Key Words: activities of daily living, Chinese Longitudinal Healthy Longevity Survey, cohort, older Chinese individuals, propensity score matching, risk, stroke, survey
Abstract
It remains unclear whether limitations in activities of daily living (ADL) increase the risk of stroke in older Chinese adults. This longitudinal study used data from the Chinese Longitudinal Healthy Longevity Survey to investigate the effects of limitations in ADL on the incidence of stroke in older adults. Between 2002 and 2011, 46,728 participants from 22 provinces in China were included in this study. Of participants, 11,241 developed limitations in ADL at baseline. A 3-year follow-up was performed to determine the incidence of stroke. During the 3-year follow-up, 929 participants (8.26%) and 2434 participants (6.86%) experienced stroke in the ADL limitations group and non-ADL limitations group, respectively. Logistic regression was used to analyze the effect of ADL limitations on the risk of stroke. The results showed that after adjusting for the confounding factors gender, age, weight, hypertension, diabetes, heart disease, natural teeth, hearing impairment, visual impairment, smoking, alcohol abuse, exercise, ethnicity, literacy, residential area, and poverty, the ADL limitations group had a 77% higher risk of developing stroke than the non-ADL limitations group. After propensity score matching, the ADL limitations group still had a 33% higher risk of developing stroke than the non-ADL limitations group (OR = 1.326, 95% CI: 1.174–1.497). These findings suggest that limitations in ADL are a stroke risk factor.
Chinese Library Classification No. R455; R741; R592
Introduction
Stroke is the leading cause of death and disability worldwide (Sacco et al., 2013). Interventions to prevent stroke have become a global public health priority (O’Donnell et al., 2016). The main known risk factors for stroke are hypertension, hyperlipidemia, smoking, diet, and physical inactivity (O’Donnell et al., 2016; Diener and Hankey, 2020). Owing to scientific knowledge about these risk factors, as well as preventive and risk factor modification strategies, the incidence of stroke is decreasing (GBD 2019 Diseases and Injuries Collaborators, 2020). However, some potential risk factors remain to be identified and used to screen high-risk populations.
Activities of daily living (ADL) are the basic tasks that an individual is able to perform to function on a day-to-day basis. These include bathing, dressing, eating, indoor transferring, toileting, and continence (Katz et al., 1963; Edemekong et al., 2021). ADL limitations are defined as difficulty or the need for assistance with at least one task (Fuller-Thomson et al., 2009; Wen and Gu, 2011). Approximately 9.7% of older people experience ADL limitations, and older age, joint/nerve pain, stroke, pelvic/femoral fractures, heart disease, and diabetes are common causes of ADL limitations (Sousa et al., 2009; Malhotra et al., 2012). Because ADL limitations can affect diet, physical inactivity, and weight (Henry-Sánchez et al., 2012), they increase the risk of stroke. Several studies have explored the association between physical dependence and stroke risk (Henry-Sánchez et al., 2012), but results are inconsistent. To further investigate the effect of ADL limitations on the risk of stroke, we conducted a large-scale population-based longitudinal study involving 46,728 individuals with a 3-year follow-up.
Materials and Methods
Data source
The study data were drawn from the Chinese Longitudinal Healthy Longevity Survey (CLHLS). The CLHLS is a high-quality international cooperative project hosted by the Center for the Study of Aging and Human Development at Duke University, the Center for Healthy Aging and Development Studies of Peking University, and other institutions. The CLHLS conducted seven surveys in 22 provinces of China in 1998, 2000, 2002, 2005, 2008, 2011, and 2014, respectively. The populations of these provinces comprise approximately 85% of the total population of China. To ensure sufficient participants for each age group, gender, and region, the CLHLS used the random sampling method of unequal probability proportions to obtain the required sample size for analysis. During the follow-up period, survivors and close relatives of deceased participants were reinterviewed, and deceased interviewees were replaced with new participants (Deng et al., 2020). The study followed the STrengthening the Reporting of OBservational Studies in Epidemiology (STROBE) statement (Additional file 1). The present study is a secondary analysis using the CLHLS data. The need for ethical approval for the study was waived by an institutional review board (IRB00001052–13074) for the CLHLS study, which was approved by the research ethics committees of Duke University and Peking University. Thus, consent for participation was deemed unnecessary for this study. The data were anonymized before use.
