Abstract
Background
Multidrug resistance (MDR), a major problem in oncology therapy, limits the effectiveness of anticancer drugs. Although p53 functions as a tumor suppressor, the associations between p53 status, autophagy, and MDR are complicated and conditional.
Method
In this report, p53-null human ovarian cancer cell line SKOV3 and its MDR phenotype SKVCR and human leukemia cell line CEM and its MDR phenotype CEM-VLB) (p53 mutant cell line) were used.
Results
Compared to parental SKOV3, the mRNA and protein levels of MAPLC3-II and Beclin1 were higher in SKVCR cells. The inhibition of autophagy by 3-MA significantly sensitized SKVCR to VCR. Conversely, in drug-resistant leukemic cells CEM-VLB, the expressions of Beclin1 and MAPLC3-II were lower than CEM. CEM and CEM-VLB cells were treated with VLB .01 or 0.5 μg/mL, respectively, and the expression of p53 and autophagy up-regulated after VLB (.01 μg/mL) treatment in CEM cells. The percentage of S-phase and G2/M phase cells up-regulated significantly by .01 μg/mL VLB in CEM, which may relate to the status of p53 of CEM cells. A combination of radiation with 3-MA significantly increased apoptosis in CEM-VLB cells.
Conclusion
Our discovery found that p53 is an important regulator controlling the balance between autophagy and MDR, as a potential drug target for ovarian cancer and leukemia.
Keywords: p53, autophagy, multidrug resistance, ionizing radiation, ovarian cancer, acute lymphocytic leukemia
Introduction
Multidrug resistance (MDR) has become an obstacle for chemotherapy of cancer.1-6 p53 is reported to participate in the regulation of MDR, but the association between p53 status and MDR is complicated and conditional. It has been verified that apoptosis is not the only mechanism for MDR regulation by p53, autophagy also seems to be involved.7-12
Autophagy is a dynamic process of protein degradation, which is typically observed during nutrient deprivation and genotoxic stress. It has been confirmed that autophagy is induced or inhibited in various tumor cells, and it is related with cell survival and drug resistance.6,13-18 Notably, p53 plays a dual role in the regulation of autophagy.19-22 The regulation of autophagy by p53 may depend on sub-cellular location of p53. Nuclear p53 can induce autophagy and promote apoptosis, while cytosolic p53 may act as a master repressor. The inhibition effect of p53 on autophagy provides different outcomes, either inhibit apoptosis, or promote tumor cells growth under autophagy-inducing conditions.23-27
In this study, we used human leukemia cell lines CEM/CEM-VLB (p53mutant) and SKOV3/SKVCR (p53-/-) to determine the role of p53-induced autophagy regulating chemotherapy and radiotherapy resistance. It has been shown that the different status of p53 influenced drug sensitivity by inducing different types of cell death, especially autophagy in ovarian cancer cells. These results will hint the individual treatment strategies based on difference p53 status in patients.
Materials and Methods
Reagents and Antibodies
3-methyladenine (3-MA) and monodansylcadaverine (MDC) are from Sigma (St. Louis, MO, United States). The primary antibodies include the following: p53 (Cell Signaling Technology, #126), p21(Cell Signaling Technology, #2947), MAPLC3 (Cell Signaling Technology, Beverly, MA, United States, #2775), Beclin1 (Santa Cruz, CA, United States), monoclonal mouse anti-GAPDH (Santa Cruz, CA, United States), and horseradish peroxidase-(HRP-) conjugated polyclonal goat anti-mouse and anti-rabbit (Santa Cruz, CA, United States).
Cell Culture
SKOV3 (human ovarian carcinoma cell lines), SKVCR (multidrug-resistant phenotype), and human leukemia cell lines CEM and CEM-VLB were obtained from Dr Ling, British Columbia Cancer Research Centre, Vancouver, BC, Canada. Cells were cultured in α-MEM (10% fetal bovine serum, 100 U/mL of penicillin/streptomycin) in incubator (37°C, 5% CO2), and α-MEM medium containing 2.0 μg/mL vincristine (VCR) was used to maintain the drug-resistant phenotype SKVCR and 0.4 μg/mL vinblastine is for CEM-VLB.
Cell Counting Kit-8 Assay
Ninety six-well culture plates were used to seed cells (4 × 105 cells/well for SKVO3/SKVCR, 1 × 104 cells/well for CEM/CEM-VLB). Cell Counting Kit (CCK-8) (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) assay was used to determine cell viability. The absorbance was measured at 450 nm using a microplate reader.
