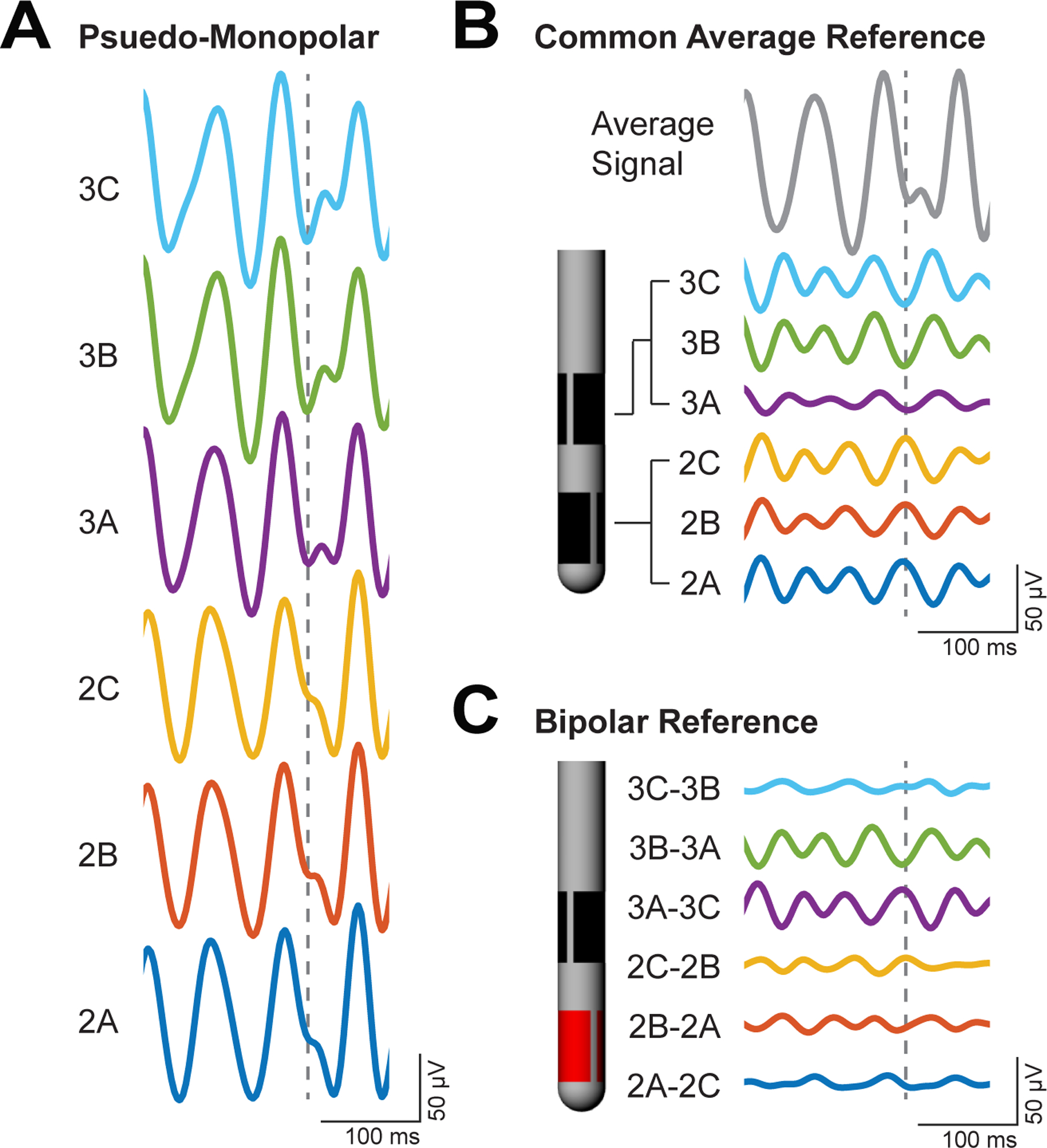
LFP beta-band activity across three referencing approaches (example from subject 4). (A) Monopolar recordings, in reference to a silver chloride wire in the DBS chamber, were filtered around the peak beta activity from 12–30 Hz. (B) CAR involved subtracting the average monopolar recording across the array from each individual monopolar recording. (C) Bipolar intra-row referencing involved subtracting monopolar recordings from adjacent electrodes within the same row. The dotted line highlights instances in which a phase reversal occurs amongst electrodes in the array indicating a dipole near electrode 3 A.
2.5. EIS and analysis
LFP instability immediately following the DBS lead implant reflects changes in the electrode–tissue interface and may stem from the reactive tissue response to neurotrauma induced by the implant [20, 21]. To track progression of this immune response we characterized the fibrous tissue encapsulation layer that forms around the lead using EIS. EIS was measured before LFP recordings to ensure viability of each electrode and to track the development of the tissue encapsulation layer surrounding the lead (AutoLab Potentiostat, NOVA 2.1 software). Electrodes were deemed un-viable if impedance measured at 1 kHz was significantly higher than anatomically possible, indicating the wire connections had come loose and neural recordings were no longer possible. Measurements used an AC signal at an amplitude of 25 mV to ensure a linear response [20]. All impedances were measured in a two-electrode configuration with a DBS electrode serving as the working electrode (figure 3(A)). A titanium head-post, attached with metallic bone screws, served as the counter electrode for subjects 1, 2, and 3. The counter electrode for subject 4 was a silver chloride wire placed inside the ipsilateral DBS chamber and submerged in 0.9% NaCl. The measured impedance spectrum ranged from 10 to 10 000 Hz, which was sufficient to capture the resistive and capacitive nature of the interface (figure 3(B)). Analysis of EIS data included a longitudinal impedance analysis specifically at 1 kHz and 20 Hz. We hypothesized that the impedance at 20 Hz may better reflect the impedance experienced by the beta-band LFP signals in the 12–30 Hz range that were recorded through the electrode–tissue interface. 1 kHz is the impedance commonly measured by investigators to ensure electrode viability [14, 20], and 20 Hz is more consistent with the spectral features of the neuronal recordings. Given the susceptibility of electrode–tissue impedances to decrease with electrical stimulation [20], no electrical stimulation was delivered during the duration of the study.
Figure 3.
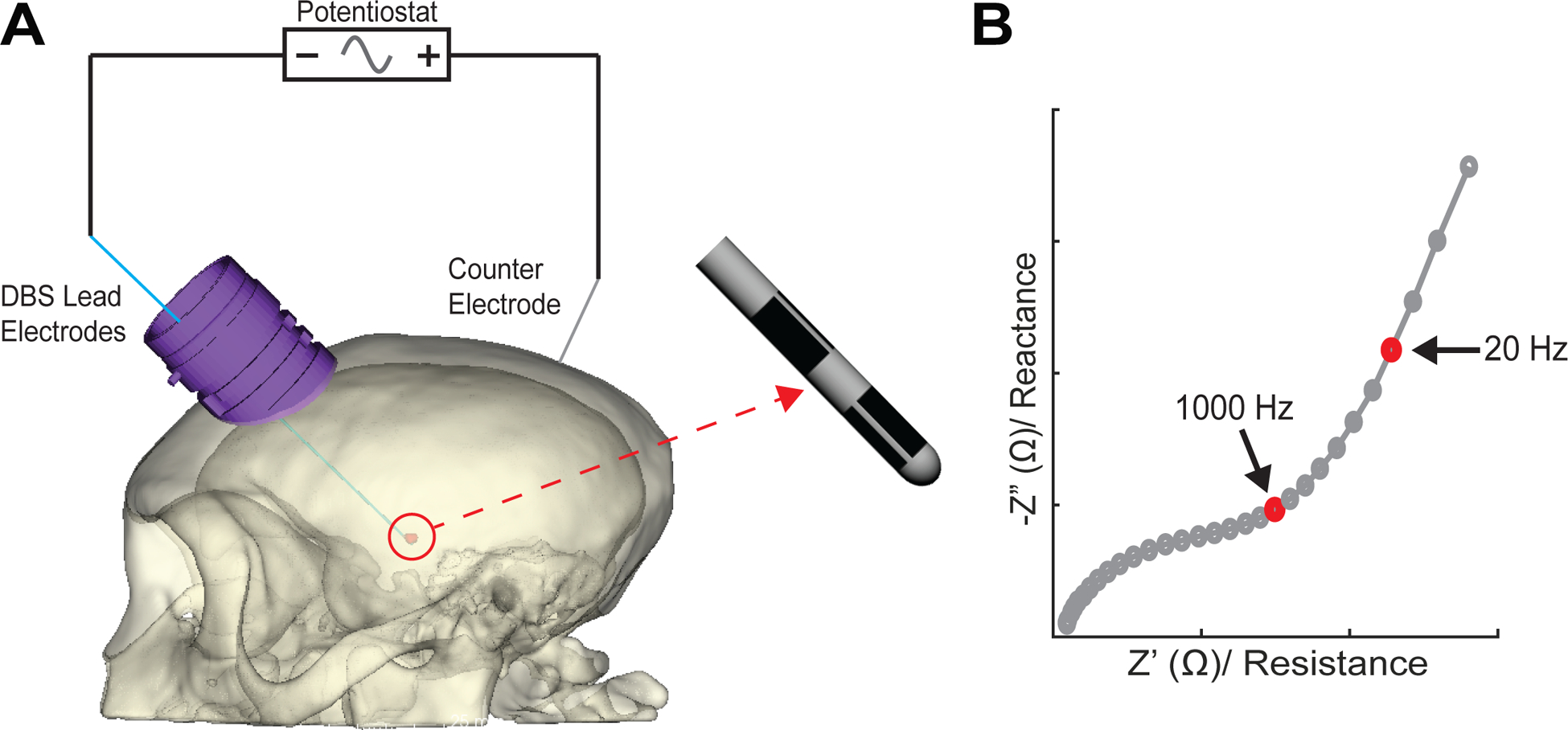
EIS measurements. (A) Impedances were measured between a working electrode on the DBS lead and a counter electrode either as a silver chloride wire in the DBS chamber or a distributed set of bone screws over the posterior cranium. (B) Shown is a Nyquist plot of an example impedance spectroscopy measurement highlighting between 1 kHz and 20 Hz resistance and reactance. The latter measurement was chosen given its relevance to typical beta-band frequencies (12–30 Hz).
