Abstract
Background:
The manifestation of multiple sclerosis (MS) in childhood and adolescence occurs in 3%−5% of all MS cases. However, the immunomodulatory and symptomatic treatment options in this population group are still limited.
Objective:
We aimed to elucidate the prescription frequency of medications used in pediatric patients with multiple sclerosis (PwMS) compared with the general population, considering the entire spectrum of medications prescribed.
Methods:
Based on nationwide outpatient drug prescription data and statutory health insurance (SHI) physicians’ claims data from 2018, we conducted a population-based cross-sectional study in Germany. Children and adolescents aged ⩽17 years (n = 11,381,939) diagnosed with MS (n = 613), and a matched (age, sex, and health insurance sector) control group (n = 6130) were included. The prescription prevalence was measured as the proportion of MS patients with ⩾1 prescription.
Results:
Of the 613 pediatric PwMS with a median age of 16 years, 403 (65.7%) were female. For 15 out of the 18 different active agents analyzed, PwMS had a significantly higher prescription prevalence than the control group (Fisher’s exact test: p ⩽ 0.037). The most frequently prescribed drugs in PwMS were ibuprofen (28.4%; anti-inflammatory drug), cholecalciferol (23.0%; vitamin D3), and interferon beta-1a (21.5%; disease-modifying drug, DMD). The proportions of DMD prescriptions and antibiotic prescriptions were higher among PwMS aged 15–17 years than among those ⩽14 years (DMD: 43.4% vs 34.2%, p = 0.05; antibiotic: 34.1% vs 24.8%, p = 0.031). In contrast, younger PwMS were more likely to receive a prescription for anti-inflammatory/anti-rheumatic drugs (36.6% vs 26.5%, p = 0.02).
Conclusion:
Our study analyzing real-world medication data showed that interferon beta, anti-inflammatory drugs, and vitamins play an essential role in the treatment of pediatric PwMS. Future research should evaluate longitudinal treatment patterns of pediatric PwMS, paying particular attention to the time of diagnosis, time of first DMD initiation, and therapy switches.
Keywords: disease-modifying drug, multiple sclerosis, pediatrics, prescription, treatment
Introduction
Multiple sclerosis (MS) is the most common neuroimmunological disease in young adults. 1 The etiology of MS is not yet clarified. There is no cure, but the clinically highly heterogeneous MS symptoms 2 can be adequately treated using an increasing number of immunomodulatory and symptomatic drugs. 1 An estimated 3%−5% of patients with multiple sclerosis (PwMS) have a disease onset before the age of 18 years and are referred to as pediatric MS patients. 3 The diagnosis prevalence of MS in German pediatric outpatients (aged ⩽ 17 years) was recently published for 2018 and is 5.4 per 100,000 insured. 4 Differences between pediatric and adult MS patients are apparent with regard to clinical symptoms and the course of MS. 5 For instance, a progressive disease course is more likely in adults than in pediatric patients. 3 The degree of disability remains relatively low in pediatric PwMS in the first 10 years after the onset of MS. However, higher relapse rates are common in the initial stage of pediatric MS. Severe disability levels are reached at a younger age in children and adolescents compared to adult patients.3,6 In this respect, the treatment regimen plays a particularly important role and differs between adult and pediatric MS.
Although an early initiation of disease-modifying drugs (DMDs) is recommended in pediatric PwMS to prevent the rapid progression of disability, 7 licensed treatment options in this age group are still limited. Relapse rate–reducing baseline DMDs with favorable safety profiles, such as interferon beta and glatiramer acetate, are common drugs for pediatric patients in Europe.6,7 In Germany, these DMD preparations are approved for MS treatment in patients aged ⩾ 12 years. Only the interferon beta-1a preparation Rebif® received approval for use in patients aged ⩾ 2 years. 8 Escalation therapeutics, such as natalizumab and fingolimod, are used when disease activity does not decrease despite baseline therapy or when highly active MS courses are present. 9 Fingolimod was the first DMD studied in a global, randomized controlled phase-III trial with 215 pediatric PwMS (PARADIGMS) and showed superiority over interferon beta in terms of efficacy. 10 However, severe side effects, such as seizures, leukopenia, and hypersensitivity reactions, occurred in some patients. 6 Fingolimod has been approved to treat highly active relapsing-remitting MS in children and adolescents aged ⩾ 10 years in 2018.11,12 To date, this is the latest DMD approval expansion regarding pediatric patients. However, the most experience in the treatment of highly active MS in pediatric patients is available for natalizumab (off-label use). 6 In addition to DMDs, targeted symptomatic therapy is an essential component of MS treatment management. The pediatric MS symptomatology is multifaceted and can range, for example, from motor disability and fatigue to cognitive as well as emotional disturbances. 13 Therefore, various therapeutic strategies to treat symptoms are required. On one hand, specific symptomatic therapy can prevent or reduce impairments in functional abilities and quality of life. 1 On the other hand, it can reduce the development of secondary physical impairment.
Adequate and individualized therapy for pediatric PwMS is challenging because children and adolescents differ significantly from adults in their pharmacokinetics (e.g. volume of distribution, mechanism, and rate of drug elimination). Real-world data are needed to gain further experience and insight into therapy efficacy and safety in pediatric MS and to improve existing therapy strategies or to develop new ones. The objective of this study was to characterize drug utilization patterns in German pediatric PwMS compared to the general population of the same age, using real-world prescription data from almost 90% of the German population. Moreover, we aimed to identify prescribing differences in subgroups of pediatric PwMS stratified by age.