STROBE Statement–Checklist of items that should be included in reports of cohort studies
| Item No | Recommendation | Reported on page No. | |
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | p.1,2 and 3 |
|
| |||
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | p.2 and 3 | ||
|
| |||
| Introduction | |||
|
| |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | p.3 |
|
| |||
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | p.3 |
|
| |||
| Methods | |||
|
| |||
| Study design | 4 | Present key elements of study design early in the paper | p.4 and 5 |
|
| |||
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | p.4 |
|
| |||
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up | p.4 and 5 |
|
| |||
| (b) For matched studies, give matching criteria and number of exposed and unexposed | p.6 | ||
|
| |||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | p.4,5 and 6 |
|
| |||
| Data sources/ measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | p.4,5 and 6 |
|
| |||
| Bias | 9 | Describe any efforts to address potential sources of bias | p.6 |
|
| |||
| Study size | 10 | Explain how the study size was arrived at | p.7 |
|
| |||
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | p.5 and 6 |
|
| |||
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | p.6 |
|
| |||
| (b) Describe any methods used to examine subgroups and interactions | p.7 | ||
|
| |||
| (c) Explain how missing data were addressed | p.6 | ||
|
| |||
| (d) If applicable, explain how loss to follow-up was addressed | Not applicable | ||
|
| |||
| (e) Describe any sensitivity analyses | p.6 | ||
|
| |||
| Results | |||
|
| |||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | p.7 |
|
| |||
| (b) Give reasons for non-participation at each stage | p.7 | ||
|
| |||
| (c) Consider use of a flow diagram | p.13 | ||
|
| |||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders | p.7 and 15 |
|
| |||
| (b) Indicate number of participants with missing data for each variable of interest | p.7 and 15 | ||
|
| |||
| (c) Summarise follow-up time (eg, average and total amount) | p.4 and 7 | ||
|
| |||
| Outcome data | 15* | Report numbers of outcome events or summary measures over time | p.7,13 and 15 |
|
| |||
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder- adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included | p.5, 7 and 17 |
|
| |||
| (b) Report category boundaries when continuous variables were categorized | p.4 | ||
|
| |||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | p.7 and 17 | ||
|
| |||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses | p.7, 17 and 18 |
|
| |||
| Discussion | |||
|
| |||
| Key results | 18 | Summarise key results with reference to study objectives | p.8 |
|
| |||
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | p.8 and 9 |
|
| |||
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | p.8 and 9 |
|
| |||
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | p.8 and 9 |
|
| |||
| Other information | |||
|
| |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | p.9 |
*Give information separately for exposed and unexposed groups.
Note: An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at http://www.strobe-statement.org.
Data collection
The CLHLS data were collected from household surveys by professionally trained investigators. They used the Katz scale to assess the ADL of older adults (Katz et al., 1963). Older adults were assessed on whether they exercised regularly by playing ball, swimming, or walking for fitness. Health data, such as vision, oral health, hearing, and previous medical history, were collected by doctors after detailed examination of participants. Details of the survey method can be found at https://sites.duke.edu/centerforaging/programs/chinese-longitudinal-healthy-longevity-survey-clhls/.
Study design and participants
Since 2002, the CLHLS has expanded the sample to include participants over 65 years old. Therefore, we used all survey waves from 2002 to 2011 and merged the data of these waves into one data set. After excluding participants who had had a stroke at baseline or had been followed for less than 3 years, we included the remaining participants in a 3-year cohort. Participants who experienced ADL limitations were included in the exposure group, and the rest were included in the control group.
The study outcome was stroke. Participants who had survived a stroke during the subsequent 3 years, those whose main cause of death was stroke, and those who had had a stroke before death were included in the outcome group with stroke. The remaining participants, whether alive or dead, were included in the outcome group without stroke.
Assessment of stroke occurrence
Stroke was defined according to the following items from the CLHLS questionnaire: “Q: Suffering from stroke or cerebrovascular diseases? A: Yes,” “Q: Name of disease suffering from for the first time. A: Stroke, Cerebrovascular diseases,” “Q: Name of disease suffering from for the second time. A: Stroke, Cerebrovascular diseases,” “Q: Name of last disease suffering from. A: Stroke, cerebrovascular diseases,” or “Q: Main cause of death? A: Cerebrovascular diseases (CVD).”
Assessment of ADL limitations
At baseline, six aspects of ADL were assessed: bathing, dressing, eating, indoor transferring, continence, and toileting (Katz et al., 1963). ADL limitations were defined as the inability to complete any ADL alone (Fuller-Thomson et al., 2009; Wen and Gu, 2011) and have been described above.
Assessment of covariates
To control for confounding factors, we included the following variables as covariates in the analysis: gender, age, weight, hypertension, diabetes, heart disease, natural teeth, hearing impairment, visual impairment, type of residential area, current smoking, drinking spirits, exercise, ethnicity, literacy, and poverty. These covariates were selected as potential confounders based on the literature (Woodward et al., 2005; Ferri et al., 2011; O’Donnell et al., 2016; Fang et al., 2019; Liccardo et al., 2019).
