Abstract
Both innate immunity and acquired immunity are involved in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. The induction of Abs that neutralize the virus has been described, and certain Abs against endemic coronaviruses may cross-react with SARS-CoV-2. Detailed mechanisms to protect against the pandemic of SARS-CoV-2 remain unresolved. We previously reported that IgG Fc-binding protein (Fcγbp), a unique, large molecular weight, and mucin-like secretory Fc receptor protein, secreted from goblet cells of human small and large intestine, mediates the transportation of serum IgG onto the mucosal surface. In this review, we show that mucous bronchial gland cells and some goblet cells are immunoreactive for Fcγbp. Fcγbp traps the cross-reactive (both neutralizing and non-neutralizing) IgG bound to the virus and can consequently eliminate the virus from the mucosal surface to decrease viral loads. Fcγbp can also suppress immune overreaction by interfering with Fc-binding by macrophages and competing with complement fixation. Fcγbp secreted from mucin-producing cells of the airway functions as an important anti-infection mucosal defense. The Fcγbp-mediated mechanism can be a key factor in explaining why SARS-CoV-2 is less infective/lethal in children, and may also be involved in the unique Ab response, recurrent infection, and effects of serum therapy and vaccination.
Keywords: Cross-reactive antibodies, endemic coronavirus, IgG Fc-binding protein (Fcγbp), mucin-secreting cells, SARS-CoV-2
Introduction
The pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has affected 193 million people around the world as of 23 July 2021. More than 4 million deaths have been recorded. COVID-19 causes clinical manifestations that range widely from no symptoms to severe acute respiratory syndrome, but the mechanisms that determine such variable outcomes remain unresolved. It has been clarified that COVID-19 is a systemic disease, involving not only the respiratory tract but also vascular endothelial cells and the gastrointestinal mucosa.1,2 However, it is also unclear whether innate or acquired immunity is required to prevent and control SARS-CoV-2 infection. Reportedly, serum neutralizing Abs (Nabs) in patients after recovery from the infection were found to be significantly higher in elderly patients than in younger ones, whereas, in some patients, Nabs were undetectable in the serum. 3 Levels of antiviral IgG and IgM were higher in the severe group than those in the non-severe group, and IgG seroconversion occurred synchronously, earlier or later than IgM seroconversion. 4 Asymptomatic individuals had a weaker immune response to SARS-CoV-2 infection, and their duration of viral shedding was significantly longer than that of the symptomatic group. 5 Immunological cross-reactivity between endemic coronaviruses causing common colds and SARS-CoV-2 may account for the reduced incidence of COVID-19 in children. 6 It is noteworthy that IgG does not necessarily reveal virus-neutralizing activity. These findings suggest the existence of previously unknown immune mechanisms that extinguish the virus from the airway.
Mucin plays a pivotal role in protecting the respiratory mucosa against microbial infections by acting as a primary contact seed to entrap and remove invading microbes. 7 One of the authors (KK) previously reported on IgG Fc-binding protein (Fcγbp) secreted from mucin-producing cells in a variety of mucosa, including the intestinal and respiratory mucosa.8–10 We postulate herein that Fcγbp binds to immune complexes of SARS-CoV-2 and IgG cross-reactive to endemic coronaviruses, and that the virus can be eliminated from the airway by non-neutralizing cross-reactive IgG coupled with Fcγbp. The immunologically neglected roles of Fcγbp in COVID-19 are proposed and discussed.
Cross-reactive epitopes on the spike protein of coronaviruses and the immune response
Four endemic (seasonal) coronaviruses, HCoV-229E, HCoV-NL63, HCoV-OC43, and HCoV-HKU1, cause seasonal common colds, usually accompanied by mild symptoms of upper airway infection. Two of these endemic coronaviruses, HCoV-OC43 and HCoV-HKU1, as well as Middle Eastern respiratory syndrome coronavirus (MERS-CoV), severe acute respiratory syndrome coronavirus (SARS-CoV), and SARS-CoV-2, belong to the β-coronaviruses, 11 whereas HCoV-229E and HCoV-NL63 are categorized as α-coronaviruses. These endemic coronaviruses may cause 10–15% of common colds encountered mainly in the wintertime. It is estimated that initial infection occurs before the age of 5 or 6 yr and that ≥ 90% of adults have serum Abs against these four viruses. 11 A recent cohort study showed that adults, but not children, suffering from a common cold (with symptoms of acute sinusitis, bronchitis, or pharyngitis) in the preceding year had a lower risk of COVID-19. 6 Sagar et al. also noticed that patients recently infected by the endemic coronaviruses suffered less severe COVID-19 infection. 12 Analysis of the amino acid sequence of the immunogenic spike glycoprotein of SARS-CoV-2 reveals homology of 76% for SARS-CoV, 42% for MERS-CoV, 30% for HCoV-OC43, and 29% for HCoV-HKU1. 13 , 14 Hicks et al. observed the cross-reactivity of IgG against the spike protein of SARS-CoV-2 with that of other β-coronaviruses, HCoV-OC43 and HCoV-HKU1, and found the strongest cross-reactivity for SARS/MERS-CoV, as expected based on the sequence homology. 14 The spike protein of β-coronaviruses is composed of two subunits, N-terminal S1 and C-terminal S2, and plays a key role in receptor recognition with the S1 subunit and the cell membrane fusion process with the S2 subunit. 15 The main antigenic target of Nabs, the receptor-binding domain (RBD), is located in the S1 subunit of the spike protein. The sequence of the RBD of SARS-CoV-2 shows 73% homology with SARS-CoV, whereas other endemic β-coronaviruses show only 7–10% sequence homology.13,16
CD4+ T cells reactive with the spike glycoprotein of SARS-CoV-2 were observed in the peripheral blood of not only patients with COVID-19 but also healthy donors unexposed to SARS-CoV-2. 17 , 18 Spike-reactive CD4+ T cells in healthy donors were reactive primarily with the S2 subunit of the spike protein. 17 Potential SARS-CoV-2 binding and the Nab precursor sequence were abundant in naive B-cell repertoires in individuals sampled before the present pandemic. 19 These latent immune responses were speculated to be a consequence of prior exposure to the endemic coronaviruses. Lv et al. analyzed cross-reactive Abs in patients and mice infected with SARS-CoV-2 or SARS-CoV. 20 Cross-reactive Abs binding to the spike protein were commonly identified, but cross-neutralization of the live virus was a rare event. Nguyen-Contant et al. reported that individuals unexposed to SARS-CoV-2 commonly had IgG against the S2 subunit, 21 suggesting that cross-reactive B cell responses against the S2 subunit might enhance broad coronavirus protection. Ng et al. documented that Nabs against the S2 subunit of the spike protein were detectable in individuals unexposed to SARS-CoV-2, 22 particularly in children and adolescents, whereas SARS-CoV-2 infection induced high titers of SARS-CoV-2 spike-reactive IgG targeting both the S1 and S2 subunits.
