Abstract
Innate immunomodulation via induction of innate memory is one mechanism to alter the host’s innate immune response to reduce or prevent disease. Microbial products modulate innate responses with immediate and lasting effects. Innate memory is characterized by enhanced (training) or depressed (tolerance) innate immune responses, including pro-inflammatory cytokine production, to secondary exposure following a priming event. To investigate the ability of β-glucans and bacillus Calmette-Guerin to induce innate training or tolerance in pig cells, porcine monocytes were cultured with priming agonist (β-glucans or bacillus Calmette-Guerin) then re-stimulated 5 d later with a heterologous microbial agonist to determine induction of innate memory. Priming with β-glucan from Saccharomyces cerevisiae depressed IL-1β and TNF-α cytokine responses to re-stimulation with LPS, indicative of a tolerized state. However, bacillus Calmette-Guerin priming induced a trained state in porcine monocytes, as LPS re-stimulation enhanced IL-1β and TNF-α gene expression and protein production. We present the first evidence of innate memory in pig monocytes, with bacillus Calmette-Guerin (training) or Saccharomyces cerevisiae β-glucan (tolerance). Induction of a trained or tolerized state in vitro is a first step to identify agonists to alter the innate immune system at the animal level with the intent of enhancing disease resistance.
Keywords: BCG, β-glucan, innate memory, innate training, porcine, monocyte
Introduction
Immunomodulation is an intervention strategy to harness the host’s immune system to prevent or reduce infection, the spread of micro-organisms, and the pathogenic consequences of infection (e.g., susceptibility to secondary infections, tissue damage, anorexia, fever). Although the Ag-specific memory of the adaptive immune system, typically induced via vaccination, is the predominate form of immunomodulation, the innate immune system has recently been the focus of modulation efforts to reduce disease against a wide range of pathogens. 1 , 2 A growing body of work supports that prior exposure of some leukocyte populations to innate agonists alters future responses to heterologous agonists and this poised state can be maintained for weeks to months. 3 , 4
Broadly termed innate memory, this altered responsiveness is characterized by either enhanced (trained) or depressed (tolerized) responses to homologous or heterologous agonists. Although initially counter to views of the innate immune system, literature long supports a memory aspect for innate immunity as decreased responsiveness to secondary stimulation. As early as the 1940s, rabbits injected with bacterial pyrogen (later called endotoxin or LPS) exhibited decreased febrile responses following a second exposure to the bacterial pyrogen d later and the decreased responsiveness to the second exposure required changes in monocyte and macrophage function. 5 In vivo exposure to other molecules or microorganisms besides LPS (e.g., zymosan, Newcastle disease virus, toxic shock syndrome toxin 1) also depressed innate responses upon homologous or heterologous re-stimulation.6–9 Within the last decade, identification of trained immunity (heightened innate responses following priming and heterologous re-stimulation) has developed into the theory of innate memory, encompassing both training and tolerance and is proposed as an explanation for the non-specific benefits observed with various vaccines. 2 , 10 , 11
The molecular basis for innate memory is epigenetic and metabolic re-programing of immune cells including monocytes, NK cells, and perhaps most importantly bone marrow progenitor cells. 12 , 13 Epigenetic re-programing for innate training and tolerance includes altered DNA accessibility and modified histones (e.g., H3K27ac, H3K4me, and H3K9me3), which enhance or repress mRNA transcription and subsequent protein production upon secondary stimulation. 3 , 7 ,14–17 Changes to the metabolic state of trained cells are critical to enhanced responses and are characterized by increased basal glycolysis and decreased basal mitochondrial respiration (the Warburg effect). 18 Innate training induces the expansion of hematopoietic stem and progenitor cells, leading to enhanced myelopoiesis and monocyte responses to secondary heterologous challenge. 19 Although the duration of the trained state is not known, epigenetic re-programing of bone marrow progenitor cells alters the state of subsequent effector cells (e.g., monocytes and NK cells) for weeks to months after the priming event, 3 , 20 and may provide a key opportunity to drive the innate immune system toward enhanced responses in agriculture animals.
Multiple agonists induce tolerance or training in innate immune cells, including LPS, muramyl dipeptide, β-glucans, and bacillus Calmette-Guerin (BCG). 13 The live-attenuated tuberculosis vaccine, BCG, was one of the earliest known inducers of what was eventually referred to as trained immunity, beginning with epidemiology reports of decreased neonatal mortality to respiratory and diarrheal diseases in BCG-vaccinated populations. 11 , 21 Innate training was later proposed as the mechanism for the observed benefits. 22 Indeed, BCG enhances in vitro monocyte cytokine production in multiple mammalian species including cattle, rodents, and humans. 4 , 23 However, it is not known if BCG, or other agonists, induce innate memory in pig monocytes. Induction of a trained or tolerized phenotype in vitro is a first step to identifying agonists that may alter the innate immune system and enhance disease resistance.
β-Glucans are a common dietary additive for food animals and are generally recognized as safe, therefore they do not require regulatory approval for supplementation. The primary source of β-glucans in food animal diets is Saccharomyces cerevisiae,24–27 which is an easily obtained, cheap source of β-glucan and yeast-derived products for supplementation. Priming of human monocytes with highly purified β-glucan from Candida albicans induces a trained state with enhanced cytokine production upon re-stimulation with LPS, a TLR4 agonist, or Pam3CSK, a TLR2 agonist. 13 However, innate training of pig monocytes by β-glucan has not been explored. The source and purity of β-glucan may influence the induction of innate memory, as highly purified β-glucan from C. albicans induces a trained state in human monocytes but zymosan (a crude S. cerevisiae β-glucan preparation) induces tolerance. 8 , 9 Identifying the impact of β-glucans at the cellular level may provide insights on the use of various β-glucan products at the animal level.
Harnessing innate training to modulate immune responses against a range of heterologous microorganisms may enhance disease resistance in swine, including the potential to reduce antibiotic usage. To determine if innate memory can be induced in swine monocytes, we used an in vitro model to test multiple sources of β-glucans as well as live and inactivated BCG (inBCG) for enhanced or depressed cytokine responses of primed monocyte to secondary TLR stimulation, a key hallmark response of innate memory.