We divided age into five 10-year age brackets. Because of the lack of appropriate parameters (e.g., height or knee height) for calculation of body mass index in the 2005 wave, we could only divide body weight into four categories by quartiles. Having natural teeth was defined as the number of natural teeth > 0. In the raw data, hearing ability was recorded as “1 = yes, without hearing aid,” “2 = yes, but needs hearing aid,” “3 = partly, despite using hearing aid,” and “4 = no.” Hearing impairment was defined as hearing ability equal to 2, 3, or 4. Visual function was recorded as “1 = can see and distinguish the break in the circle,” “2 = can see but cannot distinguish the break in the circle,” “3 = cannot see,” and “4 = blind.” Visual impairment was defined as visual function equal to 2, 3, or 4. Participants were divided into Han and other ethnic groups. Literate was defined as years of schooling > 0. Poverty was defined according to the official definition (National Bureau of Statistics, 2012) of a per capita annual income < 2300 yuan.
Statistical analysis
Multiple imputation was used to handle missing data. Frequencies were used to describe the sample and to compare the ADL limitations group with the non-ADL limitations group. A logistic regression model was used to evaluate the relationship between ADL limitations and risk of stroke and to calculate the odds ratios (ORs). The covariates were adjusted in three analysis plans. In plan 1, gender, age, and weight were adjusted (Model 1). In plan 2, hypertension, diabetes, heart disease, natural teeth, hearing impairment, and visual impairment were adjusted based on plan 1 (Model 2). In plan 3, smoking, drinking spirits, exercise, literacy, ethnicity, and poverty were adjusted based on plan 2 (Model 3).
For various reasons, bias and confounding are major problems in observational studies and can lead to incorrect results. Propensity score matching (PSM) is a statistical method used to eliminate confounding factors by balancing baseline covariates between the observation and control groups to mimic the expected effects of randomization. Briefly, PSM creates matched sets of participants for observation and control groups with similar propensity scores to control confounding. If risk factors for the outcomes are balanced at baseline, differences in outcome risk are likely to be caused by a different variable (Deb et al., 2016).
To eliminate the effects of potential confounding factors and increase the validity of the results, PSM was performed to balance the baseline characteristics of the samples (Deb et al., 2016). Logistic regression was used to build a propensity scoring model. Using 1:1 matching without replacement, the threshold was 0.02. It is not appropriate in statistical significance testing to evaluate the balance of covariates between groups (Austin, 2007; Deb et al., 2016; Benedetto et al., 2018). Standardized mean differences (SMD) were used to assess intergroup balance. SMDs < 10% are considered to indicate balance between the groups (Deb et al., 2016; Benedetto et al., 2018). The variance inflation factor was used to test whether there was multicollinearity among the independent variables. We considered a P value < 0.05 (two-sided) to be statistically significant. For cleaning, coding, and analyzing all data, we used Python (Version 3.6.10, Python Software Foundation, Wilmington, DE, USA) and the Python packages Pandas (Version 1.1.1; https://pandas.pydata.org/), Tableone (https://github.com/kaz-yos/tableone) (Pollard et al., 2018), and Statsmodels (Version 0.12.0; https://www.statsmodels.org/) (Seabold and Perktold, 2010).
Results
The merged data set comprised 58 421 participants. A total of 11 693 participants were excluded because of stroke at baseline or follow-up of less than 3 years. Finally, 46 728 participants were included in the study. Figure 1 shows the process of participant inclusion and grouping.
Figure 1.
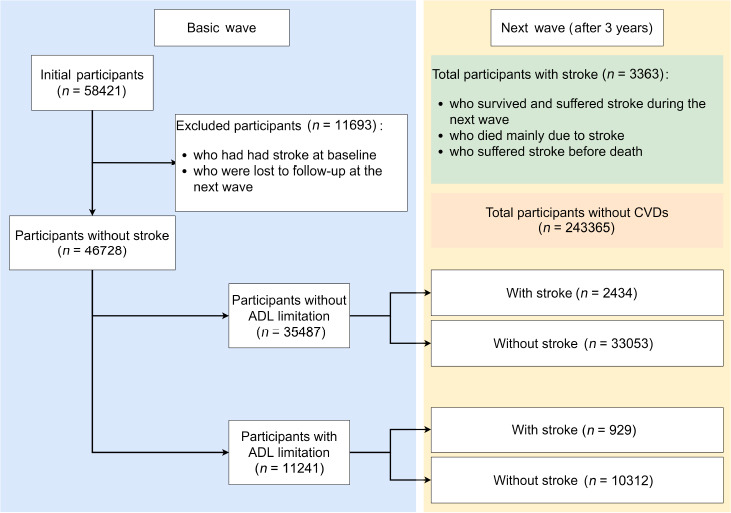
Flow chart of participant inclusion and grouping.
