Abstract
Mycotoxins are secondary metabolites produced by fungus. Many mycotoxin species are highly toxic and are frequently found in cereals and feedstuffs. So, powerful detection methods are vital and effective ways to prevent feed contamination. Traditional detection methods can no longer meet the needs of massive, real-time, simple, and fast mycotoxin monitoring. Rapid detection methods based on advanced material and sensor technology are the future trend. In this review, we highlight recent progress of mycotoxin rapid detection strategies in feedstuffs and foods, especially for simultaneous multiplex mycotoxin determination. Immunoassays, biosensors, and the prominent roles of nanomaterials are introduced. The principles of different types of recognition and signal transduction are explained, and the merits and pitfalls of these methods are compared. Furthermore, limitations and challenges of existing rapid sensing strategies and perspectives of future research are discussed.
Keywords: Biosensors, Immunoassays, Multiple detection, Mycotoxins, Nanomaterials, Rapid detection
Introduction
Topics related to food safety have been attracting significant attention all over the world. Contaminants in cereals and animal products are related closely to human health. Mycotoxins are secondary metabolites of fungus and may be produced during growth, production, processing, and storage of cereals, grains and feedstuffs [1]. There are over 300 mycotoxins identified, many of which are extremely toxic and difficult to degrade by cooking, baking or frying because of their high heat-stability [2]. The International Agency for Research on Cancer (IARC) has classified several mycotoxins into different categories based on their carcinogenic risk to human health. For example, aflatoxin B1 (AFB1) is the most carcinogenic mycotoxin and is classified as Group 1 while ochratoxin A (OTA) and fumonisin are classified into Group 2B as possibly carcinogenic in humans [3–5]. Many countries have developed standards to control mycotoxin contamination of food for safety reasons [6]. In China, maximum residue levels (MRLs) for mycotoxins in various foods have been strictly regulated in national food safety standard system. Consequently, a powerful detection method for mycotoxins is critical component in assuring food safety.
Traditional analytical methods such as enzyme-linked immunoassay (ELISA), thin layer chromatography, gas chromatography or high-performance liquid chromatography (HPLC) coupled with ultraviolet detection, fluorescence detector electron capture detectors, diode array detectors, and mass spectrometry (MS) detectors have been used to detect mycotoxins for decades [7–10]. Most of these traditional methods are accurate and sensitive but time-consuming, expensive, or require sophisticated instruments and professional technicians. They are not appropriate for a large number of samples and on-site screening. Because of demands from food industry professionals and government regulations, analytical methods that can detect multiple mycotoxins simultaneously and are fast, simple, sensitive, and low-cost with high-throughput have become the norm in the field of food safety and will continue into the future [11].
Rapid detection technology is a concept relative to traditional analysis methods and laboratory detection technology. It is often based on interdisciplinary subjects such as nanomaterials science, immunology, molecular biology, spectroscopy, and electrochemistry. Rapid detection is simple, cheap, easy to operate, and only requires portable instrument and a very short detection duration. It enables detection method to meet the need of the real-time on-site mycotoxins screening in the field of food safety. Herein, we review published research reports on rapid detection technology for mycotoxins from 2016 to 2021 including immunoassays and biosensors. Principles of recognition and signal transduction strategies are explained, and the pros and cons compared to existing method are discussed. Especially, we highlight studies on simultaneous detection of multiple mycotoxins. Limitations, challenges and perspectives of the future developments in this field are discussed.
Types of recognition strategies
Specific recognition of analytes is the primary step of rapid detection, which ensures the specificity and selectivity of the analytical method. In this section, different types of recognition elements for mycotoxin detection are introduced including antibodies, aptamers, and molecularly imprinted polymers (MIPs).
Antibodies
Antibodies are immunoglobulins produced by the immune system that specifically bind to the corresponding antigens. Based on the preparation processes used, antibodies are classified into polyclonal, monoclonal, or recombinant categories. Antibody-antigen recognition is regarded as a gold standard because of its properties of high specificity and affinity. Antibodies and antigens are the most widely commercialized recognition and capture agents applied in immunoassays and biosensors for clinical diagnosis, disease treatment, environment monitor, and food safety control [12–15]. However, there are obvious disadvantages of using antibodies. First, acquisition of high-quality antibodies requires immunization and purification which is a complex process that is time-consuming and costly. Second, antibodies are sensitive to pH and temperature conditions, which may narrow the practical application. Finally, only immunogenic and immunoreactive molecules can be identified by antibodies. Targets of small molecular weight need to conjugate with a carrier protein to enhance immunogenicity, but the chemical conjugation efficiency of mycotoxins to a protein carrier is low.
To overcome these limitations of antibodies, variable region of heavy chains antibody (nanobody) and phage-displayed mimotope peptides may be some utility in solving these antibody problems [16]. Nanobody as a naturally deficient light chain antibody is highly stable and small in size, which makes it easy to conserve and use. Phage displayed mimotope peptide can simulate the epitope of the target analytes so the hapten-carrier conjugate can be replaced by a mycotoxin-free mimic which also makes fabrication of green sensors possible [17–21].
Aptamers
Aptamers are small fragments of oligonucleotide sequences (single stranded DNA or RNA), which usually contain 10 to 100 bases. Aptamers bind to their targets by folding into specific three-dimensional structures. They are selected from a combinatorial DNA library by a technology named systematic evolution of ligands exponential enrichment (SELEX) and can bind to various targets ranging from ions to cells. Aptamers are usually considered to be ideal affinity reagents alternatives to antibodies. Compared to antibodies, aptamers are inexpensive, stable, reversible, not limited by immunogenicity of targets, and do not require immunization of animals during production. Aptamers have high bioavailability and are easy to modify. As an excellent alternative to antibodies, aptamers are being deployed in biosensor, disease diagnosis, and drug delivery [22–24]. However, acquiring aptamers with high affinity and sensitivity especially for small molecules is relatively inefficient. Over the past few years, selection techniques have been improved constantly. A series of novel SELEX-based techniques such as Cell-SELEX, Capillary electrophoresis-SELEX, Capture-SELEX, and Post-SELEX modifications have been developed. Compared with the original SELEX technique, aptamers developed with these new approaches display high affinity and specificity while selection processes are more efficient and cost-effective [25, 26]. Although aptamers are currently prevalent in scientific literatures, commercial aptamer-based mycotoxin detection kits are not yet available [27].
Molecularly imprinted polymers (MIPs)
Molecular imprinting is a technology developed based on bionic science and simulation of the interactions of enzymes with their target substrates and receptors with their antibodies in nature. Molecularly imprinted polymers (MIPs) are obtained by mixing imprinted molecules (template molecules) with appropriate functional monomers (usually small molecular compounds) and crosslinking agents. Then polymerize them and eluting the imprinted molecules by appropriate methods. Molecularly imprinted polymers have been called “artificial antibodies”. They are preserved easily, resistant to extreme conditions such as high temperature, high pressure, acid, and alkali, and difficult to destroy by biodegradation [28]. Consequently, MIPs are widely applied in optical sensors, electrochemical sensors, quartz crystal microbalances, simulated catalysis, membrane separations and solid phase extractions (SPE) [29–33]. In spite of these benefits, MIPs face several challenges in practical applications. A considerable drawback of MIPs is unspecific interactions, especially with small molecules, which requires non-imprinted polymers (NIPs) be employed to characterize nonspecific binding [34]. Selectivity and binding affinity of MIPs still require development to allow detection of target compounds in complex matrixes [33]. Commercialization of MIPs is in its infancy. Relatively few research studies focused on MIPs-based mycotoxin sensors are reported in the scientific literature.
Rapid detection strategies
Immunoassays
Immunoassays are based on immunological principles that use antibodies to recognize and capture target antigens or haptens. Enzyme-linked immunosorbent assays (ELISA) are early immunoassays that have been commercially applied in mycotoxin detection with good sensitivity and accuracy [35]. Enzyme-linked immunosorbent assays are simpler than instrumental methods such as HPLC and LC-MS/MS, but they still require complex operations and several hours of incubation and microplate washing procedures. Therefore, researchers have been working to develop various immunoassays for better analytical performance. New procedures such as fluorescent-linked immunosorbent assays (FLISA) and chemiluminescence enzyme immunoassays (CLEIA) can reduce detection duration and improves the sensitivity by relying on enhanced optical signals [36–38]. Lateral flow immunoassays (LFIA) and immunosensors have become popular analytical methods in recent years. In this part, LFIA is chiefly introduced and immunosensor is introduced in the following sensor section.
Lateral flow immunoassays
Lateral flow immunoassays (LFIA), also known as immunochromatographic assays (ICA), are the most frequently used and commercialized rapid sensing platform and widely applied in disease diagnosis, point-of-care testing (POCT) and food safety monitoring because of its low cost, rapidity and simplicity [39, 40]. The detection process is integrated on a small strip and the signal analysis requires portable instruments and very little time. Lateral flow immunoassays are highly convenient to achieve simultaneous detection of multiple mycotoxins by using multiple test lines and signal labels. There are two main detection principles used, sandwich assay (two types of antibodies are required) and competitive assay (one type of antibodies are required). Mycotoxin cannot acquire two types of recognition antibodies because it doesn’t own multiple epitopes, so the competitive LFIA is preferred.
High-quality antibodies and antigens are primary guarantees for the high sensitivity and specificity of LFIA. However, the recognition of limited immunogenic targets by antibodies has hindered development of LFIA in several fields of study. Secondly, there is a contradiction in the competitive detection mode itself. That is, within a certain range, the less quantity of antibodies makes the competition between free target analytes and the immobilized antigens more effective on the test zone. So, the lower the specific antibody concentration, the lower the detection limit. However, the amount of the label conjugated with antibody is reduced simultaneously with the low concentration of antibody, which decreases signal intensity of the test line and hinders successful detection of the targets.
To overcome these shortcomings above, researchers developed better-performed antibodies or signal labels with high luminescent intensity and stability [41]. Besides, the new detection principle based on fluorescence quenching that can directly response to changes of target analytes has been attempted [42].
Signal labels
Gold nanoparticles (AuNPs) are the most frequently used signal labels in LFIA because they are easy to synthesize, inexpensive, and emit visible red to purple light when they aggregate. However, an obvious drawback of AuNPs-based LFIA is the instability and weak optical intensity of AuNPs resulting in low sensitivity [41, 43]. Recently, non-spherical, multilayer AuNPs with varying colors such as flower-like AuNPs have been used in LFIA instead of traditional spherical AuNPs to improve the sensitivity [44–46]. Wu et al. [47] engineered gold nanoparticles to create multicolor labels such as red gold nanospheres, purple gold nanocacti, blue gold nanoflowers and black hyperbranched Au plasmonic blackbodies. Four mycotoxins (FB1, ZEN, OTA and AFB1) can be successfully detected in corn using this multicolor LFIA strip and limit of detection (LOD) for FB1, ZEN, OTA, and AFB1 were 3.27, 0.70, 0.10, and 0.06 ng/mL, respectively.
Use of various fluorescent labels with higher stability and signal intensity in LFIA to enhance the sensitivity has become commonplace. Quantum dots (QDs) are semiconductor nanoparticles that possess excellent optical properties including size-tunable emission, broad adsorption, narrow photoluminescence spectra, high photostability, large Stokes’s shift, and long fluorescence lifetime [48]. Fluorescent microspheres (FMs) are polymers that contain numerous fluorescent particles such as fluorescent dyes and quantum dots in the nanobeads. Fluorescent microspheres are more stable and have substantially higher fluorescent intensity than that of single fluorescent molecule [49]. Carbon-based nanoparticles (CNPs) such as carbon dots have desirable optical properties similar to quantum dots, and have low toxicity [50]. These materials are promising LFIA labels because they are water soluble and they are not easy to aggregate and precipitate. Functional groups like carboxyl can be easily modified on their surfaces, which helps them bind to antibodies with great stability due to covalent binding between hydroxyl and carboxyl groups.