It is likely that memory CD4+ T cells and memory B cells in healthy donors facilitate an accelerated antibody response against the spike protein, but these cross-reactive Abs may not necessarily neutralize SARS-CoV-2. The defense against SARS-CoV-2 through humoral immunity cross-reactive with endemic coronaviruses might be insufficient.
Characterization and distribution of Fcγbp in the intestine and bronchus
Goblet cells in the intestinal mucosa synthesize secretory mucin glycoprotein and bioactive molecules to form a viscous mucus layer on the mucosa. The mucus layer plays an important role in interacting with indigenous microbes and also with both innate and adaptive immunity. 10 One of the authors (KK) first identified Fcγbp in the mucin secreted by intestinal goblet cells.8,9,23 An immunohistochemical study using mAbs revealed that mucin-producing cells in the intestine, gallbladder, bile duct, bronchus, nasal mucosa, submandibular gland, and uterine cervix contained Fcγbp. 24 Secretory fluids such as the sputum, saliva, and nasal mucus were capable of binding IgG. The production of Fcγbp in the saliva has also been confirmed.25,26 Fcγbp suppressed complement-mediated hemolysis of sheep red blood cells, 10 and was detected in human serum. 27 Fcγbp was immunologically distinct from the known Fc receptor proteins on lymphocytes and macrophages. 8
Analysis of cDNA disclosed that Fcγbp shows mucin-like structures and contains 12 repeats of a 400-amino-acid-long cysteine-rich unit in a total of 5382 amino acids. 28 The amino acid sequence showed homology with MUC2, the representative intestinal goblet cell mucin, and prepro-von Willebrand factor but was distinct from other Fc-binding proteins such as bacteria-derived protein A and protein G, and neonatal Fc receptor. 29 Cysteine-mediated covalent attachment of Fcγbp with MUC2 contributes to cross-linking and stabilization of a mucin network in the mucus layer of the intestine.23,30 Fcγbp is quite unique in that it is a secretory and large-sized glycoprotein. 28 The Fcγbp gene exists in a wide variety of animals, 31 and Fcγbp expression is seen ubiquitously in vertebrates. 32
Immunohistochemical localization of Fcγbp in the colon and bronchus is illustrated in Figure 1. Formalin-fixed, paraffin-embedded sections of normal colon and lung were immunostained with a rabbit polyclonal antibody to Fcγbp, available from Abcam (Cambridge, UK, catalog no. ab121202). With a Ventana BenchMark ULTRA autostaining system (Ventana Medical Systems, Oro Valley, AZ, USA), deparaffinized sections were pre-treated with heat-induced epitope retrieval in 1 mM ethylenediamine tetrahydrochloride solution, pH 8.5, for 60 min, and the 1:500 diluted anti-Fcγbp Ab was incubated for 30 min at room temperature. The ultraView Universal kit (containing HRP-labeled long-armed linker) was used as the second layer reagent. After a diaminobenzidine coloring reaction, the nuclei were counterstained with Mayer’s hematoxylin.
Figure 1.
Immunohistochemical localization of Fcγbp in the colonic and bronchial mucosa (formalin-fixed, paraffin-embedded sections after heat-induced epitope retrieval). Goblet cells in the colon (a) and mucous cells in the bronchial gland (b) show clear positivity. The mucin secreted into the colonic lumen was also clearly immunoreactive. Some goblet cells in the ciliated bronchial mucosa are weakly immunoreactive (arrow in inset).
Goblet cells of the colonic mucosa were clearly labeled, and Fcγbp immunoreactivity was visualized in the mucinous substance secreted into the gut lumen. Fcγbp colocalizes with MUC2, an intestinal-type mucin core protein. In the lung, mucous cells of the bronchial glands were clearly labeled, and some goblet cells in the bronchial mucosa also showed weak immunoreactivity. The peripheral lung tissue was negative for Fcγbp. The distribution of Fcγbp in the bronchial mucosa is similar to that of MUC5B, another mucin core protein,7,33 and both play an important role in airway defense.7,34 MUC7 and MUC19 are other functional mucins distributed in the major and minor salivary glands. 35
Roles of Fcγbp in protecting against SARS-CoV-2 infection
We propose two kinds of function for Fcγbp as schematically illustrated in Figure 2.
Figure 2.
Schematic illustration of the functions of Fcγbp in fighting against SARS-CoV-2 infection in the bronchial mucosa. Fcγbp is produced by and secreted from mucous cells, mainly those in the bronchial gland, and some goblet cells located among the ciliated bronchial mucosa. Fcγbp steadily traps IgG, especially the IgG-SARS-CoV-2 immune complex, inside and outside the mucosa. The Fcγbp-mediated transmucosal IgG transportation system effectively inactivates the virus, even with non-neutralizing antiviral IgG. Excessive stimulation of lymphocytes and macrophages is also suppressed by Fcγbp-mediated concealment of the Fc portion of IgG.
SARS-CoV-2: severe acute respiratory syndrome coronavirus-2.
First, IgG coupled with Fcγbp actively secreted from mucin-producing cells or IgG leaked onto the mucosal surface after epithelial cell injury or apoptosis binds to microbes such as SARS-CoV-2. In the latter situation, the immune complex is captured by free Fcγbp to form a huge, mucin-coated molecular complex. 30 Abs cross-reactive to endemic coronaviruses can bind to SARS-CoV-2 but can scarcely neutralize the virus. IgG molecules can be secreted onto the mucosa when combined with Fcγbp, which is cosecreted with mucin. Interaction of Fcγbp with airway mucins (MUC5B, MUC7, and MUC19) may accelerate excretion of the virus in a large molecular complex consisting of virus-IgG-Fcγbp-mucins. 35 Supposedly, Fcγbp coupled with immune complex consisting of non-NAbs, and the virus can prevent viral entry because the virus is effectively eliminated via the flow of mucin to which Fcγbp is tightly bound (Figure 3). This process leads to effective prevention of the invasion of microbes and may compensate the mucosal immunity mediated by secretory IgA, the first defense of the mucosa, particularly when the specific IgA response is insufficient. 36 Investigators have described the roles of Fcγbp in preventing human immunodeficiency virus (Thai RV144 vaccine strain) and herpes simplex virus-1: Fcγbp functions as a viral trap for IgG-virus immune complexes on the mucosal surfaces. 37 , 38 Wang et al. emphasized the critical importance of cervicovaginal mucus gel in “muco-trapping” the virus. 38
Figure 3.
Intimate relationship between Fcγbp and mucin on the bronchial mucosa. IgG-SARS-CoV-2 immune complex is bound to Fcγbp through the Fc portion of IgG. Fcγbp is attached tightly to mucin, and SARS-CoV-2 can be eliminated by mucin secretion. (a) and (b) illustrate situations without Fcγbp production: (a) Fcγbp (–), IgG (–); (b) Fcγbp (–), IgG (+). (c) and (d) indicate situations with secretion of Fcγbp attached to the mucin flow: (c) Fcγbp (+), IgG (–); (d) Fcγbp (+), IgG (+). When IgG trapping SARS-CoV-2 is bound to Fcγbp (d), SARS-CoV-2 is effectively eliminated by Fcγbp on the mucin molecules.
