Abstract
Stress response is a cellular widespread mechanism encoded by a common protein program composed by multiple cellular factors that converge in a defense reaction to protect the cell against damage. Among many mechanisms described, heat shock proteins were proposed as universally conserved protective factors in the stress core proteome, coping with different stress stimuli through its canonical role in protein homeostasis. However, emerging evidences reveal non-canonical roles of heat shock proteins relevant for physiological and pathological conditions. Here, we review the implications of inducible heat shock proteins in the central nervous system physiology. In particular, we discuss the relevance of heat shock proteins in the maintenance of synapses, as a balanced protective mechanism in central nervous system development, pathological conditions and aging.
Key Words: aging, central nervous system, chaperones, environment, neuroprotection, pathology, stress, synapses
Stress Response and Chaperones
The stress response arises from common cellular stressors that alter the environment and impact on macromolecules such as proteins or DNA. In consequence, key aspects of the cell physiology are altered including oxidative stress production, redox state, cell cycle control, macromolecular stabilization and metabolism. As a result, if stress overcomes the limits of this response, the cell activates an apoptotic death program (Kültz, 2005). Moreover, gene expression changes contribute to maintain cellular dynamics (Gasch et al., 2000; Tukaj, 2020), but different stress conditions or stressors trigger diverse gene expression changes (Gasch et al., 2000; Girardot et al., 2004). Yeast stress response is a good example of this diversity in cellular adaptive mechanisms. Heat is the classical factor in stress response that promotes the expression of cytosolic, mitochondrial and endoplasmic reticulum chaperones genes, and induces the expression of genes involved in metabolism regulation. However, yeast exposure to H2O2 increases reactive oxygen species production and elicits a genetic program based on cellular detoxification, redox reactions genes and heat shock genes. In addition, starvation, exposure to redox agents (dithiothreitol and diamide) or osmotic variable conditions provokes dramatic gene expression changes, making the stress response unique for each stressor (Gasch et al., 2000). This diversity shapes a suitable cellular response that facilitates the adaptation to survive in a changing environment through different species. In multicellular model organisms as Drosophila melanogaster or Arabidopsis thaliana, the evidences of different gene expression profile upon different stressors support the conservation of these mechanisms in evolution (Girardot et al., 2004).
Even though each stress response is proposed to elicit a specific response, the genetic program induced by these aggressions share a common and highly conserved profile at the cellular stress proteome: the induction or activation of stress proteins including the heat shock protein (HSPs) family or molecular chaperones, DNA damage sensing/repair proteins and a set of degradation proteins (Kültz, 2005). In Saccharomyces cerevisiae, this common transcriptional profile to different stressors is known as stress core, part of the global common environmental stress response that facilitates the adaptive process (Gasch et al., 2000). Drosophila melanogaster also shows a common transcriptional profile to different environmental conditions that includes conserved genes in the common long-term stress response (Girardot et al., 2004). Heat, salinity, hypoxia, heavy metals, radiation, pesticides or even physical activity are considered stressors due to their ability to induce stress proteins production (Sørensen et al., 2003).
Search Strategy and Selection Criteria
The articles cited in this review were collected from PubMed database after a deep research in articles published from 1999 to 2020 with the following terms: “stress response” “heat shock proteins” “molecular chaperones”, the combination of “heat shock proteins” and “central nervous system” or “neurodegeneration”, the combination of “heat shock proteins” and “synapses”.
Role of Heat Shock Proteins in the Central Nervous System
HSPs proteins were first described as stress molecules that bind to destabilized proteins and assure a correct protein folding or facilitate the degradation of misfolded proteins, maintaining cellular proteostasis network (Hipp et al., 2019) (Figure 1). Thus, HSPs are a canonical cellular protection factor as stress-inducible proteins. Nevertheless, there are constitutive HSPs that are expressed ubiquitously; these constitutive HSPs have specific functions in cell physiology. In addition to protein folding, these proteins modulate cytoskeleton protection, anti-inflammatory response, autophagy, anti-apoptotic signals and developmental functions (Bakthisaran et al., 2015). Emerging evidences reveal specific activity of cellular chaperones in neural physiology, HSPs participate in synaptic maintenance, neuronal activity and protection against neurodegenerative insults under pathological conditions.