Materials and methods
Data acquisition
The findings presented in this paper are based on pseudonymized outpatient physicians’ claims data and nationwide outpatient prescription data for the year 2018 of the German statutory health insurance (SHI). The SHI covers roughly 87% of German residents (aged ⩽ 17 years (2018): n = 11,381,939). Data analyzed included sociodemographic and clinical patient characteristics, such as sex, age, place of residence, and outpatient diagnoses coded according to the International Classification of Diseases and Related Health Problems (ICD-10), including a modifier describing the diagnostic certainty (“confirmed”, “suspected”, “status post”, “excluded”). Pharmaceutical prescription information comprised the Anatomical Therapeutic Chemical (ATC) code, dates of prescription and dispensation, packaging size, strength, and formulation, as well as the generic and trade names.
The use of claims data for scientific research is regulated by the Code of Social Law (SGB V) in Germany. An ethical approval and informed consent are not required as this study used routinely collected anonymized data. The research was conducted in accordance with the Helsinki Declaration (in its current revised form: 64th WMA General Assembly, Fortaleza, Brazil, October 2013).
Inclusion criteria
PwMS were identified according to the ICD-10 GM code “G35-” (multiple sclerosis). Children and adolescents aged ⩽ 17 years (according to the age criteria of the International Pediatric MS Study Group) 14 were identified as pediatric PwMS if they had a diagnosis of MS classified as “confirmed” in ⩾1 quarter of the year 2018 (n = 613).
Drug classification
Active substances were classified using the ATC classification system and were categorized into pre-specified drug groups (Table 1). Substances not captured by pre-specified drug groups were then divided into three-digit ATC therapeutic subgroups (Table 2). Active substances, pre-specified drug groups, or three-digit ATC therapeutic subgroups prescribed to <30 patients were not analyzed due to data protection regulations. Prescription patterns in MS were investigated using a case–control design. Cases were matched to randomly selected controls by age, sex, and region of residence (17 regions of Associations of Statutory Health Insurance Physicians, ASHIPs, with a case-to-control ratio of 1:10). The controls (n = 6130) used ambulatory health care in 2018 for reasons other than MS (i.e. they were not diagnosed with MS in 2018 and previous years).
Table 1.
Pre-specified drug groups in pediatric patients with multiple sclerosis in Germany (2018).
| Pre-specified drug groups | Active substance* | ATC code |
|---|---|---|
| Analgesics | ||
| Dipyrone sodium | N02BB02 | |
| Antibiotics | ||
| Amoxicillin | J01CA04 | |
| Azithromycin | J01FA10 | |
| Cefuroxime | J01DC02 | |
| Anti-inflammatory + antirheumatic drugs | ||
| Ibuprofen | M01AE01 | |
| MS baseline drugs | ||
| Glatiramer acetate | L03AX13 | |
| Interferon beta-1a | L03AB07 | |
| Interferon beta-1b | L03AB08 | |
| MS escalation drugs | ||
| Fingolimod | L04AA27 | |
| Natalizumab | L04AA23 | |
| MS relapse drugs | ||
| Prednisolone | H02AB06 | |
ATC, anatomical therapeutic chemical; MS, multiple sclerosis; * active substances prescribed for at least 30 patients.
Table 2.
Further drug groups in pediatric patients with multiple sclerosis in Germany (2018).
| ATC-3 subgroups | ATC-3 code | Active substance* | Full ATC code |
|---|---|---|---|
| Acne drugs | D10 | ||
| Cough and cold drugs | R05 | ||
| Noscapine | R05DA07 | ||
| Drugs for acid-related disorders | A02 | ||
| Omeprazole | A02BC01 | ||
| Pantoprazole | A02BC02 | ||
| Drugs for functional gastrointestinal disorders | A03 | ||
| Drugs for obstructive airway diseases | R03 | ||
| Salbutamol | R03AC02 | ||
| Nasal preparations | R01 | ||
| Ophthalmological drugs | S01 | ||
| Sex hormones and modulators of the genital system | G03 | ||
| Levonorgestrel + ethinylestradiol | G03AA07 | ||
| Topical dermatological corticosteroids | D07 | ||
| Vaccines | J07 | ||
| Papillomavirus vaccine | J07BM03 | ||
| Vitamins | A11 | ||
| Cholecalciferol | A11CC05 |
ATC, Anatomical Therapeutic Chemical; * active substances prescribed for at least 30 patients.
Statistics
The prescription prevalence (proportion of patients and controls with ⩾1 prescription in 2018) was calculated for active substances, pre-specified drug groups, and three-digit ATC therapeutic subgroups. In addition, we calculated the mean number of drug prescriptions (for active substances and three-digit ATC therapeutic subgroups) per patient with a respective prescription. Fisher’s exact test and chi-square test were used to compare the prescription prevalence between the MS patients and the controls and between two age groups (⩽14 years vs 15–17 years). The significance level was defined as α = 0.05. In addition, to consider alpha error accumulation in multiple testing, a p value adjustment was conducted according to the false discovery rate (FDR) of 5% 15 (Supplementary Table 1).
Results
Sociodemographic and clinical characterization of the patient population
Of the 613 pediatric PwMS, 161 (26.3%) were aged ⩽ 14 years, and 452 (73.7%) were 15–17 years old. The median age was 16 years (interquartile range (IQR): 3 years), and the proportion of female patients amounted to 65.7% (n = 403). Female PwMS were significantly more often aged 15–17 years than male PwMS (77.4% vs 66.7%; p = 0.005). The median number of three-digit ICD-10-codes excluding “G35” in 2018 was 10 in PwMS (IQR: 9) and 6 in the control patients (IQR: 7).
Drugs prescribed
In total, 335 PwMS were prescribed a DMD (54.6%). The majority of those patients was aged 15–17 years (n = 280; 83.6%). In this group of 280 PwMS, MS baseline drugs were prescribed to 196 patients (70.0%), and escalation DMDs were prescribed to 84 patients (30.0%).