Quantitative Real-Time PCR
RNAiso Plus (Takara Co., Japan) was used to isolate total RNA. Ultraviolet spectrophotometry (A260/A280 ratio) was used to analyze the quantity and quality of RNA. Reverse transcription was performed using Prime Script Rt reagent Kit (Takara Co., Japan), and the samples were then analyzed using the SYBR Premix Ex TapII (Takara Co., Japan) by quantitative real-time PCR (qPCR) (Stratagene MX3000P, Japan). The procedure was as follows: initial activation step for 10 s/95°C; denaturation with 40 cycles for 20 s/95°C; annealing step for 20 s/60°C. Formula 2−(ΔΔCT) was used to determine the change in mRNA levels. GAPDH mRNA was used to normalize the relative amount of mRNA. The primers for genes were as follows: GAPDH forward: 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse: 5′-TGGTGAAGACG CCAGTGGA-3’; MAPLC3II forward: 5′-CCTAGAAGGCGCTTACAGCT-3′ and reverse: 5′-GGGACAATTTCATCCCGAAC-3’; Beclin1 forward:5′-GCGA ATTCATGGAAGGGTCTAAGACGTCCAACAACA-3′ and reverse: 5′- GCGGATCCTC ATTTGTTATAAAATTGTGAGGACACC-3'.
Western Blot Analysis
Cells were harvested after treatment, washed by phosphate buffered saline (PBS), and lyzed with radioimmunoprecipitation assay (RIPA) lysis buffer (150 mM NaCl, 1% (v/v) NP-40, 20 mM Tris HCl, 1% (v/v) Triton X-100, 50 mM NaF, 1 mM EDTA, 1 mM EGTA, .1% (w/v) SDS, protease, and phosphatase inhibitors). Whole-cell lysates were centrifuged at 12,000 rpm for 10 min, quantified using BCA Protein Assay Kit, and subjected to electrophoresis with 10–12% SDS-polyacrylamide gel. After transferred onto polyvinylidene difluoride (Bio-Rad, United States) at 90 V for 120 min, the membranes were blocked by 5% non-fat milk and incubated with primary antibodies overnight at 4°C. Then, the membranes were washed and incubated with a secondary antibody for 1 h at room temperature. The immunoreaction was visualized using Chemiluminescence (Santa Cruz).
Flow Cytometry for Quantitative Analysis of Autophagy and Apoptosis
5 × 105 of cells were collected. For autophagy analysis, cells were stained with MDC. For apoptosis detection, cells were stained using the Annexin V-FITC Apoptosis Detection Kit I (BD Biosciences, San Diego, CA) according to the manufacturer’s recommendation. The stained cells were sorted by flow cytometry (BD FACS Canto) and analyzed by using the FCS Express v2.0 software.
Cell Cycle Analysis
Cell suspension was fixed by 75% ethanol at −20°C for 8–10 h. Cells were then incubated in the dark for 30 min after the addition of 100 μL PI and 100 μL RNase. Cell cycle progression was measured by flow cytometry (BD Biosciences), and the percentage of cells at G0/G1, S, or G2/M phase was quantified.
Irradiation
An X-ray generator (X-RAD 320 ix, Precision X-ray Inc., North Branford, CT, United States) was utilized to deliver radiation at a dose rate of 1.0 Gy/min.
Statistical Analysis
SPSS 22.0 software (SPSS, Chicago, IL, United States) was utilized for statistical analyses. Differences between two groups were evaluated with Student’s t-test (two-tailed). Survival curve was generated with Kaplan–Meier method. Each experiment was conducted three times. Experimental results are presented as mean ± SD. Data were considered as statistically significant when P value was less than .05.
Results
The Multi-Drug Resistance Phenotype in VEM-VLB and SKVCR Cells
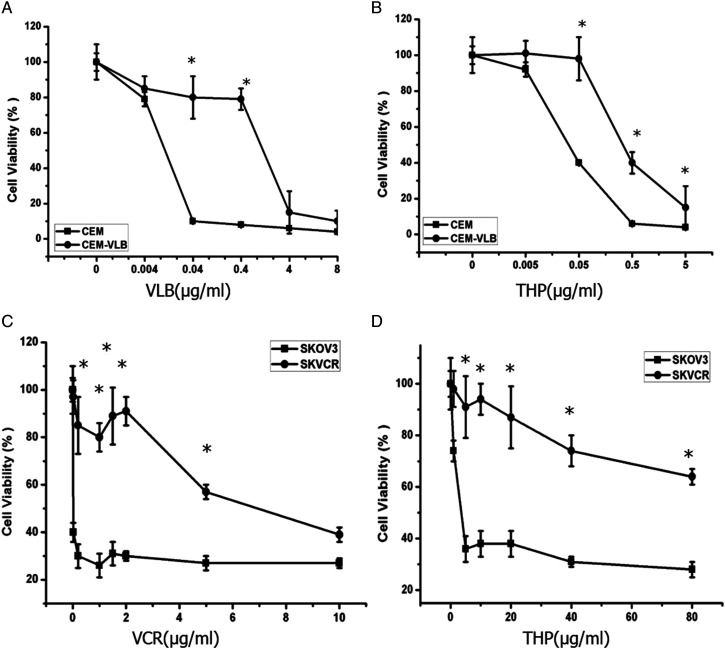
VEM and VEM-VLB cells were treated with different doses of VLB and THP for 48 h (Figures 1A and B). SKOV3 and SKVCR cells were treated with different doses of VCR and THP for 48 h (Figures 1C and D). Then CCK-8 assay was used to detect drug sensitivity. We observe a cross-resistance pattern in the assays. Cell survival decreased in a dose-dependent manner in SKOV3/SKVCR and CEM/CEM-VLB cells. However, as expected, SKVCR and CEM-VLB cells were more resistant to different chemotherapy drugs, as compared with the SKOV3 and CEM parental cells (P < .05).
