Abstract
Background and objectives:
Commercial Aspergillus IgG antibody assays have become pivotal in the current diagnosis of chronic pulmonary aspergillosis (CPA). However, diagnostic cutoffs have been found to vary from manufactures’ recommendations in different settings. This study aimed to establish the Aspergillus IgG reference range among Nigerians and determine a diagnostic cutoff for CPA.
Methods:
Sera from 519 prospectively recruited healthy blood donors and 39 previously confirmed cases of CPA were analysed for Aspergillus IgG levels using the Bordier test kit (Bordier Affinity Products SA, Crissier, Switzerland). Accuracy versus cutoff profile and receiver operating characteristics (ROC) curve were analysed for both CPA cases and controls using the R-Studio (2020), (Window desktop, version 4.0.2 software with R packages “nnet” and “ROCR”).
Results:
Among healthy blood donors, 141 (27.2%) were aged 16–25 years with median (interquartile range, IQR) of 22 (20–24) years; 304 (58.6%) were aged 26–40 years with median (IQR) of 32 (29–36) years; while 74 (14.2%) were aged 41–60 years with median (IQR) of 46 (44–49.75). Median IgG level in respective age groups were 0.069 (0.009–0.181), 0.044 (0.014–0.202) and 0.056 (0.01–0.265) with no significant difference found in the three age categories (p = 0.69). The overall diagnostic cutoff for the diagnosis of CPA was 0.821 with an accuracy of 97.1% and area under the curve (AUC) = 0.986.
Conclusion:
The optimal diagnostic cutoff for diagnosing CPA in Nigerians using the Bordier kit was 0.821 which is lower than the manufacturer’s recommended cutoff of 1.0. The determination of this cutoff among Nigerians will significantly enhance accurate identification of CPA and assessment of its true burden in Nigeria.
Keywords: Aspergillus IgG, blood donors, chronic pulmonary aspergillosis, cutoff, Nigeria
Introduction
The ubiquitous mould Aspergillus causes a wide spectrum of infectious, allergic and opportunistic lung diseases which are largely dependent on the state of the host’s immunity and pulmonary structure.1,2 One of these diseases is chronic pulmonary aspergillosis (CPA), a serious pulmonary condition estimated to affect 3 million people worldwide,3,4 with a case fatality rate of 20–33% in the short term and 50% over a span of 5 years.5,6 The disease usually occurs on a background of previous or current lung disease, such as tuberculous cavitary lesions, histoplasmosis, sarcoidosis, emphysematous bullae or fibrotic lung disease. 7 Prior tuberculosis (TB) is the single most important risk factor for CPA, and the burden of CPA is projected to be high in areas with a high burden of TB.
The diagnosis of CPA is hinged on a constellation of clinical and radiological findings combined with either direct microbiological confirmation of Aspergillus infection from culture of respiratory specimens or the presence of serological evidence in the form of elevated levels of Aspergillus-specific antibodies. 8 Due to the poor sensitivity of mycological culture methods, 9 there has been a growing reliance on Aspergillus-specific IgG assays to provide evidence of infection to the extent that these are now considered essential in diagnostic algorithms for CPA. As most adults will have some degree of antibody response due to the ubiquity of Aspergillus, the identification of an optimal cutoff to define raised levels is critical. 10 Many commercial enzyme-linked immunosorbent assays (ELISAs) are available, but data determining the optimal cutoff for their use in CPA diagnosis remains limited. 10 In those that have been studied, the optimum cutoff for CPA is often considerably different from the cutoff recommended by the manufacturers.11,12 The findings of a study comparing six Aspergillus-specific IgG assays for the diagnosis of CPA suggest that existing recommendations are sub-optimal for the diagnosis of CPA and probably require revision. 11 Nevertheless, commercial Aspergillus-specific IgG assays remain pivotal in diagnosing CPA.
Despite an estimated large burden of CPA, 13 Aspergillus antibody assays are not routinely used in clinical practice in Africa. In the only Nigerian study to incorporate Aspergillus IgG till date, Oladele et al. 14 found a CPA prevalence of 8.7% among a group of patients being managed for smear-negative TB and/or TB treatment failure. This study utilized ImmunoCAP (Thermo Fisher Scientific, Uppsala, Sweden) and diagnosis of CPA was based on the approved European cutoff of >40 mgA/L, a much higher level than the 20 mgA/L adopted in a recent study involving Ugandan controls, thus implying that CPA prevalence may have been underestimated. 11
Determining the optimal diagnostic cutoff of Aspergillus-specific IgG assays is a critical step towards describing the true burden of CPA in Nigeria. In this multi-center study, we sought to determine the optimal diagnostic cutoff for diagnosing CPA among Nigerians using the Bordier Aspergillus IgG assay (Bordier Affinity Products, Crissier, Switzerland).
Methodology
This multi-centre study involved 519 participants from a pool of healthy blood donors (HBDs) acting as controls and sera from 39 previously confirmed Nigerian cases of CPA.
Controls
Healthy controls were recruited from 9 tertiary hospitals located across Nigeria namely Lagos and Ibadan (South West); Calabar and Benin (South South); Awka (South East); Abuja and Bida (North Central); Kano (North West); and Yola (North East). Recruitment was carried out over a 12-month period spanning March 2018 to Feb 2019. A proforma was used to capture relevant data, such as age, sex, and previous exposure to TB. Five millilitres (mL) of venous blood was collected from each participant using standard universal protocols. Sera were separated and stored at −20°C and subsequently transported in a cold chain to the central research laboratory at the College of Medicine, University of Lagos for duplication and processing. Duplicates were stored at −80°C.
