Abstract
The optimal treatment strategy for patients with early prostate cancer (PCa) is unknown. We explored the feasibility of administering noni supplementation to modify gene expression of a relevant clinical signature in the prostate of men on active surveillance for PCa. A total of 6 participants with low-risk (n=5) to very low-risk (n=1) PCa who were candidates for active surveillance received 6200 mg/day of noni in capsule form for 1 year; median age was 65.5 years (range, 58–75 years). Participants were tested for serum prostate-specific antigen (PSA) levels every 3 months. At 12 months, they underwent a repeat transrectal ultrasound-guided prostate biopsy. These biopsy samples were queried for expressing 12 key genes and rates of apoptosis, angiogenesis, and proliferation. The primary outcome was the change in expression of the 12 genes that comprise the Oncotype DX prostate cancer test from baseline to 12 months of noni supplementation. Noni was well tolerated, with only 1 participant reporting side effects of grade 2 diarrhea, requiring a drug holiday of 7 days. Median serum PSA slightly increased from 7.1 ng/mL (4.4–9.7 ng/ mL) prior to therapy to 7.9 ng/mL (5.7–10.2 ng/mL) on therapy. Changes were observed in the expression levels of several genes, including FAM13C, KLK2 (associated with the androgen pathway), and GSTM2 (associated with cellular organization) at 12 months. Noni supplementation was associated with favorable clinical parameters, including stable serum PSA among most patients and no evidence of tumor on repeat biopsy, and correlated with modulation of numerous genes and proteins.
Keywords: Prostate Cancer, Active Surveillance, Noni
Introduction
Prostate cancer (PCa) is the most common malignancy affecting men, with an estimated 191 930 cases per year in the United States.1 Despite the high incidence, disease-related mortality statistics are quite favorable compared to other cancers. For example, fewer than one-third of men diagnosed with PCa will die of the disease, making it considerably less lethal than lung and colorectal cancers, which rank first and second respectively in terms of cancer-related deaths.1 Prostate-specific antigen (PSA) is a serum-based biomarker used for PCa screening and detection. The high proportion of PSA-detected cancers in current clinical practice is mainly responsible for the stage migration since patients with early PCa are now diagnosed at a lower tumor stage and grade compared to PCa patients 1 or 2 decades ago.2 Although PSA testing may allow for earlier curative therapy of potentially life-threatening disease; it also identifies a large group of patients with a relatively low risk of disease progression or relapse in whom radical therapy may be unnecessary and harmful due to its associated morbidity and costs.3 Thus, the optimal treatment strategy for these patients with early PCa (ie, very low-risk and low-risk disease) is unknown.
Morinda citrifolia (noni), a traditional medicine of Native Hawaiians, Other Pacific Islanders, and Asian populations, has been used to treat various diseases for centuries.4 Previously, noni has been reported to have significant antioxidant activity due to asperulosidic acid (an iridoid glycoside), damnacanthal (an anthraquinone), and scopoletin (a coumadin).5 These compounds have been shown in vitro cellular and molecular biologic studies to inhibit cancer development and progression. Specifically, asperuloside was reported to suppress tumor-promoting phorbal ester (TPA)- or epidermal growth factor (EGF)-induced cell transformation and associated activating protein-1 (AP-1) transcription factor activity6; damnacanthal, which has strong tyrosine kinase-inhibitory effects, can support apoptosis, and has been shown to induce normal phenotypes in ras-transformed cells7; and scopoletin possesses antiproliferative effects.8
Therefore, based on the above data, we set out to conduct a case study in which we administered oral noni to test the hypothesis that noni supplementation would modify gene expression of a relevant clinical signature in the prostate of men on active surveillance for PCa.
Methods
Participants
Patients from 2 healthcare facilities (in Honolulu and Hilo, HI) with very low risk (<5% risk of disease relapse after primary treatment, criteria; cT1c, Gleason ≤6, PSA <10 ng/mL, fewer than 3 positive biopsy cores ≤50% cancer in any biopsy core, PSA density <0.15 ng/mL/g) or low risk (10% risk of disease relapse after primary treatment, criteria; cT1-2a, Gleason ≤6, PSA <10 ng/mL)9 prostate cancer, who were considering active surveillance were approached for study enrollment. Gleason score is the most common prostate cancer grading system used and is interpreted from a core biopsy, which is a procedure where a needle is passed through the skin to take a sample of tissue from a mass or lump. If a core biopsy is noted to harbor cancer, it is considered a positive core for cancer. Also, 2 key pre-study criteria must have been met. First, the commercial Oncotype DX prostate cancer test (Genomic Health, Redwood City, CA) had to have been performed and confirmed very low-risk or low-risk disease. Second, pre-study prostate multiparametric MRI must have demonstrated no extraprostatic disease and be devoid of PIRADS >3 lesions. All participants signed an institutional review board approved informed consent. The study (NCT02648919) was approved by Western IRB (WIRB #20151535) and received an Investigational New Drug application approval from the Food and Drug Administration.
