Abstract
Collagen has a major role in the structural organization of tendons. Picrosirius red (PSR) staining viewed under polarized light microscopy is the standard method to evaluate the organization of collagen fibers in tissues. It is also used to distinguish between type I and type III collagen in tissue sections. However, accurate analysis and interpretation of PSR images are challenging because of technical factors and historical misconceptions. The aim of this study was to clarify whether collagen types I and III can be distinguished by PSR staining in rat Achilles tendons, using double immunohistochemistry as the positive control. Our findings showed that PSR staining viewed with polarized light microscopy was suitable for qualitative and quantitative assessment of total collagen but was not able to distinguish collagen types. We found it critical to use a polarizing microscope equipped with a rotating stage; tendon section orientation at 45° with respect to crossed polarizers was optimal for the qualitative and quantitative assessment of collagen organization. Immunohistochemistry was superior to PSR staining for detection of collagen type III. We also compared formalin and Bouin solution as fixatives. Both produced similar birefringence, but formalin-fixed tendons provided higher quality histological detail with both hematoxylin–eosin and immunostaining:
Keywords: Achilles tendon, collagen type, immunostaining, microscopy, polarization, Sirius red
Introduction
Collagen fibers are important for the composition and normal architecture of extracellular matrices of connective tissues such as tendons and ligaments. Tendons are largely composed of different types of collagen, which help determine the biomechanical properties of these tissues.1 Collagen makes up 60–80% of the total extracellular matrix protein of normal tendons.
Collagen type I is the major fibrillar collagen in tendon and normally forms parallel fiber bundles to provide the major tensile resistance to mechanical forces.2 It may also be involved in regulating cell proliferation, migration, and differentiation of mesenchymal stem cells.3 Tendons also contain collagen type III, which is organized as a meshwork of smaller collagen bundles. Collagen type III is also found in early-stage granulation tissue formation in wound healing, and it is believed to have a prominent role in regulating tissue regeneration.4,5 It normally accounts for less than 10% of the total weight of healthy tendons6,7 and is restricted to tendon sheaths.8 The tendon repair tissue that forms after tendon injury, however, frequently shows increased collagen type III content, although the specific role of collagen type III in repair has yet to be defined. For instance, collagen type III content is markedly increased in patients with complete Achilles tendon rupture.9,10 Importantly, the repaired tendon rarely achieves functionality equal to that of the preinjured state, with up to a 30% decrease in final tensile strength.11 Similarly, the collagen type III:type I ratio has been reported to be increased in shoulder tendinopathies,12,13 which suggests that a high collagen type III:type I ratio might account for poor matrix organization. This may not only affect tendon structure but also lead to inferior tendon repair.
As research on tendons increases, so does the need for standardized histochemical methods to identify changes in the composition and organization of collagens within tendons. This is particularly important when assessing the outcomes of tendon regeneration, tendon repair strategies, tissue engineering approaches, and the altered mechanical properties created by tendinopathies.
The van Gieson and Masson trichrome histochemical stains have been used to visualize collagen in paraffin-embedded tissue sections. However, collagen type I and type III cannot be distinguished by these stains.14 Moreover, they may fail to stain thin fibers such as collagen type III,15 leading to underestimation of the actual collagen content of the tissue.
In contrast to trichrome staining, picrosirius red (PSR, also called Sirius red) staining has been considered the standard for collagen detection and quantitative estimation in histological sections of normal and abnormal tissues.14,16–20 PSR F3BA is an anionic dye whose sulfonic acid groups react with high specificity with basic amino acids in collagen molecules. Because PSR molecules align parallel to the long axis of each collagen fiber, PSR enhances the birefringence of collagen under polarized light microscopy.17,21,22 In addition to detecting bulk collagen, PSR staining has been used to distinguish collagen type I from collagen type III in several tissues, such as those of the musculoskeletal system,23–25 liver,26 gastrointestinal tract,27 skin,28,29 and myocardium.30 With visualization of PSR-stained tissues under polarized light, termed PSR-polarization, thick collagen type I fibers appear yellowish-orange, orange, to red, whereas thin collagen type III fibers are supposed to appear green to yellowish-green against a black background.23,31
Although many studies have confirmed that PSR staining is useful for studying the collagen network under normal conditions and in pathological processes in different tissues,23–28,32,33 other studies have questioned whether this staining can identify collagen types by their colors under polarized light.29,34–36 For instance, one study using PSR-stained skin sections from patients with Ehlers–Danlos syndrome type IV showed fibers displaying the birefringence of collagen type III (greenish), although this condition is characterized by collagen type III deficiency.29 This questions the ability of the technique to accurately distinguish between collagen types.
With this as background, while preparing for a large-scale rat tendon study, we evaluated our own tendon collagen data using the PSR-polarization method. We aimed to investigate the ability of this method to distinguish collagen type I from collagen type III in rat tendons.
Materials and Methods
Animals and Collection of Achilles Tendon Specimens
Achilles tendons were obtained from Sprague Dawley rats aged 3–6 months (Charles River Laboratories; Raleigh, NC) and fixed immediately after the animals were euthanized. To determine the effect of histological fixative type, tendon specimens were fixed in either Bouin fixative (Polysciences, Inc; Warrington, PA), which has traditionally been used for PSR staining, or 10% neutral-buffered formalin solution (Thermo Scientific Richard-Allan Scientific; Waltham, MA) for 24 hr. Bouin-fixed tissues were washed thoroughly in tap water until the water ran colorless. Fixed specimens were dehydrated in a series of graded ethanols and embedded in paraffin. Specimens were sectioned longitudinally at a thickness of 5 µm using an automatic microtome (HM 355S; Thermo Scientific, Kalamazoo, MI), mounted onto positively charged slides (Fisherbrand Superfrost Plus Microscope Slides; Fisher Scientific, Pittsburgh, PA), and used for PSR, hematoxylin–eosin (H&E), and immunohistochemical staining.
