Abstract
Background
The long‐term trend in cancer death in a rapidly developing country provides information for cancer prophylaxis. Here, we aimed to identify the trends in cancer mortality in China during the 2004‐2018 period.
Methods
Using raw data from the national mortality surveillance system of China, we assessed the mortalities of all cancer and site‐specific cancers during the 2004‐2018 period. The participants were divided into three age groups: ≥65 years, 40‐64 years, and ≤39 years. Changing trends in cancer death by gender, residency, and tumor location were estimated using fitting joinpoint models to log‐transformed crude mortality rates (CMRs) and age‐standardized mortality rates (ASMRs).
Results
Cancer death accounted for 24% of all‐cause of death in China during 2014‐2018. The CMR of all cancer was 150.0 per 100,000 persons. Cancer was the leading cause of death in the population <65 years. The six major cancer types (lung/bronchus cancer, liver cancer, stomach cancer, esophagus cancer, colorectal cancer, and pancreas cancer) accounted for 75.85% of all cancer deaths. The CMR of all cancer increased while the ASMR decreased during 2014‐2018 (P < 0.001). Lung/bronchus cancer and liver cancer were the leading causes of cancer death in the population <65 years, accounting for 45.31% (CMR) and 44.35% (ASMR) of all cancer death, respectively. The ASMR of liver cancer was higher in the 40‐64 years population than in the ≥65 years population, in contrast to the other five major cancers. The ASMRs of liver cancer, stomach cancer, and esophagus cancer decreased although they were higher in rural residents than in urban residents; the ASMRs of lung/bronchus cancer, colorectal cancer, and pancreas cancer increased in rural residents although they were higher in urban residents than in rural residents during 2014‐2018.
Conclusion
Although the ASMR of all cancer decreased in China during 2004‐2018, lung/bronchus cancer and liver cancer remained the leading causes of cancer‐related premature death. Lung/bronchus cancer, colorectal cancer, and pancreas cancer increased in rural residents.
Keywords: age‐standardized mortality rate, breast cancer, colorectal cancer, crude mortality rate, demographic distribution, liver cancer, national mortality surveillance system, premature death, site‐specific cancer, stomach cancer, trend
This 15‐year longitudinal study described cancer burden of a rapid changing country with significant regional and urban‐rural disparities, which is important in evaluating the effect of population ageing, risk factor exposure, and public health efforts on cancer mortality. Lung and liver cancers were the 1st leading cause of immature death in women and men, respectively. Lung, colorectal, and pancreatic cancers kept increasing in rural areas. These findings are references for policy making to control cancer.

Abbreviations
- ASMR
age‐standardized mortality rate
- CDC
Center for Disease Control and Prevention
- CI
confidence interval
- CMR
crude mortality rate
- DSP
disease surveillance points
- GBD
Global Burden of Disease
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HP
Helicobacter pylori
- ICD‐10
International Classification of Diseases‐10th Revision
- NCCR
National Central Cancer Registry of China
1. INTRODUCTION
Cancer is one of the top two leading causes of premature death in 127 countries and may surpass cardiovascular disease as the leading cause of premature death in most countries over the course of this century [1]. China alone contributed to a quarter of the world's cancer death in 2020 [2]. The high cancer burden in China highlights the need for further improvement in etiological surveys and the building up of an anti‐cancer network for introducing and implementing sustainable actions for cancer control [3, 4]. According to the World Bank, China is one of the rapidly developing nations in the world [5]. During the era of the planned economy between 1949 and 1978 and the initial stage for the reform and opening‐up between 1979 and 1993, urban Chinese mostly worked in industry and commerce sectors. Rural Chinese mostly lived on farms. The urban–rural disparity in socioeconomic status has gradually narrowed since 1993 when China entered the market economy era [6]. However, economic development as well as its concomitant socioeconomic status and lifestyle factors varies greatly in the eastern, central, and western regions of China [7]. These rapidly changing socioeconomic features and the urban–rural disparity in China provide an opportunity to evaluate cancer burden and the effect of medical service, aging, risk factor exposure, and public health efforts on cancer mortality, which could be indispensable for cancer prophylaxis.
In China, the data on cancer registration were often collected, evaluated, and published by the National Central Cancer Registry (NCCR) of China, based on the data of all cancer and most type‐specific cancers from a given year as well as for the 11‐year trend from 2000 to 2011 [8, 9, 10]. However, the trend of cancer mortality in China after 2011 has not been reported by the NCCR, even though the number of local population‐based cancer registries in NCCR has increased from 54 in 2008 to 308, covering 300 million people, in 2014 [10]. NCCR is not the sole data resource of cancer mortality in China. The integrated national mortality surveillance system for death registration and mortality surveillance is the official source of the mortality surveillance system. This system is integrated with the disease surveillance points (DSPs) system established by the Chinese Center for Disease Control and Prevention (CDC) in 1978 and the Chinese vital registration system established by the Health Department of the People's Republic of China in the 1950s. In 2013, the government combined the two systems into an integrated national mortality surveillance system. This integrated system increased the number of DSPs from 161 to 605. The 605 DSPs were distributed to all provincial administrative regions, covering a quarter of China's population [11]. Cancer death in 1990 and 2017 was recently evaluated as one of the leading causes of death in China [12]. However, consecutive data on cancer death from 2004 to 2018 from the national mortality surveillance system have not been reported.
In this study, we evaluated trends in the mortality of total cancer and the major site‐specific cancers of mainland China from 2004 to 2018. The findings of this study could help understand the association of possible risk factor exposure, socioeconomic development, and public health efforts with the trends of cancer mortalities, thus, providing evidence for the prevention and control of cancer‐related premature death.