Materials and methods
Reagents
LPS from Escherichia coli O55:B5 was obtained from Sigma-Aldrich. The insoluble β-(1→3, 1→6)-d-glucan from S. cerevisiae (zymosan) and the soluble β-(1→3, 1→6)-d-glucan product from Laminaria digitata (laminarin) were obtained from InvivoGen. β-(1→3)-d-glucan product isolated from C. albicans was kindly provided by Dr Dave Williams at the University of Tennessee. Live BCG (lvBCG) Danish (strain 1331) were provided by Dr Mitchell Palmer as previously described. 28 BCG was inactivated by heating to 80°C for 40 min, with intermittent mixing. Inactivation of BCG was confirmed by no growth at 37°C after 3 mo on Middlebrook’s 7H11 selective media plates. Total protein concentration of inBCG was determined using the Pierce BCA Protein Assay (ThermoFisher) following the manufacturer’s instructions, and applied to lvBCG as both live and inBCG aliquots were from the same stock. Back calculations indicate approximately 3 × 106 CFU were added per well with an MOI of 6:1 (BCG:monocytes), which is similar to comparable studies in cattle. 23
Isolation of primary porcine monocytes
PBMCs were isolated from healthy, < 1-yr old, white-cross, mixed-breed pigs maintained at the National Animal Disease Center, Ames, IA with approval from the Institutional Animal Care and Use Committee. Briefly, blood was collected into acid citrate dextrose vacutainer tubes (BD Biosciences, Mountain View, CA), maintained at room temperature (RT), and diluted with RT Hanks Balanced Salt solution (HBSS) with 2% BSA (Sigma-Aldrich). Diluted blood was layered over Lymphoprep 1.077 gradient (Stemcell Technologies) on SepMate spin columns (Stemcell Technologies) and centrifuged at 1200 g for 12 min at 25°C to obtain peripheral blood mononuclear cells. Isolated PBMCs were washed twice in supplemented HBSS (ThermoFisher), 0.5% BSA (Sigma-Aldrich), and 2 mM EDTA (ThermoFisher) and viable cells enumerated using the Count and Viability Assay Kit on the MUSE® detection system (Merck Millipore). Monocytes were isolated from the PBMCs after incubation with anti-human CD14 microbeads (TUK4, Miltenyi Biotec) using the Magnetic Activated Cell Sorting system (Miltenyi Biotec). The monocyte (CD14+) enriched fraction was confirmed to be ≥90% CD14+ (FITC labeled Tuk4; ThermoFisher) by flow cytometry.
In vitro experimental design
Isolated CD14+ monocytes were seeded at 5 × 105 cells per well in 96-well flat-bottom plates and rested overnight in a humified 5% CO2, 39°C incubator in 0.25 ml of supplemented media (sMedia): advanced RPMI 1640 (Gibco) supplemented with 10% heat-inactivated, 0.22 μM filtered swine sera (HyClone, GE Healthcare Life Sciences), 2 mM l-glutamine, 1× penicillin-streptomycin (Sigma-Aldrich), and 25 mM HEPES (ThermoFisher). After overnight rest, cells were primed for 24 h with either sMedia (non-stimulated (NS) control), LPS (100 ng/ml), zymosan (100–0.1 μg/ml), laminarin (10–0.1 μg/ml), β-glucan isolated from C. albicans (100–1 μg/ml), lvBCG (100–1 μg/ml), or inBCG (100–1 μg/ml). Following the primary stimulation (24 h), cell supernatants were collected and stored at –80 °C for cytokine analysis. Fresh sMedia was added to cells, and cells were cultured for additional 5 d in sMedia. Half of the sMedia (0.15 ml) was replaced on d 3. On d 5, 0.15 ml of media was removed and the cells were re-stimulated with 0.15 ml of LPS (100 ng/ml). Cells were collected at 4 h post-secondary stimulation in TRI Reagent (Invitrogen) for quantitative RT-PCR (RT-qPCR) or supernatants were collected after 24 h and stored at −80°C for cytokine analysis.
NS cells (sMedia primed only) re-stimulated with LPS (NS/LPS) served as control cells to determine if LPS stimulation was heighted or depressed with β-glucan or BCG priming. Nomenclature for this manuscript is priming agonist/secondary stimulation. For example, non-primed cells with secondary LPS stimulation are referred to as NS/LPS, BCG primed with media as secondary as BCG/Media. Graphs or nomenclature listing only one stimulant (i.e., NS, BCG, LPS) indicate results from the priming agonist only.
Cytokine measurements
Levels of TNF-α and IL-1β in cell culture supernatants were measured by ELISA using the porcine TNF-α DuoSet ELISA kit and IL-β DuoSet ELISA kit (R&D Systems) following the manufacturer’s recommendation. Samples were diluted 1:3 with assay sample diluent and optical density was measured at 450 nm and 540 nm using a BioTek Synergy HTX plate reader (BioTek). Concentrations were calculated as described by the manufacturer using a recombinant protein as standard. Cytokine levels from the priming supernatants were reported as ng/ml of indicated cytokine. Responses following heterologous re-stimulation were analyzed by calculating the fold change of primed samples divided by the matched unprimed (NS/LPS) samples.
RNA isolation, cDNA synthesis, and RT-qPCR
RNA for RT-qPCR was isolated from monocytes stimulated as described previously. Briefly, RNA was isolated using the Direct-zol RNA Miniprep kit (Zymo Research) with on-column DNase treatment step following the manufacturer’s recommendations. RNA quality and quantity were immediately assessed using an Agilent 2200 TapeStation bioanalyzer using an RNA Analysis ScreenTape (Aglient). cDNA was synthesized from 200 ng of RNA following the manufacturer’s recommendation for the iScript Reverse Transcription Supermix for RT-qPCR kit (Bio-Rad). Real-time PCR was conducted for various mRNA targets in a 20 μl reaction with 5 ng of cDNA and 500 nM of each forward and reverse primer with the iTaq Universal SYBR Green Supermix protocol (Bio-Rad). Reactions were performed on a Quant studio 5 Real-Time PCR machine with the following amplification conditions: polymerase activation of 5 min at 95°C; amplification stage 15 s at 95°C, 1 min at 60°C for 40 cycles; followed by a melt curve analysis 95°C for 15 s, 60°C for 1 min, and 95°C for 1 s. A list of mRNA targets and primer pairs is provided in Table 1. Primer pair efficiencies were confirmed to be between 90% and 110% as recommended in the MIQE guidelines. 29 Data were analyzed using the 2–ΔΔCq method described by Livak and Schmittgen 30 with YWHAZ as the endogenous control. Samples were normalized to the gene expression of NS/media cells.
Table 1.
Primers.
| Forward (5′→3′) | Reverse (5′→3′) | Source | Accession number | |
|---|---|---|---|---|
| IL1B | TGGCCCACACATGCTGAA | CCTTGCACAAAGCTCATGCA | Liu et al., 2016 31 | NM_214055.1 |
| TNF | CTGGCCCCTTGAGCATCA | GGGCTTATCTGAGGTTTGAGAC | Liu et al., 2016 31 | NM_214022.1 |
| IL18 | CCATCTCTGTGCAGTGTAAGAA | GTCCAGGAACACTTCTCTGAAA | Liu et al., 2016 31 | NM_213997.1 |
| TLR4 | TGGTGTCCCAGCACTTCATA | CGGCATGACTCCTCAGAAAC | Liu et al., 2016 31 | NM_001113039.1 |
| CASP1 | GCCATTAAGAAAGCCCACATAGAA | AGGGATGTCGCCAAGAAACA | Liu et al., 2016 31 | NM_214162.1 |
| NLRP3 | GGAGGAGGAGGAAGAGGAGATA | AGGACTGAGAAGATGCCACTAC | Fu et al., 2018 32 | NM_001256770.2 |
| YWHAZ | AGACAGCACGCTAATAATGCA | CCTGCTTCAGCTTCATCTCC | Primer blast | NM_001315726.1 |
CASP1: caspase 1.
