Abstract
As part of the Human Biomonitoring for Europe (HBM4EU) initiative a human biomonitoring (HBM) survey is conducted in 21 countries. This survey builds on existing HBM capacity in Europe by aligning national or regional HBM studies. The survey targets 3 age groups (i) children aged 6–11 years, (ii) teenagers aged 12–19 years and (iii) young adults aged 20–39 years and includes a total of 9493 participants (3151 children, 2953 teenagers and 3389 young adults). Depending on the age group, internal exposure to phthalates and substitute Hexamoll® DINCH, brominated and organophosphorus flame retardants, per-/poly-fluorinated compounds, cadmium, bisphenols and/or polycyclic aromatic hydrocarbons are assessed. The main goal of the programme is to obtain quality controlled and comparable HBM data of exposure to chemicals, prioritized under HBM4EU, with European wide coverage to inform the development of environment and health policies. This paper describes the framework of the HBM4EU survey and the approach that has been applied to align European HBM initiatives across Europe.
Keywords: Human biomonitoring, Joint HBM4EU survey, Children, Teenagers, Adults
1. Introduction
In June 2004 the European Commission recognized in its Environment and Health Action Plan the relevance of human biomonitoring (HBM) and the need for more harmonized approaches in Europe to allow for better comparability of results and more efficient use of resources (European Commission, 2004). In 2005 the Expert team to Support BIOmonitoring in Europe (ESBIO) project was launched followed by the first joint European HBM initiative COPHES (Consortium to Perform Human Biomonitoring on a European Scale) together with the feasibility study DEMOCOPHES (Demonstration of a Study to Coordinate and Perform Human biomonitoring on a European Scale) which were conducted between 2009 and 2012 (Schindler et al., 2014). These projects were very successful and laid the foundation for future projects. In 2017 a joint European HBM initiative (HBM4EU) was launched. The HBM4EU project runs from 2017 to 2021 and is co-financed under Horizon 2020. The project's main goal is to coordinate and advance human biomonitoring in Europe to provide science based evidence for chemical policy development and improve chemical management (Ganzleben et al., 2017). As part of the HBM4EU initiative a joint HBM survey is conducted laying the foundation of a sustainable European HBM platform.
Human biomonitoring measures trace levels of multiple environmental chemicals, their metabolites, or reaction products in human biological matrices, such as blood and urine. HBM data directly reflects the actual internal chemical exposure of a sampled population at a given time covering all routes of exposure and taking into account the kinetics of chemicals in the body including bioaccumulation of persistent chemicals (Angerer et al., 2007). HBM data can be used for a number of objectives such as: establishing reference ranges for selected chemicals in the general population or in specific targeted populations such as occupational populations or pregnant women, identifying highly exposed populations, monitoring time trends and spatial patterns of internal exposure and evaluating the effect of policy measures. Connecting HBM data to personal information regarding lifestyle, diet, behavior and health enables the mapping of internal doses with potential exposure sources and/or health outcomes. It is recognized that HBM data provides an important tool to support environment and health policy development through regulatory actions but also by awareness raising campaigns or remediation actions (Bahadori et al., 2007). Some EU countries have national programs that collect, for a wide variety of chemicals, HBM data which are representative of specific characteristics of their populations such as age, sex and socio-demographic factors. Examples are the German GerES studies (Schulz et al., 2007), the Czech Environmental Health Monitoring System Czech-HBM (Cerna et al., 2012), the French national biomonitoring programme (ELFE) (Dereumeaux et al., 2017), the Italian PROBE (Alimonti, 2011), the Spanish BIOAMBIENT. ES (Perez-Gomez et al., 2013) and the Flemish Human Biomonitoring Studies (FLEHS) representative for the Flemish region (Choi et al., 2015; Schoeters et al., 2012). Most countries lack such a programme and collect HBM data in the frame of specific research projects. Hence the studies are fragmented and heterogeneous. There is no overarching strategy within Europe and current studies are not harmonized or aligned to meet common goals. Therefore, to generate comparable HBM data with European wide coverage of exposure to HBM4EU prioritized chemicals (David et al., 2020), ongoing and planned HBM studies from different countries and regions all over Europe have been aligned and brought together under the joint HBM4EU survey. All HBM4EU priority substances are summarised under https://www.hbm4eu.eu/the-substances/. In the hereby reported survey the focus is on a subset of the first set of priority substances of HBM4EU, phthalates and substitute Hexamoll® DINCH, brominated and organophosphorus flame retardants, per-/poly-fluorinated compounds (PFAS), cadmium (cd), bisphenols and polycyclic aromatic hydrocarbons (PAHs). The data resulting from the HBM4EU survey will feed into the evaluation of following research objectives: the derivation of European exposure values, geographical comparisons of the four European regions, identification of determinants of internal exposure (personal characteristics, external sources), comparing exposure levels to health based guidance values, associating exposure biomarkers with personal health data and linking these associations through effect markers in a causal pathway analysis.
2. Method: aligning European HBM surveys
2.1. Sampling frame
The main focus of the joint HBM4EU survey is to establish reference ranges for HBM4EU priority substances that are representative for the exposure distribution of the European population and to facilitate the use and translation of the scientific results into policy actions. The survey builds on existing HBM capacity in Europe by aligning HBM studies/initiatives targeting the general population. A sampling frame was developed to facilitate the selection of participating studies that could be aligned to obtain comparable HBM data across the EU. In a first step the target populations were defined for whom HBM data representative of the current exposure variability across Europe should be collected. Current exposure was defined as samples collected between 2014 and 2019 (later extended to 2020). Due to logistic and financial constraints we could only include a ‘sample’ of the European population in our study. However, representativeness means that this sample reflects the composition of the European population for some prespecified criteria. A representative study population can only be achieved by the use of a probability sampling method which is most commonly used in HBM studies. The ideal concept to obtain a representative sample of the European population would be a multistage probability sampling method among the participating EU countries with proportional representation of sex, age groups, SES and geographical spread. In all study areas we aimed for an equal participation of both sexes in the surveys cfr. National Health and Nutrition Examination Survey (NHANES) and Canadian Health Measures Survey (CHMS) (Statistics Canada, 2018; CDC, 2017). Additional sampling domains for which representation is considered important in HBM4EU are socio-economic status (SES) and residential degree of urbanisation. In each primary sampling unit (country) each level of SES and degree of urbanisation should be at least 10% represented in the participating surveys to cover variation in SES and degree of urbanisation. The categorisation of study participants in low – medium – high level of education was based on Eurostat's online tables that refer to the International Standard Classification of Education (ISCED) developed by United Nations Educational, Scientific and Cultural Organization (UNESCO) (UNESCO Institute for Statistics, 2012). Lower educational level denotes individuals with no to lower secondary education (ISCED 0–2), medium level of education includes individuals with upper secondary to post-secondary non-tertiary education (ISCED 3–4), and high level of education represents individuals with tertiary education and higher (ISCED ≥5). A subject's living environment is classified according to the degree of urbanisation (DEGURBA) classification of Eurostat distinguishing three levels of urbanisation. i.e. densely populated area (cities), intermediate density area (towns and suburbs) and thinly populated area (rural area) (Lewis Dijkstra, 2014).
2.2. Age categories
For the joint HBM4EU survey several age groups were considered for sampling including newborns (0-2y), toddlers (3-5y), children (6-11y), teenagers (12-19y), young adults (20-39y), adults (40-59y), elderly (60-79y) and 80 + y. Ideally, exposure data from all age groups should be collected. However, for this programme of work it was only feasible to sample certain age groups. Newborns and young children (age 0-5y) are considered as a vulnerable and important subgroup. However, newborns were not included since a lot of information is already available on early life stressors, including environmental chemicals, that has been collected in European birth cohorts (Maitre et al., 2018; Vrijheid et al., 2012). Toddlers were not included in this first step as the related field work for recruitment and sampling is more complex compared to older age groups. Therefore, the joint HBM4EU survey focuses on collecting recent data from three age groups: (i) children aged 6–11 years, (ii) teenagers aged 12–19 years and (iii) young adults aged 20–39 years. Restricting the adult age group to 39 years provides a more homogeneous group with a more similar health status (reproductive age group). Additionally, this classification mirrors the age stratification used in NHANES and CHMS (CDC, 2017; Statistics Canada, 2018).
