Abstract
Background:
Atypical antipsychotics are widely prescribed, yet have been associated with weight gain and metabolic syndrome.
Aim:
To study the effect of adjunct low-dose aripiprazole on weight and metabolic parameters of subjects on atypical antipsychotics (olanzapine, clozapine or risperidone).
Methods:
The study was carried out as an open-label trial with a fixed dose of 5 mg aripiprazole added to the patient’s current antipsychotic for 12 weeks. The primary outcome measure was mean change in weight, while secondary outcome measures included change in waist circumference; fasting blood glucose; HbA1c; triglycerides; total, HDL and LDL cholesterol levels; functioning; and neurocognition.
Results:
For the overall study (n = 55), there was no significant effect of adjunct aripiprazole on the weight of the subjects. However, the clozapine group achieved significant weight loss (p = 0.002) and also had significant improvements in total cholesterol (p < 0.001), HDL (p = 0.016), LDL (p = 0.044) and triglyceride levels (p = 0.038). The olanzapine group had significant improvement in triglycerides (p = 0.001), and other metabolic parameters for this group showed improvement trends, but did not reach statistical significance. The risperidone group did not show any significant improvement in weight or metabolic parameters.
Conclusions:
The study adds support to the adjunctive use of aripiprazole to clozapine for weight loss and improvement in metabolic profile, and for reduction in cardiometabolic risk for patients on olanzapine.
Trial Registration:
Clinicaltrials.gov identifier: NCT02949752
Keywords: aripiprazole, weight gain, metabolic syndrome, atypical antipsychotics
Introduction
Schizophrenia is a chronic neuropsychiatric illness, and long-term antipsychotic medications are the mainstay of treatment. 1 The first generation of antipsychotics, commonly referred to as typical antipsychotics, targeted mainly dopamine D2 receptors and led to disabling extrapyramidal side effects. 2 The antipsychotics introduced later, referred to as second generation or atypical antipsychotics (SGAs), acted on a wider range of receptors. 3 The SGAs had lower propensity for extrapyramidal side effects at clinically effective doses and showed improved efficacy on negative, cognitive and affective symptoms.4 –6 Thus, the SGAs found quick acceptance and their usage increased rapidly, such that they are now the more commonly prescribed drugs in large parts of the world.7 –11 The distinction between atypical and typical antipsychotics has since been called into question as many atypicals behave like typicals, and a new classification among atypicals has been proposed, introducing the concept of spectrum of atypia that begins with risperidone (the least atypical) and ends with olanzapine and clozapine (the most atypical). 12
Patients with schizophrenia are at risk of developing metabolic syndrome (MetS), with the prevalence of MetS being 1.5-fold higher than that of the general population.13,14 The MetS is a well-described cluster of interrelated risk factors for developing cardiovascular disease and type 2 diabetes. The main components of MetS are elevated waist circumference, hypertension, hyperglycaemia and dyslipidaemia. 15 The MetS is associated with a 2-fold increase in cardiovascular mortality and a 1.5-fold increase in all-cause mortality, 16 and individuals with MetS are five times more likely to develop type 2 diabetes. 17
The use of SGAs was associated with reports of dramatic weight gain, diabetes [including diabetic ketoacidosis (DKA)] and an atherogenic lipid profile [increased low-density lipoprotein (LDL) cholesterol and triglyceride levels and decreased high-density lipoprotein (HDL) cholesterol], such that the American Diabetes Association, the American Psychiatric Association, the American Association of Clinical Endocrinologists and the North American Association for the Study of Obesity convened a consensus development conference in 2003 on the subject. 18 The position statement from the conference concluded that there was considerable evidence, particularly in patients with schizophrenia, that treatment with SGAs can cause a rapid increase in body weight in the first few months of therapy that may not reach a plateau even after 1 year of treatment. Clozapine and olanzapine, which produced the greatest weight gain, were associated with the greatest increases in total cholesterol, LDL cholesterol, and triglycerides and with decreased HDL cholesterol. Since then, evidence of the link of SGAs with metabolic abnormalities has further accumulated.3,19,20
Several strategies have been tried to ameliorate the impact of SGAs on MetS, including dietary advice, physical exercise, counselling and enrolment in targeted health programmes.21,22 In addition to these, pharmacological strategies such as antipsychotic switching and adjunctive use of medications have been investigated for countering the metabolic side effects. 23
Studies have suggested aripiprazole as a potential candidate for switching strategies to improve metabolic parameters.24 –26 However, the rate of treatment discontinuation was higher in the aripiprazole group in the studies. Furthermore, clinicians are reluctant to switch antipsychotics solely to address metabolic side effects, particularly if the patient has achieved remission or clinically meaningful symptom reduction on their current antipsychotic. 27 In clinical practice, it is also challenging to switch when patients may have a belief of superior efficacy in their current antipsychotic. 24 Olanzapine and clozapine are two of the most effective antipsychotics, proven to have better efficacy than others.5,28 Together with risperidone, they are also among the highest prescribed antipsychotics, despite clear concerns about their impact on weight and metabolic profile.29,30
Aripiprazole as an adjunct has also been investigated for improving metabolic profile; a multicentre, randomised, double-blind placebo-controlled trial studying the effect of adjunctive aripiprazole (dose range 5–15 mg; mean 11.1 mg) on body weight and clinical efficacy in patients on clozapine found significant weight loss and reduction in body mass index (BMI) and waist circumference in favour of aripiprazole, with no significant differences in symptom improvement or adverse events (AEs). 31 A randomised, double-blind placebo-controlled study to examine aripiprazole’s effect on glucose metabolism in clozapine-treated patients with schizophrenia using the frequently sampled intravenous glucose tolerance test (a gold standard procedure to assess insulin sensitivity and the effectiveness of glucose metabolism) found that adjunctive aripiprazole treatment in clozapine-treated patients improved certain aspects of glucose metabolism including insulin sensitivity. 32 There are further studies that have explored adjunct aripiprazole with clozapine and olanzapine, with mostly positive findings.33 –35 As a result, use of aripiprazole as an adjunct has received cautious endorsement in reviews and clinical practice guidelines.23,36 –38
In this study, we set about evaluating the effect of adjunct use of aripiprazole at a fixed dose of 5 mg on metabolic profile and weight in patients stabilised on atypical antipsychotics olanzapine, clozapine and risperidone. We hypothesised that aripiprazole, due to its pharmacological profile, can be used as an adjunct to atypical antipsychotics at a low dose to induce weight loss and improve metabolic profile with fewer side effects and greater clinical acceptability. The primary objective of the study was to assess mean change in weight 12 weeks after the use of adjunct aripiprazole. The secondary end points included changes in the metabolic parameters (waist circumference; fasting blood glucose; HbA1c; total, HDL and LDL cholesterol levels; triglycerides).