PSR Staining
Serial paraffin-embedded sections of tendon tissue were stained with PSR using a commercial kit (Polysciences, Inc), according to the manufacturer’s protocol. Briefly, paraffin-embedded sections were deparaffinized and rehydrated through a series of xylene and graded alcohol washes, followed by staining with Weigert hematoxylin for 7 min. Slides were washed for 10 min in running tap water, treated with a solution of phosphomolybdic acid for 2 min (solution A), and then rinsed in distilled water. Next, the sections were stained in PSR F3BA (pH 2) (solution B) for 1 hr and then washed in 0.1 N hydrochloric acid (solution C) for 2 min. Slides were submersed in 70% ethanol for 45 sec, quickly dehydrated in graded ethyl alcohols, cleared with xylene, and mounted with xylene-based mounting medium (Thermo Scientific Richard-Allan Scientific).
H&E staining of Achilles tendon was performed using standard protocols for the histological assessment of tendon structure and collagen fiber organization.
Polarized Light Microscopy and Imaging
The tissue sections were analyzed under linear polarized light or brightfield microscopy using a microscope (IX83; Olympus, Waltham, MA) equipped with a digital camera (DP80; Olympus) with its dual charge–coupled device sensors, a rotating polarizer (IX-LWPO) built into the condenser with a fixed analyzer (U-ANT), and a high-luminosity and high-color-rendering LED. The IX83 also has a fully motorized stage with a circular rotating slide holder (H224ROTS; Prior Scientific, Cambridge, UK) capable of 360° rotation. The substage condenser height and the field iris diaphragm were aligned for proper Köhler illumination to ensure even illumination of the specimen. The optimal exposure time setting was determined by acquiring a series of images at varied lengths of time with constant illumination, choosing the exposure time that guaranteed capture of the brightest yellow/red and green birefringence possible without pixel saturation against a dark background. The exposure time was readjusted every time the objective and the slide changed.
To evaluate how the tendon orientation on the slide may influence the polarizing color of PSR-stained collagen fibers, the slide holder was rotated in the axial plane in increments of approximately 22.5° counterclockwise, with the tendon long axis oriented at 22.5°, 45°, 67.5°, and 90° and then back to the original 0° position. Images were stitched at 4× magnification using the cellSens imaging software (Olympus, version 3.2) to capture the entire cross-sectional area of the tendon. Anatomic landmarks (e.g., paratenon) on the tendon sections were used as points of reference to acquire the same region of interest in each tendon orientation, which represented the area of best tendon morphology. Two consecutive slides of each specimen were examined independently by three investigators (C.M.L.D.P., S.A.M., M.J.C.).
Immunohistochemistry
As a positive control, collagen type I and collagen type III were identified on Achilles tendon sections by double immunohistochemistry. Briefly, formalin-fixed tissue sections (5 µm) were deparaffinized and rehydrated as described above, and then washed twice for 5 min in phosphate-buffered saline (Sigma; St. Louis, MO). Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 min, followed by treatment with 0.1% hyaluronidase with 0.1% pronase in tris-buffered saline (all from Sigma) in a water bath for 30 min at 37C to unmask the collagen antigens. Slide sections were washed 3 times in tris-buffered saline/0.1% Tween-20 (TBS-T) for 5 min each. Nonspecific binding was blocked with 5% normal horse serum (Abcam; Cambridge, MA) and 1% bovine serum albumin (Sigma) in TBS-T for 30 min. Slides were incubated with a specific mouse monoclonal anti–collagen type I antibody [COL1 (ABIN957814), 1:50; Antibodies-Online, Limerick, PA] in a humidified chamber at room temperature for 1 hr, washed 3 times in TBS-T for 10 min, and incubated for 20 min with the 2-step HiDef Detection HRP Polymer System (954D-10; Cell Marque Millipore Sigma, Rocklin, CA). This was followed by incubation with horseradish peroxidase detector reagent for 10 min.
The antigen–antibody complex was detected by reaction with 3,3′-diaminobenzidine peroxidase substrate (ImmPACT DAB; Vector Laboratories, Burlingame, CA), which yields a brown product. Diaminobenzidine-stained slides were washed and subsequently incubated with the second primary antibody, a rabbit polyclonal specific to collagen III (ab7778, 1:300; Abcam) in a humidified chamber at 4C overnight. The next day, slides were washed 3 times in TBS-T for 10 min and treated with the 2-step HiDef Detection alkaline phosphatase polymer system (962D-10; Cell Marque Millipore Sigma), incubated with the amplifier solution for 10 min at room temperature, and then washed 3 times in TBS-T for 10 min, followed by incubation with alkaline phosphatase polymer detector solution for 10 min at room temperature. Collagen type III staining was developed with permanent red chromogen (960D-10; Millipore Sigma) that gives a red precipitate. Nuclei were counterstained with hematoxylin (Vector Laboratories), then quickly dehydrated in a graded ethyl alcohol series, cleared with xylene, and mounted with xylene-based mounting medium.
To verify the specificity of collagen immunolabeling, isotype controls consisting of mouse IgG1 kappa (1:50) and rabbit IgG polyclonal isotype (1:300) were included in a set of sections. Positive controls consisted of formalin-fixed, paraffin-embedded rat skin (collagen type I) and umbilical cord (collagen type III).
Quantitative Imaging
Images were quantified using the Count & Measure module of cellSens Dimension software (Olympus). Total collagen was quantified using the minimum/maximum threshold settings of 47/256 for the red channel, 15/256 for green, and 0/128 for blue. The background was quantified using minimum/maximum thresholds of 0/47, 0/29, and 0/5 for the red, green, and blue channels, respectively. Sum areas of total pixels were automatically generated via the Count & Measure module for the above settings and used to calculate the percentage of total collagen and background for each tissue.