2.6. Statistical analysis
For LFP and impedance time series data, a nonparametric one-way ANOVA was performed to confirm the presence of any significant changes across days. If p < 0.05, post-hoc analysis was done using a Wilcoxon signed rank test for zero median with Bonferroni corrections to account for multiple comparisons. Testing for correlations between different time series trends was done using a Pearson correlation test. This was applied to compare CAR beta-band peak power to bipolar beta-band peak power and impedance to beta-band peak power. Pearson correlations were also used to compare spatial beta peak power maps across days.
3. Results
3.1. General LFP beta peak power trends following STN-DBS lead implant
The CAR power spectra calculated from DBS lead electrodes in the four subjects varied over time (figure 4(A)). During the peri-implant period (day 1–4), beta peak power (12–30 Hz) fluctuated but showed consistent trends for each of the DBS leads (figures 4(B) and (C)). There was an increase in beta peak power between days 1 and 4 in S1 and S2 (Wilcoxon signed rank: p = 0.0122 and p = 0.0420), and a decrease in beta peak power in S4 (Wilcoxon signed rank: p = 0.0313) that similarly trended downward in S3. Note that the subjects with lower beta peak power on day 1 were those with the larger diameter 4 × 3 DBS lead (S1 and S2), whereas the subjects with higher beta peak power on day 1 were those with the smaller diameter 2 × 3 DBS lead (S3 and S4). For the bipolar configurations, all animals showed similar trends to those observed for the CAR configurations, but only S1 had a significant change in beta peak power between days 1 and 4 (Wilcoxon signed rank: p = 0.0269) (supplementary figure 1 (available online at stacks.iop.org/JNE/18/046050/mmedia)).
Figure 4.
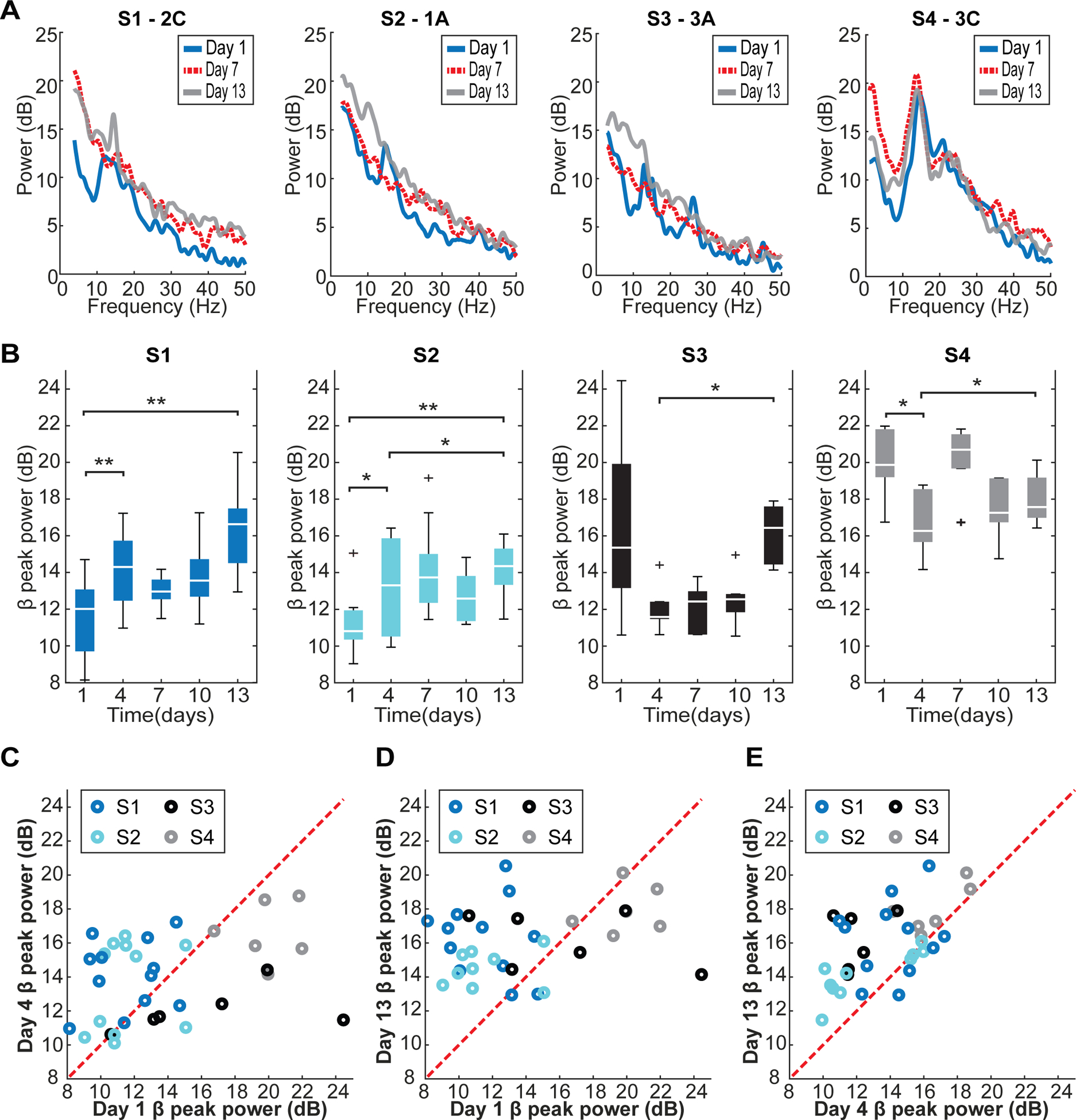
Changes in LFP spectral characteristics using a CAR scheme following implantation of a 4 × 3 DBS lead (S1 and S2) and a 2 × 3 DBS lead (S3 and S4). (A) Example power spectra with CAR from one contact of each subject’s DBS lead highlights the overall spectral variation across days and subjects. The spectra examples shown are from the contacts which were highest in beta-band peak power on day 13. (B) Beta-band peak power (between 12–30 Hz, CAR) over days for each subject showed a significant and consistent effect between peri-implant days (day 1–4) and day 13 (Wilcoxon signed rank: *p < 0.05; **p < 0.015). (C) A comparison between day 1 and day 4 showed that S1 and S2 has the bias of higher beta-band peak power towards day 4 and S3 and S4 had the bias of higher beta-band peak power towards day 1. (D) A comparison between day 1 and day 13 showed the bias of higher beta-band peak power in S1 and S2 towards day 13 across electrode sites. (E) Similarly, a comparison between day 4 and day 13 showed the bias of higher beta-band peak power in S3 and S4 towards day 13 across electrode sites.
Following the peri-implant time period, beta peak power was calculated from the CAR LFPs in the STN and compared over the two-week post-implant time period (figure 4(B)). An increase in beta peak power was observed from peri-implant days 1 (S1 and S2; Wilcoxon signed rank: p = 0.0024 and p = 0.0117) and 4 (S2, S3 and S4; Wilcoxon signed rank: p = 0.0371, p = 0.0313 and p = 0.0313). Only 3 of 34 total viable electrodes across all animals did not show this increase from either day 1 or day 4 to day 13 (figures 4(C)–(E)). Similarly, beta peak power calculated from IRBR LFPs significantly increased between the peri-implant time point and the two-week time period (supplementary figure 1). This increase was seen from days 1 (S1 and S2; Wilcoxon signed rank: p < 0.001 and p = 0.0313) and 4 (S1, S2 and S4; Wilcoxon signed rank: p < 0.001, p = 0.0078 and p = 0.0313).