2.3. EU wide coverage
To obtain complete European coverage, a maximal scenario for Europe would be to sample each of the 27 EU countries of the HBM4EU consortium. To lower the resolution and reduce the maximal scenario, Europe was stratified into four geographical regions i.e. North, East, South and West, according to the United Nations geoscheme (United Nations, 1999). The number of PSU (countries) to include per geographical region should be proportional to the number of inhabitants of that region. Given that the North, West, South and East represent 21%, 40%, 28% and 11% of the EU inhabitants respectively, we proposed to include 2 countries to represent the North, 3–4 countries to represent the West, 3 countries to represent the South and at least 1 country to represent the East of Europe. In Supplementary Figure 1 the countries contributing to the joint HBM4EU survey for at least one of the age groups are coloured according to the EU geographical region they are attributed to.
2.4. Sample size
The main objective of the HBM4EU survey is to establish EU exposure values of internal exposure to priority chemicals with their confidence intervals. As calculated by Poulsen et al. to obtain percentiles with reasonably narrow confidence intervals, at least 120 measurement values are needed (Poulsen et al., 1997). Therefore, to establish exposure values for specific subpopulations of the EU sample i.e. male vs. female, low vs. medium vs. high SES, residents of rural vs. urban vs. semi-urban areas, and residents of the 4 geographical regions, a minimum of measurements from 120 subjects per stratum is required. Since we want to be able to also compare male vs. female subjects within a region a minimum of 240 subjects (120 male and 120 female) per region is required. For Eastern Europe a region with at least 1 PSU this implies a minimum sample size of 240 subjects from the PSU. Because of EU co-funding availability the maximum contribution of samples per PSU was limited to 300. For PSU belonging to those regions with more than 1 PSU (N, S, W) an exception was granted to contribute with a reduced sample size per PSU given that the minimum of 240 is reached for their respective region (Fig. 1). The resulting sampling strategy of the joint HBM4EU survey is a compromise, in order to build maximally on already existing studies and expertise (Table 1).
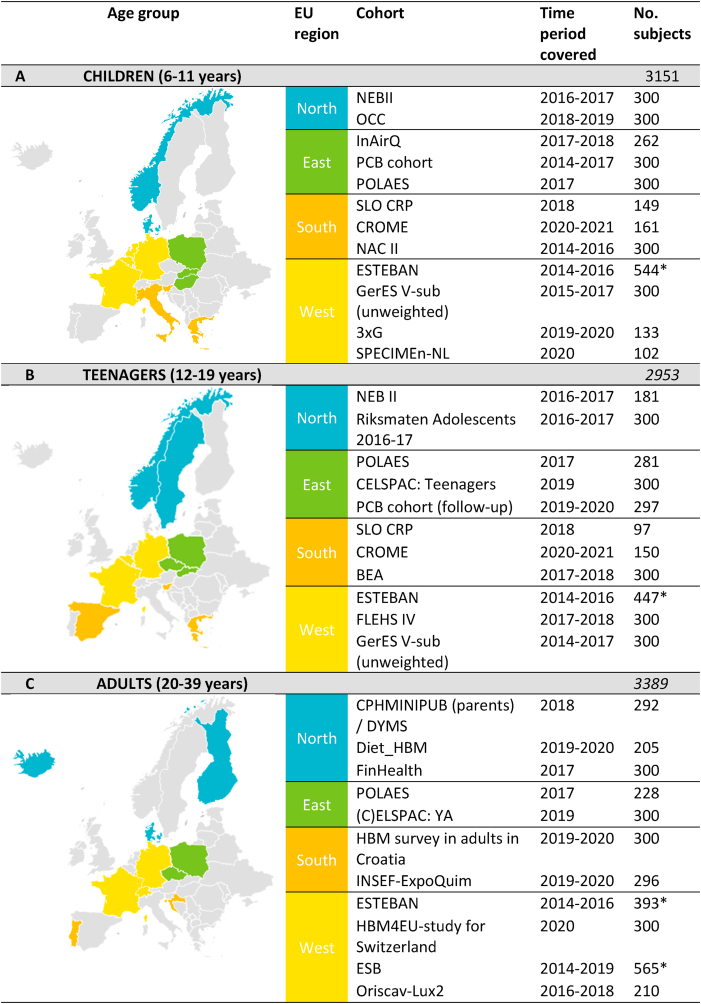
Fig. 1.
Studies participating in the joint HBM survey targeting children (A), teenagers (B) and adults (C) representing all 4 geographical subregions in Europe North, East, South and West Europe based on United Nations geoscheme subregions of Europe. * For ESTEBAN and ESB the total number of subjects >300 because the group of 300 subjects differs per substance group.
Table 1.
Sampling design: clustering of the Primary Sampling Units (PSU), selection of the PSUs, and sampling within the PSU
|
Age groups |
Target: 3 age groups:
|
|
Primary sampling unit (PSU) |
| Country based, 27* EU HBM4EU participating countries |
|
EU wide coverage (clustering of PSUs) |
PSU are clustered per EU geographical region**:
|
|
EU wide coverage (selection of PSU) |
Inclusion of number of PSU (countries) per region proportional to number of inhabitants of the region.
|
|
National representativeness |
|
|
Sampling design within the PSU |
|
*Current HBM4EU consortium consists of 30 countries including Israel (non-EU) and Estonia and Republic of North Macedonia who joined the HBM4EU consortium in a later stage, ** following the United Nations geoscheme subregion of Europe. DK = Denmark, FI = Finland, SE = Sweden, IS = Iceland, NO = Norway, LV = Latvia, LT = Lithuania, IE = Ireland, UK = United Kingdom, EE = Estonia, AT = Austria, BE = Belgium, NL = The Netherlands, FR = France, DE = Germany, CH = Switzerland, LU = Luxembourg, HR = Croatia, CY = Cyprus, EL = Greece, IT = Italy, PT = Portugal, Sl = Slovenia, ES = Spain, MK = North Macedonia, CZ = Czech Republic, PL = Poland, SK = Slovakia, HU = Hungary.
2.5. Final study selection
In practice the selection of HBM studies to be included in the HBM4EU survey started from an inventory of HBM studies in Europe composed at the start of the HBM4EU project (Institute of Environmental Health, 2019). Then inclusion and exclusion criteria presented in Table 2 were applied to evaluate the fitness of the studies to be aligned/included in the HBM4EU survey. A first selection was made to keep only studies that: (i) fit the proposed time period and age groups, (ii) targeted general population, no residents of hotspots (for the prioritized chemicals), no patient groups, no institutionalized civilians or specific occupational groups, (iii) included both male and female study subjects with a 50:50 ratio and (iv) are representative on a national (country) level. Based on these basic criteria a first overview of potential contributing studies was made. In a next step it was checked if per age group all 4 geographical regions were represented with a number of PSU (countries) per region that is proportional to the number of inhabitants of the region. 2 PSU for Northern Europe, 3–4 PSU for Western Europe, 3 PSU for Southern Europe, at least 1 PSU for Eastern Europe. At this point there were gaps for each age group. Ideally countries need to contribute with a representative sample within their country. Unfortunately, few nationally representative studies were identified that were ongoing or planned in Europe. Although national representativeness is the gold standard, it was not feasible to initiate a large number of nationally representative studies within the scope of HBM4EU. Therefore, to fill the gap we considered to also include regional studies, provided that they fulfilled all other aforementioned inclusion criteria. In an ideal scenario we would also use SES and residential degree of urbanisation as inclusion criteria i.e. each level (low, medium, high educational level and thinly, medium and highly populated areas) at least 10% represented. However, the candidate HBM studies for inclusion were limited and therefore it was decided to consider those as nice to have but not to keep them as strict criteria as it would result in too few studies that could be included.