Statistical analysis
To normalize for individual animal variation, cytokine production from re-stimulated samples were expressed as fold change over the same individual’s NS/LPS cytokine production. Statistical analyses were performed in GraphPad Prism v 8.2.0 (GraphPad Software, Inc). As the paired treatments were not normally distributed, a non-parametric ANOVA was performed (Friedman test). Differences between treatment groups and the NS-primed samples were analyzed with Dunn’s multiple comparisons test. Data were considered significant if a two-tailed P value was <0.05.
Results
β-Glucan source affected induction of a trained or tolerant state
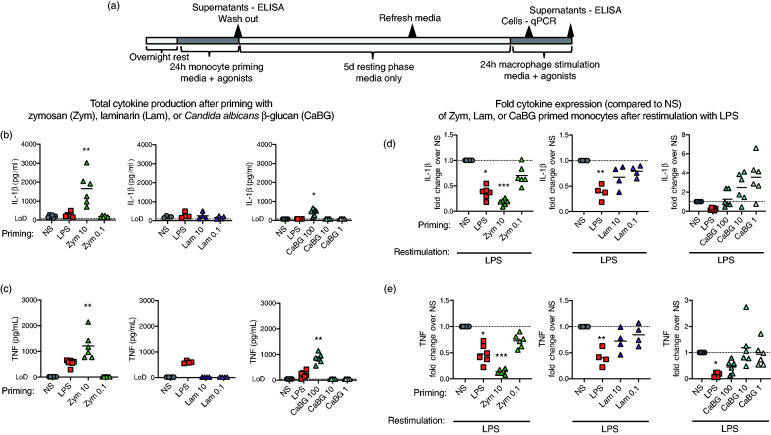
Multiple sources of β-glucan were investigated as potential innate training agonists for porcine monocytes. Primary porcine monocytes were stimulated in vitro with varying doses of β-glucans from three sources: β-glucan from C. albicans (CaBG), zymosan from S. cerevisiae (Zym), or laminarin from L. digitata (Lam). Monocyte stimulation for 24 h with either Zym (10 μg/ml) or CaBG (100 μg/ml) increased production of IL-1β (Figure 1b; 1654 ± 349 pg/ml and 438 ± 76.4 pg/ml, respectively) and TNF-α (Figure 1c; 1211 ± 214 pg/ml and 855 ± 81.2 pg/ml, respectively) compared to cytokine production by NS cells, which were at or below the limit of detection for each assay. However, no measurable TNF-α cytokine was produced with Lam stimulation of porcine monocytes, and Lam did not alter the production of IL-1β at either dose (260 ± 93.7 pg/ml and 168 ± 41 pg/ml for Lam 10 μg/ml and 0.1 μg/ml respectively) compared to NS monocytes (202 ± 23.9 pg/ml).
Figure 1.
β-Glucans induced a tolerized or trained phenotype in primary porcine monocytes. (a) In vitro monocyte culture scheme. Peripheral porcine monocytes (CD14+) were primed with zymosan (Zym; 0.1–10 µg/ml), laminarin (Lam; 0.1–10 µg/ml), β-glucan from C. albicans (CaBG; 1–10 µg/ml), LPS (0.1µg/ml), or non-stimulated (NS; media only) for 24 h, washed with warm PBS, and then cultured for 5 d in supplemented media (sMedia) with the sMedia refreshed at 3 d. After 5 d the cells were re-stimulated with LPS and supernatants (24 h) were collected for analysis of cytokine production. Total (b) IL-1β and (c) TNF-α production following Zym, Lam, or CaBG stimulation were measured by ELISA. Cytokine production for each priming group, following LPS re-stimulation were measured by ELISA and expressed relative to the media primed group (NS/LPS) for each pig, (d) IL-1β and (e) TNF-α. Data are presented as mean (bar) and significance (* P < 0.05; ** P < 0.01; *** P < 0.001) as compared to unstimulated (NS) controls. A Friedman test (one-way ANOVA) was followed by a Dunn’s multiple comparisons test. Data are representative of three independent experiments (n = 4–6 per study).
Priming agonists (Zym, Lam, or CaBG) were removed after 24 h and cells were cultured for 5 d in sMedia. After 5 d, sMedia was removed and cells were stimulated with the heterologous agonist LPS for determination of induction of innate memory by evaluating increased or decreased IL-1β and TNF-α cytokine production of primed cells compared to non-primed, LPS re-stimulated cells (NS/LPS; Figure 1d and 1e). Zymosan primed and LPS re-stimulated (Zym/LPS) cells produced less IL-1β and TNF-α (0.37 ± 0.05 fold and 0.47 ± 0.07 fold, respectively) compared to NS/LPS control cells. Neither Lam/LPS nor CaBG/LPS priming significantly altered the subsequent production of IL-1β or TNF-α upon re-simulation. However, IL-1β production numerically trended upward with the lower doses of CaBG/LPS treated cells (2.49 ± 0.61 fold with 10 μg/ml and 3.57 ± 0.81 fold with 1 μg/ml; P <0.1) (Figure 1d).