Method
Study design
The study was an open-label, 12-week prospective study conducted between May 2016 and September 2019 at the outpatient clinic at the Institute of Mental Health (IMH), Singapore. IMH is the sole tertiary psychiatric hospital in Singapore that caters to over 40,000 patients annually at its outpatient clinic. Enrolled participants were prescribed a fixed dose of 5 mg aripiprazole per day, in addition to their antipsychotic. This dose was decided based on an audit of the local clinical practice that suggested that a 5-mg adjunct dose of aripiprazole had a good balance of efficacy and side effects and lower risk of discontinuation. 39 During the 12-week study, if for any reason there was a change in the dose of the primary antipsychotic or switch of the antipsychotic, the participant was then withdrawn from the study. Medication compliance was assessed by pill counts.
Safety and tolerability were measured by Simpson Angus Scale (SAS total score), Side Effects Checklist, Barnes Akathisia Scale (BAS), Abnormal Involuntary Movement Scale (AIMS), AEs or serious AE reports.
The study was carried out in accordance with the principles of Good Clinical Practice and the relevant local health regulations. Ethics approval was obtained from the National Healthcare Group’s Domain Specific Review Board (Reference: 2016/00106). The study was registered at trial registry clinicaltrials.gov (Identifier: NCT02949752). Participants were required to provide written informed consent in English at the point of recruitment.
Subjects
The study recruited outpatients aged 21–65 years with a diagnosis of schizophrenia or schizoaffective disorder. The subjects were required to be on stable doses of atypical antipsychotics, olanzapine, clozapine, or risperidone, for at least 1 month. Antipsychotic polypharmacy was permitted, as long as there was only one of the above three atypical antipsychotics. At baseline, subjects were required to have a BMI ⩾25 kg/m2 (i.e. overweight and above) or ⩾7% increase in weight from pre-antipsychotic treatment.
The study excluded subjects who had a previous allergy to aripiprazole or contraindication to the use of aripiprazole. Participants with current substance misuse or those non-adherent to current prescribed medications were excluded. It was required that subjects had no major or unstable medical or neurological illness (such as uncontrolled diabetes and hypertension) and were not using any medications for weight loss during the preceding month. Participants with serious suicidal thoughts or who posed a serious risk of harm to self or to others were excluded. The study did not recruit women who were pregnant or breastfeeding. Participants who had clinically significant abnormalities on enrolment examination and screening that required active intervention, that is, initiation of lipid lowering agent or antidiabetic medication, were also excluded.
Metabolic measurements
Weight, BMI and waist circumference were measured at baseline and at every subsequent visit (weeks 0, 4, 8 and 12). A fasting venous blood sample was collected from each participant at baseline and at the last visit (week 12). Serum level of total cholesterol, HDL, LDL, and triglycerides and glucose levels were obtained at an accredited laboratory that analysed the samples using Roche diagnostics C702 and C513 HbA1c analysers.
Assessment and end points
Psychopathology was assessed using the Positive and Negative Syndrome Scale (PANSS) 40 and the Clinical Global Impression–Severity and Improvement scales (CGI-I and CGI-S). 41 Functioning was assessed on the Global Assessment of Functioning Scale (GAF), 42 while World Health Organization Disability Assessment Scale (WHODAS 2.0) 43 was used to assess disability across various domains. All assessments were performed by trained raters who had achieved satisfactory inter-rater reliability [i.e. intraclass correlation coefficient (ICC) >0.8]. Neurocognition was assessed using the symbol coding and digit span tasks, components of the Brief Neurocognitive Assessment, which included working memory and processing speed cognitive domains. 44
Safety and tolerability
Side effects were evaluated using the SAS, BAS, AIMS, Side Effects Checklist and AE reports.
Statistical analysis
All statistical analyses were performed using SPSS Version 22 (IBM) and SAS software. The primary outcome measure was the mean change in body weight from baseline to week 12 using last observation carried forward (LOCF) data. The sample size of 42 patients (14 in each antipsychotic arm) was designed to yield 80% power to detect a difference of 1.6 kg in the mean change from baseline in weight at week 12 (LOCF). 39 This calculation assumed a standard deviation of 2.3. Allowing for withdrawals, it was expected that 60 patients would need to be recruited. The mean changes were evaluated using generalised estimating equations model with baseline measurement, age, gender and chlorpromazine equivalent dose as covariates, and treatment group as main effects. A further analysis was carried for each individual antipsychotic group (olanzapine, clozapine and risperidone).
Results
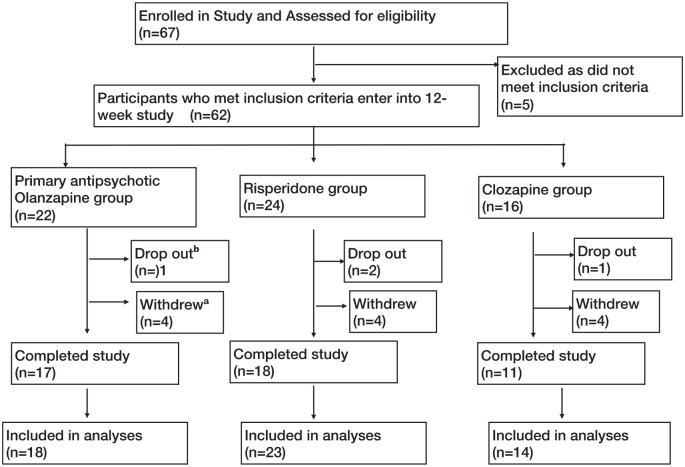
A total of 67 individuals were screened for the study; 55 patients were included in the final analysis (including those who withdrew but attended for exit visit), of which 18 were on olanzapine, 23 were on risperidone and 14 were on clozapine (see Figure 1). The clinical and demographic details of the participants are shown in Table 1. The mean dose of olanzapine was 11.9 mg (SD = 5.7) (range: 5–20), of clozapine was 292.9 mg (SD = 115.8) (range: 125–500) and of risperidone was 3.2 mg (SD = 1.6)(range: 0.5–6).
Figure 1.
Patient flow diagram through the trial as per CONSORT guidelines.
aSubjects were withdrawn from the study for any of the following reasons: non-compliance (n = 1), abnormal baseline blood results/abnormalities in the results such that medication changes were required (n = 4), social issues (n = 2), patient/family’s request (n = 4) and mental state deterioration (n = 1).
bSubjects dropped out from the study for any of the following reasons: loss to follow-up (n = 1) and side effects (n = 3).
Table 1.
