Abstract
Purpose
Phenylephrine has been shown to affect intraocular pressure (IOP) but the mechanism of action is poorly understood. However, its action as a vasoconstrictor suggests possible effects on episcleral venous pressure (EVP). In this study, we evaluated the effect of phenylephrine on EVP and IOP in healthy subjects.
Methods
Forty eyes of 20 subjects were included. Each subject received 3 drops of phenylephrine 2.5% in one eye at 1-minute intervals. The fellow eye served as control. Blood pressure, heart rate, and IOP and EVP of both eyes were measured at baseline, 15 minutes, and 60 minutes after instillation of phenylephrine. IOP was measured by pneumatonometry. EVP was assessed by using a computer-controlled episcleral venomanometer. Changes in IOP, EVP, blood pressure, and heart rate at 15 and 60 minutes were analyzed by paired t-tests.
Results
IOP increased 15 minutes after instillation of phenylephrine in both treated (P = 0.001) and control eyes (P = 0.01) and returned to baseline at 60 minutes. The change in IOP at 15 minutes was not significantly different between the 2 groups. EVP in treated eyes was unchanged at 15 minutes (P = 0.8) but decreased significantly at 60 minutes (P < 0.001). In control eyes, there was no change in EVP at any time (P > 0.6). There were no significant changes from baseline in systolic and diastolic blood pressure and heart rate after instillation of phenylephrine.
Conclusions
IOP elevation associated with topical phenylephrine is not caused by an increase in EVP in healthy subjects. Instead, EVP decreases with phenylephrine, but the mechanism remains to be determined.
Keywords: phenylephrine, episcleral venous pressure, intraocular pressure, vasoconstriction
Phenylephrine is an alpha-1 adrenergic agonist that is frequently used for pupil dilation during routine clinical fundus examinations, as well as in pre-operative regimens for intraocular surgeries. Concentrations of 2.5% and 10% phenylephrine have been used for this purpose.1,2 In the eye, phenylephrine acts locally as a potent vasoconstrictor and mydriatic by constricting ophthalmic blood vessels and the radial dilator muscle of the iris.1,2 Mydriasis begins after about 15 minutes,1,3 is maximal at 60 to 90 (mean 75) minutes, and recovers after 5 to 7 hours. Phenylephrine is also used as a decongestant by constricting conjunctival vessels, thereby decreasing eye redness. Phenylephrine solution 2.5% is considered safe for routine use in the eye.4 The US Food and Drug Administration (FDA) has approved using up to 3 drops of phenylephrine hydrochloride ophthalmic solution 2.5% or 10% for pupil dilation. However, the use of phenylephrine may affect intraocular pressure (IOP), making measurements during routine eye examinations suspect if performed after dilation.
Several studies have measured the effects of phenylephrine on IOP and aqueous outflow facility in normal and glaucomatous human eyes, but the mechanism of action remains poorly understood. In some studies, phenylephrine was found to cause a mild reduction in IOP in both normal eyes5,6 and eyes with open-angle glaucoma.6,7 Other studies have found no change8–12 or a slight increase in IOP in normal eyes or eyes with open-angle glaucoma after treatment with phenylephrine.12–17 Outflow facility has also been measured by tonography to assess the effect of phenylephrine, with some reporting an increase in normal eyes5 and in glaucomatous eyes,6 some reporting a decrease in glaucomatous eyes,12 and some reporting no effect in normal6,12,15,18 and glaucomatous eyes.15,18
The effect of phenylephrine on episcleral venous pressure (EVP), a key determinant of IOP, has not been reported. The episcleral veins are the distal portion of the conventional aqueous outflow pathway that begins at the trabecular meshwork, continues in the collector channels and aqueous veins, and drains into the superior ophthalmic vein.19–22 The specialized morphology of the episcleral vasculature, with numerous arteriovenous anastomoses (AVAs) and muscle rich arteries and veins innervated for vasodilation and vasoconstriction, allows regulation of blood flow and volume in the episcleral vessels.23,24 Phenylephrine, as a vasoconstrictor, may affect vessel tone in multiple parts of the episcleral vasculature, but the effect on EVP is unknown. In this study, we evaluated the effect of topical phenylephrine 2.5% on EVP and IOP in healthy subjects.
Methods
This study was approved by the Institutional Review Boards at Mayo Clinic. The study followed the tenets of the Declaration of Helsinki and was in accordance with Health Insurance Portability and Accountability Act (HIPAA) regulations. All subjects provided written informed consent to participate after discussion of the nature and possible risks of the study.
