Traumatic spinal cord injuries (SCI) are characterized by damage in the integrity of the spinal cord, which results in either temporary or permanent alterations in the locomotor, sensory and/or autonomic functions (Yezierski, 2009). The traumatic event leads to impairments in voluntary control of movement below the injury by affecting the connection between the brain and the neurons localized in the spinal cord. Therefore, the recovery of locomotor activity is considered one of the main goals in the search of new therapies by the scientists around the world. For many years, axon regeneration has been considered the Holy Grail in SCI research, however, now we know that the regeneration of sectioned axons is necessary but not enough to promote locomotor recovery (Raineteau and Schwab, 2001). The disruption of long motor and sensory axonal tracts as a consequence of the lesion prevents their specific interactions with their cellular targets. For this reason the goal of ongoing investigations is to promote the re-growth of axonal tracts across the lesion site and their re-connection with propiospinal neurons at different segments of the spinal cord (Figure 1A). In particular, regeneration and re-connection of corticospinal tract (CST) axons is of vital significance to regain voluntary locomotor activity after a complete spinal cord injury (Oudega and Perez, 2012). This is because the axons that integrate the CST transmit voluntary motor information to the forelimbs and hindlimbs, and the damage of this structure in humans affects directly the locomotion (Nathan, 1994).
Figure 1.
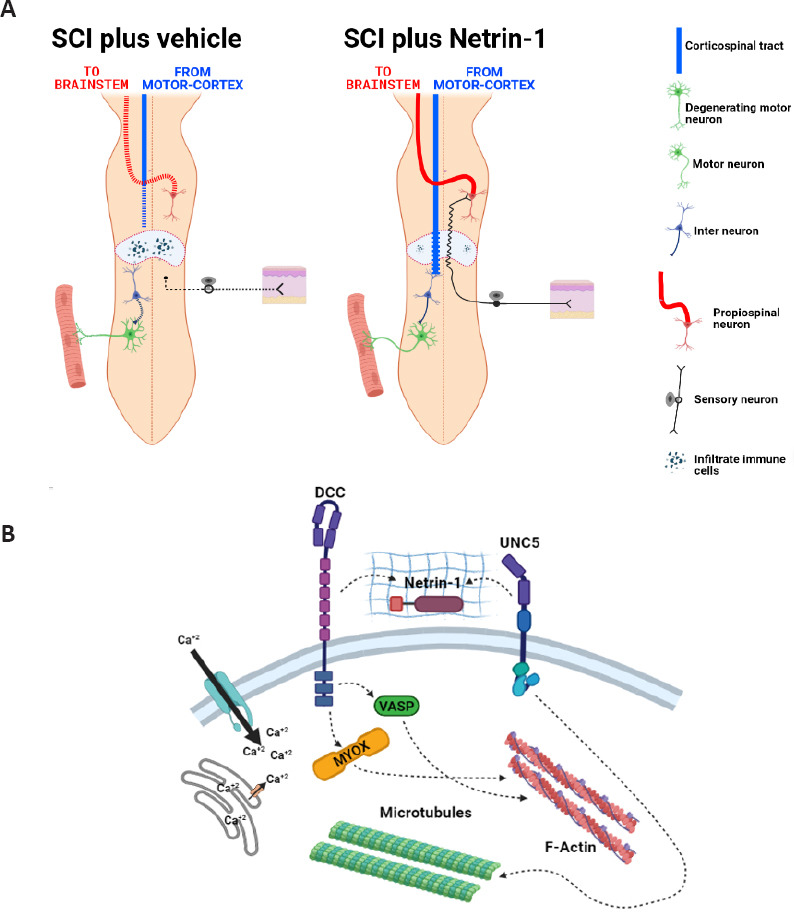
Corticospinal tract regeneration after SCI.
(A) Schematic representation of a transected spinal cord. Left panel shows de-connection of descendent and ascendant axonal tracts. CST axons show die back (blue dotted line) and ascendant axons shows degeneration (red dotted line). Right image shows CST regeneration across lesion site (wavy blue line) and re-connection. Scheme also shows regeneration of sensory axons with re-connection with propisopinal neurons. (B) Summary of possible molecular interactions between Netrin-1 and its transmembrane receptors. CST: Corticospinal tract; SCI: spinal cord injury.