ADL: Activities of daily living; CVD: cerebrovascular diseases.
Table 1 shows the participant characteristics at baseline. A total of 46,728 participants were included in the analysis: 11 241 in the ADL limitations group and 35,487 in the control group. The proportion of missing values for all variables was less than 5%. Of the 46,728 participants, 24.1% had ADL limitations, 42.6% were men, and 53.8% were 80–100 years old. Before PSM, the SMDs of gender, age, body weight, natural teeth, visual impairment, residential area, smoking, exercise, literacy, and Han ethnicity were greater than 10%. After PSM, the SMDs of the covariates were less than 10%. Figure 2 shows the SMD of each variable more intuitively.
Table 1.
Baseline characteristics before and after propensity score matching
| Variables | Missing M%)] | All participants | Propensity-matched participants | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| ADL limitations | ADL limitations | |||||||||
|
|
|
|||||||||
| Overall (n = 46728) | No (n = 35487) | Yes (n = 11241) | SMD | Overall (n = 15240) | No (n = 7620) | Yes (n = 7620) | SMD | |||
| Gender | Female | 0 | 26804 (57.4) | 18794 (53.0) | 8010 (71.3) | 0.384 | 10569 (69.4) | 5325 (69.9) | 5244 (68.8) | 0.023 |
| Male | 19924 (42.6) | 16693 (47.0) | 3231 (28.7) | 4671 (30.6) | 2295 (30.1) | 2376 (31.2) | ||||
| Age (yr) | 60 < and < 70 | 130 (0.2) | 5382 (11.5) | 5269 (14.8) | 113 (1.0) | 1.144 | 210 (1.4) | 101 (1.3) | 109 (1.4) | 0.024 |
| 70 < and < 80 | 9523 (20.4) | 9016 (25.4) | 507 (4.5) | 943 (6.2) | 462 (6.1) | 481 (6.3) | ||||
| 80 < and < 90 | 12711 (27.2) | 10476 (29.5) | 2235 (19.9) | 3831 (25.1) | 1905 (25.0) | 1926 (25.3) | ||||
| 90 < and <100 | 12452 (26.6) | 7753 (21.8) | 4699 (41.8) | 6481 (42.5) | 3283 (43.1) | 3198 (42.0) | ||||
| > 100 | 6660 (14.3) | 2973 (8.4) | 3687 (32.8) | 3775 (24.8) | 1869 (24.5) | 1906 (25.0) | ||||
| Body weight (kg) | < 41 | 527 (0.9) | 12358 (26.4) | 8005 (22.6) | 4353 (38.7) | 0.402 | 5785 (38.0) | 2892 (38.0) | 2893 (38.0) | 0.017 |
| 41 < and < 49 | 12154 (26.0) | 9190 (25.9) | 2964 (26.4) | 4114 (27.0) | 2078 (27.3) | 2036 (26.7) | ||||
| 49 < and < 56 | 11466 (24.5) | 9240 (26.0) | 2226 (19.8) | 3044 (20.0) | 1522 (20.0) | 1522 (20.0) | ||||
| > 56 | 10750 (23.0) | 9052 (25.5) | 1698 (15.1) | 2297 (15.1) | 1128 (14.8) | 1169 (15.3) | ||||
| Hypertension | No | 2287 (3.9) | 38147 (81.6) | 28754 (81.0) | 9393 (83.6) | 0.066 | 12853 (84.3) | 6463 (84.8) | 6390 (83.9) | 0.026 |
| Yes | 8581 (18.4) | 6733 (19.0) | 1848 (16.4) | 2387 (15.7) | 1157 (15.2) | 1230 (16.1) | ||||
| Diabetes | No | 2560 (4.4) | 45641 (97.7) | 34675 (97.7) | 10966 (97.6) | 0.01 | 14947 (98.1) | 7488 (98.3) | 7459 (97.9) | 0.028 |
| Yes | 1087 (2.3) | 812 (2.3) | 275 (2.4) | 293 (1.9) | 132 (1.7) | 161 (2.1) | ||||