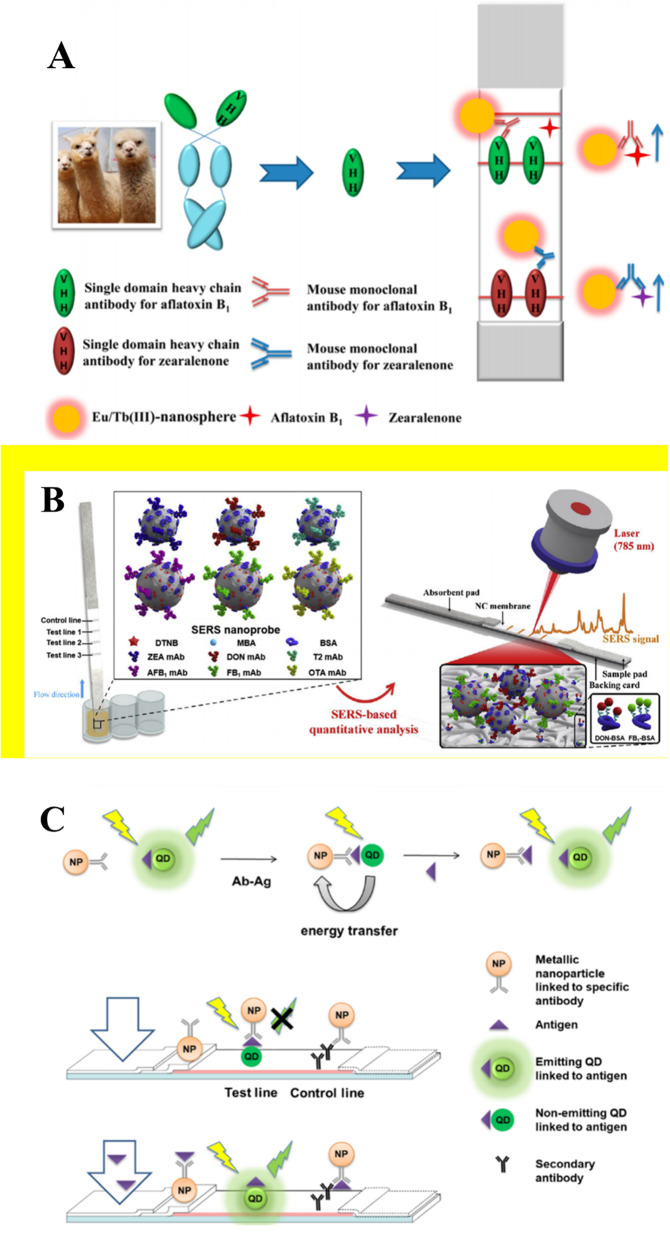
Time-resolved fluorescent microspheres (TRFMs) are recently employed as signal labels frequently. Lanthanides such as Eu (III), Tb (III) have much longer fluorescence lifetime than the previously mentioned fluorescent materials so that they can eliminate the background fluorescence interference from the matrix, thus improving the sensitivity of LFIA [51–53]. For example, Fig. 1A shows time-resolved fluorescent Eu/Tb (III) nanosphere and idiotypic nanobodies for multiplex detection of two mycotoxins simultaneously in maize [52]. Sensitivity of LFIA was improved by the enhanced fluorescence of Eu/Tb (III) nanosphere with the LOD of 0.05 and 0.07 ng/mL for AFB1 and ZEN, respectively. Another study reported novel α-Fe2O3 nanocubes used as LFIA label for simultaneously detecting two mycotoxins. These nanocubes have been employed to detect AFB1 and DON residues in mung bean, millet and corn, with the visual LOD for AFB1 and DON of 0.01 and 0.18 ng/mL, respectively [54].
Fig. 1.
Structure and test procedure of LFIA. (A) Time-resolved fluorescent Eu/Tb (III) nanosphere for detecting AFB1 and ZEN; (B) multiplex SERS-based lateral flow immunosensor for the detection of six mycotoxins; (C) detection of OVA based on donor probe (CD-OVA) and accepter probe (AgNP-Ab). Diagrams A, B, and C are adapted from Ref. [52] Ref. [60] and Ref. [42], respectively
Researchers have compared various signal labels to provide a reference for selection of appropriate labels. For instance, colloidal gold (CG) and QDs as labels for multi-mycotoxin detection in cereal matrices have been compared. The QD-LFIA was more sensitive and economical than CG-LFIA [41]. In another study, CG and FMs were compared as the LFIA labels for T-2 toxin detection in rice, fresh milk, and chicken feed. The cut-off values of the CG-LFIA and the FMs-LFIA were 400 μg/kg and 100 μg/kg, respectively. And they found CG and FMs performed differently in different matrixes. Under the same experimental conditions, CG had better tolerance to milk while FMs had better tolerance to chicken feed [55]. A comparative study of LFIA of ZAE in cereals using CG, QDs and polystyrene microspheres (PMs) as signal label was conducted [56]. Researchers reported that QD-LFIA and PM-LFIA were ten times more sensitive than CG-LFIA. Besides, a smartphone-based dual mode LFIA device for multiplex mycotoxins in cereals was developed, which was integrated with AuNPs and TRFMs as labels. The result indicated that the LOD of TRFMs mode were lower than that of AuNPs mode [57].
Gold nanoparticles and sliver nanoparticles can provide another detectable signal termed Surface-Enhanced Raman Spectroscopy (SERS). Surface-Enhanced Raman Spectroscopy effect is the phenomenon that in the excitation region of some specially prepared metal good conductor surface or colloidal sol. The Raman scattering signal of adsorbed molecules is greatly enhanced compared with the ordinary Raman scattering signal because the enhanced electromagnetic field on the sample surface. When SERS nanotags (gold, silver or Au-Ag alloy nanoparticles labelled with Raman reporter molecules) are exposed to a laser light source, the incident field is obviously enhanced at active sites (electromagnetic “hot spots”) due to localized surface plasmon resonance effects [58, 59]. Combining SERS detection and lateral flow immunoassay is also regarded as a promising choice for highly sensitive and multiplex mycotoxin detection. Fig. 1B show a SERS-based LFIA combined dual-Raman label (DTNB and MBA) with triple-line LFIA and labelled-Au@Ag core-shell nanoparticles serving as SERS nanoprobes [60]. The SERS-based LFIA can be used for the detection of six common mycotoxins (AFB1, ZEN, FB1, DON, OTA and T-2) simultaneously in maize with high sensitivity and selectivity within 20 min. Signal acquisition requires only a portable Raman System.
Fluorescence quenching LFIA
In addition to competitive assays, researchers have explored LFIA based on fluorescence quenching principle to improve sensitivity. Competitive assays need many target analytes to compete with Immobilized antigen then make the signal of test lines “turn off”. The signal is an indirect signal, which leads to relative low sensitivity. While the fluorescence quenching LFIA can provide a “turn on” signal, which responses to the amount of target analytes directly [61, 62]. Inner-filter effect (IFE) and Förster resonance energy transfer (FRET) are widely used quenching mechanisms. Quenching agents usually are metal nanoparticles such as AuNPs or AgNPs. Inner-filter effect is a radiation energy transfer mechanism while Förster resonance energy transfer is a non-radiative process. Quenching effects occur when the absorption spectrum of the accepter (quencher) overlaps with the donor’s excitation or emission spectra. Antigens (analytes) are usually linked with fluorescence donors then immobilized on the test line. Antibodies are usually linked with fluorescence accepters (quenchers) which can bind to the analytes. If there is no analyte in the sample solution, antibodies-linked accepters will bind to antigens-linked donors resulting in fluorescence quenching of the donor. Conversely, if there are many analytes in the sample solution binding with antibodies-linked accepters, the donor’s fluorescence will be restored. Fig. 1C shows a fluorescence quenching LFIA based on the CdSe/ZnS quantum dots and gold/silver nanoparticles for straightforward detection of fumonisin in maize flour [42]. Silver nanoparticles overlapped excitation bands of the QDs, and AuNPs overlapped emission bands. The QD quenching was due to IFE. Silver nanoparticles were demonstrated to be more efficient in QDs quenching because absorbing the exciting light was more efficient in IFE. Compared to conventional AgNPs-based LFIAs, the visual detection limit was four times lower. Another attempt explored a quencher system based on FRET mechanism for fluorometric LFIA for zearalenone detection in cereal samples. Carbon dots were conjugated to ovalbumin as the donor signal probe and AgNPs-Antibody served as the acceptor signal probe. The LOD of the LFIA was 10 times better than the “turn-off” AgNPs-based LFIA [63].
Based on existing studies, fluorescence quenching LFIAs exhibited a better performance than traditional AgNPs-based LFIAs but there have been hardly any researches on multiple mycotoxin detection using fluorescence quenching LFIA. Most of traditional LFIAs and fluorescence quenching LFIA can only render qualitative or semi-quantitative results at present. False positive and false negative results are still a problem. Selected studies of LFIAs for mycotoxin detection published in recent years are listed and compared in Table 1.
Table 1.
Summary of LFIA strips for detecting mycotoxins
| Target | Principle | Signal material | Sample | LOD | Ref. |
|---|---|---|---|---|---|
| FB1/ZEN/OTA/AFB1 | Competitive LFIA | Au nanospheres, Au nanocacti, Au nanoflowers and hyperbranched Au plasmonic blackbodies | Corn | 3.27/0.70/0.10/0.06 ng/mL | [47] |
| FB1/DON | Competitive LFIA | Flower-like gold nanoparticles | Chinese traditional medicine | 5.0/5.0 ng/mL | [45] |
| FB1/DON | Competitive LFIA | Au nanospheres/Au nanoflowers | Grain | 20/5 ng/mL | [44] |
| DON/AFB1 | Competitive fluorescent LFIA | α-Fe2O3 nanocubes | Food | 0.18/0.01 ng/mL | [54] |
| CIT/ZEN | Competitive fluorescent LFIA | Europium nanoparticles | Corn | 0.06/0.11 ng/mL | [64] |
| ZEN/DON | Competitive fluorescent LFIA | Near-infrared dyes | Maize | 0.55/3.8 μg/kg | [65] |
| ZEN/OTA/FB1 | Competitive fluorescent LFIA | Quantum dot nanobeads | Wheat | 5/20/10 ng/mL | [66] |
| ZEN/DON | Competitive fluorescent LFIA | Silanized quantum fots | Maize and wheat | 40 and 400 μg/kg (Cutoff value) | [67] |
| AFB1/ZEN/DON/T-2/FB1 | Competitive fluorescent LFIA | Time-resolved fluorescence microspheres | Cereals | 0.42/0.10/0.05/0.75/0.04 μg/kg | [57] |
| AFB1/ZEN | Competitive fluorescent LFIA | Time-resolved fluorescence microspheres | Maize | 0.05/0.07 ng/mL | [52] |
| AFB1/ZEN/DON | Competitive fluorescent LFIA | Quantum dot microbeads | Feedstuff | 10/80/500 pg/mL | [68] |
| DON/T-2/ZEN | Competitive fluorescent LFIA | Amorphous carbon nanoparticles | Maize | 20/13/1 μg/kg | [69] |
| AFB1/FB1/OTA | Competitive fluorescent LFIA | Quantum dot nanobeads | Cereals | 1.65 pg/mL, 1.58/0.0059 ng/mL | [70] |
| DON | Competitive fluorescent LFIA | Polydopamine coated zirconium metal-organic frameworks | Meat | 0.18 ng/mL | [71] |
| AFB1 | Fluorescence quenching LFIA (IFE) | Flower-like gold nanoparticles/quantum dots | Soybean sauce | 0.004 μg/L | [61] |
| FB1 | Fluorescence quenching LFIA (IFE) | Sliver nanoparticles/quantum dots | Maize flour | 62.5 μg/kg | [42] |
| ZEN | Fluorescence quenching LFIA (FRET) | Sliver nanoparticles/carbon dots | Cereals | 0.1 μg/L | [63] |
|
AFB1/ZEN/FB1/DON/ OTA/T-2 |
SERS-based LFIA | DTNB and MBA labelled Au@Ag coreshell nanoparticles | Maize | 0.96/6.2/0.26/0.11/15.7/8.6 pg/mL | [60] |
Biosensors
Biosensors are detection devices that can convert the information measured into electrical signals or other information output of the required form. The biosensor system mainly consists of a biorecognition (biosensing) element and a transducer element. Biorecognition elements such as antibodies, antigens, nucleic acids, and enzymes are used to identify and sense target analytes. Transducer elements convert the physical quantity signal of generated by the recognition elements into detectable signals. According to the principle of the signal transduction, biosensors can be classified into electrochemical, optical, mass-sensitive, and thermal sensors. They have become vital and powerful analytical tools widely applied in various fields including medicine, food, agriculture, environment, and industry. Biosensor systems were introduced to enhance food safety in the 1980s and provide great commercial potential currently [16, 72–74]. In this section, electrochemical and optical biosensors for mycotoxin detection are mainly introduced.
Optical biosensors
Optical sensors rely on variation in optical signals generated by transducer from molecular recognition events on sensing element. This approach excels in superiorities of simplicity, speed of detection, sensitivity and visualization [75]. On the basis of optical signals, optical biosensors are divided into many subclasses including colorimetric, fluorescent, chemiluminescent and surface plasmon resonance. Optical biosensor systems also exhibit many possibilities for determination of multiple mycotoxins simultaneously.
Colorimetric biosensors
Colorimetric biosensors are based on color changes induced by target analytes that can be easily distinguished by the naked eye. Gold or silver nanoparticles are most frequently applied as indicators to utilize their surface plasmon resonance (SPR) properties. The general principle is that recognized targets induce aggregation of AuNPs or AgNPs from well-dispersed state resulting in color change from red to blue.
A colorimetric aptasensor for simultaneously detecting ochratoxin A and aflatoxin B1 in peanut was reported [76]. Researchers fabricated a Fe3O4/GO based platform and a Fe3O4@Au based platform for AFB1 and OTA sensing. Quantitative detection of OTA and AFB1 was respectively carried out by release of thymolphthalein and 3,3′,5,5′-tetramethylbenzidine catalyzed by gold nanoparticles, respectively. In this work, OTA and AFB1 were easily and visibly detected in one system with the linear ranges of 0.5–80 and 5–250 ng/mL and did not interfere with each other because of different color reaction conditions.
In another attempt, a colorimetric sensor based on array of gold and silver nanoparticles was fabricated for simultaneous detection of AFB1, AFG1, AFM1, OTA and ZEN [77]. Mycotoxin interactions with nanoparticles induced aggregation of gold or silver nanoparticles and the color changed. Every type of mycotoxin was recognized by its unique colorimetric signatures with the LOD of 2.7, 7.3, 2.1, 3.3 and 7.0 ng/mL for AFB1, AFG1, AFM1, OTA and ZEN, respectively. The developed colorimetric method had the advantages of high throughput, simplicity, rapidity and low cost. This system was tested in pistachio, wheat, coffee, and milk samples. Most of colorimetric sensors suffer the weakness of low sensitivity which suggests optical signal intensities still need further enhancement.
Fluorescent biosensors
At present, fluorescent biosensors are the most popular optical sensors because of their high sensitivity, stable signal, and fast response. Various nano-materials such as magnetic nanoparticles (MNPs), quantum dots (QDs), carbon nanoparticles (CNPs), gold nanoparticles (AuNPs), fluorescent nanobeads, and graphene oxide (GO) have been widely applied in construction of fluorescent sensors [78–80]. Lanthanide-based luminescent materials such as upconverting nanoparticles (UCNPs) and time-resolved fluorescence materials (TRFMs) have also become popular in recent years [81, 82]. After target analytes have been identified by recognition elements, quantitative sensing of one mycotoxin is achieved by the variation of fluorescent signals based on fluorescence quenching, fluorescence enhancement, or displacement of fluorescent labels.
