Abstract
Modern scientific research has shown that Acanthopanax senticosus (AS) can regulate the innate immunity of healthy animals, thus affecting the health of animals. However, there are few systematic reports on the changes of innate immune indices of healthy animals after consuming AS. The purpose of this project was to study the effect on healthy mice’s innate immunity and changes of related immune factors induced by feeding AS root powder supplementation. The results showed that the killing rate of natural cells increased in a dose-dependent manner in a certain time period. Compared to the control group, the treatment groups (T1, T2 and T3) improved significantly in the innate immune index (lysozyme, β-defensin-2 and duodenal secretory IgA (SIgA) to varying degrees) and induced corresponding changes of immune factors at certain time periods. The correlation between SIgA and IFN-γ in mouse serum was enhanced, and the higher the concentration of AS in the diet, the stronger the correlation was. However, there was no significant difference in growth performance among groups. It is proved that AS supplementation can enhance innate immunity and change several relevant immune factors and cells of healthy mice without affecting growth performance.
Keywords: Acanthopanax senticosus, IL, lysozyme, SIgA, β-defensin-2, IFN-γ
Introduction
Innate immunity is an important function of the body, and it is characterised by the combination of defence barrier, phagocytic function of phagocytes, inflammatory reaction and cytokines in body fluid. 1 Traditional Chinese Medicine is the quintessence of China. The raw materials and extracts of Traditional Chinese Medicine have been proved to play a certain role in the disease resistance of animals by regulating innate immunity. According to the practical experience of Chinese people for many years, Traditional Chinese Medicine has an obvious effect on improving animal immunity and has played an undeniable role in the control of several large-scale human epidemic diseases in modern times. For example, Yizhe et al. 2 found that Astragalus membranaceus could repair intestinal mucosa damaged by endotoxin in mice. Hsiao et al. 3 found that the compound of camphora and Panax ginseng could play an anti-fatigue role in mice. However, there are many kinds and functions of Chinese herbal medicine, and more mechanisms still need to be studied.
Acanthopanax senticosus (AS) as a Traditional Chinese Medicine has been proved to have antibacterial, antioxidant and anti-fatigue as well as health-care effects. 4 , 5 Kong et al. fed AS extract to weaned piglets. It was found that AS extract had a certain regulatory effect on the piglets’ immune indices. 6 However, there have been few reports about the systemic effect and mechanism of AS supplement on the immune indices of healthy animals. The aim of this study was to explore the effects of AS supplementation on innate immunity indices (lysozyme, β-defensin-2 (HBD-2) and duodenal secretory IgA (SIgA)) in mice in order to provide the theoretical basis for the direction of AS in animal disease resistance and health care.
Methods
Animals and diets
The experimental animals were healthy and nulliparous mature female Kunming mice (n = 200) of similar age and body mass (28 ± 1 g), provided by Hunan SJA Laboratory Animal Co., Ltd. The mice and their care were conducted in conformity with National Institutes of Health guidelines (NIH Pub. No. 85-23, revised 1996), and the study was approved by the Animal Care and Use Committee of Hunan Agricultural University. Raw materials (AS root) were provided by Hunan Canzoho Biological Technology Co. Ltd. The raw materials were crushed through a 40-mesh sieve (sieve hole diameter: 0.425 mm), dried at 60°C to a constant mass and vacuum packed for spare use.
Experimental design
A single-factor random block test design was used for this experiment. The mice (n = 200) were randomly selected and divided into four groups: the control group (C), treatment group 1 (T1), treatment group 2 (T2) and treatment group 3 (T3). There were five replicates in each group, with 10 mice in each replicate, all housed in a cage. The preliminary experiment lasted for 7 d, and the experimental period was 28 d. Group C received a basic diet for mice, but groups T1, T2 and T3 received the basic diet plus AS root powder (0.2%, 0.35% and 0.5%, respectively) by diet mass. Two mice were randomly sampled from each cage on d 0, 7, 14 and 28 of the experiment, so a total of 40 mice were executed and sampled. At 8:00am (Beijing time) on d 0, the mice were weighed after fasting for 12 h. The mice were executed to collect 2 ml blood. The whole blood was allowed to rest for 15 min before being centrifuged for 10 min at 1006 g. The serum was then separated and stored at –20°C. To obtain duodenal SIgA, the abdominal cavity was opened to separate the duodenum and put into a test tube containing formaldehyde. Detection indicators included growth indicators (daily gain, intake and feed/gain, immune index (TNF-α, IL-1β, IL-2, IL-4, IL-6 and IL-8), lysozyme, HBD-2, duodenal SIgA, phagocytosis rate of macrophages and natural cell killing rate. At the beginning of the experiment (d 0), only the growth indicators lysozyme, HBD-2, duodenal SIgA and INF-γ were measured.