Study Subjects
Healthy subjects, age 18 years and above, were enrolled from local area residents and employees of Mayo Clinic Rochester. All participants underwent a general health interview and comprehensive ophthalmologic examination, including visual acuity, IOP by pneumatonometry, slit lamp biomicroscopy, gonioscopy, and fundoscopy. Subjects were included in the study if they had had 2 healthy eyes with normal crystalline lenses, open angles, IOP less than 22 mm Hg in each eye, and best-corrected visual acuity (BCVA) 20/50 or better in each eye. Subjects were excluded if they had a history or evidence of any clinically significant ocular pathology (including narrow angles or any form of glaucoma), intraocular surgery, laser treatment, corneal refractive surgery, a vertical cup-to-disc ratio ≥0.6 or an asymmetry of the vertical cup-to-disc ratio ≥0.2, myopia greater than −6.00 D or hyperopia greater than +2.00 D, use of any ocular medication within 30 days of study visit, known hypersensitivity to the study medications, use of systemic medications within 30 days prior to study that may affect IOP or blood pressure, use of systemic steroid, and women who were pregnant. Also excluded were participants with systemic disease that could affect vascular regulation or EVP, including severe hypertension (systolic blood pressure greater than 180 mmHg and/or diastolic blood pressure greater than 105 mm Hg), ischemic heart disease, cerebrovascular accidents, cardiac arrhythmias, cerebral or aortic aneurysms, uncontrolled diabetes mellitus, and uncontrolled hyperthyroidism.
Measurements
Intraocular Pressure
IOP was measured in both eyes in the sitting position by using a pneumatonometer (Model 30 Classic; Reichert Inc., Buffalo, NY, USA). Calibration of the tonometer was verified according to the manufacturer's instruction and the tip was cleaned before each set of measurement. Topical proparacaine 0.5% was instilled before each IOP measurement.
Episcleral Venous Pressure
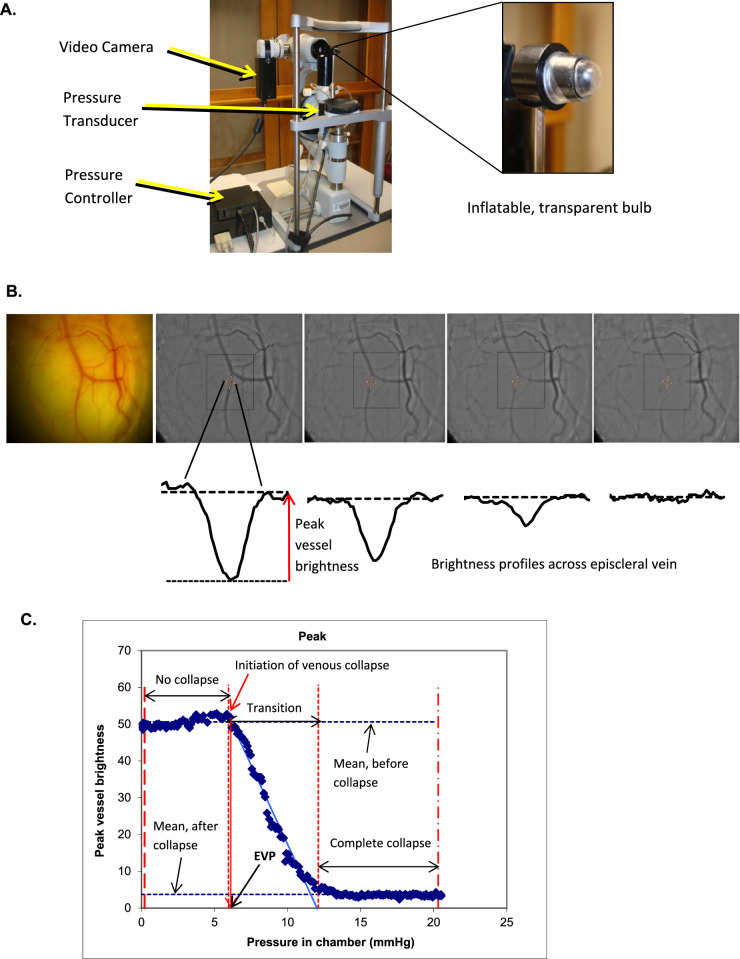
EVP was measured noninvasively by using a computer-controlled slit-lamp mounted episcleral venomanometer, with modifications designed and constructed by the Mayo Clinic Division of Engineering (Fig. A). This technique has been described previously.25,26 In brief, our system included a computer-controlled motor drive to increase pressure automatically, a transducer to record pressure, and a high-definition video camera to record vein collapse. This device utilized the pressure chamber technique, in which a clear inflatable bulb was placed against the conjunctival surface of the eye in contact with an episcleral vein, and then the pressure automatically increased at a constant rate and inflated the bulb. Images of the vein were recorded by using a video camera as the vein was compressed over 8 seconds (240 frames; Fig. B). The pressure inside the chamber was recorded and synchronized with the video stream. The brightness profile across a selected segment of the vein was calculated using custom image analysis software and the peak brightness was graphed as a function of applied pressure (Fig. C). Peak vessel brightness decreased as increasing pressure in the bulb collapsed the vein. Image analysis software was used to determine the point at which venous collapse begins, which corresponds to EVP.
Figure.
Measuring episcleral venous pressure. (A) Episcleral venomanometer. An episcleral venomanometer mounted on a slit lamp biomicroscope and modified for automated control. (B) Recording of venous collapse. The venous collapse was recorded by using a video camera. Brightness profiles across a selected segment of the vein were calculated using custom image analysis software. (C) Change in brightness profile across an episcleral vein. The peak brightness across the vein was graphed as a function of applied pressure.
One episcleral vein in the superior hemisphere was selected and a mean of three measurements was taken as the EVP. In a previous study, no significant differences in EVP were detected in different quadrants of the eye.25 All EVP measurements were performed by one of the authors (A.K.). During the measurements, the subjects were asked to relax and breathe normally, and the measurements were always made on the same vessel for each eye.
