Abstract
Parkinson’s disease, the second most prevalent neurodegenerative disorder worldwide, is characterized by a progressive loss of dopaminergic neurons in substantia nigra pars compacta, causing motor symptoms. This disorder’s main hallmark is the formation of intraneuronal protein inclusions, named Lewy bodies and neurites. The major component of these arrangements is α-synuclein, an intrinsically disordered and soluble protein that, in pathological conditions, can form toxic and cell-to-cell transmissible amyloid structures. Preventing α-synuclein aggregation has attracted significant effort in the search for a disease-modifying therapy for Parkinson’s disease. Small molecules like SynuClean-D, epigallocatechin gallate, trodusquemine, or anle138b exemplify this therapeutic potential. Here, we describe a subset of compounds containing a single aromatic ring, like dopamine, ZPDm, gallic acid, or entacapone, which act as molecular chaperones against α-synuclein aggregation. The simplicity of their structures contrasts with the complexity of the aggregation process, yet the block efficiently α-synuclein assembly into amyloid fibrils, in many cases, redirecting the reaction towards the formation of non-toxic off-pathway oligomers. Moreover, some of these compounds can disentangle mature α-synuclein amyloid fibrils. Their simple structures allow structure-activity relationship analysis to elucidate the role of different functional groups in the inhibition of α-synuclein aggregation and fibril dismantling, making them informative lead scaffolds for the rational development of efficient drugs.
Key Words: amyloid, aromatic rings, dopamine, inhibition, neurodegeneration, oligomers, Parkinson's disease, protein aggregation, α-synuclein
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder that affects > 4% of people over 60 years worldwide, being the second most prevalent neurodegenerative disease after Alzheimer’s disease. PD belongs to a group of pathologies named synucleinopathies, which also includes Dementia with Lewy Body and Multiple System Atrophy, among others. Although significant differences in terms of symptomatology, progression, and the affected cellular and anatomical compartments, these disorders share a common histopathological hallmark: proteinaceous deposits, whose main component are amyloid fibrils of α-synuclein (α-syn) (Spillantini et al., 1997). In PD, these inclusions accumulate in the body (Lewy bodies) and processes (Lewy neurites) of dopaminergic neurons in the substantia nigra pars compacta, causing progressive cell death and dopamine decline, which translates into motor symptoms like bradykinesia, rigidity, or resting tremor. Moreover, cortical Lewy bodies are also observed, especially in dementia with Lewy bodies patients (Mattila et al., 2000). Mutations and multiplications of the SNCA gene, which encodes for α-syn, are linked to early-onset and familial PD cases, evidencing the connection between α-syn aggregation and PD (Lazaro et al., 2014).
Search Strategy and Selection Criteria
For the present review, we searched the literature published on PubMed for periods between 2000 and 2020 by using key terms listed in the keywords section. In PubMed, the Related Citations function and citation tracking was used to retrieve additional articles. Additionally, the reference lists of selected articles were used to identify further significant studies. All articles were manually checked for the relevant parts by both authors. The last search was conducted in January 2021.
α-Synuclein and Parkinson’s Disease
α-Syn is an intrinsically disordered protein whose sequence can be dissected in three different regions (Fusco et al., 2014). The N-terminal domain is a highly conserved region that contains the KTKGEV repeats and an amphipathic character that regulates membrane-binding; there map all the missense mutations associated with early-onset and higher penetrance of PD. The central domain, named the non-amyloid component, is characterized by a highly hydrophobic character that drives α-syn amyloid aggregation and constitutes mature aggregates’ core. The C-terminal domain contains several negatively charged residues that seem to counteract and regulate the aggregation propensity, as it can be inferred from the higher aggregation capacity of the C-terminally truncated α-syn. In normal conditions, α-syn is a soluble monomer that can adopt a helical structure by interacting with membranes, consistent with its role in vesicle trafficking. Besides, α-syn can also be found as a soluble tetramer (Bartels et al., 2011). However, under pathological conditions, α-syn self-assembles into toxic supramolecular conformations.
