Traumatic brain injury (TBI) is a leading cause of death and disability worldwide, with estimates indicating that ~50% of the world’s population will acquire a head injury at some point in their lifetime (Maas et al., 2017). Mild TBIs account for ~80% of all reported cases, with up to 43% of all TBI patients reporting symptoms beyond 2 weeks post-injury. As of 2016, only 7% of preclinical TBI experiments included both male and female sexes, and fewer studies analyzed the data using sex as a biological variable (Spani et al., 2018). Since 2016, there has been a bigger push for TBI research to include both sexes, due to reports of sex disparities in symptom presentation, recovery, and vulnerability to other neurological disorders; emphasizing how little we know about the pathophysiology of TBI in females. In this Perspective, we discuss some preclinical and clinical sex differences, challenges addressing female inclusion in preclinical TBI research, and potential solutions towards finding a balance between female sex inclusion and sex as an independent biological variable; forging a path towards improving scientific rigor, reproducibility and inclusivity for evaluating pathophysiological sex-differences after TBI.
Sex-dependent barriers to rigor and reproducibility in experimental TBI research: In 1993, the National Institutes of Health (NIH) took a critical step towards equity in research by invoking “The Revitalization Act,” mandating women’s inclusion in clinical trials. In 2014, the mandate was emphasized for preclinical research, requiring “50:50” inclusion of both sexes unless substantial evidence can justify otherwise (Clayton and Collins, 2014). As females are included in preclinical research, the 50:50 inclusion rate may not provide sufficient power to detect important biological sex differences; as accumulating evidence indicates ‘sex’ is a crucial independent biological variable, where genetic, hormonal, metabolic, age, and environmental factors can cause a divergence in TBI outcomes.
The under-representation of females in both clinical and experimental TBI research is a barrier to scientific rigor and reproducibility. Female exclusion was originally justified by Center for Disease Control and Prevention statistics indicating TBI reports were 4-times higher in men than women in 2010. However, reports of TBI in women are rapidly increasing due to increased military and high-risk activities. Also, TBI in domestic violence has been underreported, where the National Women’s Health Network estimates that up to 20 million women per year in the US are victims of at least one TBI, exceeding the combined numbers for sports and combat-related neurotrauma in men. With increasing need for females’ inclusion in TBI research, funding became available and TBI investigators with little experience evaluating sex differences were faced with several newly identified and unidentified obstacles for including females, with extremely limited established and extensive female data (like we currently have in males) to guide the development rigorous protocols and hypothesis-based experiments.
While sex differences under nonpathological conditions are not fully understood, this is compounded during the aftermath of TBI. Mounting evidence of acute and longitudinal experiments indicate sex-differences in regard to physical, physiological, and pharmacological responses, where more data in females is necessary to elucidate how TBI (1) disrupts menstrual cycles and fertility, (2) causes increase number and severity of symptoms, and (3) increases potential for drug interactions or ineffectiveness in symptom treatment.
Here, we briefly address some of the reported sex-differences in both control and TBI circumstances, with acknowledgment this is the tip of the iceberg. The common justification for excluding females from TBI research (and neuroscience research overall) is the potential confounds of gonadal hormones, which contribute little statistical variation in some outcome measures and significant variation in other outcome measures. In addition, sexually dimorphic gene expression and other pragmatic factors can lead to physical and physiological differences that increase females’ vulnerability to TBI in the preclinical and clinical setting. Typically, females have variability in the thickness of their skulls, smaller brains, smaller average axonal diameter, different connectivity, and lesser neck strength due to differences in muscle mass. Other sex differences include body weight, body surface area, metabolism, plasma volume, muscle:fat ratio, cardiac output, classical neurotransmitter systems, response to stress, and predisposition for psychological and cognitive outcomes. Acute and chronic pharmacological treatment after TBI could also be influenced by sex differences, where differences in renal clearance (slower in females), different concentration and localization of receptors, receptor types, receptor binding affinity, and cell-dependent presence of gonadal hormone receptors can influence signal transduction pathways. In women, circulating hormones due to circadian rhythms and menstrual cycles are among other variables that can influence a drug’s pharmacokinetics and pharmacodynamics. In response to TBI, which is variable from patient-to-patient from the start, the metabolic, hormonal, and circulatory functions are altered, with the potential for differential regulation of multiple pathways that can influence the trajectory of recovery and the management of short- and long-term consequences, placing women at higher risk for medical misdiagnosis, longer or more severe disability, or adverse side effects in the aftermath of TBI (Soldin and Mattison, 2009).