Figure 1.
The chemotherapy sensitivity of CEM/CEM-VLB and SKOV3/ SKVCR cells. (A, B) Cell viability was detected by CCK-8 assay to represent the sensitivity of CEM and CEM-VLB to VLB and THP for 48 h. (C, D) Cell viability was detected by CCK-8 assay to represent the sensitivity of SKOV3 and SKVCR to VCR and THP for 48 h. Values are the mean ± S.E.M. (n = 3). *P < .05, vs control.
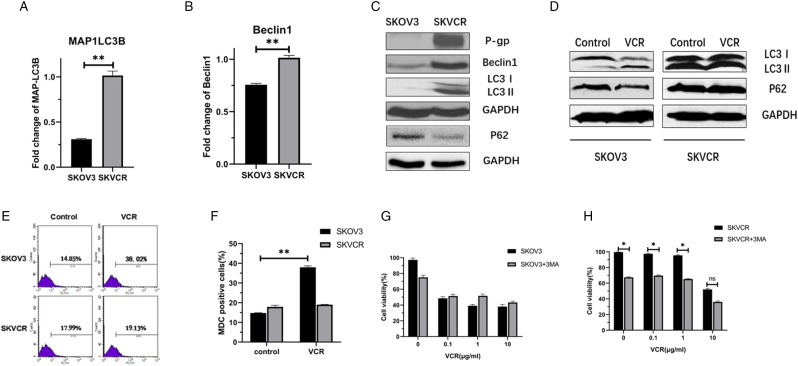
The Roles of Autophagy in Multidrug Resistance in SKOV3 and SKVCR Cells
qRT-PCR and Western blot analysis displayed that MAPLC3-II and Beclin1 were higher both at mRNA and protein levels in SKVCR cells. The ratio of MAPLC3-II/I increased to 1.68-fold in SKVCR as compared with SKOV3, suggesting the development of autophagy (Figures 2A–C). The MAPLC3-II increased and p62 decreased in SKBV3 following VCR treatment, but not in SKVCR cells. To determine the roles of autophagy in drug sensitivity, an autophagy inhibitor, 3-MA, was pretreated for 1 h following chemotherapeutic drugs treatment. The inhibition of autophagy by 3-MA significantly sensitized SKVCR to VCR (Figures 2D–F).
Figure 2.
Autophagy changes in SKOV3 and SKVCR cells. (A, B) Real-time RT-PCR was used to detect the mRNA levels of MAPLC3 and Beclin1 in SKOV3 and SKVCR cells. (C) Western blot showed P-gp, Beclin1, and MAPLC3I/II levels in SKOV3 and SKVCR cells. (D) Western blot showed MAPLC3I/II and p62 levels in SKOV3 and SKVCR cells following VCR treatment. (E, F) Autophagy was determined by flow cytometry after MDC staining. The proportion of autophagy was analyzed by using flow cytometry. (G, H). SKOV3 and SKVCR cells were pretreated with 3-MA (2 mM), then treated with VCR for 24 h. Autophagy was analyzed after MDC staining by flow cytometry. Values are the mean ± S.E.M. (n = 3). *P < .05, vs control.
