Abstract
Background:
Remnant preservation during anterior cruciate ligament (ACL) reconstruction (ACLR) is controversial, and it is unclear whether the stump aids or obscures tibial tunnel positioning.
Purpose/Hypothesis:
The aim of this study was to determine whether the rate of tibial tunnel malposition is influenced by remnant preservation. The hypothesis was that using a remnant-preserving technique to drill entirely within the tibial stump would result in a significant reduction in tibial tunnel malposition as determined by postoperative 3-dimensional computed tomography (3D-CT).
Study Design:
Cohort study; Level of evidence, 2.
Methods:
Patients undergoing ACLR between October 2018 and December 2019 underwent surgery with a remnant-preserving technique (RP group) if they had a large stump present (>50% of the native ACL length) or if there was no remnant or if it was <50% of the native length of the ACL, they underwent remnant ablation (RA group) and use of standard landmarks for tunnel positioning. The postoperative tunnel location was reported as a percentage of the overall anteroposterior (AP) and mediolateral (ML) dimensions of the tibia on axial 3D-CT. The tunnel was classified as anatomically placed if the center lay between 30% and 55% of the AP length and between 40% and 51% of the ML length.
Results:
Overall, 52 patients were included in the study (26 in each group). The mean tunnel positions were 36.8% ± 5.5% AP and 46.7% ± 2.9% ML in the RP group and 35.6% ± 4.8% AP and 47.3% ± 2.3% ML in the RA group. There were no significant differences in the mean AP (P = .134) and ML (P = .098) tunnel positions between the groups. Inter- and intraobserver reliability varied between fair to excellent and good to excellent, respectively. There was no significant difference in the rate of malposition between groups (RP group, 7.7%; RA group, 11.5%; P ≥ .999).
Conclusion:
Drilling entirely within the ACL tibial stump using a remnant-preserving reconstruction technique did not significantly change the rate of tunnel malposition when compared with stump ablation and utilization of standard landmarks.
Keywords: ACL reconstruction, computed tomography, ACL tunnel position, remnant preservation, anatomic ACL reconstruction
It is well-recognized that incorrect placement of tunnels during anterior cruciate ligament (ACL) reconstruction (ACLR) adversely influences knee kinematics and clinical outcomes, including graft failure rates. 22,25,31 Jaecker et al 17 recently reported that 40% of patients undergoing revision ACLR had a malpositioned tibial tunnel. Although there are numerous factors that could influence the rate of tunnel malposition, it is clear that it occurs frequently, and even experienced surgeons can have difficulty with correct placement intraoperatively. 1,24,34
It has been suggested that preserving the ACL tibial remnant can aid correct tibial tunnel positioning by providing an important and reliable intraoperative landmark. 4 It has also been reported that remnant preservation offers the advantages of reduced postoperative tunnel widening, 33 a greater intrinsic potential for healing, better graft vascularization, preservation of proprioceptive nerve fibers, 2,13,15,27 better knee stability, 3,35 and reduced rates of graft rupture. 13,19,39,42 In contrast, there are also reports that remnant preservation does not confer an important clinical advantage and that the preservation of a large remnant may furthermore obscure visualization and make accurate tunnel placement more difficult. 15,32,37 Besides, in the largest study to date on this specific topic, Delaloye et al 8 demonstrated that the preservation of large remnants was not significantly associated with the development of cyclops lesions or loss of full extension.
We believe that remnant preservation is a reliable way of avoiding nonanatomic tibial tunnel placement because the tunnel is drilled entirely within the stump, therefore requiring the graft to pass through the native footprint. The aim of this study was to determine whether the rate of tibial tunnel malposition is influenced by using a remnant-preserving technique. Additionally, the hypothesis was that using a remnant-preserving technique to drill entirely within the tibial stump would result in a significant reduction in tibial tunnel malposition as determined by postoperative 3-dimensional computed tomography (3D-CT).
Methods
Institutional review board approval was granted for this prospective comparative study. All patients aged 17 to 55 years undergoing outpatient ACLR with hamstring tendon autograft between October 2018 and December 2019 were considered for study eligibility. Patients were excluded if they had a history of previous knee surgery or infection, had degenerative changes on preoperative imaging, required concomitant osteotomy or reconstruction of ligaments other than the ACL, required drilling of tibial bone tunnels for meniscal repair, or did not consent to study participation. The flowchart of study patients is reported in Figure 1.
Figure 1.
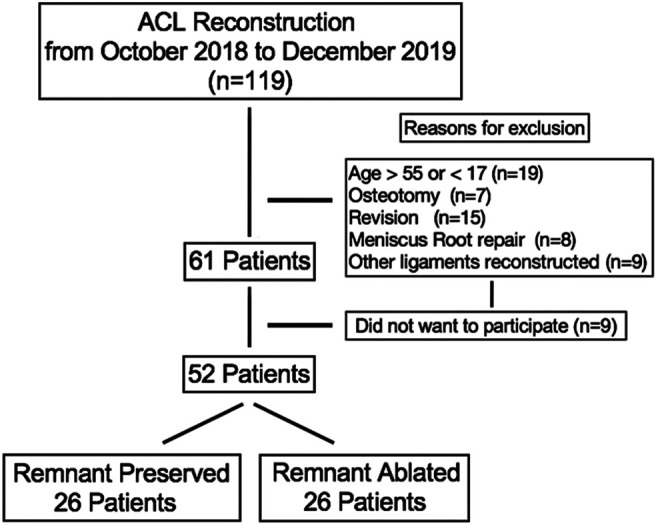
Flowchart of patients within the study. ACL, anterior cruciate ligament.