SARS-CoV-2: severe acute respiratory syndrome coronavirus-2.
It is well known that in the secretory IgA transportation system, secretory component is expressed on the basolateral plasma membrane of the columnar epithelial cells as a receptor of dimeric IgA, and secretory component-bound dimeric IgA is transported actively through their apical plasma membrane into the lumen. 36 It is intriguing to suppose that Fcγbp is expressed on the basolateral plasma membrane of the mucous epithelial cells as a receptor for IgG in tissue fluid for active and efficient transmembrane transportation of IgG into the lumen. A pre-embedding immunoelectron microscopic study of Fcγbp may help prove our assumption.
Second, Fcγbp suppresses and controls the overreaction of lymphocytes and macrophages by blocking both the Fc portion of IgG and complement fixation. Fcγbp functions mainly on the mucosal surface, so Fcγbp may inhibit the activation of Fc-receptor-positive immune cells exuded onto the mucosa. Fcγbp bound to the IgG–microbe complex may also suppress the activation of lymphocytes and macrophages within the tissue.
It is noteworthy that some bacteria express IgG Fc-binding proteins, such as protein A on Staphylococcus aureus, protein G on Streptococcus pyogenes and protein L on Peptostreptococcus magnus (Finegoldia magna). 39 Prevotella intermedia, a putative etiologic agent of adult chronic periodontitis, also shows IgG Fc-binding activity as its virulence factor. 40 The Fc-binding activity of the bacteria may compete with that of Fcγbp by hampering the binding of Fcγbp to the antibacterial IgG-bacteria complex.
Fcγbp-mediated mechanism has an aspect of innate immunity
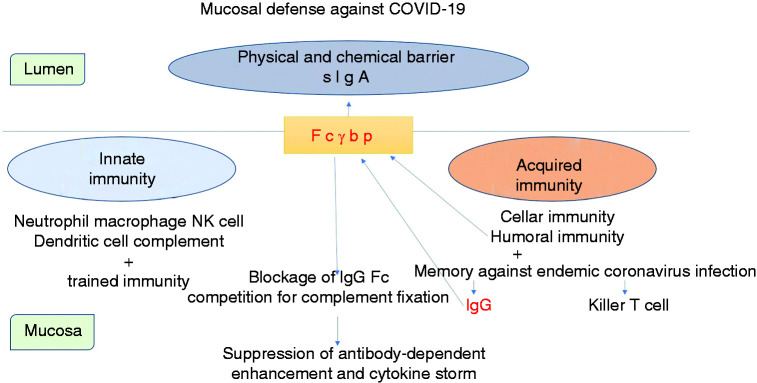
Theoretically, the immune response to SARS-CoV-2 is modulated by both IgG cross-reactive to endemic coronaviruses and IgG specific for SARS-CoV-2 secreted onto the mucosal surface via the Fcγbp-mediated mechanism. We suppose that Fcγbp-mediated transmucosal IgG transportation could be positioned intermediately between the actions of innate and acquired immunity (Figure 4).
Figure 4.
Schematic demonstration of mucosal defense against SARS-CoV-2 infection. Fcγbp effectively binds and carries the IgG-virus complex across the mucosa. The Fcγbp-mediated viral elimination can be achieved even with non-neutralizing IgG antibodies. Abs cross-reactive with endemic coronaviruses, which are particularly rich in children, can function as effective protective arms. We suppose that the Fcγbp-mediated transmucosal IgG transportation system falls between innate and acquired immunity.
SARS-CoV-2: severe acute respiratory syndrome coronavirus-2.
In addition to binding to IgG, Fcγbp is expected to be part of a net-like scaffold within mucosal barriers and can be a component of the first line of innate immune defense. 30 Fcγbp maintains MUC2 mucus structural integrity by stabilizing the mucus layer, and the integrated mucus layer responds to infection by the colonic pathogen Entamoeba histolytica. 41 Dynamic interactions between mucins and interacting proteins, including Fcγbp, contribute to the innate immune properties of gut and airway mucosal surfaces.23,42 Fcγbp has been shown to consistently form heterodimers with trefoil factor family-3 (TFF3), a cysteine-rich 59 amino acid peptide, including seven cysteine residues that allow dimerization. 43 TFF1 and TFF2 are distributed mainly in the gastric mucosa, where Fcγbp also forms a complex with TFF1. 44 TFF peptides (TFF1, TFF2, and TFF3) share a conserved 40-amino acid sequence termed the trefoil domain having three intramolecular disulfide bonds. 44 Fcγbp contains 435 cysteine residues, and this odd number may favor an intermolecular disulfide bridge with TFF1 and TFF3, as well as with the mucin molecules of MUC2 and MUC5B. TFF2 forms a tight complex with MUC6 to stabilize the gastric mucus layer. 44 TTF3 is produced by intestinal goblet cells and mucous salivary gland cells and co-secreted with Fcγbp. 45 Production of Fcγbp and TFF3 in the minor salivary gland was comparable with that in the major salivary gland. 25 Localization of TFF3 in the respiratory tract has also been proven. 46 TFF peptides are widely involved in mucosal protection and repair as a constituent of the mucus barrier.26,47 TFF3-Fcγbp heterodimers are likely a part of the innate immune defense of mucous epithelia of the gut and airway that prevent the infiltration of microorganisms. 44 In fact, TFF peptides represents a family of secretory lectins and can bind to microbial glycans. For example, a lectin activity of TFF1 enables binding to the LPS of Helicobacter pylori on the gastric mucosal surface. 48 TFF-Fcγbp heterodimers can thus contribute to the clearance of pathogenic bacteria. Fcγbp, ubiquitous in vertebrates, has a conserved N-terminal domain, and this domain is also present as an N-terminal sequence in a number of bacterial proteins. 32 Of note, Fcγbp is the highest up-regulated early defense gene in catfish skin after the occurrence of microbial infection, and Fcγbp should be viewed as a first-line responder with a distinct role in innate immunity by critically regulating pathogen attachment and disease progression on the mucosal surface. 49
Antibody responses against SARS-CoV-2
Figure 5 displays IgG responses against SARS-CoV-2 schematically. We propose the existence of three types of IgG: IgG-a, IgG-b, and IgG-c. IgG cross-reactive to endemic coronaviruses (termed IgG-a) has been repeatedly reported.6,14,20–22,50,51 IgG-a binds both to SARS-CoV-2 at the Fab portion and to Fcγbp at the Fc portion. The immune complex coupled with Fcγbp in the mucin network disturbs viral reentry into other epithelial cells. Viral attachment onto the epithelial cells is minimized, which suppresses intracellular viral proliferation. Fcγbp can thus significantly interfere with viral transmission to other persons. However, the virus may remain alive to become temporarily commensal in the mucosa. Once SARS-CoV-2 is sufficiently eliminated by IgG-a and Fcγbp, patients remain asymptomatic and have a low viral load and weak immune response specific to SARS-CoV-2.