2. METHODS AND MATERIALS
2.1. Source of data
Cancer death was derived from the death certificate data of the Chinese national mortality surveillance system [11]. Briefly, the data for the DSPs were collected using a standard protocol by trained personals. The trained staff oversaw data collection of all deaths occurring in hospitals. For deaths occurring outside a hospital setting, village health workers or community hospital professionals used verbal autopsy strategies to collect the relevant data. Local CDCs reported all data to their next‐level office (from county to prefectural, provincial, and finally to national level), and routine quality checks were conducted by coders at each center. Quality checks assessed completeness, coding, and internal logic across items reported on death certificates. Any unqualified reports detected were corrected at each DSP through a review of detailed medical records or repeated verbal autopsies. A routine national sample survey was conducted every three years at all surveillance locations [13]. The raw data are approved annually for publication by the National Center for Chronic and Non‐communicable Disease Control and Prevention (Chinese CDC, Beijing, China). The definition of cancer‐related death was based on Chapters C00–C97 of the International Classification of Diseases–10th Revision (ICD–10), as previously reported [14]. Supplementary Table S1 provides more information on the classification of cancer types by the ICD‐10 codes. Based on the administrative division of rural and urban areas, the DSPs at the counties including prefecture‐level cities were defined as rural areas, while the municipal districts were defined as urban areas. According to the classification of the National Statistics Bureau, China is divided into 22 provinces, four municipalities (Beijing, Shanghai, Tianjin, and Chongqing), five autonomous regions (Guangxi, Ningxia, Xizang, Xinjiang, and Inner Mongolia), and two special administrative regions (Hong Kong and Macau). According to the first national economic census, China is divided into three regions: the eastern (those on the eastern coastline), central (Heilongjiang, Jilin, Shanxi, Henan, Hubei, Anhui, Jiangxi, and Hunan), and western (others) regions [15]. The populations of urban and rural areas were also divided into three age groups (≤39, 40‐64, and ≥65 years).
2.2. Data analysis
The Epidata 3.1 (The Epidata Association, Odense, Syddanmark, Denmark) statistical software was used for data entry and management. The crude mortality rate (CMR) and their 95% confidence interval (CI) of total cancer and site‐specific cancer per 100,000 in the total population in all DSPs and populations stratified by the eastern, central, and western region as well as stratified by rural and urban areas were calculated every year from 2004 to 2018. The age‐standardized mortality rate (ASMR) was calculated based on the standard population from the 5th Chinese census carried out in 2000 [16]. We calculated the CMRs and the ASMRs of all cancer and type‐specific cancers in the population stratified by age, gender, region (eastern, central, and western), and residency (rural and urban residents). Temporal trends in the mortality from 2004 to 2018, as expressed by annual percent change (APC), were estimated by fitting joinpoint models (Joinpoint Regression Program Version 4.8.0.1, the National Cancer Institute, Rockville, MD, US) to log‐transformed CMRs and ASMRs. The student t‐test was employed to assess if the APC was statistically different from zero. The term “increase” or “decrease” was applied to describe the trend when the APC of the trend was statistically significant (P < 0.05). The rate ratio (RR) of rural‐urban disparity was defined as the mortality rate per cancer type rural /mortality rate per cancer type urban. RR of men–women disparity was defined as the mortality rate per cancer type men /mortality rate per cancer type women. The χ2 test and the Fisher's exact test were applied to analyze differences between urban and rural areas in cancer mortality rates. R software (Version 3.6.3, MathSoft, Cambridge, MA, US) was applied for statistical analysis. A P value of <0.05 was considered significant.
3. RESULTS
3.1. Basic information and trend in cancer death during 2004‐2018
This study included the information of all residents covered by DSPs, with 2,209,059,291 person‐years during 2004 and 2018. Cardio‐cerebrovascular disease was the first cause of death in the entire population, accounting for 43% of all‐cause death (n = 13,967,306) in this 15–year period. Cancer was the second cause of death (n = 3,314,213) in the entire population, accounting for 24% of all‐cause death. The CMR of all cancer was 150.0 per 100,000 (95% CI: 149.9 per 100,000 –150.2 per 100,000). Supplementary Table S2 shows the data of yearly cancer death among all death in the population covered by all CDC‐DSPs from 2004 to 2018. The CMR of all cancer continually increased (APC = 1.74%, P< 0.001) while the ASMRs of all cancer decreased (APC = –1.34%, P < 0.001) from 2004 to 2018 (Figure 1A–B). In the population <65 years, however, cancer was the leading cause of death; the CMR increased (APC = 0.80%, P = 0.012) while the ASMRs decreased (APC = –1.70%, P < 0.001) from 2004 to 2018 (Figure 1C‐D). In the whole population, site‐specific cancers in descending order by CMRs were lung/bronchus cancer (41.11 per 100,000), liver cancer (25.18 per 100,000), stomach cancer (20.66 per 100,000), esophagus cancer (12.74 per 100,000), colorectal cancer (9.67 per 100,000), and pancreas cancer (4.44 per 100,000) (Supplementary Figure S1A). The six major causes of cancer death (lung/bronchus cancer, liver cancer, stomach cancer, esophagus cancer, colorectal cancer, and pancreas cancer) accounted for 75.85% of all cancer‐related death. The ASMRs of each cancer type in the whole population as well as the CMRs and ASMRs of each cancer type in females and males are shown in Supplementary Figure S1B–F. In the population ≤65 years, lung/bronchus cancer and liver cancer were the major cancer types, accounting for 45.31% (in the CMR) and 44.35% (in the ASMR) of all cancer death, respectively (Supplementary Figure S2). In the whole population, the CMRs and ASMRs of all cancer were significantly higher in males than in females (RR [males vs females] = 1.792 [95% CI: 1.788–1.796], P <0.001 for the CMR; RR = 1.957 [95% CI: 1.951–1.962], P < 0.001 for the ASMR) (Figure 2A–B). In the population <65 years, lung/bronchus cancer was the leading cause of cancer death in females (Figure 2C–D) and liver cancer was the leading cause of cancer death in males (Figure 2E–F). The top 5 causes of cancer death remained similar rank from 2004 to 2018. The ranking of leukemia and uterine cancer death decreased after 2009. Pancreas cancer, cervical cancer, bladder cancer, and prostate cancer increased in rankings after 2009 (Supplementary Figure S3).