Double Ab sandwich ELISA was used to detect the immune index. Lactate dehydrogenase releasing method (LDH) was used to determine the NK cell killing rate. 7 The phagocytosis rate of macrophages was determined according to Tang et al. 8
Statistical analysis
The test data were preliminarily processed with Microsoft Excel 2016, and the system variance was analysed by two-way ANOVA using IBM SPSS Statistics for Windows v22.0 (IBM Corp., Armonk, NY). If there were significant differences between groups (P < 0.05), Duncan’s method was used for multiple comparisons. The results are expressed as the mean ± SD.
Results
Growth performance of mice
The experimental data showed that the growth performance of the mice was not significantly affected by adding different doses of AS (groups T1, T2 and T3) compared to the control group (C; P > 0.05; data not shown).
Changes in immune cells
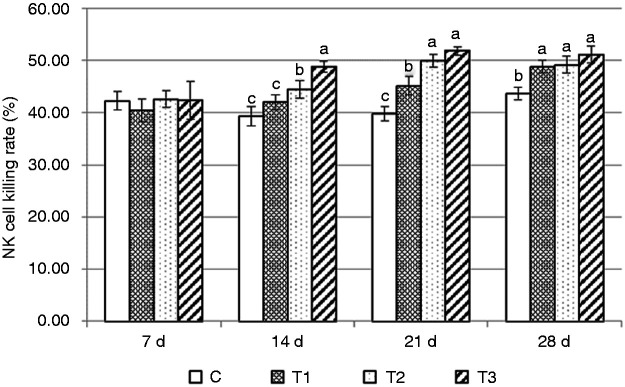
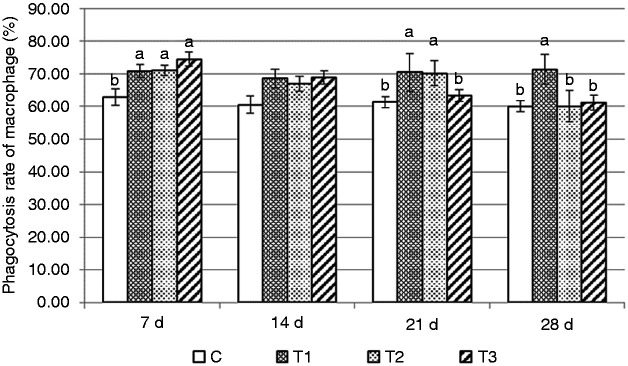
The changes in the NK cell killing rate by AS supplementation in healthy mice is shown in Figure 1. On d 14, this rate was significantly higher in group T3 than in group T2, and significantly higher in group T2 than in groups T1 and C (P < 0.05). On d 21, groups T2 and T3 were significantly higher than group T1, and group T1 was significantly higher than group C (P < 0.05). On d 28, rates in groups T1, T2 and T3 were significantly higher than group C (P < 0.05). The phagocytosis rate of macrophages is shown in Figure 2. Significant changes occurred on d 7, when groups T1, T2 and T3 were significantly higher than group C (P < 0.05). However, on d 21, the phagocytosis rate of macrophages in group T3 decreased significantly, so groups T1 and T2 were significantly higher than groups T3 and C (P < 0.05). However, on d 28, only group T1 was significantly higher than the other groups (P < 0.05).
Figure 1.
Change in killing rate of NK cells in mice. As the control group, mice in group C received a basic diet for mice, and mice in the experimental groups (T1, T2 and T3) received the basic diet plus AS root powder (0.2%, 0.35% and 0.5%, respectively) by diet mass. For the same group of data, different lower-case letters ‘abc’ represent significant differences (P < 0.05).
Figure 2.
Changes in phagocytic rate of macrophages in mice. Mice in group C received a basic diet for mice, and mice in groups T1, T2 and T3 received the basic diet plus AS root powder (0.2%, 0.35% and 0.5%, respectively) by diet mass. For the same group of data, different lower-case letters ‘abc’ represent significant differences (P < 0.05).