| Heart disease | No | 2407 (4.1) | 42713 (91.4) | 32636 (92.0) | 10077 (89.6) | 0.08 | 14087 (92.4) | 7092 (93.1) | 6995 (91.8) | 0.048 |
| Yes | 4015 (8.6) | 2851 (8.0) | 1164 (10.4) | 1153 (7.6) | 528 (6.9) | 625 (8.2) | ||||
| Have natural teeth | No | 0 | 16464 (35.2) | 10523 (29.7) | 5941 (52.9) | 0.485 | 7256 (47.6) | 3633 (47.7) | 3623 (47.5) | 0.003 |
| Yes | 30264 (64.8) | 24964 (70.3) | 5300 (47.1) | 7984 (52.4) | 3987 (52.3) | 3997 (52.5) | ||||
| Hearing impairment | No | 70 (0.1) | 31649 (67.7) | 27513 (77.5) | 4136 (36.8) | 0.903 | 7002 (45.9) | 3507 (46.0) | 3495 (45.9) | 0.003 |
| Yes | 15079 (32.3) | 7974 (22.5) | 7105 (63.2) | 8238 (54.1) | 4113 (54.0) | 4125 (54.1) | ||||
| Visual impairment | No | 486 (0.8) | 29401 (62.9) | 25333 (71.4) | 4068 (36.2) | 0.755 | 6679 (43.8) | 3334 (43.8) | 3345 (43.9) | 0.003 |
| Yes | 17327 (37.1) | 10154 (28.6) | 7173 (63.8) | 8561 (56.2) | 4286 (56.2) | 4275 (56.1) | ||||
| Residence | City | 0 | 8612 (18.4) | 5695 (16.0) | 2917 (25.9) | 0.245 | 2903 (19.0) | 1407 (18.5) | 1496 (19.6) | 0.042 |
| Town | 10519 (22.5) | 8232 (23.2) | 2287 (20.3) | 3155 (20.7) | 1544 (20.3) | 1611 (21.1) | ||||
| Rural | 27597 (59.1) | 21560 (60.8) | 6037 (53.7) | 9182 (60.2) | 4669 (61.3) | 4513 (59.2) | ||||
| Smoking | No | 138 (0.2) | 37953 (81.2) | 27851 (78.5) | 10102 (89.9) | 0.316 | 13544 (88.9) | 6820 (89.5) | 6724 (88.2) | 0.04 |
| Yes | 8775 (18.8) | 7636 (21.5) | 1139 (10.1) | 1696 (11.1) | 800 (10.5) | 896 (11.8) | ||||
| Drinking spirits | No | 0 | 41644 (89.1) | 31460 (88.7) | 10184 (90.6) | 0.064 | 13917 (91.3) | 7003 (91.9) | 6914 (90.7) | 0.041 |
| Yes | 5084 (10.9) | 4027 (11.3) | 1057 (9.4) | 1323 (8.7) | 617 (8.1) | 706 (9.3) | ||||
| Exercising | No | 209 (0.4) | 32818 (70.2) | 23362 (65.8) | 9456 (84.1) | 0.432 | 12344 (81.0) | 6195 (81.3) | 6149 (80.7) | 0.015 |
| Yes | 13910 (29.8) | 12125 (34.2) | 1785 (15.9) | 2896 (19.0) | 1425 (18.7) | 1471 (19.3) | ||||
| Literacy | No | 258 (0.4) | 29275 (62.6) | 20802 (58.6) | 8473 (75.4) | 0.362 | 11432 (75.0) | 5761 (75.6) | 5671 (74.4) | 0.027 |
| Yes | 17453 (37.4) | 14685 (41.4) | 2768 (24.6) | 3808 (25.0) | 1859 (24.4) | 1949 (25.6) | ||||
| Han ethnicity | No | 0 | 3722 (8.0) | 3128 (8.8) | 594 (5.3) | 0.138 | 880 (5.8) | 398 (5.2) | 482 (6.3) | 0.047 |
| Yes | 43006 (92.0) | 32359 (91.2) | 10647 (94.7) | 14360 (94.2) | 7222 (94.8) | 7138 (93.7) | ||||
| Poverty | No | 1129 (1.9) | 20359 (43.6) | 15701 (44.2) | 4658 (41.4) | 0.057 | 6079 (39.9) | 3015 (39.6) | 3064 (40.2) | 0.013 |
| Yes | 26369 (56.4) | 19786 (55.8) | 6583 (58.6) | 9161 (60.1) | 4605 (60.4) | 4556 (59.8) | ||||
The propensity score matching (PSM) was performed to balance the baseline characteristics of samples. The intergroup balance was assessed by standardized mean differences (SMD). If SMD is less than 10%, it is considered to be a balance between groups. ADL: Activities of daily living.
Figure 2.