Recombinant technology has allowed reproducing intracellular α-syn aggregation in vitro, revealing that it follows sigmoidal kinetics as in other amyloidogenic proteins. During this process, monomeric α-syn assembles into oligomeric and protofibrillar species, which precedes amyloid fibrils’ formation. Recent studies demonstrate that these metastable species, rather than mature fibrils, are responsible for α-syn toxicity in neuronal cells (Winner et al., 2011). Moreover, oligomeric and protofibrillar structures can be transmitted to neighbouring cells and seed the aggregation of native α-syn in a prion-like manner, thus propagating the aggregation throughout the brain. Notably, α-syn seeds could present different conformations, as previously described for the prion protein strains. Distinct α-syn strains seem to target different brain regions and cell types and might explain neuropathological differences between synucleinopathies (Lau et al., 2020).
Targeting α-Synuclein Aggregation
α-Syn aggregation has become an important target to develop disease-modifying therapies for PD. Strategies include accelerating aggregate clearance by promoting autophagy, gene-silencing to reduce protein concentration, or developing small molecules that interfere with the polymerization (Pujols et al., 2020). α-Syn is an abundant brain protein and molecules interacting with its monomeric form could impact its function; therefore, inhibitors that only interact with aggregated and toxic species are preferred.
The lack of a well-defined three-dimensional structure for monomeric, oligomeric, and protofibrillar α-syn precludes the rational design and optimization of drugs that bind these species. In this context, high-throughput screening protocols have become the primary strategy to identify such kinds of molecules. This strategy has rendered different compounds that inhibit α-syn amyloid formation in vitro and/or in animal models, such as epigallocatechin gallate, NPT200-11, CLR01, trodusquemine, or SynuClean-D (Pujols et al., 2018, 2020). Many of these molecules present complex structures characterized by a planar hydrophobic core from which they project polar ramifications. The hydrophobic moiety often has an aromatic character, and it is thought to drive the interaction with apolar exposed regions in α-syn assemblies, which are essential for fibril elongation. Polar projections would interfere with hydrophobic packing and might disrupt hydrogen bonding networks, interfering with elongation and propagation and, eventually, dismantling pre-formed aggregates. Some of the most potent inhibitors exhibit several aromatic rings, like SynuClean-D, epigallocatechin gallate, or CLR01, containing two, three, and five aromatic rings, respectively. It seems to be a certain connection between the number of aromatic rings and the molecule’s inhibitory potential, as illustrated by CLR03, a short and inactive version of the CLR01 molecular tweezer. A larger aromatic area is assumed to result in a tighter binding to α-syn hydrophobic assemblies. However, potent α-syn aggregation inhibitors consisting of a single aromatic ring have been described in recent years (Figure 1).
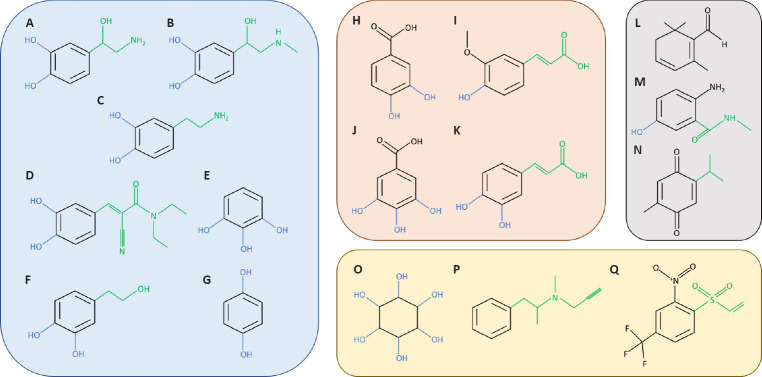
Figure 1.
Structure of single-ring inhibitors of α-synuclein (α-syn) aggregation.
In blue, catechol and catecholamine inhibitors norepinephrine (A), epinephrine (B), dopamine (C), entacapone (D), pyrogallol (E), hydroxytyrosol (F), and hydroquinone (G). In pink, phenolic acids, protocatechuic (H), ferulic (I), gallic (J), and caffeic (K) prevent α-syn amyloid aggregation. In gray, other phenolic structures as safranal (L), 576755 (M), and thymoquinone (N) with the capacity to inhibit α-syn aggregation. Non-phenolic inhibitors scyllo-inositol (O), selegiline (P), and ZPDm (Q) in yellow.