Treatment
Participants in the study were enrolled at a single dose level of noni (620 mg tablets, Healing Noni, Hilo, HI) administered 3 times a day as 3 capsules with breakfast, 3 capsules with lunch, and 4 capsules with dinner, for a total daily dose of 6200 mg/day. This dose was determined by a previous phase 1 study in cancer participants to be safe.10,11 All participants were evaluated for adverse events (AEs). AEs and laboratory values were graded using the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 5.0. Dose de-escalation was not allowed. If an AE was reported, noni was held until resolution of the AE. If the hold of noni was >21 days, the participant was removed from the study. Also, noni was also permanently discontinued for grade 3 and 4 AEs, unless not related to therapy.
During the study, participants were asked to self-administer the study medication, avoid consumption of additional noni, and complete daily drug logs to record study agent intake and concomitant medications. Participants were expected to maintain ≥85% compliance with study agent intake; comply with dietary, medication, and supplement restrictions; and complete drug logs to the best of their ability. Participants were interviewed at quarterly clinic visits, and pill counts were performed to verify compliance with study requirements.
Follow-up
Participants were seen quarterly in urology clinics, and serum PSA levels were measured. Serum for blood chemical, hematological, and clotting analyses were obtained at baseline and study conclusion (month 12). Furthermore, at 12 months, the participants underwent a repeat transrectal ultrasound-guided prostate biopsy (minimal 10 cores), similar to the original diagnostic biopsy. Disease progression was defined as an increase in Gleason score, increased number of positive cores, or increased tumor volume.
Gene Expression
Gene expression allows the measurement of expression of certain genes associated with cancers ability to grow. Briefly, histologic evaluation of hematoxylin and eosin (H&E) pathology sections confirmed that the core prostate biopsy tissue used to assess gene expression contained viable tumor. Cores between 3 and 10 mg from each participant were homogenized with a QIAGEN TissueRuptor before total RNA was extracted with the QIAGEN RNeasy Mini kit. The resulting RNA was quality checked with an Agilent Bioanalyzer. From the RNA, cDNA was synthesized using ReverTra Ace™ qPCR RT Master Mix with gDNA Remover (Toyobo, Osaka, Japan). Real-time PCR was performed with CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). Primers for the following 12 targets: androgen pathway (AZGP1, KLK2, SRD5A2, and FAM13C), cellular organization (FLNC, GSN, TPM2, and GSTM2), proliferation (TPX2), and stromal response (BGN, COL1A1, and SFRP4) along with 5 reference genes12 for normalization are reported in Supplemental Methods.
Cytokine and Serum Scopoletin Levels
Cytokines, small secreted proteins released by cells allowing interactions and communications with other cells, were quantified in the serum of study participants. Many cytokines are related to inflammation, which is common in cancer, while noni is known to have anti-inflammatory properties. Whole blood was collected in sodium heparin tubes at baseline, 3, 6, 9, and 12 months. Within 2 hours, the blood was processed, separating buffy coat from serum. Both were snapped frozen and stored at -800C until further analysis. Then, 2 aliquots of serum from each participant at each time point were thawed, 1 aliquot was used for cytokine testing, and the other for scopoletin testing. Serum samples were profiled for 22 cytokines using a customized Luminex assay (Cat # FCSTM18-22, R&D Systems, Minneapolis, MN) as per assay instructions. Measurements were performed in duplicate using a Luminex 200 instrument (Luminex Corporation, Austin, TX) and were analyzed using a standard curve for each molecule (xPONENT® software, Luminex Corporation). Serum scopoletin, a metabolite of noni and a surrogate to quantitatively assess drug compliance, was measured as previously reported.11 Briefly, serum samples collected at baseline and 9 months were subjected to High Performance Liquid Chromatography with Electrochemical Detection (HPLC-ECD) as an objective marker of compliance to noni therapy.