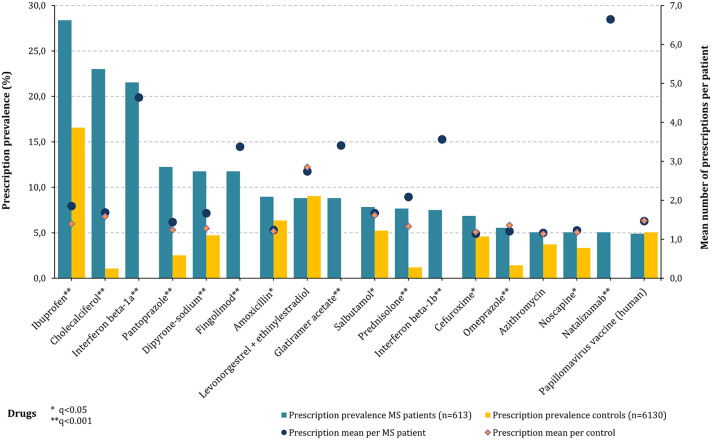
Among pediatric PwMS, a total of 5603 prescriptions were recorded in 2018. The drugs with the highest prescription prevalence among pediatric PwMS (n = 613) were ibuprofen (28.4%; non-steroidal anti-inflammatory drug, NSAID), cholecalciferol (23.0%; vitamin D3), interferon beta-1a (21.5%; baseline DMD, subcutaneous and intramuscular application), pantoprazole (12.2%; antacid), dipyrone (11.7%; analgesic), and fingolimod (11.7%; escalation DMD) (Figure 1). The average number of prescriptions per MS patient with a respective prescription was highest for the DMDs natalizumab (6.6) and interferon beta-1a (4.6). Compared with the controls, PwMS had a significantly higher prescription prevalence for 15 of the 18 active substances analyzed (FDR: q ⩽ 0.044). Only for the combination of levonorgestrel with ethinylestradiol (contraceptive), azithromycin (antibiotic), and the human papillomavirus (HPV) vaccine, differences in prescription prevalence were not significant.
Figure 1.
Prescription prevalence of single drugs in pediatric MS patients compared with the controls (2018). This figure shows the prescription prevalence of active substances in 613 pediatric PwMS (bars: blue—MS, orange—controls) and the mean numbers of prescriptions per patient with a respective prescription (bars: blue—MS, orange—controls) compared with the matched controls (n = 6130). The active substances ibuprofen (28.4%), cholecalciferol (23.0%), and interferon beta-1a (21.5%) were prescribed most frequently among the pediatric PwMS. Furthermore, these drugs were taken significantly more often by PwMS than the control patients (FDR: q < 0.001). The largest average prescription numbers per MS patient resulted in natalizumab (6.6) and interferon beta-1a (4.6). FDR: false discovery rate; MS: multiple sclerosis; q: p value adjustment according to FDR; PwMS: patients with multiple sclerosis.
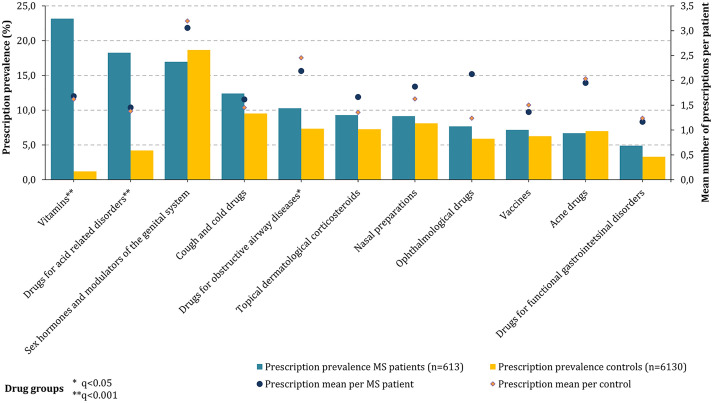
In addition, the prescription prevalence was also determined for those drugs that (a) were not included in the pre-specified drug groups of active ingredients and (b) had a patient count of ⩾30. Those active substances were classified into corresponding three-digit ATC therapeutic subgroups. Among these drugs, vitamins (A11: 23.2%), drugs for (gastric) acid-related diseases (A02: 18.3%), and hormone preparations (G03: 17.0%) were most frequently prescribed to pediatric PwMS (Figure 2). Compared with the controls, PwMS used significantly more vitamins (FDR: q < 0.001), drugs for (gastric) acid-related diseases (FDR: q < 0.001), and drugs for obstructive airway diseases (FDR: q = 0.048). The highest average number of prescriptions per patient with a respective prescription was observed for hormone preparations in both the MS cases and controls (3.1 vs 3.2).
Figure 2.
Prescription prevalence of other drugs in children and adolescents with MS compared with the controls (2018). In this diagram, the prescription prevalence of ATC therapeutic subgroups of 613 pediatric PwMS (bars: blue—MS, orange—controls) and the prescription mean values per patient with a respective prescription (dots: blue—MS, orange—controls) compared with the matched controls (n = 6130) are presented. Vitamins (A11: 23.2%), drugs for acid-related diseases (A02: 18.3%), and hormone therapy (G03: 17.0%) were prescribed most frequently among MS patients. There were significantly more prescriptions of vitamins and drugs for acid-related diseases for pediatric PwMS compared with the controls (FDR: q < 0.001). The largest average prescription value per MS patient with a respective prescription was found for hormone therapy (3.1). ATC: Anatomical Therapeutic Chemical codes; FDR: false discovery rate; MS: multiple sclerosis; q: p value adjustment according to FDR; PwMS: patients with multiple sclerosis.