Results
Histological Assessment of Tendon Structure
The Achilles tendon structure was evaluated by H&E staining. Overall, light microscopic examination showed that the normal tendon structure was maintained in both formalin- and Bouin-fixed tendon tissues. However, the formalin-fixed tendons showed higher histological quality, characterized by uniformly bright eosinophilic collagen fibers with crisper and better nuclear staining (Fig. 1A). In Bouin-fixed tendons, in contrast, the eosinophilic and basophilic cell structures were stained with a different intensity. For instance, the collagen fibers appeared intensely eosinophilic and nuclei stained darkly. In addition, shrinkage of the collagen fibers in Bouin solution caused artifactual changes in the normal appearance of tendon; the collagen fibers were more separated and assumed a wavy appearance (Fig. 1B). All slides, regardless of the fixative, were of appropriate quality for the PSR evaluation.
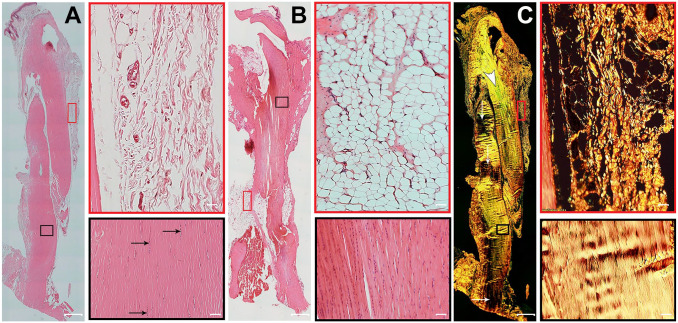
Figure 1.
Histological structure of healthy Achilles tendon. Representative serial longitudinal sections of Achilles tendons, with two fields of view of paratenon (red boxes) and a region of the mid tendon section (black boxes). (A) Formalin-fixed, hematoxylin–eosin (H&E)-stained section shows uniformly organized parallel collagen bundles and elongated tenocyte cell nuclei (black arrows). The paratenon is seen as loose connective tissue surrounding the tendon. (B) Bouin-fixed, H&E-stained tendon shows collagen fibers appearing intensely eosinophilic with nuclei stained darkly. In addition, the shrinkage effect of picric acid in Bouin solution causes artifactual changes in the normal appearance of tendon. (C) Formalin-fixed, picrosirius red staining on a sequential tissue section from the same tendon as panel A shows thick, bright yellowish-orange fibers. Another tendon area with greenish collagen fibers (white arrowhead) also contains some fibers that are at extinction (white arrows). The paratenon is seen with both thin fibers and thick fibers of yellowish-orange birefringence by polarizing microscopy. Tendon overview images were obtained with a 4× magnification lens (scale bar: 500 µm). Higher magnification images within the boxes were obtained with a 20× magnification lens (scale bar: 50 µm).
Evaluation of Collagen Fibers in PSR-stained Rat Achilles Tendon
We configured the image capture program to crossed polarized light and Köhler illumination to achieve proper light extinction and the brightest yellow/red and green birefringence without pixel saturation, as shown in Fig. 1C. In the evaluation of polarizing colors and birefringence intensities of the collagen fibers, the whole-tendon sections showed that the interference color of the collagen was not distributed homogeneously throughout the entire section, giving different patterns of color and intensity of birefringence in both formalin- and Bouin-fixed tendons, which is due to the orientation of the collagen fibers. For optical detection, collagen fibers must be aligned in line with the plane of the polarized light, which is why a rotating stage is essential for imaging.
In Bouin-fixed tendons, collagen fibers showed predominantly greenish-yellow birefringence interspersed with some amount of yellowish-orange birefringence (Fig. 2A), and few sections displayed only yellowish-orange birefringence. In formalin-fixed tendons, collagen fibers showed predominantly yellow or yellowish-orange birefringence (Fig. 3A), with some areas of greenish collagen fibers colocalized with yellowish-orange fibers in a few tendons (Fig. 1C). Overall, Bouin-fixed tendons tended to present a darker hue that was more green, whereas formalin-fixed tendons tended toward a lighter hue that was more yellow. The paratenon showed both thin and thick collagen fibers with a brilliant yellowish-orange birefringence in both Bouin- and formalin-fixed tendons (Fig. 1C); no greenish fibers were observed under polarized light.
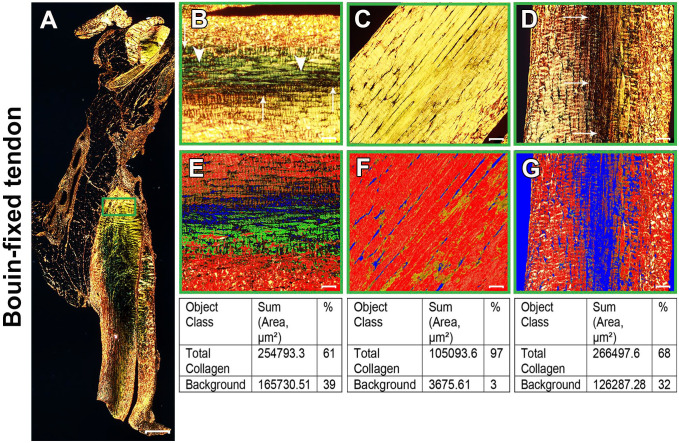
Figure 2.