Table 2.
Inclusion and exclusion criteria for participation in the joint HBM4EU survey.
| Inclusion criteria |
|
| Exclusion criteria |
|
2.6. Selection of exposure biomarkers
During the planning of HBM4EU, eight chemical groups were prioritized for studying environmental exposure in the European population. This was based on a systematic input from European and national policy makers and scientists. The specific policy needs for each of these chemical groups were identified in a consultation round (Ougier et al., 2021). Recent and comparable HBM data for Europe were requested for non-persistent organic pollutants (phthalates and Hexamoll® DINCH, bisphenols, PAHs, organophosphorus flame retardants (OPFRs)) and persistent pollutants (PFAS, brominated flame retardants (BFRs) and cadmium). To reduce costs it was decided to not analyse all exposure biomarkers in all age groups, but to analyse specific exposures in selected age groups taking into account (i) the potential exposure risk of the age groups, (ii) filling knowledge gaps and responding to policy questions, and (iii) interest of the participating countries.
The chemical exposures assessed in each age group are presented in Table 3. An expert group within HBM4EU selected the most relevant biomarkers and matrices (Vorkamp et al., 2021). Estimated exposure of children to BFRs and OPFRs via house dust and diet raises concern (Rantakokko et al., 2019; Van den Eede et al., 2011) therefore these analysis were prioritized in children in blood and urine respectively. Teenagers were selected as target group for PFAS exposure in blood because of their endocrine properties causing concern during puberty development (Terry et al., 2019). In addition, both children and teenagers were prioritized for urinary analysis of phthalates and Hexamoll® DINCH as EU wide information in this age group was lacking while modelled intake and HBM data from US and Germany suggest relatively high uptake in children (Li et al., 2019; Schwedler et al., 2020; Wang et al., 2019). In the adult age group exposure to cadmium, PAHs and bisphenols were prioritized in urine samples. Cadmium accumulates with age hence, the adults were selected as target population for analysis of cadmium. Exposure biomarkers of PAHs were measured in adults to obtain a comprehensive overview of internal exposure through air and dietary pathways. Exposure to bisphenols was measured in adults to cover a diversity of exposure pathways and vulnerability at reproductive age. Each country/HBM study could choose the age group in which they would participate. A summary of the targeted substances per PSU is shown in Table 3.
Table 3.
Overview of first set HBM4EU priority substance groups covered in joint HBM4EU survey.
| Substance group | Proposed analytes – included in QA/QC programme of HBM4EU | Age group | Sampled matrix | Country included in joint HBM4EU survey |
|---|---|---|---|---|
| Organophosphorus flame retardants | DPHP, BDCIPP, BCEP, BCIPP | children | urine | NO, DK, SK, Sl, FR, BE, DE |
| Brominated flame retardants | BDE-209, TBBPA, DBDPE, 2,4,6-TBP, BDE-47, BDE-153, DP-syn, DP-anti, α-HBCD, γ-HBCD | children | serum, plasma | NO, Sl, EL, FR |
| Phthalates | MEP, MBzP, MiBP, MnBP, MCHP, MnPeP, MEHP, 5OH-MEHP, 5oxo-MEHP, 5cx-MEPP, MnOP, OH-MiNP, cx-MiNP, OH-MiDP, cx-MiDP | children | urine | NO, DK, HU, SK*,PL, Sl, EL, IT, FR, DE*, NL, BE |
| teenagers | NO, SE, SK,PL, CZ, Sl, EL, ES, FR, DE*, BE | |||
| Hexamoll® DINCH | OH-MINCH, cx-MINCH | children | urine | NO, DK, HU, SK,PL, Sl, EL, IT, FR, DE*, NL, BE |
| teenagers | NO, SE, SK,PL, Sl, EL, ES, FR, DE*, BE | |||
| Per-/polyfluorinated compounds | PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFBS, PFHxS, PFHpS, PFOS (sum of all isomers) | teenagers | serum, plasma | NO, SE*, SK, Sl, EL, ES, FR*, DE, BE |
| Cadmium | Cd | adults | urine | DK, IS, PL, CZ, HR, PT, FR*, DE, LU |
| Polyaromatic hydrocarbons | 1-naphthol, 2-naphthol, 1,2 DHN, 2-FLUO, 3-FLUO, 9-FLUO, 1-PHEN, 2-PHEN, 3-PHEN, 4-PHEN, 9-PHEN, 1-PYR, 3-BaP | adults | urine | DK, IS, PL, CZ, HR, PT, FR*, CH, DE, LU |
| Bisphenols | BPA, BPS, BPF | adults | urine | DK, IS, FI, PL, CZ, HR, PT, FR*, CH, LU, DE |
* studies with data generated outside HBM4EU project i.e. PCB cohort (phthalates), ESTEBAN (PFAS in teenagers and PAH, bisphenols and cadmium in adults), Riksmaten Adolescents 2016–17 (PFAS), GerES V (phthalates and DINCH in children and teenagers, PFAS in teenagers). QA/QC = Quality assurance/Quality control, DK = Denmark, FI = Finland, SE = Sweden, IS = Iceland, NO = Norway, BE = Belgium, NL = The Netherlands, FR = France, DE = Germany, CH = Switzerland, LU = Luxembourg, HR = Croatia, EL = Greece, IT = Italy, PT = Portugal, Sl = Slovenia, ES = Spain, CZ = Czech Republic, PL = Poland, SK = Slovakia, HU = Hungary; MEP = Mono-ethyl phthalate, MBzP = Mono-benzyl phthalate, MiBP = Mono-isobutyl phthalate, MnBP = Mono-n-butyl phthalate, MCHP = Mono-cyclo-hexyl phthalate, MnPeP = Mono-n-pentyl phthalate, MEHP = Mono(2-ethylhexyl) phthalate, 5OH-MEHP = Mono(2-ethyl-5-hydroxyhexyl) phthalate, 5oxo-MEHP = Mono(2-ethyl-5-oxo-hexyl) phthalate, 5cx-MEPP = Mono(2-ethyl-5-carboxypentyl) phthalate, MnOP = Mono-n-octyl phthalate, OH-MiNP = 7-OH-(Mono-methyl-octyl) phthalate, cx-MiNP = 7-Carboxy-(mono-methylheptyl) phthalate, OH-MiDP = 6-OH-Mono-propyl-heptyl phthalate, cx-MiDP = Mono(2,7-methyl-7- carboxy-heptyl) phthalate, OH-MINCH = cyclohexane-1,2- dicarboxylate-mono-(7- hydroxy-4-methyl)octyl ester, cx-MINCH = cyclohexane-1,2- dicarboxylate-mono-(7- carboxylate-4- methyl)heptyl ester, DPHP = Diphenyl phosphate, BDCIPP = Bis(1,3-dichloro-2-propyl) phosphate, BCEP = Bis(2-chloroethyl) phosphate, BCIPP = bis(1-chloro-2-propyl) phosphate, BDE-209 = Polybrominated diphenylether 209, TBBPA = Tetrabromobisphenol A, DBDPE = Decabromodiphenylethane, 2,4,6-TBP = 2,4,6-Tribromophenol, BDE-47 = Polybrominated diphenylether 47, BDE-153 = Polybrominated diphenylether 153, DP-syn = Syn-dechlorane plus, DP-anti = Anti-dechlorane plus, α-HBCD = Hexabromocyclododecane alpha, γ-HBCD = Hexabromocyclododecane gamma, PFPeA = Perfluoropentanoic acid, PFHxA = Perfluorohexanoix acid, PFHpA = Perfluoroheptanoic acid, PFOA = Perfluorooctanoic acid, PFNA = Perfluorononanoic acid, PFDA = Perfluorodecanoic acid, PFUnDA = Perfluoroundecanoic acid, PFDoDA = Perfluorododecanoic acid, PFBS = Perfluorobutane sulfonic acid, PFHxS = Perfluorohexane sulfonic acid, PFHpS = Perfluoroheptane sulfonic acid, PFOS = Perfluorooctane sulfonic acid (sum of all isomers), cd = cadmium, BPA = Bisphenol A, BPS = Bisphenol S, BPF = Bisphenol F, 1-naphthol = 1-hydroxynaphthalene, 2-naphthol = 2-hydroxynaphthalene, 1,2 DHN = 1-,2-dihydroxynaphthalene, 2-FLUO = 2-hydroxyfluorene, 3-FLUO = 3-hydroxyfluorene, 9-FLUO = 9-hydroxyfluorene, 1-PHEN = 1-hydroxyphenanthrene, 2-PHEN = 2-hydroxyphenanthrene, 3-PHEN = 3-hydroxyphenanthrene, 4-PHEN = 4-hydroxyphenanthrene, 9-PHEN = 9- hydroxyphenanthrene, 1-PYR = 1-hydroxypyrene, 3-BaP = Benzo[a]pyrene.