Patients were allocated to the remnant preservation (RP) or control group (remnant ablated [RA]) during diagnostic arthroscopy (immediately before ACLR) on the basis of remnant size, and all procedures were performed by a single surgeon (V.B.C.P.). Remnant size was evaluated with knees placed at 90° of flexion and viewing performed through the anteromedial portal. The free end of the tibial ACL stump was grasped and pulled toward the femoral footprint. Remnants were categorized into ≤50% or >50% of the length of the native ACL depending on whether they extended beyond the central axis of the posterior cruciate ligament (PCL). Only patients with large remnants (>50% of the length of the native ACL), according to the classification of Buscayret et al, 4 were allocated to the RP group. Remnants ≤50% of the native ACL length were not preserved in order to avoid skewing the study results by including small remnants that were unlikely to have the potential to influence tunnel positioning.
The tibial tunnel in both groups was created by positioning the guide so that the tunnel entrance was located approximately 1 cm medial to the tibial tuberosity. In the RP group, the single anteromedial bundle biological augmentation 35 technique was used. The guide wire was positioned within the center of the ACL tibial stump (Figure 2).
Figure 2.
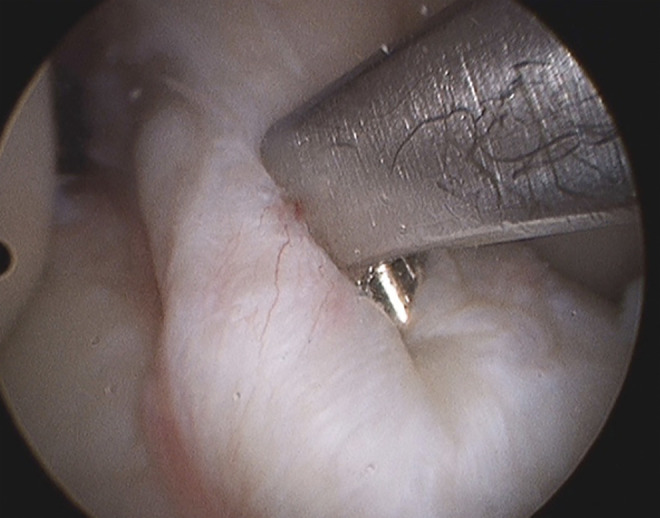
Typical position of tibial guide when using the remnant-preservation technique.
The tunnel was drilled with increasingly larger drill-bit diameters (starting at 6 mm and increasing by 1-mm increments). This sequential preparation, in combination with a minimum application of force to advance the drill, with the guide wire held in position intra-articularly with a clamp, minimized the risk of tunnel deviation. To avoid disruption of the remnant, drilling was stopped on each passage as soon as the tibial cortex was breached.
The drill remained entirely within the remnant and was not visualized within the knee. A shaver was then placed within the remnant via the tibial tunnel and used to create a pathway for the graft. The femoral tunnel was created with an outside-in technique, and the graft was shuttled from distal to proximal into the knee with a passing suture. Due to the preserved remnant, grafts could not be seen exiting the tibial tunnel and could only be observed exiting the preserved remnant more proximally.
Patients with ACL remnants that were ≤50% to the native ACL length were allocated to the control group (RA), and the entire stump was removed.
In this situation, the entire footprint and adjacent osseous (tibial spines) and soft tissue (PCL and the posterior border of the anterior horn of the lateral meniscus) structures could be clearly visualized. The aimer was placed with the intention of positioning it into the middle of the anteromedial bundle of the ACL footprint, in line with the posterior margin of the anterior horn of the lateral meniscus and approximately 5 mm lateral to the peak of the medial tibial spine. Drilling was performed in the same sequential manner as in the RP group.
Femoral tunnels were drilled with an outside-in technique, 12 and grafts were fixed with absorbable interference screws on the tibial and femoral sides with the knee positioned at 30° of flexion. Postoperatively, patients were able to mobilize brace-free and fully bear weight as tolerated (unless dictated otherwise by meniscal repair).
Between 30 and 60 days postoperatively, patients underwent a CT scan using a 16-channel Toshiba Activion multislice device, using a slice thickness of 0.5 mm and 3D-CT reconstruction by volume acquisition. No patient had any limitation of knee extension at the time of the CT scan. The most proximal tibial axial section in which it was possible to view the entire tibia was identified and used to determine the location of the center of the tibial tunnel. To record the tunnel location, a rectangle was overlaid at the maximal extents of the tibia in the anteroposterior (AP) and mediolateral (ML) directions, in accordance with the methodology described by Kosy et al, 20 and the center of the tibial tunnel was reported as a percentage of the overall AP and ML dimensions of the rectangle (Figure 3). These measurements were made independently by 3 orthopaedic surgeons (L.F.P., L.F.P., P.J.L.G.) who were blinded to all clinical and patient-identifying data at the time of the evaluations. To assess reliability, all measurements were performed twice by each observer, using the measurement tool in the institutional picture archiving and communication system software (Voxar 3D Workstation; Toshiba), with an interval of at least 30 days between the primary evaluation and re-evaluation.