Bouin-fixed normal Achilles tendon stained with picrosirius red (PSR). (A) Polarized view of representative entire longitudinal Achilles tendon section. (B–D) Magnifications of approximately the same region as the green box in panel A at three different orientations: 0°(B), 45°(C), and 90°(D). At 0°(B), collagen fibers showed a mixture of birefringence with yellowish-orange and greenish-yellow (arrowheads). As the microscope stage was rotated, the birefringence of collagen changed gradually. At 45° (C), the tendon appeared uniformly bright yellow. At 90°(D), birefringence changed to yellowish-orange with more collagen fibers at extinction (arrows in B and D). (E–G) PSR images acquired in the same regions of interest as panels B–D were quantitatively compared for the percentage of total collagen using the Count & Measure module of the cellSens software. Blue indicates background; the sum of red and yellow is total collagen, represented as red in the quantification panel. Tendon overview image (A) was obtained with a 4× magnification lens (scale bar: 500 µm). Higher magnification images within the boxes (B–F) were obtained with a 20× magnification lens (scale bar: 50 µm).
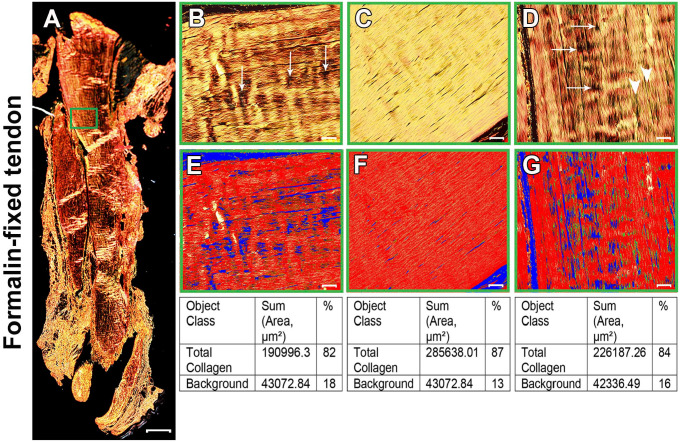
Figure 3.
Formalin-fixed normal Achilles tendon stained with picrosirius red (PSR). (A) Polarized view of representative entire longitudinal Achilles tendon section. (B–D) Magnifications of approximately the same region as the green box in panel A at three different orientations: 0°(B), 45°(C), and 90°(D). Collagen fibers showed different birefringence colors depending on the tendon orientation. Overall, collagen was displayed predominantly as a parallel arrangement of thick, yellowish-orange birefringent fibers. At 45°(C), the collagen fibers appeared uniformly bright yellow. At 0°(B) and 90°(D), a striped pattern was seen, with some fibers at extinction (arrows). White arrowheads in D show some greenish collagen fibers colocalized with thick, yellowish-orange fibers. (E–G) PSR images acquired in the same regions of interest as panels B–D were quantitatively compared for the percentage of total collagen using the Count & Measure module of the cellSens software. Blue indicates background; the sum of red and yellow is total collagen, represented as red in the quantitation panel. Tendon overview image (A) was obtained with a 4× magnification lens (scale bar: 500 µm). Higher magnification images within the boxes (B–F) were obtained with a 20× magnification lens (scale bar: 50 µm).
Tendon Orientation and Polarizing Colors of PSR-stained Collagen Fibers
We set up the microscope software user interface such that we could focus and acquire the same region on the same tendon section as the tendon slide was rotated on the microscope stage at different angles with respect to the transmission axis of either the polarizer or the analyzer. Representative examples of Bouin-fixed and formalin-fixed tendon tissues are shown in Figs. 2 and 3, respectively. The collagen fibers showed different birefringence colors according to the tendon orientation, particularly after slide rotation from 0° to 45° and 45° to 90°. In the formalin-fixed tendons, at 0° and 90° orientations, collagen was displayed in a parallel arrangement of thick and wavy collagen fibers of predominantly yellowish-orange birefringence (Fig. 3B and D), with some amount of greenish-yellow thin fibers interspersed with yellowish-orange fibers showing at 90° (Fig. 3D). At 45° orientation, collagen fibers tended to present a uniform and lighter yellow color (Fig. 3C). In addition to the change of color as the slide was rotated, the tendon acquired a striped appearance, with some areas turning black along the long axis of the tendon section at 0° and 90° because in these orientations some collagen fibers were at their extinction angle (Figs. 2B and D, and 3B and D). At 45°, no fiber appeared to be in extinction.
In the Bouin-fixed tissue, two distinct colors could be seen frequently depending on tendon orientation. At 0°, the collagen bundles showed predominantly greenish-yellow birefringence interspersed with some amount of yellowish-orange birefringence toward the edges of the tendon section (Fig. 2B). At 45°, the collagen fibers were straight, and all appeared bright yellow (Fig. 2C). At 90°, the color changed from the greenish-yellow birefringence seen at 0° to yellowish-orange (Fig. 2D), with more collagen fibers at extinction.
Quantitative Evaluation of Collagen Fibers
Because it has been claimed that PSR staining can distinguish between collagen type I (yellow-red) and collagen type III (green), areas exhibiting these colors were counted using the cellSens software. Depending on the tendon orientation, the ratio of yellow-red to green differed. Therefore, both colors were counted together and compared with the background (no red, yellow, or green) (Figs 2E–G and 3E–G). This ratio was the highest at 45° and may serve to measure collagen density as the collagen to background ratio.
Immunolocalization of Collagen Type I and Type III in the Normal Achilles Tendon
Immunostaining protocols were performed by double immunohistochemistry alone or combined with PSR. Combined immunolabeling followed by PSR staining was performed at the same time on the same section, as described by Gadd.37 In our hands, however, PSR caused appreciable overstaining by the red chromogen, which made it difficult to assess the expression of type III collagen. Staining with PSR first, followed by immunolabelling, also did not work well because PSR faded from the slide during exposure to heat-induced antigen retrieval conditions. Therefore, the immunostaining and PSR staining were carried out separately on serial sections.