FIGURE 1.
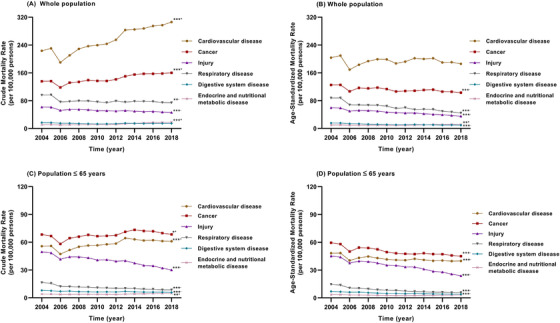
Crude and age‐standardized mortality rates for six major diseases in mainland China among the whole population and the population younger than 65 during 2004‐2018; (A) crude mortality rates for six major diseases among the whole population; (B) age‐standardized mortality rates for six major diseases among the whole population; (C) crude mortality rates for six major diseases among the population younger than 65 years; and (D) age‐standardized mortality rates for six major diseases that lead to death in China among the population younger than 65 years. * P < 0.05, ** P < 0.01, *** P < 0.001; + increased, –decreased from 2004 to 2018
FIGURE 2.
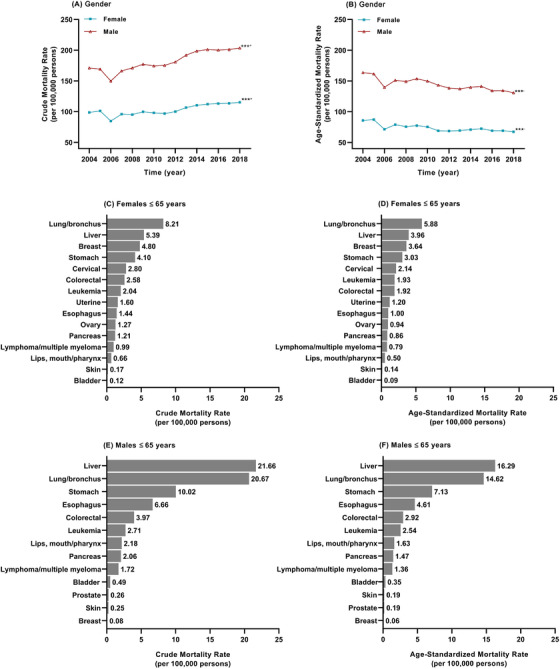
Crude and age‐standardized mortality rates for cancers of women and men in mainland China during 2004‐2018; (A) trend in the crude mortality rate of all cancer of females and males; (B) trend in the age‐standardized mortality rates of all cancer of females and males; (C) the crude mortality rate of each cancer type in females <65 years; (D) the age‐standardized mortality rate of each cancer type in females <65 years; (E) the crude mortality rate of each cancer type in males <65 years; and (F) the age‐standardized mortality rate of each cancer type in males <65 years. *** P< 0.001; + increased, –decreased from 2004 to 2018
3.2. Trends in the ASMR of the major cancers in three age groups
We divided the participants into three age groups: ≥65 years old, 40‐64 years old, and ≤39 years old. Trends in the ASMR of the six major cancer types in the three age groups during 2004‐2018 are shown in Figure 3. The ASMRs of lung/bronchus cancer and colorectal cancer did not change significantly in the ≥65 years and 40‐64 years populations, while those of both cancer types in the ≤39 years population decreased by 43.31% (APC = –2.72%, P < 0.001) and 32.48% (APC = –1.74%, P = 0.010), respectively, during 2004‐2018. The ASMRs of lung/bronchus cancer and liver cancer were higher than other cancer types in the 40‐64 years old population. In the ≤39 years, 40‐64 years, and ≥65 years populations, the ASMRs of esophagus cancer decreased by 84.01% (APC = –0.84%, P < 0.001), 57.80% (APC = –6.14%, P < 0.001), and 42.87% (APC = –3.59%, P < 0.001), respectively; those of stomach cancer decreased by 49.39% (APC = –4.05%, P < 0.001), 55.17% (APC = –5.08%, P < 0.001), and 45.26% (APC = –3.60%, P < 0.001), respectively; those of liver cancer decreased by 46.84% (APC = –3.56%, P < 0.001), 34.83% (APC = –2.74%, P < 0.001), and 30.66% (APC = –2.11%, P < 0.001), respectively. The ASMRs of pancreas cancer in the ≤39 years population did not change significantly; however, in the 40‐64 years and the ≥65 years populations they increased by 40.14% (APC = 1.87%, P < 0.001) and 45.45% (APC = 2.22%, P < 0.001), respectively. Interestingly, the ASMR of liver cancer was higher in the 40‐64 years population than in the ≥65 years population. In the ≤39 years age group, the ASMR of liver cancer was higher than that of the other four major cancer types, but it was decreasing from 2004 to 2018.
FIGURE 3.