3.2. Beta peak power spatial distribution following STN-DBS lead implant
For both CAR LFPs and IRBR of LFPs, no subjects showed a spatial consistency of beta peak power from the perioperative LFP recordings on day 1 to the post-implant LFP recordings on day 13 using Pearson’s correlation test (figure 5 and supplementary figure 3). CAR LFPs showed a re-ordering of peak beta power Z-score values across electrodes over the two-week post-implant period (supplementary figure 2(A)). A similar re-ordering over the two-week post-implant period occurred with IRBR LFPs (supplementary figure 2(B)).
Figure 5.
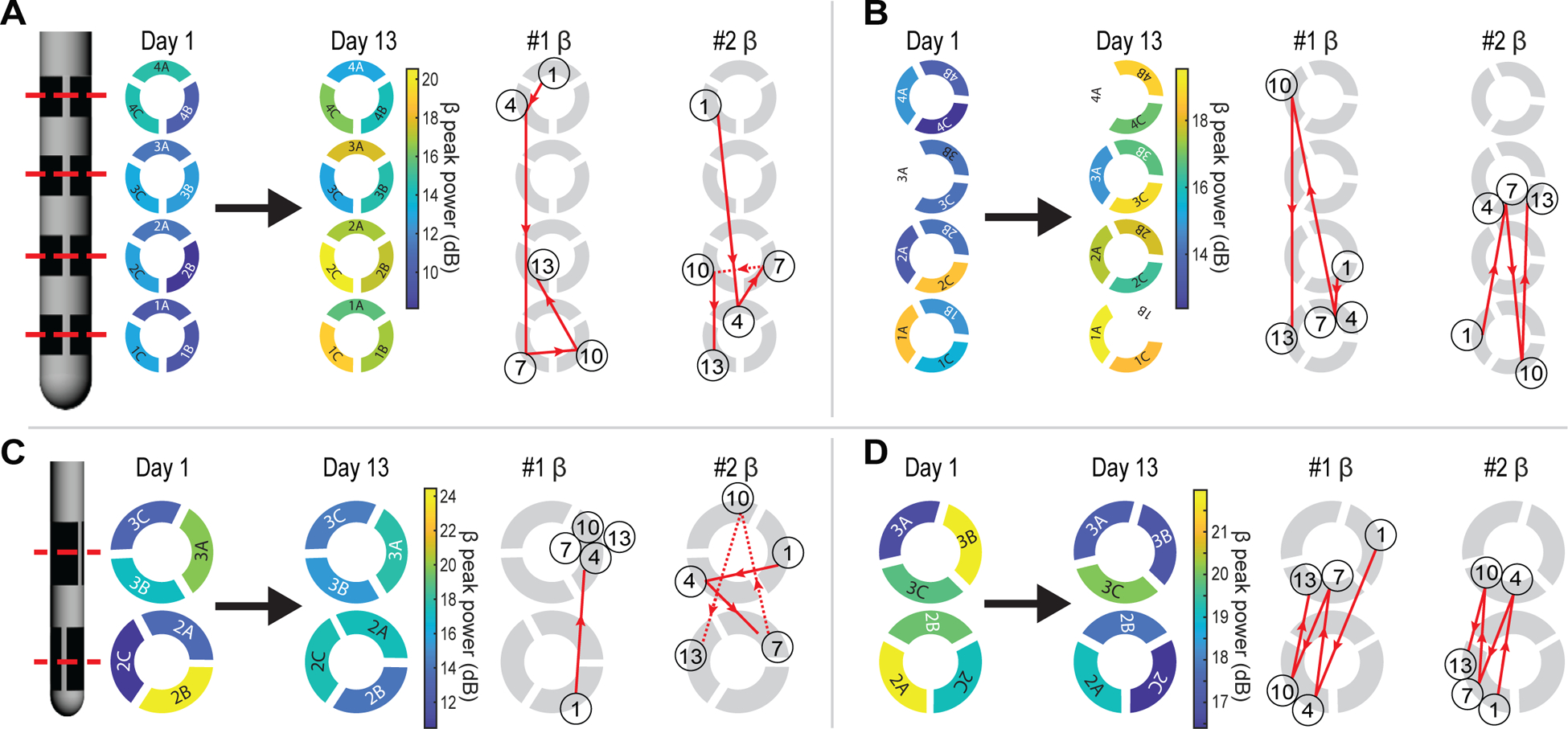
Spatiotemporal dynamics of LFP characteristics using CAR following DBS lead implantation for subjects (A) S1, (B) S2, (C) S3, and (D) S4. Left, spatial maps of beta-band (12–30 Hz) peak power on day 1 and day 13 show uncorrelated peri-implant and semi-chronic beta power distributions. White electrodes were excluded from analysis. Right, trajectories show trends of the electrodes with the highest (#1) and second highest (#2) beta-band peak power from day 1 to day 13. The number in each circle denotes the day. Circle location denotes the electrode with the highest, or second highest, beta-band peak power for that day.
The electrodes with the highest and second highest peak beta power using CAR showed varied spatial trajectories amongst inter-row and intra-row directions over the two-week post-implant period in all subjects (figure 5). In subject 3, the electrode with the highest beta peak power using CAR stabilized at day 4, whereas for subjects 1, 2, and 4, inter-row and intra-row spatial changes in the highest and second highest beta power electrodes continued throughout the two-week post-implant period. Similar trajectories were observed for the IRBR LFPs in terms of the first and second ranked bipolar pairs for beta peak power. The lengths of the trajectory of the electrode contact with highest beta peak power for each subject over the peri-implant period was compared to 100 trials of randomly generated trajectories. There was a randomness in the trajectory of the contact with the highest beta peak power for two of four subjects using both CAR (S1 and S4) and IRBR (S1 and S2) LFPs. Interestingly, the electrode with the highest day 1 beta power was more likely to remain in the top 50th percentile for beta power strength when using bipolar referencing (three of four subjects) over CAR (one of four subjects) (supplementary figure 2(B)).
3.3. Tissue impedance following STN-DBS lead implant
Nyquist plots of EIS data from example electrodes for each subject illustrated the continued development over a two-week period of the tissue response at the electrode–tissue interface for each STN-DBS lead implant (figure 6(A)). All leads developed a significant tissue impedance response over the two-week post-implant period consistent with previous studies [14, 20]. The tissue response was quantified by Nyquist plots, an increase in 1 kHz impedance for all subjects (Wilcoxon signed rank: S1 and S2, p < 0.001; S3 and S4, p = 0.0313), and an increase in 20 Hz impedance in three of the four subjects (S1, S2, and S3; Wilcoxon signed rank: p < 0.001, p = 00093 and p = 0.0313) (figure 6(B)). The two lead designs differed in that the 4 × 3 DBS leads measured a larger impedance than the 2 × 3 DBS leads at both 1 kHz and 20 Hz on post-implant days 7, 10, and 13 (unpaired two-sample t-test: p < 0.001).
Figure 6.