Figure 5.
IgG responses (IgG-a, IgG-b, and IgG-c) against SARS-CoV-2. IgG-a represents pre-existing IgG cross-reactive to endemic coronaviruses. A low level of IgG-a titer is observed in the serum as baseline immunity. IgG-b indicates boosted IgG cross-reactive to endemic coronaviruses as a secondary immune response. IgG-b production is supposedly stimulated as early as 4 or 5 d after infection. IgG-c includes IgG molecules capable of neutralizing SARS-CoV-2. The production of IgG-c is assumed to start as early as 10 d after infection.
Nabs: neutralizing Abs; SARS-CoV-2: severe acute respiratory syndrome coronavirus-2.
When the protection via IgG-a is insufficient, cross-reactive IgG (termed IgG-b) is boosted as a secondary immune response of memory cells and can protect from mucosal infection by the same mechanism as IgG-a. It is well known that the secondary Ab response occurs more quickly and forcefully than the primary response. 52 IgG-b represents Abs cross-reactive to endemic coronaviruses as a secondary response to infection of SARS-CoV-2. The boosted production of IgG-b begins as early as 4 or 5 d after infection. SARS-CoV-2 bound to IgG-b and then entrapped by Fcγbp is eliminated from the airway, thus reducing infectivity. In patients with mild and moderate disease severity, the Fc portion of IgG-a and IgG-b bound to SARS-Cov-2 is occupied by Fcγbp, and activation of lymphocytes and macrophages is limited and delayed. Therefore, binding of IgG to Fcγbp diminishes the tissue damage caused by the Fc-mediated activation of immune cells and complement fixation.
Dugas et al. noted that a less severe course of COVID-19 was associated with elevated levels of antibodies against endemic coronaviruses. 53 They concluded that prior infections with endemic coronaviruses might protect against a severe course of disease. Gouma et al. reported that Abs cross-reactive to SARS-CoV-2 elicited by past endemic coronavirus infections were not protective. 54 However, the duration of symptoms following SARS-CoV-2 infection was significantly shortened in individuals with higher Ab titers against common β-coronavirus. Zohar et al. comparatively analyzed humoral immune response to SARS-CoV-2 in survivors and non-survivors with severe disease, 55 and they emphasized the importance of the IgG-mediated Fc-binding response. IgA and IgM responses were comparable in both groups, whereas rapid and potent IgG class switching was linked to survival. The non-survivors showed attenuated IgG responses accompanied by compromised IgG Fc receptor binding and Fc effector activity.
At 10–14 d after infection, IgG-type Nabs against SARS-CoV-2 (termed IgG-c) are provoked. IgG-3, probably directed against the RBD region of the spike protein, efficiently neutralizes SARS-CoV-2 and induces the subsidence of inflammation and patient symptoms. Suthar et al. reported that Nab titers against the RBD region were detectable in all 44 patients evaluated within 6 d after confirmation of infection by PCR testing. 16 Prévost et al. found that most individuals developed RBD-specific Nabs of the IgG type within 2 wk of infection, 50 but the level of neutralizing activity decreased significantly over time. Inactivated SARS-CoV-2 or its RNA fragments may, however, be detected by PCR analysis.
Mechanism of infection of SARS-CoV-2 and sequelae
Infecting SARS-CoV-2 viral particles are attacked by lymphocytes and macrophages in the bronchial mucosa. Cytokines, including IFNs, are released as a result of activated innate immunity and provoke flu-like symptoms such as fever, fatigue, headache, and arthralgia. The coronaviruses, including SARS-CoV-2, use angiotensin-converting enzyme-2 (ACE2) as a receptor for cellular entry. The highest expression of ACE2 is seen in the ciliated epithelial cells and goblet cells in the respiratory tree, but ACE2 is also expressed in mucin-secreting glandular cells and type 2 alveolar epithelial cells. 56 Mucous bronchial glands secreting both mucin and Fcγbp are not distributed in the peripheral lung (bronchioles and alveoli). When SARS-CoV-2 viral particles reach and destroy epithelial cells of the peripheral lung, ground-glass opacities on chest computed tomography (CT) scans, 5 or interstitial lung changes (diffuse alveolar damage) occur.57,58
The maintenance of intact bronchial mucosa should be important for the secretion of Fcγbp from bronchial mucous gland cells. It should be noted that the minor salivary gland, including the bronchial gland, is functionally comparable with the major salivary gland.7,25,33–35,46 SARS-CoV-2 is entrapped by Fcγbp coupled with IgG-b or IgG-c, resulting in rapid elimination of the virus outside the body. Infection of SARS-CoV-2 may cause bronchitis that destroys the bronchial mucosal structure. In lethal cases, the bronchial mucosa is often eroded in SARS-CoV-2-infected lungs. 58 Such results may be supported by the findings of Isho et al., 59 who reported that anti-SARS-CoV-2 Ab responses were readily detected in both the serum and saliva, with peak IgG levels attained by 16–30 d post-symptom onset. Anti-SARS-CoV-2 IgA and IgM Abs decayed rapidly, whereas IgG antibodies remained relatively stable up to 105 d post-symptom onset in both biofluids. They also mentioned that IgG responses to SARS-CoV-2 spike proteins in the serum correlated positively with those in matched saliva samples.
In a systematic review of 1350 cases of recurrent COVID-19 by Gidari et al., 60 the presumptive viral reactivation occurred 34.5 d after the onset of COVID-19, and 27.5% of the patients were symptomatic. The rate of intensive care unit admission was 2.6% at the primary infection, and most patients had a good prognosis with only mild symptoms at the recurrence. In most cases, the presumptive recurrence is prolonged but the patient is not contagious, and the persistence of viral RNA in the respiratory tract might represent slow resolution of the disease. 60
SARS-CoV-2 also infects the intestinal mucosa. 2 The infectious virus is isolated readily from samples of the throat or nose but rarely from stool samples irrespective of the high concentration of viral RNA. 61 Enterocytes in the colon express the viral receptor, ACE2, but goblet cells lack it.56,62 When SARS-CoV-2 infects the intestinal mucosa, the virus bound to antiviral IgG is entrapped by Fcγbp secreted from goblet cells, richly embedded in luminal mucin (MUC2), and finally eliminated by peristaltic movement.
Gaebler et al. reported that immunofluorescence and PCR analyses of intestinal biopsies obtained from asymptomatic individuals 4 mo after the onset of COVID-19 revealed the persistence of SARS-CoV-2 nucleic acids and immunoreactivity in 7 of 14 individuals. 63 In mild cases, the level of IgG-c, the Nabs against the RBD region of the spike protein, may be low, and thus SARS-CoV-2 cannot be neutralized completely.