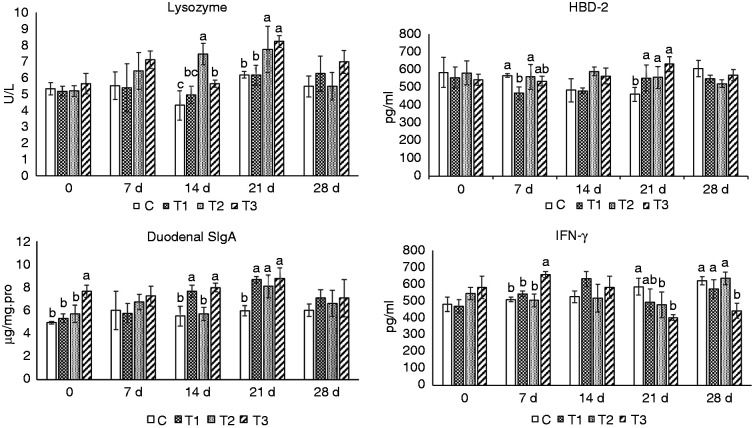
Changes in lysozyme, serum HBD-2, duodenal slgA and IFN-γ
Figure 3 shows that on d 7, compared to the group C, the level of serum HBD-2 was significantly lower in group T1 (P < 0.05). On d 14, slgA in the duodenum was significantly higher in group T1 (P < 0.05), serum lysozyme was significantly higher in group T2 (P < 0.05) and serum lysozyme and duodenal slgA were significantly higher in group T3 (P < 0.05). On d 21, the levels of serum HBD-2 and duodenal slgA were significantly higher in group T1 (P < 0.05), while the levels of serum lysozyme, HBD-2 and duodenal slgA were significantly higher in groups T2 and T3 (P < 0.05). With regard to IFN-γ, compared to group C, on d 7, the level was significantly higher in T3. However, on d 21, rates were significantly lower in groups T2 and T3, and on d 28, the rate in group T3 was significantly lower (P < 0.05).
Figure 3.
Changes in lysozyme, β-defensin-2, IFN-γ in blood and duodenal secretory IgA (SIgA) of mice. Mice in group C received a basic diet for mice, and mice in groups T1, T2 and T3 received the basic diet plus AS root powder (0.2%, 0.35% and 0.5%, respectively) by diet mass. For the same group of data, different lower-case letters ‘abc’ represent significant differences (P < 0.05).
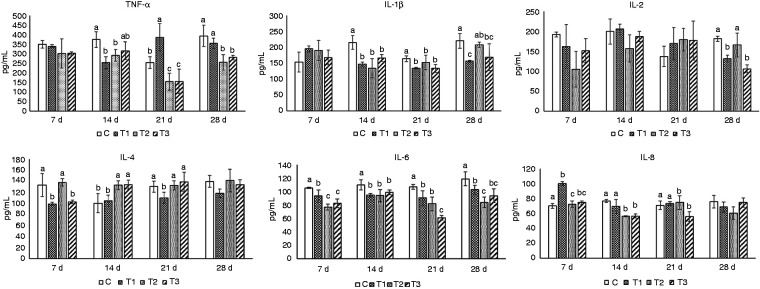
Changes in immune factors in serum
Results shown in Figure 4 can be summarised as follows. Compared to group C, levels of TNF-α in groups T1 and T2 were significantly lower on d 14, while those in groups T1, T2 and T3 were significantly lower on d 21, and those in groups T2 and T3 were significantly lower on d 28 (P < 0.05). Compared to group C, IL-1β levels in groups T1, T2 and T3 were significantly lower on d 14, while those in groups T1 and T3 were significantly lower on d 21 and 28 (P < 0.05). Compared to group C, on d 28, IL-2 levels in groups T1 and T3 were significantly lower (P < 0.05). Compared to group C, on d 14, IL-4 levels in T2 and T3 were significantly higher, while on d 7, those in groups T1 and T3 were significantly lower. On d 21, the rate in group T1 was significantly lower. Compared to group C, on d 7, 14, 21 and 28, IL-6 levels in groups T1, T2 and T3 were significantly lower (P < 0.05). Compared to group C, on d 7, IL-8 levels in group T1 were significantly higher, and on d 14, levels in groups T2 and T3 were significantly lower. On d 21, the levels in T3 were significantly lower (P < 0.05).
Figure 4.
Changes in immune indices in mice blood. Mice in group C received a basic diet for mice, and mice in groups T1, T2 and T3 received the basic diet plus AS root powder (0.2%, 0.35% and 0.5%, respectively) by diet mass. For the same group of data, different lower-case letters ‘abc’ represent significant differences (P < 0.05).
Discussion
There was no significant difference in the growth performance (body mass, daily gain, feed intake and feed–gain ratio) of mice in each group during the experiment, which proved that the AS dietary supplementation did not affect the growth performance of the mice.
NK cells are important immune cells which play a key role in anti-infection, immune regulation and anti-tumour processes. In this study, the killing activity of NK cells of mice fed AS was significantly increased in a dose-dependent manner at d 14. However, as time went on, the killing activity of NK cells tended to be stable. Ha found that AS glycoprotein can enhance the host’s non-specific immune function by activating NK cells and producing a variety of cytokines. 9 This is consistent with the results presented in this study. However, the killing activity of NK cells did not increase over time. The possible reason is that AS does not simply stimulate the increase of NK cell killing activity, but rather regulate it in many ways, so that NK cell killing activity finally tends to be stable.
