Abstract
Background:
Mounting evidence suggests disproportionate COVID-19 hospitalizations and deaths due to racial disparities. The association of race in a cohort of gynecologic oncology patients with SARS-CoV-2 infection is unknown.
Methods:
Data were abstracted from gynecologic oncology patients with COVID-19 infection among 8 New York City (NYC) area hospital systems. Multivariable mixed-effects logistic regression model accounting for county clustering was utilized to analyze COVID-19 related hospitalization and mortality.
Results:
Of 193 patients with gynecologic cancer and COVID-19, 67 (34.7%) were Black and 126 (65.3%) were non-Black. Black patients were more likely to require hospitalization compared with non-Black patients (71.6% [48/67] vs. 46.0% [58/126], P=.001). Of 34 (17.6%) patients who died from COVID-19, 14 (41.2%) were Black. Among those hospitalized, Black patients compared to non-Black patients were more likely to: have ≥ 3 comorbidities (81.1% [30/37] vs 59.2% [29/49], P=.05); reside in Brooklyn (81.0% [17/21] vs 44.4% [12/27], P=.02); live with family (69.4% [25/36] vs 41.6% [37/89], P=.009); and have public insurance (79.6% [39/49] vs 53.4% [39/73], P=.006). In multivariable analysis, for patients younger than 65 years of age, Black patients were more likely to require hospitalization compared to non-Black patients (OR, 4.87; 95% CI 1.82 to 12.99, P=.002).
Conclusions:
Although Black patients with gynecologic cancer represented only 1/3 of patients, they accounted for disproportionate rates of hospitalization (>45%) and death (>40%) due to COVID-19 infection; younger Black patients had nearly 5-fold greater risk of hospitalization. Efforts to understand and improve these disparities in COVID-19 outcomes in Black patients are critical.
Keywords: Coronavirus disease 2019 (COVID-19), gynecologic cancer, racial disparities, outcomes, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Lay Summary
In gynecologic cancer patients with COVID-19 infection, 72% of Black patients required hospitalization, compared to 46% of non-Black patients. Black patients younger than 65 years old had nearly 5 times greater risk of hospitalization compared to non-Black patients of the same age. Disparities in COVID-19 severity were driven by higher prevalence of comorbidities rather than cancer disease or treatment status, and Black patients were significantly more likely to have 3 or more comorbidities. Pre-existing racial disparities have been exacerbated during the COVID-19 pandemic and these data necessitate immediate change to enable health equity and outcomes for Black patients.
Precis
In gynecologic cancer patients with COVID-19 infection, Black patients were more likely to require hospitalization for COVID-19 infection compared to non-Black patients. Younger black patients, <65 years of age, had nearly 5-fold greater risk of hospitalization due to COVID-19 infection.
Introduction
Racial disparities in outcomes and survival of patients diagnosed with SARS-CoV-2 (COVID-19) have been observed.1–4 The overall US mortality rate is reported to be 3.2%, but preliminary US data suggests mortality among Black patients is 2.5 times higher than Whites.5 In New York State (NYS), Black or African American patients represent 14% of the population but 25% of COVID-19 related deaths.6 This discrepancy suggests Black patients are disproportionately affected by COVID-19; these data have been replicated in subsequent population-level studies throughout the country in large urban areas as well as rural regions.7–11
COVID-19 disproportionally affects vulnerable populations including cancer patients and low-income and non-English speaking populations.12,13 Currently no comprehensive guidelines inform decision-making and healthcare for these populations to ensure health equity and optimal outcomes. Although epidemiologic studies have demonstrated worse COVID-19 clinical outcomes in Black Americans, limited data explore these racial disparities in disease-specific patient populations. Improved characterization of potential contributing factors is essential to guide policy and practice towards correcting these disparities among gynecologic cancer patients.
The aim of this multicenter study is to examine whether there are racial disparities in gynecologic cancer patients with COVID-19. We compare the baseline and clinical characteristics, explore the differences in hospitalization and fatality rates, and investigate the impact that race and other socio-economic and health-related factors have on COVID-19 related outcomes.
Methods
Study Population
We conducted a multi-institutional, retrospective, observational cohort study at 8 NYC area hospital systems. The study was approved by the institutional review board at each site. Patients 18 years or older with gynecologic malignancy and confirmed SARS-CoV-2 infection from March 1, 2020 and May 20, 2020 (initial surge in NYC) were included. SARS-CoV-2 infection was defined as: a positive result with a real-time reverse transcriptase-polymerase chain reaction assay on a nasopharyngeal swab; serologic confirmation of SARS-CoV-2; or a diagnosis based on radiologic imaging by chest radiograph or chest computed tomography.14 All included subjects were de-identified prior to data review.
Data Collection
Clinical data were abstracted from the electronic medical record (EMR) for all patients meeting inclusion criteria using Research Electronic Data Capture software (Vanderbilt University).15,16 Patient characteristics included age, self-reported race and ethnicity, county of residency, employment status, essential worker status, insurance status, housing status, medical comorbidities, Eastern Cooperative Oncology Group (ECOG) performance status,17 severity of COVID-19 infection, cancer type, stage of diagnosis, current cancer disease status, and recent anti-cancer treatment. Clinical COVID-19 characteristics include symptoms of COVID-19, vital signs at admission, inpatient complications due to COVID-19, and need for supplemental oxygen including invasive mechanical ventilation.
Race and Outcome Measures
Race was the primary variable of interest. Race was classified as two groups: Black vs. non-Black (White plus Other). We grouped patients who identified themselves as Asian, American Indian or Alaska Native, and Native Hawaiian or Pacific Islander into Other because of low numbers in each category. Considering the high proportion of White in non-Black and diversity in Other group in this cohort, we also classified race as three groups, Black vs. Other vs. White in supplemental analyses (Supplemental Table 1–3). Outcomes, including hospitalization and mortality rate of Other group were similar to White group, thus were grouped together in final analysis as non-Black group to maximize the sample size in all analyses. Patients of Hispanic ethnicity were included in each racial category and represented in respective tables.
Our primary outcomes were hospitalization due to COVID-19 infection and COVID-19 related mortality. Hospitalization due to COVID-19 was stratified by COVID-19 severity, grouped as mild for cases managed on an outpatient basis and moderate or severe for cases requiring hospitalization. Severe COVID-19 cases were defined as COVID-19 infection requiring ICU admission, invasive mechanical ventilation, or resulting in COVID-19 related mortality. COVID-19 related mortality was defined as patients who died of COVID-19 related complications and not due to their cancer.