Cases
Archived sera of 39 previously confirmed Nigerian cases of CPA (Infectious Diseases Society of America definition)6,9,15 were included: 17 were from a previously published study 14 and 22 were from another set of patients being investigated for multidrug-resistant (MDR) TB (unpublished data).
Specimen analysis
All stored sera were analysed for Aspergillus-specific IgG antibodies using the Bordier Aspergillus IgG immunoassay kit (Bordier Affinity Products, Crissier, Switzerland) according to manufacturer’s instructions. An automated plate washer was used to improve accuracy. The OD index was calculated by computing the ratio of the sample OD to the OD of a cutoff provided by the manufacturer. Values of ⩾1 were considered positive, while values 0.8–1.0 were considered borderline according to the manufacturer’s instructions.
Ethical considerations
Ethical approval was obtained from the Health Research and Ethics Committee of all the participating institutions. All data were anonymized. All information obtained was kept confidential and participation in this study was entirely voluntary. Written informed consent was obtained from all participants.
Data analysis
The R-Studio statistical computing software environment was used for data analysis. 16 All 519 healthy controls were stratified by age and geographical region and the normal distribution of the stratified samples was ascertained using Shapiro–Wilk normality test (Shapiro Test).17,18 The Kruskal–Wallis test (kruskal.test) was performed to determine significant differences in the median Aspergillus IgG values of the stratified age and region. Boxplots of all profiles were created using R ggplot2 and ggpubr packages. 19 Pairwise comparisons of the regions and age groups with the same distribution (median values) were carried out using a post hoc test of the Wilcoxon Rank Sum test (pairwise.wilcox.test). 20 Receiver operating characteristics (ROC) curve analyses were performed comparing Aspergillus IgG levels in CPA cases to healthy controls. The optimal cutoff value was determined using Youden Index21,22 as described in Clinical and Laboratory Standards Institute (CLSI) guidelines C28–A3.23
Results
Descriptive analysis of the HBDs recruited
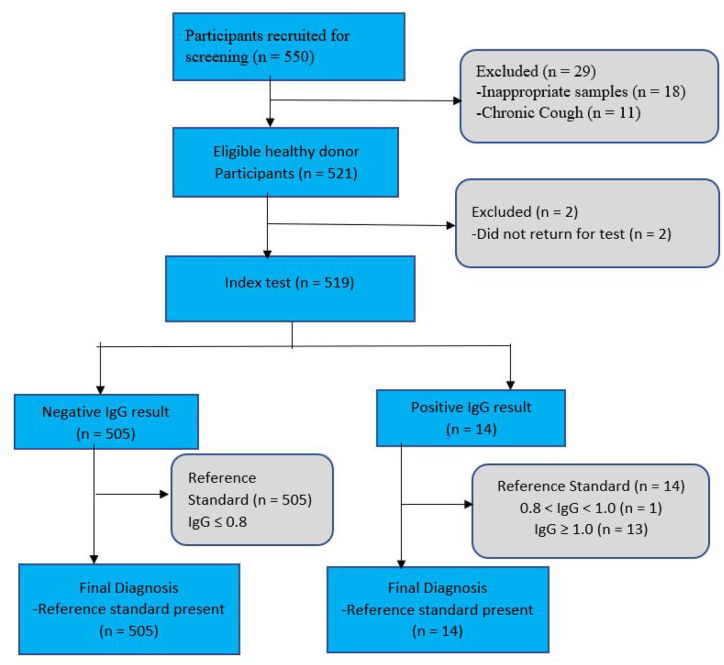
A total of 550 apparently HBDs were screened. However, only 519 eventually participated in the study as shown in Figure 1. The geographical distribution of healthy participants was as follows: Lagos (90; 17.34%), Bida (51; 9.83%), Calabar (50; 9.63%), Kano (50; 9.63%), Yola (51; 9.83%), Awka (53, 10.21%), Abuja (50, 9.63%), Benin (50, 9.63%) and Ibadan (74; 14.26%). Their age range was 17–57 years, with a median age (interquartile range (IQR)) of 31 years (25–37) years. There were 107 (20.6%) females with a median age (IQR) of 34 (26–38) and 412 (79.4%) males with a median (IQR) age of 30 (24.75–37) years. There was a statistical difference between female and male ages (p = 0.0351) (Table 1). Other sociodemographic characteristics are presented in Table 1. Most of the participants (304; 58.6%) fell within the 26–40 age group; 141 (27.2%) were in the 16–25 age group, while 74 (14.2%) were in the 41–60 age group (Figure 2(a)). The median (IQR) of age groups 16–25, 26–40 and 41–60 years were 22(20–24), 32(29–36) and 46 (44 – 49.75), respectively (Figure 2(a)).
Figure 1.
Flow diagram of healthy donor participants in the study (using STARD format). 21
Table 1.
Sociodemographic and participants characteristics.
| Participant characteristics | |||
| Overall age (years) | Median = 31, IQR = [25–37] | ||
| Female (F) age (years) | Median = 34, IQR = [26–38] | p = 0.0351 | |
| Male (M) age (years) | Median = 30, IQR = [24.75–37] | ||
| Sex (female, male) | F = 107 (20.6%), M = 412 (79.4%) | ||
| Aspergillosis (IgG) status | Neg = 505 (97.3%), Pos = 14 (2.7%) | ||
| Diabetes mellitus (DM) | No = 516 (99.4%), Yes = 3 (0.6%) | ||
| HIV status | Neg = 516 (99.4%), Pos = 3 (0.6%) | ||
| Geographical regions | |||
| North-central (NC) | 101 (19.5%) | South-East (SE) | 53 (10.2%) |
| North-east (NE) | 51 (9.8%) | South-South (SS) | 100 (19.3%) |
| North-west (NW) | 50 (9.6%) | South-West (SW) | 164 (31.6%) |
IQR, interquartile range.