The persistence of SARS-CoV-2 mRNA and Ags in small bowel goblet cells 4 mo after the onset of COVID-19, 63 and the relapse of COVID-19 more than 1 mo after disease onset, 60 allow us to propose that in mild cases with weak immune reactions, SARS-CoV-2 may become temporarily commensal probably in the intestinal mucosa, leading to recurrence of the disease. At this time, acquired immunity is re-activated as a secondary immune response, so the virus can be eliminated through the function of Fcγbp to avoid severe disease.
COVID-19 and associated disorders
The prognosis of obese patients and elderly people in nursing homes is poor. In addition to malfunctions of innate immunity, disturbed drainage of sputum due to restricted lung expansion may be responsible for the severity of the disease. SARS-CoV-2 coupled with Fcγbp is thus incompletely excreted outside the body. Pregnant women also show an increased risk of hospitalization and disease severity. 64 Disturbance of sputum drainage might occur in the third trimester because the enlarged uterus compresses the diaphragm, resulting in the restriction of thoracic cavity movement and excretion of airway exudates.
Vardavas and Nikitara calculated that smokers were 1.4 times more likely to have severe symptoms of COVID-19 and 2.4 times more likely to be admitted to an intensive care unit, need mechanical ventilation, or die compared with non-smokers. 65 Smokers showed lower saliva levels of IgG, IgA, and IgM than non-smokers. 66 Smoking resulted in increased expression of Fcγbp in the oral mucosa as a secondary response, whereas its expression was reduced in the bronchial mucosa. 67
Patients with mild-to-moderate bronchial asthma consistently present with hyperplasia of goblet cells and mucous glands (with increased storage of epithelial mucin), 68 and, thus, the comorbidity rate of COVID-19 with asthma is low, and disease severity is milder. 69 A systematic review with meta-analysis of 57 studies by Sunjaya et al. found that patients with asthma showed a 14% reduction in the risk of acquiring COVID-19 and 13% reduction in hospitalization with COVID-19 when compared with those without. 70 It is reasonable to suppose that mucous cells secreting Fcγbp may efficiently eliminate SARS-CoV-2 from the mucosal surface. Conversely, a South Korean study showed that asthma was not a risk factor for the poor prognosis of COVID-19; however, the proportion of asthmatic patients among the total COVID-19 patients was higher than the previously known prevalence in Asian-Pacific countries. 71
COVID-19 in children
Children present milder symptoms following COVID-19 when compared with adults, and only rarely develop severe respiratory disease. The susceptibility to infection in individuals under 20 yr of age is approximately half that of adults aged 20 yr or over: clinical symptoms are manifested in 21% of teenagers but in 69% of people aged over 70 yr. 72 However, children can carry high levels of the PCR-proven viral genome in the upper airway, particularly in an early phase of SARS-CoV-2 infection, yet display minimal or mild symptoms.73,74 Aran et al. emphasized the importance of Abs cross-reactive to endemic coronaviruses to reduce incidence of COVID-19 in the young. 6 Zimmermann and Curtis have reviewed extensively the variegated factors related to why children experience less severe COVID-19. 75 These include differences in innate and adaptive immunity, more frequent recurrent and concurrent infections, pre-existing immunity to coronaviruses, differences in microbiota, and lower density/affinity and different distribution of ACE2 receptors.
The presence of Abs cross-reactive to endemic coronaviruses in the serum can help us interpret these findings. It has been reported from serum sampling at regular intervals that re-infection with the same endemic coronavirus can commonly occur 12 mo after the initial infection. 76 Ab titers wane by 1 yr after initial infection, and many children become reinfected and shed the virus, even though clinical symptoms may fail to accompany the second infection. 11 Children in day-care centers and schools and young adults have more frequent social contacts than older people and are thus affected by endemic coronaviruses, often with no, or only mild, symptoms. Infants and children commonly possess a considerable amount of serum IgG-a.6,22 SARS-CoV-2 is bound to IgG-a and then coated by Fcγbp to eliminate the virus into the lumen. The viral load is controlled easily by the IgG-a/Fcγbp mechanism, which resembles innate immunity, and thus the acquired immune system is stimulated only weakly. The production of IgG-b is activated as an immune response to infection, and the cooperation of IgG-b with Fcγbp suppresses mucosal immune overreaction and prevents severe respiratory disease.
Superspreaders
Among adults, the superspreader is speculated to be a person who has no immunity cross-reactive to endemic coronaviruses and shows deficient Fcγbp functions. In patients who have no memory immunity to endemic coronaviruses and thus poor innate immunity, the elimination of SARS-CoV-2 is ineffective. Naked and active viruses not coupled to Fcγbp can be spread easily in an aerosol form to the environment. Particularly when the bronchial mucosa is destroyed, Fcγbp is lost, and, even if IgG-c is produced, the elimination of SARS-CoV-2 may be deficient because of the significant paucity of Fcγbp.
Mechanisms for the severity of COVID-19
When the SARS-CoV-2/IgG complex is not entrapped by Fcγbp, it is actively phagocytized by macrophages, which causes overproduction of cytokines and even Ab-dependent enhancement as in the case of SARS-CoV. 11 The proliferated virus particles spread to the whole body by the activated macrophages, and they are then entrapped by ACE2-expressing endothelial cells in a variety of organs and tissues. 1 The activated complement pathway may contribute to vasculitis or thrombotic microangiopathy to induce multiorgan failure.57,58 The lung epithelium shows complement deposition after inflammation. Carvelli et al. reported an increase of soluble C5a levels in proportion to the severity of COVID-19 and high expression levels of C5aR1 receptor (CD88) in blood and pulmonary myeloid cells. 77 Anti-C5aR1 mAb inhibits the acute lung injury in human C5aR1-knock-in mice.
Reportedly, some patients with severe respiratory disease due to COVID-19 pneumonia were treated successfully with the inhibitor of complement C3. 78 The complement cascade has been proposed as a possible driver of lung inflammation and cytokine storm, and blockade of the complement cascade may represent a potential therapeutic strategy. 79 In our previous study, Fcγbp inhibited complement-mediated hemolysis of sheep red blood cells in vitro, 10 and Fcγbp Ag, normally detected in the human serum, was often increased in autoimmune diseases. 27 Fcγbp may function as an intrinsic blocker of the complement cascade on the classical pathway, as well as being an immune regulator, by inhibiting the Fc receptor on lymphocytes and macrophages. Deficient Fcγbp might be one of the background mechanisms causing thrombotic angiopathy and the lethal cytokine storm seen in COVID-19.