Comparison of age groups
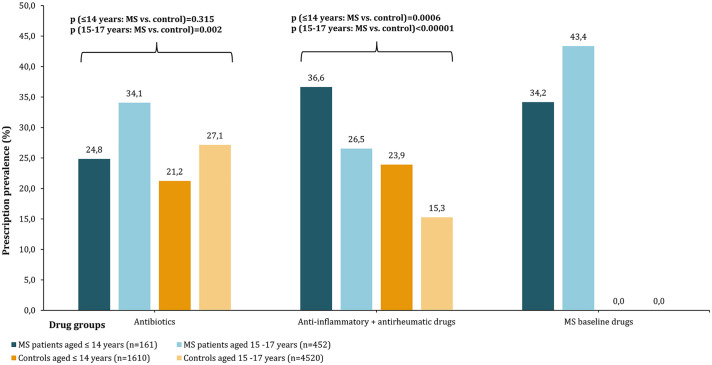
Comparing prescription rates in PwMS and the controls, stratified by age, could only be calculated for MS baseline drugs, anti-inflammatory/antirheumatic drugs, and antibiotics due to sufficient patient numbers (n ⩾ 30) in all patient subgroups. PwMS showed a higher prescription prevalence for those drug groups than the controls (Figure 3). In the age group of 15–17 years, anti-inflammatory/antirheumatic drugs were used by 26.5% of PwMS but only by 15.3% of the control patients (Fisher’s exact test: p < 0.001). Furthermore, the prescription of MS baseline drugs and antibiotics was more prevalent in the “older” pediatric PwMS compared with the younger ones (MS baseline drugs: 43.4% vs 34.2%, Fisher’s exact test: p = 0.05; antibiotics: 34.1% vs 24.8%, p = 0.031). PwMS aged ⩽ 14 years more frequently used anti-inflammatory/antirheumatic drugs than the “older” adolescents (36.6% vs 26.5%, Fisher’s exact test: p = 0.02). A comparison of the prescription rates of MS escalation drugs or overall DMDs between the two age groups was not possible due to data protection regulations (MS escalation drugs were prescribed to < 30 patients aged ⩽ 14 years).
Figure 3.
Prescription prevalence of drug groups stratified by age. In this bar chart, the prescription prevalence of antibiotics, anti-inflammatory/antirheumatic drugs, and MS baseline drugs were presented for 613 pediatric PwMS and the matched controls. The bars visualize the prescription prevalence for PwMS (blue) and the controls (orange), stratified by age. In all three drug groups, PwMS had higher prescription rates than the controls. Among PwMS aged ⩽ 14 (n = 161) years, anti-inflammatory and antirheumatic drugs were the most frequently prescribed drugs (36.6%). Adolescent PwMS aged 15–17 years (n = 452) more frequently used MS baseline drugs (43.4%) and antibiotics (34.1%). Both “younger” and “older” pediatric PwMS showed more frequent prescriptions of antibiotics (Fisher’s exact test: p (⩽14 years) = 0.315, p (15–17 years) = 0.002) and anti-inflammatory/antirheumatic drugs (Fisher’s exact test: p (⩽14 years/15–17 years) < 0.001) than the corresponding control groups. MS: multiple sclerosis; n: number of patients; PwMS: patients with multiple sclerosis.
Discussion
Pediatric and adult MS patients differ significantly in terms of pharmacokinetics and therefore require individualized and adequate treatment. Pediatric MS is a rare disease affecting 5.4 out of 100,000 German children and adolescents aged ⩽17 years. 4 To improve and extend treatment strategies of those patients, real-world therapy data are essential. Based on nationwide outpatient claims data covering a source population of about 87% of the total German population, we assessed drug utilization patterns in pediatric MS compared with non-MS controls. In our study, we identified the most common prescription medications among 613 pediatric PwMS in 2018. This includes not only the assessment regarding the prescription of DMDs but also symptomatic as well as further medications. To enhance the significance of our analyses, we conducted comparisons concerning the prescription prevalence of applied drugs with 6130 age-, sex-, and region-of-residence-matched controls without MS. There were no new indication extensions for approved DMDs to treat pediatric MS after 2018. The most recent update was in 2018 for fingolimod, which was approved for the use in children aged ⩾10 years. 11 Since then, five DMDs have been approved for the treatment of adult PwMS (ocrelizumab, ofatumumab, ozanimod, ponesimod, and siponimod), but no data exist to date on the efficacy and safety of those drugs in patients aged ⩽17 years.16–20 To our knowledge, this is the most comprehensive study in terms of population size and medication spectrum that evaluates the treatment patterns of pediatric PwMS in current pediatric practice.