Figure 1.
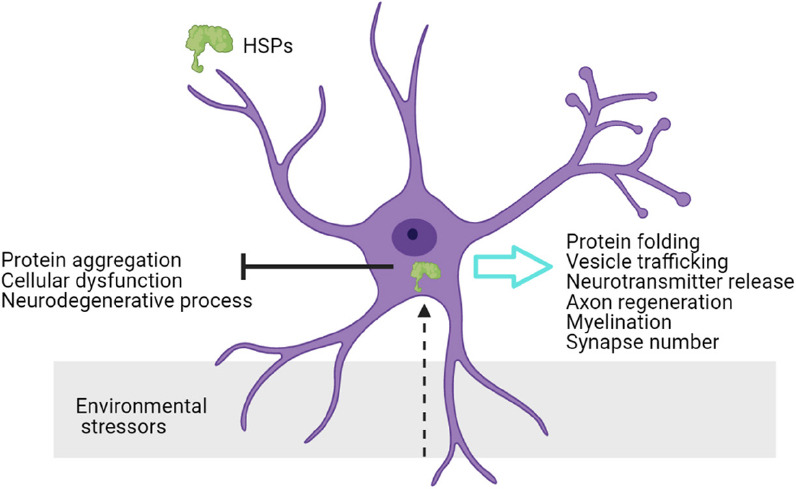
Neural functions of chaperones.
Neurons sense environmental stress and internal signals, and react through heat shock proteins (HSPs) function. HSPs functions in the nervous system regulate neuronal physiology.
HSPs in synaptic maintenance
Karunanithi et al. demonstrated that the exposure to heat stress induces Hsp70 expression in Drosophila melanogaster, and correlates with synaptic transmission maintenance in the neuromuscular junction (Karunanithi et al., 1999). This mechanism is conserved in mammals: hyperthermia induces sHsp27 and sHsp32 expression in Bergmann glial cells bodies. Their transport via radial glial fibers to the rat cerebellum (Bechtold and Brown, 2000). allows them to take part in neurotransmission regulation and neuronal protection (Bechtold and Brown, 2000). Moreover, HSP70 provokes a neuroprotective effect and increases neuronal survival under heat shock, oxidative stress, and ischemia-like conditions in vitro and in vivo models (Yenari et al., 1999; Kim et al., 2020). It has been proposed that glial cells can transfer HSP70 protein to neurons and promotes neuronal stress tolerance as a neuroprotective mechanism (Guzhova et al., 2001). Given the contribution of HSPs in neuronal survival and synapse maintenance, there is a need to understand the mechanisms underlying chaperone activity modulation in the nervous system.
HSPs are expressed in neurons and glial cells, and are involved in protein folding, vesicle trafficking and neurotransmitter release (Stetler et al., 2010). In this context, molecular chaperones mediate the turnover of receptors through uncoated vesicles in postsynaptic structures, and regulate vesicle fusion and neurotransmission in presynaptic structures (Stetler et al., 2010). Small HSPs (sHSPs) are the most extended HSPs subfamily, we have shown recently in Drosophila melanogaster that the modulation of sHSPs in neurons compromises synapse number. We described that the upregulation of sHsp23 and sHsp26 in neurons provokes an increase in synapse number and neuronal function, acting as pro-synaptogenic complex during development. Moreover, the combined downregulation of sHsp23 and sHsp26 does not alter synapse number. Genetic modifications that affect the expression of sHsp23 or sHsp26, provokes an imbalance in chaperones equilibrium that causes a reduction in synapse number, reinforcing the idea of chaperone complex and protein quality system equilibrium. Thus, sHSPs in neurons participate in synapse maintenance and modulation of neuronal function (Santana et al., 2020).