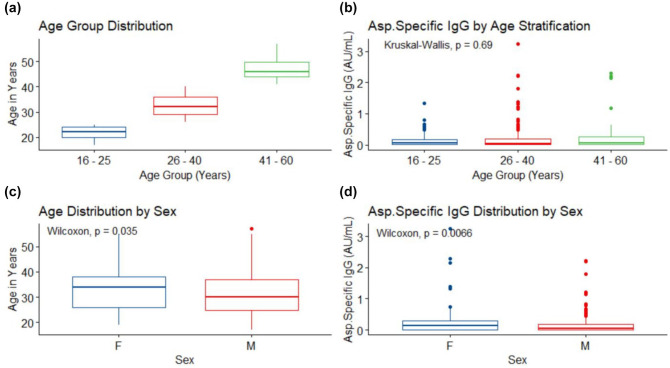
Figure 2.
Stratified age, and gender IgG distributions: (a) is stratified age distribution, age group 16–25 (141 (27.2%), min = 17, max = 25, median (interquartile range) = 22 (20–24)); age group 26–40 (304 (58.6%), min = 26, max = 40, 32 (29–36)); age group 41–60 (74 (14.2%), min = 41, max = 57, 46 (44–49.75)). (b) is IgG level distribution by age stratification, age group 16–25 (min = 0.001, max = 1.324, 0.069 (0.009–0.181)), age group 26–40 = (min = 0, max = 3.24, 0.044 (0.014–0.202)) and age group 41–60 = (min = 0, max = 2.29, 0.056 (0.01–0.265)), there was no difference in IgG level across the age group, p = 0.69. (c) is age distribution by gender, female (F) = (107 (20.6%), min = 19, max = 55, 34 (26–38)), male (M) = (412 (20.6%), min = 17, max = 57, 30 (24.75–37)), difference between female and male age is significant, p = 0.035. (d) is IgG versus gender distributions, female (F) = (min = 0, max = 3.24, 0.139 (0.013–0.299)), male (M) = (min = 0, max = 2.23, 0.044 (0.012–0.181)). There was a significant difference between female and male IgG level, p = 0.0066.
Distribution of Aspergillus IgG by age, occupation, and geography
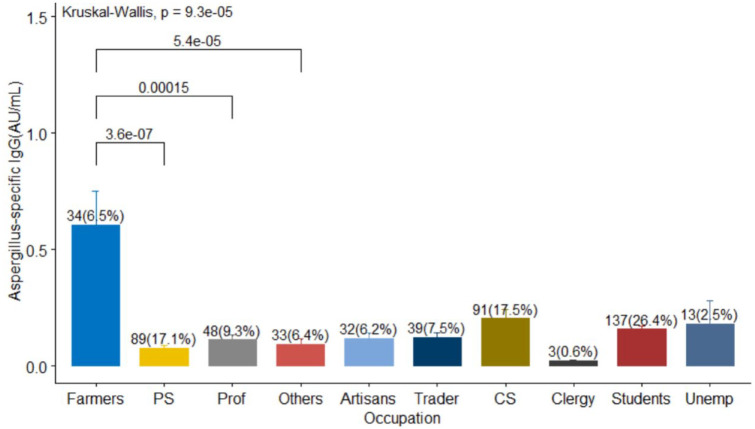
The sampling from the geographic regions in the country was largely balanced although more participants were recruited from the south-west (31.6%), south-south (19.3%), and North Central (19.5%) regions of Nigeria (Table 1). The pairwise Wilcox test revealed a statistically significant difference between the Aspergillus-specific IgG levels of farmers versus private sector (p < 0.001), farmers versus professionals (p = 0.0066) and farmers versus others (p = 0.0024). There was no substantial difference in pairwise comparisons of other occupations (p > 0.05), see Figure 3.
Figure 3.
Frequency distribution of participants occupation. The median (IQR) of Aspergillus-specific IgG of artisans, traders, civil servant (CS), clergy, farmers, private sector (PS), professionals (prof), students, unemployed (unemp), and Others were 0.069 (0.016–0.191), 0.012 (0.011–0.196), 0.047 (0.011–0.194), 0.023 (0.022–0.026), 0.152 (0.081–1.02), 0.05 (0.012–0.199), 0.043 (0.01–0.149), 0.083 (0.013–0.232), 0.051 (0.012–0.189), and 0.019 (0.0–0.089), respectively.