2.7. Standardization and harmonization of procedures for sample transport and chemical analysis
Despite national studies entering the project in different phases, harmonization and quality assurance of some key aspects was undertaken for all participating studies. Standard protocols for recruitment, sampling, questionnaire development and sample transport were developed within HBM4EU and made available online at the HBM4EU website for each of the study phases (https://www.hbm4eu.eu/deliverables/, Deliverable 7.3 and Deliverable 7.6 (for standard operating procedures (SOPs) and guidelines for recruitment, sampling and questionnaires, Deliverable 7.2 (for sample transport)) (Fiddicke et al., 2021).
Some studies already collected their samples in the period between 2014 and 2019, those samples were biobanked (frozen at min. storage temp of −20 °C preferably −80 °C) and made available to the project. Other studies were ongoing and could not adjust their protocols as they were already approved by an ethics committee, while some studies still needed to be initiated and could develop their protocols according to the HBM4EU guideline protocol.
Transport of samples to the laboratories followed the appropriate SOP, which was developed within the framework of the HBM4EU initiative (Lermen et al., 2020). Additionally, the comparability and quality of the biomarker measurements performed within the joint HBM4EU survey is controlled at the EU level. Briefly, the biomarkers must be analysed in laboratories that successfully participated in the interlaboratory comparison investigation/external quality assurance scheme (ICI/EQUAS) organized as part of the HBM4EU initiative which is described by Esteban et al. (Esteban López, 2021). Participating analytical laboratories had to participate in at least two proficiency tests. This quality assurance scheme safeguards the reliability and comparability of analytical results. Laboratories from all European countries could participate in the ICI/EQUAS programme. HBM4EU provided an opportunity to check and improve the analytical methods and facilitated capacity building in Europe. Results of the cadmium and FR ICI/EQUAS are described by Nübler et al. and Dvorakova et al. respectively (Nubler et al., 2021; Dvorakova et al., 2021). All data coming from the joint HBM4EU survey have a data quality label assigned. Compounds for which the analysing laboratory obtained successful results are labelled as “Biomarker data quality assured by HBM4EU QA/QC programme”. Specific compounds for which the analyzing laboratory did not obtain successful results are labelled as “Biomarker data not quality assured by HBM4EU QA/QC programme”. Some of the contributing studies had already analysed some of the selected substance groups outside HBM4EU context. Those studies, indicated in Table 3, provided the already available data. As no real retrospective assessment of the proficiency and comparability of the data is possible, the Quality Assurance Unit (QAU) could not consider these results at the same level as those obtained within the QA/QC programme and therefore, the QAU provided a recommendation on how to deal with these available data. Different labels were attributed to those data generated before HBM4EU to distinguish them from the data analysed under HBM4EU. If the data was analysed in laboratories that later on obtained successful results under the QA/QC programme and used the same method with continuous internal quality assurance the data are labelled “Biomarker data generated before HBM4EU QA/QC programme but deemed comparable”, data generated by laboratories that did not participate or did not obtain successful results under the QA/QC programme are labelled “Biomarker data generated before HBM4EU QA/QC programme but not deemed comparable”. For the derivation of European exposure values only data quality approved under HBM4EU will be included.
2.8. Post-harmonization of questionnaire data
All participating studies were requested to collect information on socio-demographics and on substance specific environmental- and lifestyle-related exposure sources and exposure routes through questionnaires. The method applied to conduct the questionnaires differed between the individual studies (e.g. telephone assisted interview, paper questionnaires, …). New or ongoing studies that were still in the planning phase were requested to adapt their questionnaires to the standard HBM4EU questionnaire. Since the joint HBM4EU survey aligned both new/ongoing studies and recently conducted studies, a post-harmonization approach was applied to harmonize the collected questionnaire data. A specific expert working group provided advise on the harmonization rules for each variable based on prior experience in the process of post-harmonizing variables from the OBELIX (OBesogenic Endocrine disrupting chemicals: LInking prenatal eXposure to the development of obesity later in life) (Legler et al., 2011) and HELIX (Human Early-Life Exposome) project (Maitre et al., 2018). All the studies report their individual data in a similar way using a harmonized HBM4EU codebook which was developed centrally and is available online at the HBM4EU website (https://www.hbm4eu.eu/online-library/).
2.9. Exchange of personal HBM data on EU level conform the general data protection regulation
Since human biomonitoring data is considered as sensitive personal data, the collection and exchange of these type of data are subject to the general data protection regulation (GDPR, Regulation (EU) 2016/679) as of May 2018. Each of the participating studies of the HBM4EU survey collected personal characteristics, socio-demographic and lifestyle information of their study participants and the individual exposure biomarker levels. Subsequently, the data were transferred to a central database in encrypted format through an established HBM4EU webportal to ensure GDPR compliant exchange of individual personal data. The data is subjected to an extensive quality control process, checking for consistency between the provided variables and ensuring the data is correctly harmonized according to the HBM4EU codebook. After the quality control the individual data are integrated into a central database, hosted at the Flemish Institute for Technological Research NV (VITO), to create a pooled EU wide dataset per age group. Consequently, the data are processed to create additional variables in a uniform way such as the calculation of creatinine or specific gravity corrected biomarker values, imputed values for measurements below the limit of detection or quantification or calculation of sum parameters for biomarkers belonging to the same parent compound.
The role and responsibilities of VITO as a Data Processor (for the hosting activity) are stated in a bilateral processing agreement. Access to the individual data within the HBM4EU consortium is regulated via a single collaboration agreement i.e. joint data controller agreement between all supplying data controllers and receiving data controllers (Supplementary Figure 2). Access to the single measurement data is controlled at the individual user level. Users are provided with encrypted extracts of the database via the HBM4EU webportal, that only contain those variables required for the research question defined by the user in order to adhere to the data minimization principle laid down by the GDPR (Art5) (European Commission, 2016). In addition, sample and sample associated data exchange were covered with data and material transfer agreements signed by the responsible institutions.
2.10. Joint statistical analysis at EU level
Statistical analysis plans (SAPs) were developed describing the statistical approach that will be applied on the pooled European datasets. Within HBM4EU the following research objectives were defined: derivation of European reference values of internal exposure, comparing internal exposure levels between geographical regions, examination of determinants of internal exposure (personal characteristics, exposure sources, exposure routes), associating exposure biomarkers with personal health data and linking these associations through effect markers by causal pathway analysis. The use of the data for the research purposes described in the SAPs were formulated as part of the collaboration agreement established between all parties involved in the data exchange (i.e. supplying and receiving data controllers). For testing additional research hypotheses, a request should be submitted to the supplying data controllers, and bilateral data controller agreements should be established.