Sexually dimorphic characteristics have been identified in preclinical studies indicating not only acute differences, but chronic differences that could have important implications for interventional trials. Robust pathological sex differences are demonstrated in region-dependent neurotransmitter regulation, glial activation, immune response, vascular-mediated neuronal damage, and hypothalamic-pituitary axis regulation (Ma et al., 2019; Bromberg et al., 2020). In clinical and preclinical studies, females demonstrate lower levels of neuro-glial-vascular unit pathogenesis, decreased oxidative stress, and fewer behavioral deficits in comparison to males in the acute and subacute time period; yet, females are more likely to demonstrate worse outcomes chronically (Inampudi et al., 2020). Despite available literature indicating that ovarian hormones have neuroprotective attributes, two primary caveats remain. Firstly, why do women have a disproportional number and severity of persistent neurological problems and longer recovery rates than men after TBI (Mollayeva et al., 2018)? Secondly, why have clinical trials administering ovarian hormones after TBI demonstrated little improvement in both sexes and some worse outcomes in women? Failure of clinical trials can be attributed to the heterogeneity of brain injury, peripheral injury, age, pre-morbid conditions, clinical standard operating procedures that include additional pharmacological support, and a host of other variables that are not replicated in preclinical experiments and are difficult to control in multi-center testing sites. Further, few clinical trials include longitudinal follow-up beyond a year, preventing necessary support as to whether an acute or short-term therapy improves long-term outcome in both sexes. Post-hoc analyses have been useful for identifying several of these variables and are a convenient interim solution for identifying and delineating sex as a biological variable after TBI and justifying future inclusion of sex as an independent variable. Additionally, factors such as age-at-injury, aging-with-injury, and genetic predisposition to neurological diseases are also important variables with profound sex and TBI interactions that require more detailed investigation independently and as a co-variable within clinical trials (as a subgroup analysis). With preliminary data, studies can appropriately power for sex as an independent biological variable, which may indicate important information regarding the failure and successes of interventional drug trials and provide greater indication as to mechanisms driving sex difference.
Further data requires us to consider broader implications for the role of ovarian hormones in TBI. Several clinical studies support that circulating ovarian hormones contribute to post-TBI symptoms; where women injured during the luteal phase of the menstrual cycle (with rising progesterone levels) had worse post-concussive symptoms than those injured during the follicular phase (Wunderle et al., 2014), cycling females had more adverse outcomes in comparison to age-matched females on birth control, pre-menopausal and post-menopausal women (Davis et al., 2006), and the phase of menstrual cycle can influence a woman’s neurological performance during testing (Mihalik et al., 2009). Our lab has found that female rats spent more time in diestrus and less time in estrus over 28 days after experimental TBI (Krishna et al., 2020). Since a rat cycles 5–7 times during a 28 day period, this is an indication of chronic disruption, corroborating clinical reports of irregular menstrual cycles after TBI (Colantonio et al., 2010). Together, these data indicate those circulating hormones (during and after TBI) can influence recovery and testing for chronic TBI symptoms, yet there are no reporting guidelines for the menstrual/estrous cycle phases required for publication.