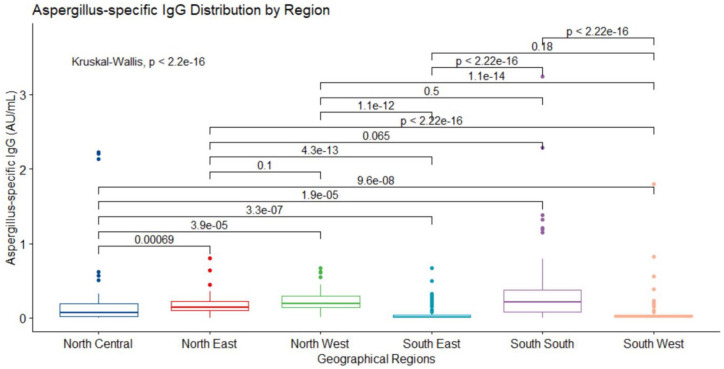
Normality test carried out on the data using the Shapiro–Wilk test17,18 (p < 0.05) revealed that central tendencies of Aspergillus IgG levels in all geographic regions of the study were not normally distributed. The median (IQR) values for the six regions are depicted in Figure 4. The Kruskal–Wallis test for difference in median values across the regions confirmed significant differences (p < 0.0001). The Pairwise Comparison of the IgG levels in the geographical regions using Wilcoxon Rank sum test revealed substantial differences (all p < 0.001) between (NC vs NE), (NC vs NW), (NC vs SE), (NC vs SS), (NC vs SW), (NE vs SE), (NE vs SW), (NW vs SE), (NW vs SW), (SE vs SS) and (SS vs SW). However, the pairs of (NE vs NW), (NE vs SS), (NW vs SS) and (SE vs SW) were not statistically significant (all p > 0.05). All the Aspergillus specific IgG median (IQR) in the regions were below manufacturer’s cut-off range of 0.8–1.0 (see Figure 4).
Figure 4.
IgG level stratified by geographical regions. Median (IQR) values in the regions are north central (NC) (n = 101 (19.5%), 0.07 (0.017–0.188)), north east (NE) (n = 51 (9.8%), 0.143 (0.103–0.227)), north west (NW) (n = 50 (9.6%), 0.189 (0.138–0.295)), south east (SE) (n = 53 (10.2%), 0.012 (0.005–0.035)), south south (SS) (n = 100 (19.3%), 0.215 (0.076–0.379)) and south west (SW) (n = 164 (13.6%), 0.018 (0.007–0.036)). Kruskal–Wallis rank sum test displayed differences in the median values (p < 0.0001) of IgG level across the regions. The Pairwise comparison of the medians using Wilcoxon rank sum test revealed significant differences between (NC – NE, p = 0.00069), (NC – NW, p < 0.0001), (NC – SE, p < 0.0001), (NC – SS, p < 0.0001), (NC – SW, p < 0.0001), (NE – SE, p < 0.0001), (NE – SW, p < 0.0001), (NW – SE, p < 0.0001), (NW – SW, p < 0.0001), (SE – SS, p < 0.0001) and (SS – SW, p < 0.0001). There were not statistically significant differences between (NE – NW, p = 0.1), (NE – SS, p = 0.065), (NW – SS, p = 0.5) and (SE – SW, p = 0.18).
The comparisons of Aspergillus IgG levels against age strata using Kruskal–Wallis Rank Sum Test showed no significant differences in the median values of IgG across the age groups (p = 0.69; Figure 2(b)). There were statistically significant differences in age versus sex, p = 0.035 (Figure 2(c)) and IgG levels versus gender, p = 0.0066 (Figure 2(d)) profiles. The median (IQR) IgG level by age stratification in 16–25 years, 26–40 years and 41–60 years were 0.069 (0.009–0.181), 0.044 (0.014–0.202) and 0.056 (0.01–0.265), respectively. There was no statistically significant difference (p = 0.69) in the IgG levels of the age groups, Figure 2(b). These analyses validated the reliability of the results.
The Aspergillus-specific IgG levels in various strata of age and region are reported (see Figures 1–3, Supplementary document). The IgG of (16–25) age, (26–40) age and (41–60) age categories in the six regions were not normally distributed (p < 0.05). The medians of (16–25) age, (26–40) age, (41–60) age groups ranged from 0.009 to 0.177, 0.019 to 0.22 and 0.025 to 0.328, respectively. There were significant differences in Aspergillus specific IgG among the regions when stratified by age. However, all the Aspergillus specific IgG levels were below the manufacturer’s recommended threshold range of 0.8 – 1.0 (Figures 1 – 3, Supplementary document).
Radiological (chest x-ray) findings and laboratory report of CPA-positive cases
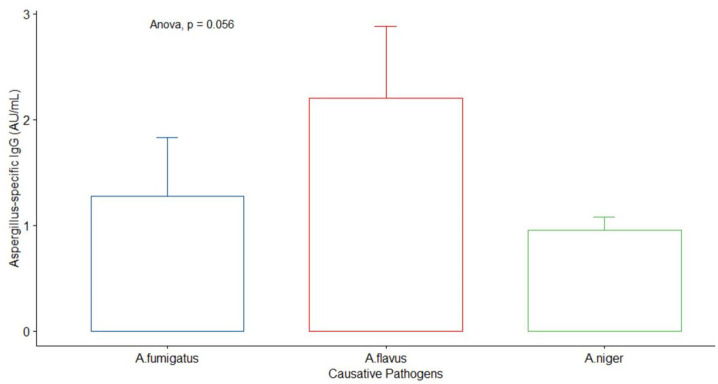
A total of 39 previously confirmed Nigerian cases of CPA were included in the study. These patients were initially managed as either smear-negative TB or treatment failure TB or MDR TB. Radiological findings revealed 21 (54%) cavitation, 20 (51%) fungal ball, 20 (51%) fibrosis and 21 (54%) pleural thickening (see Table 2). Only nine patients had sputum culture results documented; six (6) out of 17 previously published cases (PPCs) had sputum results while 3 out of 22 MDR patients had sputum results. The causative pathogens in nine positive CPA patients were Aspergillus fumigatus, A. flavus and A. niger. The mean (SD) of A. niger, A. flavus and A. fumigatus were 0.95 (0.13), 2.2 (0.68) and 1.28 (0.55), respectively. There was no significance among the three pathogens, p = 0.056 (Figure 5).