In our study, the significantly higher prevalence of prescribed drugs in pediatric PwMS compared with the controls likely reflects a high burden of MS-associated comorbidities. In general, PwMS are more often affected by comorbidities than the general population, 21 which adds to the complexity of pharmacological MS treatment. Comorbidities in PwMS can delay the initiation of DMD therapy and are associated with increased disability progression, poorer quality of life, and increased mortality. 21 The delay in therapy initiation may be due to masking or concealment of the MS diagnosis by comorbidities. This issue has already been reported in several large retrospective observational studies.22,23 Moreover, the diversity of MS symptoms (e.g. cognitive, emotional, gastrointestinal, urogenital, and musculoskeletal) is connected with an increased need for treatment compared to control patients. 1 A healthy lifestyle can optimize therapy management in pediatric PwMS (e.g. obesity avoidance, smoking, and drug abuse) as well as adequate treatment of MS symptoms and comorbidities. 24
Considering the immunomodulatory treatments, about 50% of the pediatric PwMS were treated with DMDs. Interferon beta-1a was the most frequently prescribed DMD due to its approval for patients aged ⩾2–12 years (Rebif®/Avonex®) and its favorable safety profile. However, especially in children, a subcutaneous application could pose an issue, leading to a lack of therapeutic adherence or treatment switches. In our study, the drug group of baseline DMDs (interferon beta-1a (subcutaneous and intramuscular)/-1b, glatiramer acetate) was more frequently prescribed in “older” pediatric patients (15–17 years) compared with patients aged ⩽14 years. Although children, adolescents, and adults are not expected to show differences concerning side effects, those baseline DMDs are not approved for patients younger than 12 years, except for the interferon beta-1a preparation Rebif®.7,8 This issue and the limited number of DMDs approved for the treatment in children and adolescents in general contribute to the fact that about half of the analyzed pediatric PwMS were DMD-untreated. Fingolimod was only recommended for the use in children ⩾10 years by EMA and FDA in the course of 2018 (FDA: May, EMA: November).11,12
The most prevalent prescribed substance in the pediatric PwMS analyzed was the NSAID ibuprofen. This drug is commonly used to treat mild to moderate pain as well as fever. The additional benefit of ibuprofen in MS treatment is to prevent flu-like symptoms, which can be caused by injectable DMDs, for example, by interferon beta or glatiramer acetate. 25 Another drug with high prescription prevalence in our study was cholecalciferol (vitamin D3). This vitamin is often prescribed as an add-on to the established MS immunomodulatory and symptomatic therapies. It is assumed that higher cholecalciferol levels are associated with less disability and with lower MS disease activity. However, current evidence does not allow assessment of a causal relationship between vitamin D3 supplementation and disease activity. 26 Prospective high-quality randomized controlled trials are needed to increase the evidence concerning the (long-term) effect of cholecalciferol on the disease course. The analysis of the prescription prevalence of ATC therapeutic subgroups in our study also revealed that pediatric PwMS used vitamins (A11) significantly more often than the controls. Among PwMS, the regular intake of vitamins and other dietary supplements is becoming a topic of interest. 27 In a Danish interview-structured cross-sectional study examining 384 adult PwMS, 85% took dietary supplements within the last year, with vitamin D, multivitamins, and calcium being the most frequent supplements. 28 To our knowledge, apart from our analysis based on prescription claims, no other study examined the frequency of dietary supplements, such as vitamins, in pediatric PwMS.
The only drugs prescribed with nearly equal frequencies in PwMS and the controls were oral contraceptives (levonorgestrel + ethinylestradiol) and HPV vaccines. The initiation of conceptional prophylaxis with oral contraceptives in adolescent women today usually takes place during puberty. Effective contraception is vital for PwMS, as most DMDs are contraindicated or not recommended during a planned or unplanned pregnancy. 29 The HPV vaccination is recommended for girls since 2007 and for boys since 2018 (between the ages of 9 and 14 years) by the German Standing Vaccination Committee (STIKO) of the Robert Koch Institute in Germany. 30 In general, vaccinations play an important role in PwMS. Vaccinations protect against severe systemic infections and thus also against an associated worsening of the MS disease course.31,32
Comparing our results internationally, we found similarities in the frequency of DMD use. A study of the Swedish MS Registry compared clinical and demographic data of 549 pediatric-onset and 11,933 adult-onset PwMS with disease onset between 1975 and 2014. 33 Of the pediatric PwMS ever treated with DMDs (n = 518), 24.1% only received first-line DMDs (interferon beta, glatiramer acetate, teriflunomide, or dimethyl fumarate), 17.8% only received second-line DMDs (fingolimod, daclizumab, rituximab, mitoxantrone, or natalizumab), and 58.1% switched from first-line to second-line DMDs. Using this DMD classification, our data showed 74.9% first-line DMD use and 25.1% second-line DMD use among DMD-treated pediatric PwMS. On one hand, the Swedish study presented longitudinal data on DMD switches. On the other hand, the study was limited to analyzing DMD treatment patterns; medications for MS symptoms and comorbidities were not considered. In a Danish population-based cohort study by Erdal et al., 34 interferon beta was the most commonly prescribed initial DMD, used by 64.3% of PwMS with a DMD initiation before the age of 18 years between 2011 and January 2018 (n = 70). Our study also revealed interferon beta as the most used DMD among German pediatric DMD-treated PwMS in 2018, but with a lower prescription prevalence (53.1%). This could be due in part to the fact that it was only in 2018 that the spectrum of DMDs for the treatment of pediatric MS increased (approval expansion of fingolimod). 11 There were also differences between the study by Erdal et al. and ours concerning the prescription prevalence of glatiramer acetate (4.3% vs 16.1%), natalizumab (12.9% vs 9.3%), and fingolimod (4.3% vs 21.5%) among DMD-treated PwMS. These differences may be attributed to differences in study designs and the selection of the study populations: Erdal et al. included PwMS with disease onset before the age of 18 years from 1979 to 2018. Among these 347 patients included, only 70 received DMDs before the age of 18 years between 2011 and January 2018. Furthermore, only the initiation DMD was considered in the Danish analysis. Consequently, the results by Erdal et al. are unlikely to reflect current medical practice. Our study solely included patients who had a confirmed MS diagnosis at age ⩽17 years in 2018 and thus provided more recent information on DMD treatment patterns. Moreover, in contrast to other pediatric MS studies,33–35 our research provided information on symptomatic MS drugs and non-MS-related drugs used in pediatric patients. Overall, interferon beta still plays an essential role as initiation therapy after MS diagnosis in childhood or adolescence, but the oral escalation DMD fingolimod is becoming increasingly common in pediatric patients.