Blood Pressure and Heart Rate
Each subject was seated for 5 minutes and blood pressure and heart rate were measured by using an automatic blood pressure monitor. An appropriate pressure cuff size was used for each subject.
Instillation of Phenylephrine and Order of Measurements
On the day of the study, the subjects were asked to maintain a regular schedule with normal activities. After confirming eligibility, baseline IOP and EVP in both eyes, and blood pressure and heart rate were measured. Topical proparacaine was instilled in each eye before IOP and EVP measurement. The left or right eye of each subject was then randomized to receive 3 drops of phenylephrine 2.5% instilled into the lower conjunctival cul-de-sac at 1-minute intervals between drops. The fellow eye served as a control. Subjects were instructed to close their eyelids gently after instillation of each drop and to maintain closed eyes for 1 minute. Subjects were also instructed to perform punctal occlusion by pressing a finger over the medial canthal area against the nasal bone to close the nasolacrimal duct, reducing systemic absorption of phenylephrine. Blood pressure and heart rate, IOP, and EVP were then measured 15 minutes and 60 minutes after instillation of phenylephrine. For each parameter, a mean of three measurements was accepted as a final value.
Statistical Analysis
Significance of changes in IOP, EVP, systolic and diastolic blood pressures, and heart rate from baseline and comparison of changes between treated eyes and control eyes at 15 and 60 minutes after instillation of phenylephrine eye drop were determined by using paired t-tests. Differences were considered significant for P < 0.05. All of the P values were calculated based on paired differences between groups, which provide greater power to detect differences between means than an unpaired t-test between independent samples.
Results
Forty eyes from 20 healthy subjects (7 men and 13 women), ages 19 to 60 years (35.4 ± 13.3 years, mean ± SD; Table 1) were enrolled in the study. There was one subject with moderate systemic hypertension and no subjects with diabetes. IOP in treated eyes increased from 15.0 ± 2.5 mm Hg at baseline to 16.0 ± 2.4 mm Hg (mean difference of 1.0 ± 1.2 mm Hg, P = 0.001; Table 2) at 15 minutes after instillation of phenylephrine but then decreased to baseline (15.0 ± 2.0 mm Hg, mean difference of 0.0 ± 1.4 mmHg, P = 0.9) at 60 minutes. In control eyes, IOP was 14.5 ± 2.0 mmHg at baseline, increased to 15.1 ± 1.9 mmHg at 15 minutes (mean difference of 0.6 ± 1.0 mm Hg, P = 0.01; Table 2), and then returned to baseline (14.8 ± 2.0 mm Hg, mean difference of 0.3 ± 1.0 mm Hg, P = 0.18) at 60 minutes. At 15 minutes, there was no significant difference in the change in IOP from baseline between treated and control eyes (mean difference of 0.4 ± 1.1 mm Hg, P = 0.18; Table 3).
Table 1.
Study Population Characteristics
| Characteristic | |
|---|---|
| Men | 7 |
| Women | 13 |
| Age (y) | |
| Mean ± SD | 35.4 ± 13.3 |
| Range | 19–60 |
| Race | |
| White | 16 |
| Asian | 4 |
Table 2.
Pairwise Comparison of IOP, EVP, Blood Pressure, and Heart Rate at 15 and 60 Minutes of Instillation of Phenylephrine 2.5% With Baseline Among Treated and Control Eyes
| 15 Min | 60 Min | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Baseline Mean ± SD | Mean ± SD | Pairwise Difference From Baseline ± SD | P Value | Mean ± SD | Pairwise Difference From Baseline ± SD | P Value |
| Treated IOP (mm Hg) | 15.0 ± 2.5 | 16.0 ± 2.4 | 1.0 ± 1.2 | 0.001 | 15.0 ± 2.0 | 0.0 ± 1.4 | 0.92 |
| Control IOP (mm Hg) | 14.5 ± 2.0 | 15.1 ± 1.9 | 0.6 ± 1.0 | 0.01 | 14.8 ± 2.0 | 0.3 ± 1.0 | 0.18 |
| Treated EVP (mm Hg) | 8.4 ± 1.7 | 8.3 ± 1.9 | −0.1 ± 1.5 | 0.81 | 7.3 ± 1.9 | −1.1 ± 0.9 | <0.001 |
| Control EVP (mm Hg) | 8.3 ± 2.2 | 8.3 ± 2.4 | 0.0 ± 1.0 | 0.91 | 8.2 ± 2.5 | −0.1 ± 0.9 | 0.68 |
| SBP (mm Hg) | 117.1 ± 16.4 | 118.8 ± 19.5 | 1.7 ± 6.8 | 0.26 | 119.0 ± 21.0 | 1.9 ± 8.8 | 0.35 |
| DBP (mm Hg) | 79.4 ± 8.7 | 81.0 ± 10.0 | 1.6 ± 4.2 | 0.10 | 81.7 ± 10.2 | 2.3 ± 5.4 | 0.07 |
| HR (bpm) | 74.9 ± 10.5 | 73.4 ± 11.9 | −1.5 ± 5.5 | 0.23 | 72.6 ± 11.1 | −2.3 ± 7.1 | 0.15 |
All comparisons were performed using paired t-tests.
DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure.
Table 3.
Pairwise Comparison of Baseline IOP and EVP, and Changes From Baseline at 15 and 60 Minutes of Instillation of Phenylephrine 2.5% Between Treated and Control Eyes
| Baseline | Change From Baseline at 15 min | Change From Baseline at 60 min | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Treated Eyes | Control Eyes | Pairwise Difference | P Value | Treated Eyes | Control Eyes | Pairwise Difference | P Value | Treated Eyes | Control Eyes | Pairwise Difference | P Value |
| IOP (mm Hg) | 15.0 ± 2.5 | 14.5 ± 2.0 | 0.5 ± 1.0 | 0.02 | 1.0 ± 1.2 | 0.6 ± 1.0 | 0.4 ± 1.1 | 0.18 | 0.0 ± 1.4 | 0.3 ± 1.0 | −0.3 ± 1.0 | 0.15 |
| EVP (mm Hg) | 8.4 ± 1.7 | 8.3 ± 2.2 | 0.1 ± 2.5 | 0.83 | −0.1 ± 1.5 | 0.0 ± 1.0 | −0.1 ± 1.8 | 0.90 | −1.1 ± 0.9 | −0.1 ± 0.9 | −1.0 ± 1.4 | 0.003 |
All comparisons were performed using paired t-tests.
EVP in treated eyes decreased significantly at 60 minutes from 8.4 ± 1.7 mm Hg at baseline to 7.3 ± 1.9 mm Hg (mean difference of −1.1 ± 0.9 mm Hg, P < 0.001) but there was no change at 15 minutes (8.3 ± 1.9 mm Hg, mean difference of −0.1 ± 1.5 mm Hg, P = 0.8). In control eyes, EVP was similar at all 3 times: 8.3 ± 2.2 mm Hg at baseline, 8.3 ± 2.4 mm Hg (mean difference of 0.0 ± 1.0 mm Hg, P = 0.9) at 15 minutes, and 8.2 ± 2.5 mm Hg (mean difference of −0.1 ± 0.9 mm Hg, P = 0.7) at 60 minutes. The EVP change was greater in treated eyes than in control eyes at 60 minutes (mean difference of −1.0 ± 1.4 mm Hg, P = 0.003) but not at 15 minutes (mean difference of −0.1 ± 1.8 mm Hg, P = 0.9; Table 3).
There was a trend toward an increase in both systolic and diastolic blood pressure and a decrease in heart rate after phenylephrine, but the changes were not statistically significant (P ≥ 0.07; Table 2).
Discussion
The regulation of EVP is complex, and the effects of vasoactive compounds, such as phenylephrine, can be difficult to predict. We previously investigated the effect of body position on EVP and found that changes in EVP can explain changes in IOP with postural changes.27 However, the magnitude of the changes is far less than would be expected if the pressure in the venous system were determined only by gravity and this suggests the presence of auto-regulation in the episcleral vasculature. Strohmaier et al. demonstrated that electrical stimulation at the location of the superior salivatory nucleus in rats resulted in elevation of EVP and IOP, whereas blood pressure and heart rate remained unchanged, suggesting the possibility of central nervous system (CNS) control.28 One possible mechanism for EVP control is through the arteriovenous anastomoses (AVAs), which connect the arterial and venous sides of the episcleral circulation.23,29–32 In animal studies, episcleral arteries, AVAs, and veins respond to topical vasoconstrictors and vasodilators, indicating their potential for active regulation under neural control, which may have an important role in the control of normal IOP.23,24,32–34 Vasoactive medications have been shown to affect EVP, with vasoconstrictors, such as brimonidine35 and L-NAME,36 causing a decrease, whereas vasodilators, such as nitroprusside,36 cause an increase. Curiously, netarsudil, a rho kinase inhibitor which causes vasodilation, decreases EVP in both animals and humans.37–39 Nevertheless, it is reasonable to expect that topical phenylephrine, a sympathomimetic agent with primary alpha-1 adrenergic activity known to act directly on vascular smooth muscle resulting in vasoconstriction,40,41 would affect EVP. Aqueous humor suppression with timolol or acetazolamide does not appear to affect EVP.42–44
Our study found a significant decrease (1.1 mm Hg) in EVP 60 minutes after instillation of phenylephrine eye drops in the treated eyes. EVP did not change in the control eyes at any time. Because phenylephrine is a vasoconstrictor, we expected a decrease in the diameter of episcleral veins after treatment and this was subjectively observed (but not measured) in most of the phenylephrine treated eyes. Decreased venous diameter after topical phenylephrine is in agreement with previous studies of reduction of conjunctival venous diameter with phenylephrine in human45 and rabbits.46 However, the diameter of the episcleral veins alone likely has limited influence on EVP. Instead, the effect is likely mediated by constriction and dilation of the episcleral AVAs. Funk et al. demonstrated that pharmacologically induced vasodilation and vasoconstriction of the episcleral vasculature in rabbits affected the flow of blood through the AVAs.34 Application of a topical vasoconstrictor (epinephrine) caused the AVAs to constrict, leading to a reduction of relatively high-pressure blood flow from the arterial to the venous side, which manifests as a decrease in EVP. In contrast, the application of a topical vasodilator (nitroprusside) caused dilation of the episcleral vasculature, and an increase in both EVP and IOP, likely mediated by dilation of AVAs and increased flow of high-pressure arterial blood to the venous system.34 Phenylephrine would be expected to constrict the AVAs, similar to epinephrine, causing a reduction in EVP as observed in our study subjects.
