Abstract
Helicobacter fennelliae (formerly Campylobacter fennelliae) has been reported to cause bacteremia in homosexual men with or without human immunodeficiency virus (HIV) infection. We report here a 48-year-old, non-HIV-infected, heterosexual man with diabetes mellitus and cirrhosis of the liver who developed bacteremia and septic shock due to H. fennelliae. The patient was treated successfully initially with intravenous ampicillin-sulbactam and ceftazidime, followed by ampicillin-sulbactam only. These agents were active in vitro against the isolate by E-test results. To our knowledge, this is the first documented case of septic shock due to H. fennelliae in a non-HIV-infected, heterosexual, immunocompromised patient.
Helicobacter fennelliae, formerly known as Campylobacter fennelliae, has been isolated from rectal swabs of asymptomatic and symptomatic (proctitis, proctocolitis, or enteritis) homosexual men with or without human immunodeficiency virus (HIV) infection (7, 8, 11, 13, 15, 16). Bacteremia caused by this organism in HIV-infected patients has also been previously reported (8, 9, 11). We describe here an immunocompromised patient with bacteremia associated with septic shock due to H. fennelliae. To our knowledge, this is the first documented case of septic shock due to H. fennelliae in a non-HIV-infected heterosexual patient.
Case report.
A 48-year-old heterosexual man with cirrhosis of the liver and diabetes mellitus was hospitalized with a 2-week history of fever, progressive abdominal fullness, and dyspnea. Disseminated Penicillium marneffei infection involving the lung (pneumonia and pleural effusion), peritoneum (peritonitis and ascites), and lymph nodes (cervical lymphadenitis) was diagnosed. His HIV antibody test results by enzyme immunoassay (ICE* HIV-1.O.2; Murex Biotech Limited, Dartford, United Kingdom) and Western blot (NEW LAV-BLOT 1; Sanofi Diagnostics Pasteur, Marnes-La-Coquette, France) were negative. He received amphotericin B treatment (1 mg/kg of body weight/day), and his condition remained stable. Unfortunately, 3 weeks after initiation of amphotericin B treatment (accumulative dose, 650 mg), high fever (40°C) accompanying shaking chills, drowsiness, dyspnea, and hypotension (systolic blood pressure, 60 mm Hg) developed. Laboratory data revealed leukocytosis, lactic acidosis, and deterioration of renal and liver functions. A chest radiograph showed complete resolution of previous pneumonic patches and pleural effusion, and there were no gastrointestinal symptoms. The patient was transferred to the medical intensive care unit and was treated empirically with ampicillin-sulbactam (6 g/day) and ceftazidime (6 g/day). Two sets of blood cultures obtained on the day of septic shock yielded large, elongated, gram-negative rods with spiral or helical morphology (Fig. 1) from two BACTEC 6A bottles (Becton Dickinson, Sparks, Md.) after 5 days of incubation. During treatment with ampicillin-sulbactam and ceftazidime for 5 days and ampicillin-sulbactam (6 g/day) only for another 9 days, the fever gradually subsided and clinical symptoms resolved. Blood cultures drawn 2 days after completion of antibiotic therapy were sterile. Stool and rectal swab cultures for H. fennelliae were obtained after the notification of positive blood cultures. The patient received a total of 2 g of amphotericin B, followed by itraconazole (400 mg/day) for another 3 months for P. marneffei infection. He was well at follow-up 6 months after discharge.
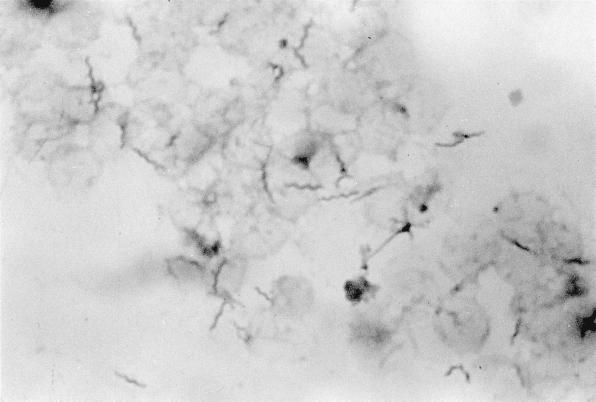
FIG. 1.