Live and inBCG priming enhanced cytokine responses to LPS re-stimulation
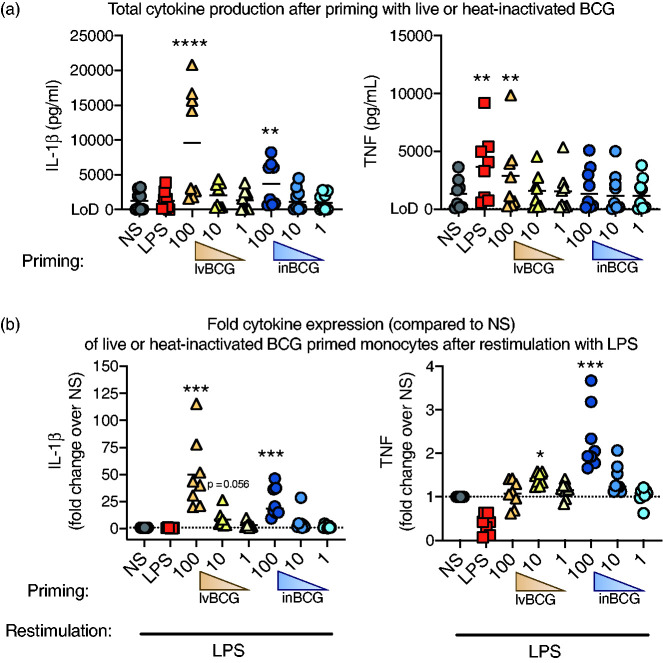
Unlike human monocytes, 13 β-glucan from C. albicans was not a strong inducer of innate training in porcine monocytes in vitro (Figure 1d and e). However, other non-β-glucan agonists (e.g., BCG) induce innate training in human monocytes both in vitro and in vivo. 3 Therefore, we assessed BCG as a training agonist in porcine monocytes (Figure 2). LvBCG priming at 100 μg/ml induced production of IL-1β by porcine monocytes (9568 ± 2835 pg/ml), but not at the lower doses (10 or 1 μg/ml) (Figure 2a). Then 24 h after secondary stimulation, lvBCG primed/LPS re-stimulated monocytes secreted more IL-1β cytokine compared to the NS/LPS monocytes. Specifically, lvBCG/LPS cells produced 50-fold more IL-1β at lvBCG 100 μg/ml (P <0.001) and 8.5 fold more at 10 μg/ml (P = 0.056) compared to NS/LPS. However, enhanced TNF-α production (P <0.05) was only observed in the lvBCG/LPS cells when primed with the lvBCG 10 μg/ml dose (1.4-fold increase; Figure 2b).
Figure 2.
Priming with live or heat-inactivated BCG increased cytokine production when re-stimulated with LPS. Primary porcine monocytes (CD14+) were stimulated with live BCG (lvBCG; 1, 10, or 100 µg/ml), inactivated BCG (inBCG; 1, 10, or 100 µg/ml), LPS (100 ng/ml), or media for 24 h, washed once with PBS, and cultured for 5 d in sMedia only. Cells were re-stimulated with LPS for 24 h. Supernatants were collected measured for IL-1β and TNF-α cytokine production after the (a) primary stimulation; expressed as total protein and (b) LPS re-stimulation; expressed as relative to the media primed group (NS/LPS) for each pig. Data are presented as mean (bar) and significance (* P < 0.05; ** P < 0.01; *** P < 0.001, **** P < 0.0001) as compared to unstimulated (NS) controls. A Friedman test (one-way ANOVA) was followed by a Dunn’s multiple comparisons test. Data are representative of three independent experiments (n = 8 per study).
To determine if live bacilli were required for the induction of trained immunity in porcine monocytes, monocytes were also primed with inBCGand re-stimulated 5 d later with LPS (inBCG/LPS; Figure 2). Monocytes primed with 100 μg/ml inBCG had significantly enhanced production of IL-1β (P < 0.01) and TNF-α (Figure 2b) with LPS re-stimulation (25- and 2.3-fold increase over NS/LPS, respectively). The lower doses of inBCG (10 μg/ml and 1 μg/ml) did not significantly alter IL-1β or TNF-α cytokine production compared to the NS monocytes. No differences were observed with 10 or 1 μg/ml doses of inBCG after either the primary stimulation or re-stimulation with LPS.
Following priming, cytokine production required re-stimulation with heterologous agonists
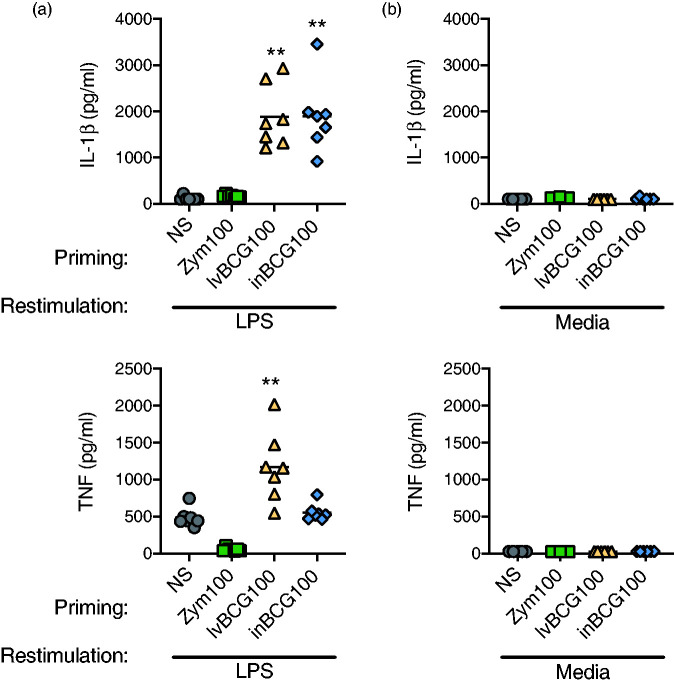
To determine if the enhanced (lvBCG/LPS and inBCG/LPS) and reduced (Zym/LPS) cytokine production was specific to the re-stimulation event or if primed cells continually produced cytokine even in the absence of exposure to heterologous agonist, a small study was performed. Primary monocytes were stimulated with either media-only (NS), zymosan, lvBCG, or inBCGand cultured as described before (24 h culture with priming agonist (NS, Zym, lvBCG, or inBCG), supernatants removed, cells washed with PBS, and cultured for 5 d in media). Priming concentrations were selected by consistency of the tolerized or trained state for each agonist across multiple experiments (Figure 2b for lvBCG and inBCG, Zym100 not shown). After the 5-d culture, cells were either re-stimulated with LPS or media alone. Cells re-stimulated with LPS displayed a similar phenotype observed with previous experiments (Figures 1d, 1e, and 2b). In brief, zymosan priming resulted in reduced cytokine production to LPS re-stimulation (55.1 ± 9.3 pg/ml of TNF-α with Zym/LPS and 487 ± 46.9 pg/ml with NS/LPS). In contrast, both lvBCG and inBCG priming with LPS re-stimulation enhanced cytokine production compared to NS/LPS (1884 ± 256 and 1897 ± 295 pg/ml of IL-1β for lvBCG/LPS and inBCG/LPS respectively, and 119 ± 17 pg/ml for NS/LPS; Figure 3a). IL-1β and TNF-α cytokine levels were below the limit of detection when primed cells were re-stimulated with media-only (no LPS re-stimulation); NS/Media, Zym/Media, lvBCG/Media, or inBCG/Media (Figure 3b). These data indicate the innate memory states observed with zymosan and BCG priming required heterologous re-stimulation and was not the result of continued cytokine production from the initial priming event.
Figure 3.