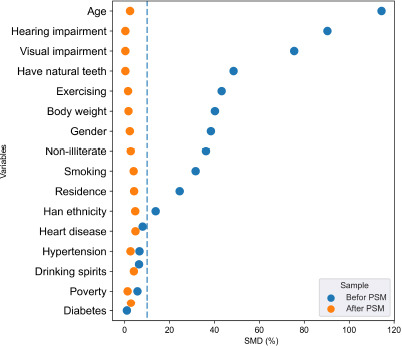
The standardized mean differences (SMD) of the variables.
The propensity score matching (PSM) was performed to balance the baseline characteristics of samples. The intergroup balance was assessed by SMD. If the SMD is less than 10%, it is considered to be a balance between groups.
During the 3-year follow-up, 929 participants (8.26%) and 2434 participants (6.86%) experienced stroke in the ADL limitations and control groups, respectively. ADL limitations were significantly related to stroke. Compared with those without ADL limitations, participants with ADL limitations had a 77% higher risk of stroke (OR = 1.77, 95% confidence interval (CI): 1.56–2.016) after adjusting for gender, age, weight, hypertension, diabetes, heart disease, natural teeth, hearing impairment, visual impairment, smoking, drinking spirits, exercising, literacy, ethnicity, and poverty. The risk increase was slightly attenuated to 33% (OR = 1.326, 95% CI: 1.174–1.497) after PSM (Table 2).
Table 2.
The result of logistic regression between ADL limitations and stroke
| Variables | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||||||
| 95% CI | 95% CI | 95% CI | 95% CI | |||||||||||||
|
|
|
|
|
|||||||||||||
| OR | P | [0.025 | 0.975] | OR | P | [0.025 | 0.975] | OR | P | [0.025 | 0.975] | OR | P | [0.025 | 0.975] | |
| Intercept | 0.04 | *** | 0.04 | 0.05 | 0.03 | *** | 0.03 | 0.04 | 0.04 | *** | 0.03 | 0.05 | 0.08 | *** | 0.04 | 0.14 |
| ADL limitations (vs. No) | 2.15 | *** | 1.91 | 2.44 | 1.79 | *** | 1.58 | 2.04 | 1.77 | *** | 1.56 | 2.02 | 1.33 | *** | 1.17 | 1.5 |
| Gender (vs. female) | 0.96 | 0.89 | 1.03 | 1.11 | ** | 1.03 | 1.2 | 1.15 | ** | 1.06 | 1.26 | 1.16 | 0.99 | 1.37 | ||
| Age (yr) (vs. 60 < and < 70 yr) | ||||||||||||||||
| 70 < and < 80 | 1.25 | *** | 1.16 | 1.35 | 1.21 | *** | 1.12 | 1.31 | 1.21 | *** | 1.12 | 1.31 | 1.09 | 0.69 | 1.71 | |
| 80 < and < 90 | 1.23 | *** | 1.09 | 1.38 | 1.16 | * | 1.03 | 1.31 | 1.15 | * | 1.02 | 1.31 | 0.86 | 0.56 | 1.33 | |
| 90 < and < 100 | 1.03 | 0.7 | 1.5 | 1 | 0.68 | 1.47 | 0.98 | 0.66 | 1.44 | 0.7 | 0.46 | 1.09 | ||||
| > 100 | 0.72 | 0.05 | 10.01 | 0.7 | 0.05 | 9.89 | 0.68 | 0.05 | 9.59 | 0.53 | ** | 0.34 | 0.84 | |||
| Body weight (kg) (vs. < 41 kg) | ||||||||||||||||
| 41 < and < 49 | 1.27 | ** | 1.1 | 1.47 | 1.23 | ** | 1.06 | 1.42 | 1.21 | * | 1.05 | 1.4 | 1.15 | 0.98 | 1.36 | |
| 49 < and < 56 | 1.66 | *** | 1.44 | 1.9 | 1.51 | *** | 1.31 | 1.74 | 1.47 | *** | 1.27 | 1.69 | 1.25 | * | 1.05 | 1.5 |
| > 56 | 2.18 | *** | 1.9 | 2.51 | 1.74 | *** | 1.51 | 2.01 | 1.64 | *** | 1.42 | 1.9 | 1.38 | ** | 1.12 | 1.69 |
| Hypertension (vs. No) | 1.58 | *** | 1.46 | 1.71 | 1.57 | *** | 1.45 | 1.7 | 1.64 | *** | 1.41 | 1.9 | ||||
| Diabetes (vs. No) | 1.97 | *** | 1.73 | 2.25 | 1.89 | *** | 1.66 | 2.16 | 1.26 | 0.9 | 1.76 | |||||
| Heart disease (vs. No) | 1.81 | *** | 1.64 | 1.98 | 1.74 | *** | 1.59 | 1.92 | 1.33 | ** | 1.09 | 1.61 | ||||
| Have natural teeth (vs. No) | 1.01 | 0.92 | 1.11 | 1.02 | 0.92 | 1.12 | 1.01 | 0.89 | 1.14 | |||||||
| Hearing impairment (vs. No) | 1.28 | *** | 1.12 | 1.46 | 1.28 | *** | 1.12 | 1.46 | 1.15 | * | 1.01 | 1.31 | ||||