Regarding the number of drugs prescribed, there was a mean of 9.1 prescriptions (including several prescriptions of the same drug) per pediatric PwMS in 2018. Although a significant proportion of prescriptions consist of DMDs, other drugs are generally prescribed to support MS management (e.g. cholecalciferol), 26 to treat comorbidities (e.g. chronic and acute infections), 36 and for contraception (e.g. levonorgestrel + ethinylestradiol). It should be noted that such complex drug regimens increase the risk of polypharmacy (the concomitant use of ⩾5 drugs) as well as of severe drug–drug interactions (DDIs), especially in those PwMS suffering from comorbidities and severe degrees of disability.37,38 Clinical decision support systems (CDSSs) represent tools for screening medication plans regarding potential DDIs. 39 However, to date, there is no CDSS that is specialized for pediatric patients. 39 In geriatrics, databases to identify potentially inappropriate medications (PIMs) are useful support tools in therapy management, such as the Beers criteria and the START/STOPP criteria. 40 A list of 67 PIM specialized for children and adolescents has been developed only since 2020 (the KIDS list). 41 To improve medication management in pediatric patients in the long term, the acquisition of real-world pharmacovigilance data of the drugs used in children and adolescents is essential.
Our study presented both strengths and limitations. To our best knowledge, there is no study that has validated the case definition G35.- (Multiple Sclerosis) in Germany to date. In other electronic health databases, for instance, the Clinical Practice Research Datalink from the United Kingdom, many endpoints are validated, for example, by sample surveys or chart reviews of patient records.42,43 Validation studies or databases that provide information at least on the positive predictive value of the case definition or also on its sensitivity as well as specificity are almost non-existent for German routine data. For data protection regulations, we were unable to present prescription frequencies of drugs and drug groups with a patient number <30. Nevertheless, our study presented real-world prescription data from over 600 children and adolescents, covering the vast majority of pediatric PwMS in Germany (approximately 87%). Another limitation is that we did not consider over-the-counter drugs and dietary supplements but only prescription drugs. However, generally, prescription drugs pose the major proportion of drugs used. Furthermore, the present spectrum of medications included not only DMDs but also drugs for the treatment of MS symptoms and comorbidities as well as prophylactic interventions, whereas other studies only focused on DMDs. In addition, the significance of our results for pediatric PwMS is greatly enhanced by the fact that we conducted comparisons with a sex- and age-matched control group of over 6000 non-MS patients.
Conclusion
In summary, our population-based cross-sectional study presented real-world data on the prescription situation in pediatric MS, covering the vast majority of affected patients in Germany. The data revealed that interferon beta plays the most crucial role in the immunomodulating therapy of pediatric MS in Germany. Our study did not focus exclusively on DMDs but covered the entire spectrum of medications prescribed, from symptomatic drugs to treat MS to therapeutic agents for comorbidities. The results showed that vitamins, especially vitamin D3 (cholecalciferol), were among the most commonly prescribed drugs. In general, PwMS had a higher prescription prevalence and more comorbidities than non-MS patients. To our best knowledge, this is the largest study of treatment patterns in pediatric PwMS with regard to the size of the covered patient population. In the coming years, new DMDs will be approved for pediatric MS therapy and the indication of DMDs approved only for adults will be expanded to include children and adolescents. To augment or innovate new therapy strategies, existing treatment patterns have to be well studied using real-world data. Therefore, future research should longitudinally assess treatment patterns of pediatric PwMS, giving particular attention to the time of diagnosis, time of first DMD initiation, and medication switches.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864211048336 for Treatment patterns in pediatric patients with multiple sclerosis in Germany—a nationwide claim-based analysis by Niklas Frahm, Melanie Peters, Jörg Bätzing, David Ellenberger, Manas K. Akmatov, Judith Haas, Paulus S. Rommer, Alexander Stahmann, Uwe K. Zettl and Jakob Holstiege in Therapeutic Advances in Neurological Disorders
Footnotes
Author contributions: Ja.Ho., M.P., N.F., A.S., U.K.Z., P.S.R., J.B., and D.E. conceptualized and designed the study. M.P., N.F., and Ja.Ho. analyzed and interpreted the data gathered. N.F. and M.P. drafted the manuscript. Ja.Ho., P.S.R., U.K.Z., J.B., Ju.Ha., A.S., M.K.A., D.E., N.F., and M.P. critically revised the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.B., Ja.Ho., Ju.Ha., and M.K.A. declare no competing interests. D.E., M.P. had no personal financial interests to disclose other than being employees of the German MS Registry funded by many public and corporate sponsors. N.F. is an employee of the MSFP. Moreover, NF is an employee of the University Medical Center Rostock and received travel funds for research meetings from Novartis. None resulted in a conflict of interest. P.S.R. has received speaking fees, honoraria from advisory boards, and financial support for research activities from AbbVie, Alexion, Almirall, Amicus, Biogen, Celgene, Daiichi-Sankyo, Merck Serono, Novartis, Roche, Sandoz, Sanofi Genzyme, and Teva. None resulted in a conflict of interest. AS has no personal financial interests to disclose, other than being the leader of the German MS Registry, which receives funding from a range of public and corporate sponsors, recently including The German Innovation Fund (G-BA), The German MS Trust, German MS Society, Biogen, Celgene (Bristol Myers Squibb), Merck, Novartis, Roche, and Sanofi. None resulted in a conflict of interest. U.K.Z. has received speaking fees, travel support, and financial support for research activities from Alexion, Almirall, Bayer, Biogen, Janssen, Merck Serono, Novartis, Octapharm, Roche, Sanofi Genzyme, Teva, as well as EU, BMBF, BMWi, and DFG. None resulted in a conflict of interest.