The joint HBM4EU survey builds further on existing capacity and expertise of human biomonitoring programs. The individual data collections are not perfectly homogeneous. There are differences in sampling year, season and for some main characteristics like age, sex and educational level. Moreover, not all HBM data are representative for their country. This has implications for the derivation of European reference values and the geographical comparison of the exposure levels between regions. To provide an estimate of the internal exposure of the European population for a specific age group, we will calculate internal exposure values for each exposure biomarker from the HBM4EU population sample as it is recruited. We will refer to these values as ‘European exposure values’ for internal exposure of the HBM4EU population. To obtain European exposure values for internal exposure the weighted geometric mean and 95th percentile (P95), and their 95% confidence interval will be calculated for each exposure biomarker. The population that has been sampled in the joint HBM4EU survey will be clearly described for characteristics that are known to influence exposure levels, i.e. age, sex, sampling year, sampling season, smoking habit and educational level. Descriptive characteristics will be presented for these parameters per participating data collection, per geographical region, and for the total population recruited. These main characteristics will be compared with Eurostat reference tables for the included countries and EU population to document observed differences between the HBM4EU sampled population and European population of the same age group (EUROSTAT, 2021). This information will allow comparison of the biomarker exposure values in HBM4EU with biomarker exposure values obtained in future monitoring campaigns in Europe or international monitoring programs, taking into account the characteristics of the sampled populations.
The joint HBM4EU survey results from a complex, stratified, multistage design survey. Due to that, data should be analysed using survey procedures that account for the complex survey design when calculating variance estimates. For the calculation of European exposure values, sample weights will be used to ensure that all geographical regions contribute proportionally to the number of inhabitants: North = 21%, East = 11%, South = 28% and West 40%. European exposure values will be calculated for the common set of analytically qualified exposure biomarkers (and sum-parameters) obtained in the contributing studies within one age group (i.e. children, teenagers, adults). For urinary biomarkers, geometric means and P95s will be calculated in μg/L and in μg/g creatinine, and μg/L corrected for specific gravity (SG) (only for teenagers); for lipid soluble blood biomarkers, geometric means and P95 will be calculated in μg/L and in μg/g lipid. The imputed biomarker data will be used, geometric means will only be estimated if at least 60% of the biomarker values are above the detection limits. For each biomarker, results will be given for the total population, and stratified by sex, educational level, degree of urbanisation and European region.
To formally test for geographical differences survey models will be fitted. Multiple linear regression models for the ln-transformed biomarkers will be built testing the fixed effect of EU region on the estimated geometric means, and quantile regression will be used to model the P95. Again, sample weights will be used to ensure that all geographical regions contribute proportionally to the number of inhabitants. As the samples of each PSU differ in population characteristics like age, sex, educational level, sampling year(s), sampling season, sampling type, etc., the models will be adjusted for those covariates that could influence the observed exposure values. Attention should be paid only to adjust for some basic covariates, and not for influencing factors that could possibly explain the differences observed between the regions. As a first step we will check if there are differences observed for the above mentioned covariates on the exposure biomarkers by regression models, per PSU and on the EU pooled database. If so, the models will be adjusted for these covariates. The overall p-value of region in the final model indicates if there is any significant difference observed for the estimated geometric mean/P95 levels of a biomarker between the EU regions. If the null hypothesis is not rejected (i.e. there were no significant differences between the regions at the 5% significance level), no further testing will be done. If the null hypothesis is rejected, further testing will be done comparing the geometric mean/P95 in each geographical region with the other geographical regions (all pairwise comparisons).
For the research questions on exposure determinants, associations between exposure and health effect, and pathway analysis, it will be evaluated whether pooled analysis of the data is suited, or if it is better to apply meta-analysis by first looking into the data per PSU and afterwards trying to combine the effect estimates. Regression models will be applied. Generalised additive models (GAMs) will be used to visualize the shape of the relationship between the exposure biomarkers and health effects. A directed acyclic graph (DAG) will be adopted for each exposure-effect association as a visual aid to check for relevant confounders and for completeness of the model. Mediation analysis will be considered to assess if a potential exposure-health effect association may be mediated by the selected molecular/clinical effect biomarkers.
2.11. Communication strategy
As HBM4EU is a collaborative research effort, at the science and policy interface, involving both the national- and the EU-level, respect for each other's role and needs is crucial, especially when communicating results. Communication of results to participants, and communication of country level results fall under the responsibility of the principle investigators of the contributing studies. For the interpretation and public communication of results on EU level a step-wise approach was developed. Key principles of the approach are: (i) results cannot be withheld or influenced, (ii) all actors involved must be informed in time and depending on their role have a say in the way results are communicated, (iii) national authorities are informed before public communication of results and (iv) respecting terms and conditions of national studies feeding their data into HBM4EU. The communication strategy should ensure that results and main key messages are supported by all partners which in turn can facilitate broad dissemination of results.
2.12. Ethics and data protection
All studies of the joint HBM4EU survey followed national and European ethics regulation. They all acquired approval from their country's ethics committees. Participation in all studies is voluntary, written informed consent was obtained from all participants and withdrawal from the study is possible at any time. Each study also confirmed that informed consent and approval were in place for secondary use of the collected data. New biomarker analysis in the frame of HBM4EU was covered by renewed ethics approvals in each country if the original approval did not cover surplus analyses. The project developed an inventory of all ethics and data protection approvals to ensure full compliance with EU requirements and also installed an Ethics Board. The data provider ensured legal and GDPR compliant use of data in pseudonymised format.
3. Results: a joint HBM4EU survey
The joint HBM4EU survey builds on the existing HBM capacity in Europe bringing together ongoing studies, that collected new samples and recently conducted studies, that provided biobanked samples. Because of these different scenarios of the participating HBM initiatives it was not feasible to implement a rigid scheme of mandatory protocols that would fit all participating countries. Instead the studies were aligned and post-harmonized as much as possible, to limit the effects on heterogeneity of the data, whilst respecting the countries individualities. Participating studies that were still in the planning/start-up phase were encouraged and supported to adhere as closely as possible to the recommendations for conducting a study developed in the frame of HBM4EU (HBM4EU D7.3 and D7.6) (Fiddicke et al., 2021).
3.1. Studies participating in the joint HBM4EU survey
An overview of the studies participating in the joint HBM survey is presented in Fig. 1. In total 34 studies from 21 countries took part in this initiative. Each participating study contributed with a maximum of 300 samples per age group. With the exception of a few studies that participated with a reduced number of subjects.
Under the joint HBM4EU survey 12 studies from 12 different countries targeting children between 6 and 11 years of age were brought together. For Northern Europe, NEBII (Norwegian Environmental Biobank II; Norway) and OCC (Odense Child Cohort; Denmark) are included. For Eastern Europe, InAirQ (Transnational Adaption Actions for Integrated Indoor Air Quality Management; Hungary), PCB cohort (Endocrine disruptors and health in children and teenagers in Slovakia; Slovakia) and POLAES (Polish Aligned Environmental Study; Poland) are included. For Southern Europe SLO CRP (Exposure of children and adolescents to selected chemicals through their habitat environment; Slovenia), CROME (Cross-Mediterranean Environment and Health Network; Greece) and NACII (Northern Adriatic cohort II; Italy) and for Western Europe, ESTEBAN (Étude de santé sur l'environnement, la biosurveillance, l'activité physique et la nutrition; France), GerES V-sub, unweighted (German Environmental Survey 2014-2017 subsample; Germany), 3xG (Gezondheid, Gemeenten, Geboorte studie; Belgium) and SPECIMEn-NL (Survey on PEstiCide Mixtures in Europe, The Netherlands) are included. Together they result in a total study population of 3151 children distributed across Europe (Fig. 1A).