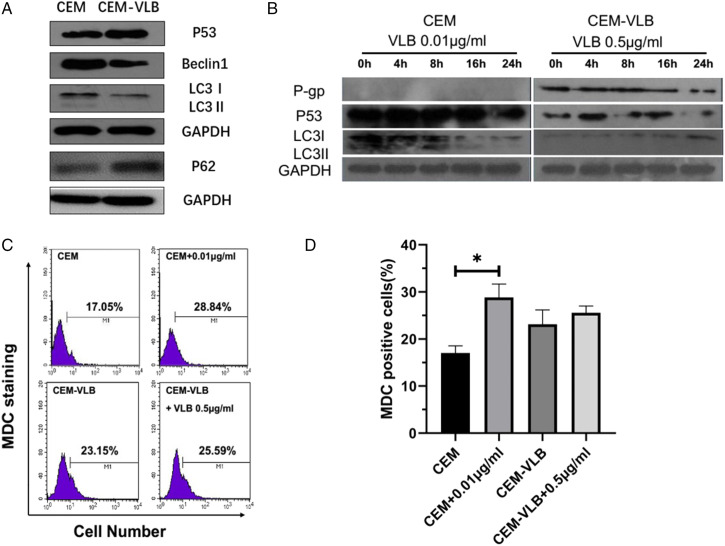
The Roles of Autophagy in Multidrug Resistance in CEM and CEM-VLB Cells
Conversely, in drug-resistant leukemic cells CEM-VLB, the expressions of Beclin1 and MAPLC3-II were lower than CEM, indicating a low level of autophagy in the CEM-VLB cells. Next, we determined the relationship between p53 and MDR. Compared to CEM, p53 expression increased significantly in CEM-VLB cells(Figure 3A).To determine the correlation between autophagy and p53 in the drug-sensitive and -resistant cells under the same pressure conditions, CEM and CEM-VLB cells were treated with VLB .01 μg/mL or 0.5 μg/mL, respectively. Then cells were harvested at 4, 8, 16, 24 hours. Western blot results showed that the expression of p53 up-regulated after VLB (.01 μg/mL) treatment in CEM cells and induced conversion of MAPLC3 (LC3I to LC3 II). The levels of MAP LC3-II rose to the peak at 8 hours in CEM cells, then decreased with the down-regulation of p53(Figure 3B).The autophagy in CEM cells increased after VLB .01 μg/mL treatment, and the chemotherapeutic drugs did not trigger biological effects in CEM-VLB cell (Figures 3C and D).
Figure 3.
The effects of autophagy on multidrug resistance in CEM and CEM-VLB cells. (A)Western blot showed p53, Beclin1, and MAPLC3I/II levels in CEM and CEM-VLB cells. (B) Western blot showed P-gp, p53, and MAPLC3I/II levels in CEM and CEM-VLB cells following VLB treatment. (C, D) Autophagy was determined by Flow Cytometry after MDC staining. The proportion of autophagy was analyzed by using flow cytometry. Values are the mean ± S.E.M. (n = 3). *P < .05, vs control.
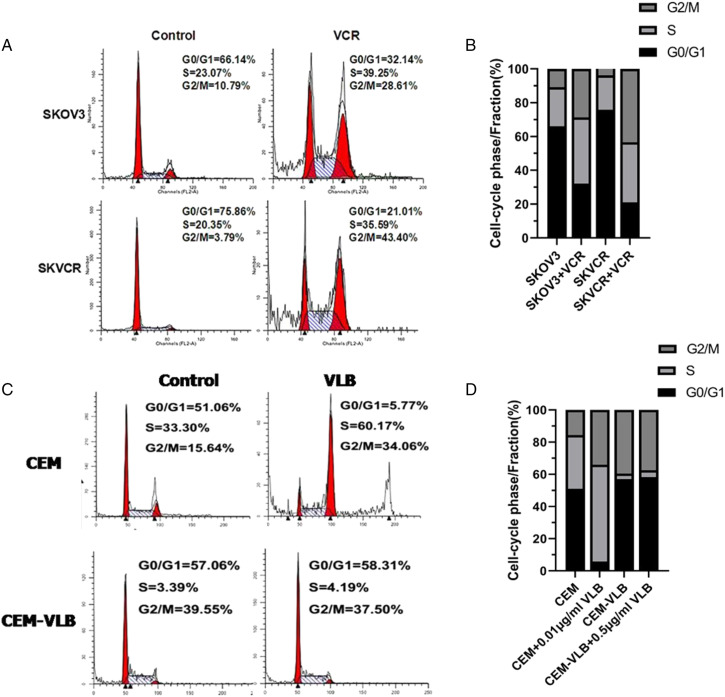
Cell Cycle Arrest in SKOV3/SKVCR and CEM/CEM-VLB Cells
Flow cytometry analysis showed that the percentage of S-phase and G2/M phase cells up-regulated significantly in SKVO3 and SKVCR (Figures 4A and B). What is more, the percentage of S-phase and G2/M phase cells up-regulated significantly in CEM + .01 μg/mL VLB (Figures 4C and D), this may be related to the p53 gene status in Cells. Some p53 target genes have been confirmed to induce G2 phase arrest, such as inhibition of CDC2 and cyclinB1. Mutant p53 could not cause G0/G1 phase arrest, and the percentage of S phase cells increased relatively.
Figure 4.
The effects of cell cycle on multidrug resistance in SKOV3/SKVCR and CEM/CEM-VLB cells. (A, B). Cell cycle was determined by flow cytometry after using PI stain in SKVO3 and SKVCR following VCR treatment. (C, D) Cell cycle was determined by flow cytometry after using PI staining CEM/CEM-VLB cells following VLB treatment. Values are the mean ± S.E.M. (n = 3). *P < .05, vs control.