Statistical Analysis
Descriptive statistics were calculated for demographic, socioeconomic, health-related, cancer-related, and COVID-19 related characteristics by Black and non-Black patients. Continuous variables were described as median and interquartile range (IQR) and compared between groups using the Wilcoxon rank-sum test. Categorical variables were presented as frequency and proportion and compared between groups using the Chi-squared test. Hospitalization and mortality rates were calculated in Black and non-Black patients in the overall population or the subpopulation stratified by other categorical covariates and compared using the Chi-squared test. To account for potential clustering effects among geographical regions, multivariable mixed-effects logistic models were fitted with county variable as a random effect. County of residency included New York (Manhattan), Brooklyn (Kings County), Queens, and Bronx. Surrounding counties including Richmond, Nassau and Westchester County were grouped into Other because of low numbers in each category. To explicitly model the interaction of race and age on COVID-19 outcomes, we defined the groups as non-Black under age 65 years (based on median age of 65), Black under age 65 years, non-Black aged 65 or older, and Black aged 65 or older. Multivariable models adjusted for insurance status (public vs. private), smoking status (never vs. former/current), performance status (score of 0–1 vs. 2–3), number of comorbidities (0–2 vs. 3 or more), initial cancer stage (I/II vs. III/IV), cancer status (evidence of disease vs. remission), and current cancer treatment (no vs. yes). For missing covariate values, 16 cases with unknown information were put into the ‘other’ county group, 5 cases with unknown smoking status were put into the ‘never’ group, 18 cases with unknown performance status were put into ‘<2’ group, and 19 cases with unknown cancer stage were put into the ‘I/II’ group. Odds ratios (OR) and 95% confidence intervals (CI) were reported for all logistic models. Statistical analyses were performed using R version 4.0.1 (https://cran.r-project.org/). All statistical tests were two-sided, and a P-value of less than .05 was considered statistically significant.
Results
Baseline Characteristics of Patients
A total of 193 patients with gynecologic malignancy and COVID-19 infection were identified, of whom 67 (34.7%) were Black and 126 (65.3%) were non-Black. The baseline demographic and cancer characteristics of the patients are shown in Table 1. Among all patients included in the analysis, the median age was 65 years. Most Black patients were non-Hispanic and Black patients were more likely to speak English as a first language compared to non-Black patients (85.1% [57/67] vs 68.3% [86/126], P=.02). Thirty eight of 67 (56.7%) Black patients resided in Brooklyn and Bronx counties; Black patients compared to non-Black patients were more likely to reside in a county with higher poverty percent based on 2018 US Census Bureau Small Area Income and Poverty Estimates18 (median [IQR]: 18.9 [15.6, 23.1] vs 15.6 [11.7, 18.9], P=.004). We observed no differences between living situation, employment status, or essential worker status between Black and non-Black patients. Black patients were less likely to have private insurance compared to non-Black patients (26.9% [18/67] vs 42.1% [53/126], P=.05).
Table 1.
Description of baseline characteristics by race.
| Characteristic | Overall | Black | Non-Black | P-value |
|---|---|---|---|---|
| (n=193) | (n=67) | (n=126) | ||
| Age – median (IQR) | 65.0 (54.0, 73.0) | 65.0 (56.5, 73.0) | 65.0 (53.3, 72.0) | .49 |
| Ethnicity – N (%) | .002 | |||
| Hispanic | 39 (20.2) | 4 (6.0) | 35 (27.8) | |
| BMI – median (IQR) | 30.0 (25.0, 36.8) | 32.0 (25.6, 38.0) | 28.7 (24.4, 36.3) | .16 |
| Smoking status – N (%) | .44 | |||
| Former/current smoker | 49 (25.4) | 18 (26.9) | 31 (24.6) | |
| Never smoker | 139 (72.0) | 46 (68.7) | 93 (73.8) | |
| Unknown | 5 (2.6) | 3 (4.5) | 2 (1.6) | |
| County of residence – N (%) | .001 | |||
| New York | 41 (21.2) | 14 (20.9) | 27 (21.4) | |
| Brooklyn | 48 (24.9) | 21 (31.3) | 27 (21.4) | |
| Queens | 17 (8.8) | 1 (1.5) | 16 (12.7) | |
| Bronx | 40 (20.7) | 17 (25.4) | 23 (18.3) | |
| Other | 31 (16.1) | 4 (6.0) | 27 (21.4) | |
| Unknown | 16 (8.3) | 10 (14.9) | 6 (4.8) | |
| Poverty percent by county – median (IQR) | 18.9 (15.6, 18.9) | 18.9 (15.6, 23.1) | 15.6 (11.7, 18.9) | .004 |
| Primary language English – N (%) | 143 (74.1) | 57 (85.1) | 86 (68.3) | .02 |
| Current living situation – N (%) | .09 | |||
| Alone | 39 (20.2) | 19 (28.4) | 20 (15.9) | |
| Family | 125 (64.8) | 36 (53.7) | 89 (70.6) | |
| Skilled nursing facility | 7 (3.6) | 2 (3.0) | 5 (4.0) | |
| Unknown | 22 (11.4) | 10 (14.9) | 12 (9.5) | |
| Employed – N (%) | 60 (31.1) | 15 (22.4) | 45 (35.7) | .14 |
| Worker in essential industry – N (%) | 22 (11.4) | 8 (11.9) | 14 (11.1) | .80 |
| Private insurance – N (%) | 71 (36.8) | 18 (26.9) | 53 (42.1) | .05 |
| Performance status – N (%) | .24 | |||
| <2 | 148 (76.7) | 47 (70.1) | 101 (80.2) | |
| ≥2 | 27 (14.0) | 13 (19.4) | 14 (11.1) | |
| Unknown | 18 (9.3) | 7 (10.4) | 11 (8.7) | |
| Number of comorbidities ≥ 3 – N (%) | 86 (44.6) | 37 (55.2) | 49 (38.9) | .04 |
| Comorbidity – N (%) | ||||
| Asthma | 21 (10.9) | 11 (16.4) | 10 (7.9) | .12 |
| COPD/emphysema | 5 (2.6) | 3 (4.5) | 2 (1.6) | .47 |
| Obstructive sleep apnea | 12 (6.2) | 4 (6.0) | 8 (6.3) | 1.0 |
| Pulmonary embolism | 11 (5.7) | 6 (9.0) | 5 (4.0) | .27 |
| Hypertension | 115 (59.6) | 50 (74.6) | 65 (51.6) | .003 |
| Coronary artery disease | 13 (6.7) | 7 (10.4) | 6 (4.8) | .23 |
| CKD/ESRD | 21 (10.9) | 11 (16.4) | 10 (7.9) | .12 |
| Diabetes mellitus | 70 (36.3) | 32 (47.8) | 38 (30.2) | .02 |
| Obesity | 98 (50.8) | 42 (62.7) | 56 (44.4) | .02 |
| Autoimmune disease | 18 (9.3) | 7 (10.4) | 11 (8.7) | .90 |
| Cancer type – N (%) | .24 | |||
| Cervical | 24 (12.4) | 9 (13.4) | 15 (11.9) | |
| Ovary | 67 (34.7) | 18 (26.9) | 49 (38.9) | |
| Uterine | 87 (45.1) | 34 (50.7) | 53 (42.1) | |
| Vaginal | 2 (1.0) | 2 (3.0) | 0 (0.0) | |
| Vulvar | 8 (4.1) | 2 (3.0) | 6 (4.8) | |
| Other | 5 (2.6) | 2 (3.0) | 3 (2.4) | |
| Cancer stage at diagnosis – N (%) | .18 | |||
| I/II | 74 (38.3) | 26 (38.8) | 48 (38.1) | |
| III/IV | 100 (51.8) | 38 (56.7) | 62 (49.2) | |
| Unknown | 19 (9.8) | 3 (4.5) | 16 (12.7) |
No differences were seen in performance status between Black and non-Black patients. Compared with non-Black patients, Black patients were more likely to have three or more comorbidities (55.2% [37/67] vs 38.9% [49/126], P=.04), with higher prevalence of hypertension (74.6% [50/67] vs 51.6% [65/126], P=.003), obesity (62.7% [42/67] vs 44.4% [56/126], P=.02), and diabetes (47.8% [32/67] vs 30.2% [38/126], P=.02). Gynecologic cancer type and stage were evenly distributed among Black and non-Black patients (Table 1). Among the cohort, 56.7% (38/67) Black and 49.2% (62/126) non-Black patients presented with stage III or IV disease.