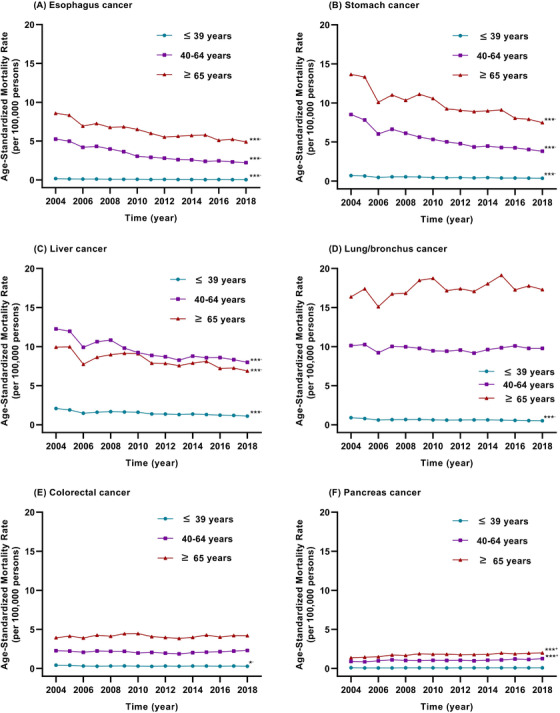
Trends in the age‐standardized mortality rates per 100,000 of six major cancer types in three age groups (younger than 39 years, 40‐64 years, 65 years or older) in mainland China during 2004‐2018; (A) trends in the age‐standardized mortality rate of esophagus cancer; (B) trends in the age‐standardized mortality rate of stomach cancer; (C) trends in the age‐standardized mortality rate of liver cancer; (D) trends in the age‐standardized mortality rate of lung/bronchus cancer; (E) trends in the age‐standardized mortality rate of colorectal cancer; and (F) trends in the age‐standardized mortality rate of pancreas cancer. *P < 0.05, ***P < 0.001; +increased, –decreased from 2004 to 2018
3.3. Differences in cancer death between urban and rural residents
Of the 3,314,213 cancer deaths in total, 1,164,937 occurred in urban areas, accounting for 26% of all death (n = 4,454,338) in urban areas; 2,149,276 occurred in rural areas, accounting for 23% of all death (n = 9,512,968) in rural areas. The CMR of all cancer was generally higher in urban residents than in rural residents. In the age groups of ≤39 and 40‐64 years old, the CMR was lower in urban residents than in rural residents; however, the situation was inversed for the ≥65 years age group (P < 0.001) (Table 1). The ASMR of all cancer in the whole population, urban population, and rural population decreased by 20.54% (APC = –1.34%, P < 0.001), 20.59% (APC = –1.38%, P < 0.001) and 20.32% (APC = –1.29%, P < 0.001), respectively (Supplementary Figure S4). The CMRs of the six major cancer types are shown in Supplementary Table S3. The ASMRs of liver cancer, stomach cancer, and esophagus cancer were higher in rural residents than those in urban residents, in contrast to lung/bronchus cancer, colorectal cancer, and pancreas cancer (P < 0.001) (Figure 4A). In urban residents, the ASMRs of liver cancer, stomach cancer, esophagus cancer, and lung/ bronchus cancer decreased by 32.98% (APC = –2.27%, P < 0.001), 44.65% (APC = –3.32%, P < 0.001), 29.71% (APC = –1.90%, P = 0.009) and 12.68% (APC = –0.82%, P = 0.009), respectively, from 2004 to 2018. However, those of colorectal cancer and pancreatic cancer did not change significantly during this period (Figure 4B). In rural residents, the ASMRs of liver cancer, stomach cancer, and esophagus cancer decreased by 35.00% (APC = −2.75%, P < 0.001), 51.25% (APC = −4.60%, P < 0.001), and 55.31% (APC = −5.68%, P < 0.001), respectively, from 2004 to 2018. However, those of lung/bronchus cancer and pancreas cancer increased by 11.96% (APC = 1.73%, P = 0.006) and 11.48% (APC = 4.18%, P < 0.001), respectively, during 2004‐2018. The ASMR of colorectal cancer did not change significantly, during this period (Figure 4C). The rate ratios of lung/bronchus cancer, stomach cancer, esophagus cancer, and pancreas cancer between urban and rural areas from 2004 to 2018 changed from 0.73 to 0.93 (APC = 2.57%, P = 0.002), 1.26 to 1.11 (APC = –1.32%, P = 0.022), 1.85 to 1.17 (APC = ‐3.86%, P < 0.001), and 0.47 to 0.76 (APC = –3.84%, P < 0.001), respectively, indicating that the urban–rural disparity became smaller. The changes in urban–rural disparity were not significant in liver cancer and colorectal cancer.
TABLE 1.
Rate ratio of cancer mortality between urban and rural residents by region, sex, and age in China, 2004‐2018*
| Urban | Rural | ||||||
|---|---|---|---|---|---|---|---|
| Subgroup | Total people | Mortality Rate (per 100,000) | Total people | Mortality Rate (per 100,000) | RR (95%CI) | P ‐ value | |
| Region | |||||||
| Eastern | 323,951,273 | 172.15 | 530,871,331 | 169.13 | 0.982 (0.979‐0.986) | <0.001 | |
| Central | 226,256,815 | 147.23 | 547,916,404 | 141.72 | 0.963 (0.959‐0.966) | <0.001 | |
| Western | 194,756,008 | 140.76 | 385,307,460 | 123.24 | 0.875 (0.871‐0.880) | <0.001 | |
| Sex | |||||||
| Male | 377,468,459 | 196.48 | 747,633,232 | 188.22 | 0.958 (0.955‐0.961) | <0.001 | |
| Female | 367,495,637 | 115.18 | 716,461,963 | 103.57 | 0.899 (0.896‐0.903) | <0.001 | |
| Age | |||||||
| ≤39 | 393,287,134 | 8.89 | 803,927,015 | 10.33 | 1.163 (1.148‐1.178) | <0.001 | |
| 40‐64 | 274,441,623 | 151.79 | 511,551,133 | 164.87 | 1.086 (1.082‐1.090) | <0.001 | |
| ≥65 | 77,235,274 | 923.68 | 148,617,047 | 822.80 | 0.890 (0.887‐0.892) | <0.001 | |
| Overall | 744,964,096 | 156.37 | 1464,095,195 | 146.80 | 0.939 (0.937‐0.941) | <0.001 | |
Data are presented as rate ratio (RR) and 95% confidence interval (CI).
FIGURE 4.