Multiplex Immunofluorescence Staining
Multiplex immunofluorescence staining allows the analysis of several key molecules, in this case, CD31, cleaved caspase-3, and Ki-67, which are surrogates for angiogenesis, apoptosis, and cellular proliferation, respectively, in a single biopsy sample from a participant. Multiplex immunofluorescence staining was performed automatically in the DISCOVERY ULTRA system (Ventana Medical Systems, Inc., Tucson, AZ) using individual tyramide signal amplification (TSA)-conjugated fluorophores to detect various targets. Paraffin-embedded tissue samples were stained with a prediluted mouse monoclonal anti-CD31 (Cat # 760-4378, Roche Diagnostics, Indianapolis, IN), a 1:2000 dilution of rabbit monoclonal anti-cleaved caspase-3 antibody (Cat # 9661, Cell Signaling, Danvers, MA), a prediluted rabbit monoclonal anti-Ki-67 antibody (Cat # 790-4286, Roche Diagnostics) and a prediluted mouse anti-cytokeratin 8/18 antibody (Cat # 760-4344, Roche Diagnostics) as previously reported.13 Secondary OmniMap antibodies were purchased from Roche Diagnostics. The tyramide signal amplification (TSA) was purchased from Perkin Elmer, and the polymer amplification system was obtained from VECTOR labs. The immunofluorescent staining was performed with DISCOVERY RED610 kit (cleaved caspase-3: #760-245, Ventana Medical Systems, Inc.), DISCOVERY FAM kit (cytokeratin 8/18: #760-243, Ventana Medical Systems, Inc.), DISCOVERY DCC Kit (Ki-67: # 760-240, Ventana Medical Systems, Inc.), and DISCOVERY Rhodamine 6G Kit (CD31: # 760-244, Ventana Medical Systems, Inc.). Lastly, the slides were mounted in ProLong™ Gold Antifade Mountant with DAPI (Life Technologies, Carlsbad, CA). The slides were digitized on the high-resolution Tissue- FAXS 200 scanner system (Tissuegnostics, Vienna, Austria) with 20x magnification, and images were sequentially analyzed with NIH ImageJ.
Power Analysis
The study was designed to test feasibility (ie, whether subjects could be identified, recruited, followed, and samples collected for correlative studies); therefore, a single-arm design was chosen over a 2-arm with placebo control. The primary outcome was the change in expression of the 12 genes that comprise the Oncotype DX prostate cancer test from baseline to 12 months of noni supplementation. This prospective case study was to generate high-quality preliminary data and demonstrate feasibility. Pearson correlation coefficient tests with a 5% significance level (2-sided) and 80% power allows sample sizes of 6 participants to detect a difference between the null hypothesis correlation of 0 and the alternative hypothesis correlation of 0.76.14 The maximum width of the 95% confidence interval for proportions associated with sample sizes of 6 is 0.63.15
Results
From January 1, 2016, to December 2017, 6 of 10 participants who were screened were enrolled. The mean age was 66.6 years, 83% (n=5) had low-risk cancer, and 17% (n=1) had very low-risk cancer. All 6 participants completed the 12-month intervention period. Baseline characteristics of the participants are depicted in Table 1. Noni supplement was well tolerated with only 1 participant (participant #5) experiencing a treatment-related AE (grade 2 diarrhea), which improved with a 7-day drug discontinuance (data not shown). Compliance with the daily administration of 6200 mg of noni was generally excellent based on the drug logs and returned drug vials, with a mean of <3% of doses missed per month per participant. Though we collected serum throughout the study for analysis, the 9-month serum was more representative of drug compliance as a noncompliant participant may begin to take the drug before the 12-month final visit, and thus reported. As such, the median serum scopoletin was 5.1 ng/mL (range, <0.5–15.0 ng/mL) at 9 months, up from a median of 2.1 ng/mL (range, <0.5–3.2 ng/mL) at baseline. One participant (participant #6) may have been noncompliant despite the drug logs and visual inspection of drug vials.
Table 1.
Patient Demographics and Clinical Presentation (N=6)
| Subject ID | Age | Race | Serum PSA | Clinical T Stagea | Risk Category | MRIb | GPSc |
|---|---|---|---|---|---|---|---|
| Ppt 1 | 62 | White | 4.4 | T2a | Low | PIRADS 3, max 13 mm without ECE | 37 |
| Ppt 2 | 74 | Asian | 5.4 | T1c | Low | PIRADS 3, max 11 mm without ECE | 34 |
| Ppt 3 | 75 | Native Hawaiian/ Other Pacific Islander | 7.3 | T1c | Very low | PIRADS 3, max 14 mm without ECE | 17 |
| Ppt 4 | 62 | White | 9.7 | T1c | Low | PIRADS 3, max 10 mm without ECE | 36 |
| Ppt 5 | 58 | Unknown | 6.9 | T1c | Low | PIRADS 3, max 12 mm without ECE | 38 |
| Ppt 6 | 69 | Asian | 7.9 | T1c | Low | PIRADS 3, max 19 mm without ECE | 39 |
Abbreviations: ECE, extracapsular extension; GPS, genomic prostate score; MRI, magnetic resonance imaging; PIRADS, Prostate Imaging–Reporting and Data System; PSA, prostate-specific antigen.