IOP increased by 1.0 mm Hg in treated eyes and by 0.6 mm Hg in control eyes 15 minutes after phenylephrine instillation but returned to baseline after 60 minutes. There are several mechanisms that could explain this IOP elevation, but the change does not appear to be related to EVP, which decreased after phenylephrine treatment. Cycloplegia can change outflow facility but phenylephrine does not appear to change ciliary muscle tone. One possible mechanism may be release of iris pigment after pharmacologically pupillary dilation,47 causing accumulation in the trabecular meshwork and decrease in outflow facility. These mechanisms are also consistent with the lack of IOP decrease at 60 minutes, which would be expected if a decrease in EVP were the only change in aqueous humor dynamics. IOP elevation observed at 15 minutes in the control eyes may be due to systemic absorption and contralateral effect of the eye drops. However, the effect of phenylephrine on IOP is inconclusive in the literature. Several studies have indicated no change in IOP in normal eyes8–12,48 or in eyes with open-angle glaucoma10,12 after treatment with phenylephrine. Other studies have reported a slight increase in IOP after phenylephrine treatment.12–17 In contrast, Becker6 reported that phenylephrine caused a mild IOP reduction in both normal eyes and eyes with open-angle glaucoma. One possible reason for the variety of results may be due to individual patient variations. Mapstone reported that phenylephrine 10% drops given to untreated ocular hypertension patients resulted in an IOP decrease in patients without pigment release, whereas no IOP change occurred in patients with visible pigment release after dosing.49
We did not measure aqueous humor flow rate but there are controversies about the effect of phenylephrine on the flow rate. An increased flow rate could increase IOP and Van Genderen et al.48 reported that flow increased significantly between 0 and 1 hour after phenylephrine instillation but decreased to normal after 2 hours. In a study by Lee et al.11 aqueous flow showed significant increases at 1 hour and 2 hours after instillation of phenylephrine but no significant effect on IOP at the same measurement times. However, some authors stated that the effect of phenylephrine on the aqueous flow in normal human eyes is considered to be negligible as a result of either an insufficient concentration of phenylephrine in the ciliary body or the lack of influence of an α-receptor system on the aqueous flow.8,9 A pharmacokinetic analysis of topical phenylephrine in the human eye revealed that phenylephrine is lost fairly rapidly from the anterior chamber.3 As well, any apparent increase in aqueous humor flow rate when determined by using fluorophotometry will be complicated by the increased fluorescein clearance from the anterior chamber through a dilated pupil.50
Cardiovascular side effects of phenylephrine include a rise in systolic and diastolic blood pressure and reflex bradycardia.51–55 However, there were no significant changes in systolic and diastolic blood pressures and heart rate after instillation of phenylephrine 2.5% in our subjects. Although some studies have reported a significant increase in blood pressure after instillation of phenylephrine 5% or 10% eye drops,56–58 controlled studies and meta-analyses have not found significant cardiovascular changes after phenylephrine 2.5% eye drops alone or in combination with tropicamide.4,59 A large randomized clinical trial found a transient increase in blood pressure after phenylephrine 2.5% topical drops, but this reverted to baseline in less than 20 minutes.59 There was also no significant increase in blood pressure or heart rate at 20 to 30 minutes or at 60 minutes or longer. Other studies that used phenylephrine 2.5% or higher concentrations did not show significant change in blood pressure.17,59–65
Our study has some limitations. We did not use a placebo eye drop in the control eyes. Unfortunately, a placebo that had similar sensation upon instillation and participant detectable effects on the eye would be difficult to develop. As well, we did not measure outflow facility or aqueous humor flow rate as the relatively short-lived effects of phenylephrine 2.5% do not provide enough time to perform those measurements in addition to EVP measurements. The most important advantage of our study was that we used an automated, computer-controlled episcleral venomanometer that can reliably detect the beginning of venous collapse, providing an objective measurement of EVP. As well, all EVP measurements were performed by one investigator (author A.K.) who is experienced in the field of episcleral venomanometry.
In conclusion, our study found that IOP increased 1.0 mm Hg 15 minutes after instillation of phenylephrine 2.5% eye drops but returned to baseline at 60 minutes. This transient elevation of IOP was not caused by an increase in EVP. Instead, there was a significant decrease, 1.1 mm Hg, of EVP in the treated eyes at 60 minutes. The mechanism for this decrease is currently unknown but vasoconstriction of the episcleral vessels, particularly the arteriovenous anastomoses is a possibility requiring further investigation. As well, future studies are required to identify the mechanism for IOP elevation and to investigate the effect of phenylephrine on IOP and EVP in glaucoma suspects and patients.
Acknowledgments
Supported by Mayo Foundation for Medical Education and Research.