Clinical and demographic characteristics of the study sample (n = 55).
| Overall sample n (%) |
Olanzapine (n = 18) n (%) |
Risperidone (n = 23) n (%) |
Clozapine (n = 14) n (%) |
|
|---|---|---|---|---|
| Age, mean (SD) | 39.3 (8.2) | 37.7 (6.8) | 43.8 (7.7) | 33.9 (6.6) |
| Sex | ||||
| Female | 27 (49.1) | 7 (38.9) | 12 (52.2) | 8 (57.1) |
| Male | 28 (50.9) | 11 (61.1) | 11 (47.8) | 6 (42.9) |
| Ethnicity | ||||
| Chinese | 39 (70.9) | 13 (72.2) | 13 (56.5) | 13 (92.9) |
| Malay | 11 (20.) | 3 (16.7) | 8 (34.8) | 0 (0) |
| Indian | 4 (7.3) | 1 (5.6) | 2 (8.7) | 1 (7.1) |
| Others | 1 (1.8) | 1 (5.6) | 0 (0) | 0 (0) |
| Diagnosis | ||||
| Schizophrenia | 46 (83.6) | 13 (72.2) | 19 (82.6) | 14 (100) |
| Schizoaffective disorder | 9 (16.4) | 5 (27.8) | 4 (17.4) | 0 (0) |
| Medication groups | ||||
| Olanzapine | 18 (32.7) | – | – | – |
| Risperidone | 23 (41.8) | – | – | – |
| Clozapine | 14 (25.5) | – | – | – |
| Dosage (mg/day), mean (SD) | 11.9 (5.7) (range 5–20) | 3.2 (1.6) (range: 0.5–6) |
292.9 (115.8) (range: 125–500) |
|
Metabolic parameters
Thirty-one (56.4%) participants recorded weight loss after 12 weeks of adjunctive aripiprazole [13 (92.9%) out of 14 for clozapine, 8 (44.4%) out of 18 for olanzapine and 10 (43.5%) out of 23 for risperidone). Among the participants with documented weight loss, the average weight loss was 1.82 kg (SD = 1.64). Overall, there was no significant change in weight, BMI or waist circumference between week 0 and week 12 (see Table 2).
Table 2.
Mean change in weight and other outcomes between baseline and 12-week follow-up in the overall sample (n = 55).
| Baseline | 12-week | Paired t test | Generalised estimating equations | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean change | p value a | β | 95% CI Lower |
95% CI Upper |
p value b | |
| Weight (kg) | 84.48 | 14.65 | 84.27 | 15.34 | -0.21 | 0.508 | −0.21 | −0.41 | 0.84 | 0.501 |
| BMI (kg/m2) | 31.23 | 4.21 | 31.26 | 4.54 | −0.03 | 0.814 | 0.03 | −0.23 | 0.30 | 0.812 |
| Waist circumference (cm) | 102.47 | 10.11 | 102.65 | 11.00 | 0.19 | 0.744 | 0.19 | −0.91 | 1.28 | 0.741 |
| Metabolic parameters | ||||||||||
| Fasting glucose (mmol/L) | 5.16 | 0.96 | 5.21 | 1.21 | 0.05 | 0.625 | 0.05 | −0.14 | 0.23 | 0.620 |
| HbA1c (%) | 5.63 | 0.66 | 5.60 | 0.75 | −0.03 | 0.509 | −0.03 | −0.10 | 0.05 | 0.502 |
| Cholesterol (mmol/L) | 4.88 | 0.97 | 4.60 | 0.94 | −0.28 | 0.002 | −0.28 | −0.44 | −0.11 | 0.001 |
| HDL (mmol/L) | 1.19 | 0.30 | 1.23 | 0.30 | 0.03 | 0.070 | 0.03 | 0.00 | 0.06 | 0.062 |
| LDL (mmol/L) | 2.83 | 0.78 | 2.65 | 0.78 | −0.16 | 0.051 | −0.17 | −0.33 | −0.01 | 0.038 |
| Triglycerides (mmol/L) | 2.07 | 1.34 | 1.79 | 1.16 | −0.28 | 0.007 | −0.28 | −0.47 | −0.08 | 0.005 |
| Neurocognition tests | ||||||||||
| Symbol coding test | 44.40 | 10.98 | 45.80 | 11.90 | 1.40 | 0.136 | 1.40 | −0.40 | 3.20 | 0.127 |
| Digit span | 16.89 | 4.07 | 16.69 | 3.40 | −0.20 | 0.552 | −0.20 | −0.85 | 0.45 | 0.545 |
| Clinical scales | ||||||||||
| GAF total | 67.87 | 8.73 | 72.91 | 8.31 | 5.04 | <0.001 | 5.04 | 2.93 | 7.14 | <0.001 |
| CGI-S | 2.24 | 0.77 | 2.02 | 0.73 | -0.22 | 0.022 | −0.22 | −0.40 | −0.04 | 0.017 |
| CGI-I | 2.15 | 0.80 | 2.35 | 1.11 | 0.17 | 0.290 | 0.15 | −0.14 | 0.44 | 0.300 |
| PANSS total | 48.53 | 9.26 | 49.36 | 11.20 | 0.84 | 0.484 | 0.84 | −1.47 | 3.14 | 0.477 |
| WHODAS | 20.35 | 7.13 | 20.61 | 8.24 | 0.27 | 0.535 | 0.45 | −1.34 | 2.25 | 0.621 |
β: beta coefficient; CI: confidence interval; BMI: body mass index; HbA1c: haemoglobin A1c; HDL: high-density lipoprotein; LDL: low-density lipoprotein; GAF: Global Assessment of Functioning; CGI-S: Clinical Global Impression–Severity; CGI-I: Clinical Global Impression–Improvement; PANSS: Positive and Negative Syndrome Scale; WHODAS: World Health Organization Disability Assessment Scale.
Paired t test.
Age, sex, dosage and baseline measurements as covariates.
Bold values denote statistical significance at the p < 0.05
In addition, there was significant reduction in total cholesterol (β = −0.28, p = 0.001), LDL (β = −0.17, p = 0.038) and triglycerides (β = −0.47, p = 0.005) in the overall study sample. An analysis of completers (n = 46) versus LOCF did not lead to a change in the findings.
Symptoms and functioning
There were statistically significant improvements in CGI-S (β = −0.22, p = 0.017) and GAF (β = 5.04, p < 0.001) scores from baseline to week 12; however, there were no significant difference in PANSS and WHODAS scores (Table 2).
Safety and tolerability
A total of 20 patients reported suffering from any kind of AE, with the most common being anxiety (n = 4), dizziness (n = 3), sedation (n = 3), constipation (n = 3), tachycardia (n = 3), restlessness (n = 2) and akathisia (n = 2) (see Table 3). After acceptance into the study and commencing the study medication, four subjects dropped out of the study: three due to side effects and one was lost to follow-up. Eight participants were withdrawn from the study: four due to patient/family request, two due to social issues (housing/work), one due to deterioration in mental state assessed to be unrelated to the study medication and one due to non-compliance. Mean total SAS, BAS and AIMS scores were very low at study entry with no significant increases at the end of the study. For AIMS, nine subjects (16.3%) had baseline scores >1 (median, 2; range, 1–5), while at visit 4, seven subjects (12.7%) had AIMS score >1 (median, 2; range, 1–3). For BAS, six subjects (10.9%) had baseline scores >1 (median, 1; range, 1–2), while at visit 4, three subjects (5.5%) had scores >1 (median, 1; range. 1–2). For SAS, eight subjects (14.5%) had scores > 0 at baseline (median, 1; range, 1–2), while four subjects (7.5%) had scores > 0 (median, 2; range, 1–3).