Based on the results of the digital rectal exam.
PIRADS score based on size of lesion(s) and whether ECE is noted on MRI.
Analysis of initial prostate biopsy.
Only 1 of 6 participants (participant #1) experienced an overall increase in PSA levels of >30%, though his total PSA was still below 10 ng/ml, the other participants showed stable PSA levels (+/−15% from baseline; Figure 1). Though rising serum PSA levels are problematic, serum PSA is not used as a marker of treatment response except when definitive therapy (eg, prostatectomy, radiation therapy) is rendered.16 No abnormalities were found in chemical, hematological, and clotting analyses in the 6 participants (data not shown).
Figure 1.
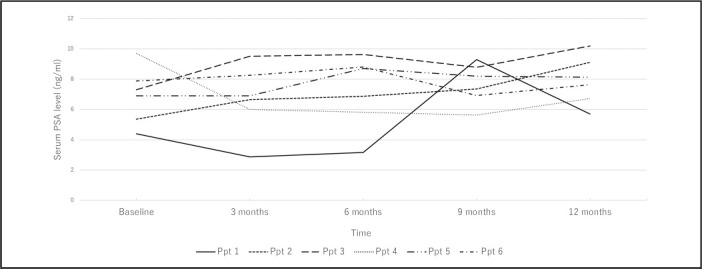
Serum PSA Spider Plot of Participants while Receiving Noni Treatment
All 6 participants underwent a repeat biopsy at 12 months, noted as post-therapy biopsy (Figure 2). At the time of biopsy, the palpable disease was not appreciated in any participant. Two participants did not have histologic evidence of cancer on biopsy, while 1 participant showed atypia. It has been reported that over 35% of men on active surveillance may have a negative prostate biopsy when a 12-month prostate biopsy is performed.17 However, in the current study, 2 participants had increases in their Gleason score (6 to 7). Both of these participants also showed an increase in the absolute number of positive cores and an increase in the percentage of positive cores (Figure 2).
Figure 2.
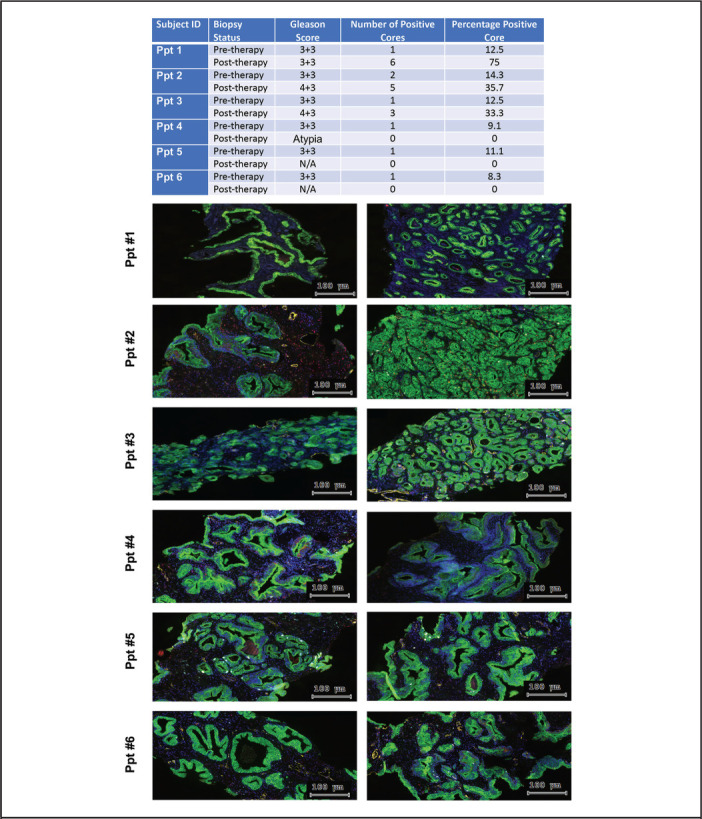
Histopathological Changes of Prostate Tissue Associated with Noni Treatment. The slides were digitized on the high-resolution TissueFAXS 200 scanner system (Tissuegnostics, Vienna, Austria). Right column, pre-therapy biopsy and left column post-therapy biopsy. 20x magnification immunofluorescence images of prostate tissues show staining with DAPI (blue), CD31 (angiogenesis marker, yellow), Ki67 (proliferation marker, sky blue), cleaved caspase 3 (apoptosis marker, red), and CK8/18 (epithelial marker, green)
Furthermore, though underpowered, there was no significant difference between pre-treatment and post-treatment levels of markers of microvessel density (MVD also termed “angiogenesis”; CD31), apoptosis (cleaved caspase-3), and proliferation (Ki-67) (data not shown). Similarly, immunofluorescent staining of the pre-treatment and post-treatment biopsies depicts the spatial relationship between MVD, apoptosis, and proliferation (Figure 2).