Figure 3.
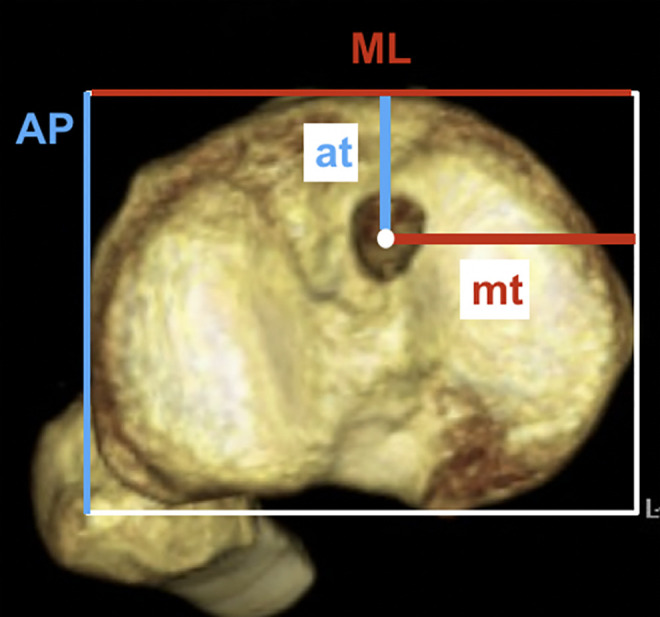
Location of tibial tunnel center, calculated as at/AP and mt/ML, where at is the distance from the anterior tibial border of the superimposed rectangle to the tunnel center, mt is the distance from the medial border of the rectangle to the tunnel center, AP is the anteroposterior border length, and ML is the mediolateral border length of the rectangle.
Determination of whether tunnels were placed anatomically was based upon whether the center of the tibial tunnel lay within the range reported in previous studies, as summarized by McConkey et al. 26 Specifically, if the center of the tibial tunnel lay between 30% and 55% in the AP direction and between 40% and 51% in the ML direction, it was classified as being anatomically placed.
Statistical Analysis
A sample-size calculation for a binary outcome superiority trial was performed using an online sample-size calculator (http://sealedenvelope.com). The calculation was based on the primary outcome measure of whether an anatomic tibial tunnel position was achieved on postoperative 3D-CT. It was determined that 38 patients were required in order to have an 80% chance of detecting, as significant at the 5% level, a decrease in the rate of nonanatomic tibial tunnel placement (based on the findings of Pedneault et al, 29 who recently reported that 30% of tibial tunnels completely miss the ACL footprint) to 0% in the RP group.
Qualitative variables were described by the distribution of relative frequency (%) and absolute value (n). The relationship between qualitative variables was analyzed using the chi-square and Fisher exact tests. Quantitative variables were described by the mean and standard deviation. The Kolmogorov-Smirnov test was used to verify normality of distribution. The Student t test was used to compare means. Inter- and intraobserver reliability was evaluated with the intraclass correlation coefficient and was classified according to the criteria of Cicchetti and Sparrow. 5 To analyze the main effect of the measure (time), of the evaluator (observers 1, 2, and 3), and group (RP and RA), a repeated-measures analysis of variance of 2 factors was performed based on the assumptions of homogeneity of the covariance matrices by the Box test, and sphericity by the Mauchly test. SPSS Version 19.0 (SPSS) was used for all analyses. The level of significance was set at P ≤ .05.
Results
A total of 52 patients were included in the study (26 in each group [RP and RA]). The mean age of the overall study population was 32.5 ± 9.5 years. There were no significant differences between groups with respect to age, sex, or mean tibial tunnel diameter (Table 1).
Table 1.
Comparison of Age, Sex, and Mean Tunnel Size Between Groups a
| RP Group (n = 26) | RA Group (n = 26) | P Value | |
|---|---|---|---|
| Age, y | 33.8 ± 7.9 | 31.1 ± 10.9 | .300 b |
| Tunnel size, mm | 8.4 ± 0.6 | 8.3 ± 0.7 | .378 b |
| Sex | .486 c | ||
| Male | 22 (84.6) | 20 (76.9) | |
| Female | 4 (15.4) | 6 (23.1) |
a Data are reported as mean ± SD or n (%). RA, remnant-ablated; RP, remnant-preserved.
b Student t test for unpaired samples.
c Chi-square association test.
There were no significant differences in the mean AP and ML tunnel positions between the RP and RA groups (Table 2). In the RP group, the mean values were 36.8 ± 5.5% AP and 46.7 ± 2.9% ML, and in the control group (RA group), they were 35.6% ± 4.8% AP and 47.3% ± 2.3% ML.
Table 2.