Disclosure: A. Kazemi, None; J.W. McLaren, None; A.J. Sit, None
References
- 1. Haddad NJ, Moyer NJ, Riley FC Jr.. Mydriatic effect of phenylephrine hydrochloride. Am J Ophthalmol. 1970; 70: 729–733. [DOI] [PubMed] [Google Scholar]
- 2. Duffin RM, Pettit TH, Straatsma BR.. 2.5% v 10% phenylephrine in maintaining mydriasis during cataract surgery. Arch Ophthalmol. 1983; 101: 1903–1906. [DOI] [PubMed] [Google Scholar]
- 3. Matsumoto S, Tsuru T, Araie M, Komuro Y.. Pharmacokinetics of topical phenylephrine hydrochloride in the normal human eye. Jpn J Ophthalmol. 1982; 26: 338–344. [PubMed] [Google Scholar]
- 4. Stavert B, McGuinness MB, Harper CA, Guymer RH, Finger RP.. Cardiovascular Adverse Effects of Phenylephrine Eyedrops: A Systematic Review and Meta-analysis. JAMA Ophthalmol. 2015; 133: 647–652. [DOI] [PubMed] [Google Scholar]
- 5. Mapstone R. Normal response to pilocarpine and phenylephrine. Br J Ophthalmol. 1977; 61: 510–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Becker B, Friedenwald JS.. Clinical aqueous outflow. AMA Arch Ophthalmol. 1953; 50: 557–571. [DOI] [PubMed] [Google Scholar]
- 7. Posner A. Neosynephrin in glaucoma simplex. Am J Ophthalmol. 1948; 31: 222–224. [PubMed] [Google Scholar]
- 8. Lee DA, Brubaker RF.. Effect of phenylephrine on aqueous humor flow. Curr Eye Res. 1982; 2: 89–92. [DOI] [PubMed] [Google Scholar]
- 9. Araie M. Acute effects of topical phenylephrine on aqueous humor dynamics and corneal endothelial permeability in man. Jpn J Ophthalmol. 1983; 27: 340–345. [PubMed] [Google Scholar]
- 10. Marchini G, Babighian S, Tosi R, Perfetti S, Bonomi L.. Comparative study of the effects of 2% ibopamine, 10% phenylephrine, and 1% tropicamide on the anterior segment. Invest Ophthalmol Vis Sci. 2003; 44: 281–289. [DOI] [PubMed] [Google Scholar]
- 11. Araie M, Mori M, Oshika T.. Effect of topical phenylephrine on the permeability of the blood-aqueous barrier in man. Graefes Arch Clin Exp Ophthalmol. 1992; 230: 171–174. [DOI] [PubMed] [Google Scholar]
- 12. Lee PF. The influence of epinephrine and phenylephrine on intraocular pressure. AMA Arch Ophthalmol. 1958; 60: 863–867. [DOI] [PubMed] [Google Scholar]
- 13. Kronfeld PC, McGarry HI, Smith HE.. The Effect of Mydriatics Upon the Intra-ocular Pressure in So-Called Primary Wide-Angle Glaucoma. Trans Am Ophthalmol Soc. 1942; 40: 127–140. [PMC free article] [PubMed] [Google Scholar]
- 14. Harris LS. Cycloplegic-induced intraocular pressure elevations a study of normal and open-angle glaucomatous eyes. Arch Ophthalmol. 1968; 79: 242–246. [DOI] [PubMed] [Google Scholar]
- 15. Schimek RA, Lieberman WJ.. The influence of Cyclogyl and Neosynephrine on tonographic studies of miotic control in open-angle glaucoma. Am J Ophthalmol. 1961; 51: 781–784. [DOI] [PubMed] [Google Scholar]
- 16. Barbee RF, Smith WO Jr.. A comparative study of mydriatic and cycloplegic agents; in human subjects without eye disease. Am J Ophthalmol. 1957; 44: 617–622. [DOI] [PubMed] [Google Scholar]
- 17. Takayama J, Mishima A, Ishii K.. Effects of topical phenylephrine on blood flow in the posterior segments of monkey and aged human eyes. Jpn J Ophthalmol. 2004; 48: 243–248. [DOI] [PubMed] [Google Scholar]
- 18. Becker B, Gage T, Kolker AE, Gay AJ.. The effect of phenylephrine hydrochloride on the miotic-treated eye. Am J Ophthalmol. 1959; 48: 313–321. [DOI] [PubMed] [Google Scholar]
- 19. McDougal DH, Gamlin PD.. Autonomic control of the eye. Compr Physiol. 2015; 5: 439–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ashton N. Anatomical study of Schlemm's canal and aqueous veins by means of neoprene casts. Part I. Aqueous veins. Br J Ophthalmol. 1951; 35: 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ashton N. Anatomical study of Schlemm's canal and aqueous veins by means of neoprene casts. II. Aqueous veins. Br J Ophthalmol. 1952; 36: 265–267; contd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ashton N, Smith R.. Anatomical study of Schlemm's canal and aqueous veins by means of neoprene casts. III. Arterial relations of Schlemm's canal. Br J Ophthalmol. 1953; 37: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Selbach JM, Schonfelder U, Funk RH.. Arteriovenous anastomoses of the episcleral vasculature in the rabbit and rat eye. J Glaucoma. 1998; 7: 50–57. [PubMed] [Google Scholar]