Gram-negative helical bacilli in a BACTEC 6A aerobic bottle cultured from a blood specimen of a 48-year-old, heterosexual, non-HIV-infected patient with septic shock (Gram stain with 1% carbolfuchsin as counterstain; magnification, ×1,000).
Microbiology.
The bacterium recovered had a positive culture signal with growth indexes of 45 and 42, respectively, in two BACTEC 6A aerobic bottles but no growth in two BACTEC 7A anaerobic bottles. Upon subculture to Trypticase soy agar supplemented with 5% sheep blood, CDC blood agar, and chocolate agar (BBL Microbiology Systems), no growth was found after 48 h of incubation at 25 or 37°C under ambient air, CO2 incubator, or anaerobic conditions. When incubating in a microaerobic atmosphere (5% O2, 10% CO2, 85% N2), the bacterium grew well at 37°C (but did not grow at 25 or 42°C) on both CDC blood agar and chocolate agar. Poor growth was noted on Trypticase soy agar supplemented with 5% sheep blood. Scanty growth at 37°C and no growth at 42°C on CAMPY BAP (containing the following five antimicrobial agents: cephalothin [15 μg/ml], trimethoprim [5 μg/ml], polymyxin [2.5 U/ml], vancomycin [10 μg/ml], and amphotericin B [2 μg/ml]; BBL Microbiology Systems) was found after a 48-h incubation in a microaerobic atmosphere. Colonies on chocolate agar were 2 to 3 mm in diameter and were gray, convex, and circular with a hypochlorite smell. Isolates were gram-negative curved or helical bacilli and were positive for both oxidase and catalase reactions. Nitrate reduction, hippurate hydrolysis, urease reactions, and H2S production in triple sugar iron agar (BBL Microbiology Systems) were negative. The organism was susceptible to cephalothin (30-μg disk) and nalidixic acid (30-μg disk). Cellular morphotypes, growth characteristics, biochemical profiles, and susceptibility results for the above two agents were in agreement with those of H. fennelliae (7, 14, 16). Stool and rectal swab cultures for this organism on chocolate agar and CDC blood agar plates at 37°C under microaerobic conditions after 5 days of incubation grew moderate amounts of several species of bacteria and yeasts but were negative for H. fennelliae.
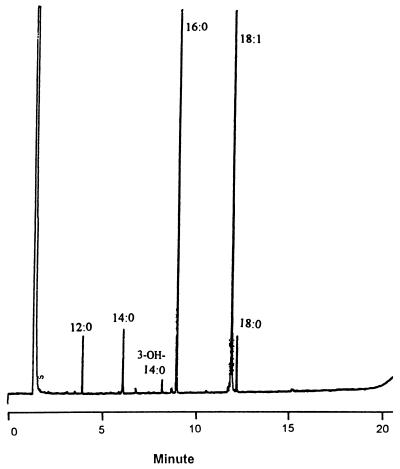
Cellular fatty acid methyl esters of the isolate were analyzed with the MIDI automated microbial identification system, as previously described (5, 6). As shown in Fig. 2, the isolate consisted of large amounts (≥3%) of hexadecanoic acid (16:0), octadecenoic acid (18:1), tetradecanoic acid (14:0), octadecanoic acid (18:0), and dodecanoic acid (12:0) and small amounts (≦3%) of 3-hydroxytetradecanoic acid (3-OH-14:0). The chromatogram (Lambert group E) of the isolate can be differentiated from that for Helicobacter cinaedi by the presence of 3-OH-14:0 and the absence of 3-OH-12:0 (3-hydroxydodecanoic acid) (10, 12).
FIG. 2.
Gas chromatogram of cellular fatty acid methyl esters of H. fennelliae. See the text for compound abbreviations and designations.