Another 11 studies targeted teenagers between 12 and 19 years of age. For Northern Europe NEBII (Norwegian Environmental Biobank II; Norway) and Riksmaten Adolescents 2016–17 (Sweden) are included. For Eastern Europe, POLAES (Polish Aligned Environmental Study; Poland), CELSPAC: Teenagers (Central European Longitudinal Studies of Parents and Children: Teenagers; Czech Republic) (Piler et al., 2017) and PCB cohort follow-up (Endocrine disruptors and health in children and teenagers in Slovakia; Slovakia) take part in the survey. For Southern Europe, SLO CRP (Exposure of children and adolescents to selected chemicals through their habitat environment; Slovenia), CROME (Cross-Mediterranean Environment and Health Network; Greece) and BEA (Biomonitorización en Adolescentes; Spain) are included and for Western Europe, ESTEBAN (Étude de santé sur l'environnement, la biosurveillance, l'activité physique et la nutrition; France), GerES V-sub, unweighted (German Environmental Survey 2014-2017 subsample; Germany) and FLEHS IV (Flemish Environment and Health Survey IV; Belgium) participate. This results in a total population of 2953 teenagers distributed across Europe (Fig. 1B).
In addition, the following 11 studies are targeting adults between 20 and 39 years of age. CPHMINIPUB/DYMS (Copenhagen Minipuberty study (parents)/Danish Young Men Study; Denmark), Diet_HBM (Icelandic National Dietary Survey; Iceland), and FinHealth (Finland) are included to represent Northern Europe. (C)ELSPAC: YA (Central European Longitudinal Studies of Parents and Children: Young Adults; Czech Republic) and POLAES (Polish Aligned Environmental Study; Poland) represent Eastern Europe. INSEF-ExpoQuim (Exposure of the Portuguese Population to Environmental Chemicals: a study nested in INSEF, 2015; Portugal) and HBM survey in adults in Croatia (Implementation of Human Biomonitoring Survey In Adults in Croatia Using HBM4EU Methodology; Croatia) are included to represent Southern Europe and ESTEBAN (Étude de santé sur l'environnement, la biosurveillance, l'activité physique et la nutrition; France), ESB (Environmental Specimen Bank; Germany), HBM4EU-study Switzerland (Human Biomonitoring for Europe Program for Switzerland; Switzerland) and Oriscav-Lux2 (Observation des Risques et de la Santé Cardiovasculaire au Luxembourg; Luxembourg) are included to represent Western Europe. Together these studies result in a total adult population of 3389 subjects distributed across Europe (Fig. 1C).
Across all three age groups, a total of 9493 participants were recruited from 21 European countries covering a time period from 2014 to 2020. In these subjects exposure to HBM4EU priority chemicals are assessed. Aligning HBM studies across Europe is a dynamic process influenced by many factors. As a result, some studies deviate to some extent from the proposed age ranges or time period. Due to unexpected delays in the execution of the study, part of the samples collected in Greece for children and teenagers were sampled in 2021, and for PCB cohort from Slovakia children were recruited at age 11 but turned 12 by the time actual sampling could take place. It was decided to accept these exceptional deviations and to not discard those samples and data from the joint HBM4EU survey.
4. Discussion
The project's main goal is to coordinate and advance human biomonitoring in Europe to provide evidence for chemical policy making and to supply data to inform the EU citizens about their exposure to chemicals and associated risks (Ganzleben et al., 2017). The importance of this work is highlighted by the announcement of the EU green deal and the European Commission's new chemicals strategy for a toxic-free environment (European Commission, 2020). Reliable and comparable HBM exposure data representative of the EU population is indispensable to feed into chemical risk assessment and support chemical policy making. HBM4EU aims to lay the foundation for a European HBM platform that serves as a framework for sustainable and harmonized HBM conduct in Europe. The resulting data of this joint HBM4EU survey provides a baseline for chemical exposure of EU citizens to evaluate existing and upcoming chemicals policies. From the perspective of establishing a sustainable framework, it was decided to adopt an approach that builds further on existing capacity in Europe, whilst improving comparability of the data. This has several advantages, but also brings challenges. By choosing this approach it was not feasible to implement a rigid scheme of mandatory protocols. Instead a sampling framework was developed that facilitates the integration of existing HBM initiatives in Europe, bringing them together in a centrally coordinated joint HBM4EU survey and aligning them to serve common goals on the EU level.
4.1. Improving comparability of HBM data across europe
When comparing and interpreting biomonitoring data several aspects should be carefully considered such as (i) overall study design including target population and sampling period, (ii) sample collection, handling, storage and transport (iii) chemical analysis and (iv) data handling and presentation. Most differences between the studies aligned under the joint HBM4EU survey originate from the early/initial stages of study conduct. Thereafter, all participating studies followed the same HBM4EU procedures from the point of transport of samples to conclusion.
There are many HBM data available within Europe. However, the data are often disparate in terms of sample collection period (years), biological matrices and targeted population (age and sex of participants). Smolders et al. (2010) did a case-study as part of the INTARESE project where they collected individual HBM data on blood-lead exposure from more than 20.000 subjects from 8 European countries to evaluate the comparability of the data. They found that it is difficult to use disparate data collections because of the inherent variability with respect to the sex and age of participants and the time period (years) of sample collection. They highlighted that the need to get data from comparable (sub-)populations is essential for appropriate use and interpretation of HBM data for environmental health impact assessment (Smolders et al., 2010). By integrating studies into the joint HBM4EU survey that fit the sampling frame and fulfill the inclusion and exclusion criteria, as defined in Table 1, we improved harmonization of those key aspects across all contributing studies. The selected age groups are also aligned with the age groups as defined in NHANES and CHMS which can facilitate international comparison of EU results. The current HBM capacity in Europe did not allow to select a more restricted time period for the joint HBM4EU survey, therefore it was decided to include samples over a 7 year period (2014–2020). Towards future HBM surveys on European scale a more restricted time period aligned to international HBM cycles such as NHANES, CHMS should be strived for.
The present survey covers all 4 geographical areas of Europe with a minimum participation of 11 PSU (countries) per selected age group. Across all age groups we have 21 out of 31 different EU countries contributing to this study. Hence there is a significant EU appetite for such a work programme, especially considering countries had to secure 50% of the study budget via national funding channels. Aligning EU and national priorities in terms of target population and substances of interests are a key prerequisite for the success of this funding scheme. Collecting EU wide HBM data was feasible by including national representative as well as regional HBM initiatives. Geographical coverage was ensured by including PSUs from different geographical regions proportional to the % of EU inhabitants living in each region. When investigating and documenting HBM initiatives in Europe, eligible for inclusion into the joint HBM4EU survey, it became clear that HBM surveys with national representativity are scarce.
Moreover, different sample types are being used to assess pollutant levels in HBM initiatives. Longnecker et al. (2003) compared polychlorinated biphenyl (PCBs) levels across studies and pointed out that the use of different specimen types for analysis (i.e. serum, breast milk) complicates the comparability of HBM data (Longnecker et al., 2003). In the joint HBM4EU survey, the selected priority chemicals are all assessed in the same biological specimen (blood or urine) across the individual participating studies. The different PSU do have a mixture of blood-based matrices (serum, plasma) collected. Ehresman et al. demonstrated a 1:1 serum to plasma ratio for PFHS, PFOS, and PFOA (Ehresman et al., 2007). Given the lipophilic character of brominated flame retardants, they are associated with blood lipids, not with blood proteins, therefore results in serum and plasma are correlated, as blood lipid concentrations in serum/plasma are similar (Cholesterol and triglycer, 1977). The present study collects information of 60 exposure biomarkers from six chemical substance groups in urine and blood samples. When searching available HBM data different studies have often analysed a different set of biomarkers within a chemical substance group. Within the joint HBM survey we aimed to analyse a selected set of biomarkers for each substance group. Unfortunately, we could not identify a minimal set of biomarkers that could be analysed by all laboratories except for urinary cadmium and Hexamoll® DINCH metabolites (OH-MINCH and cx-MINCH). This is partly because the laboratories could choose for which metabolites they took part in the QA/QC programme and because not all laboratories had the capacity to measure all metabolites included in the HBM4EU QA/QC programme (Table 3). An important aspect of the joint HBM survey is to make full use of the expertise and experience available within the individual countries and to share this with each other. On the other hand, a major aim is to produce new results not only for the well-known markers but also for novel biomarkers. Balancing these 2 priorities, capacity building on one hand and project's ambition on the other hand, means we need to make compromises. To generate reliable biomonitoring data and reduce interlaboratory variability, samples for the joint HBM4EU survey were analysed in HBM4EU QA/QC laboratories, that successfully participated in the internal proficiency testing, only.