Mean Tunnel Positions Reported for Each Group, by Each Observer a
| RP Group (n = 26) | RA Group (n = 26) | ||||
|---|---|---|---|---|---|
| Primary Evaluation | Retest Evaluation | Primary Evaluation | Retest Evaluation | P Value b | |
| %AP | |||||
| O1 | 36.3 ± 5.1 | 37.1 ± 4.7 | 35.2 ± 4 | 35.8 ± 4.9 | |
| O2 | 36.5 ± 5.9 | 36.3 ± 5.5 | 35.5 ± 4.9 | 36.1 ± 5.3 | |
| O3 | 37.3 ± 6 | 37.2 ± 6.1 | 35.6 ± 5 | 35.3 ± 4.7 | |
| Overall | 36.8 ± 5.5 | 35.6 ± 4.8 | .134 | ||
| %ML | |||||
| O1 | 47.3 ± 2.3 | 46.8 ± 3.3 | 47.7 ± 1.9 | 47.4 ± 2 | |
| O2 | 46.5 ± 2.8 | 46.0 ± 4.3 | 47.7 ± 2.9 | 47.2 ± 2.7 | |
| O3 | 46.7 ± 2 | 46.6 ± 2.1 | 46.9 ± 1.8 | 46.8 ± 1.7 | |
| Overall | 46.7 ± 2.9 | 47.3 ± 2.3 | .098 | ||
a Data are reported as mean ± SD. %AP, position of the center of the tibial tunnel as defined as the anteroposterior distance as a percentage of the overall anteroposterior length of the tibial plateau; %ML, position of the center of the tibial tunnel as defined as the mediolateral distance as a percentage of the overall mediolateral width of the tibial plateau; O1 , observer 1; O2, observer 2; O3, observer 3.
b Analysis of variance.
Intraobserver reliability varied between good and excellent, with interobserver reliability between moderate and excellent (Table 3).
Table 3.
Intra- and Interobserver ICCs for %AP and %ML a
| ICC | 95% CI | Interpretation b | |
|---|---|---|---|
| Intraobserver reliability | |||
| %AP, E1 vs E2 | |||
| O1 | 0.846 | 0.737-0.913 | Excellent |
| O2 | 0.888 | 0.805-0.936 | Excellent |
| O3 | 0.995 | 0.991-0.997 | Excellent |
| %ML, E1 vs E2 | |||
| O1 | 0.698 | 0.321-0.775 | Good |
| O2 | 0.554 | 0.225-0.744 | Good |
| O3 | 0.978 | 0.961-0.987 | Excellent |
| Interobserver reliability | |||
| %AP | |||
| O1 vs O2 | 0.971 | 0.950-0.983 | Excellent |
| O1 vs O3 | 0.897 | 0.820-0.941 | Excellent |
| O2 vs O3 | 0.951 | 0.914-0.972 | Excellent |
| %ML | |||
| O1 vs O2 | 0.857 | 0.753-0.918 | Excellent |
| O1 vs O3 | 0.497 | 0.130-0.711 | Fair |
| O2 vs O3 | 0.803 | 0.658-0.887 | Excellent |
a %AP, position of the center of the tibial tunnel as defined as the anteroposterior distance as a percentage of the overall anteroposterior length of the tibial plateau; %ML, position of the center of the tibial tunnel as defined as the mediolateral distance as a percentage of the overall mediolateral width of the tibial plateau; E1, primary evaluation; E2, re-evaluation; ICC, intraclass correlation coefficient; O1, observer 1; O2, observer 2; O3, observer 3.
b According to Cicchetti and Sparrow. 5
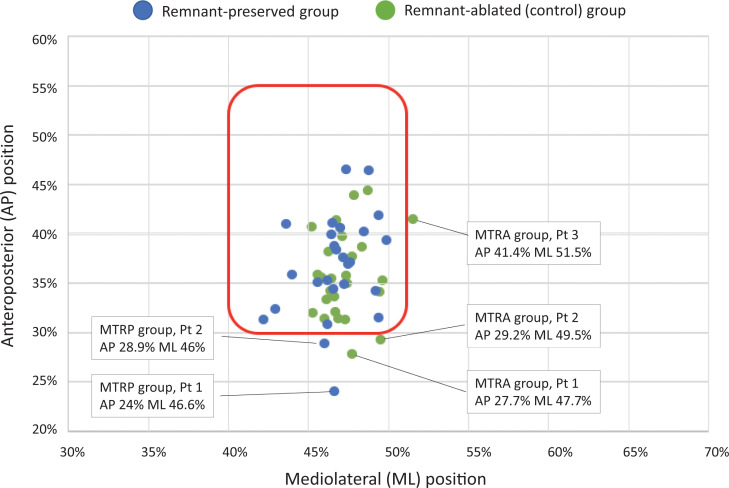
Tunnel-positioning data are graphically presented with a scatterplot of the mean locations of the center of the tibial tunnel recorded for each patient in Figure 4. Two patients (7.7%) in the RP group and 3 (11.5%) in the control group met the criteria for a malpositioned tunnel. The precise location of these malpositioned tunnels is also shown in the scatterplot. An analysis of the direction of malposition in each of these patients is summarized in Table 4. There was no significant difference between groups when considering the rate or direction of malposition.
Figure 4.
Mean tunnel location of each patient (Pt). The area within the red line identifies the positions considered anatomic according to the criteria of McConkey et al 26 (30%-55%, AP; 40%-51%, ML). AP, anteroposterior; ML, mediolateral; MTRA, malpositioned tunnel for remnant ablated; MTRP, malpositioned tunnel for remnant preserved.