Using RT-PCR, the gene expression of 12 transcripts, which comprise the Oncotype DX prostate cancer test, was queried. The alteration of androgen, cellular organization, proliferation, and stromal response pathways after noni therapy is illustrated with a heatmap (Figure 3). Two participants showed increased expression of FAM13C, KLK2 (associated with androgen pathway), and GSTM2 (associated with cellular organization) over the study period. However, no participants had reductions in common genes. Furthermore, serum analysis showed increases in IL6 and CXCL10 in 2 participants while on treatment, while most other cytokines were reduced while on treatment (Figure 4).
Figure 3.
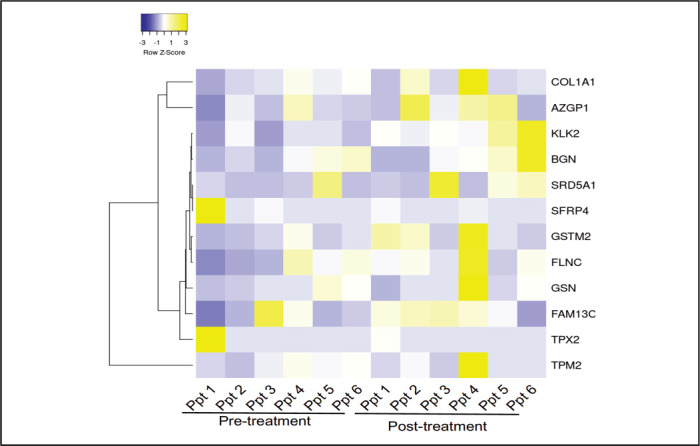
Heatmap Illustrating Differences in the 12-Transcript Expression Among the Six Participants. Cluster analysis of RT-PCR data profiling in six participants. Each row represents one of the 12 transcripts present in Oncotype Dx Prostate Cancer test and each column a participant. The scale represents standard deviations from the mean after a Z-transformation of signal values of a gene across all samples. Yellow represents a higher level of gene expression and blue a lower level, relative to the mean across all samples for each gene.
Figure 4.
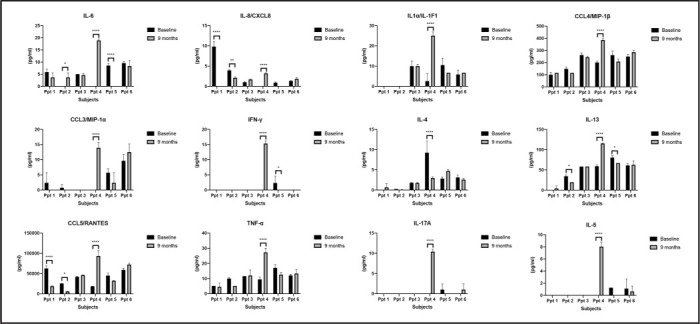
Changes in Serum Cytokine Profiles following Noni Treatment. A panel of cytokines were queried in serum samples collected at 9 months and compared to samples collected at baseline using the 22-plex customized human cytokine Luminex assay (R&D Systems).
Discussion
Identification of the suitable patient population is fundamental to the development of an appropriate treatment strategy. For patients with PCa, the outcome after therapy has been shown to be dependent on tumor stage, serum PSA, and Gleason score (grade).18–20 Because of this association, PCa can be stratified as very low risk (<5% risk of disease relapse after primary therapy, criteria; cT1c, Gleason <6, PSA <10 ng/mL, fewer than 3 positive biopsy cores <50% cancer in any core, PSA density <0.15 ng/mL/g); low risk (10% risk of disease relapse after primary therapy, criteria; cT1-2, Gleason <6, PSA <10 ng/mL); intermediate risk (25% risk of disease relapse after primary therapy, criteria; cT2b-2c, Gleason 7, PSA 10–20 ng/mL) and high risk (>50% risk of disease relapse after primary therapy, criteria; cT3a, Gleason 8–10, PSA >20 ng/mL). Thus, it is important to tell patients with very low-risk and low-risk PCa that long-term survival is possible with conservative management. Despite this information, 56% to 91% of very low-risk or low-risk PCa are reluctant only to surveil their PCa.21 Thus, an opportunity exists to incorporate a therapeutic option, with minimal side effects, into the armamentarium of physicians caring for patients with very low-risk and low-risk PCa.