The change in macrophages in mice fed with AS was very significant, which was significantly higher on d 7 compared to group C. Ryu proved that the water extract of AS can promote the proliferation of spleen cells and activate peritoneal macrophages. 10 Yoon mentioned that AS can inhibit tumour activity by activating macrophages and promoting cytokines such as TNF-α, IL-12 and IFN-γ. 11 However, it is surprising that with the increase of time, the macrophage activity of mice fed with the high dose of AS gradually decreased, even in the inhibition state, and the higher the dose, the earlier the inhibition time. This phenomenon may be related to the two-way immune regulation of AS. Yi confirmed that AS can inhibit mast cell–dependent anaphylaxis. 12 The results in this experiment showed that AS could enhance the early activity of macrophages, and inhibit its activity over time. It is suggested that AS has a bidirectional effect on immune regulation.
Lysozyme is a type of antimicrobial protein existing widely in biological organisms. It often decomposes the cell wall peptidoglycan (PG) of bacteria to achieve resistance to bacteria. As a congenital immune factor, lysozyme plays a vital role in the immune function of animals. 13
As shown in Figure 3, lysozyme concentrations were significantly higher in groups T2 and T3 than in group C on d 14 and 21 (P < 0.05). The results showed that AS could induce the expression of lysozyme in mice at a specific dose and time. The possible reason is that AS contains polysaccharides. Jufen et al. 14 used Astragalus polysaccharide injection to inject tilapia, and it was found that Astragalus polysaccharide could induce the expression of lysozyme in the liver, spleen and other tissues of tilapia, and enhanced the body immunity of tilapia. Lysozyme has the ability to dissolve PG which is produced only by bacteria and not by eukaryotes. So, PG represents an excellent target for lysozyme. 15 One of the functions of lysozyme is to induce the generation of pro-inflammatory factors such as IL-8 and antimicrobial molecules through the destruction of PG after bacteria have invaded the body. 16 , 17 Wolf et al. 18 found that lysozyme-sensitive Staphylococcus aureus was more susceptible to macrophages, and then increased the release of inflammatory factors, such as IL-6 and TNF-α, through the receptors for bacterial-derived lipoproteins and DNA. However, contrary to the above results, in this experiment, the lysozyme of mice in groups T2 and T3 was significantly higher than that of group C on d 14 and 21, while IL-6, IL-8 and TNF-α levels of mice in groups T2 and T3 were significantly lower than those of group C (P < 0.05) or there was no significant difference (P > 0.05). Our results suggest that AS in this experiment had an immunomodulatory effect on healthy mice in the natural environment and can induce the expression of lysozyme, it but did not increase the release of pro-inflammatory factors such as IL-6, IL-8 and TNF-α.
Defensins are cationic polypeptides rich in disulphide bonds, widely distributed in fungi, plants and animals, and are important regulators in the biological immune system. 19 HBD-2 is widely distributed in the skin and mucosa and other epithelial tissues of animals which have direct bactericidal function, and HBD-2 represents an important class of antimicrobial peptides. 20
Although HBD-2 is an important congenital immune factor, it has been reported that it is characterised by inducible expression, and its content is mainly regulated by local inflammation and microbial stimulation, such as Gram-negative and Gram-positive bacteria, fungi, symbiotic bacteria in vivo, TNF-α and IL-1β, all of which can up-regulate the expression of HBD-2, leading to an increase in its content in the area of inflammation.21–23 McDermott et al. 24 found that both TNF-α and IL-1β could stimulate the expression of HBD-2 in human corneal epithelial cell line human corneal endothelial cells. Bajajelliott et al. 25 confirmed that IL-1β could significantly up-regulate the expression of HBD-2 in gastric epithelial cell lines AGS and MKN7, and when Perregaux et al. 26 studied the changes of IL-1β transcription level, it was found that HBD-2 may act as a secondary effector molecule to stimulate cells to produce IL-1β continuously. According to the above reports, the interaction between HBD-2 and pro-inflammatory factors such as TNF-α and IL-1β may have a positive correlation with the content change effect. According to the results of this experiment, the content of HBD-2 in the serum of mice in groups T1 and T2 on d 7 was significantly lower than that of group C, and was significantly higher in all treatment groups on d 21 compared to group C (P < 0.05). However, the results of TNF-α and IL-1β are different from those above. On d 7 and 21, the levels of these two cytokines were not higher than those in group C; some were even significantly lower (P < 0.05). Therefore, AS can increase the content of HBD-2 in serum at a certain time and to some extent, but the relationship between the expression of HBD-2 and TNF-α, IL-1β in serum is different from that in local tissues. The specific interaction between HBD-2 and cytokines in serum still needs further experimental verification and discussion.