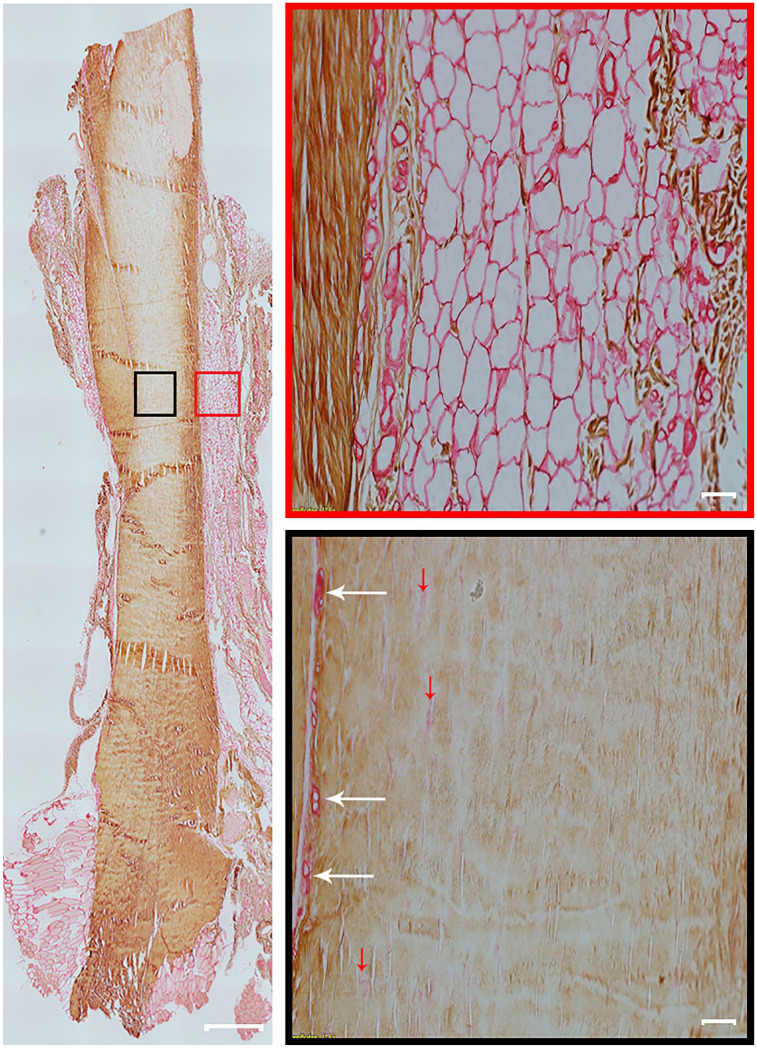
As shown in Fig. 4, the tendon core was strongly stained for collagen type I (thick brown collagen fibers) in formalin-fixed tendon, with low expression of collagen type III (thin reddish collagen fibers). Collagen type III was most highly expressed in the paratenon, endotenon, and blood vessels (Fig. 4). Bouin-fixed tendon sections lacked immunoreactivity for collagen type III in most tendons, which suggests that the antigen retrieval did not reverse the denaturing effect of Bouin fixation. None of the isotype controls showed any staining for either collagen type I or type III.
Figure 4.
Collagen type I and type III expression in rat Achilles tendon. A representative entire longitudinal Achilles tendon section is double immunolabeled for collagen type I and collagen type III. Positive immunolabelling for collagen type I is brown (diaminobenzidine chromogen) and for collagen type III is red (permanent red chromogen). Two high-magnification fields of view are shown of paratenon (red box) and the mid tendon section (black box). Collagen type I is present in tendon. Collagen type III is found mostly in the paratenon (red box) and blood vessels (black box, white arrows). However, a small amount of collagen type III is also found in the tendon core and indicated with red arrows (black box). Tendon overview image was obtained with a 4× magnification lens (scale bar: 500 µm). Higher magnification images within boxes obtained with a 20× magnification lens (scale bar: 50 µm).
Discussion
Collagen has an important role in maintaining the structural integrity of tendons and in determining their tensile strength.2 Therefore, a better understanding of the relevant molecular and mechanical properties of collagen is an important objective in tendon research. For several decades, the group of Junqueira17,18,21–23 promoted the use of PSR-polarization for the detection and analysis of collagen, and it has become widely used to distinguish between collagen type I and type III in different tissues. However, Pierard34 questioned its utility for this purpose because PSR-stained collagen bundles in dermis sections changed from orange-red to green, and vice versa, in different slides originating from the same biopsy when the slides were rotated on the microscope stage. This observation was, for many years, cited only sporadically, and Piérard failed to report many aspects of his methods, such as the optical configuration and details about the specimen orientation with respect to the polarizing filters, which are crucial for establishing the method’s reproducibility. Lattouf et al.29 also subsequently described how the polarization colors in skin strictly depended on the orientation of the collagen bundles and did not specifically reveal collagen types.
Nevertheless, the idea that a spectrum of yellow/orange/red birefringence accurately identifies collagen type I, and that green birefringence identifies collagen type III, has almost universally persisted since the early development of this method. At the time of this writing, querying PubMed for the phrase “picrosirius red staining AND collagen type I OR collagen type III” retrieved 4010 records, whereas searching for a conjunction of query terms (picrosirius red staining AND collagen type I AND collagen type III) retrieved 163 records. A cursory glance at these published studies shows that most identify a mixture of yellow, orange, and red for collagen type I and that some identify green fibers in tissue areas where collagen type III is not expected at all.