COVID-19 Related Characteristics of Patients
Table 2 presents clinical COVID-19 related characteristics of patients. 106 (54.9%) patients required hospitalization. Black patients were more likely to require hospitalization compared with non-Black patients (71.6% [48/67] vs. 46.0% [58/126], P=.001). The majority of hospitalized patients in Brooklyn and Bronx were Black, while a higher percentage of non-Black patients were hospitalized in Manhattan (Figure 1). A higher percentage of Black patients compared to non-Black patients presented with shortness of breath (49.3% [33/67] vs 31.7% [40/126], P=.03). Black patients were also more likely to require supplemental oxygen (49.3% [33/67] vs 31.0% [39/126], P=.04). Black patients experienced higher rates of renal (16.4% [11/67] vs 7.9% [10/126], P=.12), cardiovascular (10.4% [7/67] vs 7.1% [9/126], P=.64), and pulmonary (44.8% [30/67] vs 28.6% [36/126], P=.04) complications during hospitalization compared to non-Black patients. Six of the 13 (46.2%) patients who required mechanical ventilation were Black. Of the 34 patients who died due to COVID-19 related complications, 14 (41.2%) were Black. The case-fatality rate for Black patients was 20.9% (14/67), compared to 15.9% (20/126) among non-Black patients, with the highest percentage of COVID-19 related deaths in Brooklyn, followed by Bronx. (Figure 1).
Table 2.
Description of COVID-19 characteristics by race.
| Characteristic | Overall | Black | Non-Black | P-value |
|---|---|---|---|---|
| (n=193) | (n=67) | (n=126) | ||
| COVID-19 severity – N (%) | .002 | |||
| Mild | 87 (45.1) | 19 (28.4) | 68 (54.0) | |
| Moderate | 67 (34.7) | 33 (49.3) | 34 (27.0) | |
| Severe | 39 (20.2) | 15 (22.4) | 24 (19.0) | |
| Admitted from – N (%) | .33 | |||
| Home | 104 (53.9) | 42 (62.7) | 62 (49.2) | |
| Hospital transfer | 3 (1.6) | 1 (1.5) | 2 (1.6) | |
| Skilled nursing facility | 11 (5.7) | 4 (6.0) | 7 (5.6) | |
| Other | 4 (2.1) | 2 (3.0) | 2 (1.6) | |
| Unknown | 71 (36.8) | 18 (26.9) | 53 (42.1) | |
| COVID-19 symptoms – N (%) | ||||
| Fatigue | 67 (34.7) | 24 (35.8) | 43 (34.1) | .94 |
| Fever | 99 (51.3) | 32 (47.8) | 67 (53.2) | .57 |
| Cough | 94 (48.7) | 30 (44.8) | 64 (50.8) | .52 |
| Shortness of breath | 73 (37.8) | 33 (49.3) | 40 (31.7) | .03 |
| Myalgia | 30 (15.5) | 9 (13.4) | 21 (16.7) | .73 |
| Sore throat | 11 (5.7) | 2 (3.0) | 9 (7.1) | .39 |
| Headache | 12 (6.2) | 5 (7.5) | 7 (5.6) | .83 |
| Anosmia | 9 (4.7) | 1 (1.5) | 8 (6.3) | .24 |
| Ageusia | 7 (3.6) | 1 (1.5) | 6 (4.8) | .45 |
| Rhinorrhea | 13 (6.7) | 2 (3.0) | 11 (8.7) | .23 |
| Nausea or vomiting | 26 (13.5) | 5 (7.5) | 21 (16.7) | .12 |
| Diarrhea | 33 (17.1) | 11 (16.4) | 22 (17.5) | 1.0 |
| Abdominal pain | 11 (5.7) | 5 (7.5) | 6 (4.8) | .66 |
| Asymptomatic | 28 (14.5) | 9 (13.4) | 19 (15.1) | .93 |
| Hospitalization – N (%) | 106 (54.9) | 48 (71.6) | 58 (46.0) | .001 |
| Vital signs on ED admission* – | ||||
| Oxygen saturation % | 95.0 (91.0, 98.0) | 95.0 (91.0, 98.0) | 95.0 (92.0, 98.0) | .64 |
| Temperature, °F | 98.8 (98.1, 100.2) | 98.6 (98.0, 100.2) | 98.9 (98.2, 100.2) | .69 |
| Heart rate, beats/min | 101.0 (82.5, 114.3) | 107.0 (95.25, 117.0) | 94.0 (76.3, 109.0) | .002 |
| Respiratory rate, breaths/min | 20.0 (18.0, 22.0) | 21.0 (19.0, 24.0) | 19.0 (18.0, 20.5) | .006 |
| Required supplemental oxygen – N (%) | 72 (37.3) | 33 (49.3) | 39 (31.0) | .04 |
| Required invasive mechanical ventilation – N (%) | 13 (6.7) | 6 (9.0) | 7 (5.6) | .52 |
| COVID-19 complications – N (%) | ||||
| Bleeding | 3 (1.6) | 2 (3.0) | 1 (0.8) | .58 |
| Disseminated intravascular coagulation | 1 (0.5) | 0 (0.0) | 1 (0.8) | 1.0 |
| Multiorgan failure | 9 (4.7) | 2 (3.0) | 7 (5.6) | .65 |
| Sepsis | 12 (6.2) | 5 (7.5) | 7 (5.6) | .83 |
| Pulmonary | 66 (34.2) | 30 (44.8) | 36 (28.6) | .04 |
| Cardiac | 16 (8.3) | 7 (10.4) | 9 (7.1) | .64 |
| Renal | 21 (10.9) | 11 (16.4) | 10 (7.9) | .12 |
| None | 105 (54.4) | 25 (37.3) | 80 (63.5) | .01 |
| Current COVID-19 status – N (%) | .40 | |||
| Died of COVID-19 related complications | 34 (17.6) | 14 (20.9) | 20 (15.9) | |
| Ongoing infection | 20 (10.4) | 6 (9.0) | 14 (11.1) | |
| Recovered with complications | 16 (8.3) | 8 (11.9) | 8 (6.3) | |
| Fully recovered | 123 (63.7) | 39 (58.2) | 84 (66.7) |
vital signs of hospitalized patients only
Figure 1. COVID-19 hospitalizations (A) and mortality (B) data in Black patients.