Modern medical research has found that there are a large number of immune factors in the mucosal mucus of mammals, which represent an important part of the body’s immune system, in which SIgA plays a major role. 27 Although SIgA is the product of specific immunity, its main function is to block the contact between the pathogen and mucosal epithelial cells through innate physical barriers, thus preventing the invasion of pathogens. 28
When exogenous pathogenic micro-organisms invade animal mucosa, the related lymphoid tissues will be activated. Specific B and T cell reactions will be induced by Ag presentation. When T cells are activated, IgM+B cells will be transformed into IgA+B cells. Activated lymphocytes leave the induction part and home to the lymphoid tissue of the effector part. During the homing process, IgA+B cells differentiate and mature into IgA+effector B cells. When IgA+effector B cells are colonised at the effector site, aggregated IgA is produced and covalently bound with secretory slices (SC) on the surface of epithelial cells to form sIgA. 27 , 29 It has been reported that sIgA has the functions of an immune barrier, immune clearance and neutralisation of viruses and toxins. 30 In this study, the level of SIgA in the duodenum of mice fed an AS diet was significantly higher than that of group C on d 14 (groups T1 and T3) and d 21 (groups T1, T2 and T3; P < 0.05). On d 28, the level of SIgA in the duodenum of mice fed an AS diet was higher than that of group C, but the difference was not significant.
IFN is an important immune response factor, of which type I IFN (IFN-α, IFN-β) can significantly enhance the killing function of NK cells, promoting cell secretion of a variety of cytokines and playing a broad-spectrum anti-tumour and antiviral role. 31 IFN-γ is a type II IFN, which is mainly produced by activated NK cells and T cells, including Th1 cells in CD4+ T cells and Ag-stimulated cytotoxic T cells (CTL) in CD8+ T cells.31–33 Recent studies suggest that regulatory T cells (Treg) secrete IFN-γ rapidly and transiently at the early stage of contact Ag and such early-produced IFN-γ can prevent the initiation of an over-immune response by inhibiting the apoptosis of initial T cells. 34 However, Lin et al. 35 found that RAW264.7 macrophages exposed to AS polysaccharides (ASP) could inhibit the production of NO induced by LPS and IFN-γ in a dose-dependent manner. From this point, we can deduce that in the middle and early stage of this study, because the amount of ASP was low in the blood of the mice, IFN-γ could be produced rapidly on d 7, and on d 21, IFN-γ was inhibited by the increase in the concentration of ASP.
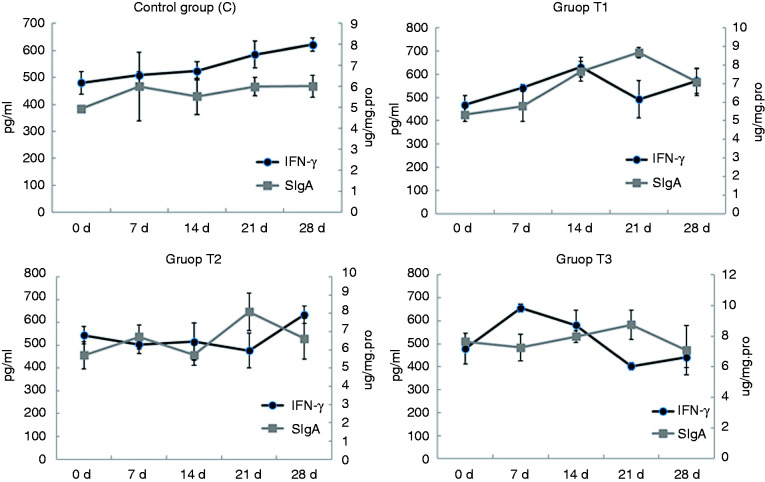
IFN-γ is thought to have two-way immunomodulatory effects on animal organisms. 36 Interestingly, in this study, the levels of IFN-γ and SIgA in the duodenum showed an alternating trend (Figure 5). Because the mice in this study were exposed to Ags, they were not tested in a sterile environment. It can be inferred that when the SIgA content increases, the protective effect on the mucosa is enhanced. So, the Ag content of the body decreases, and the secretion of IFN-γ initiates the corresponding mechanism, leading to the decrease of IFN-γ content to a certain level. This implies that the diet of AS supplementation may have a correlative effect on IFN-γ and SIgA in the duodenum.
Figure 5.
Relationship between serum IFN-γ and duodenal SIgA in mice. Mice in group C received a basic diet for mice, and mice in groups T1, T2 and T3 received the basic diet plus AS root powder (0.2%, 0.35% and 0.5%, respectively) by diet mass. For the same group of data, different lower-case letters ‘abc’ represent significant differences (P < 0.05).