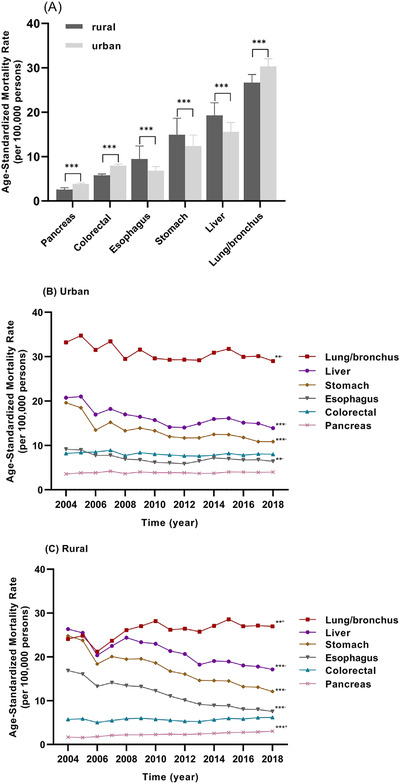
The age‐standardized mortality rates per 100,000 for six major cancer types in urban and rural populations in mainland China during 2004‐2018; (A) comparison of the age‐standardized mortality rates per 100,000 of the six major cancer types in urban and rural populations; (B) trends in the age‐standardized mortality rates of six major cancer types in urban population, 2004‐2018; (C) trends in the age‐standardized mortality rates of six major cancer types in rural population, 2004‐2018. ** P < 0.01, *** P < 0.001; + increased, –decreased from 2004 to 2018
3.4. Trends in cancer death among the three geographic regions
The CMR of all cancer decreased from the eastern to western regions, whereas the urban‐rural disparity increased from the eastern to western regions (Table 1). The CMR of all cancer increased significantly except for the central urban residents. However, the ASMR decreased significantly except for the western rural residents (Supplementary Figure S5). In rural residents, the ASMR of all cancer decreased by 21.24% (APC = –1.40%, P < 0.001) and 27.61% (APC = ‐2.28%, P < 0.001) in the eastern and central regions, respectively; in urban residents, it decreased by 16.92% (APC = –1.17%, P < 0.001), 26.84% (APC = –1.69%, P = 0.001), and 18.79% (APC = –1.27%, P = 0.014) in the eastern, central, and western regions, respectively. The ASMR of lung/bronchus cancer decreased in the urban population in the eastern and central regions, while the rate increased in the rural population in the eastern and western regions. The ASMR of liver cancer in the eastern and central regions, as well as the ASMR of stomach cancer in the three regions, decreased significantly. The ASMRs of colorectal cancer and pancreas cancer continually increased in the rural residents of the three regions (Figure 5).
FIGURE 5.
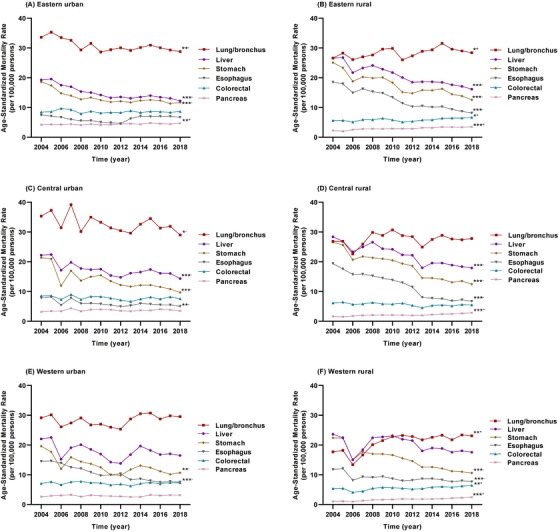
Trends in the age‐standardized mortality rates per 100,000 of six major cancer types in the eastern, central, and western regions of mainland China during 2004‐2018; (A) trends in the age‐standardized mortality rates of six major cancer types in eastern urban population; (B) trends in the age‐standardized mortality rates of six major cancer types in eastern rural population; (C) trends in the age‐standardized mortality rates of six major cancer types in central urban population; (D) trends in the age‐standardized mortality rates of six major cancer types in central rural population; (E) trends in the age‐standardized mortality rates of six major cancer types in western urban population; and (F) trends in the age‐standardized mortality rates of six major cancer types in western rural population. *P < 0.05, **P < 0.01, ***P < 0.001; +increased, –decreased from 2004 to 2018
4. DISCUSSION
In this study, the overall profile and trends of cancer death from 2004 to 2018 were evaluated. The rankings of the five leading causes of cancer death in the annual reports from NCCR were different between 2011 and 2015, possibly because the number of population‐based cancer registries increased from 177 in 2011 [10] to 308 in 2014 [8]. The ranking remained consistent during 2004‐2018 in this study, possibly because the national mortality surveillance system is highly representative in China after several rounds of representativeness evaluation and adjustment [11]. Cancer was the second leading cause of death in the whole population and the first leading cause of death in those <65 years and was consistent with a study we previously performed in the Shanghai population only [17]. Thus, cancer has been the major cause of premature death during the past 15 years. The CMR of all cancer continually increased while their ASMR decreased, indicating that aging contributed greatly to the increased cancer death.
Most type‐specific cancers are aging‐related diseases. However, each type‐specific cancer causes a distinct decrease in life expectancy. In contrast to other cancer types, liver cancer caused more death in the 40‐64 years old population than in the ≥65 years old population. Our previous study has also demonstrated that liver cancer was the leading cause of cancer death in the population <75 years [17]. This might be related to the cause of liver cancer. Although hepatitis C virus infection and alcoholic and non‐alcoholic fatty liver disease contribute to the occurrence of liver cancer, chronic hepatitis B virus (HBV) infection contributes to 87.5% of hepatocellular carcinoma (HCC), the major subtype accounting for 94.6% of liver cancer in Eastern China [18, 19]. HBV infection has been significantly associated with 10 years earlier onset, more cirrhosis, higher α‐fetoprotein, and more microvascular invasion in HCC [18]. Chronic HBV infection is more prevalent in the eastern region of China than in the central or western regions; thus, it could be the major cause of HCC burden in the next several decades [20]. The ASMR of liver cancer in the ≥65 years population and the 40‐64 years population continued to decrease possibly because antiviral treatment for chronic hepatitis B has significantly decreased HCC occurrence since antiviral agents nucleotide/nucleoside analogs were introduced into China in 1998 [21, 22]. The CMR of liver cancer was significantly higher in rural residents than in urban residents (Figure 4A), possibly because of the difference in the availability and adherence of receiving antiviral treatment [23]. The ASMR of liver cancer in the ≤39 years population continued to decrease, possibly because of HBV vaccination in newborns enforced in mainland China since 1992 [24]. Thus, the data of cancer death reflect not only the natural course of each cancer type but also the effects of public health actions.