We report that the administration of noni in men with very low-risk or low-risk PCa was feasible and well tolerated in most patients, which is aligned with previous studies from our group.10,11 Critical molecular modulation associated with treatment includes reduced tumor vasculature (MVD) in Ppt 3 (P =.013) and 5 (P =.00011), reduced cellular proliferation in post-treatment decreased in Ppt 2 (P =.070) and 3 (P =.0036) and increased apoptosis, program cell death in Ppt 2 (P =.074). Furthermore, we noted modification in gene expression profile while on treatment. Specifically, FAM13C and KLK2 (markers of androgen metabolism) and GSTM2 (a marker of cellular organization) were increased over time in 2 participants, which may hint at noni-induced changes. Identifying a difference in gene expression with the initiation of noni will support the design of future studies with adequate power.
Diet has long been associated with PCa etiology.22 The most provocative data supporting the influence of dietary factors on the incidence of PCa come from international studies and studies of immigrant populations in the United States. For example, historically, the incidence of PCa in Japan has been extremely low. However, as Japanese men migrate to Hawai‘i or the US mainland and subsequently adopt western culture, their incidence of PCa rapidly approaches that of whites.23,24 The diets of native Japanese and other Asians are rich in fiber and low in saturated fat. These cultural differences may contribute to the lower rates of clinical PCa in Asia compared to Northern Europe and North America.
Furthermore, the influence of dietary interventions and their role in prevention and treatment have been highly speculated. Recently, the Men's Eating and Living (MEAL) Study (CALGB 70807 [Alliance]), a randomized clinical trial conducted at 91 US urology and medical oncology clinics that enrolled 478 men aged 50 to 80 years with biopsy-proven very low-risk or low-risk PCa, was reported. Patients were randomized to a counseling behavioral intervention promoting 7 or more daily vegetable servings or a control group. Among 478 patients randomized (mean age [SD], 64 [7] years; mean PSA level [SD], 4.9 [2.1] ng/mL), 443 (93%) eligible patients were included in the primary analysis. There were 245 progression events (intervention: 124; control: 121) with no significant difference in time to progression (unadjusted hazards ratio, 0.96 [95% confidence interval {CI}, 0.75–1.24]; adjusted hazard ratio, 0.97 [95% CI, 0.76 –1.25]) between the 2 groups.25 We anxiously await novel correlative analysis from this study as it could shed light on the association between diet and cancers.
In our case study, we explored a well-known medicinal fruit from the Pacific, Morinda citrifolia or noni, in men with very low-risk or low-risk PCa. We treated 6 men with 6200 mg per day for 12 months. Our dose regimen was based on a previous phase 1 study, which we conducted.10,11 Overall, the treatment was well tolerated. Serum PSA levels at study entry were not significantly different after 12 months of therapy (median, 7.1 ng/mL, [range, 4.4–9.7] versus median, 7.9 ng/mL [range, 5.7–10.23], respectively). Furthermore, 50% of participants had a negative prostate biopsy at 12 months.
Currently, gene expression CLIA assays (Oncotype DX®, Genomic Health, Redwood City, CA and Prolaris®, Myriad, Salt Lake City, UT) are available to assist clinicians in determining the likelihood of more aggressive cancer26,27 or determining the likelihood of having metastatic disease23 and have been incorporated into current National Comprehensive Cancer Network (NCCN) guidelines.9 Thus, we sought to study the ability of noni supplementation to modify mRNA expression levels of the genes related to determining the likelihood of aggressive disease. Initially, Genomic Health was to perform the analysis on baseline tissue and then tissue obtained at 12-month biopsy. Unfortunately, Genomic Health could not run the assay on 12-month prostate biopsy samples in which active cancer was not identified. Even though Genomic Health performed GPS assay on all baseline prostate biopsies, our laboratory performed RT-PCR on 12-month and baseline (comparator) tissue. As evident in Figure 3, noni treatment was associated with increases in expression of FAM13C (Ppt 1 and 4), KLK2 (androgen signaling, Ppt 2 and 4), and GSTM2 (cellular structure, Ppt 1 and 3), and increases in serum cytokines IL6 and CXCL10.
Scopoletin is 1 of many metabolites of noni. Perhaps its presence in the blood occurs in patients who can better metabolize noni into its active components. A similar phenomenon has been reported with isoflavone supplements, with higher levels of free serum equol reported in patients who responded to isoflavone therapy.28 If effective metabolism is the key, then serum scopoletin levels may be a valuable surrogate to monitor molecular changes associates with noni.
There are several weaknesses of this study. First, the study is only a single-arm, pilot study, demonstrating the feasibility of deploying a dietary intervention to this population. Second, we could not verify any positive or negative clinical or molecular trends due to noni ingestion, mainly due to small sample size and interpatient variability.