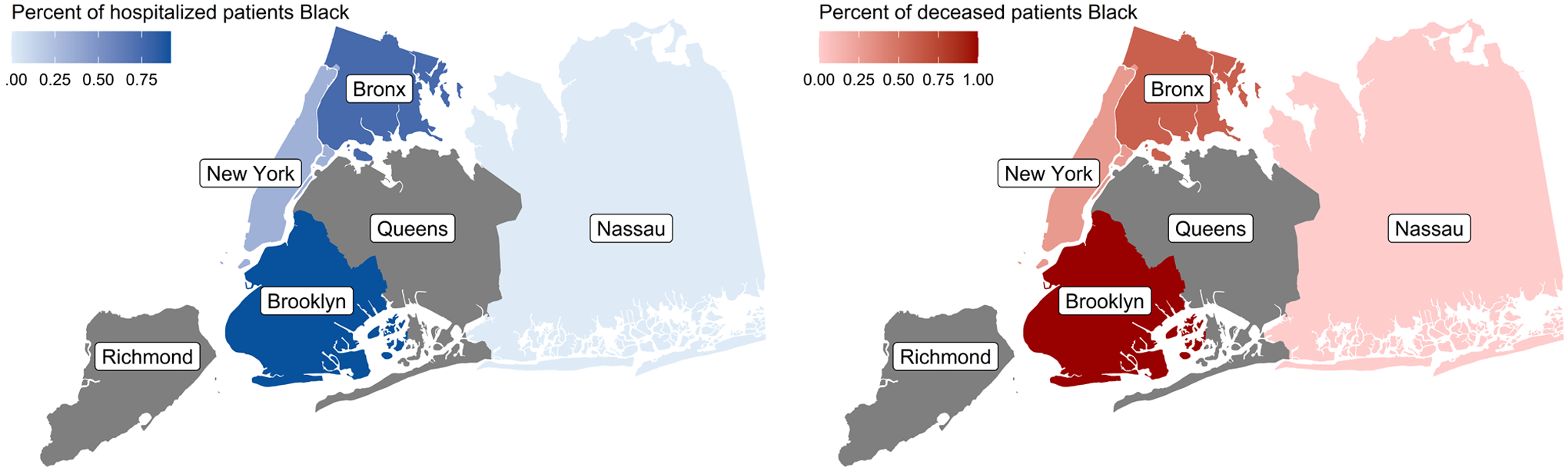
Percentages reflect the percentage of Black patients of total patients in our cohort who were hospitalized or died due to COVID-19 infection. Grey areas indicate no COVID-19 hospitalization or mortality cases.
Hospitalization and Mortality between Racial Groups
Table 3 presents outcome rates in Black and non-Black patients separately and compares them between racial groups. The higher rates of hospitalization seen in Black patients compared with non-Black patients was most pronounced age <65 years (68.8% [22/32] vs 29.0% [18/62], P<.001), obese (80.0% [32/40] vs 50.0% [27/54], P=.006), never smokers (67.4% [31/46] vs 43.0% [40/93], P=.01), residents of Brooklyn (81.0% [17/21] vs 44.4% [12/27], P=.02), primarily English speakers (73.7% [42/57] vs 46.5% [40/86], P=.002), currently living with family (69.4% [25/36] vs 41.6% [37/89], P=.009), employed (73.3% [11/15] vs 35.6% [16/45], P=.02), with public insurance (79.6% [39/49] vs 53.4% [39/73], P=.006), and performance status score <2 (70.2% [33/47] vs 37.6% [38/101], P<.001). Black patients who were essential workers during the COVID-19 pandemic were more likely to die than non-Black essential workers (50.0% [4/8] vs 7.1% [1/14], P=.04).
Table 3.
Comparison of hospitalization and mortality between racial groups.
| Characteristic | Hospitalization | Mortality | |||||
|---|---|---|---|---|---|---|---|
| Black | Non-Black | P-value | Black | Non-Black | P-value | ||
| Overall – N (%) | 48/67 (71.6) | 58/126 (46.0) | .001 | 14/67 (20.9) | 20/126 (15.9) | .50 | |
| Ethnicity – N (%) | |||||||
| Hispanic | 2/4 (50.0) | 16/35 (45.7) | 1.0 | 2/4 (50.0) | 6/35 (17.1) | .18 | |
| Age – N (%) | |||||||
| <65 years | 22/32 (68.8) | 18/62 (29.0) | <.001 | 6/32 (18.8) | 5/62 (8.1) | .23 | |
| ≥65 years | 26/35 (74.3) | 40/64 (62.5) | .33 | 8/35 (22.9) | 15/64 (23.4) | 1.0 | |
| BMI – N (%) | |||||||
| Normal | 11/15 (73.3) | 15/34 (44.1) | .07 | 5/15 (33.3) | 8/34 (23.5) | .71 | |
| Overweight | 5/11 (45.5) | 15/35 (42.9) | 1.0 | 1/11 (9.1) | 3/35 (8.6) | 1.0 | |
| Obese | 32/40 (80.0) | 27/54 (50.0) | .006 | 8/40 (20.0) | 8/54 (14.8) | .70 | |
| Smoking status – N (%) | |||||||
| Former/current smoker | 15/18 (83.3) | 17/31 (54.8) | .06 | 6/18 (33.3) | 9/31 (29.0) | 1.0 | |
| Never smoker | 31/46 (67.4) | 40/93 (43.0) | .01 | 6/46 (13.0) | 11/93 (11.8) | 1.0 | |
| Unknown | 2/3 (66.7) | 1/2 (50.0) | 1.0 | 2/3 (66.7) | 0/2 (0.0) | .40 | |
| County of residence – N (%) | |||||||
| New York | 7/14 (50.0) | 16/27 (59.3) | .81 | 1/14 (7.1) | 7/27 (25.9) | .31 | |
| Brooklyn | 17/21 (81.0) | 12/27 (44.4) | .02 | 5/21 (23.8) | 1/27 (3.7) | .10 | |
| Queens | 0/1 (0.0) | 5/16 (31.2) | 1.0 | 0/1 (0.0) | 2/16 (12.5) | 1.0 | |
| Bronx | 13/17 (76.5) | 11/23 (47.8) | .10 | 7/17 (41.2) | 4/23 (17.4) | .19 | |
| Other | 3/4 (75.0) | 12/27 (44.4) | .33 | 0/4 (0.0) | 5/27 (18.5) | 1.0 | |
| Unknown | 8/10 (80.0) | 2/6 (33.3) | .12 | 1/10 (10.0) | 1/6 (16.7) | 1.0 | |
| Primary language English – N (%) | 42/57 (73.7) | 40/86 (46.5) | .002 | 13/57 (22.8) | 12/86 (14.0) | .25 | |
| Current living situation – N (%) | |||||||
| Alone | 13/19 (68.4) | 9/20 (45.0) | .25 | 4/19 (21.1) | 4/20 (20.0) | 1.0 | |
| Family | 25/36 (69.4) | 37/89 (41.6) | .009 | 4/36 (11.1) | 8/89 (9.0) | .98 | |
| Skilled nursing facility | 2/2 (100.0) | 4/5 (80.0) | 1.0 | 1/2 (50.0) | 3/5 (60.0) | 1.0 | |
| Unknown | 8/10 (80.0) | 8/12 (66.7) | .65 | 5/10 (50.0) | 5/12 (41.7) | 1.0 | |
| Employed – N (%) | 11/15 (73.3) | 16/45 (35.6) | .02 | 5/15 (33.3) | 6/45 (13.3) | .18 | |
| Worker in essential industry – N (%) | 6/8 (75.0) | 4/14 (28.6) | .07 | 4/8 (50.0) | 1/14 (7.1) | .04 | |
| Insurance – N (%) | |||||||
| Public | 39/49 (79.6) | 39/73 (53.4) | .006 | 11/49 (22.4) | 14/73 (19.2) | .83 | |
| Private | 9/18 (50.0) | 19/53 (35.8) | .43 | 3/18 (16.7) | 6/53 (11.3) | .86 | |
| Performance status – N (%) | |||||||
| <2 | 33/47 (70.2) | 38/101 (37.6) | <.001 | 9/47 (19.1) | 11/101 (10.9) | .27 | |
| ≥2 | 11/13 (84.6) | 12/14 (85.7) | 1.0 | 4/13 (30.8) | 6/14 (42.9) | .80 | |