Susceptibilities of the isolate to seven antimicrobial agents were tested by the E test (PDM Epsilometer; AB Biodisk, Solna, Sweden) on CDC blood agar and chocolate agar plates (BBL Microbiology Systems), and the results were read after 48 h of incubation at 37°C in a microaerobic atmosphere (4). The isolate was β-lactamase negative by cefinase disk (BBL Microbiology Systems). The MICs of the seven agents were identical on the two media and were as follows: ampicillin-sulbactam, 0.38 μg/ml; ceftazidime, 0.75 μg/ml; ceftriaxone, 0.094 μg/ml; ciprofloxacin, 0.5 μg/ml; clarithromycin, 4 μg/ml; trimethoprim-sulfamethoxazole, 0.5 μg/ml; gentamicin, 1.5 μg/ml.
This is the first documented case of H. fennelliae bacteremia in a non-HIV-infected patient or in a heterosexual patient and the first reported case of septic shock due to infection caused by this organism. The nearly complete resolution of systemic fungal infection prior to acquisition of H. fennelliae, the absence of other newly developed infections, and the patient’s apparent response to antimicrobial therapy suggest that H. fennelliae caused the severe septic complication. Though ours was not an AIDS patient or an HIV-infected homosexual or bisexual man, his underlying immunoincompetence due to cirrhosis of the liver and diabetes mellitus, as well as preexisting disseminated fungal infections, might have contributed to the occurrence of bacteremia complicated with septic shock caused by this organism.
The portal of entry of H. fennelliae in our patient was not readily apparent. H. cinaedi has been reported to gain access to the host through the gastrointestinal tract or via direct inoculation into skin (cellulitis) and joints (arthritis) (1, 3, 6, 11, 13). This organism is found in 8% of stool cultures from homosexual men without diarrhea, but it is not found in stool cultures from heterosexual men and women (2, 14). Despite the original association between H. cinaedi bacteremia and diarrhea, gastrointestinal symptoms have rarely been encountered in patients with bacteremia due to H. fennelliae or H. cinaedi (1, 14). Our patient had no gastrointestinal symptoms or skin and soft-tissue lesions prior to or during the bacteremic episode, and microbiological investigations of fecal specimens and rectal swab samples were all negative for the organism.
Several investigators have noted difficulty in initial staining of H. cinaedi and H. fennelliae in blood culture bottles (2, 11). In these studies, the organisms were demonstrated only upon dark-field examination or acridine orange staining smears. We did not experience difficulty visualizing these organisms in the nonradiometric BACTEC blood culture system (6). However, the replacement of safranin by 1% carbolfuchsin in the Gram-staining preparation improved visualization of these organisms.
Susceptibility tests for H. cinaedi or H. fennelliae are not standardized. However, a wide variety of antimicrobial agents, including ceftriaxone, gentamicin, rifampin, doxycycline, and ciprofloxacin have in vitro activity against these organisms and have also been demonstrated to be effective in vivo (2, 6, 11–13). The susceptibility pattern of our isolate partly agreed with the previous observations of H. fennelliae.
In conclusion, this report describes H. fennelliae bacteremia in a non-HIV-infected heterosexual man and demonstrates the capacity of this organism to cause septic shock. H. fennelliae should be considered in clinical contexts, including HIV-infected homosexual men with proctocolitis or bacteremia and patients with other immunocompromised conditions manifesting bacteremia with or without severe septic complications.