Secondary cytokine response required LPS exposure. (a) Primed monocytes (NS, zymosan 100 μg/ml, live BCG 100 μg/ml, or heat-inactivated BCG 100 μg/ml) when re-stimulated with LPS produced IL-1β and TNF-α as seen before. No cytokine production was observed with Zym100 LPS re-stimulated monocytes due to the tolerant state. (b) In contrast, matched samples produced neither IL-1β nor TNF-α cytokine when re-stimulated with media only. Data are presented as mean (bar) and significance (* P < 0.05; ** P < 0.01) as compared to unstimulated (NS) controls. A Friedman test (one-way ANOVA) was followed with a Dunn’s multiple comparison test. Data are representative of two independent experiments (n = 8 per study).
Zymosan and BCG priming did not alter IL1B or TNF-α, but did alter IL18, CASP1, TLR4, and NLRP3 expression to LPS
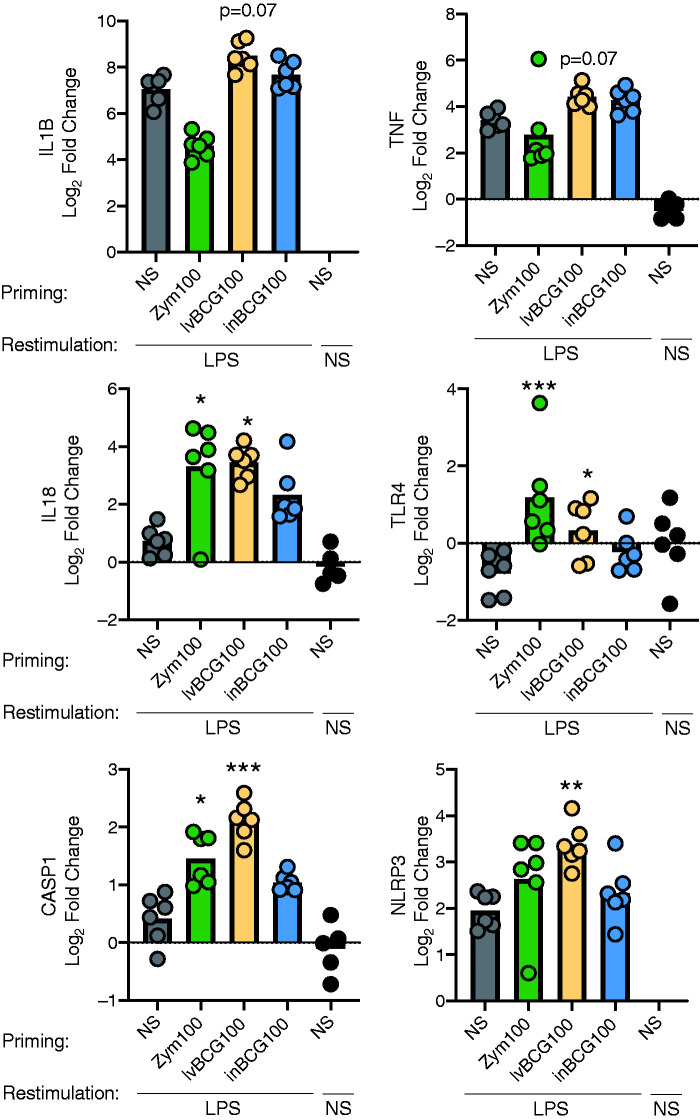
To further examine changes associated with training and tolerance, porcine monocytes were primed and cultured as described in the materials and methods section, and gene expression was analyzed 4 h after re-stimulation to generate the following groups: Zym100/LPS, lvBCG100/LPS, inBCG100/LPS, and NS/LPS (see Supplemental Table 1). Gene expression was normalized to NS/Media cells and data expressed as log2 fold change. No significant differences were observed in IL1B or TNF-α mRNA expression with Zym100/LPS or inBCG100/LPS groups when compared to NS/LPS. LvBCG100/LPS cells trended (P = 0.07) toward enhanced IL1B and TNF-α gene expression (Figure 4). A numerical decrease in IL1B and TNF-α expression was observed in Zym100/LPS group compared to NS/LPS, although the difference was not statistically significant (P > 0.1; Figure 4).
Figure 4.
Zymosan and BCG priming alters gene expression following LPS re-stimulation. BCG (live or heat-inactivated) up-regulates expression of both IL1B and CASP1 genes. Primary porcine monocytes (CD14+) were primed for 24 h with media only (NS), zymosan (Zym100), live BCG (lvBCG100), or inactivated BCG (inBCG100) at 100 μg/ml; cultured for 5 d in sMedia and re-stimulated for 4 h with either LPS 0.1 µg/ml or Media. Supernatant was removed and cells were lysed for gene expression analysis. Gene expression was normalized to NS/Media cells with YWHAZ as the endogenous control and are expressed as the log2 of the fold change. Data are presented as mean (bar) and significance (* P < 0.05; ** P < 0.01, *** P < 0.001) as compared to NS primed, LPS re-stimulated monocytes. A Friedman test was followed with a Dunn’s multiple comparison test. NS-primed, NS re-stimulated samples were included on graph, but excluded from statistical analysis. n = 6 pigs per group (data representative of two independent studies).
Gene expression of the LPS receptor (TLR4) was 2-fold higher in the Zym100/LPS group compared to NS/LPS. However, TLR4 gene expression was down-regulated in NS/LPS cells, contributing to the significant (P <0.001) difference in TLR4 gene expression in Zym100/LPS compared to NS/LPS cells. TLR4 gene expression was increased in the Zym100/LPS group when compared to the NS/media group (P = 0.04; Figure 4). Interestingly, despite increased expression of TLR4 at 4 h in Zym100/LPS treated cells, there was no corresponding increase in production of inflammatory cytokines (IL-1β and TNF-α; Figure 3a) following TLR4 stimulation with LPS.
Expression of IL18 mRNA was also up-regulated in Zym100/LPS and lvBCG100/LPS cells (P <0.05), but not in inBCG100/LPS cells (Figure 4). Likewise, caspase 1 (CASP1) expression was increased with lvBCG100/LPS and Zym100/LPS cells compared with NS/LPS, but not with inBCG100/LPS cells (Figure 4). Of the primed cells, expression of NLRP2 mRNA was only significantly up-regulated with lvBCG100/LPS stimulated cells compared to NS/LPS.
Discussion
Although innate training is well established in human and rodent literature, there is a paucity of information on innate training in agricultural animals. A trained phenotype has been shown in vitro and ex vivo with lvBCG in cattle, 23 and there are reports of “heterologous protection” in swine inoculated with inactivated Mycobacterium paratuberculosis, 33 a mycobacterium related to BCG (Mycobacterium bovis). Our research presents the first evidence for in vitro monocyte training and tolerance to multiple priming agonists for pigs, and that re-stimulation of cells with LPS was required for IL-1β and TNF-α cytokine production after priming with lvBCG, inBCG, or zymosan. Two very different products were able to induce innate memory, and both have relevance for use in humans and food animals. However, individual variation in response to priming stimulation (particularly BCG) were noted and may be due to prior life exposure altering the poised state of the cells for certain individuals toward heightened or depressed responses. The loss of high and low responders following priming and LPS re-stimulation may suggest that priming with specific agonists realigned the cells toward a uniform state.