Similar to liver cancer, the ASMRs of stomach cancer and esophagus cancer continued to decrease from 2004 to 2018. An NCCR study demonstrated that the trends in ASMRs of lung/bronchus cancer, liver cancer, stomach cancer, esophagus cancer, and colorectal cancer were quite consistent with their age‐standardized incidence rates (ASIRs) during 2000 to 2011 [8]. The decrease in the ASMRs could be largely related to the decrease in the ASIRs of the five major cancer types. Changes in the ASMRs could reflect different exposures to risk factors, socioeconomic settings, lifestyles, and access to care and screening for cancers [25, 26]. The rapid progresses in economic development and optimization of public health policy in China greatly increase the socioeconomic status, improve healthy lifestyles to decrease the risk factor exposure via health education, and increase medical insurance coverage for access to care and screening for cancers in Chinese, resulting in the decreases in the ASMRs of stomach cancer, esophagus cancer, and liver cancer.
The difference in cancer mortality between rural and urban residents and among the three regions during the last 15 years might provide useful information regarding the exposure of cancer risk factors, health care access, and the effects of public health action for cancer control. Urban‐rural disparities in cancer incidence and mortality were found although the gaps in health care utilization, socioeconomic status, and even lifestyle have gradually narrowed in China since 1993 [27]. In this study, we found that the CMR of all cancer was higher in rural residents than in urban residents in the population <65 years; however, the situation was inversed for the ≥65 years population (Table 1). This finding indicates that health care access could have been weaker in rural areas than in urban areas. The disparity in socioeconomic status contributes to the difference in cancer occurrence and subsequent cancer mortality [28, 29]. The ASMRs of stomach cancer and esophagus cancer were higher in rural than in urban residents, possibly because of poor health care access including lack of active screening and facilities for such screening, unhealthy lifestyle‐related conditions including familial clustering of Helicobacter pylori (HP) infection, increased intake of salted and hot food, and higher prevalence of tobacco smoking in rural residents. Chronic HP infection and increased intake of salted food increase the risk of stomach cancer [30, 31]. Long‐term intake of hot food and tobacco smoking increase the risk of esophagus cancer [32, 33].
The ASMRs of lung/bronchus cancer and colorectal cancer were higher in urban residents than in rural residents, possibly because of greater exposure to air pollution in urban areas [34, 35], less consumption of coarse food, and a sedentary lifestyle in urban residents [36]. Interestingly, the trend of ASMR of lung cancer decreased in urban residents but increased in rural residents (Figure 4C). This might be caused by the following aspects: (1) polluted industry had been increasingly moved from urban areas to rural areas; and (2) tobacco and cigarette smoking and indoor air pollution caused by smoky coal usage contribute to the sustained level of lung cancer in rural areas [25, 37]. The ASMR of lung/bronchus cancer decreased from the eastern to western regions, which was consistent with a decreasing level of industrialization during and before the study period. Thus, industrialization‐related air pollution could be one of the major reasons for the increased mortality of lung/bronchus cancer.
Tobacco smoking has been demonstrated to be the single most important carcinogenic risk factor in China, contributing to approximately 24.5% of cancers in males, especially for lung/bronchus cancer [4]. Thus, tobacco control, as well as indoor and ambient air pollution reduction, could be critical in reducing the disease burden of lung/bronchus cancer. We also observed that the ASMR of colorectal cancer remained stable in urban residents and increased in rural residents during 2004‐2018 (Figure 5). As the urban‐rural disparity in socioeconomic status has gradually narrowed since 1993, the incidence of westernized lifestyle‐related cancers including colorectal cancer has risen in rural residents. To reduce the burden of colorectal cancer, the prevention and management of modifiable risk factors through national public health policies are recommended [36]. It has been demonstrated in the US that the mortality of colorectal cancer was greatly reduced by second‐grade prophylaxis, namely, early screening to remove precancerous polyps, early diagnosis, and early surgical resection [38, 39]. The government‐supported second‐grade prophylaxis has been carried out for years in several areas in China, resulting in a significant decrease in the incidence and mortality of colorectal cancer in these areas [40, 41]. Thus, second‐grade prophylaxis for colorectal cancer should be encouraged.
The ASMRs of liver cancer, stomach cancer, and esophagus cancer consecutively decreased in urban and rural areas of the three regions during 2004‐2018, indicating that public health actions including active treatment of hepatitis B, eradication of HP infection, and health education for giving up hot food have been effective in the past decades. In addition to lung/bronchus cancer and colorectal cancer, the ASMR of pancreas cancer increased in rural residents. Dietary habits such as high intake of alcohol, fructose, and red and processed meat, pathological conditions including diabetes, obesity, and infections, stress, and smoking behavior have been associated with increased risk of developing pancreas cancer [42]. Public health interventions to these modifiable risk factors might be applied to prevent pancreas cancer, a deadly malignancy with a limited screening opportunity. Pollution control including indoor and ambient air pollution reduction and lifestyle intervention including tobacco and alcohol control and obesity control remain challenges in rural areas.