Table 4.
Total Number of Patients in Each Group With a Malpositioned Tunnel and the Direction in Which the Malposition Occurred a
| Direction of Malposition | RP Group | RA Group | P Value b |
|---|---|---|---|
| Anterior only | 2 | 2 | ≥.999 |
| Medial only | 0 | 0 | |
| Lateral only | 0 | 1 | ≥.999 |
| Both anterior and lateral | 0 | 0 | |
| Both anterior and medial | 0 | 0 | |
| Total | 2 | 3 | ≥.999 |
a RA, remnant-ablated; RP, remnant-preserved.
b Fisher exact test.
Discussion
The main finding of this study was that there was no significant difference in the rate of nonanatomic tibial tunnel placement, determined by postoperative 3D-CT, regardless of whether a tibial remnant was preserved. Although not directly comparable, the rates of nonanatomic placement based upon mean tunnel positions (7.7% in the RP group and 11.5% in the RA group) appear to be low when considered alongside the 22% rate of nonanatomic tibial tunnels reported by McConkey et al 26 when using the same criteria. The low rate of nonanatomic tunnels in the RP group is consistent with Buscayret et al, 4 who demonstrated that preserving large remnants does not compromise tunnel positioning. However, in contrast to the study hypothesis, preservation of ACL remnants did not result in a reduced rate of nonanatomic tibial tunnels when compared with stump ablation and utilization of standard surgical landmarks. It was an unexpected finding that 7.7% (2/26) of patients in the remnant-preservation group had tunnels that were classified as nonanatomic because intraoperatively the graft is shuttled within the remnant, and therefore, the tunnel must lie within the footprint. Upon that basis, it should be considered that in 3 patients, the tunnels were identified to be just outside the cutoff boundaries (within <1.5%). It could be argued that such small deviations from the cutoff fall within, or close to, the expected error of 3D-CT measurement tools, which have a reported accuracy of approximately 0.3 mm 18 and are therefore unlikely to be clinically relevant. However, it should also be noted that in both patients with a malpositioned tunnel in the RP group, the tunnels were anteriorly placed according to 3D-CT. Another potential explanation for these findings is that the most anterior fibers of the ACL fan out. 10,11,14,42 It is therefore possible that an anteriorly malpositioned tunnel could be concealed by these anterior fibers but still allow shuttling of the graft entirely within the remnant. This seems unlikely because these fibers are only observed immediately at the attachment and empirically are very susceptible to disruption by reaming if a tunnel is malpositioned. Furthermore, the rate of anterior malposition was not significantly different in the control group, despite complete ablation of the remnant, therefore suggesting that an alternative explanation, potentially including the variety of tibial footprint shapes and sizes, should be considered.
Perhaps a more robust explanation for the disparity between intraoperative observation and tunnel malposition identified by 3D-CT lies in the lack of both normative data and consensus regarding what exactly constitutes an anatomic position. Although criteria recently published 26 were utilized to classify anatomic and nonanatomic positions, the unexpected result prompted a review of the correlation between the anatomic ACL footprint and imaging criteria. To our knowledge, only 3 previous studies 23,28,40 comprising 46 patients have mapped the tibial ACL footprint to 3D-CT (Appendix Table A1). This small number precludes a reliable estimate of the true range that might be encountered in clinical practice, particularly when several different morphologies of tibial footprint have been reported (eg, triangular, oval, and c-shaped). Furthermore, when all imaging modalities are considered, the range of means varies from 24.6% to 62.1% from anterior to posterior and 40% to 55% from medial to lateral. If the current study had used this broader range, every tunnel would have been classified as anatomic. However, using such a broad range may result in classifying a tunnel as anatomic when individual variation in footprint morphology and location means that a tunnel may completely miss, or only partly overlap, the footprint but still lie within this broad range of values. An alternative strategy is to use magnetic resonance imaging (MRI) of both knees to evaluate tunnel position. Pedneault et al 29 reported that, using this strategy, they identified that in 30% of patients the tibial tunnel missed the footprint completely, and in an additional 25% of patients, there was <50% overlap with the footprint. Pedneault et al concluded that there is room for improvement in tunnel positioning and that this should be individualized to the patient. These findings, along with those of the current study, suggest that despite its widespread clinical use and acceptance as the gold standard for determining postoperative tunnel position, 3D-CT may not be a reliable method to determine whether a tunnel is anatomically placed for an individual patient, and clearly, further study is needed in this regard.
In a recent study by Kosy et al, 20 the authors evaluated the accuracy and precision of the tibial tunnel in the RP and RA groups using the mean AP (38.7%) and ML (49.1%) positions determined by Lertwanich et al 21 as the reference standard. Those authors reported no significant differences between groups regarding these metrics, and their work is therefore broadly consistent with the findings of the current study. However, in light of the reported variation in the center of the anatomic footprint when correlated with 3D-CT, it is unsurprising that Kosy et al 20 reported accuracies between 4.8% and 6.1% and a precision between 2.8% and 3.9% when using a specific point as a reference standard. It could perhaps be argued that these metrics are incorrectly used because none of the intraoperative steps taken or landmarks used actually sought to achieve this specific position that was identified only on postoperative CT. Specifically, ML and AP tibial plateau widths are not measured during surgery, and instead, intraoperative landmarks are utilized. Recently, Cremer et al 7 attempted to address this issue by evaluating postoperative tunnel position using a grid positioned according to the intracondylar bony landmarks seen intraoperatively. Although the technique is of interest, there are currently limited data to support its validity and further study is needed.
