Abstract
The global COVID-19 pandemic remains challenging with efforts for community vaccination the primary strategy to control transmission and disease sequalae in the mid to long term. While several candidate vaccines have been approved for use, there is an ongoing discussion regarding potential vaccine-related adverse events. Notably, thrombotic thrombocytopaenia has been reported following ChAdOx1 nCov-19 (AstraZeneca) vaccination. We report the first known case of takotsubo (stress) cardiomyopathy 4 days after administration of the ChAdOx1 nCoV-19 vaccine in a 72-year-old man. While this condition remains one primarily seen in females, our case represents a new trigger that warrants careful consideration when assessing patients presenting with acute coronary syndromes following ChAdOx1 nCoV-19 vaccination.
Keywords: Heart failure, COVID-19, Drug interactions, Global Health, Vaccination/immunisation
Background
Vaccines remain the greatest hope in combating the global SARS-CoV-2 pandemic. To date, the Therapeutic Goods Administration of Australia has provisionally approved the use of the BNT162b2 (Pfizer–BioNTech) and ChAdOx1 nCov-19 (AstraZeneca) vaccines.
Case presentation
A 72-year-old man was admitted to our institution 4 days after receiving his first dose of the ChAdOx1 nCov-19 vaccine. Having been well prior to his vaccination, he experienced mild symptoms of fatigue and myalgias on the first day post vaccination that then developed into significant lethargy, low-grade fever, myalgias and arthralgias. On day 4 after vaccination, he also presented with retrosternal chest pain with associated dyspnoea and was referred to the emergency department. On clinical assessment, he was tachypnoeic with normal oxygen saturations, a heart rate of 99 beats/min and blood pressure of 130/65 mm Hg, and was afebrile with a temperature of 36·1°C. The 12-lead ECG showed sinus tachycardia with first degree and right bundle branch block without acute or dynamic ischaemic changes. Physical examination did not show any evidence of pulmonary oedema. His laboratory findings were significant for a serially elevated cardiac troponin I, from 32 ng/L to a peak of 1868 ng/L (reference value:<26 ng/L) and D-dimer level of 0·87 mg/L (reference value: <0·5 mg/L). All other laboratory investigations were normal, including a platelet count of 203 ×109/L (reference value: 150–400×109/L).
The patient had a history of ischaemic heart disease, undergoing elective coronary artery bypass grafting 14 years earlier (left internal mammary to left anterior descending artery and radial to first marginal artery), hypertension, hypercholesterolaemia, type 2 diabetes mellitus and ulcerative colitis. His ischaemic heart disease was asymptomatic and stable post-surgical revascularisation, on acetylsalicylic acid, angiotensin receptor antagonist and statin therapy. Serial transthoracic echocardiograms, including 5 weeks prior to vaccination, demonstrated completely normal left ventricular size and systolic function (ejection fraction: 60%), without regional wall motion abnormalities.
Investigations
As a result of dyspnoea and elevated D-dimer level, a CT angiography of the pulmonary arteries was performed and excluded any pulmonary embolism. A SARS-CoV-2 reverse-transcriptase PCR assay was negative. Given his premorbid history, the patient was managed for their acute coronary syndrome with dual antiplatelet therapy, including a P2Y12 antagonist.
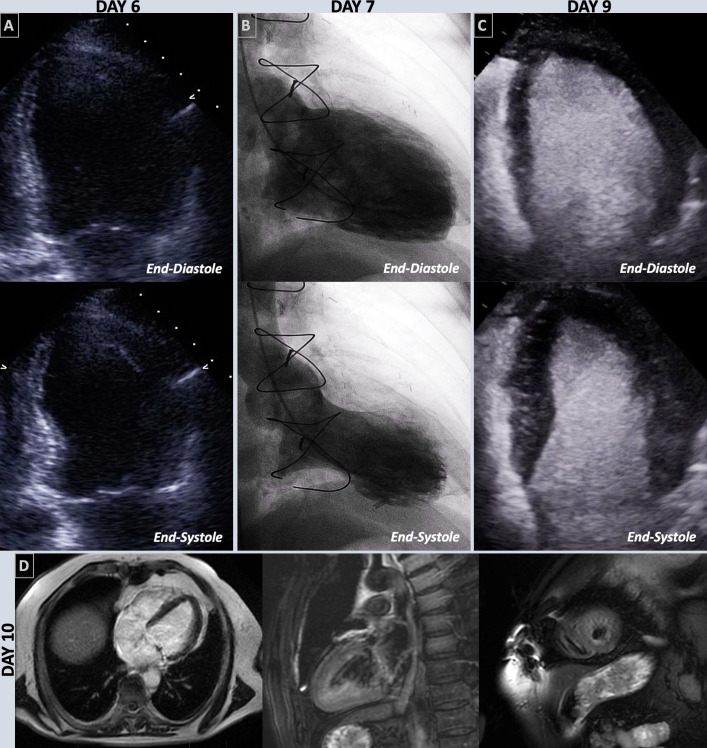
Transthoracic echocardiogram (6 days after vaccination) revealed new moderately severe segmental systolic dysfunction with an estimated ejection fraction of 37%–39% (reference value: >50%) with hyperdynamic base, akinesis of the mid-distal left ventricular segments and severe hypokinesis of the apical cap with apical ballooning, consistent with takotsubo cardiomyopathy (figure 1A, video 1). The differential diagnoses that we considered included possible left anterior descending artery ischaemia or myocarditis.