| Visual impairment (vs. No) | 1.07 | 0.98 | 1.17 | 1.08 | 0.98 | 1.19 | 0.9 | 0.79 | 1.02 | |||||||
| Residence (vs. City) | ||||||||||||||||
| Town | 0.78 | *** | 0.69 | 0.87 | 0.76 | ** | 0.63 | 0.91 | ||||||||
| Rural | 0.82 | *** | 0.74 | 0.9 | 0.8 | ** | 0.68 | 0.94 | ||||||||
| Smoking (vs. No) | 0.95 | 0.87 | 1.04 | 0.89 | 0.73 | 1.09 | ||||||||||
| Drinking spirits (vs. No) | 0.99 | 0.88 | 1.11 | 0.85 | 0.68 | 1.07 | ||||||||||
| Exercising (vs. No) | 1 | 0.93 | 1.08 | 0.88 | 0.75 | 1.03 | ||||||||||
| Literate (vs. No) | 0.99 | 0.91 | 1.07 | 1.06 | 0.91 | 1.24 | ||||||||||
| Han ethnicity (vs. No) | 1.18 | * | 1 | 1.38 | 1.18 | 0.88 | 1.57 | |||||||||
| Poverty (vs. No) | 0.98 | 0.91 | 1.06 | 0.91 | 0.8 | 1.03 | ||||||||||
Model 1: Gender, age, and weight were adjusted. Model 2: Hypertension, diabetes, heart disease, natural teeth, hearing impairment, and visual impairment were additionally adjusted. Model 3: Smoking, drinking hard alcohol, exercising, literacy, ethnicity, and poverty were additionally adjusted; Model 4: A model that uses propensity score matching (PSM) to balance the baseline before adjusting for covariates. *P < 0.05, **P < 0.01, ***P < 0.001. ADL: Activities of daily living.
In addition to ADL limitations, several covariates were associated with stroke. In the PSM model, participants older than 100 years had a lower OR than those aged ≥ 60 years and ≤ 70 years. Participants from rural or town areas had lower ORs of stroke than those from cities. The ORs and 95% CIs of body weight, hypertension, heart disease, and hearing impairment were greater than 1. The risk of ADL limitations ranked fourth after hypertension (OR = 1.64, 95% CI: 1.41–1.9), body weight over 56 kg (OR = 1.38, 95% CI: 1.12–1.69), and heart disease (OR = 1.33, 95% CI: 1.09–1.61).
We also conducted a subgroup analysis based on gender, age, and residential area. ADL limitations significantly increased the risk of stroke in subgroups of different genders, subgroups of different residential areas, and subgroups of participants ≤ 90 years old. In the two subgroups of participants older than 90 years, ADL limitations did not increase the risk of stroke (Table 3).
Table 3.
The relationship between ADL limitations and stroke in each subgroup
| Variables | OR | 95% CI | P | ||
|---|---|---|---|---|---|
|
| |||||
| [0.025 | 0.975] | ||||
| All participants |
|
1.56 | 1.77 | 2.02 | *** |
| Gender | |||||
| Female | 1.98 | 1.67 | 2.35 | *** | |
| Male | 1.53 | 1.26 | 1.86 | *** | |
| Age (yr) | |||||
| 60 ≤ and ≤ 70 | 1.67 | 1.28 | 2.19 | *** | |
| 70 < and ≤ 80 | 2.04 | 1.70 | 2.45 | *** | |
| 80 < and ≤ 90 | 1.62 | 1.26 | 2.09 | *** | |
| 90 < and ≤ 100 | 1.22 | 0.54 | 2.78 | ||
| 100 < | 1.22 | 0.00 | 392.75 | ||
| Residence | |||||
| City | 1.67 | 1.29 | 2.17 | *** | |
| Town | 2.02 | 1.50 | 2.70 | *** | |
| Rural | 1.71 | 1.44 | 2.03 | *** | |
***P < 0.001. ADL: Activities of daily living.
The variance inflation factor of each variable was less than 10, which indicated that there was no serious multicollinearity among the independent variables (Table 4).