sHSPs balance in synapse maintenance
The chaperone quality system is critical for protein folding or degradation, in consequence it is under a fine-tuned regulation. A balance is established between folding or degradation chaperone activity and a failure in this system provokes pathological conditions (Muller et al., 2013). Different regulatory mechanisms have been proposed to modulate chaperone activity as the presence of unfolded or partially folded substrates, changes in temperature, posttranslational modifications and hetero-oligomers formation (Haslbeck and Vierling, 2015). Among these mechanisms of regulation, kinase proteins play a central role through posttranslational modifications that regulate HSPs dimer/oligomer formation balance and hence, HSPs activity (Muller et al., 2013; Haslbeck and Vierling, 2015) (Figure 2).
Figure 2.

Diagram of small chaperones sHSP23, sHSP26 and Pkm mechanisms of function.
sHSP23 (blue) and sHSP26 (green) accumulate in neuronal cytoplasm. Pkm Kinase protein (yellow) promotes the inhibition of sHsp23 transcription. In consequence, sHSP23/26 complex modulates synapse number and neuronal activity. sHSP: Small heat shock protein.
HSPs proteins typically contain phosphorylation sites susceptible to kinase proteins modifications (Muller et al., 2013; Katsogiannou et al., 2014). Un-phosphorylated sHSP27 can stablish multimers that act as molecular chaperones, besides phosphorylated sHSP27 leads to a significant decrease of oligomer size and promote complex dissociation (Rogalla et al., 1999). Moreover, sHSP27 expression correlates with tumour cells resistance to chemotherapy and tumor progression (Matsushima-Nishiwaki et al., 2008; Kuramitsu et al., 2012). These results strongly suggest that the control of the phosphorylated HSP27 levels could be a new therapeutic strategy specially to counter cancer recurrence. In this context, Muller et al. (2013) modifications in the phosphorylation sites of the C-terminal region of HSP70 and HSP90. These alterations are critical for HSPs phosphorylation, activity and for the folding/degradation dynamic equilibrium that control the proliferation rate of cancer cells (Muller et al., 2013). Thus, the phosphorylation state can alter protein activity and interactions with chaperones clients as a key-mechanism to sHSPs regulation. In our recent work, we propose that that sHSP23 and 26 neuronal functions are mediated by a novel putative kinase protein CG1561, named Pinkman (Pkm) (Santana et al., 2020).
We decided to study the common interactome of sHSP23 and 26 to explore for potential modulators of their function as a complex. Both proteins share three common interactors predicted as protein kinase activity molecules: CG11534, CG43755 and CG1561 (Santana et al., 2020). Pkm is physically interacts with sHSP23 and sHSP26 (Guruharsha et al., 2011), and the absence of pkm does not alter sHSP23-26 complex formation (Santana et al., 2020). However, pkm knockdown provokes an increase in sHsp23 expression. Pkm is involved in the transcriptional regulation of sHSP23 that, in consequence, alters sHSPs complex stability, synapse number and neuronal activity. We hypothesized that Pkm-sHSPs balance is relevant as central regulatory mechanism for the correct formation, function and response of the nervous system to environmental conditions.
sHSPs in pathology
Internal environmental stressors such as pathophysiological stress, nerve injury, axon damage or ischemia, modulate HSPs expression in the nervous system. HSPs promote neuronal survival, axon regeneration, myelination and functional locomotor recovery (Ousman et al., 2017). In particular, HSP70 and HSP27 chaperones are well studied in nervous system damage, and have emerged as promising therapeutic target (Ousman et al., 2017). Further, increasing evidences associate chaperones strategies with a neuroprotective mechanism in neurodegeneration events (Stetler et al., 2010; Bakthisaran et al., 2015).