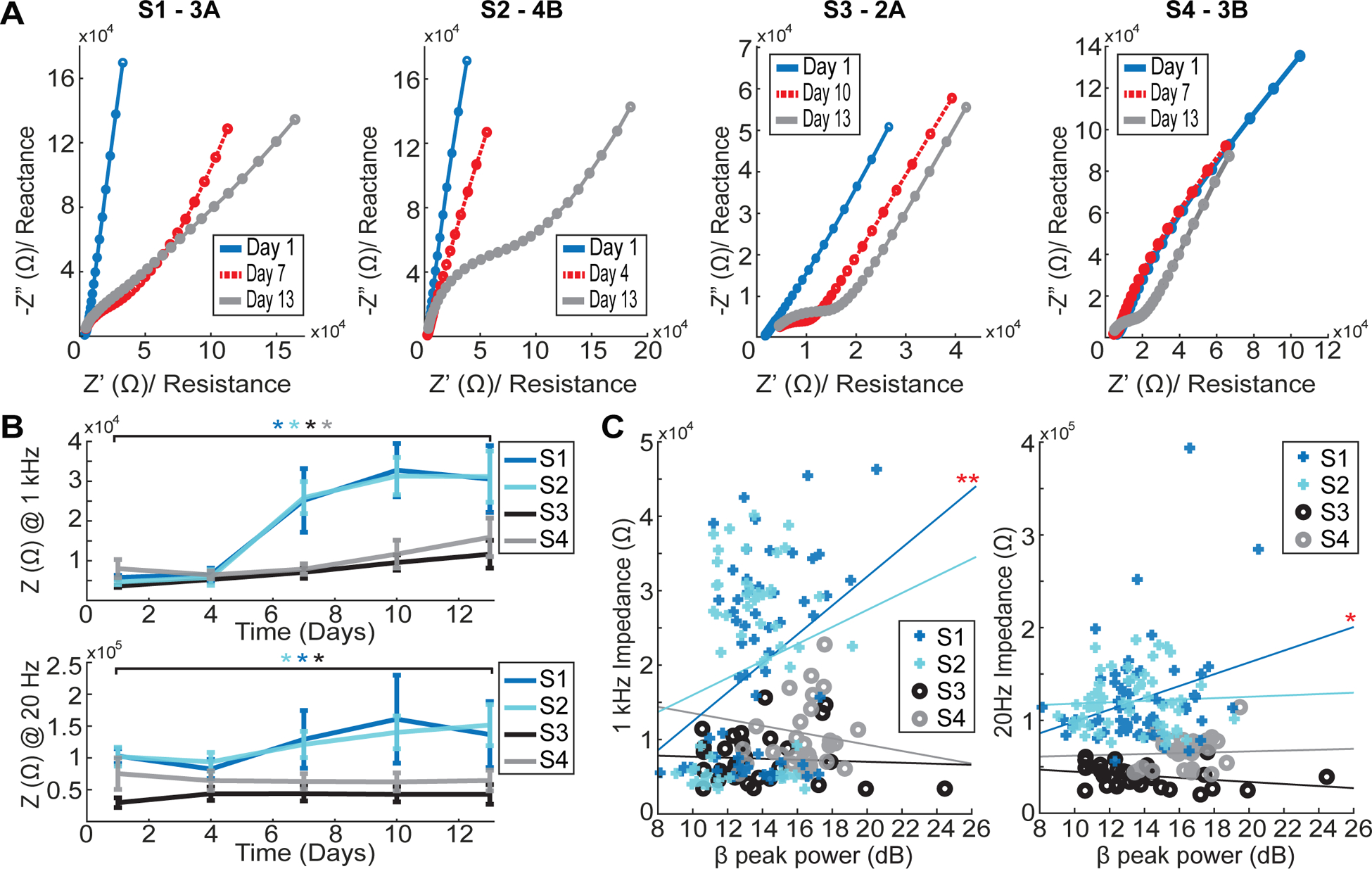
Dynamics of tissue impedance following DBS lead implantation. (A) Nyquist plot examples for one electrode of each subject showed development of a reactive tissue response at the electrode–tissue interface. (B) Longitudinal trends of impedance measured at 1 kHz (top) and 20 Hz (bottom) showed significant increased impedances over time across subjects (Wilcoxon signed rank: *p < 0.05). (C) A comparison between beta-band (12–30 Hz, CAR) peak power and impedance measured at 1 kHz (left) and 20 Hz (right) only revealed a significant correlation in one subject (S1) (Pearson correlation: *p < 0.05; **p < 0.0125).
Despite significant trends in short term days across all subjects, only one subject (S1) showed a relationship between beta peak power trends and 1 kHz and 20 Hz impedance trends, which was weakly correlated (Pearson correlation: p = 0.0045 and p = 0.0292) (figure 6(C)). A Pearson correlation test including all subjects across all days did not show a significant relationship between either 1 kHz or 20 Hz impedance and beta peak power.
3.4. Longer term beta peak power dynamics
The spatial variance of CAR beta peak power across electrodes decreased between the semi-chronic (day 1–13) and chronic (day 35+) periods for subjects 1, 2 and 3 (Wilcoxon rank sum: p = 0.0141, p < 0.001 and p = 0.0022) (figures 7(A) and (B)). IRBR beta peak power showed the same decrease of variance in the day 35+ period for S2 and S3 (Wilcoxon rank sum: p < 0.001 and p = 0.0022) (supplementary figures 4(A) and (B)). Subject 4 was euthanized before long-term data could be collected. There was no significant change in average beta-band peak power from the semi-chronic (day 1–13) to chronic (day 35+) periods.
Figure 7.
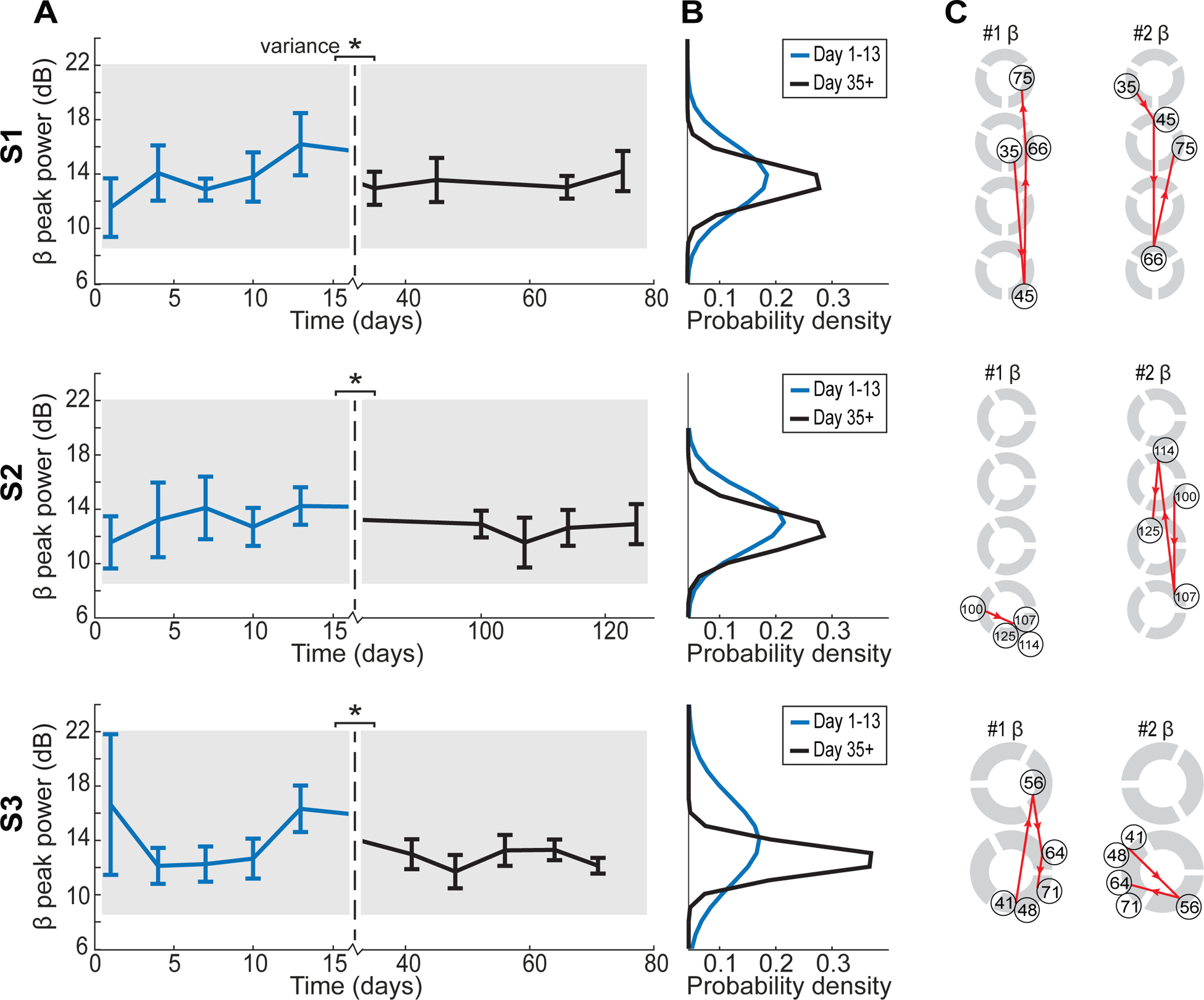
Longer term beta-band (12–30 Hz) peak power trends. (A) Average temporal trends revealed significant decreases in the variance of beta peak power across all electrodes after 35 d (Wilcoxon rank sum: *p < 0.05). (B) Variance is plotted as probability density functions. (C) Trajectories showed trends of the electrodes with the highest (left) and second highest (right) beta-band peak power for days 35+ for S1, S2, and S3.
In subjects 1, 2, and 3, spatial changes in the CAR highest, and second highest, beta peak power electrodes continued to occur after 35+ d across and within rows (figure 7(C)). The dynamics observed in the peri-implant time period (days 1–13) of the electrode with the highest beta peak power (figure 5(C)) are similar to the dynamics seen in the later days (day 35+) with both time periods showing variability (figure 7(C)). Spatial beta peak power maps from perioperative day 1 and the two-week time point (day 13) did not show a significant correlation to the more chronic beta peak power maps.