Table 2.
Radiological features of CPA-positive cases.
| Radiological findings (n = 39) | Positive | Negative |
|---|---|---|
| Cavitation | 21 (53.8%) | 18 (46.2%) |
| Consolidation | 20 (51.3%) | 19 (48.7%) |
| Fibrosis | 20 (51.3%) | 19 (48.7%) |
| Fungal ball | 20 (51.3%) | 19 (48.7%) |
| Pleural thickening | 21 (53.8%) | 18 (46.2%) |
| Causative pathogens | ||
| Pathogens | A. flavus | 3 (7.7%) |
| A. fumigatus | 3 (7.7%) | |
| A. niger | 3 (7.7%) | |
CPA, chronic pulmonary aspergillosis.
Figure 5.
Comparison of the causative pathogens in nine positive CPA-confirmed patients.
Aspergillus-specific IgG levels in HBDs and CPA cohort
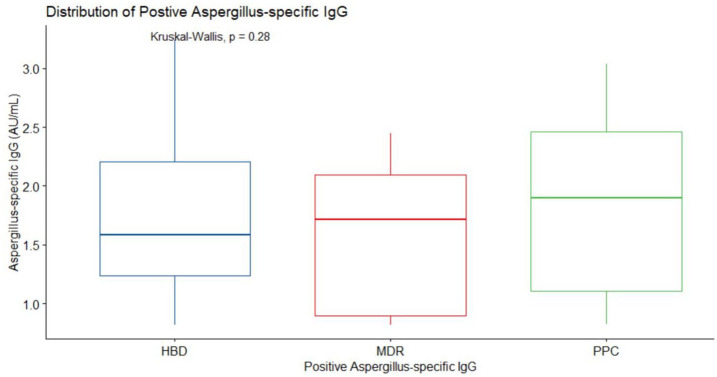
The O.D. in 14 (2.7%) of the HBDs recruits exceeded 1.0, the recommended cutoff for CPA specified by the test kit manufacturers. The mean (standard error) IgG level was 1.749 (0.176). All 39 radiologically confirmed CPA cases were also positive by the Bordier test kit manufacturer’s cutoff. These were comprised of MDR TB patients (n = 22, median (IQR) = 1.718 (0.898–2.095)) and previous published cases (PPC) (n = 17, mean (standard error) = 1.88 (0.19)). There was no statistical difference (p = 0.28) in the middle values of HBD, MDR, and PPC-positive IgG levels as shown in Figure 6.
Figure 6.
Distribution of positive Aspergillus-specific IgG level. Radiological confirmed positive IgG cases of multidrug-resistant (MDR) TB patients (n = 22, min = 0.822, max = 2.499, median = 1.718, IQR = (0.898–2.095)), radiological confirmed positive IgG cases that was previously published (PPC) ([n = 17, min = 0.828, max = 3.034, median = 1.896, IQR = (1.109–2.464)), radiologically unconfirmed positive IgG cases of healthy blood donors (HBD) = (n = 14, min = 0.82, max = 0.324, median = 1.588, IQR = (1.238–2.205)).
Determination of the Aspergillus IgG cutoff
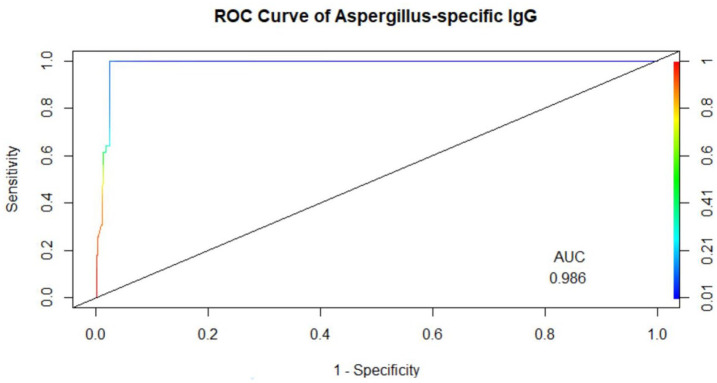
The overall cutoff value was 0.821 at an accuracy of 97.1%. Females had a slightly lower value of 0.782 with an accuracy of 87%. The sensitivity and specificity of the test were 100% (95% CI: 93.28–100%) and 100% (95% CI: 99.27–100%), respectively. The area under the curve (AUC) of the ROC curve was 0.986 (Figure 7). The negative predictive value was 100% (see Table 3).
Figure 7.
Receiving operating characteristics curve (ROC), AUC = 0.986, sensitivity = 100% (95% CI: 91.0–100%), specificity = 96.9% (95% CI: 95.0–98.2%) and accuracy = 97.1% (95% CI: 95.4–98.4%), positive predictive value (PPV) = 71%, negative predictive value (NPV) = 100%.
Table 3.