At the cellular level, an intrinsic neuronal mechanism has been described, where cortical neurons activate a regenerative program in response to injury. This cellular mechanism reflects an attempt to restore the loss of function; however this self-recovery mechanism is completely inefficient. Taking into account this premise, researchers around the world are working on the development of biological therapies to heal the lesioned spinal cord. For example, several approaches target the cytoskeleton of damaged axons, enhancing the re-polimerization of actin, tubulin and neurofilaments and preventing the production of oxidative species that contribute to the degenerative process (Ruschel et al., 2015; Quinta et al., 2016). Other approaches act on the metabolism and energy balance of neurons, restoring cellular energy, in particular enhancing the mitocondrial transport to promote axonal regeneration and synaptic re-connection (Han et al., 2020). However, axonal re-growth after SCI does not ensure a proper guidance and navigation of regenerated axons across the lesion site, and synaptic re-connection with their specific neuronal effectors.
To address this limitation, in a recent study I examined the effect of Netrin-1 treatment on axonal regeneration across the lesion, and synaptic re-connection to re-establish locomotion after SCI (Quinta, 2021).
Netrin-1 is a developmental axon guidance cue involved in CST growth, CST 3-dimensional navigation, and neuronal connection. In particular, this molecule regulates the degree of decussation of the CST at the medulla oblongata (Meneret et al., 2017). The mechanism involves two major Netrin-1 receptors, known as UNC5h and DCC (Deleted in Colorectal Cancer), which could act as trigger of repulsive or attractive signaling during development (Keino-Masu et al., 1996).
The therapeutic effect of Netrin-1 was addressed using a rat model of complete spinal cord transection (T10–11). This model is suitable to investigate axonal regeneration after SCI, since the precision of the lesion causes minimal tissue loss but severs all axons, disconnecting spinal circuits below the injury site from all supra-spinal input, as well as all ascending axons.
This study showed that Netrin-1 administered at the time of injury in the epicenter of the lesion, promotes a significant regeneration of axonal tracts localized at the white matter segment across the transected area [Figure 4 from Quinta (2021)]. In particular re-grown CST axons were identified by three-dimnesional imaging on cleared whole spinal cord. It was also shown that regenerated axons that crossed the lesion presented trans-synaptic interactions with their targets below the injury. This interaction between regenerated axons and their neuronal targets in the spinal cord prevented the trans-synaptic degeneration of spinal neurons. This effect was reflected in the preservation of axonal structures in peripheral nerves, like the sciatic nerve. Finally, rats treated with Netrin-1 after complete SCI recovered their locomotion, in contrast to what was observed in vehicle-treated injured rats [Figure 1 from Quinta (2021)].
The locomotor recovery observed is dependent on the regeneration of ascendant/descendent axons across the lesion and their re-connection with specific neuronal targets, rather than due to a compensatory response below the lesion site triggered by spinal circuit plasticity. To confirm this, rats treated with Netrin-1 after SCI were re-transected in the same anatomical location. As a consequence, rats showed a complete paralysis of the hindlims. This result confirmed that the recovery of locomotion was the result of the regeneration of axonal tracts across the lesion and their re-connection with spinal neurons.
The complete molecular mechanism by which acute Netrin-1 administration promotes locomotor recovery is far from being elucidated yet. We know that after SCI, Netrin-1 is expressed by neurons and oligodendrocytes that are surrounding the lesion. However, in the epicenter of the lesion, a sharp decrease in the level of Netrin-1 is observed in both neurons and oligodendrocytes. These low levels of Netrin-1 persist at least 7 months after SCI (Manitt et al., 2006). In line with this, a recent research shows that cortical neurons attempt to activate a regenerative transcriptomic program after SCI (Poplawski et al., 2020). This regenerative state is defined by the up-regulation of several genes involved in cellular growth, and the down-regulation of growth-inhibitory pathways. Interesting in the context of this perspective, both NTN1 gene and its receptor UNC5b are up-regulated after injury. Therefore, the effect of exogenous Netrin-1 administration could mimic this transcriptional program, promoting an embryonic regenerative state. Moreover, exogenous Netrin-1 addition also could prevent the sharp drop in Netrin-1 levels at the epicenter of the lesion. Together, these two effects could overcome the low efficiency of spontaneous re-growth, improving the regeneration process.