Nervous system protection by sHSPs
In addition to the previously described stressors, proteostasis network is vulnerable to collapse in neurons under certain conditions as aging or neurodegeneration, what is known as chaperone-overload model. In this context, an imbalance of cellular proteostasis is a hallmark in aging that induces protein aggregation, cellular dysfunction and the onset of neurodegenerative processes. HSPs system plays a central role in proteostasis network function, and participates as a defense barrier against disruptions in protein dynamics. Besides, recent publications propose HSPs function and protein levels as a brain quality system that declines with aging and neurodegeneration (Hipp et al., 2019). Within the HSPs family, sHSPs are upregulated in aged tissues in different species, and are proposed as a conserved anti-aging mechanism (Hipp et al., 2019). Our recent results and others show that overexpression of sHSPs increases synapse number (Santana et al., 2020), co-localize with aggregates in neurodegenerative-aggregated diseases and contribute to restore neural function (Stetler et al., 2010). Moreover, mutations that disrupt sHSPs structure and function are responsible of neuropathies and myopathies, known as chaperonopathies (Bakthisaran et al., 2015). For example, mutations in sHSP27 are commonly inherited as autosomal dominant mutations related to Charcot–Marie–Tooth disease (Bakthisaran et al., 2015). Besides, mutations in HSPB4 are related to retina degeneration and cataract disease (Frankfater et al., 2020). Moreover, mutations in sHSP20 sequence that affect protein phosphorylation state, affect smooth muscle contraction in cardiomiocytes (Gardner et al, 2019).
Trans Generational Regulation of Chaperones
Stressors change environmental conditions that induce the modulation of genetic programs, including changes in HSPs expression. These changes in the DNA code facilitate the adaptation to the new conditions and therefore, the heritable information can affect the features of the next generation (Maggert, 2019). In this context, a revolutionary field is emerging based on the impact of stress in the organism, and the inheritance of genetic and epigenetic modifications across generations that compromise fitness and evolution (Heard and Martienssen, 2014; Cappucci et al., 2019; McGreevy et al., 2019). Artemia is a genus of crustacean that after heat exposure increases HSP70 levels, this upregulation improves environmental stress resistance, and it is observed in three subsequent generations, (Norouzitallab et al., 2014). Moreover, in Drosophila ovaries and testis, Hsp70 chaperone interacts with the chaperone complex and factors involved in piRNAs biogenesis after heat shock, and induces displacement of these factors to the lysosomes (Cappucci et al., 2019). Besides, stress exposure in Caenorhabditis elegans alters the expression of Heat Shock Factor 1 (HSF-1) in the germline, this transcription factor modulates epigenetics mechanisms and induces inheritable marks that improve stress resistance in the progeny (Kishimoto et al., 2017). All these evidences indicate that HSPs cope with environmental conditions, and inheritable factors that modulate gene expression condition the adaptation of the next generations to stress. This form of adaptation claims for inheritable acquired features in evolution, consistent with other studies that link stress inheritance and CNS modulation (McGreevy et al., 2019).
Conclusions
The stress response has been a hot field of study during last decades linked to the challenge of better understanding role of HSPs in cell physiology. Proteotasis network arises as a key factor in the central nervous system and includes the non-canonical roles described here. We have recently described a balanced system between sHSP23 and sHSP26 that act under the modulation of Pinkman kinase-like protein in the synapse formation and modulation (Santana et al., 2020). This implication is consistent with other studies that propose HSPs as therapeutic molecules to neuronal disturbance (Stetler et al., 2010; Bakthisaran et al., 2015; Ousman et al., 2017; Hipp et al., 2019). Here we discuss a perspective for HSPs as a sensor to the environmental conditions that targets neural function and synaptogenesis in development, aging or neurodegenerative stages. Synapse disruption is associated with morphological impairments but also with cognitive and psychiatric disorders (Das et al., 2019). Thus, HSPs emerge as a new central mechanism to assist synapse formation and maintenance, but also a potential target to drive therapeutic approaches to CNS malignancies.