As shown in Table 1, the correlation between serum SIgA and IFN-γ showed no significant positive correlation between the two indices (P = 0.071) in mice without AS. However, there was no significant negative correlation in the low-dose group (T1; P = 0.482), while there was a significant negative correlation between the middle-dose group (T2) and the high-dose group (T3; P = 0.039 and P = 0.011), and the significance of group T3 was greater than that of group T2. In innate immunity, IFN-γ is produced by NK cells stimulated by IL-18 and IL-12, but in adaptive immunity, IFN-γ is produced by CD4+ T helper cells (Th1) and CD8+ CTL stimulated by MHC when presenting Ag. 37 Although IFN-γ has been proved to play an important role in promoting macrophage activation, mediating antiviral and antimicrobial immune responses, enhancing Ag presentation and activating the innate immune system, IFN-γ also has a two-way regulatory role. 38 It has been reported that IFN-γ produced by Treg can affect the activity of APCs, especially dendritic cells (DC). The principle is that Treg in peripheral tissues receive rapid release of IFN-γ by allo-Ag stimulation, and induce nearby macrophages to express NO synthase (iNOS), resulting in a large amount of NO production which spreads to the surrounding T cells, affecting their function and triggering their apoptosis. IFN-γ produced by Treg in drainage lymph nodes not only promotes the expression of iNOS in DC, but also stimulates the expression of indoleamine-2,3-dioxygenase (IDO) in DC and accelerates the degradation of tryptophan. By-products of Treg’s metabolism can promote the apoptosis of T cells and affect the function of T cells and the balance of Th1 and Th2 cells. 39 , 40 In addition, IFN-γ has a negative regulatory effect on the migration of DC. Xiaodong et al. 41 found that the migration ability of DC in IFN-γ knockout mice was significantly enhanced, and the migration ability of DC was significantly decreased after intraperitoneal injection of IFN-γ to normal levels. Meanwhile, the activity of T cells and the production of IL-2 in IFN-γ knockout mice were decreased. Concerning DC cells, some studies have shown that DC can induce the phenotype transformation of B cells from the IgM phenotype to IgA, and co-culture of DC with B cells and T cells can promote the production of SigA. 42 However, Mora et al. 43 found that Peyer′s patches DC could also induce the type change in IgA production in B cells without T cell dependence. Thus, the functional activity of DC is a key factor in the formation of SIgA. In addition, DC can regulate the function of NK cells by ligand-receptor and release cytokines such as IL-12 and IL-18, thus affecting the production of IFN-γ. 44 , 45
Table 1.
Analysis of the correlation between SIgA and IFN-γ in serum with different doses of AS supplement in mice.
| C |
T1 |
T2 |
T3 |
||||
|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P |
| 0.603 | 0.071 | –0.221 | 0.482 | –0.615 | 0.039 | –0.704 | 0.011 |
P < 0.05 represents significant difference.
SIgA: secretory IgA; AS: Acanthopanax senticosus; C: control group; T1, T2, T3: experimental groups.
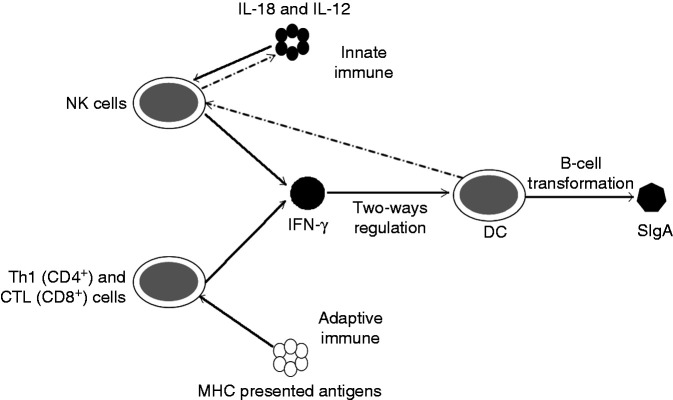
Comprehensive analysis of the results of the above studies and experiments shows that, as shown in Figure 6, healthy mice fed a diet supplemented with AS could induce IFN-γ production, while in vivo DC cells were two-way regulated by IFN-γ, thus affecting SIgA production. However, the release of IL-18 and IL-12 weakens when DC activity decreases, which negatively regulates the content of IFN-γ. Therefore, the level of the two factors showed an alternating trend, and the higher the content of AS in the diet of the mice, the stronger the correlation between these changes. The results suggest that AS dietary supplementation can have certain relevance to the immune factors of animals. This correlation and the ability of two-way regulation between immune factors are of great significance for the innate immunity and disease resistance of animals.
Figure 6.
Correlation diagram of serum immune factors in mice. In innate immune factors, IFN-γ and SIgA form bidirectional regulation with the participation of NK cells and dendritic cells.