Single-Ring Polyphenolic Inhibitors of α-Synuclein Aggregation
Polyphenols constitute one of the primary sources of α-syn aggregation inhibitors, and most of the single-ring inhibitors fall into this group. Dopamine, epinephrine, and norepinephrine, along with several analogues, were the first single-ring inhibitors described (Li et al., 2004). These catecholamines interfered with the in vitro and in vivo aggregation of both Aβ and α-syn, driving the reaction towards off-pathway oligomers’ formation. However, the lack of inhibition in anaerobic and acidic conditions demonstrated that these catecholamines’ oxidative products were the most active structures, consistent with catecholamines’ susceptibility to oxidation (Li et al., 2004). The mechanism behind this effect is still unclear, and probably different processes coexist. Some studies indicate that inhibition is due to a covalent interaction of the compound with tyrosine or lysine residues, while others suggest that the compound-mediated oxidation of methionine in α-syn is necessary for inhibition and off-pathway oligomers formation (Oliveri, 2019). Non-covalent interactions with the non-amyloid component domain and the C-terminal YEMPS α-syn sequence have also been proposed for dopamine and norepinephrine. The lower potency of tyramine, which lacks one of the two hydroxyl groups of dopamine, illustrates aromatic ring polar projections’ critical role in inhibition (Latawiec et al., 2010). Single-ring phenolic acids such as ferulic acid, caffeic acid, protocatechuic acid, and gallic acid were also found to be active molecules (Ono and Yamada, 2006; Di Giovanni et al., 2010; Hornedo-Ortega et al., 2016). Ferulic acid is a potent inhibitor of α-syn aggregation in vitro, and the first phenolic compound described to destabilize mature fibrils; further analysis demonstrated that it drives α-syn aggregation towards off-pathway structures (Ono and Yamada, 2006). Similarly, caffeic acid, gallic acid, and protocatechuic acid, described as inhibitors of α-syn and Aβ aggregation, all promoted the formation of stable off-pathway amorphous aggregates, consistent with their lack of effect in the lag phase of the reaction (Hornedo-Ortega et al., 2016). Nevertheless, only in the case of α-syn seeding was prevented, indicating protein-specific activity. NMR detected no direct interaction with the monomeric form; instead, gallic and protocatechuic acids’ showed fibril-destabilization capacity. Docking analysis demonstrated the interaction of caffeic acid with aggregated α-syn (PDB: 1XQ8). However, as this interaction was analyzed using micelle-bound α-syn, it may not mimic the contacts occurring in vivo. Gallic acid also inhibited the aggregation of the familial A53T variant of α-syn by transient and low-affinity interactions. Structure-Activity Relationship (SAR) analysis with several molecules’ derivates demonstrates that the phenyl group is not sufficient by itself to inhibit fibril formation. Besides, the number (trihydroxybenzoic acid > dihydroxybenzoic acid > monohydroxybenzoic acid), position, and conjugation of the hydroxyl groups at the benzoic acid appendage are crucial activity determinants. Notably, a higher effect could be observed when these polar moieties were displayed consecutively.