Application of serum therapy
Passive Ab therapy using the serum of patients recovered from COVID-19 has been tried as a therapeutic and prophylactic option. In randomized trials, however, no significant differences were observed in clinical status or overall mortality between patients treated with the convalescent plasma and control patients.80,81 In the subgroup analysis of their control study, Salazar et al. reported that patients transfused with the high-titered plasma within 72 h of admission experienced a significant decrease in mortality rate. 82 A randomized, double-blind, placebo-controlled trial showed that early administration of the convalescent high-titered plasma to mildly ill elderly adults reduced the progression of COVID-19 with a relative risk reduction of 48%. 83 A high percentage of COVID-19 patients with severe respiratory disease possess detectable levels of Nabs 7 d after disease onset. Supposedly, in severe cases, advanced destruction of the bronchial mucosa may result in the decrease of Fcγbp, and, thus, IgG against SARS-CoV-2 may be unable to function due to the paucity of Fcγbp. Bryce et al. described autopsy lung findings of lethal COVID-19. 58 In addition to diffuse alveolar damage and thromboembolism as direct causes of death, the epithelium of the mainstem bronchi was largely denuded and the airways showed only minimal lymphocytic infiltration. Ulceration and acute inflammation were seen in 2 of 99 cases. It is thus reasonable to suppose that convalescent plasma therapy might be effective before extensive destruction of the bronchial mucosa occurs. A multicenter retrospective cohort study indicated that early intravenous administration of high-dose immunoglobulins improved the prognosis of patients with critically severe COVID-19. 84 Immunoglobulins pooled from healthy donors may contain antibodies to endemic coronaviruses, and the infused cross-reactive IgG can work in conjunction with Fcγbp to protect the bronchial mucosa from the viral insult. Fcγbp has critical roles in the elimination of SARS-CoV-2 and blockage of complement as long as the bronchial mucosa remains intact.
Variant viruses, vaccination, and Fcγbp
Fcγbp might also play an important role in the process of vaccination against SARS-CoV-2. A new variant of SARS-CoV-2 of United Kingdom (UK) origin is accompanied by 14 mutations and three deletions, including an amino acid substitution in six key residues in the RBD region of the spike protein. 85 The variant virus is 43–90% more transmissible than the predecessor lineage, but without clear evidence showing a change in disease severity, and its enhanced transmission may lead to higher disease incidence and more frequent hospital admissions. 86 Children are more susceptible to infection with the variant than with the preexisting virus. It is speculated that enhanced virus binding affinity to human ACE2 results in the increased transmissibility. We suppose the following mechanisms for the susceptibility in children and young adults. The mutation and deletion in the spike protein contribute to an immune escape from cross-reactive antibodies (IgG-a and IgG-b) against endemic coronaviruses. The generation time is shorter and the number of viruses reproduced is higher when compared with those against the predecessor virus. The variant viruses induce inflammation in the bronchial mucosa more quickly, and more frequently cause children and young adults to be symptomatic before IgG-b can be produced. Namely, the higher disease incidence and more frequent hospital admissions in these age groups may be related to earlier damage of the lung due to the accelerated production of viruses.
Vaccines commonly target the spike protein of SARS-CoV-2, particularly at its RBD region. IgG and IgA responses to SARS-CoV-2 mRNA vaccines were detected in saliva 87 : within 1–2 wk after the second dose, all mRNA vaccine recipients had saliva IgG Abs against S-protein. However, mutation of the spike protein might lead to poor responsiveness of the Nabs generated by vaccination. Xie et al. reported that vaccine-elicited sera effectively neutralized three (UK, South African, and Brazilian) variants of SARS-CoV-2 having mutations of the spike protein. 88 In contrast, Chen et al. noted that most convalescent sera, as well as mRNA vaccine-induced immune sera, showed reduced inhibitory activity against the variant virus containing an E484K spike mutation. 89 The estimated effectiveness of the vaccine against infection with the B.1.1.7 variant was 89.5% ≥14 d after the second vaccine dose, whereas the effectiveness against infection with the B.1.351 variant was 75.0%. The effectiveness of the vaccine against severe, critical, or fatal disease due to infection with the variant viruses (the B.1.1.7 and B.1.351 variants being predominant in Qatar) was very high, at 97.4%. 90 Vaccine-induced Abs that polyclonally recognize plural viral epitopes can facilitate the elimination of SARS-CoV-2 with the aid of Fcγbp. It seems that the vaccine-induced polyclonal Abs against the spike protein can keep patients minimally symptomatic even when the neutralization of SARS-CoV-2 fails to work due to mutation of the RBD region.
Conclusions
IgG Fc-binding protein (Fcγbp), a unique high molecular mass mucin-like secretory protein that transports IgG across the mucosa, supposedly plays an important role in the defense against SARS-CoV-2 on the bronchial mucosal surface. Fcγbp effectively binds and carries the IgG-virus complex across the mucosa. Importantly, Fcγbp-mediated viral elimination can be achieved with non-neutralizing IgG Abs. Abs cross-reactive with endemic coronaviruses, which are particularly rich in children, can function as effective protection against SARS-CoV-2. The Fcγbp-mediated transmucosal transportation of IgG may be critical in understanding the trajectory of SARS-CoV2 disease and the effects of serum therapy and vaccination, in addition to innate immunity, Nab response and T-cell-mediated cellular immunity. Further pathobiological analysis is needed to prove our hypothesis regarding the neglected functions of Fcγbp to reveal novel and marvelous immune mechanisms in COVID-19.
Contributors
Each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content. KK designed the study and drafted the manuscript. MT planned the study framework and performed immunohistochemical analysis. YT provided in-depth discussion on our hypothetical proposal regarding the functions of Fcγbp and improved the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We cordially thank Naoki Ooishi, and Kuniaki Muramatsu, Department of Diagnostic Pathology, Shimada General Medical Center, Shimada, Shizuoka, Japan, for their skillful technical assistance. We also cordially acknowledge the valuable advice and suggestions given by Takashi Hashimoto, Department of Dermatology, Osaka City University, Graduate School of Medicine, Osaka, Japan and Toshifumi Hibi, IBD Center, Kitasato University Kitasato Institute Hospital, Tokyo, Japan. The manuscript was kindly checked by a native English speaker and simultaneous interpreter, Tina Hiroko Tajima at St. Marianna University School of Medicine, Kawasaki, Kanagawa, Japan.
Conflicts of interest: None declared.
Ethics approval: All procedures were performed in accordance with the ethical standards of the responsible institutional committee on human experimentation and with the Helsinki Declaration of 1964 and later versions. The study was approved in August 2020 by the Ethics Committee for Clinical Research of Shimada General Medical Center, Shimada, Shizuoka, Japan (approval number: R02-03).
Funding: The authors received no financial support for the research, authorship and/or publication of the present article.