Effects of Radiation on the Regulations of p53 and Autophagy in CEM and CEM-VLB Cells
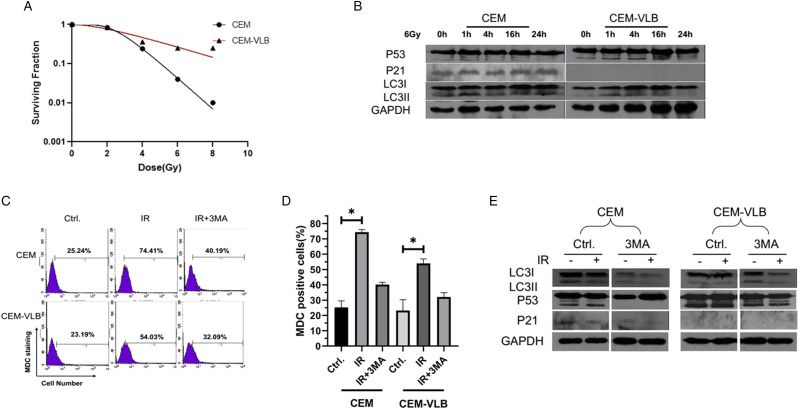
VEM and VEM-VLB cells were treated with different doses of radiation (0, 2.0, 4.0, 6.0, 8.0 Gy), and after 96 h, the radiosensitivity was detected by CCK-8 assay. Cell survival decreased in a dose-dependent manner in CEM/CEM-VLB cells. However, as expected, the CEM-VLB cells were more resistant to radiation, as compared with the parental CEM cells, especially after 6.0, 8.0 Gy treatment (P < .05) (Figure 5A).
Figure 5.
Effects of radiation on the regulations of p53 and autophagy in CEM and CEM-VLB cells. VEM and VEM-VLB cells were treated with different doses of radiation (0, 2.0, 4.0, 6.0, 8.0 Gy) for 96 h, and CCK-8 assay was used to detect radiation sensitivity. (B) Western blot showed p53, p21, and MAPLC3I/II levels following 6 Gy x-ray treated for .1, 4, 16, 24 h in CEM and CEM-VLB cells. (C, D) The autophagy rates were tested by MDC staining following radiation treatment with or without 3-MA in CEM and CEM-VLB cells. (E) Western blot showed p53, p21, and MAPLC3I/II levels following radiation treatment with or without 3-MA in CEM and CEM-VLB cells. Values are the mean ± S.E.M. (n = 3). *P < .05, vs control.
In CEM cells, p53 continuously activated its downstream gene p21 expression after radiation treatment, while the high level of p53 in CEM-VLB did not activate p21. In addition, radiation-induced autophagy was more obvious in CEM than CEM-VLB cells (Figure 5B). Then, we used MDC staining to analyze the autophagy rates. After radiation, the percentages of MDC positive cells increased from 25.24% to 74.41%, 3-MA could decrease it from 74.41% to 40.19% in CEM cells. Meanwhile, in CEM-VLB cells, the MDC positive cells increased from 23.19% to 54.03%, and decreased from 54.03% to 32.09% following 3-MA treatment (Figures 5C and D). Western blot data displayed that the conversion of MAP LC3 (LC3I to LC3II) in 3-MA + 6.0 Gy X-rays decreased significantly as compared with control group, indicating the inhibition of autophagy. CEM cells treated with 3-MA + 6.0 Gy X-ray for 16 h resulted in the disappearance of p53 abnormal band and the reduction of p21, which supported a critical role for p53 in the regulation of autophagy (Figure 5E).
Effects of Radiation on the Regulations of Autophagy and Apoptosis in CEM and CEM-VLB Cells
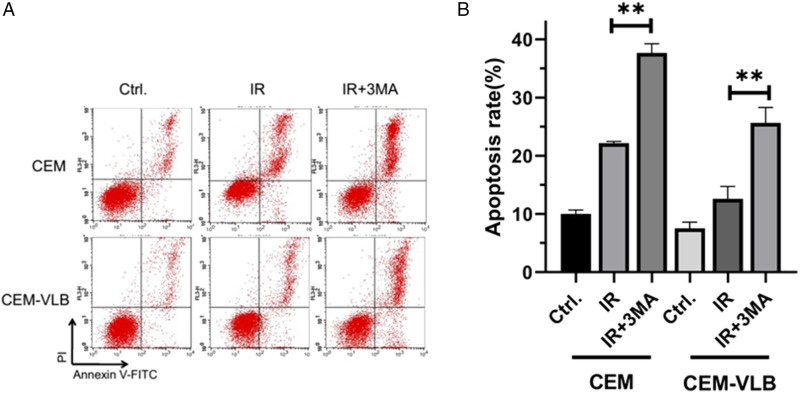
CEM and CEM-VLB cells were pretreated with 2 mM 3-MA for 1 h followed 6.0 Gy X-rays, and after 16 h, apoptotic percentages were analyzed by using Annexin V/PI staining. Results showed that apoptotic rate of CEM and CEM-VLB cells increased to 22.03 and 12.11%, respectively, (P < .05). A combination of IR with autophagy inhibitors significantly increased apoptosis in CEM and CEM-VLB (Figures 6A and B).