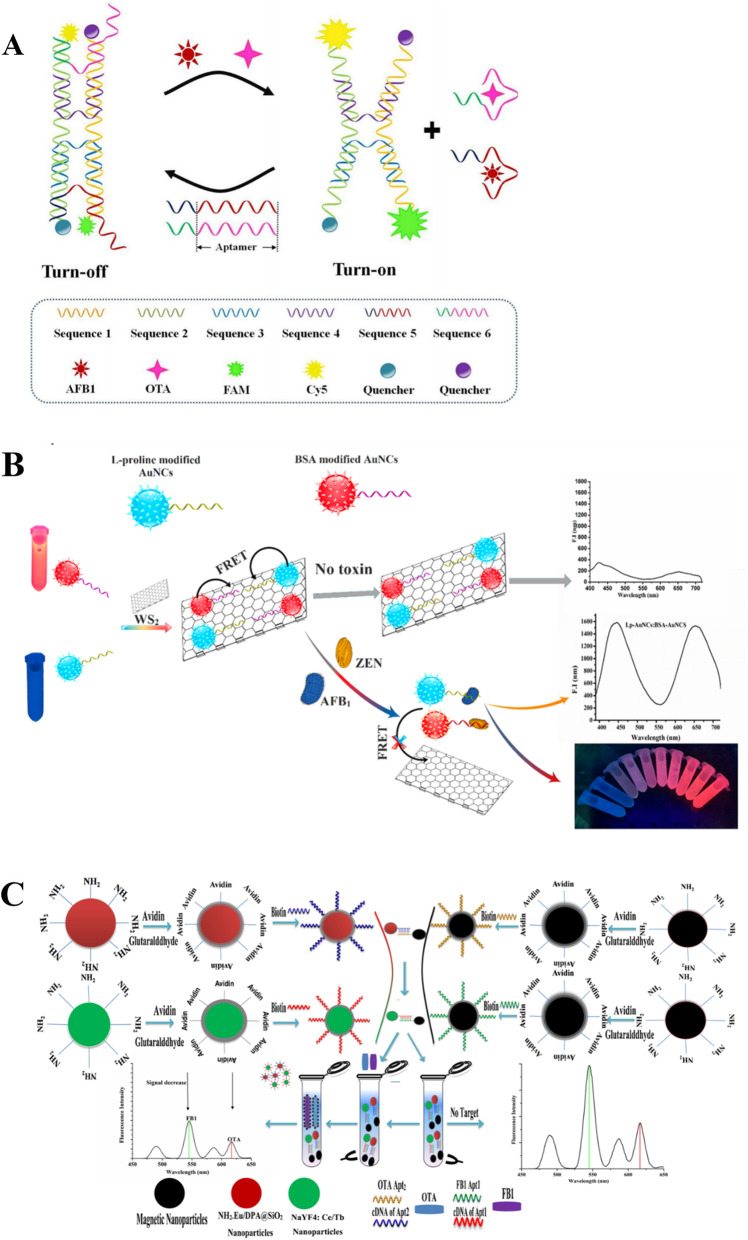
Figure 2A shows dual DNA tweezers nanomachine has been utilized for simultaneous detection of AFB1 and OTA [83]. The fluorophores were quenched by the quencher when DNA tweezers were closed. In the presence of AFB1 and OTA, DNA tweezers opened after the aptamer strands bind with their corresponding targets resulting in the activated fluorescent signals. The aptasensor exhibited satisfactory applicability in food samples including corn, peanut, olive oil, peanut oil, and coffee.
Fig. 2.
Schematic illustration of the optical sensing platforms. (A) Dual DNA tweezers nanomachine for simultaneous detection of AFB1 and OTA; (B) a FRET-based platform using WS2 nanosheet and dual-color gold nanoclusters (AuNCs) for simultaneous detection of AFB1 and ZEN; (C) a fluorescence aptasensor for FB1 and OTA detection by using TRF-NPs and MNPs; Diagrams A, B, and C are adapted from Ref. [83], Ref. [84], and Ref. [81], respectively
A FRET-based platform was developed by WS2 nanosheet and dual-color gold nanoclusters (AuNCs) for simultaneous detection of AFB1 and ZEN in maize [84]. Gold nanoclusters and WS2 were connected by AFB1/ZEN-aptamer-BSA resulting in fluorescent quenching of AuNCs. Fluorescence turned on when target analytes were present (Fig. 2B). The sensor achieved multiplex target determinations in a homogeneous system with excellent simplicity, sensitivity, and selectivity. However, this system can only provide semiquantitative detection. Another FRET-based platform was proposed using GO/Fe3O4 as a single energy acceptor, which can quench the dual aptamer-modified QDs simultaneously and eliminate background interference effectively by magnetic separation [85]. This system successfully detected AFB1 and FB1 in peanut with the LOD of 6.7 and 16.2 pg/mL, respectively.
Time-resolved fluorescence materials are also excellent materials for fluorescent probes design because of its long fluorescence lifetime, which provides anti-interference capability. For example, Fig. 2C shows a fluorescence aptasensor for FB1 and OTA detection in maize samples by using time-resolved fluorescence nanoparticles (TRF-NPs) and MNPs [81]. The aptamer-MNPs and cDNA-TRF-NPs formed the duplex structure. With the addition of FB1 and OTA, the aptamer-MNPs bound to FB1 and OTA then dissociated from its cDNA, leading to a decrease in fluorescent intensity when magnetic separation. The sensor was user-friendly with LOD for FB1 and OTA were 0.019 and 0.015 pg/mL, respectively. The aptasensor exhibited better practicability than common fluorescent system in complex metrics because of the unique merits of TRFM-NPs.
Chemiluminescence and electrochemiluminescence biosensors
Chemiluminescence is a light radiation phenomenon resulting from a chemical reaction, which is also an important tool for molecular sensing. But, low luminescence intensity of chemiluminescence needs to be enhanced by enzymes or metal nanoparticles in practical applications [86]. For instance, a AgNPs modified surface-plasmon-coupled chemiluminescence immunosensor was developed for aflatoxin B1 and ochratoxins A detection in red yeast rice [87]. The generated chemiluminescence signal on the chip was amplified by AgNPs through the SPR phenomenon, which greatly enhanced sensitivity of the biosensor. The chip array offered a high-throughput detection mode for multiple targets. But several incubations and washing operations were needed, which complicated the assay. The problem of uneven brightness made advanced instruments and equipment necessary to achieve acceptable readings.
Electrochemiluminescence (ECL), as a subclass of chemiluminescence or electrochemistry, is regarded as the reverse of photoelectron chemistry. Voltage or current serves as the excitation source and the generated chemiluminescence is used as the detectable signal. With ECL, the electro-active species generated in the vicinity of the electrode surface are in excited states emitting light that arises from the high-energy electron-transfer reactions of these species. Luminol and Ru (bpy)32+ are classic ECL luminophores while QDs are beginning to be applied to ECL sensors to achieve higher efficiency and stability of ECL [88, 89]. Electrochemiluminescence-based sensors have fast response, high sensitivity and low background interference, which is a promising strategy for applying in rapid detection [90]. However, ECL biosensors have similar drawbacks observed with electrochemical biosensors, such as instability of electrode including the degradation biosensing reagents and the instable optical signal of ECL luminophores. And few ECL instruments have been reported. Advanced and portable equipment is urgently needed.
A nicking endonuclease-powered DNA walking machine was proposed to fabricate an ECL aptasensor for OTA detection [91]. Quantum dots produced ECL signal and was greatly amplified by DNA walking machine, which increased the sensitivity of this ECL sensor. This ECL aptasensor showed satisfactory recoveries of OTA in beer and wine with the LOD of 12 pmol/L. Other researchers developed a label-free ZnCdS@ZnS QDs-based ECL immunosensor for AFB1 detection in lotus seed [92]. Quantum dots and Nafion were assembled on Au electrode surface as ECL signal probes and anti-AFB1 antibodies were coupled as the capturing element. Using of Nafion and core-shell QDs greatly enhanced the ECL signal thus improved the sensitivity. The label-free design simplified the synthesis steps and reduced detection time.
Surface plasmon resonance biosensors
Surface plasmon resonance (SPR) sensors are optical sensors based on changes in the refractive index due to the mass changes that occur when molecules bind to the sensor surface. These changes in refractive index can provide direct recognition information between a target and a probe on the sensor surface. Therefore, SPR sensor enables monitoring the real-time interaction among molecules on a tiny chip which can be a powerful analytical tool for purpose of medical diagnostics, food safety analysis and environmental monitoring [19, 93].
A competitive-type SPR sensor for DON and OTA detection by imaging nanoplasmonics was developed [94]. A portable nanostructured imaging surface plasmon resonance (iSPR) chip was immobilized with 3-dimensional carboxymethylated dextran. Afterwards, target mycotoxins and immobilized mycotoxins competitively bound to the corresponding antibodies producing SPR signals. The SPR-based sensor achieved portable detection for DON and OTA in beer with the LOD of 17 and 7 ng/mL, respectively.
Another study described a SPR-based aptasensor in a direct assay format for AFB1 detection. The aptamers were modified on the commercial sensor chip surface, and the SPR signals increased when AFB1 bound to aptamers to achieve quantification with the LOD of 0.4 nmol/L (124.8 pg/mL) [95]. A label-free microfluidic SPR biosensor based on nanoparticles integrated gold chip for Aflatoxin B1 detection was developed. A self-assembled monolayer (SAM) Au chip was modified with AFB1 antibodies and functionalized lipoic acid AuNPs were deposited on it. This multilayer functionalized AuNPs modified Au chip exhibited better sensing performance than bare self-assembled Au chip with the LOD of 0.003 nmol/L (0.93 pg/mL) [96].
By comparing these studies, we can infer that direct SPR-sensors are simpler and more sensitive than indirect SPR-sensors. However, because the small molecule analytes (such as mycotoxins) cannot cause obvious mass concentration changes at the sensor surface, the direct SPR-sensor’s chip surface must be modified with noble metal NPs for signal amplification. More advanced portable SPR instruments are required for practical application.
Optical biosensor platforms that recently reported for mycotoxin determinations are listed in Table 2.
Table 2.
Summary of optical biosensor platforms for detecting mycotoxins
| Target | Principle | Materials | Sample | LOD | Ref. |
|---|---|---|---|---|---|
| OTA/AFB1 | Colorimetric | Aptamer, Fe3O4/graphene oxide and Fe3O4@Au | Agricultural products | 0.5/5 ng/mL | [76] |
| AFB1/AFG1/AFM1/OTA/ZEN | Colorimetric | gold and silver nanoparticles | Pistachio, wheat, coffee and milk | 2.7/ 7.3/ 2.1/3.3/ 7.0 ng/mL | [77] |
| AFB1 | Colorimetric | Aptamer and AuNPs | Animal feed and milk | 10 nmol/L | [97] |
| ZEN | Colorimetric | Aptamer and AuNPs with peroxidase-like activity | Corn and corn oil | 10 ng/mL | [98] |
| AFB1/OTA | Fluorescent | Aptamer, DNA tweezers | Food | 0.035 ng/mL | [83] |
| AFB1/OTA/FB1 | Fluorescent protein microarray | Antibody, TiO2-modified porous silicon | Rice, corn and wheat | 0.243/0.433/0.093 ng/mL | [99] |
| ZEN/OTA/FB1 | Fluorescent | Aptamer, upconversion nanoparticle and gold nanoparticle | Corn | 30/10/0.02 pg/mL | [100] |
| ZEN/FB1 | Fluorescent | Aptamer, gold nanorods and upconversion nanoparticles | Corn | 0.01/0.003 ng/mL | [101] |
| AFB1/FB1 | Fluorescent | Aptamer, graphene oxide/Fe3O4 and CdTe quantum dots | Peanut | 6.7/16.2 pg/mL | [85] |
| OTA/OTB | Fluorescent | Nanobody, Eu/Tb nanosphere | Rice | 0.06/0.12 ng/mL | [20] |
| FB1/OTA | Fluorescent | Aptamer, time-resolved nanoparticles and magnetic nanoparticles | Maize | 0.019/0.015 pg/mL | [81] |
| OTA/AFB1 | Fluorescent | Aptamer, SiO2@QDs and magnetic nanoparticles | Corn | 0.067/1.7 pg/mL | [102] |
| OTA/ZEN | Fluorescent | Antibody, upconversion-encoded microspheres and phycoerythrin | Corn | 0.34/0.41 ng/mL | [82] |
| AFB1/ZEN | Fluorescent quenching | Aptamer, AuNCs and WS2 nanosheet | Maize | 0.34/0.53 pg/mL | [84] |
| ZEN/T-2/AFB1 | Fluorescent quenching | Aptamer, time-resolved nanoparticles and WS2 nanosheet | Maize | 0.51/0.33/0.40 pg/mL | [103] |
| AFB1 | Fluorescent quenching | Aptamer, CdZnTe QDs and AuNPs | Peanut | 20 pg/mL | [79] |
| OTA | Fluorescent quenching | Aptamer, nitrogen doped carbon dots and AgNPs | Flour and beer | 8.7 nmol/L | [80] |
| AFB1/OTA | Chemiluminescence | Antibody and AgNPs | Red yeast rice | 0.44/0.83 pg/mL | [87] |
| DON | Electrochemiluminescence | Antibody, NPCo/Co3O4–Au and RuSi@Ru (bpy)32+ | Wheat flour | 1 pg/mL | [104] |
| OTA | Electrochemiluminescence | MIP, CdTe QDs and [Ru (bpy)3]2+ | Starch | 0.25 fg/mL | [90] |
| AFB1 | Electrochemiluminescence | Antibody and ZnCdS@ZnS quantum dots | Lotus seed | 0.01 ng/mL | [92] |
| AFM1 | Electrochemiluminescence | Aptamer, AuNPs-magnetic nanoparticles and luminol-functionalized silver nanoparticle-decorated graphene oxide | Milk | 0.05 ng/mL | [105] |
| OTA | Electrochemiluminescence | CdS QDs, Cy5-labeled DNA | Beer and wine | 0.012 nmol/L | [91] |
| AFB1/OTA/ZEN/DON | SPR | Antibody and self-assembled monolayer SPR chips | Cereal | 0.59 /1.27 /7.07/ 3.26 ng/mL | [106] |
| DON/OTA | SPR | Antibody, nanostructured gold chips | Beer | 17/7 ng/mL | [94] |
| AFB1 | SPR | Antibody, AuNPs and self-assembled monolayer Au chips | Wheat | 0.003 nmol/L | [96] |
| AFB1 | SPR | Antibody, gold chips | Grains | 2.51 ppb | [107] |
| AFB1 | SPR | Aptamer, gold chips | Red wine | 0.4 nmol/L | [108] |
Electrochemical biosensors
Electrochemical biosensors are based on changes in outputted electrical signals that produced by the chemical reactions between electrode-immobilized recognition elements and target analytes. According to the types of detectable electrical signals, electrochemical sensors can be categorized into amperometric, potentiometric, conductometric, impedimetric and voltammetric methods. Besides, the electrode system (working electrode, reference electrode and counter electrode) is vital to the sensors because identification of target analytes needs to be finished on the electrode. The electrode is also used to conduct electrical signals [12, 109].