3.5. Referencing scheme relationships
While CAR is useful to interpret the spatial location of peak beta-band activity, we also investigated IRBR given its utility in clinical devices [8, 24] (see supplementary figures 1–4). Comparing the two referencing approaches across all days in all four subjects, there was a correlation between CAR and IRBR beta peak power trends for S1, S2 and S3 (Pearson correlation: p < 0.001, p = 0.0041 and p = 0.0012) (figure 8(A)). In the semi-chronic period (days 1–13), subjects 1 and 4 showed a significant bias towards higher beta peak power using CAR LFPs (Wilcoxon signed rank: p = 0.0067 and p < 0.001), while S2 showed a significant bias towards higher beta peak power using IRBR LFPs (Wilcoxon signed rank: p < 0.001) (figure 8(B)). In the chronic period (days 35+), subjects 1, 2 and 3 showed a significant bias towards higher beta peak power using IRBR LRPs (Wilcoxon signed rank: p < 0.001, p < 0.001 and p = 0.0047) (figure 8(A)).
Figure 8.
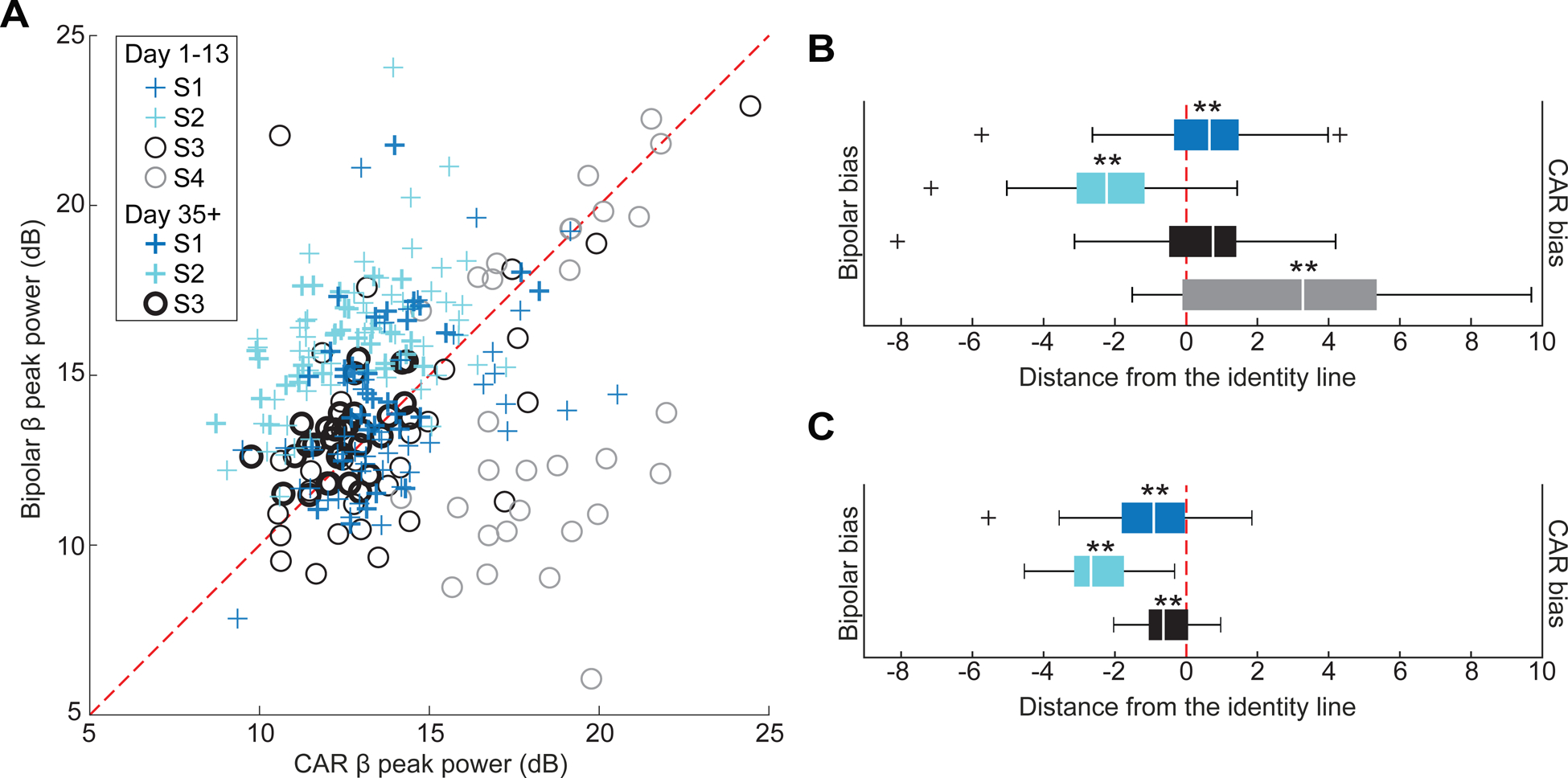
Comparison between CAR and bipolar referencing approaches. (A) A comparison of CAR and bipolar reference beta-band peak power over all short term and long-term measurements showed a significant correlation across all days for S1, S2 and S3 (Pearson correlation: p < 0.001). (B) Bias in the semi-chronic period towards CAR or bipolar referencing was revealed by calculating the shortest distance to the identity line from each point in (A). S1 and S4 were significantly biased with higher CAR beta peak power and S2 was significantly biased with higher bipolar referencing beta peak power (Wilcoxon signed rank: **p < 0.001; *p < 0.05). (C) Bias in the chronic period was towards bipolar referencing for S1, S2 and S3 (Wilcoxon signed rank: **p < 0.001).
4. Discussion
While beta-band oscillations in the STN have shown promising utility as feedback signals for closed-loop DBS technologies [1–3] and post-operative programming based on the spatial mapping of beta-band power [4–8], the spectral features of LFPs are known to change between intraoperative and 30 d post-implant time periods [33]. There remains a knowledge gap in the literature of how LFPs change immediately following implantation given that many LFP recording studies in humans are constrained to externalization of wires during implant (day 1) and during neurostimulator implant surgeries (day 30+). Such information would be useful, especially in the context of (a) deriving chronic neurostimulator settings from intraoperative LFP recordings [4], (b) investigating closed-loop DBS approaches in intra-operative settings [3], and (c) performing longer-term externalized recordings [40, 41]. Here, we more closely examined the LFP beta peak power dynamics following directional STN-DBS lead implantation in an animal model. Such DBS leads have a higher spatial resolution than the macro-ring electrode contacts used in previous studies.
4.1. Average beta peak power varies during the initial two weeks following implantation
The resting state LFP recordings showed that initially upon implant, there was a strong degree of variability in beta peak power. In the two subjects (S1 and S2) with the larger lead diameter, there was a steady significant increase in beta peak power over the two-week post-implant period. However, in the subjects (S3 and S4) with the smaller lead diameter, there was a significant drop in beta peak power between day 1 and day 4 followed by a slight increase over the two-week post-implant period. This suggests that the neural tissue can still exhibit strong oscillations immediately following the implantation process and during the initial phase of vasogenic edema [42]. Over the course of two weeks, beta peak power increased for many electrodes suggesting that the tissue adjacent to the DBS lead was slowly able to regain oscillatory function. These results agree with studies that show significant changes in beta power between intraoperative and 30+ d time periods [33]. The results also suggest that fluctuations in beta peak power can occur during the initial 4 d following implantation and that at least two weeks are required for the adjacent tissue to stabilize [33]. These findings were observed for both CAR and IRBR schemes which suggests that the time-varying effects on beta peak power occur across multiple oscillatory dipole orientations reflecting putatively different pathways within the STN or potentially different behavioral states [43].
4.2. Variance of beta peak power is largest during the initial two weeks following implant
The directional DBS leads provided opportunities to investigate and track the spatial variance of beta peak power within and across daily recording sessions. During the initial two-week post-implant time period, the beta peak power showed greater variability across electrodes than for the 35+ d time period. Further, when analyzing individual electrodes, beta peak power variance across days in the initial two-week period was significantly higher than that in the longer-term 35+ d time period suggesting that beta peak power measurements are more stable 1 month post-implant, which aligns with previous literature for other types of electrode arrays [44]. Similar trends were observed for CAR and IRBR schemes, suggesting that the post-implant beta peak power variability resulted from larger-scale reactive tissue responses to the DBS lead implantation and continued presence of the DBS lead in the neuropil.
4.3. Spatial arrangement of beta peak power changes post-implant
The spatial distribution of beta peak power in the perioperative period (day 1) did not match that in the two-week post-implant period nor in the 35+ d post-implant period. This could explain the variability observed when comparing intraoperative beta oscillation measurements to delayed programming sessions identifying the optimal monopolar electrode for DBS therapy for parkinson’s disease [4]. Tinkhauser et al showed that the directional DBS lead electrode with the strongest intraoperative CAR beta power was consistently rated in the top 50th percentile for predicting the optimal stimulation contact for delivering motor symptom therapy for parkinson’s disease. In our study, at least one of the two electrodes with the strongest perioperative beta power remained in the top 50th percentile for beta power strength at the end of the initial two weeks post-implant for three out of four subjects using both CAR and bipolar referencing schemes. While there was some spatial consistency, a substantial amount of day-to-day variability in relative beta power strength across all electrodes occurred, and there was a randomness to the location of the highest beta activity along the DBS lead for two of the four subjects using CAR and IRBR LFPs.