It is our opinion that the disparity between the intraoperative observation of a tibial tunnel drilled entirely within the remnant and postoperative CT, suggesting malposition is due to a lack of normative data and highlights one of the limitations of evaluating malposition in this manner. This is of particularly importance from a medicolegal perspective due to the ramifications of incorrectly classifying a tunnel as nonanatomic. Surgeons and radiologists should be aware of this limitation of 3D-CT criteria in the assessment of tunnel positioning and also that anatomic variations may exist outside of the normal range.
The main limitation of the current study was that there are limited published data correlating 3D-CT measurements and the actual tibial ACL footprint. This is compounded by the fact that large interindividual variation in footprint morphology and position is reported. This suggests that although 3D-CT is frequently used to determine tunnel position, it may not be reliable in evaluating malposition. A further limitation is that bilateral postoperative MRI according to the methodology of Pedneault et al 29 was not undertaken, and this may have helped to clarify whether tunnels were anatomic. Additionally, other potential risks of bias were that the study was not randomized and that only a single experienced surgeon decided group allocation for patients. However, attempts to minimize bias were made by using the classification system of Buscayret et al 4 to determine group allocation. Further limitations include that clinical outcomes were not compared between groups and that differences in the size of patients were not considered in any evaluations. Finally, the literature demonstrates a lack of agreement on the ideal single-bundle tibial tunnel position. As a result, the findings of this study may not extrapolate well to other techniques for localizing tunnel position.
Conclusion
Drilling entirely within the ACL tibial stump using a remnant-preserving reconstruction technique does not significantly change the rate of tunnel malposition determined by postoperative 3D-CT when compared with stump ablation and utilization of standard landmarks.
APPENDIX
Table A1.
Anatomic Studies a
| Tibial ACL Center | ||||
|---|---|---|---|---|
| Lead Author (year) | Measurement | N | at/AP b | mt/ML c |
| Lorenz (2009) 23 | CT | 12 | 37 ± 3 (31-41) | 52 ± 2 (47-55) |
| Tampere (2017) 40 | CT | 8 | 39.7 ± 2.9 | 49.3 ± 2.1 |
| Parkinson (2017) 28 | CT | 26 | 38 ± 2 | 48 ± 2 |
| Parkinson (2017) 28 | MRI | 76 | 39 ± 3 | 48 ± 2 |
| Colombet (2006) 6 | XR | 7 | 36 ± 3.8 | — |
| Zantop (2008) 42 | XR | 20 | 30 | — |
| Pietrini (2011) 30 | XR | 12 | 37.7 ± 6.6 | 48 ± 3 |
| Tsukada (2008) 41 | Photograph | 36 | 37.6 ± 3.6 | 46.5 ± 3.2 |
| Iriuchishima (2010) 16 | Photograph | 15 | 31 ± 3 | 49 ± 4 |
| Edwards (2007) 9 | Photograph | 55 | 36 (29-46) | 43 |
| Takahashi (2006) 38 | Photograph | 31 | 28.6 ± 5.3 | 44.2 ± 2.4 |
| Takahashi (2006) 38 | MRI | 23 M | 44.1 (28.3-59.9) | — |
| Takahashi (2006) 38 | MRI | 12 F | 43.7 (27.4-60.0) | — |
| Stäubli (1994) 36 | MRA | 5 | 43 (24.6-62.1) | — |
a Data are reported as mean ± SD (range where provided). Dashes indicate that in that study those measurements were not performed. ACL, anterior cruciate ligament; CT, computed tomography; F, female; M, male; MRA, magnetic resonance arthrography; MRI, magnetic resonance imaging; XR, radiography.
b at is the distance from the anterior tibial border of the superimposed rectangle to the tunnel center; AP is the anteroposterior border length of the rectangle.
c mt is the distance from the medial border of the rectangle to the tunnel center; ML is the mediolateral border length of the rectangle.
Footnotes
Final revision submitted April 15, 2021; accepted May 4, 2021.
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the University of Marilia.