Cutoff points and performance for overall, female and male participants.
| Performance | Overall | Female | Male |
|---|---|---|---|
| Cutoff | 0–0.821 | 0–0.782 | 0–0.821 |
| Accuracy (95% CI) | 97.1 (95.4–98.2) | 87 (80.4–92) | 97.6 (95.7–98.8) |
| Sensitivity (95% CI) | 100 (91–100) | 100 (91–100) | 100 (91–100) |
| Specificity (95% CI) | 96.9 (95–98.2) | 82.2 (73.7–89) | 97.3 (95.3–86.4) |
| PPV (%) | 71 (60–79.8) | 67.2 (57.7–75.5) | 78 (66.4–86.4) |
| NPV (%) | 100 | 100 | 100 |
| AUC | 0.986 | 0.966 | 0.991 |
| DOR | >1 | >1 | >1 |
| Youden Index | 0.975 | 0.822 | 0.973 |
AUC, area under the curve; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
Discussion
We determined the optimal diagnostic cutoff value for Bordier Aspergillus-specific IgG antibody to be 0.821 with an accuracy of 97.1% by comparing Aspergillus IgG levels in healthy Nigerians drawn from the six geopolitical zones of the country and previously diagnosed CPA cases. Sensitivity and specificity of testing at this cutoff were 100% and 96.9%, respectively. We chose the Bordier assay for our study because it had been previously shown to be of superior performance – 97% sensitivity and 90.3% specificity – using the manufacturer’s recommended cutoff of >1.0. 24 Compared to two other kits (Bio-Rad’s Platelia and Virion/Serion), Dumollard et al. 24 found the Bordier assay had the best balance of specificity and sensitivity and an AUC of 0.977 in a prospective multi-centre study.
To the best of our knowledge, this is the first study to ascertain a cutoff value for any Aspergillus-specific IgG antibody assay among a Nigerian cohort. The cutoff in our study is lower than the diagnostic cutoff of 1.0 recommended by the kit manufacturer as well as the optimum cutoff of 0.9 determined by Wilopo et al. 12 , who studied the assay’s performance in UK CPA patients and healthy controls. The difference in cutoffs could be due to a number of factors including age and environmental exposure. The age range of controls in our study was 17–57 years compared with 36–64 years in the UK cohort. However, when we stratified the healthy controls in the index study into age groups, there was no significant difference in IgG levels, so the effect of age is likely to be minimal.
Occupation also played a role, with farmers more likely to have higher Aspergillus-specific IgG (p < 0.05). Differences in environmental exposure to A. fumigatus in the different settings may account for this. Unpublished data from Lagos, Nigeria, has shown preponderance of A. niger and A. flavus compared to A. fumigatus. Likewise, in a multi-centred study, A. niger was also higher in proportion, although this study was confined to south-Western Nigeria. 14 Females had a slightly lower cutoff value (0.782) compared males; however, this should be interpreted with caution because of the much lower sample size of 107, as against the recommended minimum sample size of 120.
To date, there have been very few studies to determine the diagnostic cutoff levels of Aspergillus-specific IgG assays for CPA diagnosis. The widely approved UK cutoff for the commonly used ThermoFisher Scientific ImmunoCAP was derived from an unpublished study involving 20 Europeans with either allergic or CPA. 25 In a study using sera from 100 blood donors in Uganda and 241 CPA patients from UK, Page et al. assessed the comparative performance of six assays and used ROC curve analysis to determine their optimal diagnostic cutoffs. The results were as follows: ImmunoCAP 20 mgA/L; Dynamiker 65 AU/mL; Genesis 20 U/mL; Immulite 10 mg/L; and Serion 35 U/mL varied from values recommended by the manufacturers. 11 In a subsequent study, the same researchers assessed the Bio-Rad, Immulite, ImmunoCAP, and Serion assays using the same 214 CPA cases while the Ugandan blood donors were replaced with a variety of healthy controls from across Europe. 26 This time, the optimal diagnostic cutoffs – ImmunoCAP 50 mgA/L, Serion 50 U/mL and Immulite 25 mgA/L – were considerably higher than previously reported. The researchers inferred that Aspergillus IgG levels may be generally higher among Europeans than Africans and attributed this to differences in environmental distribution of Aspergillus species, bearing in mind that available Aspergillus IgG assays are specific for A. fumigatus.
Using CPA cases and diseased controls in India, Seghal et al. 27 determined an optimal cutoff of 27 mgA/L at a sensitivity and specificity of 91.3% and 100%, respectively, for the ImmunoCAP system. The varied optimal diagnostic cutoffs determined for ImmunoCAP alone in varied contexts supports the need for context-specific data to improve diagnostic accuracy.
The inclusion of a control arm to determine the diagnostic cutoff constitutes a strength of our study as only a few studies have done this. 27 However, there were several limitations in this study. First, a relatively small number (39) of CPA cases was included in determining the cutoff. Studies involving larger numbers of CPA cases are desirable to improve the generalizability of the results. It is noteworthy that unpublished observations involving just 20 European patients informed the United Kingdom approved cutoff of 40 mgA/L for the ImmunoCAP method which has been used widely for decades. 25 Second, no imaging studies were done for the HBDs to exclude asymptomatic cases of CPA; indeed 14 of the samples from supposedly healthy controls had Aspergillus-specific IgG levels above 1.0, and these may have represented undiagnosed cases of CPA. Finally, the use of healthy controls may have resulted in falsely high specificity. Ideally, the describe cutoffs should have been described in relation to diseased controls in whom CPA had been effectively ruled out.26,28 However, in one study that compared optimal cutoffs for the Siemens Immulite assay in both healthy and diseased controls, the outcomes were similar. 29
In conclusion, the Bordier Aspergillus-specific IgG test kit at a cutoff value of 0.821 had high sensitivity and accuracy for diagnosing CPA among Nigerians. This is a significant step towards accurately identifying CPA cases and understanding the true burden of this disease in Nigeria and similar contexts.