On a more pragmatic level, there are additional considerations when adding female subjects to a research proposal optimized for males. The influence of pheromones on behavioral outcomes specific to the rodent experimental models should be considered in experimental design, especially when carrying out behavior on both sexes in the same room, using the same equipment, and on the same day. Females may respond differently in behavioral paradigms, requiring additional expertise for appropriate interpretation. A particularly important point to consider is the fact that even seemingly identical outcomes in males and females could be been driven by various underlying mechanisms where they may respond differently to intervention. While estrous cycle tracking is not mandatory, knowledge that TBI can chronically influence the cyclicity of hormones, thereby underlying mechanisms, should not be overlooked.
Solutions towards finding a balance between female sex inclusion and sex as an independent biological variable: In our experience, over the past 4 years at least one sex difference or sex × injury interaction has been detected in most of our experiments, supporting the inclusion of female sex as an independent biological variable. Additional evidence in clinical and preclinical neurotrauma research indicates similarities, but in many cases, profound differences, that should direct experimental design, guide constructive conversations with peers and sponsored research officers, and evolve how translational research is carried forward (Clayton and Collins, 2014). Given sex-specific central nervous system vulnerability, injury parameters in experimental TBI models need a priori considerations for females or to be justified and realistically evaluated for relevance and potential caveats in the discussion. For example, experimental TBI studies in male Sprague-Dawley rats are typically weight-matched (~350 g; ~3 months old) because there is an interaction between the injury force and the rat’s size, resulting in different pathology. Since females weigh 35–50% less than males in most rodent strains, a weight-matched female would be substantially older (9–12 months old), where age and diminishing ovarian hormone levels could impact outcome. If these rats are age-matched, the female has a smaller, thinner skull and brain, where the same force of hit could cause more significant injury to the females than males. Several laboratories, including ours, have addressed this issue by changing parameters of injury force between the sexes to match acute pathology or loss of righting reflex times. Regardless of whether injury parameters are changed to accommodate for sex-related size differences or not, clearly stated and justified methods are needed to address whether changed parameters (or lack of changes) could influence sex-differences in the outcome measures. Investigators can also administer the injury and associated behavioral tasks only during diestrus, when circulating ovarian hormones are lowest.
As such, additional training in tracking estrous cycles is advisable when newly incorporating females with males into an experimental design, if it does not interfere with outcome measures. This will allow for follow-up analysis to determine whether estrous phase at time-of-injury or time-of-behavior is associated with data variability. When tracking or controlling for estrous cycles, it is important to record and report beyond the current Animal Research: Reporting of In Vivo Experiments guidelines, with the duration and frequency of tracking, time of day when estrous cycle was tracked, or protocols used for controlling for phase of estrous or synchronization. Further, it is important to separately report the number of animals, ages, and weights for males and females, as these factors guide injury parameters and regularity of cycles. Also, daily tracking of estrous cycle in females habituates the females to handling and therefore the males should be handled for an equivalent amount of time.
Combining data from both sexes requires careful planning of analysis and appropriate consideration in the interpretation. Power calculations can indicate if groups sizes need to be adjusted to accommodate females. In the least, experiments combining both sexes should include follow-up analyses to evaluate for any evidence of sex-related dichotomy in outcome measures in clinical and translational studies. If a dichotomy is identified, the average of both sexes may not be representative of the biological or pathological processes taking place. Care is needed to ensure data are appropriately represented to avoid misdirected follow-up experiments and conflicting data in the field. The dichotomy can guide the interpretation of the data and the next steps in future work, which is fundamental for moving the field forward. In this case, publishing supplementary data where potential sex-differences are indicated would provide access to others considering similar experiments.
Most importantly, the support of a neuroendocinologist with clinical and preclinical expertise is helpful in implementation, evaluation, and translational relevance of sex differences and the influence of sex hormones. In TBI, this is especially critical since the HPA axis function can be chronically dysregulated, influencing sex hormones on multiple levels (Rowe et al., 2016). Collaboratively, the TBI community needs to adopt new approaches to experimental design, statistics, and data interpretation. Communication regarding obstacles, optimizations, and successes should be prioritized, with documentation and dissemination of information via public forums for rapid communication of gained knowledge that is reliable and available to other investigators to move the field forward. As knowledge accumulates, the development of algorithms for handling sex differences and analysis should become a readily available resource. Participation in annual meetings, like the Organization for the Study of Sex Differences, could promote feedback, knowledge, and exposure to effective collaborators. Public recognition of manuscript peer reviewers by some journals could also fill an important niche for more insiqhtful or alternative interpretations of neurotrauma investigator’s data as more publications including both sexes are being subrnitted.