Data availability: The data sets analyzed during this study are not publicly available due to data protection regulations by the German Social Security Code (Sozialgesetzbuch (SGB) V).
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Niklas Frahm  https://orcid.org/0000-0002-4655-774X
https://orcid.org/0000-0002-4655-774X
Paulus S. Rommer  https://orcid.org/0000-0001-5209-6647
https://orcid.org/0000-0001-5209-6647
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Niklas Frahm, MS Forschungs- und Projektentwicklungs- gGmbH (MS Research and Project Development gGmbH [MSFP]), Krausenstr. 50, Hannover, 30171, Germany.
Melanie Peters, Gesellschaft für Versorgungsforschung mbH (Society for Health Care Research [GfV]), Hannover, Germany.
Jörg Bätzing, Department 5, Epidemiology & Health Care Atlas, Central Research Institute of Ambulatory Health Care in the Federal Republic of Germany (Zi), Berlin, Germany.
David Ellenberger, MS Forschungs- und Projektentwicklungs- gGmbH (MS Research and Project Development gGmbH [MSFP]), Hannover, Germany.
Manas K. Akmatov, Department 5, Epidemiology & Health Care Atlas, Central Research Institute of Ambulatory Health Care in the Federal Republic of Germany (Zi), Berlin, Germany
Judith Haas, Deutsche Multiple Sklerose Gesellschaft, Bundesverband e.V. (German Multiple Sclerosis Society [DMSG]), Hannover, Germany.
Paulus S. Rommer, Department of Neurology, Medical University of Vienna, Vienna, AustriaNeuroimmunological Section, Department of Neurology, University Medical Center of Rostock, Rostock, Germany
Alexander Stahmann, MS Forschungs- und Projektentwicklungs- gGmbH (MS Research and Project Development gGmbH [MSFP]), Hannover, Germany.
Uwe K. Zettl, Neuroimmunological Section, Department of Neurology, University Medical Center of Rostock, Rostock, Germany
Jakob Holstiege, Department 5, Epidemiology & Health Care Atlas, Central Research Institute of Ambulatory Health Care in the Federal Republic of Germany (Zi), Berlin, Germany.
References
- 1. Thompson AJ, Baranzini SE, Geurts J, et al. Multiple sclerosis. Lancet 2018; 391: 1622–1636. [DOI] [PubMed] [Google Scholar]
- 2. Rommer PS, Eichstädt K, Ellenberger D, et al. Symptomatology and symptomatic treatment in multiple sclerosis: results from a nationwide MS registry. Mult Scler 2019; 25: 1641–1652. [DOI] [PubMed] [Google Scholar]
- 3. Alroughani R, Boyko A. Pediatric multiple sclerosis: a review. BMC Neurol 2018; 18: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frahm N, Peters M, Bätzing J, et al. Prevalence of pediatric multiple sclerosis in Germany: a nationwide population-based analysis. Eur J Neurol 2021; 28: 3173–3176. [DOI] [PubMed] [Google Scholar]
- 5. Huppke DP, Gärtner DJ. S1 guideline pediatric multiple sclerosis, https://www.awmf.org/leitlinien/detail/ll/022-014.html (accessed 23 August 2021).
- 6. Otallah S, Banwell B. Pediatric multiple sclerosis: an update. Curr Neurol Neurosci Rep 2018; 18: 76. [DOI] [PubMed] [Google Scholar]
- 7. Ghezzi A, Amato MP, Makhani N, et al. Pediatric multiple sclerosis: conventional first-line treatment and general management. Neurology 2016; 87: S97–S102. [DOI] [PubMed] [Google Scholar]
- 8. European Medicines Agency. Rebif—summary of product characteristics, https://www.ema.europa.eu/en/medicines/human/EPAR/rebif (accessed 23 August 2021).
- 9. Huppke P, Huppke B, Ellenberger D, et al. Therapy of highly active pediatric multiple sclerosis. Mult Scler 2019; 25: 72–80. [DOI] [PubMed] [Google Scholar]
- 10. Chitnis T, Arnold DL, Banwell B, et al. Trial of fingolimod versus interferon beta-1a in pediatric multiple sclerosis. N Engl J Med 2018; 379: 1017–1027. [DOI] [PubMed] [Google Scholar]
- 11. European Medicines Agency. Gilenya—Summary of product characteristics, https://www.ema.europa.eu/en/medicines/human/EPAR/gilenya (accessed 23 August 2021).
- 12. Novartis. Gilenya—summary of product characteristics (Food and Drug Administration), https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022527s26lbl.pdf (accessed 23 August 2021).
- 13. Wilbur C, Yeh EA. Improving outcomes in pediatric multiple sclerosis: current and emerging treatments. Paediatr Drugs 2019; 21: 137–152. [DOI] [PubMed] [Google Scholar]
- 14. Krupp LB, Banwell B, Tenembaum S, et al. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology 2007; 68: S7–S12. [DOI] [PubMed] [Google Scholar]
- 15. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995; 57: 289–300. [Google Scholar]
- 16. European Medicines Agency. Ocrevus—summary of product characteristics, https://www.ema.europa.eu/en/medicines/human/EPAR/ocrevus (accessed 23 August 2021).
- 17. European Medicines Agency. Kesimpta—summary of product characteristics, https://www.ema.europa.eu/en/medicines/human/EPAR/kesimpta (accessed 23 August 2021).
- 18. European Medicines Agency. Zeposia—summary of product characteristics, https://www.ema.europa.eu/en/medicines/human/EPAR/zeposia (accessed 23 August 2021).
- 19. European Medicines Agency. Ponvory—summary of product characteristics, https://www.ema.europa.eu/en/medicines/human/EPAR/ponvory (accessed 23 August 2021).