ORCID iDs: Mitsuhiro Tachibana https://orcid.org/0000-0002-1041-0391
Yutaka Tsutsumi https://orcid.org/0000-0002-4136-9678
References
- 1.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020; 395: 1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devaux CA, Lagier J-C, Didier R. New insights into the physiopathology of COVID-19: SARS-CoV-2-associated gastrointestinal illness. Front Med (Lausanne) 2021: 8: 640073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu F, Liu M, Wang A, et al. Evaluating the association of clinical characteristics with neutralizing antibody levels in patients who have recovered from mild COVID-19 in Shanghai, China. JAMA Intern Med 2020; 180: 1356–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 2020; 26:845–848. [DOI] [PubMed] [Google Scholar]
- 5.Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020; 26:1200–1204. [DOI] [PubMed] [Google Scholar]
- 6.Aran D, Beachler DC, Lanes S, et al. Prior presumed coronavirus infection reduces COVID-19 risk: a cohort study. J Infect 2020; 81: 923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatterjee M, van Putten JPM, Strijbis K. Defensive properties of mucin glycoproteins during respiratory infections. Relevance for SARS-CoV-2. mBio 2020; 11:e02374–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi K, Blaser MJ, Brown WR. Identification of IgG Fc binding site in human intestinal epithelium. J Immunol 1989; 143: 2567–2574. [PubMed] [Google Scholar]
- 9.Kobayashi K, Hamada Y, Blaser MJ, et al. The molecular configuration and ultrastructural locations of an IgG Fc binding site in human colonic epithelium. J Immunol 1991; 146: 68–74. [PubMed] [Google Scholar]
- 10.Kobayashi K, Ogata H, Morikawa M, et al. Distribution and partial characterisation of IgG Fc binding protein in various mucin producing cells and body fluids. Gut 2002; 51: 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sariol A, Perlman S. Lessons for COVID-19 immunity from other coronavirus infections. Immunity 2020; 53: 248–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sagar M, Reifler K, Rossi M, et al. Recent endemic coronavirus infection is associated with less severe COVID-19. J Clin Invest 2021; 131: e143380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaimes JA, André NM, Chappie JS, et al. Phylogenetic analysis and structural modeling of SARS-CoV-2 spike protein reveals an evolutionary distinct and proteolytically sensitive activation loop. J Mol Biol 2020; 432: 3309–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hicks J, Klumpp-Thomas C, Kalish H, et al. Serologic cross-reactivity of SARS-CoV-2 with endemic and seasonal Betacoronaviruses. J Clin Immunol 2021; 41: 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Yang C, Xu XF, et al. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin 2020; 41: 1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suthar MS, Zimmerman MG, Kauffman RC, et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep Med 2020; 1:10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braun J, Loyal L, Frentsch M, et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature 2020; 587: 270–274. [DOI] [PubMed] [Google Scholar]
- 18.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T Cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020; 181:1489–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreer C, Zehner M, Weber T, et al. Longitudinal isolation of potent near-germline SARS-CoV-2-neutralizing antibodies from COVID-19 patients. Cell 2020; 182: 843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lv H, Wu NC, Tsang OT, et al. Cross-reactive antibody response between SARS-CoV-2 and SARS-CoV infections. Cell Rep 2020; 31: 107725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen-Contant P, Embong AK, Kanagaiah P, et al. S protein-reactive IgG and memory B cell production after human SARS-CoV-2 infection includes broad reactivity to the S2 subunit. mBio 2020; 11: e01991–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng KW, Faulkner N, Cornish GH, et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 2020; 370: 1339–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi K, Brown WR. Study of colonic IgG Fc binding site in cultured epithelial cells. Digest Dis Sci 1994; 39: 526–533. [DOI] [PubMed] [Google Scholar]
- 24.Kim YS, Ho SB. Intestinal goblet cells and mucins in human and disease: recent insights and prognosis. Curr Gastroenterol Rep 2010; 12: 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kouznetsova I, Gerlach KL, Zahl C, et al. Expression analysis of human salivary glands by laser microdissection: differences between submandibular and labial glands. Cell Physiol Biochem 2010; 26: 375–382. [DOI] [PubMed] [Google Scholar]
- 26.Houben T, Harder S, Schlüter H, et al. Different forms of TFF3 in the human saliva: heterodimerization with IgG Fc binding protein (FCGBP). Int J Mol Sci 2019; 20: 5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi K, Yagasaki M, Harada N, et al. Detection of Fcgamma binding protein antigen in human sera and its relation with autoimmune diseases. Immunol Lett 2001; 79: 229–235. [DOI] [PubMed] [Google Scholar]
- 28.Harada N, Iijima S, Kobayashi K, et al. Human IgGFc binding protein (FcgammaBP) in colonic epithelial cells exhibits mucin-like structure. J Biol Chem 1997; 272: 15232–15234. [DOI] [PubMed] [Google Scholar]
- 29.Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature 1989; 337: 184–187. [DOI] [PubMed] [Google Scholar]
- 30.Johansson MEV, Thomsson KA, Hansson GC. Proteomic analyses of the two mucus layers of the colon barrier reveal that their main component, the Muc2 mucin, is strongly bound to the Fcgbp protein. J Proteome Res 2009; 8: 3549–3557. [DOI] [PubMed] [Google Scholar]
- 31.Gene ID: 8857. FCGBP: Fc fragment of IgG binding protein [Homo sapiens (human)], https://www.ncbi.nlm.nih.gov/gene/?term=fcgbp (2021, accessed July 2021).
- 32.Lang T, Klasson S, Larsson E, et al. Searching the evolutionary origin of epithelial mucus protein components-mucins and FCGBP. Mol Biol Evol 2016; 33: 1921–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okuda K, Chen G, Subramani DB, et al. Localization of secretory mucins MUC5AC and MUC5B in normal/healthy human airways. Am J Respir Crit Care Med 2019; 199: 715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy MG, Livraghi-Butrico A, Fletcher AA, et al. Muc5b is required for airway defense. Nature 2014; 505: 412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frenkel ES, Ribbeck K. Salivary mucins in host defense and disease prevention. J Oral Microbiol 2015; 7: 29759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantis N, Rol N, Corthésy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol 2011; 4: 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz JL. Fcgbp. A potential viral trap in RV144. Open AIDS J 2014; 8: 21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang YY, Kannan A, Nunn K, et al. IgG in cervicovaginal mucus traps HSV and prevents vaginal Herpes infections. Mucosal Immunol 2014; 7: 1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyle MDP. (ed) Bacterial immunoglobulin-binding proteins. 1989, Cambridge, MA: Academic Press, 1989. [Google Scholar]
- 40.Labbé S, Grenier D. Characterization of the human immunoglobulin G Fc-binding activity in Prevotella intermedia. Infect Immun 1995; 63:2785–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorman H, Moreau F, Kim A, et al. FCGBP maintains MUC2 mucus structural integrity by stabilizing the mucus layer in response to the colonic pathogen, Entamoeba histolytica. J Can Assoc Gastroenterol 2021; 4: 212–213. [Google Scholar]
- 42.Radicioni G, Cao R, Carpenter J, et al. The innate immune properties of airway mucosal surfaces are regulated by dynamic interactions between mucins and interacting proteins: the mucin interactome. Mucosal Immunol 2016; 9: 1442–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albert TK, Laubinger W, Müller S, et al. Human intestinal TFF3 forms disulfide-linked heteromers with the mucus-associated FCGBP protein and is released by hydrogen sulfide. J Proteome Res 2010; 9: 3108–3117. [DOI] [PubMed] [Google Scholar]