Traditional electrochemical immunosensors using general electrodes have some defects of low sensitivity, low stability, and inability to detect multiple targets simultaneously. To address these issues, improving performance of working electrodes and achieving signal amplification have become necessary. Commonly used working electrodes such as glass carbon, gold, and silver electrodes have recently been modified with various nanomaterials (carbon nanotubes, graphene oxide, metal nanoparticles, thin-layer MoS2, and porous metal organic framework) to increase their surface area [110–113]. As a result, the nanostructured rough electrode surface can be immobilized with more recognition reagents and more sufficient contact with the analytes so that sensitivity and conductivity are greatly improved [114]. For example, a facile electrochemical immunosensor was constructed for rapid detection of ZEN using thin-layer molybdenum disulfide and thionin composites (MoS2-Thi). Thin-layer MoS2 is an important graphene analog that is used frequently as a supporting substrate for stabilizing nanoparticles [112]. In this research, ZEN monoclonal antibodies were modified with platinum (Pt) nanoparticles then immobilized on MoS2-Thi composites to obtain synergistic signal amplification. Therefore, this immunosensor was easy to operate as well as offered a higher sensitivity compared with the original ZEN monoclonal antibodies.
Screen-printed electrodes have become popular for their advantages of reliability, reproducibility, ductility, ease of mass production, and low costs. They are variable in shape and small enough to be combined with miniaturized devices. Similarly, screen-printed electrodes are also modified with various nanomaterials to enhance sensitivity of electrochemical sensors [115–117].
Microfluidic systems employed in electrochemical immunosensor is an ideal choice to reduce detection time, improve stability and detect multiple targets simultaneously [118]. For example, Lu et al. [119] reported a dual-channel microfluidic electrochemical immunosensor for FB1 and DON detection in corn. Three-electrodes were etched on transparent indium tinoxide (ITO)-coated glass. A sample solution was introduced in the capillary-driven polydimethylsiloxane (PDMS) microfluidic channel. This microfluidic electrochemical immunosensor exhibited high potential for practical application with the LOD of 97 and 35 pg/mL for FB1 and DON, respectively. However, there are few reports on electrochemical immunosensors for detection of multiple mycotoxins.
Since antibody’s degradation is still a hinder for regeneration of electrochemical immunosensor sensing elements, aptamer-based sensors (aptasenor) have attracted more and more attention recently. Aptasensors exhibit greater diversity and universality than immunosensors. They are easier to construct for multiple analytes detection systems and sensitivity of aptasensor can be greatly improved by a ratiometric mode. Fig. 3A shows a hairpin DNA-based ratiometric electrochemical aptasensor for simultaneous detection of AFB1 and OTA [120]. Ferrocene-labelled AFB1 aptamer (Fc-Apt1) and methylene blue-labelled OTA aptamer (MB-Apt2) served as binding probes and current signal indicators. Both aptamers were complementary to the carboxylic acid (AQ)-labelled hairpin DNA that served as reference signal. It was applied to analyze AFB1 and OTA in corn and wheat samples with high sensitivity and reliability. Similarly, Wei et al. [121] designed a unique Y-shaped complementary DNA structure for simultaneously hybridizing with OTA and FB1 aptamer. OTA and FB1 aptamers were immobilized with thionine and thiolated ferrocene as signal indicators. It was applied to detect OTA and FB1 in beer with the LOD of 0.47 and 0.26 pg/mL, respectively.
Fig. 3.
Schematic illustration of the electrochemical sensing platforms. (A) Hairpin DNA-based ratiometric electrochemical aptasensor for simultaneous detection of AFB1 and OTA; (B) dual-target electrochemical aptasensor was developed based on co-reduced molybdenum disulfide and gold nanoparticles (rMoS2-Au) modified electrodes. Diagrams A and B are adapted from Ref. [120] and Ref. [122], respectively
Another dual-target electrochemical aptasensor was developed based on co-reduced molybdenum disulfide and gold nanoparticles (rMoS2-Au) modified electrodes [122]. Aptamers of AFB1 and ZEN were binding with thionine and 6-(Ferrocenyl) hexanethiol modified complementary strands, respectively (Fig. 3B). This platform obtained satisfactory recoveries of AFB1 and ZEN in maize with the LOD as low as 0.4 pg/mL. Li et al. reported a ratiometric electrochemical aptasensor for AFB1 detection in peanut. Ferrocene-labelled aptamer (Fc-apt) served as the response signal and reduced graphene oxide (THI-rGO) functionalized with thionine was used as the reference signal. In this study, ratiometric detection was achieved by formation of Fc-apt-AFB1 complex which resulted in decreased current intensity of Fc (IFc), and increased current intensity of THI (ITHI) [123]. It is worth mentioning that sensitivity of this sensor can be adjusted by changing the assembly of Fc-apt, which exhibited high sensitivity and selectivity.
Molecularly imprinted polymers (MIPs) can serve as the recognition elements in electrochemical sensors which are favorable alternatives to antibodies. MIPs are mechanical and chemical stable and provide thickness-controlled MIP films on electrode surfaces, which permit direct communication between the films and the transducer. A novel MIP-sensor with two functionalization methods for AFB2 detection in milk was developed. Researches compared ZnO-NPs/chitosan (CS)/AFB2 and ZnO-NPs/Cs/polypyrrole (PPy)/AFB2 composite that electrodeposited on the screen-printed electrode [124]. They showed that ZnO-NPs/CS/PPy/AFB2 functionalized composite had greater sensitivity than ZnO-NPs/CS/AFB2. This MIP-sensor exhibited good specificity, reproducibility and stability as well as extremely low LOD (0.2 fg/mL). Differential pulse voltammetry (DPV) techniques used in this study had a faster and more sensitive response compared to the electrochemical impedance spectroscopy.
In general, electrochemical biosensors show reliable sensitivity and selectivity as well as great potential for miniaturization and portability. But the modification steps involved in the electrochemical biosensors’ preparation are complicated. Reproducibility and interference resistance of traditional electrodes in complex matrices still is problematic. Compared with optical detection methods, there are no huge advantages for development of multiple target detection systems. Table 3 summarizes the important parameters of electrochemical biosensor platforms that have been published in recent years for mycotoxin determinations.
Table 3.
Summary of electrochemical sensor platforms for detecting mycotoxins
| Target | Method | Principle | Electrode | Sample | LOD | Ref. |
|---|---|---|---|---|---|---|
| FB1/DON | Immunosensor | Differential pulse voltammetry | Indium tin oxide electrode integrated with PDMS microfluidic channel | Corn | 97/35 pg/mL | [119] |
| OTA | Immunosensor | Differential pulse voltammetry | Gold electrode | Medicinal and edible malt | 0.08 ng/mL | [125] |
| ZEN | Immunosensor | Square wave voltammetry | MoS2-Thi composite-modified glass carbon electrode | Human biofluids | 0.005 ng/mL | [112] |
| ZEN | Immunosensor | Differential pulse voltammetry | Screen-printed electrode | Beer and wine | 0.25 ng/mL | [117] |
| AFB1 | Immunosensor | Impedimetric | Cysteine/carbon nanotubes- modified gold electrode | Corn flour | 0.79 pg/g | [126] |
| ZEN | Immunosensor | Differential pulse voltammetry | Multi-walled carbon nanotubes and chitosan-modified glass carbon electrode | Cereal and feedstuff | 4.7 pg/mL | [127] |
| OTA | Immunosensor | Differential pulse voltammetry | Palladium nanoparticles-modified carbon felt electrode | Coffee | 0.096 ng/mL | [128] |
| AFB1 | Immunosensor | Cyclic voltammetry | Graphene quantum dots and AuNPs-modified Indium tin oxide electrode | Maize | 0.1 ng/mL | [129] |
| ZEN/FB1 | Aptasensor | Differential pulse voltammetry | Co-reduced molybdenum disulfide/AuNPs-modified glass carbon electrode | Maize | 0.5 pg/mL | [122] |
| OTA/FB1 | Aptasensor | Differential pulse voltammetry | Gold electrode | Beer | 0.47/0.26 pg/mL | [121] |
| AFB1/OTA | Ratiometric aptasensor | Differential pulse voltammetry | Gold electrode | Corn and wheat | 4.3/13.3 pg/mL | [120] |
| OTA/FB1 | Magneto-controlled aptasensor | Square wave voltammetry | Glassy carbon electrode | Maize | 20/5 pg/mL | [130] |
| AFB1 | Ratiometric aptasensor | Alternating current voltammetry | Thionine functionalized reduced graphene oxide/AuNPs-modified glass carbon electrode | Peanut | 0.016 ng/mL | [123] |
| T-2 | Aptasensor | Differential pulse voltammetry | Molybdenum disulfide/polyaniline/chitosan AuNPs-modified glassy carbon electrode | Beer | 1.79 fg/mL | [113] |
| AFB1 | Aptasensor | Square wave voltammetry | Gold electrode | Beer | 2 nmol/L | [131] |
| ZEN | Aptasensor | Square wave voltammetry | Cysteamine-hydrochloride/1,4-phenylene diisocyanate-modified gold electrode | Maize grain | 0.017 ng/mL | [132] |
| AFM1 | Aptasensor | Impedance voltammetry | PtNPs/Fe-based metal organic frameworks-modified glassy carbon electrode | Powder and pasteurized milk | 2 pg/mL | [133] |
| ZEN | Molecularly imprinted sensor | Impedimetric | Poly (o-phenylenediamine)- modified screen-printed gold electrode | Corn flakes | 0.2 ng/mL | [134] |
| DON | Molecularly imprinted sensor | Impedimetric | Poly o-phenylenediamine-modified screen-printed gold electrode | Food | 0.3 ng/mL | [135] |
| AFB2 | Molecularly imprinted sensor | Differential pulse voltammetry | ZnO-NPs/chitosan/polypyrrole modified screen-printed electrode | Fresh and pasteurized milk | 0.2 and 0.7 fg/mL | [124] |
Others
Photoelectrochemical sensors
Photoelectrochemical (PEC) sensors are emerging as an analytical technology that combines features of both optics and electrochemistry. In PEC sensors, light source, electrochemical workstation, and signal acquisition system are main components. Light is used as the excitation source and the generated photocurrent is used as the detection signal, which significantly reduces background interference [136]. Photoactive material, as a transducer for the conversion from biological recognition events to observable PEC signals, plays a crucial role in the PEC biosensing platform. Semiconductors (such as QDs) and semiconductor-based heterojunctions (such as graphene and reduced graphene oxide) are commonly used [137–139]. However, PEC biosensors have no obvious advantages over common electrochemical biosensors in sensitivity, simplicity, and practicability at present. Multiple mycotoxins detected by PEC sensors have not been found yet. The detection instruments used for PEC sensor still need a simplification [140].
Metal−organic frameworks (MOFs)
In recent years, a novel material has been be applied in fluorescent and electrochemical sensing, termed metal−organic frameworks (MOFs). Metal−organic frameworks are porous coordination polymers with huge surface area, that exhibit excellent optical, electrochemical and catalytic properties [111, 141]. Luminescent metal−organic frameworks (LMOFs), a subclass of MOFs, display outstanding optical properties such as large stokes shifts, high quantum yield, characteristically narrow emission spectra, and long fluorescence lifetime. They also function in recognition and enrichment of targets due to the porous and easily modified structure, which are considered as promising chemical sensor materials [142, 143]. Hu et al. [144] firstly reported a highly luminescent Zn-based metal−organic framework for AFB1 sensing in 2015. The blue luminescence of MOFs can be quenched by AFB1 efficiently and specifically with the LOD of 46 ppb. Another water-stable LMOFs was developed for sensitive and rapid detection of AFB1 in walnut and almond beverages [145]. The LMOF was synthesized by 1,2,4,5-tetrakis (4-carboxyphenyl) benzene (H4TCPB) and highly water-stable Zr, which exhibited raging fluorescence quenching when AFB1 existed with the LOD of 19.97 ppb. These proposed MOFs provide a sensor platform that is very easy to synthesize and operate, but the sensitivity of MOF sensors was not as good as that of other electrochemical and optical biosensors. Anti-interference capability and specific selectivity in complex matrix related to MOFs still requires investigation.