PSR-polarization can be considered the most direct method for collagen detection in tissue sections owing to the simplicity of the procedure. However, insufficient understanding of the polarization properties and polarization microscope configuration may introduce errors during image acquisition, which can lead to misinterpretation of the experimental data. In this study, we have made important observations that can be of practical value for the analysis of collagen fibers in PSR-stained tendons. We capitalized on acquiring stitched images of the entire tendon section, which allowed visualization of every portion of each collagen bundle.
We found that it is critical to use a polarizing microscope equipped with a rotating stage. Our results showed that a tendon section oriented at 45° with respect to the crossed polarizers provided the best qualitative and quantitative assessment of collagen organization in both Bouin- and formalin-fixed tendon tissues. At 45°, each individual collagen bundle appears as uniformly bright yellow birefringence against the dark background, rather than collagen bundles with a mixture of yellowish and greenish birefringence observed on the same tendon area at different angles. Collagen bundles usually appear crimped or wavy with alternating transverse dark bands under polarized light microscopy; it is said that the dark collagen bands are at extinction.38 This collagen crimp pattern was also seen in PSR-stained Achilles tendons, particularly in Bouin-fixed tendon sections, and could result in underestimation of total collagen content, especially in tendon sections containing a large amount of wavy fibers where portions of the fibers appear dark.
Figures 2 and 3 demonstrate that smaller areas of collagen bundles were at extinction when the long axis of the tendon sections was oriented at 45°, compared with 0° and 90°. In addition, higher total collagen content values were obtained in PSR-stained tendon sections analyzed at the 45° orientation, which indicates that a 45° orientation may be more favorable if total collagen quantification is intended. Because the birefringent colors of the collagen bundles varied in shades of yellow/orange and green when the Achilles tendon slide was rotated on the microscope stage with respect to crossed polarizers, we conclude that PSR staining cannot accurately identify collagen type I and collagen type III fibers in Achilles tendon. This conclusion was confirmed by immunohistochemical mapping of collagen type I and type III in the same tendons.
Bouin fixative has historically been recommended as a fixative for collagen on paraffin-embedded tissues.39,40 Although both formalin and Bouin fixatives have occupational exposure risks, Bouin fixative consists of a mixture of saturated picric acid, acetic acid, and formaldehyde. Picric acid is related to trinitrotoluene, which has been shown to be a high-power explosive compound; thus, it poses a potential explosion hazard when it dries. Because of these safety concerns, our institution has encouraged its replacement with an alternative fixative whenever possible.
The quality of PSR staining was compared between tendon tissues prepared separately in both fixatives. On the basis of our findings, both Bouin- and formalin-fixed tendon sections produced similar birefringence. However, compared with Bouin fixative, formalin fixation of rat Achilles tendon showed superior quality for histological detail on H&E staining with regard to fiber structure, fiber arrangement, and morphology of the nuclei. In addition, Bouin- and formalin-fixed Achilles tendons were examined for immunohistochemical localization of collagen type I and collagen type III under similar conditions. The enzymatic antigen retrieval treatment of formalin-fixed Achilles tendon sections satisfactorily retrieved both collagen type I and type III antigens, but it was ineffective for unmasking collagen type III on Bouin-fixed tendon sections. Bedossa et al.41 reported that enzymatic treatment with pepsin was also ineffective for retrieving collagen type I and III antigens but effective for visualizing the type IV collagen antigen on Bouin-fixed liver tissue. Gala et al.42 reported a list of antibodies that work well in Bouin-fixed bone marrow biopsies. Therefore, a different antigen retrieval protocol may be needed to expose the collagen type III epitope in Bouin-fixed tissue. Our data showed that formalin fixation is a suitable alternative to Bouin fixation of tendon when various histochemical procedures are intended in the same specimen.
In summary, this study clarifies the distribution of type III collagen in tendon and demonstrates that PSR-polarization alone does not enable the distinction of collagen type I and collagen type III in tendon tissue. Even when the polarizer filters are accurately crossed, the core tendon, which mainly consists of densely packed bundles of collagen type I, showed more than one color in the shades of yellow/orange and green. Only a small amount of type III collagen is located within the tendon core, whereas more type III collagen is found within the epitenon and paratenon, and blood vessels of uninjured tendons. PSR can be used along with double immunohistochemical labeling when precise distinction of collagen type I and type III is necessary. PSR staining is an effective and low-cost tool to investigate collagen fiber content, organization, and orientation and, possibly, total collagen density and quantification, depending on the orientation of the tendon section on the microscope stage. Correct and standardized use of PSR staining is important for reliable and reproducible results.
Supplemental Material
Supplemental material, sj-pdf-1-jhc-10.1369_00221554211046777 for Picrosirius Red Staining: Revisiting Its Application to the Qualitative and Quantitative Assessment of Collagen Type I and Type III in Tendon by Consuelo M. López De Padilla, Michael J. Coenen, Alejandro Tovar, Rodolfo E. De la Vega, Christopher H. Evans and Sebastian A. Müller in Journal of Histochemistry & Cytochemistry
Acknowledgments
The authors acknowledge Chris Johnson from Olympus for his scientific and technical assistance with the polarized light microscopy configuration and analysis.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: CMLDP was involved in conceptualization, methodology, investigation, formal analysis, writing—original draft preparation, and visualization. MJC was involved in conceptualization, formal analysis, resources, and review and editing. AT was involved in methodology, and review and editing. REDV was involved in formal analysis. CHE was involved in conceptualization, formal analysis, writing—review and editing, visualization, supervision, and funding acquisition. SAM was involved in conceptualization, formal analysis, investigation, writing—review and editing, visualization, and supervision.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by the John and Posy Krehbiel Professorship in Orthopedics and by the Mayo Clinic Rehabilitation Medicine Research Center.