Although BCG may induce innate training, in vivo administration of lvBCG as an immunomodulator poses regulatory issues that may be circumvented with the use of inBCG. Vaccination of humans or animals with BCG to protect against tuberculosis infection is not practiced in the United States, and many countries around the world prohibit BCG vaccination of humans and food animals as to allow monitoring for Mycobacterium tuberculosis infection via intradermal tuberculin skin testing.34–36 However, administration of inBCG does not result in a positive skin test 37 and therefore may serve to induce innate training without the potential regulatory conflicts of lvBCG administration. In humans, γ-irradiated BCG was studied as an alternative agonist to stimulate innate training both in vitro and in vivo. 38 , 39 An in vivo γ-irradiated BCG vaccination in humans failed to alter cytokine expression when PBMCs were stimulated ex vivo with various agonists, 38 in contrast to previous studies with lvBCG. 3 , 40 In vitro γ-irradiated BCG induces trained immunity but to a lesser degree than lvBCG; Arts et al. 39 theorize that the reduced state of innate training with γ-irradiated BCG compared to lvBCG may be due to changes to the immunostimulatory component of BCG (i.e., muramyl dipeptide) or that intracellular replication of live bacterium is necessary to induce trained immunity. However, our results suggest replication of the bacterium was not required for induction of trained immunity in pig monocytes, as both live and heat-inBCG primed porcine monocytes for enhanced cytokine production to LPS re-stimulation. Changes to the bacterial DNA induced by γ-irradiation, but not through heat inactivation, may be responsible for the differences between the studies. We cannot rule out other differences, such as study species (human vs swine), dose, source of BCG, and others as potential causes. Further research is warranted to characterize the specific component(s) of BCG that maximize induction of trained immunity but do not interfere with regulatory compliance.
In light of the potential limitations of using lvBCG in animal agriculture and the well-studied role of C. albicans β-glucan induced trained immunity in humans and rodents, 13 we investigated β-glucans as an innate immune modulator in pig cells. As pigs and other food animals are commonly fed β-glucans from non-C. albicans sources, we also investigated β-glucans from S. cerevisiae and L. digitata as potential inducers of innate memory. We acknowledge the limitation of comparison between stimulated circulating monocytes and the complex multi-cellular intestinal environment where dietary β-glucans is delivered in pigs. The soluble β-glucan, laminarin, failed to induce IL-1β or TNF-α cytokine production following primary stimulation. Upon re-stimulation with LPS, no differences between the NS/LPS cells were observed when compared to laminarin/LPS cells. However, stimulation of porcine monocytes with insoluble β-glucan from S. cerevisiae (zymosan) induced cytokine production following primary exposure. Upon re-stimulation with LPS, zymosan-primed cells exhibited depressed cytokine responses, indicative of a tolerized state. Similar results have been observed with human monocytes. 8 , 9 Upon exposure to β-glucans, human myeloid cells require cross-linkage of multiple Dectin-1 receptors to form a phagocytic synapse for downstream signaling. 41 Soluble β-glucans such as laminarin can bind to the Dectin-1 receptor but are incapable of cross-linking multiple receptors and thereby fail to initiate an immune response. 41 , 42 Porcine monocytes, macrophages, and neutrophils respond to soluble or insoluble β-glucan in a similar manner, as soluble β-glucan does not induce an immune response. 43 , 44 Thus, the differences in innate memory in porcine monocytes with various β-glucans was likely in part due to the structure and solubility of the β-glucan molecules.
Other factors, such as preparation and purity of the β-glucan, may influence the trained versus tolerized phenotype observed with priming monocytes with β-glucans. Although Dectin-1 is the primary phagocytosis-inducing receptor for β-glucan, complement receptor 3, and TLR2/6 also play a major role in the inflammatory response of cells to β-glucans. 42 , 44 , 45 For example, although both S. cerevisiae and C. albicans are heated and chemically treated to isolate the β-glucan fraction, zymosan is a crude prep containing mannan and proteins along with β-glucan. 46 , 47 Also, the C. albicans β-glucan used for innate training studies 13 , 14 , 48 , 49 is partially phosphorylated to increase solubility, 50 which may alter distribution and recognition of the β-glucan molecule by immune cells. However, innate training in human monocytes is also induced by inactivated C. albicans, 51 suggesting that a trained or tolerized state may be due to purity or structural differences in C. albicans β-glucan and zymosan. β-Glucan from C. albicans did not induce innate training or tolerance in pig monocytes. In fact, none of the β-glucan products studied herein induced a trained state in pig monocytes. With the wide array of β-glucan products in the agricultural market with varying sources, purities, and isolation methods, consideration is warranted before selecting a product for in vivo use.
Innate training is characterized in part by chromatin remodeling to open regions of DNA allowing for rapid and enhanced mRNA synthesis, and thus, enhanced cytokine production upon secondary stimulation. 3 The opposite is true with tolerance, as chromatin remodeling leads to limited expression of certain genes upon re-stimulation. 16 Although we did not investigate changes at the chromatin level, our lvBCG primed and LPS re-stimulated protein and gene expression data suggest an open chromatin structure, as IL1B and TNF trended toward enhanced gene expression in BCG/LPS cells compared to NS/LPS cells. BCG/LPS led to enhanced expression of IL18 mRNA, a closely related member of the IL-1 family. These data suggest a more permissive chromatin structure at these cytokine genes following BCG priming of porcine monocytes, as with human responses to BCG priming. 3 LPS-induced tolerance in human monocytes is mediated by closed chromatin structures for many inflammatory genes including IL1B and IL18. 16 Contrary to expectations based on decreased IL-1β and TNF-α protein production we observed and human chromatin work, expression of IL1B and TNF mRNA in Zym/LPS cells was not statistically lower than the NS/LPS group, whereas IL18 gene expression was elevated.
Furthermore, NS/LPS cells also exhibited enhanced IL1B and TNF gene expression compared to the NS/Media that was not matched with enhanced IL1B or TNF-α protein production (active IL-18 protein was not measured). We can conclude that LPS stimulation alone may be enough to induce expression of IL1B and TNF mRNA, but that additional control mechanisms likely regulate mRNA translation or protein processing for release from the cell.