Conclusion and perspectives
Various mycotoxin contamination occurs frequently and unavoidably in feedstuffs and cereals, which poses enormous risks to public health and leads to economic losses. This paper introduces advanced analytical methods and nanomaterials for mycotoxin detection in recent years including immunoassays and biosensors, especially for multiple mycotoxin detections. Target recognition, signal transduction, and nanoparticles are keys to rapid detection techniques. Analytical methods introduced in this paper are divided into many categories based on these three aspects.
Colorimetric immunoassays are simple, visible, and low-cost methods without the requirement of expensive instruments but the instability, background interference, and poor sensitivity are considerable drawbacks. Therefore, many fluorescent nanoparticles have been introduced to rapid detection and greatly enhance sensitivity and stability. However, some fluorescent signal labels are susceptible to fluorescent bleaching, autofluorescence or environmental interference and require complex labeling steps. Electrochemical biosensors are classic and powerful sensing strategy because of their high sensitivity and ability to be miniaturized, but they are hard to resist interferences, complex electrode modifications are required and electrode fouling and degradation still need to be solved. Photoelectrochemical and electrochemiluminescence sensors are regarded as combining the merits of optical and electrochemical strategies, which show low background, fast response, and high sensitivity. However, the absence of advanced PEC and ECL instrumentation limits practical application. And signal stability and ability to analyze multiple targets of PEC and ECL sensors is not sufficient. Label-free SPR biosensors simplify preparation and detection procedures. They have excellent analytical performance and offers direct and real-time detection platforms by using tiny chips, which make them have great potential for commercial detection of mycotoxins. But SPR sensors are not sensitive to binding events of low molecular weight molecules so that some modifications on chips and sophisticated instruments are still required. Both advantages and disadvantages exist in each kind of detection strategy. We need to choose the appropriate approach according to the conditions and requirements.
In spite of numerous successes with laboratory-based mycotoxin detection, there are still many limitations and challenges to conquer in achieving practical applications.
Firstly, nanomaterials are widely applied almost in all sensing strategies but the function of nanomaterials currently available are not perfect. There are many studies improving material properties and biosensor performances by using hybrid nanostructured materials, but they are not convenient for practical application and mass manufacturing. Developing promising nanomaterials that have excellent optical or electrochemical properties, but also are inexpensive, easy to prepare and environmentally friendly is still a focus of future research. This is also one of the core driving forces behind development of biosensor and immunoassay-based analytical methods.
Secondly, most of the existing sensing strategies are based on single signal output, which are susceptible to instrument conditions and environmental interferences resulting in poor reproducibility and stability. To overcome this drawback, ratiometric sensor with dual signals is an ideal solution. Detection results are based on the ratio of two signals enable self-built-in corrections thus greatly improving sensitivity and accuracy of sensing method. Two different luminescent particles connected together to form a FRET system in optical sensors or two signal labels served as response/reference system in electrochemical sensors are mainstream of ratiometric sensor design. But there are few reports on the detection of mycotoxins by ratiometric biosensors at present.
Lastly, simplicity, sensitivity, and high throughput have not really been implemented simultaneously in a rapid detection method. High-sensitive methods such as electrochemical biosensors often require complex modification operations, while simple and portable LFIA tend to produce qualitative or semi-quantitative detection results. Nowadays, with the development of micro-electro-mechanical systems (MEMS), sensor devices can be miniaturized at a tiny size. There are more and more reports on smartphone-based microfluidic biosensor systems which provide the portable, rapid, and sensitive sensing platforms. This is also a future trend that makes biosensors to better serve humans. Moreover, faced with a wide variety of mycotoxins, there have been many reports on the LFIA strip with multiplex test lines, multiplex SPR biochips, biosensors with multiple labels and integrating biosensors with microarrays and microfluidic systems. Most examples discussed in this paper are appropriate for up to three to six mycotoxins and involved complex designs. But achieving high-throughput quantitative detection of a dozen to dozens of analytes under the premise of guaranteed sensitivity still needs hard work.
Despite many challenges, rapid detection methods based on cross-discipline play an increasingly important role in food safety, industrial manufacture, environmental monitoring, and clinical diagnoses. Traditional determination technology has not been adapted to the demands of rapid, on-site, and large-scale screening. Fast, simple, sensitive, low-cost, and high-throughput analytical methods with portable instrument are growing need. With development of nanomaterials and biosensor technology, rapid detection will receive much more attention in the future.
Acknowledgments
Not applicable.
Abbreviations
- AFB1
Aflatoxin B1
- OTA
Ochratoxin A
- MRLs
Maximum residue levels
- ELISA
Enzyme-linked immunoassay
- HPLC
High-performance liquid chromatography
- LC-MS/MS
Liquid chromatography-tandem mass spectrometry
- MS
Mass spectrometry
- MIPs
Molecularly imprinted polymers
- SELEX
Systematic evolution of ligands exponential enrichment
- SPE
Solid phase extractions
- NIPs
Non-imprinted polymers
- FLISA
Fluorescent-linked immunosorbent assays
- CLEIA
Chemiluminescence enzyme immunoassays
- LFIA
Lateral-flow immunoassays
- POCT
Point-of-care testing
- AuNPs
Gold nanoparticles
- FB1
Fumonisin B1
- ZEN
Zearalenone
- FMs
Fluorescent microspheres
- CNPs
Carbon-based nanoparticles
- TRFMs
Time-resolved fluorescent microspheres
- CG
Colloidal gold
- PMs
Polystyrene microspheres
- SERS
Surface-enhanced raman spectroscopy
- IFE
Inner-filter effect
- FRET
Förster resonance energy transfer
- DON
Deoxynivalenol
- T−2
T-2 toxin
- SPR
Surface plasmon resonance
- MNPs
Magnetic nanoparticles
- GO
Graphene oxide
- QDs
Quantum dots
- UCNPs
Upconverting nanoparticles
- AuNCs
Gold nanoclusters
- LOD
Limit of detection
- PEC
Photoelectrochemical
- MOFs
Metal−organic frameworks
Authors’ contributions
RL wrote the manuscript. YW and FW revised the manuscript. PL revised and finalized the manuscript. All authors read and approved the final manuscript.
Funding
The financial support from the National Key Research and Development Program of China (2017YFC1600300).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no conflict of interest to disclose.
References
- 1.Yang Y, Li G, Wu D, Liu J, Li X, Luo P, Hu N, Wang H, Wu Y. Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends Food Sci Technol. 2020;96:233–252. doi: 10.1016/j.tifs.2019.12.021. [DOI] [Google Scholar]
- 2.Zhou SY, Xu LG, Kuang H, Xiao J, Xu CL. Immunoassays for rapid mycotoxin detection: state of the art. Analyst. 2020;145(22):7088–7102. doi: 10.1039/D0AN01408G. [DOI] [PubMed] [Google Scholar]
- 3.Xue Z, Zhang Y, Yu W, Zhang J, Wang J, Wan F, Kim Y, Liu Y, Kou X. Recent advances in aflatoxin B1 detection based on nanotechnology and nanomaterials-a review. Anal Chim Acta. 2019;1069:1–27. doi: 10.1016/j.aca.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 4.Xu L, Zhang Z, Zhang Q, Li P. Mycotoxin determination in foods using advanced sensors based on antibodies or aptamers. Toxins. 2016;8:239. 10.3390/toxins8080239. [DOI] [PMC free article] [PubMed]
- 5.Gallo A, Giuberti G, Frisvad JC, Bertuzzi T, Nielsen KF. Review on mycotoxin issues in ruminants: occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects. Toxins. 2015;7(8):3057–3111. doi: 10.3390/toxins7083057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Egmond HP, Schothorst RC, Jonker MA. Regulations relating to mycotoxins in food. Anal Bioanal Chem. 2007;389(1):147–157. doi: 10.1007/s00216-007-1317-9. [DOI] [PubMed] [Google Scholar]
- 7.Njumbe Ediage E, Van Poucke C, De Saeger S. A multi-analyte LC-MS/MS method for the analysis of 23 mycotoxins in different sorghum varieties: the forgotten sample matrix. Food Chem. 2015;177:397–404. doi: 10.1016/j.foodchem.2015.01.060. [DOI] [PubMed] [Google Scholar]
- 8.Pei SC, Lee WJ, Zhang GP, Hu XF, Eremin SA, Zhang LJ. Development of anti-zearalenone monoclonal antibody and detection of zearalenone in corn products from China by ELISA. Food Control. 2013;31(1):65–70. doi: 10.1016/j.foodcont.2012.09.006. [DOI] [Google Scholar]
- 9.Tkaczyk A, Jedziniak P. Dilute-and-shoot HPLC-UV method for determination of urinary creatinine as a normalization tool in mycotoxin biomonitoring in pigs. Molecules. 2020;25:13. doi: 10.3390/molecules25102445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grippi F, Crosta L, Aiello G, Tolomeo M, Oliveri F, Gebbia N, Curione A. Determination of stilbenes in Sicilian pistachio by high-performance liquid chromatographic diode array (HPLC-DAD/FLD) and evaluation of eventually mycotoxin contamination. Food Chem. 2008;107(1):483–488. doi: 10.1016/j.foodchem.2007.07.079. [DOI] [Google Scholar]
- 11.Chauhan R, Singh J, Sachdev T, Basu T, Malhotra BD. Recent advances in mycotoxins detection. Biosens Bioelectron. 2016;81:532–545. doi: 10.1016/j.bios.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Alhamoud Y, Yang D, Fiati Kenston SS, Liu G, Liu L, Zhou H, Ahmed F, Zhao J. Advances in biosensors for the detection of ochratoxin a: bio-receptors, nanomaterials, and their applications. Biosens Bioelectron. 2019;141:111418. doi: 10.1016/j.bios.2019.111418. [DOI] [PubMed] [Google Scholar]
- 13.Ding L, Chen X, He L, Yu F, Yu S, Wang J, Tian Y, Wang Y, Wu Y, Liu LE, Qu L. Fluorometric immunoassay for the simultaneous determination of the tumor markers carcinoembryonic antigen and cytokeratin 19 fragment using two kinds of CdSe/ZnS quantum dot nanobeads and magnetic beads. Mikrochim Acta. 2020;187(3):171. doi: 10.1007/s00604-019-3914-7. [DOI] [PubMed] [Google Scholar]
- 14.Lv Y, Wang F, Li N, Wu R, Li J, Shen H, Li LS, Guo F. Development of dual quantum dots-based fluorescence-linked immunosorbent assay for simultaneous detection on inflammation biomarkers. Sensor Actuat B Chem. 2019;301:127118. doi: 10.1016/j.snb.2019.127118. [DOI] [Google Scholar]
- 15.Li M, Ma M, Hua X, Shi H, Wang Q, Wang M. Quantum dots-based fluoroimmunoassay for the simultaneous detection of clothianidin and thiacloprid in environmental and agricultural samples. RSC Adv. 2015;5(4):3039–3044. doi: 10.1039/C4RA13305F. [DOI] [Google Scholar]
- 16.Bueno D, Istamboulie G, Munoz R, Marty JL. Determination of mycotoxins in food: a review of bioanalytical to analytical methods. Appl Spectrosc Rev. 2015;50(9):728–774. doi: 10.1080/05704928.2015.1072092. [DOI] [Google Scholar]
- 17.Ren W, Xu Y, Huang Z, Li Y, Tu Z, Zou L, He Q, Fu J, Liu S, Hammock BD. Single-chain variable fragment antibody-based immunochromatographic strip for rapid detection of fumonisin B1 in maize samples. Food Chem. 2020;319:126546. doi: 10.1016/j.foodchem.2020.126546. [DOI] [PubMed] [Google Scholar]