- 24. Selbach JM, Rohen JW, Steuhl KP, Lutjen-Drecoll E.. Angioarchitecture and innervation of the primate anterior episclera. Curr Eye Res. 2005; 30: 337–344. [DOI] [PubMed] [Google Scholar]
- 25. Sit AJ, Ekdawi NS, Malihi M, McLaren JW.. A novel method for computerized measurement of episcleral venous pressure in humans. Exp Eye Res. 2011; 92: 537–544. [DOI] [PubMed] [Google Scholar]
- 26. Sit AJ, McLaren JW.. Measurement of episcleral venous pressure. Exp Eye Res. 2011; 93: 291–298. [DOI] [PubMed] [Google Scholar]
- 27. Arora N, McLaren JW, Hodge DO, Sit AJ.. Effect of Body Position on Epsicleral Venous Pressure in Healthy Subjects. Invest Ophthalmol Vis Sci. 2017; 58: 5151–5156. [DOI] [PubMed] [Google Scholar]
- 28. Strohmaier CA, Reitsamer HA, Kiel JW.. Episcleral venous pressure and IOP responses to central electrical stimulation in the rat. Invest Ophthalmol Vis Sci. 2013; 54: 6860–6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gaasterland DE, Jocson VL, Sears ML.. Channels of aqueous outflow and related blood vessels. 3. Episcleral arteriovenous anastomoses in the rhesus monkey eye (Macaca mulatta). Arch Ophthalmol. 1970; 84: 770–775. [DOI] [PubMed] [Google Scholar]
- 30. Rohen JW, Funk RH.. Functional morphology of the episcleral vasculature in rabbits and dogs: presence of arteriovenous anastomoses. J Glaucoma. 1994; 3: 51–57. [PubMed] [Google Scholar]
- 31. Funk RH, Rohen JW.. In vivo observations of the episcleral vasculature in the albino rabbit. J Glaucoma. 1994; 3: 44–50. [PubMed] [Google Scholar]
- 32. Funk RH, Rohen JW.. Scanning electron microscopic study of episcleral arteriovenous anastomoses in the owl and cynomolgus monkey. Curr Eye Res. 1996; 15: 321–327. [DOI] [PubMed] [Google Scholar]
- 33. Ascher KW. Aqueous veins and their significance for pathogenesis of glaucoma. Arch Ophthal. 1949; 42: 66–76. [DOI] [PubMed] [Google Scholar]
- 34. Funk RH, Gehr J, Rohen JW.. Short-term hemodynamic changes in episcleral arteriovenous anastomoses correlate with venous pressure and IOP changes in the albino rabbit. Curr Eye Res. 1996; 15: 87–93. [DOI] [PubMed] [Google Scholar]
- 35. Reitsamer HA, Posey M, Kiel JW.. Effects of a topical alpha2 adrenergic agonist on ciliary blood flow and aqueous production in rabbits. Exp Eye Res. 2006; 82: 405–415. [DOI] [PubMed] [Google Scholar]
- 36. Zamora DO, Kiel JW.. Episcleral venous pressure responses to topical nitroprusside and N-Nitro-L-arginine methyl ester. Invest Ophthalmol Vis Sci. 2010; 51: 1614–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kazemi A, McLaren JW, Kopczynski CC, Heah TG, Novack GD, Sit AJ.. The Effects of Netarsudil Ophthalmic Solution on Aqueous Humor Dynamics in a Randomized Study in Humans. J Ocul Pharmacol Ther. 2018; 34: 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kiel JW, Kopczynski CC.. Effect of AR-13324 on episcleral venous pressure in Dutch belted rabbits. J Ocul Pharmacol Ther. 2015; 31: 146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sit AJ, Gupta D, Kazemi A, et al.. Netarsudil Improves Trabecular Outflow Facility in Patients with Primary Open Angle Glaucoma or Ocular Hypertension: A Phase 2 Study. Am J Ophthalmol. 2021; 226: 262–269. [DOI] [PubMed] [Google Scholar]
- 40. Heath P. Neo-Synephrin: Some Uses and Effects in Ophthalmology. Trans Am Ophthalmol Soc. 1936; 34: 231–235. [PMC free article] [PubMed] [Google Scholar]
- 41. Faber JE. In situ analysis of alpha-adrenoceptors on arteriolar and venular smooth muscle in rat skeletal muscle microcirculation. Circ Res. 1988; 62: 37–50. [DOI] [PubMed] [Google Scholar]
- 42. Berson FG, Epstein DL.. Separate and combined effects of timolol maleate and acetazolamide in open-angle glaucoma. Am J Ophthalmol. 1981; 92: 788–791. [DOI] [PubMed] [Google Scholar]
- 43. Brubaker RF. Flow of aqueous humor in humans [The Friedenwald Lecture]. Invest Ophthalmol Vis Sci. 1991; 32: 3145–3166. [PubMed] [Google Scholar]