This current study had several limitations: First, the national mortality surveillance system increased the number of surveillance points from 161 in 2004 to 605 in 2013, covering the surveillance population from 6% to 24% of the Chinese population [11]. The CMRs and ASMRs during 2004‐2012 were not as stable as those during 2013‐2018, although the DSPs were highly representative. Second, relatively poor health care access in rural areas might reduce the data quality of the mortality, thus, affecting the trends in the mortalities of lung/bronchus cancer and liver cancer in the western rural areas (Figure 5F). Third, the study population was originally divided into four age groups. We found that the mortality rates of all cancer in children/adolescents group and young adults group were too low to perform statistical analysis. Therefore, we merged the children/adolescents group and young adults group, resulting in loss of the mortality data in each of the two groups. Fourth, malignant disease in the central nervous system (C69‐C72 in ICD10) was classified as “other cancers” based on the Global Burden of Disease code U077 [12], resulting in a loss of data.
In summary, the ASMR of all cancer continually decreased although their CMRs increased in China during 2004‐2018. Lung/bronchus cancer and liver cancer remain the leading causes of cancer‐related death. Lung/bronchus cancer, colorectal cancer, and pancreatic cancer increased in rural residents, urging major interventions for authorities to control cancer growth and fatalities.
DECLARATIONS
AUTHOR CONTRIBUTIONS
Study concept and design: DMJ, LJZ, and GWC. Data organization and administration: DMJ, LJZ, RBR, and QL. Statistical analysis: DMJ and WBL. Technical supports for analysis: YFC, JYS, YBD, JHY. Interpretation of the data: GWC, XJT, and HWZ. Drafting of the manuscript: GWC. Study supervision: GWC. Final approval: all authors
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The raw data we used in this study are published annually in the form of yearbook by the National Center for Non‐communicable Disease Control and Prevention, Chinese CDC. We bought the yearbook for this analysis. Institutional Review Board (IRB) was not needed for using these data.
CONSENT FOR PUBLICATION
The data can be published according to the law of the People's Republic of China on Government Procurement.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
Authors are grateful to colleagues involved in the construction of the national mortality surveillance system and journal reviewers whose comments are greatly helpful for us to improve the manuscript. This work was supported by grants 81673250, 81520108021, and 91529305 from the National Natural Science Foundation of China (GC); Key discipline from the “3‐year public health promotion” program of Shanghai Municipal Health Commission (GC).
Jiang D, Zhang L, Liu W, Ding Y, Yin J, Ren R, et al. Trends in cancer mortality in China from 2004 to 2018: A nationwide longitudinal study. Cancer Commun. 2021;41:1024–1036. 10.1002/cac2.12195
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Yearbook of the National Death Cause Surveillance Data Set between 2004 and 2018, compiled by the Chinese Center for Disease Control and Prevention (CDC) collected through the Disease Surveillance Points system.
REFERENCES
- 1. Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever‐increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021. [DOI] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 3. Cao M, Li H, Sun D, Chen W. Cancer burden of major cancers in China: A need for sustainable actions. Cancer Commun (Lond). 2020;40(5):205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond). 2019;39(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lim SS, Updike RL, Kaldjian AS, Barber RM, Cowling K, York H, et al. Measuring human capital: a systematic analysis of 195 countries and territories, 1990‐2016. Lancet. 2018;392(10154):1217–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun J, Lyu S, Dai Z. The impacts of socioeconomic status and lifestyle on health status of residents: Evidence from Chinese General Social Survey data. Int J Health Plann Manage. 2019;34(4):1097–108. [DOI] [PubMed] [Google Scholar]
- 7. Li J, Shi L, Liang H, Ding G, Xu L. Urban‐rural disparities in health care utilization among Chinese adults from 1993 to 2011. BMC Health Serv Res. 2018;18(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. [DOI] [PubMed] [Google Scholar]
- 9. Chen W, Zheng R, Zhang S, Zhao P, Zeng H, Zou X, et al. Annual report on status of cancer in China, 2010. Chin J Cancer Res. 2014;26(1):48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27(1):2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu S, Wu X, Lopez AD, Wang L, Cai Y, Page A, et al. An integrated national mortality surveillance system for death registration and mortality surveillance, China. Bull World Health Organ. 2016;94(1):46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang L, Wu Y, Yin P, Cheng P, Liu Y, Schwebel DC, et al. Poisoning deaths in China, 2006‐2016. Bull World Health Organ. 2018;96(5):314–26A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang S, Du X, Han X, Yang F, Zhao J, Li H, et al. Influence of socioeconomic events on cause‐specific mortality in urban Shanghai, China, from 1974 to 2015: a population‐based longitudinal study. CMAJ. 2018;190(39):E1153–E61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang L, Li Z, Li X, Zhang J, Zheng L, Jiang C, et al. Study on the trend and disease burden of injury deaths in Chinese population, 2004‐2010. PLoS One. 2014;9(1):e85319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fan C. Cindy. Population Change and Regional Development in China: Insights Based on the 2000 Census. Eurasian Geography and Economics. 2002;43(6):425–442. https://www.tandfonline.com/doi/abs/10.2747/1538‐7216.43.6.425 [Google Scholar]