Acknowledgements
We are grateful to Xiang Li, YouFu Yang, XiaoJuan Bi and HuiWang Liu for animal care and their helpful assistance in the experiment.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iDs
YunQiang Zhang https://orcid.org/0000-0003-3172-9120
References
- 1.Hawn TR and Underhill DM. Toll-like Receptors in Innate Immunity. Measuring Immunity 2005; 17: 80–90. [Google Scholar]
- 2.Yizhe C, Qiuju W, Rui S, et al. Astragalus membranaceus (Fisch.) Bunge repairs intestinal mucosal injury induced by LPS in mice. BMC Complement Altern Med 2018; 18: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsiao CY, Hsu YJ, Tung YT, et al. Effects of Antrodia camphorata and Panax ginseng supplementation on anti-fatigue properties in mice. J Vet Med Sci 2018; 80: 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee S, Shin DS, Oh KB, et al. Antibacterial compounds from the leaves of Acanthopanax senticosus. Arch Pharm Res 2003; 26: 40–42. [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Son D, Ryu J, et al. Anti-oxidant activities of Acanthopanax senticosus stems and their lignan components. Arch Pharm Res 2004; 27: 106–110. [DOI] [PubMed] [Google Scholar]
- 6.Kong X, Yin Y, Guoyao WU, et al. Dietary supplementation with Acanthopanax senticosus extract modulates cellular and humoral immunity in weaned piglets. Asian-Australas J Anim Sci 2007; 20: 1453–1461. [Google Scholar]
- 7.Yang HU, Guodong Z, Jianer YU, et al. Effects of Bushen Gubiao Granules on serum IL-2 and IFN-γ, NK cell activity and splenic lymphocytes proliferation in hypoimmune mice. Shanghai J Tradit Chin Med 2019; 53: 77–80. [Google Scholar]
- 8.Tang QJ, Zhang JS, Pan YJ, et al. Activation of mouse macrophages by the alkali-extracted polysaccharide from spore of Ganoderma lucidum. Chin J Cell Mol Immunol 2004; 20: 142–144. [PubMed] [Google Scholar]
- 9.Ha ES, Hwang SH, Shin KS, et al. Anti-metastatic activity of glycoprotein fractionated from Acanthopanax senticosus, involvement of NK-cell and macrophage activation. Arch Pharm Res 2004; 27: 217–224. [DOI] [PubMed] [Google Scholar]
- 10.Ryu H-S. Enhancing effect of Acanthopanax senticosus extracts on mouse spleen and macrophage cells activation. Korean J Food Nutr 2015; 28: 253–257. [Google Scholar]
- 11.Yoon TJ, Yoo YC, Lee SW, et al. Anti-metastatic activity of Acanthopanax senticosus extract and its possible immunological mechanism of action. J Ethnopharmacol 2004; 93: 247–253. [DOI] [PubMed] [Google Scholar]
- 12.Yi JM, Hong SH, Kim JH, et al. Effect of Acanthopanax senticosus stem on mast cell-dependent anaphylaxis. J Ethnopharmacol 2002; 79: 347–352. [DOI] [PubMed] [Google Scholar]
- 13.Callewaert L, Michiels CW. Lysozymes in the animal kingdom. J Biosci 2010; 35: 127–160. [DOI] [PubMed] [Google Scholar]
- 14.Jufen T, Jichang J, Zaohe W, et al. Effect of Astragalus polysaccharides (APS) on the expression of lysozyme-c gene in GIFT strain of Nile tilapia (Oreochromis niloticus). J Guangdong Ocean U 2011; 31: 58–61. [Google Scholar]
- 15.Ragland SA, Criss AK. From bacterial killing to immune modulation: recent insights into the functions of lysozyme. PLoS Pathog 2017; 13: e1006512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masumoto J. Nod1 acts as an intracellular receptor to stimulate chemokine production and neutrophil recruitment in vivo. J Exp Med 2006; 203: 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caruso R, Warner N, Inohara N, et al. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity 2014; 41: 898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf AJ, Arruda A, Reyes CN, et al. Phagosomal degradation increases TLR access to bacterial ligands and enhances macrophage sensitivity to bacteria. J Immunol 2011; 187: 6002–6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hancock REW, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 2006; 24: 1551–1557. [DOI] [PubMed] [Google Scholar]
- 20.Mathews M, Jia HP, Guthmiller JM, et al. Production of β-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect Immunol 1999; 67: 2740–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harder J, Bartels JH, Christophers E, et al. A peptide antibiotic for human skin. Nature 1997; 387: 861. [DOI] [PubMed] [Google Scholar]