| Number of comorbidities ≥3 – N (%) | 30/37 (81.1) | 29/49 (59.2) | .05 | 8/37 (21.6) | 12/49 (24.5) | .96 | |
| Comorbidity – N (%) | |||||||
| Asthma | 9/11 (81.8) | 6/10 (60.0) | .36 | 1/11 (9.1) | 3/10 (30.0) | .51 | |
| COPD/emphysema | 2/3 (66.7) | 2/2 (100.0) | 1.0 | 0/3 (0.0) | 2/2 (100.0) | .10 | |
| Obstructive sleep apnea | 4/4 (100.0) | 6/8 (75.0) | .52 | 1/4 (25.0) | 3/8 (37.5) | 1.0 | |
| Pulmonary embolism | 5/6 (83.3) | 3/5 (60.0) | .55 | 1/6 (16.7) | 1/5 (20.0) | 1.0 | |
| Hypertension | 37/50 (74.0) | 37/65 (56.9) | .09 | 12/50 (24.0) | 13/65 (20.0) | .77 | |
| Coronary artery disease | 7/7 (100.0) | 3/6 (50.0) | .07 | 3/7 (42.9) | 1/6 (16.7) | .56 | |
| CKD/ESRD | 9/11 (81.8) | 9/10 (90.0) | 1.0 | 2/11 (18.2) | 5/10 (50.0) | .28 | |
| Diabetes mellitus | 24/32 (75.0) | 25/38 (65.8) | .56 | 8/32 (25.0) | 8/38 (21.1) | .92 | |
| Autoimmune disease | 4/7 (57.1) | 6/11 (54.5) | 1.0 | 1/7 (14.3) | 4/11 (36.4) | .63 | |
| Cancer stage – N (%) | |||||||
| I/II | 20/26 (76.9) | 19/48 (39.6) | .005 | 5/26 (19.2) | 8/48 (16.7) | 1.0 | |
| III/IV | 25/38 (65.8) | 26/62 (41.9) | .03 | 9/38 (23.7) | 10/62 (16.1) | .50 | |
| Unknown | 3/3 (100.0) | 13/16 (81.2) | 1.0 | 0/3 (0.0) | 2/16 (12.5) | 1.0 | |
Factors Associated with Hospitalization and Death
Table 4 presents the estimated odds ratios and p-values for factors associated with hospitalization and mortality. In the unadjusted analysis, Black patients had a 2.7 fold higher risk of hospitalization compared with non-Black patients (OR=2.69; 95% CI= 1.37 to 5.26; P=.004), but no statistically significant racial differences in the risk of dying were observed (OR=1.57; 95% CI= 0.71 to 3.46; P=.26). The effect of race on hospitalization and mortality varied between patient groups age <65 years and ≥65, indicating an interaction between race and age. In adjusted mixed-effects logistic models, compared with non-Black patients aged <65 years, Black patients aged <65 years had the highest risk of hospitalization (OR=4.87; 95% CI= 1.82 to 12.99; P=.002). Hospitalization risk was also increased for Black patients aged ≥65 years (OR=3.44; 95% CI= 1.17 to 10.11; P=.02) and non-Black patients aged ≥65 years (OR=2.64; 95% CI= 1.13 to 6.18; P=.02). Poorer performance status (score ≥2) (OR=3.58; 95% CI= 1.12 to 11.39; P=.03) and ≥3 comorbidities (OR=1.97; 95% CI= 1.01 to 3.85; P=.05) were also statistically significant risk factors for hospitalization in the multivariable model.
Table 4.
Odds ratios from logistic models.
| Hospitalization | Mortality | ||||
|---|---|---|---|---|---|
| OR (95%CI) | P-value | OR (95%CI) | P-value | ||
| Unadjusted model | |||||
| Non-Black race | 1.00 (Referent) | 1.00 (Referent) | |||
| Black race | 2.69 (1.37, 5.26) | .004 | 1.57 (0.71, 3.46) | .26 | |
| Adjusted Model | |||||
| Interaction as categorical (cut at 65 years) | |||||
| Age < 65, Non-Black race | 1.00 (Referent) | 1.00 (Referent) | |||
| Age < 65, Black race | 4.87 (1.82, 12.99) | .002 | 2.08 (0.54, 8.03) | .29 | |
| Age ≥ 65, Non-Black race | 2.64 (1.13, 6.18) | .02 | 2.58 (0.76, 8.74) | .13 | |
| Age ≥ 65, Black race | 3.44 (1.17, 10.11) | .02 | 1.95 (0.46, 8.26) | .36 | |
| Private insurance | 0.60 (0.28, 1.26) | .18 | 0.97 (0.36, 2.65) | .96 | |
| Former/current smoker | 1.56 (0.74, 3.31) | .25 | 2.93 (1.28, 6.70) | .01 | |
| Performance status ≥ 2 | 3.58 (1.12, 11.39) | .03 | 2.55 (0.97, 6.70) | .06 | |
| Number of comorbidities ≥ 3 | 1.97 (1.01, 3.85) | .05 | 1.70 (0.74, 3.93) | .21 | |
| Cancer stage III/IV | 0.79 (0.37, 1.7) | .54 | 1.32 (0.50, 3.50) | .58 | |
| Cancer status in remission | 1.15 (0.43, 3.03) | .78 | 0.55 (0.17, 1.73) | .30 | |
| Current cancer treatment | 0.83 (0.34, 2.05) | .69 | 0.58 (0.20, 1.67) | .32 | |
The risk of death from COVID-19 infection was higher in Black aged <65, non-Black aged ≥65, and Black aged ≥65 compared with non-Black aged <65 years patients but this was not statistically significant. The only risk factor significantly associated with mortality after adjustment was former/current smoking status (OR=2.93; 95% CI= 1.28 to 6.70; P=.01).