Studies that show more substantial correlations between the electrode with the highest beta peak power and the optimal electrode for therapy contain several experimental decisions, such as the choice of bipolar referencing over CAR, which may account for the difference in outcome [5, 8]. This suggests that, in some cases, it may be beneficial to use one referencing scheme over another. Different referencing may be more insightful depending upon the spatial orientation of oscillatory sources, with IRBR more sensitive to sources arranged horizontally and nearer to the DBS lead and CAR more sensitive to multiple orientations both around and tangential to the DBS lead. For example, in the context of STN-DBS, the IRBR LFPs may reflect axons projecting into STN from the external globus pallidus and hyperdirect pathways [45–47]. Beyond referencing schemes, the concept of oscillatory source localization has been commonly used in EEG literature [48]. This study observed several instances where clear dipole sources were apparent (e.g. figure 2). Source localization techniques leveraging directional DBS leads will be useful in future studies to investigate the precise locations of oscillatory sources within deep brain targets [7, 38, 49].
4.4. Tissue impedance does not directly affect LFP signals
The significant increase in impedance seen in the later days following STN-DBS lead implant with no stimulation agrees with previous studies across a broad range of neural probe implants [14, 20, 22, 33]. Our study did not show a significant correlation between beta peak power and 1 kHz or 20 Hz impedance. While the rise in electrode–tissue interface impedance generally followed a rise in beta peak power over the initial two weeks post-implant, there was no significant correlation when considering individual electrodes. A similar study found no significant correlation between 30 Hz impedance and beta power [33] and others have shown no consistent correlation between 1 kHz impedance and microelectrode spike recordings [50]. Based on these results, tissue impedance is not likely responsible for early LFP beta power signal dynamics. Other factors may include the ‘microlesion effect’ [51–55] or a functional reorganization of the location of the oscillatory source(s) adjacent to the leads.
In the peri-implant time period, one noticeable difference in LFP dynamics was found between the two DBS lead designs. Subjects 1 and 2 had lower beta peak power on day 1 compared to day 4, whereas subjects 3 and 4 had higher beta peak power on day 4 compared to day 1. The geometric surface area of the electrodes differs in subjects 1 and 2 (0.36 mm2) compared to subjects 3 and 4 (0.41 mm2). Additionally, subjects 1 and 2 were implanted with a larger diameter DBS lead (0.8 mm) than subjects 3 and 4 (0.5 mm). It may be that the larger diameter leads in subjects 1 and 2 caused a larger ‘microlesion effect’ which suppressed the initial LFP signals that were visible in subjects 1 and 2 on day 1. Another possibility could be the micro-dynamics immediately following the DBS lead implant, as a relevant study noted a significant difference in low beta (8–20 Hz) peak power between zero- and two-hours post-implant [33].
4.5. Implications for clinical and preclinical research
Beta oscillations have long been used intraoperatively to guide DBS lead placement, and are known to be robustly expressed in the sensorimotor STN of patients with parkinson’s disease [29–32, 43, 56]. Previous studies have reported that beta-band spectral power can relate to bradykinesia severity [2, 57–59] and other motor signs [57, 60, 61]. Based on this, beta power has been advanced as a feedback signal for closed-loop DBS systems for parkinson’s disease with the goal of reducing the amount of stimulation to limit side effect emergence and provide more consistent treatment of parkinsonian motor signs (over continuous, fixed stimulation) [3]. Further, programming of DBS systems for treating parkinson’s disease requires meticulous clinical examination of patients’ response to stimulation through various electrode configurations, amplitudes, frequencies, and pulse widths [62]. With the availability of directional DBS leads, the clinical parameter space becomes greater, prompting the investigation of beta power to guide the spatial targeting and programming of DBS leads [4–8].
Translation of novel closed-loop DBS systems [3, 25, 63–65], directional DBS lead designs [7, 66], and LFP-guided programming strategies [4, 8] in humans have relied on intraoperative and peri-implant state experiments while the DBS lead wires are externalized. This study showed that there can be significant differences in perioperative and post-implant LFP characteristics (spatial variation). While perioperative LFP recordings can be generally predictive of which electrode to use for chronic DBS therapy [1, 4], the electrode recording with the strongest peri-operative beta power was not always the most therapeutic electrode contact at the subsequent clinical programming visit. Similar to these previous studies, the data in this study also showed that at least one of the two electrodes with the highest beta peak power in the peri-operative state remained in the top 50% of electrodes with the highest power in the chronic recording condition. The data presented here also suggest that there is variability in the spatial distribution of beta peak power between perioperative and chronic time points, which may help explain those clinical observations. This research suggests the importance and utility of chronically implanted bidirectional DBS system [67, 68].
The same precautions should be warranted in preclinical experiment design, as intra- and peri-operative studies are common. The study herein suggests that chronic DBS recording studies may result in more directly translatable data. If the reactive-tissue response and ‘micro-lesion effect’ are not accounted for in these experiments, these effects could play a role in the dynamics observed in the data. Additionally, as suggested in this study (figures 6(A) and (B)), varying lead diameters used in preclinical studies may influence the initial reactive tissue response [21].
4.6. Limitations
There are several limitations that should be considered when interpreting the results of this study, including the lack of a disease condition and the low sample number of subjects with variability in LFP recordings. The inter-subject variability seen herein is typical in studies measuring LFPs [2, 26, 49]. Factors within this study that could have added to typical inter-subject variability include lead implant location and lead design. Subjects 1 and 2 were implanted with a larger diameter DBS lead (0.8 mm) than subjects 3 and 4 (0.5 mm). This could play a role in inducing a larger ‘microlesion effect’ which suppressed the initial LFP signals that were visible in subjects 1 and 2 on day 1.
The LFP recordings were performed in a non-parkinsonian state, which resulted in an observable, but attenuated beta-band peak power from that observed in the parkinsonian state [27, 28]. The rationale for choosing a non-parkinsonain state was (a) beta power is present in the naive state, and (b) one can block out variability in beta peak power due to (1) potential changes in overall parkinsonian condition that can occur from day to day [49] and (2) potential lesion-like effects due to lead implantation that can affect overall parkinsonian motor scores [51–55]. Therefore, the non-parkinsonian state of the subjects allowed for an investigation focused on LFP dynamics related to the reactive tissue response. Similarly, this study did not address any behavioral correlates in order to control for day-to-day fluctuations in oscillatory power due to motor behaviors such as desynchronization of beta during movement [69] and focus on changes that may be due to the reactive-tissue response. In the future, preclinical and clinical studies that can control for the same severity of parkinsonism prior to implant will aid in further investigation of LFP dynamics immediately following DBS lead implantation.
5. Conclusions
This study showed that beta peak power may be spatially and temporally dynamic in the first two weeks following implantation of a directional DBS lead in the sensorimotor STN. These oscillatory dynamics did not correlate with post-implant impedance changes, which suggests that beta power dynamics may be due to additional electrode–tissue factors of the reactive tissue response. Referencing schemes may affect LFP oscillatory features following directional DBS lead implantation. Such insights will be important for interpreting the results from intraoperative and externalized closed-loop DBS studies and post-op programming studies, as well as acute preclinical DBS studies, investigating the physiological mechanisms of DBS. Future studies in humans leveraging longer-term externalization procedures and chronic bidirectional systems will be helpful to further elucidate the dynamic changes in LFPs following DBS lead implantation in parkinson’s disease as well as other brain disorders.
Supplementary Material
Acknowledgments
This work was funded by the National Institutes of Health under Grants R44-NS103714, R01-NS094206, and P50-NS098573. The authors would like to thank Josh Rosing and Joan Dao for their contributions to data collection and histological analysis, respectively. We thank Noam Harel, Essa Yacoub, Remi Patriat, and the Center for Magnetic Resonance Research (CMRR) for their assistance with collecting the pre-operative 7T MRI data for surgical planning (CMRR funding under NIH Grants P41-EB015894, P30-076408, U54-MH091657). We also thank Abbott Neuromodulation (Filippo Agnesi) and Heraeus Medical Components (Mark Hjelle, Robert Cass, Ben Probiel, Mitch Lark, Paul Noffke, Cullen Boyd, David Ohmann, John Warling, Vu Tran, and Alan Carlson) for their collaborations on developing the scaled-down directional DBS leads. We also thank Melanie Graham and Luke Mutch for help implementing the cooperative behavioral training program.
Footnotes
Supplementary material for this article is available online
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.