Production and secretion of active IL-1β protein is a tightly controlled, complex process requiring multiple signaling events. 52 , 53 Briefly, an initial stimulus signals for the transcription of IL1B mRNA which is translated into the precursor protein, pro-IL-1β. A second signal is required to assemble the NLRP3 inflammasome complex and activate CASP1 to cleave the pro-IL-1β protein into the biologically active IL-1β protein 52 , 54 which is then secreted. As with IL-1β, production of bioactive IL-18 requires cleavage of the pro-IL-18 protein by NLRP3 inflammasome and CASP1; 53 , 55 however, a different mechanism controls expression of IL18 mRNA and pro-IL-1b protein expression in human PBMCs. 55 Our data indicate LPS stimulation was a sufficient signal to induce expression of IL1B mRNA regardless of prior priming (NS, BCG, or Zym). However, LPS stimulation only enhanced IL18 mRNA expression in BCG- or zymosan-primed cells, but not in the NS/LPS cells, indicating the priming event modulated IL18 transcription. Although LPS stimulation enhanced expression of IL1B mRNA regardless of previous priming (NS, BCG, or Zym), IL-1β protein was only secreted by BCG/LPS cells, suggesting a mechanism besides limited IL1B gene accessibility for mRNA synthesis. It is unclear if IL1B mRNA was translated into the pro-IL-1β protein in cells in which IL1B mRNA was detected, but the data suggest regulation of active IL-1β production at a step after transcription in NS/LPS cells. The second step in the production of active IL-1β protein (NLRP3 inflammasome and CASP1 cleavage of pro-IL-1β) may be involved. Similar levels of NLRP3 and CASP1 mRNA were detected in both zymosan and lvBCG primed, LPS re-stimulated cells, but not non-primed cells, suggesting the primed cells up-regulated the expression of NLRP3 and CASP1 in response to LPS. We attempted to assess functional CASP1 activity in BCG/LPS and NS/LPS cells, but were limited by lack of pig-specific reagents and necessary amounts of starting material for activity assays. The development of reagents for these assays in swine will be critical for clarifying the mechanism.
Utilizing immunomodulation, including innate memory, in animal agriculture may provide a method to limit disease burden and reduce the use of antibiotics, improving both animal and human health, as reviewed in Byrne et al. (2020). 56 Our research presents the first example of innate training in pigs and suggests that consideration of β-glucan should be utilized when selecting a product for immunomodulatory benefits. With a model for inducing innate memory in pig monocytes, numerous avenues of research are open to better understand the mechanistic background of innate memory as well as the role of specific molecules and their effect on the innate immune system. Furthermore, with their physiological and immunological similarities to humans, pigs are an excellent biomedical model for human immune studies, such as innate modulation of neonatal immune responses where ethical and practical aspects limit such studies in human neonates. 57 Although in vitro human adult and neonate innate training has been described, 58 pigs provide a model for in vivo studies. Innate memory opens a broad field of immunomodulation for pigs and other agricultural species, but caution is warranted as we need to better understand the immunological cost and disease interactions before extensive use in live animal production.
Acknowledgements
We greatly appreciate Dr David Williams for providing the β-glucan from C. albicans, NADC animal care staff for providing swine blood samples, and Dr Mitchell Palmer and Shelly Zimmerman for assistance propagating and preparing BCG and technical advice. We also thank Dr Juber Herrera-Uribe, Zahra Bond, Jayne Wiarda, and Amber Miranda for technical advice and support.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by appropriated funds from United States Department of Agriculture-Agricultural Research Service (USDA-ARS) Current Research Information System (CRIS) project 5030-31320-004-00D. All opinions expressed in this paper are the authors’ and do not necessarily reflect the policies and views of USDA-ARS.
ORCID iD
Kristen A Byrne https://orcid.org/0000-0003-4910-732X
Supplemental material
Supplemental material for this article is available online.
References
- 1.Levy O, Levy O. Ready to benefit from training: Heterologous effects of early life immunization. Trans R Soc Trop Med Hyg 2015; 109: 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Meer JW, Joosten LA, Riksen N, et al. Trained immunity: A smart way to enhance innate immune defence. Mol Immunol 2015; 68: 40–44. [DOI] [PubMed] [Google Scholar]
- 3.Kleinnijenhuis J, Quintin J, Preijers F, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA 2012; 109: 17,537–17,542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arts RJW, Carvalho A, La Rocca C, et al. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep 2016; 17: 2562–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beeson PB. Development of tolerance to typhoid bacterial pyrogen and its abolition by reteculo-endothelial blockade. Proc Soc Experimental Biol Med 1946; 61: 248–250. [DOI] [PubMed] [Google Scholar]
- 6.Park SH, Park-Min KH, Chen J, et al. Tumor necrosis factor induces GSK3 kinase-mediated cross-tolerance to endotoxin in macrophages. Nat Immunol 2011; 12: 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seeley JJ, Ghosh S. Molecular mechanisms of innate memory and tolerance to LPS. J Leukoc Biol 2017; 101: 107–119. [DOI] [PubMed] [Google Scholar]
- 8.Cavaillon JM, Pitton C, Fitting C. Endotoxin tolerance is not a LPS-specific phenomenon: Partial mimicry with IL-1, IL-10 and TGFβ. J Endotoxin Res 2016; 1: 21–29. [Google Scholar]
- 9.Cavaillon JM. The nonspecific nature of endotoxin tolerance. Trend Microbiol 1995; 3. [DOI] [PubMed] [Google Scholar]
- 10.Jensen KJ, Benn CS, van Crevel R. Unravelling the nature of non-specific effects of vaccines: A challenge for innate immunologists. Semin Immunol 2016; 28: 377–383. [DOI] [PubMed] [Google Scholar]
- 11.Aaby P, Roth A, Ravn H, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: Beneficial nonspecific effects in the neonatal period? J Infect Dis 2011; 204: 245–252. [DOI] [PubMed] [Google Scholar]
- 12.Kleinnijenhuis J, Quintin J, Preijers F, et al. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin Immunol 2014; 155: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ifrim DC, Quintin J, Joosten LA, et al. Trained immunity or tolerance: Opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors. Clin Vaccine Immunol 2014; 21: 534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novakovic B, Habibi E, Wang SY, et al. beta-glucan reverses the epigenetic state of LPS-induced immunological tolerance. Cell 2016; 167: 1354–1368 e1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saeed S, Quintin J, Kerstens HHD, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014; 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 2007; 447: 972–978. [DOI] [PubMed] [Google Scholar]
- 17.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: New mechanisms, molecules and clinical significance. Trend Immunol 2009; 30: 475–487. [DOI] [PubMed] [Google Scholar]