To further understand the interaction of race and age, clinico-demographic characteristics among patients under age 65 years are detailed in Table 5. Black patients <65 years were more likely than all other patients to live in Brooklyn and Bronx counties (62.5% [20/32], P=.01), and to reside in a county with higher poverty percent (median [ICR] 18.9 [18.9,27.3], P=.02). Black patients <65 years of age were just as likely as Non-Black patients ≥ 65 years to have more ≥ 3 comorbidities (46.9% [15/32] vs 48.4% [31/64]).
Table 5.
Description of characteristics of patient stratified by age and race.
| Characteristic | Overall | Non-Black, Age < 65 | Black, Age < 65 | Non-Black, Age ≥ 65 | Black, Age ≥ 65 | P-value |
|---|---|---|---|---|---|---|
| (n=193) | (n=62) | (n=32) | (n=64) | (n=35) | ||
| Age – median (IQR) | 65.0 [54.0, 73.0] | 53.0 [43.0, 59.0] | 55.0 [50.0, 61.0] | 72.0 [69.0, 75.3 | 73.0 [68.0, 78.0] | <.001 |
| Ethnicity – N (%) | .03 | |||||
| Hispanic | 39 (20.2) | 17 (27.4) | 1 (3.1) | 18 (28.1) | 3 (8.6) | |
| BMI – median (IQR) | 30.0 [25.0, 36.8] | 30.2 [25.3, 38.0] | 31.9 [26.9, 39.8] | 27.0 [23.9, 35.5] | 32.9 [24.5, 37.6] | .15 |
| Smoking status – N (%) | .79 | |||||
| Former/current smoker | 49 (25.4) | 13 (21.0) | 8 (25.0) | 18 (28.1) | 10 (28.6) | |
| Never smoker | 139 (72.0) | 48 (77.4) | 23 (71.9) | 45 (70.3) | 23 (65.7) | |
| Unknown | 5 (2.6) | 1 (1.6) | 1 (3.1) | 1 (1.6) | 2 (5.7) | |
| County of residence – N (%) | .01 | |||||
| New York | 41 (21.2) | 9 (14.5) | 5 (15.6) | 18 (28.1) | 9 (25.7) | |
| Brooklyn | 48 (24.9) | 12 (19.4) | 11 (34.4) | 15 (23.4) | 10 (28.6) | |
| Queens | 17 (8.8) | 11 (17.7) | 0 (0.0) | 5 (7.8) | 1 (2.9) | |
| Bronx | 40 (20.7) | 11 (17.7) | 9 (28.1) | 12 (18.8) | 8 (22.9) | |
| Other | 31 (16.1) | 16 (25.8) | 3 (9.4) | 11 (17.2) | 1 (2.9) | |
| Unknown | 16 (8.3) | 3 (4.8) | 4 (12.5) | 3 (4.7) | 6 (17.1) | |
| Poverty percent by county – median (IQR) | 18.9 [15.6, 18.9] | 15.6 [11.6, 18.9] | 18.9 [18.9, 27.3] | 15.6 [15.6, 18.9] | 18.9 [15.6, 18.9] | .02 |
| Primary language English – N (%) | 143 (74.1) | 40 (64.5) | 29 (90.6) | 46 (71.9) | 28 (80.0) | .04 |
| Current living situation – N (%) | .07 | |||||
| Alone | 39 (20.2) | 6 (9.7) | 10 (31.2) | 14 (21.9) | 9 (25.7) | |
| Family | 125 (64.8) | 49 (79.0) | 19 (59.4) | 40 (62.5) | 17 (48.6) | |
| Skilled nursing facility | 7 (3.6) | 1 (1.6) | 0 (0.0) | 4 (6.2) | 2 (5.7) | |
| Unknown | 22 (11.4) | 6 (9.7) | 3 (9.4) | 6 (9.4) | 7 (20.0) | |
| Employed – N (%) | 60 (31.1) | 31 (50.0) | 14 (43.8) | 14 (21.9) | 1 (2.9) | <0.001 |
| Worker in essential industry – N (%) | 22 (11.4) | 9 (14.5) | 8 (25.0) | 5 (7.8) | 0 (0.0) | .03 |
| Private insurance – N (%) | 71 (36.8) | 40 (64.5) | 16 (50.0) | 13 (20.3) | 2 (5.7) | <0.001 |
| Performance status – N (%) | .01 | |||||
| <2 | 148 (76.7) | 57 (91.9) | 26 (81.2) | 44 (68.8) | 21 (60.0) | |
| ≥2 | 27 (14.0) | 2 (3.2) | 3 (9.4) | 12 (18.8) | 10 (28.6) | |
| Unknown | 18 (9.3) | 3 (4.8) | 3 (9.4) | 8 (12.5) | 4 (11.4) | |
| Number of comorbidities ≥ 3 – N (%) | 86 (44.6) | 18 (29.0) | 15 (46.9) | 31 (48.4) | 22 (62.9) | .01 |
| Comorbidity – N (%) | ||||||
| Asthma | 21 (10.9) | 6 (9.7) | 6 (18.8) | 4 (6.2) | 5 (14.3) | .27 |
| COPD/emphysema | 5 (2.6) | 0 (0.0) | 0 (0.0) | 2 (3.1) | 3 (8.6) | .06 |
| Obstructive sleep apnea | 12 (6.2) | 3 (4.8) | 2 (6.2) | 5 (7.8) | 2 (5.7) | .92 |
| Pulmonary embolism | 11 (5.7) | 3 (4.8) | 2 (6.2) | 2 (3.1) | 4 (11.4) | .39 |
| Hypertension | 115 (59.6) | 20 (32.3) | 21 (65.6) | 45 (70.3) | 29 (82.9) | <0.001 |
| Coronary artery disease | 13 (6.7) | 0 (0.0) | 0 (0.0) | 6 (9.4) | 7 (20.0) | .001 |
| CKD/ESRD | 21 (10.9) | 2 (3.2) | 3 (9.4) | 8 (12.5) | 8 (22.9) | .03 |
| Diabetes mellitus | 70 (36.3) | 17 (27.4) | 12 (37.5) | 21 (32.8) | 20 (57.1) | .03 |
| Obesity | 98 (50.8) | 33 (53.2) | 20 (62.5) | 23 (35.9) | 22 (62.9) | .02 |
| Autoimmune disease | 18 (9.3) | 5 (8.1) | 5 (15.6) | 6 (9.4) | 2 (5.7) | .54 |
| Cancer stage – N (%) | .20 | |||||
| I/II | 74 (38.3) | 27 (43.5) | 10 (31.2) | 21 (32.8) | 16 (45.7) | |
| III/IV | 100 (51.8) | 30 (48.4) | 20 (62.5) | 32 (50.0) | 18 (51.4) | |
| Unknown | 19 (9.8) | 5 (8.1) | 2 (6.2) | 11 (17.2) | 1 (2.9) | |
| COVID-19 severity – N (%) | <0.001 | |||||
| Mild | 87 (45.1) | 44 (71.0) | 10 (31.2) | 24 (37.5) | 9 (25.7) | |
| Moderate | 67 (34.7) | 12 (19.4) | 16 (50.0) | 22 (34.4) | 17 (48.6) | |
| Severe | 39 (20.2) | 6 (9.7) | 6 (18.8) | 18 (28.1) | 9 (25.7) | |
| Hospitalization – N (%) | 106 (54.9) | 18 (29.0) | 22 (68.8) | 40 (62.5) | 26 (74.3) | <0.001 |
| COVID-19 complications – N (%) | ||||||
| Bleeding | 3 (1.6) | 0 (0.0) | 0 (0.0) | 1 (1.6) | 2 (5.7) | .14 |
| Disseminated intravascular coagulation | 1 (0.5) | 0 (0.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | .57 |
| Multiorgan failure | 9 (4.7) | 1 (1.6) | 1 (3.1) | 6 (9.4) | 1 (2.9) | .18 |
| Sepsis | 12 (6.2) | 4 (6.5) | 2 (6.2) | 3 (4.7) | 3 (8.6) | .90 |
| Pulmonary | 66 (34.2) | 13 (21.0) | 14 (43.8) | 23 (35.9) | 16 (45.7) | .04 |
| Cardiac | 16 (8.3) | 1 (1.6) | 5 (15.6) | 8 (12.5) | 2 (5.7) | .05 |
| Renal | 21 (10.9) | 3 (4.8) | 2 (6.2) | 7 (10.9) | 9 (25.7) | .01 |
| None | 105 (54.4) | 45 (72.6) | 12 (37.5) | 35 (54.7) | 13 (37.1) | .001 |
| Current COVID-19 status – N (%) | .11 | |||||
| Died of COVID-19 related complications | 34 (17.6) | 5 (8.1) | 6 (18.8) | 15 (23.4) | 8 (22.9) | |
| Ongoing infection | 123 (63.7) | 48 (77.4) | 18 (56.2) | 36 (56.2) | 21 (60.0) | |
| Recovered with complications | 20 (10.4) | 8 (12.9) | 3 (9.4) | 6 (9.4) | 3 (8.6) | |
| Fully recovered | 16 (8.3) | 1 (1.6) | 5 (15.6) | 7 (10.9) | 3 (8.6) |
Discussion
This study examined clinical characteristics and outcomes of a diverse cohort of gynecologic cancer patients with COVID-19 across 8 hospital systems in New York City. Over 70% of Black patients in this study required hospitalization for COVID-19 infection, compared to only 46% of non-Black patients. In addition to race and age, poor performance status and increasing number of comorbidities were associated with increased odds of hospital admission. Notably, Black patients less than 65 years of age were nearly 5 times more likely to require hospitalization for COVID-19 compared with non-Black patients younger than 65 years. Black race was not associated with increased mortality due to COVID-19 before or after adjustment for clinical and socioeconomic characteristics.