References
- 1. Abebe ES, Moorman CT, 3rd, Dziedzic TS, et al. Femoral tunnel placement during anterior cruciate ligament reconstruction: an in vivo imaging analysis comparing transtibial and 2-incision tibial tunnel-independent techniques. Am J Sports Med. 2009;37(10):1904–1911. [DOI] [PubMed] [Google Scholar]
- 2. Adachi N, Ochi M, Takazawa K, et al. Morphologic evaluation of remnant anterior cruciate ligament bundles after injury with three-dimensional computed tomography. Knee Surg Sports Traumatol Arthrosc. 2016;24(1):148–153. [DOI] [PubMed] [Google Scholar]
- 3. Adachi N, Ochi M, Uchio Y, Sumen Y. Anterior cruciate ligament augmentation under arthroscopy: a minimum 2-year follow-up in 40 patients. Arch Orthop Trauma Surg. 2000;120(3-4):128–133. [DOI] [PubMed] [Google Scholar]
- 4. Buscayret F, Temponi EF, Saithna A, Thaunat M, Sonnery-Cottet B. Three-dimensional CT evaluation of tunnel positioning in ACL reconstruction using the single anteromedial bundle biological augmentation (SAMBBA) technique. Orthop J Sports Med. 2017;5(5):2325967117706511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cicchetti DVS, Sparrow SA. Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic. 1981;86(2):127–137. [PubMed] [Google Scholar]
- 6. Colombet P, Robinson J, Christel P, et al. Morphology of anterior cruciate ligament attachments for anatomic reconstruction: a cadaveric dissection and radiographic study. Arthroscopy. 2006;22(9):984–992. [DOI] [PubMed] [Google Scholar]
- 7. Cremer P, Peltier A, Maubisson L, Neyret P, Lustig S, Servien E. Positioning of the tibial tunnel after single-bundle ACL primary reconstruction on 3D CT scans: a new method. Sports Med Arthrosc Rehabil Ther Technol. 2020;2(5):e615–e622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delaloye J-R, Murar J, Vieira TD, et al. Knee extension deficit in the early postoperative period predisposes to cyclops syndrome after anterior cruciate ligament reconstruction: a risk factor analysis in 3633 patients from the SANTI study group database. Am J Sports Med. 2020;48(3):565–572. [DOI] [PubMed] [Google Scholar]
- 9. Edwards A, Bull AMJ, Amis AA. The attachments of the anteromedial and posterolateral fibre bundles of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1414–1421. [DOI] [PubMed] [Google Scholar]
- 10. Ferretti M, Doca D, Ingham SM, Cohen M, Fu FH. Bony and soft tissue landmarks of the ACL tibial insertion site: an anatomical study. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):62–68. [DOI] [PubMed] [Google Scholar]
- 11. Fu FH, Jordan SS. The lateral intercondylar ridge---a key to anatomic anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2007;89(10):2103–2104. [DOI] [PubMed] [Google Scholar]
- 12. Garofalo R, Mouhsine E, Chambat P, Siegrist O. Anatomic anterior cruciate ligament reconstruction: the two-incision technique. Knee Surg Sports Traumatol Arthrosc. 2006;14(6):510–516. [DOI] [PubMed] [Google Scholar]
- 13. Georgoulis AD, Pappa L, Moebius U, et al. The presence of proprioceptive mechanoreceptors in the remnants of the ruptured ACL as a possible source of re-innervation of the ACL autograft. Knee Surg Sports Traumatol Arthrosc. 2001;9(6):364–368. [DOI] [PubMed] [Google Scholar]
- 14. Guenther D, Irarrázaval S, Nishizawa Y, et al. Variation in the shape of the tibial insertion site of the anterior cruciate ligament: classification is required. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2428–2432. [DOI] [PubMed] [Google Scholar]
- 15. Inderhaug E, Larsen A, Strand T, Waaler PA, Solheim E. The effect of feedback from post-operative 3D CT on placement of femoral tunnels in single-bundle anatomic ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2016;24(1):154–160. [DOI] [PubMed] [Google Scholar]
- 16. Iriuchishima T, Ingham SJM, Tajima G, et al. Evaluation of the tunnel placement in the anatomical double-bundle ACL reconstruction: a cadaver study. Knee Surg Sports Traumatol Arthrosc. 2010;18(9):1226–1231. [DOI] [PubMed] [Google Scholar]
- 17. Jaecker V, Zapf T, Naendrup J-H, et al. High non-anatomic tunnel position rates in ACL reconstruction failure using both transtibial and anteromedial tunnel drilling techniques. Arch Orthop Trauma Surg. 2017;137(9):1293–1299. [DOI] [PubMed] [Google Scholar]
- 18. Kim G, Jung H-J, Lee H-J, Lee J-S, Koo S, Chang S-H. Accuracy and reliability of length measurements on three-dimensional computed tomography using open-source OsiriX software. J Digit Imaging. 2012;25(4):486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koga H, Muneta T, Yagishita K, et al. Effect of femoral tunnel position on graft tension curves and knee stability in anatomic double-bundle anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2014;22(11):2811–2820. [DOI] [PubMed] [Google Scholar]
- 20. Kosy JD, Walmsley K, Gordon EA, et al. Remnant preservation does not affect accuracy of tibial tunnel positioning in single-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2021;29(4):1157–1163. [DOI] [PubMed] [Google Scholar]