Evidence shows that the molecular pathway of axonal regeneration could involve extracellular calcium influx in response to Netrin-1/Netrin-1 receptors interaction, since this molecular mediator participates in the actin and tubulin cytoskeleton assembly in the growth cone (Figure 1B). Therefore the future work will be oriented to identify the mechanism by which this mediator interacts with voltage-gated calcium and/or TRP channels in response to Netrin-1 treatment.
Finally, the biological approaches where the restorative mechanisms involve the re-growth of axons after a mechanical lesion could be useful in other neuropathologies where axonal trauma takes place as well. For example traumatic brain injury or peripheral nerve injuries, could be excellent models to explore new potential applications in the future since both require the regeneration of the axonal shaft.
Additional file: Open peer review report 1 (89.7KB, pdf) .
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by the author before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: David Romeo-Guitart, Universitat Autònoma de Barcelona, Spain.
P-Reviewer: Romeo-Guitart D; C-Editors: Zhao M, Liu WJ, Li JY; T-Editor: Jia Y
References
- 1.Han Q, Xie Y, Ordaz JD, Huh AJ, Huang N, Wu W, Liu N, Chamberlain KA, Sheng ZH, Xu XM. Restoring cellular energetics promotes axonal regeneration and functional recovery after spinal cord injury. Cell Metab. 2020;31:623–641. doi: 10.1016/j.cmet.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 3.Manitt C, Wang D, Kennedy TE, Howland DR. Positioned to inhibit: netrin-1 and netrin receptor expression after spinal cord injury. J Neurosci Res. 2006;84:1808–1820. doi: 10.1002/jnr.21070. [DOI] [PubMed] [Google Scholar]
- 4.Méneret A, Franz EA, Trouillard O, Oliver TC, Zagar Y, Robertson SP, Welniarz Q, Gardner RJM, Gallea C, Srour M, Depienne C, Jasoni CL, Dubacq C, Riant F, Lamy JC, Morel MP, Guérois R, Andreani J, Fouquet C, Doulazmi M, et al. Mutations in the netrin-1 gene cause congenital mirror movements. J Clin Invest. 2017;127:3923–3936. doi: 10.1172/JCI95442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan PW. Effects on movement of surgical incisions into the human spinal cord. Brain. 1994;117:337–346. doi: 10.1093/brain/117.2.337. [DOI] [PubMed] [Google Scholar]
- 6.Oudega M, Perez MA. Corticospinal reorganization after spinal cord injury. J Physiol. 2012;590:3647–3663. doi: 10.1113/jphysiol.2012.233189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poplawski GHD, Kawaguchi R, Van Niekerk E, Lu P, Mehta N, Canete P, Lie R, Dragatsis I, Meves JM, Zheng B, Coppola G, Tuszynski MH. Injured adult neurons regress to an embryonic transcriptional growth state. Nature. 2020;581:77–82. doi: 10.1038/s41586-020-2200-5. [DOI] [PubMed] [Google Scholar]
- 8.Quinta HR. Intra spinal administration of Netrin-1 promotes locomotor recovery after complete spinal cord transection. J Neurotrauma. 2021 doi: 10.1089/neu.2020.7571. doi: 10.1089/neu.2020.7571. [DOI] [PubMed] [Google Scholar]
- 9.Quintá HR, Wilson C, Blidner A, González-Billault C, Pasquini L, Rabinovich G, Pasquini JM. Ligand-mediated Galectin-1 endocytosis prevents intraneural H2O2 production promoting F-actin dynamics reactivation and axonal re-growth. Exp Neurol. 2016;283:165–78. doi: 10.1016/j.expneurol.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- 11.Ruschel J, Hellal F, Flynn KC, Dupraz S, Elliott DA, Tedeschi A, Bates M, Sliwinski C, Brook G, Dobrindt K, Peitz M, Brustle O, Norenberg MD, Blesch A, Weidner N, Bunge MB, Bixby JL, Bradke F. Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science. 2015;348:347–352. doi: 10.1126/science.aaa2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yezierski RP. Spinal cord injury pain: spinal and supraspinal mechanisms. J Rehabil Res Dev. 2009;46:95–107. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