Data Availability Statement: More supplemental data that support the findings of this study are available at: https://figshare.com/s/097a7ae29d5f75051608.
Contributor Information
Consuelo M. López De Padilla, Musculoskeletal Gene Therapy Research Laboratory, Mayo Clinic, Rochester, Minnesota
Michael J. Coenen, Musculoskeletal Gene Therapy Research Laboratory, Mayo Clinic, Rochester, Minnesota
Alejandro Tovar, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, Minnesota.
Rodolfo E. De la Vega, Musculoskeletal Gene Therapy Research Laboratory, Mayo Clinic, Rochester, Minnesota Department cBITE, MERLN Institute, Maastricht University, The Netherlands.
Christopher H. Evans, Musculoskeletal Gene Therapy Research Laboratory, Mayo Clinic, Rochester, Minnesota
Sebastian A. Müller, Department of Orthopedic Surgery, Kantonsspital Baselland, University of Basel, Basel, Switzerland (Research Collaborator [limited tenure], Mayo Clinic, Rochester, Minnesota).
Literature Cited
- 1. Kirkendall DT, Garrett WE. Function and biomechanics of tendons. Scand J Med Sci Sports. 1997;7(2):62–6. [DOI] [PubMed] [Google Scholar]
- 2. Wang JH. Mechanobiology of tendon. J Biomech. 2006; 39(9):1563–82. [DOI] [PubMed] [Google Scholar]
- 3. Somaiah C, Kumar A, Mawrie D, Sharma A, Patil SD, Bhattacharyya J, Swaminathan R, Jaganathan BG. Collagen promotes higher adhesion, survival and proliferation of mesenchymal stem cells. PLoS ONE. 2015; 10(12):e0145068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Merkel JR, DiPaolo BR, Hallock GG, Rice DC. Type I and type III collagen content of healing wounds in fetal and adult rats. Proc Soc Exp Biol Med. 1988;187(4):493–7. [DOI] [PubMed] [Google Scholar]
- 5. Volk SW, Wang Y, Mauldin EA, Liechty KW, Adams SL. Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells Tissues Organs. 2011;194(1):25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanson AN, Bentley JP. Quantitation of type I to type III collagen ratios in small samples of human tendon, blood vessels, and atherosclerotic plaque. Anal Biochem. 1983;130(1):32–40. [DOI] [PubMed] [Google Scholar]
- 7. Miller EJ. Biochemical characteristics and biological significance of the genetically-distinct collagens. Mol Cell Biochem. 1976;13(3):165–92. [DOI] [PubMed] [Google Scholar]
- 8. Birk DE, Mayne R. Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur J Cell Biol. 1997;72(4):352–61. [PubMed] [Google Scholar]
- 9. Eriksen HA, Pajala A, Leppilahti J, Risteli J. Increased content of type III collagen at the rupture site of human Achilles tendon. J Orthop Res. 2002;20(6):1352–7. [DOI] [PubMed] [Google Scholar]
- 10. Pajala A, Melkko J, Leppilahti J, Ohtonen P, Soini Y, Risteli J. Tenascin-C and type I and III collagen expression in total Achilles tendon rupture. An immunohistochemical study. Histol Histopathol. 2009;24(10):1207–11. [DOI] [PubMed] [Google Scholar]
- 11. Sharma P, Maffulli N. The future: rehabilitation, gene therapy, optimization of healing. Foot Ankle Clin. 2005; 10(2):383–97. [DOI] [PubMed] [Google Scholar]
- 12. Thankam FF, Evan DK, Agrawal DK, Dilisio MF. Collagen type III content of the long head of the biceps tendon as an indicator of glenohumeral arthritis. Mol Cell Biochem. 2019;454(1–2):25–31. [DOI] [PubMed] [Google Scholar]
- 13. Lui PP, Chan LS, Lee YW, Fu SC, Chan KM. Sustained expression of proteoglycans and collagen type III/type I ratio in a calcified tendinopathy model. Rheumatology. 2010;49(2):231–9. [DOI] [PubMed] [Google Scholar]
- 14. Constantine VS, Mowry RW. Selective staining of human dermal collagen. II. The use of picrosirius red F3BA with polarization microscopy. J Invest Dermatol. 1968;50(5):419–23. [DOI] [PubMed] [Google Scholar]
- 15. Kvasnicka HM, Beham-Schmid C, Bob R, Dirnhofer S, Hussein K, Kreipe H, Kremer M, Schmitt-Graeff A, Schwarz S, Thiele J, Werner M, Stein H. Problems and pitfalls in grading of bone marrow fibrosis, collagen deposition and osteosclerosis — a consensus-based study. Histopathology. 2016;68(6):905–15. [DOI] [PubMed] [Google Scholar]
- 16. James J, Bosch KS, Aronson DC, Houtkooper JM. Sirius red histophotometry and spectrophotometry of sections in the assessment of the collagen content of liver tissue and its application in growing rat liver. Liver. 1990;10(1):1–5. [DOI] [PubMed] [Google Scholar]
- 17. Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11(4):447–55. [DOI] [PubMed] [Google Scholar]
- 18. Junquiera LC, Junqueira LC, Brentani RR. A simple and sensitive method for the quantitative estimation of collagen. Anal Biochem. 1979;94(1):96–9. [DOI] [PubMed] [Google Scholar]
- 19. Malkusch W, Rehn B, Bruch J. Advantages of Sirius Red staining for quantitative morphometric collagen measurements in lungs. Exp Lung Res. 1995;21(1):67–77. [DOI] [PubMed] [Google Scholar]
- 20. Segnani C, Ippolito C, Antonioli L, Pellegrini C, Blandizzi C, Dolfi A, Bernardini N. Histochemical detection of collagen fibers by sirius red/fast green is more sensitive than van gieson or sirius red alone in normal and inflamed rat colon. PLoS ONE. 2015;10(12):e0144630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Junqueira LC, Montes GS, Bezerra MS. Do Schwann cells produce collagen type III? Experientia. 1979;35(1):114. [DOI] [PubMed] [Google Scholar]