- 18.Hou SL, Ma ZE, Meng H, Xu Y, He QH. Ultrasensitive and green electrochemical immunosensor for mycotoxin ochratoxin a based on phage displayed mimotope peptide. Talanta. 2019;194:919–924. doi: 10.1016/j.talanta.2018.10.081. [DOI] [PubMed] [Google Scholar]
- 19.Mahmoudpour M, Ezzati Nazhad Dolatabadi J, Torbati M, Pirpour Tazehkand A, Homayouni-Rad A, de la Guardia M. Nanomaterials and new biorecognition molecules based surface plasmon resonance biosensors for mycotoxin detection. Biosens Bioelectron. 2019;143:111603. doi: 10.1016/j.bios.2019.111603. [DOI] [PubMed] [Google Scholar]
- 20.Tang Z, Liu X, Wang Y, Chen Q, Hammock BD, Xu Y. Nanobody-based fluorescence resonance energy transfer immunoassay for noncompetitive and simultaneous detection of ochratoxin a and ochratoxin B. Environ Pollut. 2019;251:238–245. doi: 10.1016/j.envpol.2019.04.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peltomaa R, Farka Z, Mickert MJ, Brandmeier JC, Pastucha M, Hlavacek A, et al. Competitive upconversion-linked immunoassay using peptide mimetics for the detection of the mycotoxin zearalenone. Biosens Bioelectron. 2020;170:112683. doi: 10.1016/j.bios.2020.112683. [DOI] [PubMed] [Google Scholar]
- 22.Xiang WW, Lv QX, Shi HX, Xie B, Gao L. Aptamer-based biosensor for detecting carcinoembryonic antigen. Talanta. 2020;214:17. doi: 10.1016/j.talanta.2020.120716. [DOI] [PubMed] [Google Scholar]
- 23.Sinha K, Das MC. Quantitative detection of neurotransmitter using aptamer: from diagnosis to therapeutics. J Biosci. 2020;45:12. doi: 10.1007/s12038-020-0017-x. [DOI] [PubMed] [Google Scholar]
- 24.He F, Wen NC, Xiao DP, Yan JH, Xiong HJ, Cai SD, Liu Z, Liu Y. Aptamer-based targeted drug delivery systems: current potential and challenges. Curr Med Chem. 2020;27(13):2189–2219. doi: 10.2174/0929867325666181008142831. [DOI] [PubMed] [Google Scholar]
- 25.Darmostuk M, Rimpelova S, Gbelcova H, Ruml T. Current approaches in SELEX: An update to aptamer selection technology. Biotechnol Adv. 2015;33(6):1141–1161. doi: 10.1016/j.biotechadv.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Guo X, Wen F, Zheng N, Saive M, Fauconnier ML, Wang J. Aptamer-based biosensor for detection of mycotoxins. Front Chem. 2020;8:195. doi: 10.3389/fchem.2020.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen A, Yang S. Replacing antibodies with aptamers in lateral flow immunoassay. Biosens Bioelectron. 2015;71:230–242. doi: 10.1016/j.bios.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 28.Gaudin V. Advances in biosensor development for the screening of antibiotic residues in food products of animal origin - a comprehensive review. Biosens Bioelectron. 2017;90:363–377. doi: 10.1016/j.bios.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Bezdekova J, Zemankova K, Hutarova J, Kociova S, Smerkova K, Adam V, et al. Magnetic molecularly imprinted polymers used for selective isolation and detection of Staphylococcus aureus. Food Chem. 2020;321:8. doi: 10.1016/j.foodchem.2020.126673. [DOI] [PubMed] [Google Scholar]
- 30.Cao YR, Feng TY, Xu J, Xue CH. Recent advances of molecularly imprinted polymer-based sensors in the detection of food safety hazard factors. Biosens Bioelectron. 2019;141:18. doi: 10.1016/j.bios.2019.111447. [DOI] [PubMed] [Google Scholar]
- 31.Ahmadi R, Noroozian E, Jassbi AR. Molecularly imprinted polymer solid-phase extraction for the analysis of 1,8-cineole in thyme and sagebrush distillates. J Iran Chem Soc. 2020;17(5):1153–1161. doi: 10.1007/s13738-019-01840-x. [DOI] [Google Scholar]
- 32.Choi SW, Chang HJ, Lee N, Chun HS. A surface plasmon resonance sensor for the detection of deoxynivalenol using a molecularly imprinted polymer. Sensors. 2011;11:8654–8664. doi: 10.3390/s110908654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu H, Xu W, Wang H, Liao S, Chen G. Preparation of magnetic molecularly imprinted polymers for the identification of zearalenone in grains. Anal Bioanal Chem. 2020;412(19):4725–4737. doi: 10.1007/s00216-020-02729-y. [DOI] [PubMed] [Google Scholar]
- 34.Ahmad OS, Bedwell TS, Esen C, Garcia-Cruz A, Piletsky SA. Molecularly imprinted polymers in electrochemical and optical sensors. Trends Biotechnol. 2019;37(3):294–309. doi: 10.1016/j.tibtech.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Kolosova AY, Shim WB, Yang ZY, Eremin SA, Chung DH. Direct competitive ELISA based on a monoclonal antibody for detection of aflatoxin B1. Stabilization of ELISA kit components and application to grain samples. Anal Bioanal Chem. 2006;384(1):286–294. doi: 10.1007/s00216-005-0103-9. [DOI] [PubMed] [Google Scholar]
- 36.Zhang F, Liu B, Sheng W, Zhang Y, Liu Q, Li S, Wang S. Fluoroimmunoassays for the detection of zearalenone in maize using CdTe/CdS/ZnS quantum dots. Food Chem. 2018;255:421–428. doi: 10.1016/j.foodchem.2018.02.060. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Liu G, Fu X, He J, Wang Z, Hou J, et al. High-sensitive chemiluminescent elisa method investigation for the determination of deoxynivalenol in rice. Food Anal Methods. 2014;8:656–660. doi: 10.1007/s12161-014-9941-4. [DOI] [Google Scholar]
- 38.Lu T, Zhan S, Zhou Y, Chen X, Huang X, Leng Y, Xiong Y, Xu Y. Fluorescence ELISA based on CAT-regulated fluorescence quenching of CdTe QDs for sensitive detection of FB1. Anal Methods. 2018;10(48):5797–5802. doi: 10.1039/C8AY02065E. [DOI] [Google Scholar]
- 39.Gowri A, Ashwin Kumar N, Suresh Anand BS. Recent advances in nanomaterials based biosensors for point of care (PoC) diagnosis of Covid-19 - a minireview. TrAC Trend Anal Chem. 2021;137:116205. doi: 10.1016/j.trac.2021.116205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian JQ, He QQ, Liu LL, Wang M, Wang BM, Cui LW. Rapid quantification of artemisinin derivatives in antimalarial drugs with dipstick immunoassays. J Pharm Biomed Anal. 2020;191:8. doi: 10.1016/j.jpba.2020.113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foubert A, Beloglazova NV, De Saeger S. Comparative study of colloidal gold and quantum dots as labels for multiplex screening tests for multi-mycotoxin detection. Anal Chim Acta. 2017;955:48–57. doi: 10.1016/j.aca.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 42.Anfossi L, Di Nardo F, Cavalera S, Giovannoli C, Spano G, Speranskaya ES, et al. A lateral flow immunoassay for straightforward determination of fumonisin mycotoxins based on the quenching of the fluorescence of CdSe/ZnS quantum dots by gold and silver nanoparticles. Mikrochim Acta. 2018;185(2):94. doi: 10.1007/s00604-017-2642-0. [DOI] [PubMed] [Google Scholar]
- 43.Yao J, Sun Y, Li Q, Wang F, Teng M, Yang Y, Deng R, Hu X. Colloidal gold-McAb probe-based rapid immunoassay strip for simultaneous detection of fumonisins in maize. J Sci Food Agric. 2017;97(7):2223–2229. doi: 10.1002/jsfa.8032. [DOI] [PubMed] [Google Scholar]
- 44.Huang X, Huang X, Xie J, Li X, Huang Z. Rapid simultaneous detection of fumonisin B1 and deoxynivalenol in grain by immunochromatographic test strip. Anal Biochem. 2020;606:113878. doi: 10.1016/j.ab.2020.113878. [DOI] [PubMed] [Google Scholar]
- 45.Huang X, Huang T, Li X, Huang Z. Flower-like gold nanoparticles-based immunochromatographic test strip for rapid simultaneous detection of fumonisin B1 and deoxynivalenol in Chinese traditional medicine. J Pharm Biomed Anal. 2020;177:112895. doi: 10.1016/j.jpba.2019.112895. [DOI] [PubMed] [Google Scholar]
- 46.Di Nardo F, Baggiani C, Giovannoli C, Spano G, Anfossi L. Multicolor immunochromatographic strip test based on gold nanoparticles for the determination of aflatoxin B1 and fumonisins. Microchim Acta. 2017;184(5):1295–1304. doi: 10.1007/s00604-017-2121-7. [DOI] [Google Scholar]
- 47.Wu Y, Zhou Y, Huang H, Chen X, Leng Y, Lai W, Huang X, Xiong Y. Engineered gold nanoparticles as multicolor labels for simultaneous multi-mycotoxin detection on the immunochromatographic test strip nanosensor. Sensor Actuat B Chem. 2020;316:128107. doi: 10.1016/j.snb.2020.128107. [DOI] [Google Scholar]
- 48.Guo L, Shao Y, Duan H, Ma W, Leng Y, Huang X, Xiong Y. Magnetic quantum dot nanobead-based fluorescent immunochromatographic assay for the highly sensitive detection of aflatoxin b1 in dark soy sauce. Anal Chem. 2019;91(7):4727–4734. doi: 10.1021/acs.analchem.9b00223. [DOI] [PubMed] [Google Scholar]
- 49.Liu M, Zeng L-F, Yang Y-J, Hu L-M, Lai W-H. Fluorescent microsphere immunochromatographic assays for detecting bone alkaline phosphatase based on biolayer interferometry-selected antibody. RSC Adv. 2017;7(52):32952–32959. doi: 10.1039/C7RA03756B. [DOI] [Google Scholar]
- 50.Tang DP, Lin YX, Zhou Q. Carbon dots prepared from Litchi chinensis and modified with manganese dioxide nanosheets for use in a competitive fluorometric immunoassay for aflatoxin B-1. Microchim Acta. 2018;185:9. doi: 10.1007/s00604-017-2581-9. [DOI] [PubMed] [Google Scholar]
- 51.Altunbas O, Ozdas A, Yilmaz MD. Luminescent detection of Ochratoxin a using terbium chelated mesoporous silica nanoparticles. J Hazard Mater. 2020;382:121049. doi: 10.1016/j.jhazmat.2019.121049. [DOI] [PubMed] [Google Scholar]
- 52.Tang X, Li P, Zhang Q, Zhang Z, Zhang W, Jiang J. Time-resolved fluorescence immunochromatographic assay developed using two idiotypic nanobodies for rapid, quantitative, and simultaneous detection of aflatoxin and zearalenone in maize and its products. Anal Chem. 2017;89(21):11520–11528. doi: 10.1021/acs.analchem.7b02794. [DOI] [PubMed] [Google Scholar]
- 53.Wang D, Zhang Z, Li P, Zhang Q, Zhang W. Time-resolved fluorescent immunochromatography of aflatoxin b1 in soybean sauce: a rapid and sensitive quantitative analysis. Sensors. 2016;16:1094. 10.3390/s16071094. [DOI] [PMC free article] [PubMed]
- 54.Zhao S, Bu T, He K, Bai F, Zhang M, Tian Y, Sun X, Wang X, Zhangsun H, Wang L. A novel α-Fe2O3 nanocubes-based multiplex immunochromatographic assay for simultaneous detection of deoxynivalenol and aflatoxin B1 in food samples. Food Control. 2021;123:107811. doi: 10.1016/j.foodcont.2020.107811. [DOI] [Google Scholar]
- 55.Zhang X, Wu C, Wen K, Jiang H, Shen J, Zhang S, et al. Comparison of fluorescent microspheres and colloidal gold as labels in lateral flow immunochromatographic assays for the detection of T-2 toxin. Molecules. 2015;21:E27. doi: 10.3390/molecules21010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li SJ, Sheng W, Wen W, Gu Y, Wang JP, Wang S. Three kinds of lateral flow immunochromatographic assays based on the use of nanoparticle labels for fluorometric determination of zearalenone. Mikrochim Acta. 2018;185(4):238. doi: 10.1007/s00604-018-2778-6. [DOI] [PubMed] [Google Scholar]
- 57.Liu Z, Hua Q, Wang J, Liang Z, Li J, Wu J, Shen X, Lei H, Li X. A smartphone-based dual detection mode device integrated with two lateral flow immunoassays for multiplex mycotoxins in cereals. Biosens Bioelectron. 2020;158:112178. doi: 10.1016/j.bios.2020.112178. [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Park SG, Ko J, Xiao X, Giannini V, Maier SA, et al. Sensitive and reproducible immunoassay of multiple mycotoxins using surface-enhanced raman scattering mapping on 3d plasmonic nanopillar arrays. Small. 2018;14:e1801623. doi: 10.1002/smll.201801623. [DOI] [PubMed] [Google Scholar]
- 59.Ko J, Lee C, Choo J. Highly sensitive SERS-based immunoassay of aflatoxin B1 using silica-encapsulated hollow gold nanoparticles. J Hazard Mater. 2015;285:11–17. doi: 10.1016/j.jhazmat.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 60.Zhang W, Tang S, Jin Y, Yang C, He L, Wang J, Chen Y. Multiplex SERS-based lateral flow immunosensor for the detection of major mycotoxins in maize utilizing dual Raman labels and triple test lines. J Hazard Mater. 2020;393:122348. doi: 10.1016/j.jhazmat.2020.122348. [DOI] [PubMed] [Google Scholar]