Figure 6.
Effects of radiation on the regulations of autophagy and apoptosis in CEM and CEM-VLB cells. (A, B) CEM and CEM-VLB cells were pretreated with 2 mM 3-MA for 1 h and then treated with 6.0 Gy X-rays for 16 h, and apoptotic percentages were analyzed by using Annexin V/PI staining. Values are the mean ± S.E.M. (n = 3). *P < .05, vs control.
Discussion
Autophagy, a highly regulated cell “self-eating” pathway, is controlled by the action of over 34 autophagy-related proteins (collectively termed Atgs). One of the major challenges in cancer therapy is the drug resistance. Recently, several studies have shown that autophagy constitutes a potential target for cancer therapy, which contributes to the anticancer efficacy of drugs as well as drug resistance.28-30
Tumor suppressor p53 is closely related to the occurrence of autophagy, which can regulate autophagy from many aspects.31-41 While little is known about the relationship between MDR, p53, and autophagy. It has been reported that p53 localized to the nucleus through transcriptional activation of target genes to enhance autophagy.31-35 Studies have shown that autophagy can be activated by inhibiting the function of p53 by genetic methods or pharmacological inhibitors.42,43 The expression of endogenous p53 in human leukemia cell line CEM, which is a kind of heterozygous mutation, is known as the pseudo wild type p53. 44 In CEM cells, VLB increased the expression of p53, MAPLC3-I conversion to MAPLC3-II. After that, MAPLC3-II decreased with the decrease of p53 expression. But, CEM-VLB does not appear to have biological effects on the drug’s stimulating effect, which may be due to the difference in the drug sensitivity of CEM and CEM-VLB cells.
We found that S phase and G2/M block are most obvious in CEM, which may be related to p53 status in CEM cells. Some p53 target genes have been confirmed to induce G2 arrest, for example, inhibition of CDC2 and G2 could occur after cyclinB1 block. 45 The mutant p53 could not induce G0/G1 phase arrest, cells entered S phase and resulted in the increased S phase percentage. More importantly, cell cycle inhibitors, such as genotoxic drugs that cause DNA damage and cell cycle arrest to inhibit tumor cell division, always activate autophagy, which delays cell death and may therefore lead to chemoresistance
Since p53 is a radiation-sensitive gene, 6.0 Gy X-ray was administrated, and radiation induced high expression of p53 in CEM and CEM-VLB cells. In CEM cells, the p53–p21 pathway has been activated, but not in CEM-VLB cell. Although p21 is a direct p53 response gene, only one has evaluated p21 expression as a function of sequenced p53 gene mutation. The reasons may have two aspects: First is CEM-VLB radiation resistance is higher than CEM cells, so CEM-VLB is less sensitive to IR than CEM; second, CEM-VLB cells of p53 function are different from the parental CEM, although IR induced the expression of p53 protein, p53 may not have the regulatory function of autophagy. Our previous detection of protein also found that wt p53 protein was not abnormal bands, and mutation of p53 gene will appear with abnormal migration in 53kDa either up or down, and the anomalies may be for mutant p53 expression subtypes. Morgan SE 46 reported, although drug-sensitive CEM cells and multidrug resistant cell CEM/VM-1 contain the same p53 mutant forms, in CEM/VM-1 cells half-life of p53 protein decreased, and MDM2-mediated degradation of p53 pathway in resistant cells has changed.
The mutant form of p53 can confer resistance to apoptosis, thereby reducing tumor cell susceptibility to cell death. Mutant p53 not only interferes with the transcriptional activity of wt p53 in the nucleus but also abolishes the interaction between wt p53 and BCL-2 family proteins in the cytoplasm. 6.0 Gy X-ray irradiation increased apoptosis in CEM and CEM-VLB cells, and the apoptosis rate in 3-MA combined with IR group was much higher than that of in single irradiation group. Therefore, we believe that when CEM-VLB cells are not sensitive to chemotherapy, radiotherapy combined with autophagy inhibitors can be used to improve the therapeutic effect.
In summary, p53 plays a crucial role in autophagy and MDR regulation. A better understanding of the relationship between autophagy and multidrug resistance will improve the efficacy of chemotherapy and radiotherapy.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China grant (81673092, 81773363, 81872558, and 81972969).