The addition of females as an independent variable directly addresses the NIH’s primary mission to promote equity in preclinical research, with broader implications on overall health impact of disease and therapies. Adding females as an independent variable can double the cost, resources, and time allocations. Recognizing the importance, the NIH has a funding mechanism for contingencies to provide additional funds when the results of the studies dictate that additional animals are required to satisfy the originally approved and funded aims. Therefore, it is hopeful that other funding agencies will adopt similar policies and promote their utilization.
Concluding remarks: Biological sex-dependent outcomes emphasize the need for sex equity in preclinical research with the potential for important implications for both sexes in overall health impact of disease and therapies. However, proceed with caution, as numerous biological processes have basic sex differences with potential interactions that can confound or skew the results if data from both sexes are combined. From the translational perspective, interacting sex-related competing variables (i.e., phases of estrous) need to be reported and publication policies refined to incorporate more details associated with each sex. Continued candid discussions and adaption of the strategies for the inclusion of both sexes, formation of multi-disciplinary collaborative teams for designing experiments and interpreting results, and transparency in reporting the methods will provide the necessary knowledge to guide improvements in how females are integrated into TBI research. A broader appreciation and support in the scientific community to balance inclusion of biologically intact females and sex as an independent variable is needed to facilitate forging a path toward fulfilling the mandate’s mission and, ultimately, the improving quality of life for both women and men after TBI (Figure 1).
Figure 1.
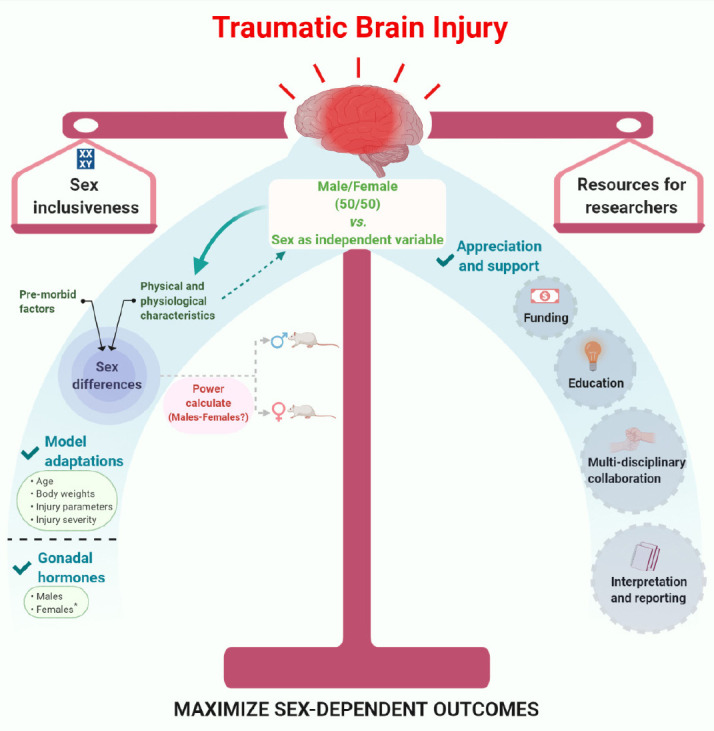
Traumatic brain injury and sex differences.
Striking a balance between inclusion of females and sex as an independent variable – Are we maximizing our resources? (*Diestrus and Estrus). Created with BioRender.com.
The content is solely the authors’ responsibility and does not necessarily represent the official views of the NIH.
The authors thank Ms. Carol A. Haussler (University of Arizona College of Medicine-Phoenix) for editing this article.