- 21. Lertwanich P, Martins CAQ, Asai S, Ingham SJM, Smolinski P, Fu FH. Anterior cruciate ligament tunnel position measurement reliability on 3-dimensional reconstructed computed tomography. Arthroscopy. 2011;27(3):391–398. [DOI] [PubMed] [Google Scholar]
- 22. Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40(7):1551–1557. [DOI] [PubMed] [Google Scholar]
- 23. Lorenz S, Elser F, Mitterer M, Obst T, Imhoff AB. Radiologic evaluation of the insertion sites of the 2 functional bundles of the anterior cruciate ligament using 3-dimensional computed tomography. Am J Sports Med. 2009;37(12):2368–2376. [DOI] [PubMed] [Google Scholar]
- 24. Marchant BG, Noyes FR, Barber-Westin SD, Fleckenstein C. Prevalence of nonanatomical graft placement in a series of failed anterior cruciate ligament reconstructions. Am J Sports Med. 2010;38(10):1987–1996. [DOI] [PubMed] [Google Scholar]
- 25. MARS Group, Wright RW, Huston LJ, et al. Descriptive epidemiology of the Multicenter ACL Revision Study (MARS) cohort. Am J Sports Med. 2010;38(10):1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McConkey MO, Amendola A, Ramme AJ, et al. Arthroscopic agreement among surgeons on anterior cruciate ligament tunnel placement. Am J Sports Med. 2012;40(12):2737–2746. [DOI] [PubMed] [Google Scholar]
- 27. Ochi M, Iwasa J, Uchio Y, Adachi N, Kawasaki K. Induction of somatosensory evoked potentials by mechanical stimulation in reconstructed anterior cruciate ligaments. J Bone Joint Surg Br. 2002;84(5):761–766. [DOI] [PubMed] [Google Scholar]
- 28. Parkinson B, Gogna R, Robb C, Thompson P, Spalding T. Anatomic ACL reconstruction: the normal central tibial footprint position and a standardised technique for measuring tibial tunnel location on 3D CT. Knee Surg Sports Traumatol Arthrosc. 2017;25(5):1568–1575. [DOI] [PubMed] [Google Scholar]
- 29. Pedneault C, Laverdière C, Hart A, Boily M, Burman M, Martineau PA. Evaluating the accuracy of tibial tunnel placement after anatomic single-bundle anterior cruciate ligament reconstruction. Am J Sports Med. 2019;47(13):3187–3194. [DOI] [PubMed] [Google Scholar]
- 30. Pietrini SD, Ziegler CG, Anderson CJ, et al. Radiographic landmarks for tunnel positioning in double-bundle ACL reconstructions. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):792–800. [DOI] [PubMed] [Google Scholar]
- 31. Pinczewski LA, Salmon LJ, Jackson WFM, von Bormann RBP, Haslam PG, Tashiro S. Radiological landmarks for placement of the tunnels in single-bundle reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br. 2008;90(2):172–179. [DOI] [PubMed] [Google Scholar]
- 32. Rayan F, Nanjayan SK, Quah C, Ramoutar D, Konan S, Haddad FS. Review of evolution of tunnel position in anterior cruciate ligament reconstruction. World J Orthop. 2015;6(2):252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rothrauff BB, Kondo E, Siebold R, Wang JH, Yoon KH, Fu FH. Anterior cruciate ligament reconstruction with remnant preservation: current concepts. J ISAKOS. 2020;5(3):128–133. [Google Scholar]
- 34. Sommer C, Friederich NF, Müller W. Improperly placed anterior cruciate ligament grafts: correlation between radiological parameters and clinical results. Knee Surg Sports Traumatol Arthrosc. 2000;8(4):207–213. [DOI] [PubMed] [Google Scholar]
- 35. Sonnery-Cottet B, Freychet B, Murphy CG, Pupim BHB, Thaunat M. Anterior cruciate ligament reconstruction and preservation: the single-anteromedial bundle biological augmentation (SAMBBA) technique. Arthrosc Tech. 2014;3(6):e689–e693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Staubli H-U, Rauschning W. Anatomy and cryosections in vitro complemented by magnetic resonance arthrography in vivo. Knee Surg Sports Traumatol Arthrosc. 1994;2:138–146. [DOI] [PubMed] [Google Scholar]
- 37. Sutter EG, Anderson JA, Garrett WE, Jr. Direct visualization of existing footprint and outside-in drilling of the femoral tunnel in anterior cruciate ligament reconstruction in the knee. Arthrosc Tech. 2015;4(2):e107–e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takahashi M, Doi M, Abe M, Suzuki D, Nagano A. Anatomical study of the femoral and tibial insertions of the anteromedial and posterolateral bundles of human anterior cruciate ligament. Am J Sports Med. 2006;34(5):787–792. [DOI] [PubMed] [Google Scholar]
- 39. Takazawa Y, Ikeda H, Kawasaki T, et al. ACL reconstruction preserving the ACL remnant achieves good clinical outcomes and can reduce subsequent graft rupture. Orthop J Sports Med. 2013;1(4):2325967113505076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tampere T, Van Hoof T, Cromheecke M, et al. The anterior cruciate ligament: a study on its bony and soft tissue anatomy using novel 3D CT technology. Knee Surg Sports Traumatol Arthrosc. 2017;25(1):236–244. [DOI] [PubMed] [Google Scholar]
- 41. Tsukada H, Ishibashi Y, Tsuda E, Fukuda A, Toh S. Anatomical analysis of the anterior cruciate ligament femoral and tibial footprints. J Orthop Sci. 2008;13(2):122–129. [DOI] [PubMed] [Google Scholar]
- 42. Zantop T, Wellmann M, Fu FH, Petersen W. Tunnel positioning of anteromedial and posterolateral bundles in anatomic anterior cruciate ligament reconstruction: anatomic and radiographic findings. Am J Sports Med. 2008;36(1):65–72. [DOI] [PubMed] [Google Scholar]