ORCID iD
Xiaodong Liu https://orcid.org/0000-0002-6879-990X
References
- 1.Spolitu S, Uda S, Deligia S, et al. Multidrug resistance P-glycoprotein dampens SR-BI cholesteryl ester uptake from high density lipoproteins in human leukemia cells. Am J Cancer Res. 2016;6(3):615-627. [PMC free article] [PubMed] [Google Scholar]
- 2.Chen CY, Liu NY, Lin HC, Lee CY, Hung CC, Chang CS. Synthesis and bioevaluation of novel benzodipyranone derivatives as P-glycoprotein inhibitors for multidrug resistance reversal agents. Eur J Med Chem. 2016;118:219-229. [DOI] [PubMed] [Google Scholar]
- 3.Yu Y, Xiang K, Xu M, et al. Prodrug nanomedicine inhibits chemotherapy-induced proliferative burst by altering the deleterious intercellular communication. ACS Nano. 2021;15(1):781-796. [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Liang Z, Ma P, et al. Application of CRISPR/cas9 system to reverse ABC-mediated multidrug resistance. Bioconjug Chem. 2021;32(1):73-81. [DOI] [PubMed] [Google Scholar]
- 5.Li B, Jiang J, Assaraf YG, Xiao H, Chen ZS, Huang C. Surmounting cancer drug resistance: New insights from the perspective of N6-methyladenosine RNA modification. Drug Resist Updates. 2020;53:100720. [DOI] [PubMed] [Google Scholar]
- 6.Zamame Ramirez JA, Romagnoli GG, Kaneno R. Inhibiting autophagy to prevent drug resistance and improve anti-tumor therapy. Life Sci. 2021;265:118745. [DOI] [PubMed] [Google Scholar]
- 7.Ye S, Shen J, Choy E, et al. p53 overexpression increases chemosensitivity in multidrug-resistant osteosarcoma cell lines. Canc Chemother Pharmacol. 2016;77(2):349-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature. 2009;458(7242):1127-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris SL, Levine AJ. The p53 pathway: Positive and negative feedback loops. Oncogene. 2005;24(17):2899-2908. [DOI] [PubMed] [Google Scholar]
- 10.Wu D, Zhang Z, Chu H, et al. Intron 3 sixteen base pairs duplication polymorphism of p53 contributes to breast cancer susceptibility: Evidence from meta-analysis. PLoS One. 2013;8(4):e61662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goh AM, Coffill CR, Lane DP. The role of mutant p53 in human cancer. J Pathol. 2011;223(2):116-126. [DOI] [PubMed] [Google Scholar]
- 12.Xu J, Patel NH, Gewirtz DA. Triangular relationship between p53, autophagy, and chemotherapy resistance. Int J Mol Sci. 2020;21(23):8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macintosh RL, Ryan KM. Autophagy in tumour cell death. Semin Canc Biol. 2013;23(5):344-351. [DOI] [PubMed] [Google Scholar]
- 14.Cesen MH, Pegan K, Spes A, Turk B. Lysosomal pathways to cell death and their therapeutic applications. Exp Cell Res. 2012;318(11):1245-1251. [DOI] [PubMed] [Google Scholar]
- 15.Hara T, Nakamura K, Matsui M, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441(7095):885-889. [DOI] [PubMed] [Google Scholar]
- 16.Wirawan E, Berghe TV, Lippens S, Agostinis P, Vandenabeele P. Autophagy: For better or for worse. Cell Res. 2012;22(1):43-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.An Y, Zhang Z, Shang Y, et al. miR-23b-3p regulates the chemoresistance of gastric cancer cells by targeting ATG12 and HMGB2. Cell Death Dis. 2015;6:e1766-e1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camuzard O, Trojani MC, Santucci-Darmanin S, et al. Autophagy in osteosarcoma cancer stem cells is critical process which can be targeted by the antipsychotic drug thioridazine. Cancers. 2020;12(12):3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim WK, Pyee Y, Chung HJ, et al. Antitumor activity of spicatoside a by modulation of autophagy and apoptosis in human colorectal cancer cells. J. Nat. Prod. 2016;79(4):1097-1104. [DOI] [PubMed] [Google Scholar]
- 20.White E. Autophagy and p53. Cold Spring Harb Perspect Med. 2016;6(4):a026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cordani M, Oppici E, Dando I, et al. Mutant p53 proteins counteract autophagic mechanism sensitizing cancer cells to mTOR inhibition. Mol Oncol. 2016;10(7):1008-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui L, Song Z, Liang B, Jia L, Ma S, Liu X. Radiation induces autophagic cell death via the p53/DRAM signaling pathway in breast cancer cells. Oncol Rep. 2016;35(6);3639-3647. [DOI] [PubMed] [Google Scholar]