The findings of this study are consistent with prior studies from across the country that have demonstrated increased COVID-19 hospitalization rates in minority groups. We were unable to demonstrate a mortality difference among Black and non-Black patients likely due to our limited cohort size. Studies have found that Black patients are two to three times as likely as White patients to require hospitalization after adjusting for confounders including age, sex, comorbidities, and income and are over five times as likely to die from COVID-19 infection. On a nationwide level, an analysis across 2,886 US counties found a positive correlation between the percentage of African Americans in a county and the number of confirmed cases and deaths in the county.11 Here we report Black patients with COVID-19 were more likely to have public insurance, live in a county below the poverty index, and live with family. Hospitalized Black patients were more likely to be essential workers during the COVID-19 pandemic. These findings are consistent with previous reports that Black Americans and other minorities are more likely to work essential, front-line jobs at risk for exposure.21 These structural factors prevent minority populations from practicing social distancing, increasing their risk of COVID-19 infection.
The underlying causes of racial disparities are multifactorial and include limited access to healthcare, structural and social determinants of health, racism, and discrimination. The COVID-19 pandemic has exacerbated these baseline inequalities. Black Americans are more likely to suffer from medical comorbidities known to be risk factors for severe COVID-19 infection, including hypertension, diabetes, kidney disease, and respiratory disease.22–25 In this cohort, a higher percentage of Black patients less than 65 years of age had three or more comorbidities, with a higher prevalence of hypertension, obesity, and diabetes compared with non-Black patients in this age group. We also noted that in the younger Black (< 65 years) cohort the majority resided in Brooklyn and Bronx and were from counties with higher poverty index. We hypothesize that the development of chronic conditions in Black patients at younger ages drives the increased risk of hospitalization. Furthermore, while less Black patients were former or current smokers at baseline, those that did smoke were at increased risk of mortality secondary to COVID-19 infection. This represents a modifiable risk factor which stresses the urgent need for community wide policies to eliminate disparities through equitable access to health care resources and improved education specifically in younger patients.
Higher rates of comorbidities seen in Black Americans may be due to weathering, a phenomenon in which chronic economic and social stressors caused by systemic racism result in increased activation of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis.26 When these physiologic stress responses are chronically activated over a lifetime, Black Americans are put at greater risk for developing health conditions, placing them at increased risk for poor COVID-19 outcomes. Additionally, structural biases embedded in healthcare systems decrease minority access to healthcare and implicit biases by healthcare providers can adversely impact the quality of care.27 Together, these factors create an environment where Black Americans are at increased risk for contracting COVID-19 infection, have a greater chance of developing severe disease, and are then more likely to receive substandard care.
This study has several limitations. In our small cohort, an evaluation of outcomes and risk factors associated with COVID-19 in other minority groups was not feasible. Our sample size is limited by the number of patients with gynecologic cancer who developed COVID-19 infection in NYC, which hampers our ability to detect mortality differences between Black and non-Black patients. Our sample may also be biased because we included patients who sought care for cancer treatment at academic institutions and their affiliates. Our patients therefore have proven to have some degree of access to the health care system that might not be true for all Black or minority patients in the US. Since lack of health care access is one explanation for COVID-19 disparities, our study may have underestimated disparities in outcomes. Interestingly, even with access to care, we still demonstrate disparities among this relatively well-connected patient cohort. It is possible that this cohort of patients did not include those with milder symptoms who did not present for COVID-19 testing as well as patients with more severe symptoms who did not seek care and died at home.
Strengths of this study include our diverse population. The population served by these NYC institutions is racially, ethnically, and socioeconomically diverse. This population has a higher proportion of Black (23% vs 13% US overall), and Hispanic (27% vs 18% US overall) individuals. Significant variability is observed in racial and ethnic compositions within and across neighborhoods in NYC and Long Island. Black individuals make up 43.6% of the Bronx, 34.1% of Brooklyn, 20.7% of Queens, 17.9% of Manhattan, 13.0% of Nassau County, and 11.7% of Staten Island. Additionally, this study presents poverty estimate data based on county of residence. These data show that Black patients had higher percentages of hospitalization and mortality in counties with a higher poverty percent (Bronx 27.3% and Brooklyn 18.9% vs US overall 13.1%).18
In summary, this study highlighted the racial disparities that exist in patients with gynecologic cancer diagnosed with COVID-19. Racial differences in both the severity and outcomes of COVID-19 infection seem to reflect differences in chronic conditions rather than cancer disease or treatment status. Younger (<65 years) Black patients had the highest risk of hospitalization in our cohort. Pre-existing racial disparities have been exacerbated during the COVID-19 pandemic and these data necessitate immediate change to enable health equity and outcomes in vulnerable populations. COVID-19 infection outcomes experienced by Black women highlight pre-existing disparities and call for multifaceted attention to address these longstanding differences in health outcomes among gynecologic cancer patients.