TCT was supported by the National Institutes of Health (R01NS100793) and Phoenix Children’s Hospital Mission Support.
Additional file: Open peer review report 1 (75.1KB, pdf) .
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Zachary M. Weil, West Virginia University, USA.
P-Reviewer: Weil ZM; C-Editors: Zhao M, Liu WJ, Li JY; T-Editor: Jia Y
References
- 1.Bromberg CE, Condon AM, Ridgway SW, Krishna G, Garcia-Filion PC, Adelson PD, Rowe RK, Thomas TC. Sex-dependent pathology in the HPA axis at a sub-acute period after experimental traumatic brain injury. Front Neurol. 2020;11:946. doi: 10.3389/fneur.2020.00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colantonio A, Harris JE, Ratcliff G, Chase S, Ellis K. Gender differences in self reported long term outcomes following moderate to severe traumatic brain injury. BMC Neurol. 2010;10:102. doi: 10.1186/1471-2377-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis DP, Douglas DJ, Smith W, Sise MJ, Vilke GM, Holbrook TL, Kennedy F, Eastman AB, Velky T, Hoyt DB. Traumatic brain injury outcomes in pre-and post-menopausal females versus age-matched males. J Neurotrauma. 2006;23:140–148. doi: 10.1089/neu.2006.23.140. [DOI] [PubMed] [Google Scholar]
- 5.Inampudi C, Ciccotosto GD, Cappai R, Crack PJ. Genetic modulators of traumatic brain injury in animal models and the impact of sex-dependent effects. J Neurotrauma. 2020;37:706–723. doi: 10.1089/neu.2019.6955. [DOI] [PubMed] [Google Scholar]
- 6.Krishna G, Bromberg C, Connell EC, Mian E, Hu C, Lifshitz J, Adelson PD, Thomas TC. Traumatic brain injury-induced sex-dependent changes in late-onset sensory hypersensitivity and glutamate neurotransmission. Front Neurol. 2020;11:749. doi: 10.3389/fneur.2020.00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma C, Wu X, Shen X, Yang Y, Chen Z, Sun X, Wang Z. Sex differences in traumatic brain injury: a multi-dimensional exploration in genes, hormones , cells, individuals, and society. Chin Neurosurg J. 2019;5:24. doi: 10.1186/s41016-019-0173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Büki A, Chesnut RM, Citerio G, Coburn M, Cooper DJ, Crowder AT, Czeiter E, Czosnyka M, Diaz-Arrastia R, Dreier JP, Duhaime AC, Ercole A, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 9.Mihalik JP, Ondrak KS, Guskiewicz KM, McMurray RG. The effects of menstrual cycle phase on clinical measures of concussion in healthy college-aged females. J Sci Med Sport. 2009;12:383–387. doi: 10.1016/j.jsams.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Mollayeva T, Mollayeva S, Colantonio A. Traumatic brain injury: sex, gender and intersecting vulnerabilities. Nat Rev Neurol. 2018;14:711–722. doi: 10.1038/s41582-018-0091-y. [DOI] [PubMed] [Google Scholar]
- 11.Rowe RK, Rumney BM, May HG, Permana P, Adelson PD, Harman SM, Lifshitz J, Thomas TC. Diffuse traumatic brain injury affects chronic corticosterone function in the rat. Endocr Connect. 2016;5:152–166. doi: 10.1530/EC-16-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48:143–157. doi: 10.2165/00003088-200948030-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Späni CB, Braun DJ, Van Eldik LJ. Sex-related responses after traumatic brain injury: Considerations for preclinical modeling. Front Neuroendocrinol. 2018;50:52–66. doi: 10.1016/j.yfrne.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wunderle K, Hoeger KM, Wasserman E, Bazarian JJ. Menstrual phase as predictor of outcome after mild traumatic brain injury in women. J Head Trauma Rehabil. 2014;29:E1–8. doi: 10.1097/HTR.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