- 61.Jiang H, Zhang W, Li J, Nie L, Wu K, Duan H, Xiong Y. Inner-filter effect based fluorescence-quenching immunochromotographic assay for sensitive detection of aflatoxin B1 in soybean sauce. Food Control. 2018;94:71–76. doi: 10.1016/j.foodcont.2018.06.030. [DOI] [Google Scholar]
- 62.Oh HK, Joung HA, Jung M, Lee H, Kim MG. Rapid and simple detection of ochratoxin a using fluorescence resonance energy transfer on lateral flow immunoassay (FRET-LFI). Toxins. 2019;11. [DOI] [PMC free article] [PubMed]
- 63.Li S, Wang J, Sheng W, Wen W, Gu Y, Wang S. Fluorometric lateral flow immunochromatographic zearalenone assay by exploiting a quencher system composed of carbon dots and silver nanoparticles. Mikrochim Acta. 2018;185(8):388. doi: 10.1007/s00604-018-2916-1. [DOI] [PubMed] [Google Scholar]
- 64.Xu Y, Ma B, Chen E, Yu X, Ye Z, Sun C, Zhang M. Dual fluorescent immunochromatographic assay for simultaneous quantitative detection of citrinin and zearalenone in corn samples. Food Chem. 2021;336:127713. doi: 10.1016/j.foodchem.2020.127713. [DOI] [PubMed] [Google Scholar]
- 65.Jin Y, Chen Q, Luo S, He L, Fan R, Zhang S, Yang C, Chen Y. Dual near-infrared fluorescence-based lateral flow immunosensor for the detection of zearalenone and deoxynivalenol in maize. Food Chem. 2021;336:127718. doi: 10.1016/j.foodchem.2020.127718. [DOI] [PubMed] [Google Scholar]
- 66.Duan H, Li Y, Shao Y, Huang X, Xiong Y. Multicolor quantum dot nanobeads for simultaneous multiplex immunochromatographic detection of mycotoxins in maize. Sensors Actuators B Chem. 2019;291:411–417. doi: 10.1016/j.snb.2019.04.101. [DOI] [Google Scholar]
- 67.Goryacheva OA, Guhrenz C, Schneider K, Beloglazova NV, Goryacheva IY, De Saeger S, et al. Silanized luminescent quantum dots for the simultaneous multicolor lateral flow immunoassay of two mycotoxins. ACS Appl Mater Interfaces. 2020;12(22):24575–24584. doi: 10.1021/acsami.0c05099. [DOI] [PubMed] [Google Scholar]
- 68.Li R, Meng C, Wen Y, Fu W, He P. Fluorometric lateral flow immunoassay for simultaneous determination of three mycotoxins (aflatoxin B1, zearalenone and deoxynivalenol) using quantum dot microbeads. Mikrochim Acta. 2019;186(12):748. doi: 10.1007/s00604-019-3879-6. [DOI] [PubMed] [Google Scholar]
- 69.Zhang X, Yu X, Wen K, Li C, Mujtaba Mari G, Jiang H, Shi W, Shen J, Wang Z. Multiplex lateral flow immunoassays based on amorphous carbon nanoparticles for detecting three fusarium mycotoxins in maize. J Agric Food Chem. 2017;65(36):8063–8071. doi: 10.1021/acs.jafc.7b02827. [DOI] [PubMed] [Google Scholar]
- 70.Shao Y, Duan H, Zhou S, Ma T, Guo L, Huang X, Xiong Y. Biotin-streptavidin system-mediated ratiometric multiplex immunochromatographic assay for simultaneous and accurate quantification of three mycotoxins. J Agric Food Chem. 2019;67(32):9022–9031. doi: 10.1021/acs.jafc.9b03222. [DOI] [PubMed] [Google Scholar]
- 71.Li R, Bu T, Zhao Y, Sun X, Wang Q, Tian Y, Bai F, Wang L. Polydopamine coated zirconium metal-organic frameworks-based immunochromatographic assay for highly sensitive detection of deoxynivalenol. Anal Chim Acta. 2020;1131:109–117. doi: 10.1016/j.aca.2020.07.052. [DOI] [PubMed] [Google Scholar]
- 72.Evans GP, Briers MG, Rawson DM. Can biosensors help to protect drinking-water. Biosensors. 1986;2(5):287–300. doi: 10.1016/0265-928X(86)80008-6. [DOI] [PubMed] [Google Scholar]
- 73.Guilbault GG, Schmid RD. Biosensors for the determination of drug substances. Biotechnol Appl Biochem. 1991;14(2):133–145. [PubMed] [Google Scholar]
- 74.Evtugyn G, Hianik T. Electrochemical immuno- and aptasensors for mycotoxin determination. Chemosensors. 2019;7:29. doi: 10.3390/chemosensors7010010. [DOI] [Google Scholar]
- 75.Chen H, Zhang L, Hu Y, Zhou C, Lan W, Fu H, She Y. Nanomaterials as optical sensors for application in rapid detection of food contaminants, quality and authenticity. Sensors Actuators B Chem. 2021;329:129135. doi: 10.1016/j.snb.2020.129135. [DOI] [Google Scholar]
- 76.Zhu W, Li L, Zhou Z, Yang X, Hao N, Guo Y, Wang K. A colorimetric biosensor for simultaneous ochratoxin a and aflatoxins B1 detection in agricultural products. Food Chem. 2020;319:126544. doi: 10.1016/j.foodchem.2020.126544. [DOI] [PubMed] [Google Scholar]
- 77.Sheini A. Colorimetric aggregation assay based on array of gold and silver nanoparticles for simultaneous analysis of aflatoxins, ochratoxin and zearalenone by using chemometric analysis and paper based analytical devices. Mikrochim Acta. 2020;187:167. doi: 10.1007/s00604-020-4147-5. [DOI] [PubMed] [Google Scholar]
- 78.Wang X, Gao X, He J, Hu X, Li Y, Li X, Fan L, Yu HZ. Systematic truncating of aptamers to create high-performance graphene oxide (GO)-based aptasensors for the multiplex detection of mycotoxins. Analyst. 2019;144(12):3826–3835. doi: 10.1039/C9AN00624A. [DOI] [PubMed] [Google Scholar]
- 79.Lu X, Wang C, Qian J, Ren C, An K, Wang K. Target-driven switch-on fluorescence aptasensor for trace aflatoxin B1 determination based on highly fluorescent ternary CdZnTe quantum dots. Anal Chim Acta. 2019;1047:163–171. doi: 10.1016/j.aca.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 80.Wang C, Tan R, Chen D. Fluorescence method for quickly detecting ochratoxin a in flour and beer using nitrogen doped carbon dots and silver nanoparticles. Talanta. 2018;182:363–370. doi: 10.1016/j.talanta.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 81.Niazi S, Khan IM, Yan L, Khan MI, Mohsin A, Duan N, Wu S, Wang Z. Simultaneous detection of fumonisin B1 and ochratoxin a using dual-color, time-resolved luminescent nanoparticles (NaYF4: Ce, Tb and NH2-Eu/DPA@SiO2) as labels. Anal Bioanal Chem. 2019;411(7):1453–1465. doi: 10.1007/s00216-019-01580-0. [DOI] [PubMed] [Google Scholar]
- 82.Yang M, Cui M, Wang W, Yang Y, Chang J, Hao J, Wang H. Background-free upconversion-encoded microspheres for mycotoxin detection based on a rapid visualization method. Anal Bioanal Chem. 2020;412(1):81–91. doi: 10.1007/s00216-019-02206-1. [DOI] [PubMed] [Google Scholar]
- 83.Xiong Z, Wang Q, Xie Y, Li N, Yun W, Yang L. Simultaneous detection of aflatoxin B1 and ochratoxin a in food samples by dual DNA tweezers nanomachine. Food Chem. 2021;338:128122. doi: 10.1016/j.foodchem.2020.128122. [DOI] [PubMed] [Google Scholar]
- 84.Khan IM, Niazi S, Yu Y, Mohsin A, Mushtaq BS, Iqbal MW, Rehman A, Akhtar W, Wang Z. Aptamer induced multicolored auncs-WS2 “turn on” FRET nano platform for dual-color simultaneous detection of aflatoxinb (1) and zearalenone. Anal Chem. 2019;91(21):14085–14092. doi: 10.1021/acs.analchem.9b03880. [DOI] [PubMed] [Google Scholar]
- 85.Wang C, Huang X, Tian X, Zhang X, Yu S, Chang X, Ren Y, Qian J. A multiplexed FRET aptasensor for the simultaneous detection of mycotoxins with magnetically controlled graphene oxide/Fe3O4 as a single energy acceptor. Analyst. 2019;144(20):6004–6010. doi: 10.1039/C9AN01593K. [DOI] [PubMed] [Google Scholar]
- 86.Sharma R, Ragavan KV, Abhijith KS, Akanksha, Thakur MS. Synergistic catalysis by gold nanoparticles and metal ions for enhanced chemiluminescence. RSC Adv. 2015;5:31434–31438. doi: 10.1039/C5RA01078K. [DOI] [Google Scholar]
- 87.Jiang F, Li P, Zong C, Yang H. Surface-plasmon-coupled chemiluminescence amplification of silver nanoparticles modified immunosensor for high-throughput ultrasensitive detection of multiple mycotoxins. Anal Chim Acta. 2020;1114:58–65. doi: 10.1016/j.aca.2020.03.052. [DOI] [PubMed] [Google Scholar]
- 88.Ma C, Cao Y, Gou X, Zhu JJ. Recent progress in electrochemiluminescence sensing and imaging. Anal Chem. 2020;92(1):431–454. doi: 10.1021/acs.analchem.9b04947. [DOI] [PubMed] [Google Scholar]
- 89.Chen X, Liu Y, Ma Q. Recent advances in quantum dot-based electrochemiluminescence sensors. J Mater Chem C. 2018;6(5):942–959. doi: 10.1039/C7TC05474B. [DOI] [Google Scholar]
- 90.Chen MM, Wang Y, Cheng SB, Wen W, Zhang X, Wang S, Huang WH. Construction of highly efficient resonance energy transfer platform inside a nanosphere for ultrasensitive electrochemiluminescence detection. Anal Chem. 2018;90(8):5075–5081. doi: 10.1021/acs.analchem.7b05074. [DOI] [PubMed] [Google Scholar]
- 91.Wei M, Wang C, Xu E, Chen J, Xu X, Wei W, Liu S. A simple and sensitive electrochemiluminescence aptasensor for determination of ochratoxin a based on a nicking endonuclease-powered DNA walking machine. Food Chem. 2019;282:141–146. doi: 10.1016/j.foodchem.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 92.Sun C, Liao X, Jia B, Shi L, Zhang D, Wang R, Zhou L, Kong W. Development of a ZnCdS@ZnS quantum dots-based label-free electrochemiluminescence immunosensor for sensitive determination of aflatoxin B1 in lotus seed. Mikrochim Acta. 2020;187(4):236. doi: 10.1007/s00604-020-4179-x. [DOI] [PubMed] [Google Scholar]
- 93.Homola J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem Rev. 2008;108(2):462–493. doi: 10.1021/cr068107d. [DOI] [PubMed] [Google Scholar]
- 94.Joshi S, Annida RM, Zuilhof H, van Beek TA, Nielen MW. Analysis of mycotoxins in beer using a portable nanostructured imaging surface plasmon resonance biosensor. J Agric Food Chem. 2016;64(43):8263–8271. doi: 10.1021/acs.jafc.6b04106. [DOI] [PubMed] [Google Scholar]
- 95.Sun L, Wu L, Zhao Q. Aptamer based surface plasmon resonance sensor for aflatoxin B1. Microchim Acta. 2017;184(8):2605–2610. doi: 10.1007/s00604-017-2265-5. [DOI] [Google Scholar]
- 96.Bhardwaj H, Sumana G, Marquette CA. A label-free ultrasensitive microfluidic surface Plasmon resonance biosensor for aflatoxin B1 detection using nanoparticles integrated gold chip. Food Chem. 2020;307:125530. doi: 10.1016/j.foodchem.2019.125530. [DOI] [PubMed] [Google Scholar]
- 97.Kasoju A, Shrikrishna NS, Shahdeo D, Khan AA, Alanazi AM, Gandhi S. Microfluidic paper device for rapid detection of aflatoxin B1 using an aptamer based colorimetric assay. RSC Adv. 2020;10(20):11843–11850. doi: 10.1039/D0RA00062K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun S, Zhao R, Feng S, Xie Y. Colorimetric zearalenone assay based on the use of an aptamer and of gold nanoparticles with peroxidase-like activity. Mikrochim Acta. 2018;185(12):535. doi: 10.1007/s00604-018-3078-x. [DOI] [PubMed] [Google Scholar]
- 99.Li J, Cai T, Li W, Li W, Song L, Li Q, Lv G, Sun J, Jiao S, Wang S, Jin Y, Zheng T. Highly sensitive simultaneous detection of multiple mycotoxins using a protein microarray on a tio2-modified porous silicon surface. J Agric Food Chem. 2021;69(1):528–536. doi: 10.1021/acs.jafc.0c06859. [DOI] [PubMed] [Google Scholar]
- 100.Wu Z, He D, Cui B, Jin Z, Xu E, Yuan C, Liu P, Fang Y, Chai Q. Trimer-based aptasensor for simultaneous determination of multiple mycotoxins using SERS and fluorimetry. Mikrochim Acta. 2020;187(9):495. doi: 10.1007/s00604-020-04487-1. [DOI] [PubMed] [Google Scholar]
- 101.He D, Wu Z, Cui B, Jin Z, Xu E. A fluorometric method for aptamer-based simultaneous determination of two kinds of the fusarium mycotoxins zearalenone and fumonisin B1 making use of gold nanorods and upconversion nanoparticles. Mikrochim Acta. 2020;187(4):254. doi: 10.1007/s00604-020-04236-4. [DOI] [PubMed] [Google Scholar]
- 102.Qian J, Ren C, Wang C, Chen W, Lu X, Li H, Liu Q, Hao N, Li H, Wang K. Magnetically controlled fluorescence aptasensor for simultaneous determination of ochratoxin a and aflatoxin B1. Anal Chim Acta. 2018;1019:119–127. doi: 10.1016/j.aca.2018.02.063. [DOI] [PubMed] [Google Scholar]
- 103.Niazi S, Khan IM, Yu Y, Pasha I, Shoaib M, Mohsin A, Mushtaq BS, Akhtar W, Wang Z. A “turnon” aptasensor for simultaneous and time-resolved fluorometric determination of zearalenone, trichothecenes a and aflatoxin B1 using WS2 as a quencher. Mikrochim Acta. 2019;186(8):575. doi: 10.1007/s00604-019-3570-y. [DOI] [PubMed] [Google Scholar]