Table 3.
Mean change in weight and other outcomes between baseline and 12-week follow-up within olanzapine (n = 18).
| Baseline | 12-week | Paired t test | Generalised estimating equations | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean change | p value a | β | 95% CI Lower |
95% CI Upper |
p value b | |
| Weight (kg) | 81.56 | 16.58 | 81.99 | 17.62 | 0.43 | 0.352 | 0.43 | −0.42 | 1.28 | 0.325 |
| BMI (kg/m2) | 30.24 | 4.21 | 30.31 | 4.44 | −0.07 | 0.695 | 0.07 | −0.27 | 0.42 | 0.681 |
| Waist circumference (cm) | 97.94 | 9.52 | 98.67 | 11.06 | 0.73 | 0.530 | 0.73 | −1.44 | 2.89 | 0.510 |
| Metabolic parameters | ||||||||||
| Fasting glucose (mmol/L) | 4.95 | 0.38 | 5.10 | 0.53 | 0.15 | 0.247 | 0.15 | −0.09 | 0.39 | 0.217 |
| HbA1c (%) | 5.54 | 0.38 | 5.53 | 0.44 | −0.01 | 0.926 | −0.01 | −0.12 | 0.11 | 0.922 |
| Cholesterol (mmol/L) | 5.12 | 1.16 | 4.94 | 1.15 | −0.18 | 0.167 | −0.18 | −0.41 | 0.06 | 0.138 |
| HDL (mmol/L) | 1.19 | 0.26 | 3.06 | 0.92 | 0.05 | 0.105 | 0.05 | −0.01 | 0.11 | 0.079 |
| LDL (mmol/L) | 3.13 | 0.92 | 1.24 | 0.30 | −0.07 | 0.491 | −0.07 | −0.27 | 0.13 | 0.469 |
| Triglycerides (mmol/L) | 1.77 | 0.81 | 1.43 | 0.59 | −0.34 | 0.006 | −0.34 | −0.55 | −0.14 | 0.001 |
| Neurocognition tests | ||||||||||
| Symbol coding | 45.94 | 10.23 | 47.83 | 10.61 | 1.89 | 0.302 | 1.89 | −1.49 | 5.27 | 0.273 |
| Digit span | 16.83 | 4.16 | 16.39 | 3.50 | −0.44 | 0.420 | −0.44 | −1.47 | 0.58 | 0.395 |
| Clinical scales | ||||||||||
| GAF total | 69.11 | 7.44 | 73.44 | 7.51 | 4.33 | 0.030 | 4.33 | 0.85 | 7.82 | 0.015 |
| CGI-S | 2.11 | 0.47 | 2.17 | 0.71 | 0.06 | 0.749 | 0.06 | −0.27 | 0.38 | 0.738 |
| CGI-I | 2.00 | 0.50 | 1.94 | 0.80 | 0.00 | 1.000 | −0.02 | −0.30 | 0.27 | 0.914 |
| PANSS total | 46.89 | 9.47 | 47.89 | 9.69 | 1.00 | 0.627 | 1.00 | −2.85 | 4.85 | 0.611 |
| WHODAS | 23.06 | 9.14 | 22.35 | 10.65 | 0.12 | 0.948 | −0.05 | −3.42 | 3.32 | 0.977 |
β: beta coefficient; CI: confidence interval; BMI: body mass index; HbA1c: haemoglobin A1c; HDL: high-density lipoprotein; LDL: low-density lipoprotein; GAF: Global Assessment of Functioning; CGI-S: Clinical Global Impression–Severity; CGI-I: Clinical Global Impression–Improvement; PANSS: Positive and Negative Syndrome Scale; WHODAS: World Health Organization Disability Assessment Scale.
Paired t test.
Age, sex, dosage and baseline measurements as covariates.
Bold values denote statistical significance at the p < 0.05
Groupwise analyses
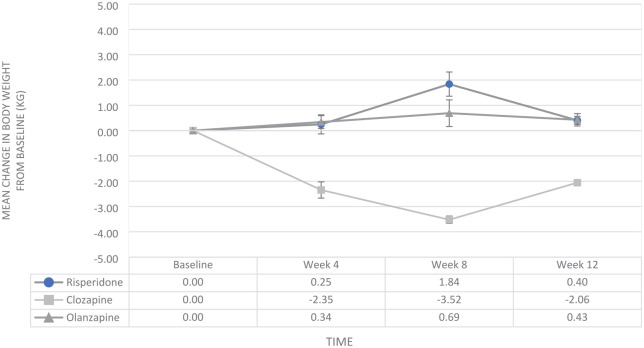
In groupwise analyses, only the group on clozapine had significant weight loss (β = −2.06, p = 0.002) after 12 weeks of adjunctive aripiprazole (see Figure 2). The clozapine group also had significant improvements in total cholesterol (β = −0.47, p < 0.001), HDL (β = 0.08, p = 0.016), LDL (β = −0.28, p = 0.044) and triglycerides (β = −0.58, p = 0.038). There were significant improvements in symptom severity (β = −0.43, p = 0.010) and functioning (β = 8.29, p < 0.001) (see Table 4).
Figure 2.
Mean change in body weight (kg) from baseline for atypicals (olanzapine, risperidone and clozapine) and adjunct aripiprazole 5 mg.
Table 4.
Mean change in weight and other outcomes between baseline and 12-week follow-up within clozapine (n = 14).