Other Phenolic and Non-Phenolic Modulators of α-Synuclein Amyloid Aggregation
Other single-ring phenols, such as catechols and quinones, have also been described as anti-aggregational compounds. Hydroxytyrosol, but not tyrosol and hydroxyphenyl acetic acid, both lacking a single hydroxyl group, inhibits the aggregation of α-syn, and as the above-described phenolic acids, they mainly stabilize off-pathway oligomers (Hornedo-Ortega et al., 2018). Entacapone and pyrogallol, which present multiple consecutive hydroxyl groups, prevented the aggregation and seeding of α-syn up to 90% at high compound: protein ratios, although entacapone was more effective at low ratios (Di Giovanni et al., 2010). These molecules act by diverting toxic intermediates towards off-pathway nontoxic species, which is confirmed by the lack of NMR binding signature to the monomeric protein. Another catechol structure that presents anti-aggregational capacity is hydroquinone, one of the tobacco components (Hong et al., 2009). It acts on both wild-type and A30P familial variants, inducing the formation of stable off-pathway oligomers. Interaction studies revealed that hydroquinone establishes covalent interactions via Michael additions with the side chains of neighboring lysine residues placed at the interface of two α-syn molecules in an oligomer (Hong et al., 2009). Thymoquinone, a structurally related quinone, also inhibited the aggregation and seeding of α-syn in vitro and an MPTP-induced mice model of PD (Ardah et al., 2019). Another compound that deserves mention is safranal, a carotenoid with inhibitory and disaggregation capacity, at high doses, whose hydrophobic interactions with α-syn increase its solubility (Save et al., 2019). One of the last single-ring phenolic compounds found is compound 576755, a quinone-based structure discovered by applying a new high-throughput strategy based on chemical microarray surface plasmon resonance imaging (Toth et al., 2019). The compound exhibited a significant but undeciphered inhibitory effect in fibril and oligomers formation, also acting in seeded reactions. Treatment with 576755 attenuated the A53T α-syn mediated dopaminergic loss in cellular models, without impacting cell-to-cell transmission.
Nevertheless, phenolic compounds are not the only one-ring structures with α-syn anti-aggregation capacity. Scyllo-inositol, a natural-sugar derivate displaying a planar structure that inhibits aggregation of α-syn and Aβ, by diverting the reaction towards the formation of off-pathway structures, exerts a neuroprotective effect in cell cultures (Ibrahim and McLaurin, 2016). The absence of activity in chiro-inositol suggests that the planar structure plays a fundamental role in masking aggregates’ self-recognition sites (Ibrahim and McLaurin, 2016). Phenethylamine-based structures, as selegiline, also prevent the formation of α-syn aggregates. This particular single-ring compound significantly delays the lag phase, impacting the nuclei’s formation and inducing the accumulation of amorphous and annular structures of both wild-type and A30P α-syn variants (Braga et al., 2011). Also, a destabilizing fibril capacity has been described for this compound. One of the latest single-ring inhibitor compounds discovered is ZPDm (Pena-Diaz et al., 2020), which shares these molecules’ typical architecture, with polar, non-hydroxylic projections coming out from a benzene ring. This minimal structure is highly related to that of the three-rings containing ZPD-2 aggregation inhibitor (Pena-Diaz et al., 2019), but their action mechanisms are different. ZPDm inhibits the aggregation of α-syn at substoichiometric ratios, displaying a better pharmacological profile than ZPD-2. The compound was very active against the oligomer-promoting A30P and H50Q α-syn variants, suggesting that it could interact with these low-molecular-weight species. Besides, the compound significantly blocked seeded transmission in protein misfolding cyclic amplification assays and exhibited a strong fibril disassembling capacity, with the reactive vinyl sulfone group likely involved in this last activity. Noteworthy, the in vitro inhibitory and remodeling activities of ZPDm could be recapitulated in a Caenorhabditis elegans model of PD.