Acknowledgments:
We would like to thank biorender (www.biorender.com) for the open access platform to create Illustrations.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Bakthisaran R, Tangirala R, Rao CM. Small heat shock proteins: role in cellular functions and pathology. Biochim Biophys Acta. 2015;1854:291–319. doi: 10.1016/j.bbapap.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 2.Bechtold DA, Brown IR. Heat shock proteins Hsp27 and Hsp32 localize to synaptic sites in the rat cerebellum following hyperthermia. Mol Brain Res. 2000;75:309–320. doi: 10.1016/s0169-328x(99)00323-x. [DOI] [PubMed] [Google Scholar]
- 3.Cappucci U, Noro F, Casale AM, Fanti L, Berloco M, Alagia AA, Grassi L, Le Pera L, Piacentini L, Pimpinelli S. The Hsp70 chaperone is a major player in stress-induced transposable element activation. Proc Natl Acad Sci U S A. 2019;116:17943–17950. doi: 10.1073/pnas.1903936116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das SC, ChenD , Callor WB, Christensen E, Coon H, Williams ME. DiI-mediated analysis of presynaptic and postsynaptic structures in human postmortem brain tissue. J Comp Neurol. 2019;527:3087–3098. doi: 10.1002/cne.24722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frankfater C, Bozeman SL, Hsu FF, Andley UP. Alpha-crystallin mutations alter lens metabolites in mouse models of human cataracts. PLoS One. 2020;15:e0238081. doi: 10.1371/journal.pone.0238081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner GT, Travers JG, Qian J, Liu GS, Haghighi K, Robbins N, Jiang M, Li Y, Fan GC, Rubinstein J, Blaxall BC, Kranias EG. Phosphorylation of Hsp20 promotes fibrotic remodeling and heart failure. JACC Basic Transl Sci. 2019;4:188–199. doi: 10.1016/j.jacbts.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girardot F, Monnier V, Tricoire H. Genome wide analysis of common and specific stress responses in adult drosophila melanogaster. BMC Genomics. 2004;5:74. doi: 10.1186/1471-2164-5-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guruharsha KG, Rual JF, Zhai B, Mintseris J, Vaidya P, Vaidya N, Beekman C, Wong C, Rhee DY, Cenaj O, McKillip E, Shah S, Stapleton M, Wan KH, Yu C, Parsa B, Carlson JW, Chen X, Kapadia B, VijayRaghavan K, et al. A protein complex network of Drosophila melanogaster. Cell. 2011;147:690–703. doi: 10.1016/j.cell.2011.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guzhova I, Kislyakova K, Moskaliova O, Fridlanskaya I, Tytell M, Cheetham M, Margulis B. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914:66–73. doi: 10.1016/s0006-8993(01)02774-3. [DOI] [PubMed] [Google Scholar]
- 11.Haslbeck M, Vierling E. A first line of stress defense: Small heat shock proteins and their function in protein homeostasis. J Mol Biol. 2015;427:1537–1548. doi: 10.1016/j.jmb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heard E, Martienssen RA. Transgenerational epigenetic inheritance: myths and mechanisms. Cell. 2014;157:95–109. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hipp MS, Kasturi P, Hartl FU. The proteostasis network and its decline in ageing. Nat Rev Mol Cell Biol. 2019;20:421–435. doi: 10.1038/s41580-019-0101-y. [DOI] [PubMed] [Google Scholar]
- 14.Karunanithi S, Barclay JW, Robertson RM, Brown IR, Atwood HL. Neuroprotection at Drosophila synapses conferred by prior heat shock. J Neurosci. 1999;19:4360–4369. doi: 10.1523/JNEUROSCI.19-11-04360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katsogiannou M, Andrieu C, Rocchi P. Heat shock protein 27 phosphorylation state is associated with cancer progression. Front Genet. 2014;5:346. doi: 10.3389/fgene.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JY, Barua S, Huang MY, Park J, Yenari MA, Lee JE. Heat shock protein 70 (HSP70) induction: chaperonotherapy for neuroprotection after brain injury. Cells. 