Figure 1.
End-diastolic and end-systolic focused view of the left ventricle on day 6 echocardiography (A), left ventriculography on day 7 (B) and contrast-enhanced echocardiography on day 9 (C). Cardiac MRI performed on day 10 in the horizontal, and vertical long axis, and short axis stack of the apex post gadolinium contrast (D).
Video 1.
Coronary angiography
Subsequent coronary angiography on day 9 demonstrated patent grafts, no new flow limiting coronary disease and left ventriculography consistent with transthoracic echocardiogram finding with apical ballooning and reduced cardiac function in the antero-apical regions (figure 1B, video 2). His left ventricular end-diastolic pressure was elevated at 22 mm Hg (reference range: ≤11 mm Hg).
Video 2.
Further imaging: contrast enhanced echocardiograph and cardiac MRI
Repeat contrast-enhanced transthoracic echocardiography was performed (day 9 after vaccination), demonstrating complete resolution of systolic dysfunction and wall motion abnormalities (figure 1C, video 3). Cardiac MRI performed on day 10 showed normalisation of left ventricular systolic function with an ejection fraction of 52% (reference range: ≥50%). The tissue characterisation with T2 weighted imaging revealed oedema involving the subendocardium of the apical segments sparing the basal segments (figure 1D; online supplemental files 1–3). Apical ballooning and akinetic segments seen previously on echocardiogram had resolved. There was no infarct-like hyperenhancement on late gadolinium enhancement to indicate fibrosis. These features supported the diagnosis of recovered takotsubo cardiomyopathy.
Video 3.
bcr-2021-246580supp001.mp4 (3.7MB, mp4)
bcr-2021-246580supp002.mp4 (3.2MB, mp4)
bcr-2021-246580supp003.mp4 (2.9MB, mp4)
Outcome and follow-up
With the exclusion of coronary ischaemia and myocarditis and confirmation of the diagnosis of takotsubo cardiomyopathy, our patient received appropriate introduction and titration of therapy. He was discharged on day 10, following a 7-day admission to our coronary care unit, with complete resolution of dyspnoea, chest pain and non-specific fatigue and myalgias.
Discussion
Takotsubo cardiomyopathy is an acute and transient syndrome characterised by both systolic and diastolic left ventricular dysfunction with new regional wall-motion abnormalities extending beyond the distribution of a single coronary artery.1 While its pathogenesis is incompletely understood, catecholamine excess mediated myocardial stunning is favoured, with excess release precipitated by a physical or emotional triggering event.2 Primarily seen in ageing women, the diagnosis of takotsubo cardiomyopathy is challenging and relies on the recognition of presentation and triggers, characteristic imaging and exclusion of acute coronary syndrome and myocarditis.3
The clinical course and investigations in our patient support the diagnosis of takotsubo cardiomyopathy. Not only occurring soon after a triggering event, the resolution of this transient cardiomyopathy between day 4 and day 9, as demonstrated on repeat contrast-enhanced transthoracic echocardiography, is also consistent with this diagnosis. For our patient, with the exception of the ChAdOx1 nCov-19 vaccine, no physical or emotional trigger was identified, although we accept this cannot establish causality. Furthermore, cardiac MRI supported the exclusion of an acute ischaemic event or myocarditis, due to the absence of late gadolinium enhancement.3
Noteworthy is the speed of recovery of our patient’s myocardial dysfunction, evident with normalisation of cardiac function on both serial echocardiography and on cardiac MRI. Recovery of takotsubo cardiomyopathy 9 days after triggering event and 5 days after presentation is atypical, with median recovery time up to 60 days.1 It is unclear if the rapidity of our patient’s recovery could be a feature of possible ChAdOx1 nCov-19 induced takotsubo cardiomyopathy and no doubt future cases may help shed more light onto the pathogenesis.