| Baseline | 12-week | Paired t test | Generalised estimating equations | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean change | p value a | β | 95% CI Lower |
95% CI Upper |
p value b | |
| Weight (kg) | 84.22 | 12.50 | 82.16 | 12.32 | −2.06 | 0.010 | −2.06 | −3.34 | −0.78 | 0.002 |
| BMI (kg/m2) | 31.01 | 3.10 | 30.40 | 3.48 | 0.61 | 0.072 | −0.61 | −1.20 | −0.02 | 0.042 |
| Waist circumference (cm) | 104.86 | 10.44 | 103.95 | 11.28 | −0.91 | 0.479 | −0.91 | −3.26 | 1.44 | 0.449 |
| Metabolic parameters | ||||||||||
| Fasting glucose (mmol/L) | 4.89 | 0.31 | 4.84 | 0.39 | −0.05 | 0.519 | −0.05 | −0.19 | 0.09 | 0.491 |
| HbA1c (%) | 5.29 | 0.33 | 5.21 | 0.30 | −0.08 | 0.159 | −0.08 | −0.18 | 0.02 | 0.121 |
| Cholesterol (mmol/L) | 4.82 | 0.85 | 4.35 | 0.78 | −0.47 | 0.002 | −0.47 | −0.71 | −0.23 | <0.0001 |
| HDL (mmol/L) | 1.11 | 0.17 | 2.29 | 0.55 | 0.08 | 0.037 | 0.08 | 0.01 | 0.14 | 0.016 |
| LDL (mmol/L) | 2.57 | 0.53 | 1.19 | 0.21 | −0.27 | 0.094 | −0.28 | −0.54 | −0.01 | 0.044 |
| Triglycerides (mmol/L) | 2.95 | 1.85 | 2.37 | 1.75 | −0.58 | 0.066 | −0.58 | −1.13 | −0.03 | 0.038 |
| Neurocognition tests | ||||||||||
| Symbol coding | 49.50 | 13.55 | 49.00 | 14.28 | −0.50 | 0.782 | −0.50 | −3.84 | 2.84 | 0.769 |
| Digit span | 16.00 | 2.99 | 16.00 | 3.21 | 0.00 | 1.000 | 0.00 | −1.17 | 1.17 | 1.000 |
| Clinical scales | ||||||||||
| GAF total | 62.43 | 9.76 | 70.71 | 9.64 | 8.29 | 0.003 | 8.29 | 4.01 | 12.56 | < 0.001 |
| CGIS | 2.57 | 0.94 | 2.14 | 0.77 | −0.43 | 0.028 | −0.43 | −0.75 | −0.10 | 0.010 |
| CGII | 3.00 | 1.00 | 3.00 | 1.18 | 0.00 | 1.000 | 0.22 | −0.85 | 1.29 | 0.687 |
| PANSS total | 53.93 | 8.86 | 55.21 | 12.94 | 1.29 | 0.640 | 1.29 | −3.78 | 6.35 | 0.619 |
| WHODAS | 21.00 | 4.85 | 21.86 | 7.93 | 0.86 | 0.712 | 0.86 | −3.43 | 5.15 | 0.696 |
β: beta coefficient; CI: confidence interval; BMI: body mass index; HbA1c: haemoglobin A1c; HDL: high-density lipoprotein; LDL: low-density lipoprotein; GAF: Global Assessment of Functioning; CGI-S: Clinical Global Impression–Severity; CGI-I: Clinical Global Impression–Improvement; PANSS: Positive and Negative Syndrome Scale; WHODAS: World Health Organization Disability Assessment Scale.
Paired t test.
Age, sex, dosage and baseline measurements as covariates.
Bold values denote statistical significance at the p < 0.05
The olanzapine group reported significant improvement in triglycerides (β = −0.34, p = 0.001); the other metabolic parameters showed similar improvement trends, but did not reach statistical significance (see Table 3). In the clinical measures, there was a significant improvement in functioning (β = 4.33, p = 0.015), but no significant change in symptom severity.
In the risperidone group, there were no significant changes to any metabolic indicators except the BMI, which increased from baseline to end of the study (β = −0.39, p = 0.019); however, there were significant improvements in symptom severity (β = −0.30, p = 0.039) and functioning (β = 3.61, p = 0.022) (see Table 5).
Table 5.
Mean change in weight and other outcomes between baseline and 12-week follow up within risperidone (n = 23).
| Baseline | 12-week | Paired t-test | Generalised estimating equations | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean change | p value a | β | 95% CI Lower |
95% CI Upper |
p value b | |
| Weight (kg) | 86.93 | 14.44 | 87.33 | 15.21 | 0.40 | 0.372 | 0.40 | −0.45 | 1.26 | 0.352 |
| BMI (kg/m2) | 32.13 | 4.74 | 32.53 | 5.02 | −0.39 | 0.056 | 0.39 | 16.30 | 48.69 | 0.039 |
| Waist circumference (cm) | 104.56 | 9.56 | 104.98 | 10.38 | 0.43 | 0.550 | 0.43 | −0.92 | 1.77 | 0.535 |
| Metabolic parameters | ||||||||||
| Fasting glucose (mmol/L) | 5.49 | 1.37 | 5.52 | 1.75 | 0.03 | 0.900 | 0.03 | −0.37 | 0.42 | 0.897 |
| HbA1c (%) | 5.90 | 0.86 | 5.89 | 0.99 | −0.01 | 0.907 | −0.01 | −0.15 | 0.13 | 0.904 |
| Cholesterol (mmol/L) | 4.73 | 0.87 | 4.50 | 0.81 | −0.23 | 0.147 | −0.23 | −0.53 | 0.06 | 0.124 |
| HDL (mmol/L) | 1.25 | 0.38 | 2.53 | 0.62 | −0.01 | 0.699 | −0.01 | −0.06 | 0.04 | 0.688 |
| LDL (mmol/L) | 2.71 | 0.70 | 1.24 | 0.35 | −0.19 | 0.245 | −0.19 | −0.49 | 0.11 | 0.221 |
| Triglycerides (mmol/L) | 1.76 | 1.10 | 1.73 | 0.95 | −0.04 | 0.754 | −0.04 | −0.27 | 0.19 | 0.745 |
| Neurocognition tests | ||||||||||
| Symbol coding | 40.09 | 8.30 | 42.26 | 10.82 | 2.17 | 0.125 | 2.17 | −0.44 | 4.79 | 0.103 |
| Digit span | 17.48 | 4.59 | 17.35 | 3.46 | −0.13 | 0.824 | −0.13 | −1.24 | 0.98 | 0.818 |
| Clinical scales | ||||||||||
| GAF total | 70.22 | 7.90 | 73.83 | 8.18 | 3.61 | 0.036 | 3.61 | 0.52 | 6.70 | 0.022 |
| CGIS | 2.13 | 0.81 | 1.83 | 0.72 | −0.30 | 0.031 | −0.30 | −0.56 | −0.05 | 0.019 |
| CGII | 1.91 | 0.68 | 2.26 | 1.14 | 0.36 | 0.119 | 0.36 | −0.06 | 0.78 | 0.096 |
| PANSS total | 46.52 | 8.36 | 46.96 | 10.32 | 0.43 | 0.810 | 0.43 | −2.99 | 3.86 | 0.804 |
| WHODAS | 17.83 | 5.79 | 18.57 | 6.00 | 0.74 | 0.508 | 0.74 | −1.37 | 2.84 | 0.491 |
β: beta coefficient; CI: confidence interval; BMI: body mass index; HbA1c: haemoglobin A1c; HDL: high-density lipoprotein; LDL: low-density lipoprotein; GAF: Global Assessment of Functioning; CGI-S: Clinical Global Impression–Severity; CGI-I: Clinical Global Impression–Improvement; PANSS: Positive and Negative Syndrome Scale; WHODAS: World Health Organization Disability Assessment Scale.