4.2. Establishing a sustainable HBM platform for europe built on existing capacity
By making use of existing HBM capacity within Europe, a bottom-up rather than top-down approach is used in developing a joint HBM4EU survey. Including existing HBM initiatives can ensure a more sustainable engagement of member states. Countries with a stronger tradition in HBM that have regular HBM campaigns can integrate and align their HBM initiative on EU level. They can continue their research activities and pursue goals on national level whilst expanding the study to meet specific objectives on EU level. This facilitates the comparison of their country results with EU results. The HBM4EU project provides the opportunity for countries without strong HBM tradition to initiate a new HBM study that fulfilled the inclusion criteria. They can make use of the HBM4EU developed guidelines and support of HBM experts. This is both time and budget wise a more efficient approach than initiating a completely new EU level HBM study as was done in the DEMOCOPHES project (Schindler et al., 2014). Moreover, this approach allows for the integration of HBM into existing Health Examination Surveys (HES) such as in Portugal (INSEF-ExpoQuim) or into nutritional surveys such as in Sweden (Riksmaten Adolescents, 2016–17) and Iceland (Diet_HBM) which can also be a cost effective method of implementing HBM (Moraeus et al., 2018).
As a result, the joint HBM4EU survey aligns a combination of (i) conducted studies with available biobanked samples, (ii) ongoing studies and (iii) studies in the planning phase. This poses some challenges: limited opportunities for upfront alignment and post-harmonization of the questionnaires is required. This is a very time-consuming process and has its limitations. Basic variables, required for each study participant, as listed in Supplementary Table 1 can be harmonized across all studies. More specific information e.g. consumption of canned food could not be harmonized for all studies since the information was not available in all studies. This is because the studies developed their questionnaire with the original study objectives in mind. When additional objectives such as analysing exposure to additional substance groups are set at a later stage, the questionnaires lack those more specific questions related to these new chemical exposures. This limits the possibilities for data analysis when studying for example, exposure determinants.
In addition, as a consequence of working with this combination of studies, the timelines of the different studies are not well synchronized at EU level. Getting results delivered by a common deadline is much more complicated when working with existing HBM initiatives as they all have their own priorities, timing and procedures in releasing/communicating results.
To date there are few EU countries that have a regular HBM survey at national level that can benefit from structured research funding. Most EU countries depend on non-recurrent project based funding for their HBM activities which complicates the development of a structured HBM monitoring system for Europe. Regular monitoring cycles every 2–3 years would be beneficial for evaluating time trends. EU support can help to boost HBM research in the member states. However, continued efforts are required to put HBM on the agenda of the EU member states, to ensure sustainable funding in order to safeguard success at both the national and EU level in providing current human exposure data to better protect citizens and the environment.
5. Conclusions
Although there are many HBM initiatives in Europe a harmonized, coordinated and sustainable European approach is currently lacking. There are several good practices of national HBM programs in Europe but they are aimed at national interests in terms of (sub-)populations included and measured biomarkers. However, as the responsibility for chemical policy lays at the EU level, national initiatives are less efficient to protect the respective citizens against chemical risks without cooperation at EU level. No overarching strategy exists, studies are not harmonized and aligned to common goals on EU level. To improve the comparability of European HBM data in support of European and national environment and health policy and measures, ideally European studies should follow similar protocols. Within the HBM4EU project it was one of the priorities to combine EU and national interests and build on existing HBM knowledge and capacities present in the member states rather than initiating new HBM studies as was done in the COPHES/DEMOCOPHES project (Schindler et al., 2014). Therefore, within the joint HBM4EU survey ongoing European HBM initiatives are aligned and harmonized to determine internal exposure levels to priority chemicals in the European population. The HBM4EU project and in particular the joint HBM4EU survey provides a platform to exchange best practices and improve and align HBM in Europe including data exchange and analysis. It also enables additional aspects to be included in this framework such as exploring the added value of effect biomarkers and measuring additional exposure biomarkers in the same sampling frame. The newly collected HBM data can also be used to improve and validate exposure modelling. Several aspects such as target population(s), biomarker analysis, data handling, statistical analysis, transparency on ethics requirements and protocols for reporting of results are aligned thereby improving inter-study/inter-country comparability of the data and acceptability of the results by policy makers. Furthermore, this joint survey might convince national authorities to further expand human biomonitoring within their country from regional level to national level. In conclusion, with this first large scale joint HBM4EU survey ongoing HBM initiatives in Europe are aligned and harmonized in an effort to take the first steps towards a sustainable European HBM platform in support of environment and health chemicals policy. Via the current approach we sampled 9493 participants across three age groups, and a total of 60 individual biomarkers have been assessed in subsets of this population, the resulting data will provide a baseline for chemical exposure of EU citizens to evaluate existing and upcoming chemicals policies.
Declaration of competing interest
The Authors declare that there is no conflict of interest. All co-authors have seen and agree with the contents of the manuscript and there is no financial interest to report. We certify that the submission is original work and is not under review at any other publication.
Acknowledgements
HBM4EU is co-financed under Horizon 2020 (grant agreement No 733032). The authors thank all principle investigators of the contributing studies for their participation and contribution to the joint HBM4EU survey and the national programme owners for their financial support. In addition we want to thank Dr. Liesbeth Bruckers and Dr. Michael Schümann.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijheh.2021.113809.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alimonti A. 2011. Programme for biomonitoring the Italian population exposure (PROBE): internal dose of metals; pp. 1–85. Rapp ISTISAN: [Google Scholar]
- Angerer J., Ewers U., Wilhelm M. Human biomonitoring: state of the art. Int. J. Hyg Environ. Health. 2007;210(3–4):201–228. doi: 10.1016/j.ijheh.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Bahadori T. Making sense of human biomonitoring data: findings and recommendations of a workshop. J. Expo. Sci. Environ. Epidemiol. 2007;17(4):308–313. doi: 10.1038/sj.jes.7500581. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) National health and nutrition examination survey. 2017. https://www.cdc.gov/nchs/data/factsheets/factsheet_nhanes.htm December 2017; Available from.