- 18.Cheng SC, Quintin J, Cramer RA, et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014; 345: 1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitroulis I, Ruppova K, Wang B, et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 2018; 172: 147–161 e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Heijden C, Noz MP, Joosten LAB, et al. Epigenetics and trained immunity. Antioxid Redox Signal 2018; 29: 1023–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biering-Sorensen S, Aaby P, Napirna BM, et al. Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guerin vaccination at first health center contact. Pediatr Infect Dis J 2012; 31: 306–308. [DOI] [PubMed] [Google Scholar]
- 22.Blok BA, Arts RJW, van Crevel R, et al. Trained innate immunity as underlying mechanism for the long-term, nonspecific effects of vaccines. J Leukocyte Biol 2015; 98: 347–356. [DOI] [PubMed] [Google Scholar]
- 23.Guerra-Maupome M, Vang DX, McGill JL. Aerosol vaccination with bacillus Calmette-Guerin induces a trained innate immune phenotype in calves. PLoS One 2019; 14: e0212751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn TW, Lohakare JD, Lee SL, et al. Effects of supplementation of B-glucans on growth performance, nutrient digestibility, and immunity in weanling pigs. J Animal Sci 2006; 84: 1422–1428. [DOI] [PubMed] [Google Scholar]
- 25.Zanello G, Meurens F, Berri M, et al. Saccharomyces cerevisiae decreases inflammatory responses induced by F4+ enterotoxigenic Escherichia coli in porcine intestinal epithelial cells. Vet Immunol Immunopathol 2011; 141: 133–138. [DOI] [PubMed] [Google Scholar]
- 26.Eicher SD, McKee CA, Carroll JA, et al. Supplemental vitamin C and yeast cell wall β-glucan as growth enhancers in newborn pigs and as immunomodulators after an endotoxin challenge after weaning1. J Animal Sci 2006; 84: 2352–2360. [DOI] [PubMed] [Google Scholar]
- 27.Xiao Z, Trincado CA, Murtaugh MP. Beta-glucan enhancement of T cell IFNgamma response in swine. Vet Immunol Immunopathol 2004; 102: 315–320. [DOI] [PubMed] [Google Scholar]
- 28.Palmer MV, Thacker TC, Waters WR, et al. Oral vaccination of white-tailed deer (Odocoileus virginianus) with Mycobacterium bovis bacillus Calmette-Guerin (BCG). PLoS One 2014; 9: e97031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin Chem 2009; 55: 611–622. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Nguyen YT, Nettleton D, et al. Post-weaning blood transcriptomic differences between Yorkshire pigs divergently selected for residual feed intake. BMC Genomics 2016; 17: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu S, Liu H, Xu L, et al. Baicalin modulates NF-kappaB and NLRP3 inflammasome signaling in porcine aortic vascular endothelial cells infected by Haemophilus parasuis causing Glasser's disease. Sci Rep 2018; 8: 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen KJ, Hansen MS, Heegaard PMH, et al. The effect of inactivated Mycobacterium paratuberculosis vaccine on the response to a heterologous bacterial challenge in pigs. Front Immunol 2019; 10: 1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. Global Tuberculosis Report 2017. Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
- 35.World Health Organization. Roadmap for zoonotic tuberculosis. Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
- 36.Olea-Popelka F, Muwonge A, Perera A, et al. Zoonotic tuberculosis in human beings caused by Mycobacterium bovis: A call for action. Lancet Infectious Dis 2017; 17: e21 World Health e25. [DOI] [PubMed] [Google Scholar]
- 37.Pritchard DG. A century of bovine tuberculosis 1888–1988: conquest and controversy. J Comparative Pathol 1988; 99. [DOI] [PubMed] [Google Scholar]
- 38.Hamers LA, Kox M, Arts RJ, et al. Gamma-irradiated bacille Calmette-Guerin vaccination does not modulate the innate immune response during experimental human endotoxemia in adult males. J Immunol Res 2015; 2015: 261864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arts RJ, Blok BA, Aaby P, et al. Long-term in vitro and in vivo effects of gamma-irradiated BCG on innate and adaptive immunity. J Leukoc Biol 2015; 98: 995–1001. [DOI] [PubMed] [Google Scholar]
- 40.Netea MG, van Crevel R. BCG-induced protection: Effects on innate immune memory. Semin Immunol 2014; 26: 512–517. [DOI] [PubMed] [Google Scholar]
- 41.Goodridge HS, Reyes CN, Becker CA, et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse'. Nature 2011; 472: 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodridge HS, Wolf AJ, Underhill DM. B-glucan recognition by the innate immune system. Immunological Reviews 2009; 2: 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sonck E, Devriendt B, Goddeeris B, et al. Varying effects of different beta-glucans on the maturation of porcine monocyte-derived dendritic cells. Clin Vaccine Immunol 2011; 18: 1441–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baert K, Sonck E, Goddeeris BM, et al. Cell type-specific differences in beta-glucan recognition and signalling in porcine innate immune cells. Dev Comp Immunol 2015; 48: 192–203. [DOI] [PubMed] [Google Scholar]
- 45.Gantner BN, Simmons RM, Canavera SJ, et al. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med 2003; 197: 1107–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonck E, Stuyven E, Goddeeris B, et al. The effect of beta-glucans on porcine leukocytes. Vet Immunol Immunopathol 2010; 135: 199–207. [DOI] [PubMed] [Google Scholar]
- 47.Underhill DM. Macrophage recognition of zymosan particles. J Endotoxin Res 2003; 9: 176–180. [DOI] [PubMed] [Google Scholar]
- 48.Leentjens J, Quintin J, Gerretsen J, et al. The effects of orally administered Beta-glucan on innate immune responses in humans, a randomized open-label intervention pilot-study. PLoS One 2014; 9: e108794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia-Valtanen P, Guzman-Genuino RM, Williams DL, et al. Evaluation of trained immunity by beta-1, 3 (d)-glucan on murine monocytes in vitro and duration of response in vivo. Immunol Cell Biol 2017; 95: 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams DL, McNamee RB, Jones EL, et al. A method for the solubilization of a (1->2)-B-D-glucan isolated from Saccharomyces cerevisiae. Carbohydrate Research 1991; 219: 203–213. [DOI] [PubMed] [Google Scholar]
- 51.Quintin J, Saeed S, Martens JHA, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012; 12: 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev 2011; 22: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dinarello CA, Novick D, Kim S, et al. Interleukin-18 and IL-18 binding protein. Front Immunol 2013; 4: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elliott EI, Sutterwala FS. Monocytes take their own path to IL-1beta. Immunity 2016; 44: 713–715. [DOI] [PubMed] [Google Scholar]
- 55.Puren AJ, Fantuzzi G, Dinarello CA. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1beta are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc Natl Acad Sci USA 1999; 96: 2256–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Byrne KA, Loving CL, McGill JL. Innate immunomodulation in food animals: evidence for trained immunity? Front Immunol 2020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walters EM, Prather RS. Advancing swine models for human health and diseases. Missouri Medicine 2013; 110: 212–215. [PMC free article] [PubMed] [Google Scholar]
- 58.Namakula R, de Bree LCJ, Tvedt TH, et al. Monocytes from neonates and adults have a similar capacity to adapt their cytokine production after previous exposure to BCG and beta-glucan. PLoS One 2020; 15: e0229287. [DOI] [PMC free article] [PubMed] [Google Scholar]