Paired t test.
Age, sex, dosage and baseline measurements as covariates.
Bold values denote statistical significance at the p < 0.05
Discussion
This study was initiated to identify an effective and pragmatic strategy to tackle the growing epidemic of MetS associated with the use of atypical antipsychotics in schizophrenia. 45 Although our study found no significant weight reduction with adjunctive use of aripiprazole after 12 weeks, there were significant improvements in total cholesterol, LDL and triglycerides. The fixed dose of 5 mg aripiprazole was tolerated well and did not lead to side effects for the majority of the patients within the trial. The study also highlighted between group differences in favour of adjunctive aripiprazole use in individuals on olanzapine and clozapine, the two antipsychotics that are deemed more ‘atypical’ and most often associated with metabolic abnormalities (see Table 6).
Table 6.
Side effects (frequency and severity) from use of adjunct aripiprazole with atypical antipsychotics olanzapine, clozapine and risperidone.
| Side effect | Frequency | Severity |
|---|---|---|
| Anxiety | 4 | Mild |
| Dizziness | 3 | Mild |
| Sedation | 3 | Mild |
| Nausea | 3 | Mild-2, moderate-1 |
| Increased appetite | 3 | Mild |
| Restlessness | 2 | Mild |
| Dystonia | 2 | Mild-1, moderate-1 |
| Somnolence | 2 | Mild |
| Dry mouth | 2 | Mild-1, moderate-1 |
| Insomnia | 1 | Moderate |
| Depression | 1 | Mild |
| Tremor | 1 | Mild |
| Headache | 1 | Severe |
| Blurred vision | 1 | Mild |
| Vomiting | 1 | Moderate |
| Constipation | 1 | Mild |
| Salivary hypersecretion | 1 | Moderate |
| Fatigue | 1 | Mild |
| Rash | 1 | Mild |
| Shortness of breath | 1 | Mild |
| Reduced appetite | 1 | Mild |
| Urinary frequency | 1 | Mild |
| Confusion | 1 | Moderate |
The group on clozapine appears to benefit most from adjunctive use of aripiprazole. An improvement in lipid profile and weight but no significant change in the fasting glucose levels was also seen in previous trials with aripiprazole and clozapine.32,33,46 It is likely that the duration of the study was too short for detecting the small change in glucose levels, as a similar study combining metformin and atypical antipsychotics for weight loss also did not detect a significant change in fasting glucose. 47 Importantly, 13 out of the 14 patients on clozapine achieved weight reduction in this 12-week trial, adding support to the adjunctive use of aripiprazole to clozapine for weight loss and improvement in metabolic profile.
Our study results do not make a case for use of fixed low-dose adjunct aripiprazole for olanzapine and risperidone patients as there was no weight loss. However, there was a significant reduction in triglycerides and increase in HDL levels that did not reach significance in the olanzapine group. These results are consistent with reports indicating improvements in triglycerides, but we did not observe the corresponding weight loss effects.34,35 Taken together, the evidence does suggest improvement in cardiometabolic risk profile in individuals on olanzapine as hypertriglyceridaemia is an independent risk factor for cardiovascular disease. 48
Aripiprazole is described as a partial agonist at D2 receptors although its complex mechanism of action based on findings of large variations in both intrinsic activity and potency also suggests that aripiprazole is ‘functionally selective’ at D2 receptors.49 –51 Aripiprazole also works as a partial agonist at the serotonin 5-HT1A and 5-HT2C receptors and as an antagonist at the serotonin 5-HT2A receptor. 52 Dopamine D2 agonists are reported to reduce food intake by acting on hypothalamic areas, 53 while agonism of 5-HT2C receptors has been linked with decreased appetite and weight loss. 54 5-HT2C receptors may also play a role in the peripheral dysregulation of leptin and ghrelin pathways and may impair neural processing of glucose and fat metabolism. 55
Adjunctive use of aripiprazole in individuals on clozapine significantly improved clinical symptoms and functioning. Effectiveness of clozapine–aripiprazole combination in clozapine-resistant individuals has previously been reported 33 and is one of the strategies recommended by an expert consensus group. 56 This study findings are congruent with what was previously reported on this topic. At minimum, we do not observe any relapse or significant worsening of psychotic symptoms with the adjunctive use of aripiprazole.
Aripiprazole functions as a partial D2 agonist at mesocortical pathway, where reduced dopaminergic activity is proposed to lead to negative symptoms and cognitive impairment. 57 While an 8-week open-label trial of aripiprazole’s effect on cognition showed significant improvement in several neurocognitive domains and PANSS scores, 58 adjunct use of aripiprazole in a 24-week double-blind randomised placebo-controlled trial study, that added 10–15 mg aripiprazole to clozapine, found no significant effect on negative symptoms and executive cognitive functions. 59 In our study too, we did not see any significant changes in scores within the two neuropsychological tests of digit span and symbol coding and the PANSS scores.
Current treatment guidelines are usually critical of antipsychotic polypharmacy, with strong recommendation for monotherapy. However, in the case of aripiprazole, there seems to be evidence supporting its use as an adjunct, for its clinical effectiveness or improving metabolic risks. 46 In addition, adjunctive use of aripiprazole has also been recommended to reduce hyperprolactinaemia in patients on risperidone and other prolactin-raising antipsychotics 52 and for reduction in obsessive compulsive symptoms for patients on clozapine and olanzapine.60,61
There are several limitations to this study. First, the overall sample size is small, particularly as the study included three different atypical antipsychotics, with distinct pharmacological profiles. The study used a fixed dose of 5 mg of aripiprazole, which is lowest among the published studies that have mostly used a variable dosing strategy (up to 20 mg daily). 62 A low fixed dose strategy reduces the likelihood of AEs and increases acceptability, yet it may have been inadequate to achieve significant improvement. The duration of the study was 12 weeks, which may not be sufficiently long to see significant change in the outcomes. The study did not identify patients who were pre-morbidly obese; thus, the study may have included patients whose weight gain was not linked to atypical antipsychotics. Concurrent medications like mood stabilisers may have contributed to weight and metabolic profile changes. Last but not least, this is an open-label study which lacks randomisation, placebo control and blinding.
Nonetheless, this study is an independent pragmatic trial, closely aligned to clinical practice and with broad inclusion criteria. The outcomes of the study are mostly objective (weight measurement and laboratory parameters), reducing the likelihood of bias. The other strengths of the study include the relatively low dropout rate. This may be the first open-label study aiming to assess the effect of adjunctive aripiprazole for weight reduction and improvement in metabolic profiles comparing the atypical antipsychotics risperidone, olanzapine and clozapine.