- 23.Lu M, Boschetti C, Tunnacliffe A. Long term aggresome accumulation leads to DNA damage, p53-dependent cell cycle arrest, and steric interference in mitosis. J Biol Chem. 2015;290(46):27986-28000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mrschtik M, O’Prey J, Lao LY, et al. DRAM-3 modulates autophagy and promotes cell survival in the absence of glucose. Cell Death Differ. 2015;22(10):1714-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comel A, Sorrentino G, Capaci V, Del Sal G. The cytoplasmic side of p53’s oncosuppressive activities. FEBS Lett. 2014;588(16):2600-2609. [DOI] [PubMed] [Google Scholar]
- 26.Rosenfeldt MT, O’Prey J, Morton JP, et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504(7479):296-300. [DOI] [PubMed] [Google Scholar]
- 27.Kong D, Ma S, Liang B, et al. The different regulatory effects of p53 status on multidrug resistance are determined by autophagy in ovarian cancer cells. Biomed Pharmacother. 2012;66(4):271-278. [DOI] [PubMed] [Google Scholar]
- 28.Yang Yp, Liang Zq, Gu Zl, Qin Zh. Molecular mechanism and regulation of autophagy1. Acta Pharmacol Sin. 2005;26(12):1421-1434. [DOI] [PubMed] [Google Scholar]
- 29.Ng G, Huang J. The significance of autophagy in cancer. Mol Carcinog. 2005;43(4):183-187. [DOI] [PubMed] [Google Scholar]
- 30.Nelson DA, White E. Exploiting different ways to die. Genes & Development. 2004;18(11):1223-1226. [DOI] [PubMed] [Google Scholar]
- 31.Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci Unit States Am. 2005;102(23):8204-8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones RG, Plas DR, Kubek S, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18(3):283-293. [DOI] [PubMed] [Google Scholar]
- 33.Feng Z, Hu W, de Stanchina E, et al. The regulation of AMPK β1, TSC2, and PTEN expression by p53: Stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Canc Res. 2007;67(7):3043-3053. [DOI] [PubMed] [Google Scholar]
- 34.Tabancay AP, Jr., Gau C-L, Machado IMP, et al. Identification of dominant negative mutants GTof Rheb Pase and their use to implicate the involvement of human Rheb in the activation of p70S6K. J Biol Chem. 2003;278(41):39921-39930. [DOI] [PubMed] [Google Scholar]
- 35.Scott RC, Schuldiner O, Neufeld TP. Role and regulation of starvation-induced autophagy in the drosophila fat body. Dev Cell. 2004;7(2):167-178. [DOI] [PubMed] [Google Scholar]
- 36.Crighton D, Wilkinson S, O’Prey J, et al. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126(1):121-134. [DOI] [PubMed] [Google Scholar]
- 37.Zalckvar E, Berissi H, Eisenstein M, Kimchi A. Phosphorylation of beclin 1 by DAP-kinase promotes autophagy by weakening its interactions with Bcl-2 and Bcl-XL. Autophagy. 2009;5(5):720-722. [DOI] [PubMed] [Google Scholar]
- 38.Guo Xl, Hu F, Zhang SS., et al. Inhibition of p53 increases chemosensitivity to 5-FU in nutrient-deprived hepatocarcinoma cells by suppressing autophagy. Canc Lett. 2014;346(2):278-284. [DOI] [PubMed] [Google Scholar]
- 39.Stambolic V, MacPherson D, Sas D, et al. Regulation of PTEN transcription by p53. Mol Cell. 2001;8(2):317-325. [DOI] [PubMed] [Google Scholar]
- 40.Foster FM, Traer CJ, Abraham SM, Fry MJ. The phosphoinositide (PI) 3-kinase family. J Cell Sci. 2003;116(15):3037-3040. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Kan H, Liu Y, Ding W. Plumbagin induces Ishikawa cell cycle arrest, autophagy, and apoptosis via the PI3K/Akt signaling pathway in endometrial cancer. Food Chem Toxicol. 2021;148:111957. [DOI] [PubMed] [Google Scholar]
- 42.Tasdemir E, Maiuri MC, Galluzzi L, et al. Regulation of autophagy by cytoplasmic p53. Nat Cell Biol. 2008;10(6):676-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maiuri MC, Galluzzi L, Morselli E, Kepp O, Malik SA, Kroemer G. Autophagy regulation by p53. Curr Opin Cell Biol. 2010;22(2):181-185. [DOI] [PubMed] [Google Scholar]
- 44.Cheng J, Haas M. Frequent mutations in the p53 tumor suppressor gene in human leukemia T-cell lines. Mol Cell Biol. 1990;10(10):5502-5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20(15):1803-1815. [DOI] [PubMed] [Google Scholar]
- 46.Morgan SE, Kim R, Wang PC, et al. Differences in mutant p53 protein stability and functional activity in teniposide-sensitive and -resistant human leukemic CEM cells. Oncogene. 2000;19(43):5010-5019. [DOI] [PubMed] [Google Scholar]