We report in a case study that long-term noni treatment in men with very low-risk or low-risk PCa was feasible to perform and was associated with favorable gene expression changes. Consideration should be given for additional testing of noni in human clinical trials involving men with very low-risk and low-risk PCa.
Supplementary Method
Preamplification
The cDNA was preamplified using SsoAdvanced™ PreAmp Supermix (Bio-Rad Laboratories, Hercules, CA) and PrimePCR™ PreAmp for SYBR® Green Assays for each target gene according to the manufacturer's instructions (Supplementary Table 1). The preamplification was performed in a thermal cycler under the following conditions: 95°C for 10 min followed by 8 cycles of 95°C for 15 sec and 60°C for 4 min.
Quantitative Reverse Transcriptase-PCR
The amplified product was mixed with iTaq Universal SYBR Green Supermix, and 20 μl of the PCR reaction mix was applied into each well. qPCR was performed for 45 cycles in a CFX96 Touch Real-Time PCR Detection System (Bio-Rad) under the following conditions: enzyme activation (95°C, 15 min), amplification (95°C for 20 sec and 60°C for 45 sec; 45 cycles in total), and cooling (40°C, 5 sec). The level of expression was calculated with the crossing point (Cp) method. All gene assays were measured in duplicate.
Supplementary Table 1.
List of Primers
| Genes | Unique Assay ID |
|---|---|
| Target genes | |
| AZGP1 | qHsaCED0056827 |
| BGN | qHsaCID0014636 |
| COL1A1 | qHsaCED0043248 |
| FAM13C | qHsaCID0010924 |
| FLNC | qHsaCID0012034 |
| GSN | qHsaCID0021416 |
| GSTM2 | qHsaCED0038361 |
| KLK2 | qHsaCID0014394 |
| SFRP4 | qHsaCID0022180 |
| SRD5A1 | qHsaCID0012570 |
| TPM2 | qHsaCED0002140 |
| TPX2 | qHsaCID0016024 |
| Reference genes | |
| ARF1 | qHsaCED0002471 |
| ATP5E | qHsaCID0038009 |
| CLTC | qHsaCID0017813 |
| GPS1 | qHsaCED0038152 |
| PGK1 | qHsaCED0042912 |
Acknowledgment
The following cores for services provided: Analytical Biochemistry Core University of Hawai‘i Cancer Center, Biostatistics Core University of Hawai‘i Cancer Center, Molecular and Cellular Immunology Core University of Hawai‘i, and Biobank and Translational Research Core Cedars-Sinai Medical Center.
Abbreviations and Acronyms
- AE
adverse events
- AP-1
activating protein-1
- EGF
epidermal growth factor
- MVD
microvessel density
- NCCN
National Comprehensive Cancer Network
- PIRADS
Prostate Imaging–Reporting and Data System
- PSA
prostate-specific antigen
- RT-PCR
reverse transcription polymerase chain reaction
- TPA
tumor-promoting phorbal ester
- TSA
tyramide signal amplification
Conflict of Interest
None of the authors identify any conflict of interest.
Disclosure Statement
This work was supported by a research grant from NIH/NCI, R01 CA1988887 (CJR).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70((1)):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Ohmann EL, Loeb S, Robinson D, Bill-Axelson A, Berglund A, Stattin P. Nationwide, populationbased study of prostate cancer stage migration between and within clinical risk categories. Scand J Urol. 2014;48((5)):426–435. doi: 10.3109/21681805.2014.892150. Epub 2014 Mar 11. [DOI] [PubMed] [Google Scholar]
- 3.Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65((6)):1046–1055. doi: 10.1016/j.eururo.2013.12.062. Epub 2014 Jan 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClatchey W. From Polynesian healers to health food stores: changing perspectives of Morinda citrifolia (Rubiaceae) Integr Cancer Ther. 2002;1((2)):110–120. doi: 10.1177/1534735402001002002. discussion 120. [DOI] [PubMed] [Google Scholar]
- 5.Akihisa T, Matsumoto K, Tokuda H, et al. Anti-inflammatory and potential cancer chemopreventive constituents of the fruits of Morinda citrifolia (Noni) J Nat Prod. 2007;70((5)):754–757. doi: 10.1021/np068065o. Epub 2007 May 5. [DOI] [PubMed] [Google Scholar]
- 6.Liu G, Bode A, Ma WY, Sang S, Ho CT, Dong Z. Two novel glycosides from the fruits of Morinda citrifolia (noni) inhibit AP-1 transactivation and cell transformation in the mouse epidermal JB6 cell line. Cancer Res. 2001;61((15)):5749–5756. [PubMed] [Google Scholar]
- 7.Hiwasa T, Arase Y, Chen Z, et al. Stimulation of ultraviolet-induced apoptosis of human fibroblast UVr-1 cells by tyrosine kinase inhibitors. FEBS Lett. 1999;444((2-3)):173–176. doi: 10.1016/s0014-5793(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 8.Liu XL, Zhang L, Fu XL, Chen K, Qian BC. Effect of scopoletin on PC3 cell proliferation and apoptosis. Acta Pharmacol Sin. 2001;22((10)):929–933. [PubMed] [Google Scholar]