References
- [1].Priori A, Foffani G, Rossi L and Marceglia S 2013. Adaptive deep brain stimulation (aDBS) controlled by local field potential oscillations Exp. Neurol 245 77–86 [DOI] [PubMed] [Google Scholar]
- [2].Rosa M, Giannicola G, Servello D, Marceglia S, Pacchetti C, Porta M, Sassi M, Scelzo E, Barbieri S and Priori A 2011. Subthalamic local field beta oscillations during ongoing deep brain stimulation in parkinson’s disease in hyperacute and chronic phases Neurosignals 19 151–62 [DOI] [PubMed] [Google Scholar]
- [3].Little S et al. 2013. Adaptive deep brain stimulation in advanced parkinson disease Ann. Neurol 74 449–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tinkhauser G et al. 2018. Directional local field potentials: a tool to optimize deep brain stimulation Mov. Disorders 33 159–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ince NF, Gupte A, Wichmann T, Ashe J, Henry T, Bebler M, Eberly L and Abosch A 2010. Selection of optimal programming contacts based on local field potential recordings from subthalamic nucleus in patients with parkinson’s disease Neurosurgery 67 390–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Connolly AT, Kaemmerer WF, Dani S, Stanslaski SR, Panken E, Johnson MD and Denison T 2015. Guiding deep brain stimulation contact selection using local field potentials sensed by a chronically implanted device in parkinson’s disease patients 2015 7th Int. IEEE/EMBS Conf. on Neural Engineering (NER) (Montpellier: IEEE; ) pp 840–3 [Google Scholar]
- [7].Bour LJ, Lourens MAJ, Verhagen R, de Bie RMA, van den Munckhof P, Schuurman PR and Contarino MF 2015. Directional recording of subthalamic spectral power densities in parkinson’s disease and the effect of steering deep brain stimulation Brain Stimul. 8 730–41 [DOI] [PubMed] [Google Scholar]
- [8].Yoshida F et al. 2010. Value of subthalamic nucleus local field potentials recordings in predicting stimulation parameters for deep brain stimulation in parkinson’s disease J. Neurol. Neurosurg. Psychiatr 81 885–9 [DOI] [PubMed] [Google Scholar]
- [9].Connolly AT, Vetter RJ, Hetke JF, Teplitzky BA, Kipke DR, Pellinen DS, Anderson DJ, Baker KB, Vitek JL and Johnson MD 2016. A novel lead design for modulation and sensing of deep brain structures IEEE Trans. Biomed. Eng 63 148–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kozai TDY, Du Z, Gugel ZV, Smith MA, Chase SM, Bodily LM, Caparosa EM, Friedlander RM and Cui XT 2015. Comprehensive chronic laminar single-unit, multi-unit, and local field potential recording performance with planar single shank electrode arrays J. Neurosci. Methods 242 15–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ludwig KA, Miriani RM, Langhals NB, Joseph MD, Anderson DJ and Kipke DR 2009. Using a common average reference to improve cortical neuron recordings from microelectrode arrays J. Neurophysiol 101 1679–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vetter RJ, Williams JC, Hetke JF, Nunamaker EA and Kipke DR 2004. Chronic neural recording using silicon-substrate microelectrode arrays implanted in cerebral cortex IEEE Trans. Biomed. Eng 51 896–904 [DOI] [PubMed] [Google Scholar]
- [13].Ward MP, Rajdev P, Ellison C and Irazoqui PP 2009. Toward a comparison of microelectrodes for acute and chronic recordings Brain Res. 1282 183–200 [DOI] [PubMed] [Google Scholar]
- [14].Williams JC, Hippensteel JA, Dilgen J, Shain W and Kipke DR 2007. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants J. Neural Eng 4 410–23 [DOI] [PubMed] [Google Scholar]
- [15].Woolley AJ, Desai HA and Otto KJ 2013. Chronic intracortical microelectrode arrays induce non-uniform, depth-related tissue responses J. Neural Eng 10 026007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Barz F, Livi A, Lanzilotto M, Maranesi M, Bonini L, Paul O and Ruther P 2017. Versatile, modular 3D microelectrode arrays for neuronal ensemble recordings: from design to fabrication, assembly, and functional validation in non-human primates J. Neural Eng 14 036010. [DOI] [PubMed] [Google Scholar]
- [17].Suner S, Fellows MR, Vargas-Irwin C, Nakata GK and Donoghue JP 2005. Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex IEEE Trans. Neural Syst. Rehabil. Eng 13 524–41 [DOI] [PubMed] [Google Scholar]
- [18].House PA, MacDonald JD, Tresco PA and Normann RA 2006. Acute microelectrode array implantation into human neocortex: preliminary technique and histological considerations Neurosurg. Focus 20 1–4 [DOI] [PubMed] [Google Scholar]
- [19].Ferńandez E, Greger B, House PA, Aranda I, Botella C, Albisua J, Soto-Śanchez C, Alfaro A and Normann RA 2014. Acute human brain responses to intracortical microelectrode arrays: challenges and future prospects Front. Neuroeng 7 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lempka SF, Miocinovic S, Johnson MD, Vitek JL and McIntyre CC 2009. In vivo impedance spectroscopy of deep brain stimulation electrodes J. Neural. Eng 6 046001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kozai TDY, Jaquins-Gerstl AS, Vazquez AL, Michael AC and Cui XT 2015. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies ACS Chem. Neurosci 6 48–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lungu C, Malone P, Wu T, Ghosh P, McElroy B, Zaghloul K, Patterson T, Hallett M and Levine Z 2014. Temporal macrodynamics and microdynamics of the postoperative impedance at the tissue–electrode interface in deep brain stimulation patients J. Neurol. Neurosurg. Psychiatr 85 816–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sillay KA, Ondoma S, Wingeier B, Schomberg D, Sharma P, Kumar R, Miranpuri GS and Williams J 2018. Long-term surface electrode impedance recordings associated with gliosis for a closed-loop neurostimulation device Ann. Neurosci 25 289–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Abosch A, Lanctin D, Onaran I, Eberly L, Spaniol M and Ince NF 2012. Long-term recordings of local field potentials from implanted deep brain stimulation electrodes Neurosurgery 71 804–14 [DOI] [PubMed] [Google Scholar]
- [25].Arlotti M et al. 2018. Eight-hours adaptive deep brain stimulation in patients with parkinson disease Neurology 90 e971–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bronte-Stewart H, Barberini C, Koop MM, Hill BC, Henderson JM and Wingeier B 2009. The STN beta-band profile in parkinson’s disease is stationary and shows prolonged attenuation after deep brain stimulation Exp. Neurol 215 20–28 [DOI] [PubMed] [Google Scholar]
- [27].Connolly AT, Jensen AL, Bello EM, Netoff TI, Baker KB, Johnson MD and Vitek JL 2015. Modulations in oscillatory frequency and coupling in globus pallidus with increasing parkinsonian severity J. Neurosci 35 6231–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Connolly AT, Jensen AL, Baker KB, Vitek JL and Johnson MD 2015. Classification of pallidal oscillations with increasing parkinsonian severity J. Neurophysiol 114 209–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kuhn AA, Trottenberg T, Kivi A, Kupsch A, Schneider GH and Brown P 2005. The relationship between local field potential and neuronal discharge in the subthalamic nucleus of patients with parkinson’s disease Exp. Neurol 194 212–20 [DOI] [PubMed] [Google Scholar]
- [30].Holdefer RN, Cohen BA and Greene KA 2010. Intraoperative local field recording for deep brain stimulation in parkinson’s disease and essential tremor Mov. Disorders 25 2067–75 [DOI] [PubMed] [Google Scholar]
- [31].Kolb R, Abosch A, Felsen G and Thompson JA 2017. Use of intraoperative local field potential spectral analysis to differentiate basal ganglia structures in parkinson’s disease patients Physiol. Rep 5 e13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen CC, Pogosyan A, Zrinzo LU, Tisch S, Limousin P, Ashkan K, Yousry T, Hariz MI and Brown P 2006. Intra-operative recordings of local field potentials can help localize the subthalamic nucleus in parkinson’s disease surgery Exp. Neurol 198 214–21 [DOI] [PubMed] [Google Scholar]
- [33].Rosa M et al. 2010. Time dependent subthalamic local field potential changes after DBS surgery in parkinson’s disease Exp. Neurol 222 184–90 [DOI] [PubMed] [Google Scholar]
- [34].Graham ML, Rieke EF, Mutch LA, Zolondek EK, Faig AW, DuFour TA, Munson JW, Kittredge JA and Schuurman H-J 2012. Successful implementation of cooperative handling eliminates the need for restraint in a complex nonhuman primate disease model J. Med. Primatol 41 89–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McMillan JL, Perlman JE, Galvan A, Wichmann T and Bloomsmith MA 2014. Refining the pole and collar method of restraint: emphasizing the use of positive training techniques with rhesus macaques (Macaca mulatta) J. Am. Assoc. Lab. Anim. Sci 53 61–68 [PMC free article] [PubMed] [Google Scholar]