- 17. Li M, Wang S, Han X, Liu W, Song J, Zhang H, et al. Cancer mortality trends in an industrial district of Shanghai, China, from 1974 to 2014, and projections to 2029. Oncotarget. 2017;8(54):92470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang F, Ma L, Yang Y, Liu W, Zhao J, Chen X, et al. Contribution of Hepatitis B Virus Infection to the Aggressiveness of Primary Liver Cancer: A Clinical Epidemiological Study in Eastern China. Front Oncol. 2019;9:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nevola R, Rinaldi L, Giordano M, Marrone A, Adinolfi LE. Mechanisms and clinical behavior of hepatocellular carcinoma in HBV and HCV infection and alcoholic and non‐alcoholic fatty liver disease. Hepatoma Research. 2018;4:55. https://hrjournal.net/article/view/2790 [Google Scholar]
- 20. Liu J, Zhang S, Wang Q, Shen H, Zhang M, Zhang Y, et al. Seroepidemiology of hepatitis B virus infection in 2 million men aged 21‐49 years in rural China: a population‐based, cross‐sectional study. Lancet Infect Dis. 2016;16(1):80–6. [DOI] [PubMed] [Google Scholar]
- 21. Yin J, Wang J, Pu R, Xin H, Li Z, Han X, et al. Hepatitis B Virus Combo Mutations Improve the Prediction and Active Prophylaxis of Hepatocellular Carcinoma: A Clinic‐Based Cohort Study. Cancer Prev Res (Phila). 2015;8(10):978–88. [DOI] [PubMed] [Google Scholar]
- 22. Lin C‐L, Kao J‐H. Prevention of hepatitis B virus‐related hepatocellular carcinoma. Hepatoma Research. 2021;7:9. [Google Scholar]
- 23. Xu K, Liu LM, Farazi PA, Wang H, Rochling FA, Watanabe‐Galloway S, et al. Adherence and perceived barriers to oral antiviral therapy for chronic hepatitis B. Glob Health Action. 2018;11(1):1433987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang X, Allain JP, Wang H, Rong X, Chen J, Huang K, et al. Incidence of hepatitis B virus infection in young Chinese blood donors born after mandatory implementation of neonatal hepatitis B vaccination nationwide. J Viral Hepat. 2018;25(9):1008–16. [DOI] [PubMed] [Google Scholar]
- 25. Liu X, Yu Y, Wang M, Mubarik S, Wang F, Wang Y, et al. The mortality of lung cancer attributable to smoking among adults in China and the United States during 1990‐2017. Cancer Commun (Lond). 2020;40(11):611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Global Burden of Disease Cancer C , Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd‐Allah F, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability‐Adjusted Life‐Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5(12):1749–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li X, Deng Y, Tang W, Sun Q, Chen Y, Yang C, et al. Urban‐Rural Disparity in Cancer Incidence, Mortality, and Survivals in Shanghai, China, During 2002 and 2015. Front Oncol. 2018;8:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoebel J, Kroll LE, Fiebig J, Lampert T, Katalinic A, Barnes B, et al. Socioeconomic Inequalities in Total and Site‐Specific Cancer Incidence in Germany: A Population‐Based Registry Study. Front Oncol. 2018;8:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benhammou JN, Lin J, Hussain SK, El‐Kabany M. Emerging risk factors for nonalcoholic fatty liver disease associated hepatocellular carcinoma. Hepatoma Research. 2020;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holleczek B, Schottker B, Brenner H. Helicobacter pylori infection, chronic atrophic gastritis and risk of stomach and esophagus cancer: Results from the prospective population‐based ESTHER cohort study. Int J Cancer. 2020;146(10):2773–83. [DOI] [PubMed] [Google Scholar]
- 31. Fang X, Wei J, He X, An P, Wang H, Jiang L, et al. Landscape of dietary factors associated with risk of gastric cancer: A systematic review and dose‐response meta‐analysis of prospective cohort studies. Eur J Cancer. 2015;51(18):2820–32. [DOI] [PubMed] [Google Scholar]
- 32. Shen Y, Xie S, Zhao L, Song G, Shao Y, Hao C, et al. Estimating Individualized Absolute Risk for Esophageal Squamous Cell Carcinoma: A Population‐Based Study in High‐Risk Areas of China. Front Oncol. 2020;10:598603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y, Ueda J, et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23(4):233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coleman NC, Burnett RT, Higbee JD, Lefler JS, Merrill RM, Ezzati M, et al. Cancer mortality risk, fine particulate air pollution, and smoking in a large, representative cohort of US adults. Cancer Causes Control. 2020;31(8):767–76. [DOI] [PubMed] [Google Scholar]
- 35. Wang H, Li J, Gao M, Chan TC, Gao Z, Zhang M, et al. Spatiotemporal variability in long‐term population exposure to PM2.5 and lung cancer mortality attributable to PM2.5 across the Yangtze River Delta (YRD) region over 2010‐2016: A multistage approach. Chemosphere. 2020;257:127153. [DOI] [PubMed] [Google Scholar]
- 36. Yang Y, Han Z, Li X, Huang A, Shi J, Gu J. Epidemiology and risk factors of colorectal cancer in China. Chin J Cancer Res. 2020;32(6):729–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu W, Downward G, Wong JYY, Reiss B, Rothman N, Portengen L, et al. Characterization of outdoor air pollution from solid fuel combustion in Xuanwei and Fuyuan, a rural region of China. Sci Rep. 2020;10(1):11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xirasagar S, Li YJ, Hurley TG, Tsai MH, Hardin JW, Hurley DM, et al. Colorectal cancer prevention by an optimized colonoscopy protocol in routine practice. Int J Cancer. 2015;136(6):E731–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16(12):713–32. [DOI] [PubMed] [Google Scholar]
- 40. Wang J, Liu L, Cai Y, Gao Y, Guo Z, Yu F, et al. Trends in the age‐related incidence of colon and rectal cancers in China, 2005‐2015. Dig Liver Dis. 2021. [DOI] [PubMed] [Google Scholar]
- 41. Li X, Zhou Y, Luo Z, Gu Y, Chen Y, Yang C, et al. The impact of screening on the survival of colorectal cancer in Shanghai, China: a population based study. BMC Public Health. 2019;19(1):1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zanini S, Renzi S, Limongi AR, Bellavite P, Giovinazzo F, Bermano G. A review of lifestyle and environment risk factors for pancreatic cancer. Eur J Cancer. 2021;145:53–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Data Availability Statement
The data that support the findings of this study are available from Yearbook of the National Death Cause Surveillance Data Set between 2004 and 2018, compiled by the Chinese Center for Disease Control and Prevention (CDC) collected through the Disease Surveillance Points system.