2020;9:2020. doi: 10.3390/cells9092020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishimoto S, Uno M, Okabe E, Nono M, Nishida E. Environmental stresses induce transgenerationally inheritable survival advantages via germline-to-soma communication in Caenorhabditis elegans. Nat Commun. 2017;8:14031. doi: 10.1038/ncomms14031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kültz D. Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol. 2005;67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- 19.Kuramitsu Y, Wang Y, Taba K, Suenaga S, Ryozawa S, Kaino S, Sakaida I, Nakamura K. Heat-shock protein 27 plays the key role in gemcitabine-resistance of pancreatic cancer cells. Anticancer Res. 2012;32:2295–2299. [PubMed] [Google Scholar]
- 20.Maggert KA. Stress: an evolutionarymutagen. Proc Natl Acad Sci U S A. 2019;116:17616–17618. doi: 10.1073/pnas.1912725116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsushima-Nishiwaki R, Takai S, Adachi S, Minamitani C, Yasuda E, Noda T, Kato K, Toyoda H, Kaneoka Y, Yamaguchi A, Kumada T, Kozawa O. Phosphorylated heat shock protein 27 represses growth of hepatocellular carcinoma via inhibition of extracellular signal-regulated kinase. J Biol Chem. 2008;283:18852–18860. doi: 10.1074/jbc.M801301200. [DOI] [PubMed] [Google Scholar]
- 22.McGreevy KR, Tezanos P, Ferreiro-Villar I, Pallé A, Moreno-Serrano M, Esteve-Codina A, Lamas-Toranzo I, Bermejo-Álvarez P, Fernández-Punzano J, Martín-Montalvo A, Montalbán R, Ferrón SR, Radford EJ, Fontán-Lozano Á, Trejo JL. Intergenerational transmission of the positive effects of physical exercise on brain and cognition. Proc Natl Acad Sci U S A. 2019;116:10103–10112. doi: 10.1073/pnas.1816781116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller P, Ruckova E, Halada P, Coates PJ, Hrstka R, Lane DP, Vojtesek B. C-terminal phosphorylation of Hsp70 and Hsp90 regulates alternate binding to co-chaperones CHIP and HOP to determine cellular protein folding/degradation balances. Oncogene. 2013;32:3101–3110. doi: 10.1038/onc.2012.314. [DOI] [PubMed] [Google Scholar]
- 24.Norouzitallab P, Baruah K, Vandegehuchte M, Van Stappen G, Catania F, Vanden Bussche J, Vanhaecke L, Sorgeloos P, Bossier P. Environmental heat stress induces epigenetic transgenerational inheritance of robustness in parthenogenetic Artemia model. FASEB J. 2014;28:3552–3563. doi: 10.1096/fj.14-252049. [DOI] [PubMed] [Google Scholar]
- 25.Ousman SS, Frederick A, Lim EF. Chaperone proteins in the central nervous system and peripheral nervous system after nerve injury. Front Neurosci. 2017;11:79. doi: 10.3389/fnins.2017.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, Paul C, Wieske M, Arrigo AP, Buchner J, Gaestel M. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor by phosphorylation. J Biol Chem. 1999;274:18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- 27.Santana E, de Los Reyes T, Casas-Tintó S. Small heat shock proteins determine synapse number and neuronal activity during development. PLoS One. 2020;15:e0233231. doi: 10.1371/journal.pone.0233231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sørensen JG, Kristensen TN, Loeschcke V. The evolutionary and ecological role of heat shock proteins. Ecol Lett. 2003;6:1025–1037. [Google Scholar]
- 29.Stetler RA, Gan Y, Zhang W, Liou AK, Gao Y, Cao G, Chen J. Heat shock proteins: cellular and molecular mechanisms in the central nervous system. Prog Neurobiol. 2010;92:184–211. doi: 10.1016/j.pneurobio.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tukaj S. Heat shock protein 70 as a double agent acting inside and outside the cell: insights into autoimmunity. Int J Mol Sci. 2020;21:5298. doi: 10.3390/ijms21155298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yenari MA, Giffard RG, Sapolsky RM, Steinberg GK. The neuroprotective potential of heat shock protein 70 (HSP70) Mol Med Today. 1999;5:525–531. doi: 10.1016/s1357-4310(99)01599-3. [DOI] [PubMed] [Google Scholar]