- 44.Hoffmann W. Trefoil factor family (TFF) peptides and their diverse molecular functions in mucus barrier protection and more: changing the paradigm. Int J Mol Sci 2020; 21: 4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Devine DA, High AS, Owen PJ, et al. Trefoil factor expression in normal and diseased salivary glands, Hum Pathol 2000; 31: 500–515. [DOI] [PubMed] [Google Scholar]
- 46.Wiede A, Jagla W, Welte T, et al. Localization of TFF3, a new mucus-associated peptide of the human respiratory tract. Am J Respir Crit Care Med 1999; 159: 1330–1335. [DOI] [PubMed] [Google Scholar]
- 47.Braga Emidio N, Hoffmann W, Brierley SM, et al. Trefoil factor family: unresolved questions and clinical perspectives. Trends Biochem Sci 2019; 44: 387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reeves EP, Ali T, Leonard P, et al. Helicobacter pylori lipopolysaccharide interacts with TFF1 in a pH-dependent manner. Gastroenterology 2008; 135: 2043–2054. [DOI] [PubMed] [Google Scholar]
- 49.Li C, Wang R, Su B, et al. Evasion of mucosal defenses during Aeromonas hydrophila infection of channel catfish (Ictalurus punctatus) skin. Dev Comp Immunol 2013; 39: 447–455. [DOI] [PubMed] [Google Scholar]
- 50.Prévost J, Gasser R, Beaudoin-Bussières G, et al. Cross-sectional evaluation of humoral responses against SARS-CoV-2 spike. Cell Rep Med 2020; 1: 100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson EM, Goodwin EC, Verma A, et al. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell 2021; 184: 1858–1864.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goins CL, Chappell CP, Shashidharamurthy R, et al. Immune complex-mediated enhancement of secondary antibody responses. J Immunol 2010; 184: 6293–6298. [DOI] [PubMed] [Google Scholar]
- 53.Dugas M, Grote-Westrick T, Vollenberg R, et al. Less severe course of COVID-19 is associated with elevated levels of antibodies against seasonal human coronaviruses OC43 and HKU1 (HCoV OC43, HCoV HKU1). Int J Infect Dis 2021; 105: 304–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gouma S, Weirick ME, Marcus J, et al. Sero-monitoring of health care workers reveals complex relationships between common coronavirus antibodies and SARS-CoV-2 severity. medRxiv. Epub ahead of print 19 April 2021. DOI: 10.1101/2021.04.12.21255324.
- 55.Zohar T, Loos C, Fischinger S, et al. Compromised humoral functional evolution tracks with SARS-CoV-2 mortality. Cell 2020; 183:1508–1519.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sungnak W, Huang N, Bécavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020; 26: 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med 2020; 173: 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bryce C, Grimes Z, Pujadas E, et al. Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Mod Pathol 2021; 34: 1456–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Isho B, Abe KT, Zuo M, et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol 2020; 5: eabe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gidari A, Nofri M, Saccarelli L, et al. Is recurrence possible in coronavirus disease 2019 (COVID-19)? Case series and systematic review of literature. Eur J Clin Microbiol Infect Dis 2021; 40: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581: 465–469. [DOI] [PubMed] [Google Scholar]
- 62.Zhang H, Li HB, Lyu JR, et al. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int J Infect Dis 2020; 96: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021; 591: 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ellington S, Strid P, Tong VT, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status. United States, January 22–June 7, 2020. MMWR 2020; 69: 769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vardavas CI, Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob Induc Dis 2020; 18: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giuca MR, Pasini M, Tecco S, et al. Levels of salivary immunoglobulins and periodontal evaluation in smoking patients. BMC Immunol 2014; 15: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boyle JO, Gümüş ZH, Kacker A, et al. Effects of cigarette smoke on the human oral mucosal transcriptome. Cancer Prev Res 2010; 3: 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ordoñez CL, Khashayar R, Wong HH, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med 2001; 163: 517–523. [DOI] [PubMed] [Google Scholar]
- 69.Matsumoto K, Saito H. Does asthma affect morbidity or severity of COVID-19? J Allergy Clin Immunol 2020; 146: 55–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sunjaya AP, Allida SM, Di Tanna GL, et al. Asthma and risk of infection, hospitalization, ICU admission and mortality from COVID-19: systematic review and meta-analysis. J Asthma. Epub ahead of print 1 April 2021. DOI: 10.1080/02770903.2021.1888116. [DOI] [PMC free article] [PubMed]
- 71.Lee SC, Son KJ, Han CH, et al. Impact of comorbid asthma on severity of coronavirus disease (COVID-19). Sci Rep 2020; 10: 21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davies NG, Klepac P, Liu Y, et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med 2020; 26: 1205–1211. [DOI] [PubMed] [Google Scholar]
- 73.Heald-Sargent T, Muller WJ, Zheng X, et al. Age-related differences in nasopharyngeal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) levels in patients with mild to moderate coronavirus disease 2019 (COVID-19). JAMA Pediatr 2020; 174: 902–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yonker LM, Neilan AM, Bartsch Y, et al. Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, and immune responses. J Pediatr 2020; 227: 45–52e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zimmermann P, Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child 2021; 106: 429–439. [DOI] [PubMed] [Google Scholar]
- 76.Edridge AWD, Kaczorowska J, Hoste ACR, et al. Seasonal coronavirus protective immunity is short-lasting. Nat Med 2020; 26: 1691–1693. [DOI] [PubMed] [Google Scholar]
- 77.Carvelli J, Demaria O, Vély F, et al. Association of COVID-19 inflammation with activation of the C5a–C5aR1 axis. Nature 2020; 588:146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mastaglio S, Ruggeri A, Risitano AM, et al. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin Immunol 2020; 215: 108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woodruff TM, Shukla AK. The complement C5a-C5aR1 GPCR axis in COVID-19 therapeutics. Trends Immunol 2020; 41: 965–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med 2021; 384: 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Agarwal A, Mukherjee A, Kumar G, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicenter randomized controlled trial (PLACID Trial). BMJ 2020; 371: m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salazar E, Christensen PA, Graviss EA, et al. Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol 2020; 190: 2290–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Libster R, Pérez Marc G, Wappner D, et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med 2021; 384: 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID‐19: a multicenter retrospective cohort study. Clin Trans Immunol 2020; 9: e1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rambaut A, Loman N, Pybus O, et al. Preliminary genomic characterization of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations. ATRIC Network. https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563 (2020, accessed December 2020).
- 86.Davies NG, Abbott S, Barnard RC, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 2021; 372: eabg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ketas TJ, Chaturbhuj D, Cruz-Portillo VM, et al. Antibody responses to SARS-CoV-2 mRNA vaccines are detectable in saliva. Pathog Immun 2021; 6: 116–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xie X, Liu Y, Liu J, et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat Med 2021; 27: 620–621. [DOI] [PubMed] [Google Scholar]
- 89.Chen RE, Zhang X, Case JB, et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med 2021; 27: 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abu-Raddad LJ, Chemaitelly H, Butt AA, et al. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med 2021; 385: 187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]