Supplementary Material
Acknowledgements
The authors thank NYU Gynecologic Oncology practitioners at the Tisch, Brooklyn and Winthrop campuses (Kari Hacker, Ghadir Salame, Franco Muggia, Edward Jimenez and Kathleen Lutz) and the Gynecologic Medical Oncology attendings at Memorial Sloan Kettering Cancer Center for identifying patients and details for this analysis.
Funding Support
R.OC and J.J. were supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748 (Memorial Sloan Kettering Cancer Center support group).
Conflict of Interest Disclosures
B.P. reports grants, personal fees and non-financial support outside the submitted work; institutional PI for industry sponsored trials from Tesaro/GSK, AstraZeneca, Merck, Genentech/ Roche, and Clovis Oncology. Compensated advisory boards include Tesaro/GSK, AstraZeneca, Merck and Eisai. J.J. reports a patent license from MDSeq Inc. R.OC reports personal fees from Tesaro, GlaxoSmithKline, Regeneron and Genentech USA, outside the submitted work and non-compensated steering committee member for the PRIMA, Moonstone (Tesaro/GSK) and DUO-O (AstraZeneca) studies. R.OC’s institute receives funding for clinical research from Celgene/Juno, Tesaro/GSK, Ludwig Cancer Institute, Abbvie, Regeneron, TCR2 Therapeutics, Atara Biotherapeutics, Marker Therapeutics, Syndax Pharmaceuticals, Genmab Therapeutics, Sellas Therapeutics, Genentech, Kite Pharma, Gynecologic Oncology Foundation. S.V.B. has research collaborations with Roche/Genentech, Tesaro/GK, Seattle Genetics, Merck and Asta Zeneca.
References
- 1.Yancy CW. COVID-19 and African Americans. Jama 2020;323:1891–2. [DOI] [PubMed] [Google Scholar]
- 2.Webb Hooper M, Napoles AM, Perez-Stable EJ. COVID-19 and Racial/Ethnic Disparities. Jama 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chowkwanyun M, Reed AL, Jr. Racial Health Disparities and Covid-19 - Caution and Context. The New England journal of medicine 2020;383:201–3. [DOI] [PubMed] [Google Scholar]
- 4.Dorn AV, Cooney RE, Sabin ML. COVID-19 exacerbating inequalities in the US. Lancet (London, England) 2020;395:1243–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). at https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-detection-instructions.html.)
- 6.New York City Health: COVID-19 Data. (Accessed May 15, 2020, at https://www1.nyc.gov/site/doh/covid/covid-19-data.page.)
- 7.Wadhera RK, Wadhera P, Gaba P, et al. Variation in COVID-19 Hospitalizations and Deaths Across New York City Boroughs. Jama 2020;323:2192–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SJ, Bostwick W. Social Vulnerability and Racial Inequality in COVID-19 Deaths in Chicago. Health education & behavior : the official publication of the Society for Public Health Education 2020;47:509–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azar KMJ, Shen Z, Romanelli RJ, et al. Disparities In Outcomes Among COVID-19 Patients In A Large Health Care System In California. Health affairs (Project Hope) 2020;39:1253–62. [DOI] [PubMed] [Google Scholar]
- 10.Henning-Smith C, Tuttle M, Kozhimannil KB. Unequal Distribution of COVID-19 Risk Among Rural Residents by Race and Ethnicity. The Journal of rural health : official journal of the American Rural Health Association and the National Rural Health Care Association 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahajan UV, Larkins-Pettigrew M. Racial demographics and COVID-19 confirmed cases and deaths: a correlational analysis of 2886 US counties. Journal of public health (Oxford, England) 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 Transmission in Patients With Cancer at a Tertiary Care Hospital in Wuhan, China. JAMA oncology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Annals of oncology : official journal of the European Society for Medical Oncology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A. Coronavirus (COVID-19) Outbreak: What the Department of Radiology Should Know. Journal of the American College of Radiology : JACR 2020;17:447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. Journal of biomedical informatics 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American journal of clinical oncology 1982;5:649–55. [PubMed] [Google Scholar]
- 18.U.S. Census Bureau (2018). Small Area Income and Poverty Estimates (SAIPE). .
- 19.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and Mortality among Black Patients and White Patients with Covid-19. The New England journal of medicine 2020;382:2534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holtgrave DR, Barranco MA, Tesoriero JM, Blog DS, Rosenberg ES. Assessing racial and ethnic disparities using a COVID-19 outcomes continuum for New York State. Annals of epidemiology 2020;48:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawkins D Differential occupational risk for COVID-19 and other infection exposure according to race and ethnicity. American journal of industrial medicine 2020;63:817–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laster M, Shen JI, Norris KC. Kidney Disease Among African Americans: A Population Perspective. American journal of kidney diseases : the official journal of the National Kidney Foundation 2018;72:S3–s7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ejike CO, Dransfield MT, Hansel NN, et al. Chronic Obstructive Pulmonary Disease in America’s Black Population. American journal of respiratory and critical care medicine 2019;200:423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carnethon MR, Pu J, Howard G, et al. Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circulation 2017;136:e393–e423. [DOI] [PubMed] [Google Scholar]
- 25.Barnes KC, Grant AV, Hansel NN, Gao P, Dunston GM. African Americans with asthma: genetic insights. Proceedings of the American Thoracic Society 2007;4:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forde AT, Crookes DM, Suglia SF, Demmer RT. The weathering hypothesis as an explanation for racial disparities in health: a systematic review. Annals of epidemiology 2019;33:1–18.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milam AJ, Furr-Holden D, Edwards-Johnson J, et al. Are Clinicians Contributing to Excess African American COVID-19 Deaths? Unbeknownst to Them, They May Be. Health equity 2020;4:139–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bristow RE, Powell MA, Al-Hammadi N, et al. Disparities in ovarian cancer care quality and survival according to race and socioeconomic status. Journal of the National Cancer Institute 2013;105:823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fairfield KM, Lucas FL, Earle CC, Small L, Trimble EL, Warren JL. Regional variation in cancer-directed surgery and mortality among women with epithelial ovarian cancer in the Medicare population. Cancer 2010;116:4840–8. [DOI] [PubMed] [Google Scholar]
- 30.Mundt AJ, Connell PP, Campbell T, Hwang JH, Rotmensch J, Waggoner S. Race and clinical outcome in patients with carcinoma of the uterine cervix treated with radiation therapy. Gynecologic oncology 1998;71:151–8. [DOI] [PubMed] [Google Scholar]
- 31.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA: a cancer journal for clinicians 2019. [DOI] [PubMed] [Google Scholar]
- 32.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 33.Terplan M, Schluterman N, McNamara EJ, Tracy JK, Temkin SM. Have racial disparities in ovarian cancer increased over time? An analysis of SEER data. Gynecologic oncology 2012;125:19–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