- Cerna M. Human biomonitoring in the Czech Republic: an overview. Int. J. Hyg Environ. Health. 2012;215(2):109–119. doi: 10.1016/j.ijheh.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Choi J. Major national human biomonitoring programs in chemical exposure assessment. Aims Environmental Science. 2015;2(3):782–802. [Google Scholar]
- Cholesterol and triglyceride concentrations in serum/plasma pairs. Clin. Chem. 1977;23(1):60–63. [PubMed] [Google Scholar]
- David M. Learning from previous work and finding synergies in the domains of public and environmental health: EU-funded projects BRIDGE Health and HBM4EU. Arch. Publ. Health. 2020;78:78. doi: 10.1186/s13690-020-00460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereumeaux C. The French human biomonitoring program: first lessons from the perinatal component and future needs. Int. J. Hyg Environ. Health. 2017;220(2 Pt A):64–70. doi: 10.1016/j.ijheh.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Dvorakova Darina. Interlaboratory comparison investigations (ICIs) and external quality assurance schemes (EQUASs) for flame retardant analysis in biological matrices: Results from the HBM4EU project. Environ. Res. 2021;202 doi: 10.1016/j.envres.2021.111705. [DOI] [PubMed] [Google Scholar]
- Ehresman D.J. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ. Res. 2007;103(2):176–184. doi: 10.1016/j.envres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Esteban López Marta. The European human biomonitoring platform - design and implementation of a laboratory quality assurance/quality control (QA/QC) programme for selected priority chemicals. Int. J. Hyg Environ. Health. 2021:234. doi: 10.1016/j.ijheh.2021.113740. May 2021. [DOI] [PubMed] [Google Scholar]
- European Commission Communication from the commission to the council, the European parliament, the European economic and social committee - The European Environment & Health Action Plan 2004-2010", in Official Journal C. 2004;49 [Google Scholar]
- European Commission . General Data Protection Regulation); 2016. Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons with Regard to the Processing of Personal Data and on the Free Movement of Such Data and Repealing Directive 95/46/EC; p. 88. [Google Scholar]
- European Commission Roadmap: chemicals – strategy for sustainability (toxic-free EU environment) 2020. https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/12264-Chemicals-strategy-for-sustainability- Available from.
- EUROSTAT . EUROSTAT Database On Population Demography. EUROSTAT; 2021. [Google Scholar]
- Fiddicke U. A Phased Approach for preparation and organization of human biomonitoring studies. Int. J. Hyg Environ. Health. 2021;232:113684. doi: 10.1016/j.ijheh.2020.113684. [DOI] [PubMed] [Google Scholar]
- Ganzleben C. Human biomonitoring as a tool to support chemicals regulation in the European Union. Int. J. Hyg Environ. Health. 2017;220(2 Pt A):94–97. doi: 10.1016/j.ijheh.2017.01.007. [DOI] [PubMed] [Google Scholar]
- UNESCO Institute for Statistics . vol. 88. UNESCO Institute for Statistics; Montreal, Quebec H3C 3J7 Canada: 2012. (International Standard Classification of Education ISCED 2011). [Google Scholar]
- Institute of Environmental Health, F.o.M.o.t.U.o.L. Portugal. HBM4EU Registry of HBM studies. 2019. http://hbmjps.topick.pt/ Available from.
- Legler J. The OBELIX project: early life exposure to endocrine disruptors and obesity. Am. J. Clin. Nutr. 2011;94(6 Suppl. l):1933S–1938S. doi: 10.3945/ajcn.110.001669. [DOI] [PubMed] [Google Scholar]
- Lermen D. Towards harmonized biobanking for biomonitoring: a comparison of human biomonitoring-related and clinical biorepositories. Biopreserv. Biobanking. 2020;18(2):122–135. doi: 10.1089/bio.2019.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis Dijkstra H.P. Working Papers. 2014. European Commission Directorate-General for Regional and Urban Policy (DG REGIO) A harmonised definition of cities and rural areas: the new degree of urbanisation. [Google Scholar]
- Li N. Lifelong exposure to multiple stressors through different environmental pathways for European populations. Environ. Res. 2019;179(Pt A):108744. doi: 10.1016/j.envres.2019.108744. [DOI] [PubMed] [Google Scholar]
- Longnecker M.P. Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environ. Health Perspect. 2003;111(1):65–70. doi: 10.1289/ehp.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre L. Human Early Life Exposome (HELIX) study: a European population-based exposome cohort. BMJ Open. 2018;8(9) doi: 10.1136/bmjopen-2017-021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraeus L. Riksmaten Adolescents 2016-17: a national dietary survey in Sweden - design, methods, and participation. Food Nutr. Res. 2018:62. doi: 10.29219/fnr.v62.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nubler S. Interlaboratory comparison investigations (ICI) and external quality assurance schemes (EQUAS) for cadmium in urine and blood: results from the HBM4EU project. Int. J. Hyg Environ. Health. 2021;234:113711. doi: 10.1016/j.ijheh.2021.113711. [DOI] [PubMed] [Google Scholar]
- Ougier E. Chemical prioritisation strategy in the European human biomonitoring initiative (HBM4EU) - development and results. Int. J. Hyg Environ. Health. 2021;236:113778. doi: 10.1016/j.ijheh.2021.113778. [DOI] [PubMed] [Google Scholar]
- Perez-Gomez B. BIOAMBIENT.ES study protocol: rationale and design of a cross-sectional human biomonitoring survey in Spain. Environ. Sci. Pollut. Res. Int. 2013;20(2):1193–1202. doi: 10.1007/s11356-012-1320-3. [DOI] [PubMed] [Google Scholar]
- Piler P. Cohort profile: the European longitudinal study of pregnancy and childhood (ELSPAC) in the Czech republic. Int. J. Epidemiol. 2017;46(5):1379. doi: 10.1093/ije/dyw091. 1379f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen O.M. Calculation and application of coverage intervals for biological reference values (Technical Report) Pure Appl. Chem. 1997;69(7) doi: 10.1351/pac199769071601. [DOI] [Google Scholar]
- Rantakokko P. Concentrations of brominated and phosphorous flame retardants in Finnish house dust and insights into children's exposure. Chemosphere. 2019;223:99–107. doi: 10.1016/j.chemosphere.2019.02.027. [DOI] [PubMed] [Google Scholar]
- Schindler B.K. The European COPHES/DEMOCOPHES project: towards transnational comparability and reliability of human biomonitoring results. Int. J. Hyg Environ. Health. 2014;217(6):653–661. doi: 10.1016/j.ijheh.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Schoeters G. Concept of the Flemish human biomonitoring programme. Int. J. Hyg Environ. Health. 2012;215(2):102–108. doi: 10.1016/j.ijheh.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Schulz C. Twenty years of the German Environmental Survey (GerES): human biomonitoring--temporal and spatial (West Germany/East Germany) differences in population exposure. Int. J. Hyg Environ. Health. 2007;210(3–4):271–297. doi: 10.1016/j.ijheh.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Schwedler G. Phthalate metabolites in urine of children and adolescents in Germany. Human biomonitoring results of the German Environmental Survey GerES V, 2014-2017. Int. J. Hyg Environ. Health. 2020;225:113444. doi: 10.1016/j.ijheh.2019.113444. [DOI] [PubMed] [Google Scholar]
- Smolders R. Availability and comparability of human biomonitoring data across Europe: a case-study on blood-lead levels. Sci. Total Environ. 2010;408(6):1437–1445. doi: 10.1016/j.scitotenv.2009.11.025. [DOI] [PubMed] [Google Scholar]
- Statistics Canada Canadian health measures survey (CHMS) 2018. https://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=5071 10-23; Available from.
- Terry M.B. Environmental exposures during windows of susceptibility for breast cancer: a framework for prevention research. Breast Cancer Res. 2019;21(1):96. doi: 10.1186/s13058-019-1168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations, S.D Standard country or area codes for statistical use (M49) 1999. https://unstats.un.org/unsd/methodology/m49/ Available from.
- Van den Eede N. Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environ. Int. 2011;37(2):454–461. doi: 10.1016/j.envint.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Vorkamp K. Biomarkers, matrices and analytical methods targeting human exposure to chemicals selected for a European human biomonitoring initiative. Environ. Int. 2021;146:106082. doi: 10.1016/j.envint.2020.106082. [DOI] [PubMed] [Google Scholar]
- Vrijheid Martine. European birth cohorts for environmental health research. Environ. Health Perspect. 2012;120:29–37. doi: 10.1289/ehp.1103823. https://pubmed.ncbi.nlm.nih.gov/21878421/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhu H., Kannan K. A review of biomonitoring of phthalate exposures. Toxics. 2019;7(2) doi: 10.3390/toxics7020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.