Supplemental Material
Supplemental material, sj-docx-1-tai-10.1177_20499361211050158 for Standardization of Aspergillus IgG diagnostic cutoff in Nigerians by Rita O. Oladele, Akaninyene A. Otu, Oluwaseyi J. Balogun, Oladayo M. Babalola, Augustina O. Nwosu, Iriagbonse Iyabo Osaigbovo, Titilayo Gbajabiamila, Nicholas K. Irurhe, Samuel A. Fayemiwo, Shuwaram A. Shettima, Nkolika Stella Uwaezuoke, Chinagozi Precious Edwin, Toyese Stephen Ayanbeku, Joy U. Okaa, Charles John Elikwu, David W. Denning, Phyllis J. Kanki and Folasade T. Ogunsola in Therapeutic Advances in Infectious Disease
Footnotes
Author contributions: ROO and AAO conceptualized the study. ROO, AAO, IIO, SAF, SAS and CJE designed the study. ROO, AAO, OJB, OMB, AON, IIO, NKI, SAF, SAS, SNU, CPE, TSA, JUO and CJE were involved in data collection, data analysis and interpretation. ROO and AO wrote the first draft of the article. DWD, PJK and FTO critically revised the first draft. All the co-authors reviewed, edited and approved the version of the article to be published.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DWD and family hold Founder shares in F2G Ltd, a University of Manchester spin-out antifungal discovery company. He acts or has recently acted as a consultant to Scynexis, Pulmatrix, Pulmocide, Zambon, iCo Therapeutics, Mayne Pharma, Biosergen, and Fujifilm. In the last 3 years, he has been paid for talks on behalf of Hikma, Gilead, Merck, Mylan and Pfizer. He is a long-standing member of the Infectious Disease Society of America Aspergillosis Guidelines group, the European Society for Clinical Microbiology, and Infectious Diseases Aspergillosis Guidelines group. The other co-authors declare no conflict of interests.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Fogarty International Centre of the National Institutes of Health under Award Number D43TW010134. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ORCID iDs: Akaninyene A. Otu  https://orcid.org/0000-0002-6009-2707
https://orcid.org/0000-0002-6009-2707
Oladayo M. Babalola  https://orcid.org/0000-0003-4106-3035
https://orcid.org/0000-0003-4106-3035
Iriagbonse Iyabo Osaigbovo  https://orcid.org/0000-0001-8111-0743
https://orcid.org/0000-0001-8111-0743
Nkolika Stella Uwaezuoke  https://orcid.org/0000-0003-1059-5637
https://orcid.org/0000-0003-1059-5637
Charles John Elikwu  https://orcid.org/0000-0003-2721-2903
https://orcid.org/0000-0003-2721-2903
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Rita O. Oladele, Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, PMB 12003, Lagos, Nigeria.
Akaninyene A. Otu, Department of Internal Medicine, University of Calabar, Calabar, Nigeria Department of Infection and Travel Medicine, Leeds Teaching Hospitals NHS Trust, Leeds, UK.
Oluwaseyi J. Balogun, Department of Biomedical Engineering, College of Medicine, University of Lagos, Lagos, Nigeria
Oladayo M. Babalola, Department of Biochemistry, College of Medicine, University of Lagos, Lagos, Nigeria
Augustina O. Nwosu, Central Research Laboratory, College of Medicine, University of Lagos, Lagos, Nigeria
Iriagbonse Iyabo Osaigbovo, Department of Medical Microbiology, College of Medical Sciences, University of Benin, Benin City, Nigeria.
Titilayo Gbajabiamila, National Institute for Medical Research, Lagos, Nigeria.
Nicholas K. Irurhe, Department of Radiology, College of Medicine, University of Lagos, Lagos, Nigeria
Samuel A. Fayemiwo, Department of Medical Microbiology & Parasitology, College of Medicine, University of Ibadan, University College Hospital, Ibadan, Nigeria
Shuwaram A. Shettima, Department of Medical Microbiology, Parasitology and Immunology, Federal Medical Centre, Yola, Nigeria
Nkolika Stella Uwaezuoke, Department of Medical Microbiology, Federal Medical Centre, Abuja, Nigeria.
Chinagozi Precious Edwin, Department of Medical Microbiology, Aminu Kano Teaching Hospital, Kano, Nigeria.
Toyese Stephen Ayanbeku, Department of Medical Microbiology, Federal Medical Centre, Bida, Nigeria.
Joy U. Okaa, Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Awka, Nigeria
Charles John Elikwu, Department of Medical Microbiology, Benjamin Carson College of Medicine, Babcock University, Ilishan-Remo, Nigeria.
David W. Denning, National Aspergillosis Centre, Manchester University NHS Foundation Trust, Manchester, UK
Phyllis J. Kanki, Department of Immunology and Infectious Diseases, Harvard T.H Chan School of Public Health, Harvard University, Boston, MA, USA
Folasade T. Ogunsola, Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Lagos, Nigeria
References
- 1. Soubani AO, Chandrasekar PH. The clinical spectrum of pulmonary aspergillosis. Chest 2002; 121: 1988–1999. [DOI] [PubMed] [Google Scholar]
- 2. Hogan C, Denning DW. Allergic bronchopulmonary aspergillosis and related allergic syndromes. Semin Respir Crit Care Med 2011; 32: 682–692. [DOI] [PubMed] [Google Scholar]
- 3. Page ID, Richardson M, Denning DW. Antibody testing in aspergillosis – quo vadis? Med Mycol 2015; 53: 417–439. [DOI] [PubMed] [Google Scholar]
- 4. Leading International Fungal Education. LIFE chronic pulmonary aspergillosis factsheet, http://www.lifeworldwide.org/chronic-pulmonary-aspergillosis1 (accessed 19 February 2017).