REFERENCES
- 1.Burman W J, Cohn D L, Reves R R, Wilson M L. Multifocal cellulitis and monoarticular arthritis as manifestations of Helicobacter cinaedi bacteremia. Clin Infect Dis. 1995;20:564–570. doi: 10.1093/clinids/20.3.564. [DOI] [PubMed] [Google Scholar]
- 2.Cimolai N, Gill M J, Jones A, Flores B, Stamm W E, Laurie W, Madden B, Shahrabadi M S. “Campylobacter cinaedi” bacteremia: case report and laboratory findings. J Clin Microbiol. 1987;25:942–943. doi: 10.1128/jcm.25.5.942-943.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flores B M, Fennell C L, Kuller L, Bronsdon M A, Morton W R, Stamm W E. Experimental infection of pig-tailed macaques (Macaca nemestrina) with Campylobacter cinaedi and Campylobacter fennelliae. Infect Immun. 1990;58:3947–3953. doi: 10.1128/iai.58.12.3947-3953.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsueh P R, Teng L J, Yang P C, Ho S W, Luh K T. Indwelling device-related bacteremia caused by serum-susceptible Campylobacter coli. J Clin Microbiol. 1997;35:2178–2180. doi: 10.1128/jcm.35.8.2178-2180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsueh P R, Teng L J, Yang P C, Wang S K, Chang S C, Ho S W, Hsieh W C, Luh K T. Bacteremia caused by Arcobacter cryaerophilus 1B. J Clin Microbiol. 1997;35:489–491. doi: 10.1128/jcm.35.2.489-491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hung C C, Hsueh P R, Chen M Y, Teng L J, Chen Y C, Luh K T, Chuang C Y. Bacteremia caused by Helicobacter cinaedi in an AIDS patient. J Formos Med Assoc. 1997;96:558–560. [PubMed] [Google Scholar]
- 7.Jerris R C. Helicobacter. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: American Society for Microbiology; 1995. pp. 492–498. [Google Scholar]
- 8.Kemper C A, Mickelsen P, Morton A, Walton B, Deresinski S C. Helicobacter (Campylobacter) fennelliae-like organisms as an important but occult cause of bacteraemia in a patient with AIDS. J Infect. 1993;26:97–101. doi: 10.1016/0163-4453(93)97128-k. [DOI] [PubMed] [Google Scholar]
- 9.Kiehlbauch J A, Brenner D J, Cameron D N, Steigerwalt A G, Makowski J M, Baker C N, Patton C M, Wachsmuth I K. Genotypic and phenotypic characteristics of Helicobacter cinaedi and Helicobacter fennelliae strains isolated from humans and animals. J Clin Microbiol. 1995;33:2940–2947. doi: 10.1128/jcm.33.11.2940-2947.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert M A, Patton C M, Barrett T J, Moss C W. Differentiation of Campylobacter and Campylobacter-like organisms by cellular fatty acid composition. J Clin Microbiol. 1987;25:706–713. doi: 10.1128/jcm.25.4.706-713.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng V L, Hadley W K, Fennell C L, Flores B M, Stamm W E. Successive bacteremia with “Campylobacter cinaedi” and “Campylobacter fennelliae” in a bisexual male. J Clin Microbiol. 1987;25:2008–2009. doi: 10.1128/jcm.25.10.2008-2009.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orlicek S L, Welch D F, Kuhls T L. Septicemia and meningitis caused by Helicobacter cinaedi in a neonate. J Clin Microbiol. 1993;31:569–571. doi: 10.1128/jcm.31.3.569-571.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Totten P A, Fennell C L, Tenover F C, Wezenberg J M, Perine P L, Stamm W E, Holmes K K. Campylobacter cinaedi (sp. nov.) and Campylobacter fennelliae (sp. nov.): two new Campylobacter species associated with enteric disease in homosexual men. J Infect Dis. 1985;151:131–139. doi: 10.1093/infdis/151.1.131. [DOI] [PubMed] [Google Scholar]
- 14.Trivett-Moore N L, Rawlinson W D, Yuen M, Gilbert G L. Helicobacter westmeadii sp. nov., a new species isolated from blood cultures of two AIDS patients. J Clin Microbiol. 1997;35:1144–1150. doi: 10.1128/jcm.35.5.1144-1150.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandamme P, Falsen E, Pot B, Kersters K, de Ley J. Identification of Campylobacter cinaedi isolated from blood and feces of children and adult females. J Clin Microbiol. 1990;28:1016–1020. doi: 10.1128/jcm.28.5.1016-1020.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandamme P, Falsen E, Rossau R, Hoste B, Segers P, Tytgat R, de Ley J. Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: emendation of generic descriptions and proposal of Arcobacter gen. nov. Int J Syst Bacteriol. 1991;41:88–103. doi: 10.1099/00207713-41-1-88. [DOI] [PubMed] [Google Scholar]