- 104.Lv X, Li Y, Yan T, Pang X, Cao W, Du B, et al. Electrochemiluminescence modified electrodes based on RuSi@Ru (bpy)3(2+) loaded with gold functioned nanoporous CO/Co3O4 for detection of mycotoxin deoxynivalenol. Biosens Bioelectron. 2015;70:28–33. doi: 10.1016/j.bios.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 105.Khoshfetrat SM, Bagheri H, Mehrgardi MA. Visual electrochemiluminescence biosensing of aflatoxin M1 based on luminol-functionalized, silver nanoparticle-decorated graphene oxide. Biosens Bioelectron. 2018;100:382–388. doi: 10.1016/j.bios.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 106.Wei T, Ren P, Huang L, Ouyang Z, Wang Z, Kong X, Li T, Yin Y, Wu Y, He Q. Simultaneous detection of aflatoxin B1, ochratoxin a, zearalenone and deoxynivalenol in corn and wheat using surface plasmon resonance. Food Chem. 2019;300:125176. doi: 10.1016/j.foodchem.2019.125176. [DOI] [PubMed] [Google Scholar]
- 107.Moon J, Byun J, Kim H, Lim EK, Jeong J, Jung J, et al. On-site detection of aflatoxin b1 in grains by a palm-sized surface plasmon resonance sensor. Sensors. 2018;18:598. 10.3390/s18020598. [DOI] [PMC free article] [PubMed]
- 108.Zhu Z, Feng M, Zuo L, Zhu Z, Wang F, Chen L, Li J, Shan G, Luo SZ. An aptamer based surface plasmon resonance biosensor for the detection of ochratoxin a in wine and peanut oil. Biosens Bioelectron. 2015;65:320–326. doi: 10.1016/j.bios.2014.10.059. [DOI] [PubMed] [Google Scholar]
- 109.Li Y, Wang Z, Sun L, Liu L, Xu C, Kuang H. Nanoparticle-based sensors for food contaminants. TrAC Trend Anal Chem. 2019;113:74–83. doi: 10.1016/j.trac.2019.01.012. [DOI] [Google Scholar]
- 110.Yang X, Zhou X, Zhang X, Qing Y, Luo M, Liu X, Li C, Li Y, Xia H, Qiu J. A highly sensitive electrochemical immunosensor for fumonisin b1detection in corn using single-walled carbon nanotubes/chitosan. Electroanalysis. 2015;27(11):2679–2687. doi: 10.1002/elan.201500169. [DOI] [Google Scholar]
- 111.Jiang M, Braiek M, Florea A, Chrouda A, Farre C, Bonhomme A, et al. Aflatoxin B1 detection using a highly-sensitive molecularly-imprinted electrochemical sensor based on an electropolymerized metal organic framework. Toxins. 2015;7:3540–3553. doi: 10.3390/toxins7093540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jiang K, Nie D, Huang Q, Fan K, Tang Z, Wu Y, Han Z. Thin-layer MoS2 and thionin composite-based electrochemical sensing platform for rapid and sensitive detection of zearalenone in human biofluids. Biosens Bioelectron. 2019;130:322–329. doi: 10.1016/j.bios.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 113.Zhong H, Yu C, Gao R, Chen J, Yu Y, Geng Y, Wen Y, He J. A novel sandwich aptasensor for detecting T-2 toxin based on rGO-TEPA-au@Pt nanorods with a dual signal amplification strategy. Biosens Bioelectron. 2019;144:111635. doi: 10.1016/j.bios.2019.111635. [DOI] [PubMed] [Google Scholar]
- 114.Rusling JF, Bishop GW, Doan NM, Papadimitrakopoulos F. Nanomaterials and biomaterials in electrochemical arrays for protein detection. J Mater Chem B. 2014;2(1):12–30. doi: 10.1039/C3TB21323D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Beitollahi H, Mohammadi SZ, Safaei M, Tajik S. Applications of electrochemical sensors and biosensors based on modified screen-printed electrodes: a review. Anal Methods. 2020;12(12):1547–1560. doi: 10.1039/C9AY02598G. [DOI] [Google Scholar]
- 116.Ben Abdallah Z, Grauby-Heywang C, Beven L, Cassagnere S, Moroté F, Maillard E, Sghaier H, Cohen Bouhacina T. Development of an ultrasensitive label-free immunosensor for fungal aflatoxin B1 detection. Biochem Eng J. 2019;150:107262. doi: 10.1016/j.bej.2019.107262. [DOI] [Google Scholar]
- 117.Yugender Goud K, Sunil Kumar V, Hayat A, Vengatajalabathy Gobi K, Song H, Kim KH, Marty JL. A highly sensitive electrochemical immunosensor for zearalenone using screen-printed disposable electrodes. J Electroanal Chem. 2019;832:336–342. doi: 10.1016/j.jelechem.2018.10.058. [DOI] [Google Scholar]
- 118.Reverte L, Prieto SB, Campas M. New advances in electrochemical biosensors for the detection of toxins: nanomaterials, magnetic beads and microfluidics systems. A review. Anal Chim Acta. 2016;908:8–21. doi: 10.1016/j.aca.2015.11.050. [DOI] [PubMed] [Google Scholar]
- 119.Lu L, Gunasekaran S. Dual-channel ITO-microfluidic electrochemical immunosensor for simultaneous detection of two mycotoxins. Talanta. 2019;194:709–716. doi: 10.1016/j.talanta.2018.10.091. [DOI] [PubMed] [Google Scholar]
- 120.Zhu C, Liu D, Li Y, Ma S, Wang M, You T. Hairpin DNA assisted dual-ratiometric electrochemical aptasensor with high reliability and anti-interference ability for simultaneous detection of aflatoxin B1 and ochratoxin a. Biosens Bioelectron. 2021;174:112654. doi: 10.1016/j.bios.2020.112654. [DOI] [PubMed] [Google Scholar]
- 121.Wei M, Xin L, Feng S, Liu Y. Simultaneous electrochemical determination of ochratoxin a and fumonisin B1 with an aptasensor based on the use of a Y-shaped DNA structure on gold nanorods. Mikrochim Acta. 2020;187(2):102. doi: 10.1007/s00604-019-4089-y. [DOI] [PubMed] [Google Scholar]
- 122.Han Z, Tang Z, Jiang K, Huang Q, Meng J, Nie D, Zhao Z. Dual-target electrochemical aptasensor based on co-reduced molybdenum disulfide and au NPs (rMoS2-au) for multiplex detection of mycotoxins. Biosens Bioelectron. 2020;150:111894. doi: 10.1016/j.bios.2019.111894. [DOI] [PubMed] [Google Scholar]
- 123.Li Y, Liu D, Zhu C, Shen X, Liu Y, You T. Sensitivity programmable ratiometric electrochemical aptasensor based on signal engineering for the detection of aflatoxin B1 in peanut. J Hazard Mater. 2020;387:122001. doi: 10.1016/j.jhazmat.2019.122001. [DOI] [PubMed] [Google Scholar]
- 124.El Alami El Hassani N, Bouchikhi B, El Bari N. Recent development of an electrochemical imprinted sensor for the detection of trace-level of unmetabolized aflatoxin B2 in dairy milk. J Electroanal Chem. 2020;865:114123. 10.1016/j.jelechem.2020.114123.
- 125.Sun C, Liao X, Huang P, Shan G, Ma X, Fu L, Zhou L, Kong W. A self-assembled electrochemical immunosensor for ultra-sensitive detection of ochratoxin a in medicinal and edible malt. Food Chem. 2020;315:126289. doi: 10.1016/j.foodchem.2020.126289. [DOI] [PubMed] [Google Scholar]
- 126.Costa MP, Frías IAM, Andrade CAS, Oliveira MDL. Impedimetric immunoassay for aflatoxin B1 using a cysteine modified gold electrode with covalently immobilized carbon nanotubes. Microchim Acta. 2017;184(9):3205–3213. doi: 10.1007/s00604-017-2308-y. [DOI] [Google Scholar]
- 127.Xu W, Qing Y, Chen S, Chen J, Qin Z, Qiu J, Li CR. Electrochemical indirect competitive immunoassay for ultrasensitive detection of zearalenone based on a glassy carbon electrode modified with carboxylated multi-walled carbon nanotubes and chitosan. Microchim Acta. 2017;184(9):3339–3347. doi: 10.1007/s00604-017-2342-9. [DOI] [Google Scholar]
- 128.Kunene K, Weber M, Sabela M, Voiry D, Kanchi S, Bisetty K, Bechelany M. Highly-efficient electrochemical label-free immunosensor for the detection of ochratoxin a in coffee samples. Sensors Actuators B Chem. 2020;305:127438. doi: 10.1016/j.snb.2019.127438. [DOI] [Google Scholar]
- 129.Bhardwaj H, Pandey MK, Rajesh, Sumana G. Electrochemical aflatoxin B1 immunosensor based on the use of graphene quantum dots and gold nanoparticles. Mikrochim Acta. 2019;186:592. doi: 10.1007/s00604-019-3701-5. [DOI] [PubMed] [Google Scholar]
- 130.Wang C, Qian J, An K, Huang X, Zhao L, Liu Q, Hao N, Wang K. Magneto-controlled aptasensor for simultaneous electrochemical detection of dual mycotoxins in maize using metal sulfide quantum dots coated silica as labels. Biosens Bioelectron. 2017;89:802–809. doi: 10.1016/j.bios.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 131.Wang C, Li Y, Zhao Q. A signal-on electrochemical aptasensor for rapid detection of aflatoxin B1 based on competition with complementary DNA. Biosens Bioelectron. 2019;144:111641. doi: 10.1016/j.bios.2019.111641. [DOI] [PubMed] [Google Scholar]
- 132.Azri FA, Eissa S, Zourob M, Chinnappan R, Sukor R, Yusof NA, Raston NHA, Alhoshani A, Jinap S. Electrochemical determination of zearalenone using a label-free competitive aptasensor. Mikrochim Acta. 2020;187(5):266. doi: 10.1007/s00604-020-4218-7. [DOI] [PubMed] [Google Scholar]
- 133.Jahangiri-Dehaghani F, Zare HR, Shekari Z. Measurement of aflatoxin M1 in powder and pasteurized milk samples by using a label-free electrochemical aptasensor based on platinum nanoparticles loaded on Fe-based metal-organic frameworks. Food Chem. 2020;310:125820. doi: 10.1016/j.foodchem.2019.125820. [DOI] [PubMed] [Google Scholar]
- 134.Radi AE, Eissa A, Wahdan T. Molecularly imprinted impedimetric sensor for determination of mycotoxin zearalenone. Electroanalysis. 2020;32(8):1788–1794. doi: 10.1002/elan.201900528. [DOI] [Google Scholar]
- 135.Radi AE, Eissa A, Wahdan T. Impedimetric sensor for deoxynivalenol based on electropolymerised molecularly imprinted polymer on the surface of screen-printed gold electrode. Int J Environ Anal Chem. 2019:1–12. 10.1080/03067319.2019.1699548.
- 136.Zhou Q, Tang D. Recent advances in photoelectrochemical biosensors for analysis of mycotoxins in food. TrAC Trend Anal Chem. 2020;124:115814. doi: 10.1016/j.trac.2020.115814. [DOI] [Google Scholar]
- 137.Zhang B, Lu Y, Yang C, Guo Q, Nie G. Simple “signal-on” photoelectrochemical aptasensor for ultrasensitive detecting AFB1 based on electrochemically reduced graphene oxide/poly (5-formylindole)/au nanocomposites. Biosens Bioelectron. 2019;134:42–48. doi: 10.1016/j.bios.2019.03.048. [DOI] [PubMed] [Google Scholar]
- 138.Mao L, Wang X, Guo Y, Yao L, Xue X, Wang HX, Xiong C, Wen W, Zhang X, Wang S. A synergistic approach to enhance the photoelectrochemical performance of carbon dots for molecular imprinting sensors. Nanoscale. 2019;11(16):7885–7892. doi: 10.1039/C9NR01675A. [DOI] [PubMed] [Google Scholar]
- 139.Mao L, Ji K, Yao L, Xue X, Wen W, Zhang X, Wang S. Molecularly imprinted photoelectrochemical sensor for fumonisin B1 based on GO-CdS heterojunction. Biosens Bioelectron. 2019;127:57–63. doi: 10.1016/j.bios.2018.11.040. [DOI] [PubMed] [Google Scholar]
- 140.Ge L, Liu Q, Hao N, Kun W. Recent developments of photoelectrochemical biosensors for food analysis. J Mater Chem B. 2019;7(46):7283–7300. doi: 10.1039/C9TB01644A. [DOI] [PubMed] [Google Scholar]
- 141.Xu Z, Long LL, Chen YQ, Chen ML, Cheng YH. A nanozyme-linked immunosorbent assay based on metal-organic frameworks (MOFs) for sensitive detection of aflatoxin B1. Food Chem. 2021;338:128039. doi: 10.1016/j.foodchem.2020.128039. [DOI] [PubMed] [Google Scholar]
- 142.Dong J, Zhao D, Lu Y, Sun W-Y. Photoluminescent metal–organic frameworks and their application for sensing biomolecules. J Mater Chem A. 2019;7(40):22744–22767. doi: 10.1039/C9TA07022B. [DOI] [Google Scholar]
- 143.Safaei M, Foroughi MM, Ebrahimpoor N, Jahani S, Omidi A, Khatami M. A review on metal-organic frameworks: synthesis and applications. TrAC Trend Anal Chem. 2019;118:401–425. doi: 10.1016/j.trac.2019.06.007. [DOI] [Google Scholar]
- 144.Hu Z, Lustig WP, Zhang J, Zheng C, Wang H, Teat SJ, Gong Q, Rudd ND, Li J. Effective detection of mycotoxins by a highly luminescent metal-organic framework. J Am Chem Soc. 2015;137(51):16209–16215. doi: 10.1021/jacs.5b10308. [DOI] [PubMed] [Google Scholar]
- 145.Li Z, Xu X, Fu Y, Guo Y, Zhang Q, Zhang Q, Yang H, Li Y. A water-stable luminescent metal–organic framework for effective detection of aflatoxin B1 in walnut and almond beverages. RSC Adv. 2019;9(2):620–625. doi: 10.1039/C8RA07804A. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.