- 5. Ohba H, Miwa S, Shirai M, et al. Clinical characteristics and prognosis of chronic pulmonary aspergillosis. Respir Med 2012; 106: 724–729. [DOI] [PubMed] [Google Scholar]
- 6. Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J 2016; 47: 45–68. [DOI] [PubMed] [Google Scholar]
- 7. Aspergillosis: from diagnosis to treatment. J Bras Pneumol 2009; 35: 1238–1244. [DOI] [PubMed] [Google Scholar]
- 8. Ma X, Wang K, Zhao X, et al. Prospective study of the serum Aspergillus specific IgG, IgA and IgM assays for chronic pulmonary aspergillosis diagnosis. BMC Infect Dis 2019; 19: 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takazono T, Izumikawa K. Recent advances in diagnosing chronic pulmonary aspergillosis. Front Microbiol 2018; 9: 1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richardson MD, Page ID. Aspergillus serology: have we arrived yet? Med Mycol 2017; 55: 48–55. [DOI] [PubMed] [Google Scholar]
- 11. Page ID, Richardson MD, Denning DW. Comparison of six Aspergillus-specific IgG assays for the diagnosis of chronic pulmonary aspergillosis (CPA). J Infect 2015; 75: 240–249. [DOI] [PubMed] [Google Scholar]
- 12. Wilopo BAP, Hunter ES, Richardson MD, et al. Optimising the cut-off of the Bordier Aspergillus IgG ELISA for the diagnosis of chronic pulmonary aspergillosis. J Microbiol Meth 2020; 176: 106021. [DOI] [PubMed] [Google Scholar]
- 13. Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis complicating sarcoidosis. Eur Respir J 2013; 41: 621.e6. [DOI] [PubMed] [Google Scholar]
- 14. Oladele RO, Irurhe NK, Foden P, et al. Chronic pulmonary aspergillosis as a cause of smear-negative TB and/or TB treatment failure in Nigerians. Int J Tuberc Lung Dis 2017; 21: 1056–1061. [DOI] [PubMed] [Google Scholar]
- 15. Denning DW, Page ID, Chakaya J, et al. Case definition of chronic pulmonary Aspergillosis in resource-constrained settings. Emerg Infect Dis 2018; 24: e171312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2018, https://www.R-project.org/ [Google Scholar]
- 17. Kwak SG, Park S-H. Normality test in clinical research. J Rheum Dis 2019; 26: 5–11. [Google Scholar]
- 18. Das KR, Rahmatullah Imon AHM. A brief review of tests for normality. Am J Theor Appl Stat 2016; 5: 5–12. [Google Scholar]
- 19. McDonald JH. Handbook of biological statistics. 3rd ed. Baltimore, MD: Sparky House Publishing, 2014, www.biostathandbook.com/kruskalwallis.html [Google Scholar]
- 20. Harris T, Hardin JW. Exact Wilcoxon signed-rank and Wilcoxon Mann-Whitney rank sum tests. Stata J 2013; 13: 337–343. [Google Scholar]
- 21. Jérémie F, Cohen Daniël A, Korevaar Douglas G, et al. STARD 2015 guidelines for reporting diagnostic accuracy: explanation and elaboration. BMJ Open 2016; 6: e012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smits N. A note on Youden’s Jand its cost ratio. BMC Med Res Methodol 2010; 10: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. CLSI. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline. 3rd ed. CLSI document C28-A3. Wayne, PA: CLSI, 2008. [Google Scholar]
- 24. Dumollard C, Bailly S, Perriot S, et al. Aspergillus Committee (2016) prospective evaluation of a new Aspergillus IgG enzyme immunoassay kit for diagnosis of chronic and allergic pulmonary Aspergillosis. J Clin Microbiol 2016; 54: 1236–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Hoeyveld E, Dupont L, Bossuyt X. Quantification of IgG antibodies to Aspergillus fumigatus and pigeon antigens by ImmunoCAP technology: an alternative to the precipitation technique? Clin Chem 2006; 52: 1785–1793. [DOI] [PubMed] [Google Scholar]
- 26. Page ID, Baxter C, Hennequin C, et al. Receiver operating characteristic curve analysis of four Aspergillus-specific IgG assays for the diagnosis of chronic pulmonary aspergillosis. Diag Microbio Infect Dis 2018; 91: 47–51. [DOI] [PubMed] [Google Scholar]
- 27. Seghal IS, Choudhary H, DhooriaS Aggarwal AN, et al. Diagnostic cut-off of Aspergillus fumigatus-specific IgG in the diagnosis of chronic pulmonary aspergillosis. Mycoses 2018; 61: 770–776. [DOI] [PubMed] [Google Scholar]
- 28. Cui J, Zhao N. Consideration in selection of the control group in diagnostic tests. Int J Biol Sci 2012; 8: 1418–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Page ID, Richardson MD, Denning DW. Siemens immulite Aspergillus-specific IgG assay for chronic pulmonary aspergillosis diagnosis. Med Mycol 2019; 57: 300–307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tai-10.1177_20499361211050158 for Standardization of Aspergillus IgG diagnostic cutoff in Nigerians by Rita O. Oladele, Akaninyene A. Otu, Oluwaseyi J. Balogun, Oladayo M. Babalola, Augustina O. Nwosu, Iriagbonse Iyabo Osaigbovo, Titilayo Gbajabiamila, Nicholas K. Irurhe, Samuel A. Fayemiwo, Shuwaram A. Shettima, Nkolika Stella Uwaezuoke, Chinagozi Precious Edwin, Toyese Stephen Ayanbeku, Joy U. Okaa, Charles John Elikwu, David W. Denning, Phyllis J. Kanki and Folasade T. Ogunsola in Therapeutic Advances in Infectious Disease