- 44. Kiel JW, Hollingsworth M, Rao R, Chen M, Reitsamer HA.. Ciliary blood flow and aqueous humor production. Prog Retin Eye Res. 2011; 30: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Houben AJ, Burgwinkel JP, de Leeuw PW.. A novel approach to the study of human microcirculation: Reactivity to locally applied angiotensin II in the conjunctival microvascular bed. J Hypertens. 2006; 24: 2225–2230. [DOI] [PubMed] [Google Scholar]
- 46. Gaynes B, Teng PY, Wanek J, Shahidi M.. Feasibility of conjunctival hemodynamic measurements in rabbits: reproducibility, validity, and response to acute hypotension. Microcirculation. 2012; 19: 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. El-Harazi SM, Ruiz RS, Feldman RM, Chuang AZ, Villanueva G.. Quantitative assessment of aqueous flare: the effect of age and pupillary dilation. Ophthalmic Surg Lasers. 2002; 33: 379–382. [PubMed] [Google Scholar]
- 48. van Genderen MM, van Best JA, Oosterhuis JA.. The immediate effect of phenylephrine on aqueous flow in man. Invest Ophthalmol Vis Sci. 1988; 29: 1469–1473. [PubMed] [Google Scholar]
- 49. Mapstone R. Pigment release. Br J Ophthalmol. 1981; 65: 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McLaren JW, Herman DC, Brubaker RF, et al.. Effect of ibopamine on aqueous humor production in normotensive humans. Invest Ophthalmol Vis Sci. 2003; 44: 4853–4858. [DOI] [PubMed] [Google Scholar]
- 51. Wellwood M, Goresky GV.. Systemic hypertension associated with topical administration of 2.5% phenylephrine HCl. Am J Ophthalmol. 1982; 93: 369–370. [DOI] [PubMed] [Google Scholar]
- 52. Fraunfelder FT, Meyer SM.. Possible cardiovascular effects secondary to topical ophthalmic 2.5% phenylephrine. Am J Ophthalmol. 1985; 99: 362–363. [DOI] [PubMed] [Google Scholar]
- 53. Fraunfelder FT, Scafidi AF.. Possible adverse effects from topical ocular 10% phenylephrine. Am J Ophthalmol. 1978; 85: 447–453. [DOI] [PubMed] [Google Scholar]
- 54. Kumar V, Schoenwald RD, Chien DS, Packer AJ, Choi WW.. Systemic absorption and cardiovascular effects of phenylephrine eyedrops. Am J Ophthalmol. 1985; 99: 180–184. [DOI] [PubMed] [Google Scholar]
- 55. Wilensky JT, Woodward HJ.. Acute systemic hypertension after conjunctival instillation of phenylephrine hydrochloride. Am J Ophthalmol. 1973; 76: 156–157. [DOI] [PubMed] [Google Scholar]
- 56. Samantary S, Thomas A.. Systemic effects of topical phenylephrine. Indian J Ophthalmol. 1975; 23: 16–17. [PubMed] [Google Scholar]
- 57. Chawdhary S, Angra SK, Zutshi R, Sachdev MS.. Mydriasis–use of phenylephrine (a dose-response concept). Indian J Ophthalmol. 1984; 32: 213–216. [PubMed] [Google Scholar]
- 58. Chin KW, Law NM, Chin MK.. Phenylephrine eye drops in ophthalmic surgery–a clinical study on cardiovascular effects. Med J Malaysia. 1994; 49: 158–163. [PubMed] [Google Scholar]
- 59. Brown MM, Brown GC, Spaeth GL.. Lack of side effects from topically administered 10% phenylephrine eyedrops. A controlled study. Arch Ophthalmol. 1980; 98: 487–489. [DOI] [PubMed] [Google Scholar]
- 60. Lam PT, Poon BT, Wu WK, Chi SC, Lam DS.. Randomized clinical trial of the efficacy and safety of tropicamide and phenylephrine in preoperative mydriasis for phacoemulsification. Clin Exp Ophthalmol. 2003; 31: 52–56. [DOI] [PubMed] [Google Scholar]
- 61. Motta MM, Coblentz J, Fernandes BF, Burnier MN Jr.. Mydriatic and cardiovascular effects of phenylephrine 2.5% versus phenylephrine 10%, both associated with tropicamide 1%. Ophthalmic Res. 2009; 42: 87–89. [DOI] [PubMed] [Google Scholar]
- 62. Heath P, Geiter CW.. Use of phenylephrine hydrochloride, neo-synephrine hydrochloride, in ophthalmology. Arch Ophthal. 1949; 41: 172–177. [DOI] [PubMed] [Google Scholar]
- 63. Trinavarat A, Pituksung A.. Effective pupil dilatation with a mixture of 0.75% tropicamide and 2.5% phenylephrine: A randomized controlled trial. Indian J Ophthalmol. 2009; 57: 351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Symons RC, Walland MJ, Kaufman DV.. A comparative study of the efficacy of 2.5% phenylephrine and 10% phenylephrine in pre-operative mydriasis for routine cataract surgery. Eye (Lond). 1997; 11(Pt 6): 946–948. [DOI] [PubMed] [Google Scholar]
- 65. Malhotra R, Banerjee G, Brampton W, Price NC.. Comparison of the cardiovascular effects of 2.5% phenylephrine and 10% phenylephrine during ophthalmic surgery. Eye (Lond). 1998; 12(Pt 6): 973–975. [DOI] [PubMed] [Google Scholar]