Concluding Remarks and Future Perspectives
Overall, single-ring inhibitors represent small (< 300 g/mol, < 200 Å2), easy-to-synthesize structures, which often display better ADME properties than complex anti-aggregational compounds. Nevertheless, they can fail to cross the blood-brain barrier or even induce significant toxicity, as shown for some polyphenols (Kobayashi et al., 2020). As a general trend, these compounds’ effect seems to be biased towards the generation of off-pathway aggregates, likely because the single-ring structure interacts with a common protein hydrophobic patch, despite some of them being also potent fibril disruptors and propagation inhibitors. Despite some debate, these off-pathways structures are generally considered to hold low intrinsic toxicity and immunogenicity. Overall, they are, in our opinion, under-investigated molecules that constitute excellent tools to decipher which groups and in which position are required for the long-pursued goal of developing disease-modifying compounds to treat PD and/or other synucleinopathies.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Qiu Y; T-Editor: Jia Y
References
- 1.Ardah MT, Merghani MM, Haque ME. Thymoquinone prevents neurodegeneration against MPTP in vivo and modulates alpha-synuclein aggregation in vitro. Neurochem Int. 2019;128:115–126. doi: 10.1016/j.neuint.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Bartels T, Choi JG, Selkoe DJ. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braga CA, Follmer C, Palhano FL, Khattar E, Freitas MS, Romao L, Di Giovanni S, Lashuel HA, Silva JL, Foguel D. The anti-Parkinsonian drug selegiline delays the nucleation phase of alpha-synuclein aggregation leading to the formation of nontoxic species. J Mol Biol. 2011;405:254–273. doi: 10.1016/j.jmb.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 4.Di Giovanni S, Eleuteri S, Paleologou KE, Yin G, Zweckstetter M, Carrupt PA, Lashuel HA. Entacapone and tolcapone, two catechol O-methyltransferase inhibitors, block fibril formation of alpha-synuclein and beta-amyloid and protect against amyloid-induced toxicity. J Biol Chem. 2010;285:14941–14954. doi: 10.1074/jbc.M109.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fusco G, De Simone A, Gopinath T, Vostrikov V, Vendruscolo M, Dobson CM, Veglia G. Direct observation of the three regions in alpha-synuclein that determine its membrane-bound behaviour. Nat Commun. 2014;5:3827. doi: 10.1038/ncomms4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong DP, Fink AL, Uversky VN. Smoking and Parkinson’s disease: does nicotine affect alpha-synuclein fibrillation? Biochim Biophys Acta. 2009;1794:282–290. doi: 10.1016/j.bbapap.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hornedo-Ortega R, Cerezo AB, Troncoso AM, Garcia-Parrilla MC. Protective effects of hydroxytyrosol against alpha-synuclein toxicity on PC12cells and fibril formation. Food Chem Toxicol. 2018;120:41–49. doi: 10.1016/j.fct.2018.06.059. [DOI] [PubMed] [Google Scholar]
- 8.Hornedo-Ortega R, Alvarez-Fernandez MA, Cerezo AB, Richard T, Troncoso AMA, Garcia-Parrilla MAC. Protocatechuic acid: inhibition of fibril formation, destabilization of preformed fibrils of amyloid-beta and alpha-synuclein, and neuroprotection. J Agric Food Chem. 2016;64:7722–7732. doi: 10.1021/acs.jafc.6b03217. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim T, McLaurin J. alpha-Synuclein aggregation, seeding and inhibition by scyllo-inositol. Biochem Biophys Res Commun. 2016;469:529–534. doi: 10.1016/j.bbrc.2015.12.043. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi H, Murata M, Kawanishi S, Oikawa S. Polyphenols with anti-amyloid beta aggregation show potential risk of toxicity via pro-oxidant properties. Int J Mol Sci. 2020;21:3561. doi: 10.3390/ijms21103561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latawiec D, Herrera F, Bek A, Losasso V, Candotti M, Benetti F, Carlino E, Kranjc A, Lazzarino M, Gustincich S, Carloni P, Legname G. Modulation of alpha-synuclein aggregation by dopamine analogs. PLoS One. 2010;5:e9234. doi: 10.1371/journal.pone.0009234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau A, So RWL, Lau HHC, Sang JC, Ruiz-Riquelme A, Fleck SC, Stuart E, Menon S, Visanji NP, Meisl G, Faidi R, Marano MM, Schmitt-Ulms C, Wang Z, Fraser PE, Tandon A, Hyman BT, Wille H, Ingelsson M, Klenerman D, et al. alpha-Synuclein strains target distinct brain regions and cell types. Nat Neurosci. 