- 9.Carroll PR, Parsons JK, Andriole G, et al. NCCN guidelines insights: prostate cancer early detection, Version 2.2016. J Natl Compr Canc Netw. 2016;14((5)):509–519. doi: 10.6004/jnccn.2016.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Issell BF, Gotay CC, Pagano I, Franke AA. Using quality of life measures in a Phase I clinical trial of noni in patients with advanced cancer to select a Phase II dose. J Diet Suppl. 2009;6((4)):347–359. doi: 10.3109/19390210903280272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Issell BF, Franke A, Fielding RM. Pharmacokinetic study of Noni fruit extract. J Diet Suppl. 2008;5((4)):373–382. doi: 10.1080/19390210802519671. [DOI] [PubMed] [Google Scholar]
- 12.Knezevic D, Goddard AD, Natraj N, et al. Analytical validation of the Oncotype DX prostate cancer assay - a clinical RT-PCR assay optimized for prostate needle biopsies. BMC Genomics. 2013;14:690–0. doi: 10.1186/1471-2164-14-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akfirat C, Zhang X, Ventura A, et al. Tumour cell survival mechanisms in lethal metastatic prostate cancer differ between bone and soft tissue metastases. J Pathol. 2013;230((3)):291–297. doi: 10.1002/path.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guenther WC. Desk calculation of probabilities for the distribution of the sample correlation coefficient. The American Statistician. 1977;31((1)):45–48. [Google Scholar]
- 15.Fleiss J, Levin B, Paik M. Statistical Methods for Rates and Proportions. 3rd ed. John Wiley & Sons; 2003. [Google Scholar]
- 16.Rosser CJ. Prostate cancer--to screen, or not to screen, is that the question? BMC Urol. 2008;8:20–0. doi: 10.1186/1471-2490-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anai S, Nakamura K, Chang MN, Pendleton J, Yacoub S, Rosser CJ. The feasibility of expectant management with inner-city men with newly diagnosed localized prostate cancer. J Health Care Poor Underserved. 2008;19((1)):164–170. doi: 10.1353/hpu.2008.0024. [DOI] [PubMed] [Google Scholar]
- 18.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280((11)):969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 19.Freedland SJ, Terris MK, Csathy GS, et al. Preoperative model for predicting prostate specific antigen recurrence after radical prostatectomy using percent of biopsy tissue with cancer, biopsy Gleason grade and serum prostate specific antigen. J Urol. 2004;171((6 Pt 1)):2215–2220. doi: 10.1097/01.ju.0000124463.13319.0a. [DOI] [PubMed] [Google Scholar]
- 20.Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90((10)):766–771. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 21.Fang AM, Glaser ZA, Rais-Bahrami S. Increasing the use of active surveillance for prostate cancer in younger men. Cancer. 2019;125:3292–3295. doi: 10.1002/cncr.32333. [DOI] [PubMed] [Google Scholar]
- 22.Pienta KJ, Esper PS. Risk factors for prostate cancer. Ann Intern Med. 1993;118((10)):793–803. doi: 10.7326/0003-4819-118-10-199305150-00007. [DOI] [PubMed] [Google Scholar]
- 23.Haenszel W, Kurihara M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. J Natl Cancer Inst. 1968;40((1)):43–68. [PubMed] [Google Scholar]
- 24.Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63((6)):963–966. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsons JK, Zahrieh D, Mohler JL, et al. Effect of a behavioral intervention to increase vegetable consumption on cancer progression among men with early-stage prostate cancer: The meal randomized clinical trial. JAMA. 2020;323((2)):140–148. doi: 10.1001/jama.2019.20207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol. 2014;66((3)):550–560. doi: 10.1016/j.eururo.2014.05.004. Epub 2014 May 16. [DOI] [PubMed] [Google Scholar]
- 27.Oderda M, Cozzi G, Daniele L, et al. Cell-cycle progression-score might improve the current risk assessment in newly diagnosed prostate cancer patients. Urology. 2017;102:73–78. doi: 10.1016/j.urology.2016.11.038. Epub 2016 Nov 25. [DOI] [PubMed] [Google Scholar]
- 28.Pendleton JM, Tan WW, Anai S, et al. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer. 2008;8:132. doi: 10.1186/1471-2407-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]