In conclusion, the results from this study support the adjunctive use of aripiprazole in individuals on clozapine and olanzapine in a bid to improve metabolic profile. Further studies on long-term effects of adjunctive aripiprazole to atypical antipsychotics are required to examine the sustainability of gains from early use as well as long-term safety and tolerability of antipsychotic polypharmacy.
Footnotes
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the National Medical Research Council (NMRC), Singapore Centre Grant research seed funding.
ORCID iDs: Bhanu Gupta  https://orcid.org/0000-0002-9515-0559
https://orcid.org/0000-0002-9515-0559
Edimansyah Abdin  https://orcid.org/0000-0002-1016-3298
https://orcid.org/0000-0002-1016-3298
Contributor Information
Bhanu Gupta, Institute of Mental Health, Singapore 539747.
Kok-Seng Chee, Institute of Mental Health, Singapore.
Li-Qi Neo, Institute of Mental Health, Singapore.
Charmaine Tang, Institute of Mental Health, Singapore.
Jayaraman Hariram, Institute of Mental Health, Singapore.
Geoffrey Chern-Yee Tan, Institute of Mental Health, Singapore.
Swapna Verma, Institute of Mental Health, Singapore.
Sutapa Basu, Institute of Mental Health, Singapore.
Deva-Priya Appan, Institute of Mental Health, Singapore.
Chan-Chun Ting, Institute of Mental Health, Singapore.
Edimansyah Abdin, Institute of Mental Health, Singapore.
Jimmy Lee, Institute of Mental Health, Singapore.
References
- 1. Keepers GA, Fochtmann LJ, Anzia JM, et al. The American psychiatric association practice guideline for the treatment of patients with schizophrenia. Am J Psych 2020; 177: 868–872. [DOI] [PubMed] [Google Scholar]
- 2. Meltzer HY, Gadaleta E. Contrasting typical and atypical antipsychotic drugs. Focus 2021; 19: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carli M, Kolachalam S, Longoni B, et al. Atypical antipsychotics and metabolic syndrome: from molecular mechanisms to clinical differences. Pharmaceuticals 2021; 14: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 2019; 394: 939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013; 382: 951–962. [DOI] [PubMed] [Google Scholar]
- 6. Grinchii D, Dremencov E. Mechanism of action of atypical antipsychotic drugs in mood disorders. Int J Mol Sci 2020; 21: 9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gaviria AM, Franco JG, Aguado V, et al. A non-interventional naturalistic study of the prescription patterns of antipsychotics in patients with schizophrenia from the Spanish province of Tarragona. PLoS ONE 2015; 10: e0139403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marston L, Nazareth I, Petersen I, et al. Prescribing of antipsychotics in UK primary care: a cohort study. BMJ Open 2014; 4: e006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keating D, McWilliams S, Boland F, et al. Prescribing pattern of antipsychotic medication for first-episode psychosis: a retrospective cohort study. BMJ Open 2021; 11: e040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park S-C, Lee M-S, Kang S-G, et al. Patterns of antipsychotic prescription to patients with schizophrenia in Korea: results from the health insurance review & assessment service-national patient sample. J Korean Med Sci 2014; 29: 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seshadri M, Elsemary A, Thalitaya MD, et al. Study on the prescribing patterns of antipsychotic medication in a rural England Community Mental Health Team. Psychiatr Danub 2017; 29: 524–529. [PubMed] [Google Scholar]
- 12. Aringhieri S, Carli M, Kolachalam S, et al. Molecular targets of atypical antipsychotics: from mechanism of action to clinical differences. Pharmacol Ther 2018; 192: 20–41. [DOI] [PubMed] [Google Scholar]
- 13. Lee J, Nurjono M, Wong A, et al. Prevalence of metabolic syndrome among patients with schizophrenia in Singapore. Ann Acad Med Singap 2012; 41: 457–462. [PubMed] [Google Scholar]
- 14. Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry 2015; 14: 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCracken E, Monaghan M, Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin Dermatol 2018; 36: 14–20. [DOI] [PubMed] [Google Scholar]
- 16. Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk: a systematic review and meta-analysis. J Am Coll Cardiol 2010; 56: 1113–1132. [DOI] [PubMed] [Google Scholar]
- 17. Regufe VM, Pinto CM, Perez PMVHC. Metabolic syndrome in type 2 diabetic patients: a review of current evidence. Porto Biomed J 2020; 5: e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Association AD. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004; 27: 596–601. [DOI] [PubMed] [Google Scholar]
- 19. De Hert M, Detraux J, Van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 2012; 8: 114–126. [DOI] [PubMed] [Google Scholar]
- 20. Perez Rodriguez A, Tajima-Pozo K, Lewczuk A, et al. Atypical antipsychotics and metabolic syndrome. Cardiovasc Endocrinol 2015; 4: 132–137. [Google Scholar]
- 21. Papanastasiou E. The prevalence and mechanisms of metabolic syndrome in schizophrenia: a review. Ther Adv Psychopharmacol 2013; 3: 33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tumiel E, Wichniak A, Jarema M, et al. Nonpharmacological interventions for the treatment of cardiometabolic risk factors in people with schizophrenia – a systematic review. Front Psychiatry 2019; 10: 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cooper SJ, Reynolds GP, Barnes T, et al. BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol 2016; 30: 717–748. [DOI] [PubMed] [Google Scholar]
- 24. Stroup TS, McEvoy JP, Ring KD, et al. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry 2011; 168: 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stroup TS, Byerly MJ, Nasrallah HA, et al. Effects of switching from olanzapine, quetiapine, and risperidone to aripiprazole on 10-year coronary heart disease risk and metabolic syndrome status: results from a randomized controlled trial. Schizophr Res 2013; 146: 190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Newcomer JW, Campos JA, Marcus RN, et al. A multicenter, randomized, double-blind study of the effects of aripiprazole in overweight subjects with schizophrenia or schizoaffective disorder switched from olanzapine. J Clin Psychiatry 2008; 69: 1046–1056. [DOI] [PubMed] [Google Scholar]
- 27. Weiden PJ. Switching antipsychotics as a treatment strategy for antipsychotic-induced weight gain and dyslipidemia. J Clin Psychiatry 2007; 68: 34–39. [PubMed] [Google Scholar]
- 28. Citrome L. A systematic review of meta-analyses of the efficacy of oral atypical antipsychotics for the treatment of adult patients with schizophrenia. Expert Opin Pharmacother 2012; 13: 1545–1573. [DOI] [PubMed] [Google Scholar]