2020;23:21–31. doi: 10.1038/s41593-019-0541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazaro DF, Rodrigues EF, Langohr R, Shahpasandzadeh H, Ribeiro T, Guerreiro P, Gerhardt E, Krohnert K, Klucken J, Pereira MD, Popova B, Kruse N, Mollenhauer B, Rizzoli SO, Braus GH, Danzer KM, Outeiro TF. Systematic comparison of the effects of alpha-synuclein mutations on its oligomerization and aggregation. PLoS Genet. 2014;10:e1004741. doi: 10.1371/journal.pgen.1004741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Zhu M, Manning-Bog AB, Di Monte DA, Fink AL. Dopamine and L-dopa disaggregate amyloid fibrils: implications for Parkinson’s and Alzheimer’s disease. FASEB J. 2004;18:962–964. doi: 10.1096/fj.03-0770fje. [DOI] [PubMed] [Google Scholar]
- 15.Mattila PM, Rinne JO, Helenius H, Dickson DW, Roytta M. Alpha-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson’s disease. Acta Neuropathol. 2000;100:285–290. doi: 10.1007/s004019900168. [DOI] [PubMed] [Google Scholar]
- 16.Oliveri V. Toward the discovery and development of effective modulators of alpha-synuclein amyloid aggregation. Eur J Med Chem. 2019;167:10–36. doi: 10.1016/j.ejmech.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 17.Ono K, Yamada M. Antioxidant compounds have potent anti-fibrillogenic and fibril-destabilizing effects for alpha-synuclein fibrils in vitro. J Neurochem. 2006;97:105–115. doi: 10.1111/j.1471-4159.2006.03707.x. [DOI] [PubMed] [Google Scholar]
- 18.Pena-Diaz S, Pujols J, Conde-Gimenez M, Carija A, Dalfo E, Garcia J, Navarro S, Pinheiro F, Santos J, Salvatella X, Sancho J, Ventura S. ZPD-2, a small compound that inhibits alpha-Synuclein amyloid aggregation and its seeded polymerization. Front Mol Neurosci. 2019;12:306. doi: 10.3389/fnmol.2019.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pena-Diaz S, Pujols J, Pinheiro F, Santos J, Pallares I, Navarro S, Conde-Gimenez M, Garcia J, Salvatella X, Dalfo E, Sancho J, Ventura S. Inhibition of alpha-Synuclein aggregation and mature fibril disassembling with a minimalistic compound, ZPDm. Front Bioeng Biotechnol. 2020;8:588947. doi: 10.3389/fbioe.2020.588947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pujols J, Pena-Diaz S, Pallares I, Ventura S. Chemical chaperones as novel drugs for Parkinson’s disease. Trends Mol Med. 2020;26:408–421. doi: 10.1016/j.molmed.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Pujols J, Pena-Diaz S, Lazaro DF, Peccati F, Pinheiro F, Gonzalez D, Carija A, Navarro S, Conde-Gimenez M, Garcia J, Guardiola S, Giralt E, Salvatella X, Sancho J, Sodupe M, Outeiro TF, Dalfo E, Ventura S. Small molecule inhibits alpha-synuclein aggregation, disrupts amyloid fibrils, and prevents degeneration of dopaminergic neurons. Proc Natl Acad Sci U S A. 2018;115:10481–10486. doi: 10.1073/pnas.1804198115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Save SS, Rachineni K, Hosur RV, Choudhary S. Natural compound safranal driven inhibition and dis-aggregation of alpha-synuclein fibrils. Int J Biol Macromol. 2019;141:585–595. doi: 10.1016/j.ijbiomac.2019.09.053. [DOI] [PubMed] [Google Scholar]
- 23.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 24.Toth G, Neumann T, Berthet A, Masliah E, Spencer B, Tao J, Jobling MF, Gardai SJ, Bertoncini CW, Cremades N, Bova M, Ballaron S, Chen XH, Mao W, Nguyen P, Tabios MC, Tambe MA, Rochet JC, Junker HD, Schwizer D, et al. Novel small molecules targeting the intrinsically disordered structural ensemble of alpha-Synuclein protect against diverse alpha-Synuclein mediated dysfunctions. Sci Rep. 2019;9:16947. doi: 10.1038/s41598-019-52598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, Tzitzilonis C, Soragni A, Jessberger S, Mira H, Consiglio A, Pham E, Masliah E, Gage FH, Riek R. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci U S A. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]