- 22.Paulsen F, Pufe T, Conradi L, et al. Antimicrobial peptides are expressed and produced in healthy and inflamed human synovial membranes. J Pathol 2002; 198: 369–377. [DOI] [PubMed] [Google Scholar]
- 23.Liu L, Wang L, Jia HP, et al. Structure and mapping of the human beta-defensin HBD-2 gene and its expression at sites of inflammation. Gene 1998; 222: 237. [DOI] [PubMed] [Google Scholar]
- 24.Mcdermott AM, Redfern RL, Zhang B, et al. Defensin expression by the cornea: multiple signalling pathways mediate IL-1beta stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophthalmol Vis Sci 2003; 44: 1859–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bajajelliott M, Fedeli P, Smith GV, et al. Modulation of host antimicrobial peptide (β-defensins 1 and 2) expression during gastritis. Gut 2002; 51: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perregaux DG, Bhavsar K, Contillo L, et al. Antimicrobial peptides initiate IL-1β posttranslational processing: a novel role beyond innate immunity. J Immunol 2002; 168: 3024–3032. [DOI] [PubMed] [Google Scholar]
- 27.Corthésy B. Role of secretory immunoglobulin A and secretory component in the protection of mucosal surfaces. Future Microbiol 2010; 5: 817–829. [DOI] [PubMed] [Google Scholar]
- 28.Matsumura T, Fujinaga Y, Jin Y, et al. Human milk SIgA binds to botulinum type B 16S toxin and limits toxin adherence on T84 cells. Biochem Biophys Res Commun 2007; 352: 867–872. [DOI] [PubMed] [Google Scholar]
- 29.Schroeder HW, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol 2010; 125: S41–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonner A, Furtado PB, Almogren A, et al. Implications of the near-planar solution structure of human myeloma dimeric IgA1 for mucosal immunity and IgA nephropathy. J Immunol 2008; 180: 1008. [DOI] [PubMed] [Google Scholar]
- 31.Baron S, Tyring SK, Fleischmann WR, Jr, et al. The interferons. Mechanisms of action and clinical applications. JAMA 1991; 266: 1375–1383. [DOI] [PubMed] [Google Scholar]
- 32.Farrar MA, Schreiber RD. The molecular cell biology of Interferon-gamma and its receptor. Annu Rev Immunol 1993; 11: 571–611. [DOI] [PubMed] [Google Scholar]
- 33.Myers L, Croft M, Kwon BS, et al. Peptide-specific CD8 T regulatory cells use IFN-γ to elaborate TGF-β-based suppression. J Immunol 2005; 174: 7625–7632. [DOI] [PubMed] [Google Scholar]
- 34.Wood KJ, Sawitzki B. Interferon gamma: a crucial role in the function of induced regulatory T cells in vivo. Trends Immunol 2006; 27: 183–187. [DOI] [PubMed] [Google Scholar]
- 35.Lin QY, Cao ZH, Jiang CS, et al. Inhibitory effect of Acanthopanax senticosus on the production of nitric oxide and tumor necrosis factor-α in RAW 264.7 macrophages. J Anhui Agric Sci 2007; 26: 8088–8089 + 8113. [Google Scholar]
- 36.Malmberg KJ, Levitsky V, Norell H, et al. IFN-γ protects short-term ovarian carcinoma cell lines from CTL lysis via a CD94/NKG2A-dependent mechanism. J Clin Invest 2002; 110: 1515–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robertsen B. The interferon system of teleost fish. Fish Shellfish Immunol 2006; 20: 172–191. [DOI] [PubMed] [Google Scholar]
- 38.Tau G, Rothman P. Biologic functions of the IFN‐γ receptors. Allergy 1999; 54: 1233–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mellor AL, Munn DH. Ido expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 2004; 4: 762–774. [DOI] [PubMed] [Google Scholar]
- 40.Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ 2002; 9: 1069–1077. [DOI] [PubMed] [Google Scholar]
- 41.Xiaodong W, Wanqiu H, Shuhui S, et al. Novel function of IFN-gamma: negative regulation of dendritic cell migration and T cell priming. J Immunol 2006; 177: 934–943. [DOI] [PubMed] [Google Scholar]
- 42.Bessa J, Jegerlehner A, Hinton H J, et al. Alveolar macrophages and lung dendritic cells sense RNA and drive mucosal IgA responses. J Immunol 2009; 183: 3788–3799. [DOI] [PubMed] [Google Scholar]
- 43.Mora JR, Iwata M, Eksteen B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 2006; 314: 1157–1160. [DOI] [PubMed] [Google Scholar]
- 44.Ferlazzo G, Münz C. NK cell compartments and their activation by dendritic cells. J Immunol 2004; 172: 1333–1339. [DOI] [PubMed] [Google Scholar]
- 45.Walzer T, Dalod M, Vivier E, et al. Natural killer cell–dendritic cell crosstalk in the initiation of immune responses. Expert Opin Biol Ther 2005; 5: S49–S59. [DOI] [PubMed] [Google Scholar]