- 22. Montes GS, Junqueira LC. The use of the Picrosirius-polarization method for the study of the biopathology of collagen. Mem Inst Oswaldo Cruz. 1991;86:1–11. [DOI] [PubMed] [Google Scholar]
- 23. Junqueira LC, Cossermelli W, Brentani R. Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch Histol Jpn. 1978;41(3):267–74. [DOI] [PubMed] [Google Scholar]
- 24. Majewski M, Betz O, Ochsner PE, Liu F, Porter RM, Evans CM. Ex vivo adenoviral transfer of bone morphogenetic protein 12 (BMP-12) cDNA improves Achilles tendon healing in a rat model. Gene Ther. 2008;15(16): 1139–46. [DOI] [PubMed] [Google Scholar]
- 25. Sugiyama Y, Naito K, Goto K, Kojima Y, Furuhata A, Igarashi M, Nagaoka I, Kaneko K. Effect of aging on the tendon structure and tendon-associated gene expression in mouse foot flexor tendon. Biomed Rep. 2019;10(4):238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Praktiknjo M, Lehmann J, Nielsen MJ, Schierwagen R, Uschner FE, Meyer C, Thomas D, Strassburg CP, Bendtsen F, Moller S, Krag A, Karsdal MA, Leeming DJ, Trebicka J. Acute decompensation boosts hepatic collagen type III deposition and deteriorates experimental and human cirrhosis. Hepatol Commun. 2018;2(2):211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown SR, Melman L, Jenkins E, Deeken C, Frisella MM, Brunt LM, Eagon JC, Matthews BD. Collagen type I:III ratio of the gastroesophageal junction in patients with paraesophageal hernias. Surg Endosc. 2011;25(5): 1390–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumari K, Ghosh S, Patil S, Augustine D, Samudrala Venkatesiah S, Rao RS. Expression of type III collagen correlates with poor prognosis in oral squamous cell carcinoma. J Investig Clin Dent. 2017;8(4):e12253. [DOI] [PubMed] [Google Scholar]
- 29. Lattouf R, Younes R, Lutomski D, Naaman N, Godeau G, Senni K, Changotade S. Picrosirius red staining: a useful tool to appraise collagen networks in normal and pathological tissues. J Histochem Cytochem. 2014;62(10):751–8. [DOI] [PubMed] [Google Scholar]
- 30. Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol. 1994;89(5):397–410. [DOI] [PubMed] [Google Scholar]
- 31. Dayan D, Hiss Y, Hirshberg A, Bubis JJ, Wolman M. Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry. 1989;93(1):27–9. [DOI] [PubMed] [Google Scholar]
- 32. Wolman M, Kasten FH. Polarized light microscopy in the study of the molecular structure of collagen and reticulin. Histochemistry. 1986;85(1):41–9. [DOI] [PubMed] [Google Scholar]
- 33. Puchtler H, Waldrop FS, Valentine LS. Polarization microscopic studies of connective tissue stained with picro-sirius red FBA. Beitr Pathol. 1973;150(2):174–87. [DOI] [PubMed] [Google Scholar]
- 34. Pierard GE. Sirius red polarization method is useful to visualize the organization of connective tissues but not the molecular composition of their fibrous polymers. Matrix. 1989;9(1):68–71. [DOI] [PubMed] [Google Scholar]
- 35. van Wyk CW, Philips VM. Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? J Oral Pathol Med. 1991; 20(2):96. [PubMed] [Google Scholar]
- 36. Coleman R. Picrosirius red staining revisited. Acta Histochem. 2011;113(3):231–3. [DOI] [PubMed] [Google Scholar]
- 37. Gadd VL. Combining immunodetection with histochemical techniques: the effect of heat-induced antigen retrieval on picro-sirius red staining. J Histochem Cytochem. 2014;62(12):902–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Whittaker P, Canham PB. Demonstration of quantitative fabric analysis of tendon collagen using two-dimensional polarized light microscopy. Matrix. 1991;11(1):56–62. [DOI] [PubMed] [Google Scholar]
- 39. Scarano A, Iezzi G, Piattelli A. Common fixatives in hard-tissue histology. In: An YH, Martin KL, editors. Handbook of histology methods for bone and cartilage. Totowa, NJ: Humana Press; 2010. p. 159–65. [Google Scholar]
- 40. Howat WJ, Wilson BA. Tissue fixation and the effect of molecular fixatives on downstream staining procedures. Methods. 2014;70(1):12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bedossa P, Bacci J, Lemaigre G, Martin E. Effects of fixation and processing on the immunohistochemical visualization of type-I, -III and -IV collagen in paraffin-embedded liver tissue. Histochemistry. 1987;88(1):85–9. [DOI] [PubMed] [Google Scholar]
- 42. Gala JL, Chenut F, Hong KB, Rodhain J, Camby P, Philippe M, Scheiff JM. A panel of antibodies for the immunostaining of Bouin’s fixed bone marrow trephine biopsies. J Clin Pathol. 1997;50(6):521–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jhc-10.1369_00221554211046777 for Picrosirius Red Staining: Revisiting Its Application to the Qualitative and Quantitative Assessment of Collagen Type I and Type III in Tendon by Consuelo M. López De Padilla, Michael J. Coenen, Alejandro Tovar, Rodolfo E. De la Vega, Christopher H. Evans and Sebastian A. Müller in Journal of Histochemistry & Cytochemistry