- 29. Meyer JM, Davis VG, Goff DC, et al. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: prospective data from phase 1. Schizophr Res 2008; 101: 273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res 2010; 123: 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fleischhacker WW, Heikkinen ME, Olié JP, et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol 2010; 13: 1115–1125. [DOI] [PubMed] [Google Scholar]
- 32. Fan X, Borba CP, Copeland P, et al. Metabolic effects of adjunctive aripiprazole in clozapine-treated patients with schizophrenia. Acta Psychiatr Scand 2013; 127: 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang JS, Ahn Y, Park HJ, et al. Aripiprazole augmentation in clozapine-treated patients with refractory schizophrenia: an 8-week, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2008; 69: 720–731. [DOI] [PubMed] [Google Scholar]
- 34. Henderson DC, Fan X, Copeland PM, et al. Aripiprazole added to overweight and obese olanzapine-treated schizophrenia patients. J Clin Psychopharmacol 2009; 29: 165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang L-J, Ree S-C, Huang Y-S, et al. Adjunctive effects of aripiprazole on metabolic profiles: comparison of patients treated with olanzapine to patients treated with other atypical antipsychotic drugs. Prog Neuropsychopharmacol Biol Psychiatry 2013; 40: 260–266. [DOI] [PubMed] [Google Scholar]
- 36. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull 2014; 40: 1385–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taylor DM, Barnes TR, Young AH. The Maudsley prescribing guidelines in psychiatry. Newark, NJ: John Wiley & Sons, 2021. [Google Scholar]
- 38. Galling B, Roldan A, Hagi K, et al. Antipsychotic augmentation vs. monotherapy in schizophrenia: systematic review, meta-analysis and meta-regression analysis. World Psychiatry 2017; 16: 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gupta B, LYS Chua, Ong J, Tan GCY, et al. Aripiprazole as an adjunct for weight loss in patient’s established on clozapine and olanzapine. In: Singapore health and biomedical congress, Singapore, 2& 3rd October 2015, p. S315. Singapore: Annals of the Academy of Medicine. [Google Scholar]
- 40. Kay SR, Opler LA, Lindenmayer JP. The positive and negative syndrome scale (PANSS): rationale and standardisation. Br J Psychiatry Suppl 1989; 7: 59–67. [PubMed] [Google Scholar]
- 41. Guy W, editor. Clinician global impression (CGI). In: ECDEU assessment manual for psychopharmacology. US Department of Health, Education, and Welfare Publication (ADM). Rockville, MD: National Institute of Mental Health, 1976, pp. 76–338. [Google Scholar]
- 42. Association AP. Diagnostic criteria from DSM-IV-TR. Washington, DC: American Psychiatric Publishing, 2000. [Google Scholar]
- 43. Üstün TB, Kostanjsek N, Chatterji S, et al. Measuring health and disability: manual for WHO disability assessment schedule WHODAS 2.0. Geneva: World Health Organization, 2010. [Google Scholar]
- 44. Fervaha G, Agid O, Foussias G, et al. Toward a more parsimonious assessment of neurocognition in schizophrenia: a 10-minute assessment tool. J Psychiatr Res 2014; 52: 50–56. [DOI] [PubMed] [Google Scholar]
- 45. Pramyothin P, Khaodhiar L. Metabolic syndrome with the atypical antipsychotics. Curr Opin Endocrinol Diabetes Obes 2010; 17: 460–466. [DOI] [PubMed] [Google Scholar]
- 46. Fleischhacker WW, Uchida H. Critical review of antipsychotic polypharmacy in the treatment of schizophrenia. Int J Neuropsychopharmacol 2014; 17: 1083–1093. [DOI] [PubMed] [Google Scholar]
- 47. Wang M, Tong J-H, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res 2012; 138: 54–57. [DOI] [PubMed] [Google Scholar]
- 48. Boullart A, de Graaf J, Stalenhoef AF. Serum triglycerides and risk of cardiovascular disease. Biochim Biophys Acta 2012; 1821: 867–875. [DOI] [PubMed] [Google Scholar]
- 49. Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des 2010; 16: 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. de Bartolomeis A, Tomasetti C, Iasevoli F. Update on the mechanism of action of aripiprazole: translational insights into antipsychotic strategies beyond dopamine receptor antagonism. CNS Drugs 2015; 29: 773–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tuplin EW, Holahan MR. Aripiprazole, a drug that displays partial agonism and functional selectivity. Cur Neuropharmacol 2017; 15: 1192–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shapiro DA, Renock S, Arrington E, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology 2003; 28: 1400–1411. [DOI] [PubMed] [Google Scholar]
- 53. Deng C, Chen J, Hu C, et al. What is the mechanism for aripiprazole’s effect on reducing olanzapine-associated obesity? J Clin Psychopharmacol 2010; 30: 480–481. [DOI] [PubMed] [Google Scholar]
- 54. Bickerdike MJ. 5-HT2C receptor agonists as potential drugs for the treatment of obesity. Curr Top Med Chem 2003; 3: 885–897. [DOI] [PubMed] [Google Scholar]
- 55. Nonogaki K, Nozue K, Oka Y. Hyperphagia alters expression of hypothalamic 5-HT2C and 5-HT1B receptor genes and plasma des-acyl ghrelin levels in Ay mice. Endocrinology 2006; 147: 5893–5900. [DOI] [PubMed] [Google Scholar]
- 56. Wagner E, Kane JM, Correll CU, et al. Clozapine combination and augmentation strategies in patients with schizophrenia – recommendations from an international expert survey among the treatment response and resistance in psychosis (TRRIP) working group. Schizophr Bull 2020; 46: 1459–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mitsonis CI, Dimopoulos NP, Mitropoulos PA, et al. Aripiprazole augmentation in the management of residual symptoms in clozapine-treated outpatients with chronic schizophrenia: an open-label pilot study. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31: 373–377. [DOI] [PubMed] [Google Scholar]
- 58. Riedel M, Spellmann I, Schennach-Wolff R, et al. Effect of aripiprazole on cognition in the treatment of patients with schizophrenia. Pharmacopsychiatry 2010; 43: 50–57. [DOI] [PubMed] [Google Scholar]
- 59. Muscatello MRA, Bruno A, Pandolfo G, et al. Effect of aripiprazole augmentation of clozapine in schizophrenia: a double-blind, placebo-controlled study. Schizophr Res 2011; 127: 93–99. [DOI] [PubMed] [Google Scholar]
- 60. Englisch S, Esslinger C, Inta D, et al. Clozapine-induced obsessive-compulsive syndromes improve in combination with aripiprazole. Clin Neuropharmacol 2009; 32: 227–229. [DOI] [PubMed] [Google Scholar]
- 61. Schönfelder S, Schirmbeck F, Waltereit R, et al. Aripiprazole improves olanzapine-associated obsessive compulsive symptoms in schizophrenia. Clin Neuropharmacol 2011; 34: 256–257. [DOI] [PubMed] [Google Scholar]
- 62. Zheng W, Zheng Y-J, Li X-B, et al. Efficacy and safety of adjunctive aripiprazole in schizophrenia: meta-analysis of randomized controlled trials. J Clin Psychopharmacol 2016; 36: 628–636. [DOI] [PubMed] [Google Scholar]