- [36].Miocinovic S, Noecker AM, Maks CB, Butson CR and McIntyre CC 2007. Cicerone: stereotactic neurophysiological recording and deep brain stimulation electrode placement software system Acta Neurochir. Suppl 97 561–7 [DOI] [PubMed] [Google Scholar]
- [37].Agnesi F, Muralidharan A, Baker KB, Vitek JL and Johnson MD 2015. Fidelity of frequency and phase entrainment of circuit-level spike activity during DBS J. Neurophysiol 114 825–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang S, Connolly AT, Madden LR, Vitek JL and Johnson MD 2018. High-resolution local field potentials measured with deep brain stimulation arrays J. Neural Eng 15 046019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bokil H, Andrews P, Kulkarni JE, Mehta S and Mitra PP 2010. Chronux: a platform for analyzing neural signals J. Neurosci. Methods 192 146–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Aman JE. et al. Directional deep brain stimulation leads reveal spatially distinct oscillatory activity in the globus pallidus internus of parkinson’s disease patients. Neurobiol. Dis. 2020;139:104819. doi: 10.1016/j.nbd.2020.104819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Duprez J, Houvenaghel J-F, Dondaine T, Péron J, Haegelen C, Drapier S, Modolo J, Jannin P, Vérin M and Sauleau P 2019. Subthalamic nucleus local field potentials recordings reveal subtle effects of promised reward during conflict resolution in parkinson’s disease Neuro Image 197 232–42 [DOI] [PubMed] [Google Scholar]
- [42].Unterberg AW, Stover J, Kress B and Kiening KL 2004. Edema and brain trauma Neuroscience 129 1021–9 [DOI] [PubMed] [Google Scholar]
- [43].Marmor O, Valsky D, Joshua M, Bick AS, Arkadir D, Tamir I, Bergman H, Israel Z and Eitan R 2017. Local vs volume conductance activity of field potentials in the human subthalamic nucleus J. Neurophysiol 117 2140–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sun FT, Arcot Desai S, Tcheng TK and Morrell MJ 2018. Changes in the electrocorticogram after implantation of intracranial electrodes in humans: the implant effect Clin. Neurophysiol 129 676–86 [DOI] [PubMed] [Google Scholar]
- [45].Sato F, Lavallée P, Lévesque M and Parent A 2000. Single-axon tracing study of neurons of the external segment of the globus pallidus in primate J. Comp. Neurol 417 17–31 [PubMed] [Google Scholar]
- [46].Hazrati LN and Parent A 1992. Differential patterns of arborization of striatal and subthalamic fibers in the two pallidal segments in primates Brain Res. 598 311–5 [DOI] [PubMed] [Google Scholar]
- [47].Coudé D, Parent A and Parent M 2018. Single-axon tracing of the corticosubthalamic hyperdirect pathway in primates Brain Struct. Funct 223 3959–73 [DOI] [PubMed] [Google Scholar]
- [48].Michel CM and He B 2019. Chapter 6-EEG source localization Handbook of Clinical Neurology (Clinical Neurophysiology: Basis and Technical Aspects) ed Levin KH and Chauvel P vol 160 (Amsterdam: Elsevier; ) pp 85–101 [DOI] [PubMed] [Google Scholar]
- [49].Connolly AT, Muralidharan A, Hendrix C, Johnson L, Gupta R, Stanslaski S, Denison T, Baker KB, Vitek JL and Johnson MD 2015. Local field potential recordings in a non-human primate model of parkinsons disease using the activa PC + S neurostimulator J. Neural. Eng 12 066012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Malaga KA, Schroeder KE, Patel PR, Irwin ZT, Thompson DE, Nicole Bentley J, Lempka SF, Chestek CA and Patil PG 2016. Data-driven model comparing the effects of glial scarring and interface interactions on chronic neural recordings in non-human primates J. Neural. Eng 13 016010. [DOI] [PubMed] [Google Scholar]
- [51].Schmidt SL, Peters JJ, Turner DA and Grill WM 2020. Continuous deep brain stimulation of the subthalamic nucleus may not modulate beta bursts in patients with parkinson’s disease Brain Stimul 13 433–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cersosimo MG, Raina GB, Benarroch EE, Piedimonte F, Alemán GG and Micheli FE 2009. Micro lesion effect of the globus pallidus internus and outcome with deep brain stimulation in patients with parkinson’s disease and dystonia Mov. Disorders 24 1488–93 [DOI] [PubMed] [Google Scholar]
- [53].Koop MM, Andrzejewski A, Hill BC, Heit G and Bronte-Stewart HM 2006. Improvement in a quantitative measure of bradykinesia after microelectrode recording in patients with parkinson’s disease during deep brain stimulation surgery Mov. Disorders 21 673–8 [DOI] [PubMed] [Google Scholar]
- [54].Rezai AR, Kopell BH, Gross RE, Vitek JL, Sharan AD, Limousin P and Benabid A-L 2006. Deep brain stimulation for parkinson’s disease: surgical issues Mov. Disorders 21 S197–218 [DOI] [PubMed] [Google Scholar]
- [55].Mann JM et al. 2009. Brain penetration effects of microelectrodes and DBS leads in STN or GPi J. Neurol. Neurosurg. Psychiatr 80 794–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pogosyan A, Yoshida F, Chen CC, Martinez-Torres I, Foltynie T, Limousin P, Zrinzo L, Hariz MI and Brown P 2010. Parkinsonian impairment correlates with spatially extensive subthalamic oscillatory synchronization Neuroscience 171 245–57 [DOI] [PubMed] [Google Scholar]
- [57].Kuhn AA, Kupsch A, Schneider GH and Brown P 2006. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in parkinson’s disease Eur. J. Neurosci 23 1956–60 [DOI] [PubMed] [Google Scholar]
- [58].Ray NJ et al. 2009. Abnormal thalamocortical dynamics may be altered by deep brain stimulation: using magnetoencephalography to study phantom limb pain J. Clin. Neurosci 16 32–36 [DOI] [PubMed] [Google Scholar]
- [59].Steiner LA, Neumann W-J, Staub-Bartelt F, Herz DM, Tan H, Pogosyan A, Kuhn AA and Brown P 2017. Subthalamic beta dynamics mirror parkinsonian bradykinesia months after neurostimulator implantation Mov. Disorders 32 1183–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sharott A et al. 2014. Activity parameters of subthalamic nucleus neurons selectively predict motor symptom severity in parkinson’s disease J. Neurosci 34 6273–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Little S, Pogosyan A, Kuhn AA and Brown P 2012. Beta band stability over time correlates with parkinsonian rigidity and bradykinesia Exp. Neurol 236 383–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hunka K, Suchowersky O, Wood S, Derwent L and Kiss ZHT 2005. Nursing time to program and assess deep brain stimulators in movement disorder patients J. Neurosci. Nurs 37 204–10 [DOI] [PubMed] [Google Scholar]
- [63].Little S et al. 2016. Adaptive deep brain stimulation for parkinson’s disease demonstrates reduced speech side effects compared to conventional stimulation in the acute setting J. Neurol. Neurosurg. Psychiatr 87 1388–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rosa M et al. 2017. Adaptive deep brain stimulation controls levodopa-induced side effects in parkinsonian patients Mov. Disord 32 628–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Iturrate I. et al. Beta-driven closed-loop deep brain stimulation can compromise human motor behavior in parkinson’s disease. bioRxiv. 2019:696385. [Google Scholar]
- [66].Pollo C, Kaelin-Lang A, Oertel MF, Stieglitz L, Taub E, Fuhr P, Lozano AM, Raabe A and Schüpbach M 2014. Directional deep brain stimulation: an intraoperative double-blind pilot study Brain 137 2015–26 [DOI] [PubMed] [Google Scholar]
- [67].Velisar A, Syrkin-Nikolau J, Blumenfeld Z, Trager MH, Afzal MF, Prabhakar V and Bronte-Stewart H 2019. Dual threshold neural closed loop deep brain stimulation in parkinson disease patients Brain Stimul. 12 868–76 [DOI] [PubMed] [Google Scholar]
- [68].Stanslaski S, Afshar P, Cong P, Giftakis J, Stypulkowski P, Carlson D, Linde D, Ullestad D, Avestruz A-T and Denison T 2012. Design and validation of a fully implantable, chronic, closed-loop neuromodulation device with concurrent sensing and stimulation IEEE Trans. Neural Syst. Rehabil. Eng 20 410–21 [DOI] [PubMed] [Google Scholar]
- [69].Johnson LA, Nebeck SD, Muralidharan A, Johnson MD, Baker KB and Vitek JL 2016. Closed-loop deep brain stimulation effects on parkinsonian motor symptoms in a non-human primate—is beta enough? Brain Stimul 9 892–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the authors.