- 20. European Medicines Agency. Mayzent—summary of product characteristics, https://www.ema.europa.eu/en/medicines/human/EPAR/mayzent (accessed 23 August 2021).
- 21. Marrie RA. Comorbidity in multiple sclerosis: implications for patient care. Nat Rev Neurol 2017; 13: 375–382. [DOI] [PubMed] [Google Scholar]
- 22. Zhang T, Tremlett H, Leung S, et al. Examining the effects of comorbidities on disease-modifying therapy use in multiple sclerosis. Neurology 2016; 86: 1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maric GD, Pekmezovic TD, Mesaros ST, et al. The prevalence of comorbidities in patients with multiple sclerosis: population-based registry data. Neurol Sci 2021; 42: 1887–1893. [DOI] [PubMed] [Google Scholar]
- 24. Wells E, Hacohen Y, Waldman A, et al. Neuroimmune disorders of the central nervous system in children in the molecular era. Nat Rev Neurol 2018; 14: 433–445. [DOI] [PubMed] [Google Scholar]
- 25. Filipi M, Jack S. Interferons in the treatment of multiple sclerosis: a clinical efficacy, safety, and tolerability update. Int J MS Care 2020; 22: 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miclea A, Bagnoud M, Chan A, et al. A brief review of the effects of vitamin d on multiple sclerosis. Front Immunol 2020; 11: 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evans E, Piccio L, Cross AH. Use of vitamins and dietary supplements by patients with multiple sclerosis: a review. JAMA Neurol 2018; 75: 1013–1021. [DOI] [PubMed] [Google Scholar]
- 28. Bergien SO, Petersen CM, Lynning M, et al. Use of natural medicine and dietary supplements concomitant with conventional medicine among people with Multiple Sclerosis. Mult Scler Relat Disord 2020; 44: 102197. [DOI] [PubMed] [Google Scholar]
- 29. Rommer PS, Zettl UK. Managing the side effects of multiple sclerosis therapy: pharmacotherapy options for patients. Expert Opin Pharmacother 2018; 19: 483–498. [DOI] [PubMed] [Google Scholar]
- 30. German Standing Committee on Vaccination (STIKO). Epidemiologisches Bulletin—Wissenschaftliche Begründung für die Empfehlung der HPV- Impfung für Jungen im Alter von 9 bis 14 Jahren. Robert Koch Inst 2018; 26: 233–250. [Google Scholar]
- 31. Zrzavy T, Kollaritsch H, Rommer PS, et al. Vaccination in multiple sclerosis: friend or foe? Front Immunol 2019; 10: 1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Monschein T, Hartung HP, Zrzavy T, et al. Vaccination and multiple sclerosis in the era of the COVID-19 pandemic. J Neurol Neurosurg Psychiatry. Epub ahead of print 5 August 2021. DOI: 10.1136/jnnp-2021-326839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McKay KA, Hillert J, Manouchehrinia A. Long-term disability progression of pediatric-onset multiple sclerosis. Neurology 2019; 92: e2764–e2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Erdal JL, Kopp TI, Blinkenberg M, et al. Clinical characteristics and use of disease modifying therapy in the nationwide Danish cohort of paediatric onset multiple sclerosis. Mult Scler Relat Disord 2020; 37: 101431. [DOI] [PubMed] [Google Scholar]
- 35. Bizjak N, Osredkar D, Perković Benedik M, et al. Epidemiological and clinical characteristics of multiple sclerosis in paediatric population in Slovenia: a descriptive nation-wide study. Mult Scler Relat Disord 2017; 18: 56–59. [DOI] [PubMed] [Google Scholar]
- 36. Persson R, Lee S, Ulcickas Yood M, et al. Infections in patients diagnosed with multiple sclerosis: a multi-database study. Mult Scler Relat Disord 2020; 41: 101982. [DOI] [PubMed] [Google Scholar]
- 37. Frahm N, Hecker M, Zettl UK. Polypharmacy among patients with multiple sclerosis: a qualitative systematic review. Expert Opin Drug Saf 2020; 19: 139–145. [DOI] [PubMed] [Google Scholar]
- 38. Frahm N, Hecker M, Zettl UK. Polypharmacy in chronic neurological diseases: multiple sclerosis, dementia and Parkinson’s disease. Curr Pharm Des. Epub ahead of print 27 July 2021. DOI: 10.2174/1381612827666210728102832. [DOI] [PubMed] [Google Scholar]
- 39. Kheshti R, Aalipour M, Namazi S. A comparison of five common drug-drug interaction software programs regarding accuracy and comprehensiveness. J Res Pharm Pract 2016; 5: 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Halli-Tierney AD, Scarbrough C, Carroll D. Polypharmacy: evaluating risks and deprescribing. Am Fam Physician 2019; 100: 32–38. [PubMed] [Google Scholar]
- 41. Meyers RS, Thackray J, Matson KL, et al. Key potentially inappropriate drugs in pediatrics: the KIDs list. J Pediatr Pharmacol Ther 2020; 25: 175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ghosh RE, Crellin E, Beatty S, et al. How clinical practice research datalink data are used to support pharmacovigilance. Ther Adv Drug Saf 2019; 10: 2042098619854010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol 2015; 44: 827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864211048336 for Treatment patterns in pediatric patients with multiple sclerosis in Germany—a nationwide claim-based analysis by Niklas Frahm, Melanie Peters, Jörg Bätzing, David Ellenberger, Manas K. Akmatov, Judith Haas, Paulus S. Rommer, Alexander Stahmann, Uwe K. Zettl and Jakob Holstiege in Therapeutic Advances in Neurological Disorders