Vaccination is not classically associated with takotsubo cardiomyopathy. Three previous case reports exist, one recently reported case associated with the mRNA-1273 or Moderna SARS-CoV-2 vaccine,4 and two in which influenza vaccination triggered this syndrome.5 6 As such, the mechanism by which the ChAdOx1 nCov-19 vaccine in our patient may have resulted in transient myocardial stunning is unknown. Our patient experienced 4 days of increasingly severe symptoms post vaccination; it is postulated that this excessive systemic inflammatory response may have induced excess catecholamine release resulting in the evident myocardial dysfunction. Importantly, we present no evidence to suggest a transient immune-related thrombosis related injury secondary to the ChAdOx1 nCov-19 vaccination, as previously described.7 Of note, our patient’s platelet count remained within the normal reference range during both the acute and recovery phase of his illness.
The importance of SARS-CoV-2 vaccination in controlling the global pandemic cannot be understated. High levels of population uptake are required to control or eradicate the SARS-CoV-2 virus,8 yet in Australia, vaccine hesitancy remains a barrier.9 Rigorous scrutiny of vaccine-related side effects to ensure safe and effective vaccine delivery to the appropriate population is, therefore, vital. While the clinical outcomes for our patient were ultimately favourable, takotsubo cardiomyopathy is not a benign condition and is associated with 5·6% per patient-year mortality and 9·9% per patient-year risk of major adverse cardiac events.1 This index case of possible ChAdOx1 nCov-19 associated takotsubo cardiomyopathy demonstrates the need for vigilance when assessing acute coronary syndrome presentations post ChAdOx1 nCov-19 vaccination.
Learning points.
This is the first known case of takotsubo (stress) cardiomyopathy following ChAdOx1 nCoV-19 vaccination.
While causality cannot be established, takotsubo (stress) cardiomyopathy should be considered a differential in acute coronary syndrome presentations following ChAdOx1 nCoV-19 vaccination.
ChAdOx1 nCoV-19 side effects remain rare with their overall safety and efficacy profile established.
While ChAdOx1 nCoV-19 side effects remain rare, takotsubo (stress) cardiomyopathy is not benign and can carry a high mortality.
Acknowledgments
Authors would like to acknowledge Dr Anastasia Vlachadis Castles, Ms Matilda Malos and Dr Rifly Rafiudeen for their contribution to the investigation of this case.
Footnotes
Contributors: PC was involved in the conception, design, acquisition of data and drafting of the article. CW was involved in data and imaging acquisition and analysis, and final approval of version published. NM involved in care of patient, imaging investigation, exclusion of differentials and final approval of version published. PB was supervising author involved in all stages of case, from formulation, drafting, interpretation and final approval of version published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med 2015;373:929–38. 10.1056/NEJMoa1406761 [DOI] [PubMed] [Google Scholar]
- 2. Ghadri J-R, Wittstein IS, Prasad A, et al. International expert consensus document on takotsubo syndrome (Part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J 2018;39:2032–46. 10.1093/eurheartj/ehy076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghadri J-R, Wittstein IS, Prasad A, et al. International expert consensus document on takotsubo syndrome (Part II): diagnostic workup, outcome, and management. Eur Heart J 2018;39:2047–62. 10.1093/eurheartj/ehy077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jani C, Leavitt J, Al Omari O, et al. COVID-19 vaccine-associated takotsubo cardiomyopathy. Am J Ther 2021;28:361–4. 10.1097/MJT.0000000000001379 [DOI] [PubMed] [Google Scholar]
- 5. Santoro F, Ieva R, Ferraretti A, et al. Tako-Tsubo cardiomyopathy after influenza vaccination. Int J Cardiol 2013;167:e51–2. 10.1016/j.ijcard.2013.03.147 [DOI] [PubMed] [Google Scholar]
- 6. Singh K, Marinelli T, Horowitz JD. Takotsubo cardiomyopathy after anti-influenza vaccination: catecholaminergic effects of immune system. Am J Emerg Med 2013;31:1627.e1–1627.e4. 10.1016/j.ajem.2013.06.039 [DOI] [PubMed] [Google Scholar]
- 7. Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092–101. 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bartsch SM, O'Shea KJ, Ferguson MC, et al. Vaccine efficacy needed for a COVID-19 coronavirus vaccine to prevent or stop an epidemic as the sole intervention. Am J Prev Med 2020;59:493–503. 10.1016/j.amepre.2020.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Edwards B, Biddle N, Gray M, et al. COVID-19 vaccine hesitancy and resistance: correlates in a nationally representative longitudinal survey of the Australian population. PLoS One 2021;16:e0248892. 10.1371/journal.pone.0248892 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bcr-2021-246580supp001.mp4 (3.7MB, mp4)
bcr-2021-246580supp002.mp4 (3.2MB, mp4)
bcr-2021-246580supp003.mp4 (2.9MB, mp4)