Table 4.
Variance inflation factor of each variable
| Variables | Variance inflation factor | Variables | Variance inflation factor |
|---|---|---|---|
| Intercept | 41.5 | Visual impairment | 1.3 |
| ADL limitation | 1.3 | Residence | 1.2 |
| Gender | 1.7 | Smoking | 1.2 |
| Age | 1.7 | Drinking spirits | 1.1 |
| Body weight | 1.5 | Exercising | 1.1 |
| Hypertension | 1.1 | Literacy | 1.5 |
| Diabetes | 1 | Han ethnicity | 1 |
| Heart disease | 1.1 | Poverty | 1.1 |
| Have natural teeth | 1.1 | ||
| Hearing impairment | 1.5 |
ADL: Activities of daily living.
Discussion
In this study, we found a significant correlation between ADL limitations and stroke. When covariates were adjusted or subjected to PSM, having ADL limitations increased the odds of stroke by 77% or 33%, respectively, compared with having no ADL limitations. Therefore, ADL limitations increase the risk of stroke.
ADL limitations are associated with aging and chronic diseases, such as heart disease and diabetes (Sousa et al., 2009; Hou et al., 2018; Fong, 2019), which may also be stroke risk factors (O’Donnell et al., 2016). ADL limitations restrict activities and increase financial burden. Older adults with ADL limitations may delay the treatment of related chronic diseases, such as hypertension and diabetes, because of difficulties in seeing a doctor. ADL limitations may also increase the prevalence of depression (Wada et al., 2004) and further increase the prevalence of stroke (Van der Kooy et al., 2007). ADL limitations may increase the risk of stroke through the above mechanisms.
Previous large prospective studies have reported a relationship between ADL and stroke risk. In a study by Heshmatollah et al. (2020), 489 of 8519 individuals had stroke, and 20 ADL items (per standard deviation decrease) were associated with higher stroke risk. In a study by Capistrant et al. (2013), of 18 441 participants, those who developed stroke had worse ADL independence (five items) than those who remained stroke-free throughout the follow-up period. Colantonio et al. (1992) measured physical function using ADL and the Rosow scale, and found an association between impairment of physical function and stroke risk among 2812 participants. However, in another prospective study of 9451 participants, ADL limitations had no effect on the odds of stroke (Clarke et al., 2011). There are several differences between the above studies and our study. First, this study had a larger sample (46,728 participants). Second, 16 covariates were considered and subjected to PSM. Third, the participants in this study were Asian people from developing countries, which further confirmed that ADL limitations are a risk factor for different groups of people. Moreover, in the present study, a simpler definition of ADL limitations was used that was more suitable for stroke screening.
When the baseline was balanced using PSM, participants older than 100 years had lower stroke odds than participants aged 60–70 years. This may be because the overall life expectancy of cardiovascular disease patients is relatively short (Wang et al., 2014), and it is difficult for them to live beyond 100 years. As a result, the incidence of stroke is lower in adults older than 100 years. In all models adjusted for hypertension and heart disease, the OR of these variables was greater than 1 and P < 0.001. These risk factors have long been confirmed by many studies (Turin et al., 2016; Zhang et al., 2020). These findings also indicate that early intervention for these risk factors is essential. In all models adjusted for hearing impairment, this variable was a risk factor for stroke. In model 3, hearing impairment increased participants’ odds of stroke by 28%. This finding is similar to the results of a study by Fang et al. (2019). However, the mechanism underlying the interaction between hearing impairment and stroke needs further research. Compared with participants who lived in cities, participants who lived in towns or rural areas had lower stroke odds. Participants living in cities may have greater life pressures. Additionally, air pollution in urban areas is more severe, and air pollution is another risk factor for stroke (Li et al., 2020). Compared with nonurban participants, urban participants have better economic status, a better medical environment, and better health awareness, which may explain why participants in urban areas have a higher stroke diagnosis rate. Nonurban participants may have experienced a stroke without knowing it.
This study had the following shortcomings. First, because of the lack of an exact diagnosis date for stroke, accurate survival analysis could not be performed. Second, the diagnosis of stroke was based on participant self-reports or reports from relatives of deceased individuals. This may have led to bias in the entry results. Third, although we used a variety of methods to correct for confounding factors, there is no perfect correction method for retrospective data.
In summary, after adjusting for age, gender, chronic diseases, and other covariates, a significant correlation between ADL limitations and stroke incidence remained. Therefore, ADL limitations are a risk factor for stroke. The ADL scale is simple, easy to learn, and inexpensive. It is recommended that ADL limitations be used as a screening tool for stroke to quickly identify high-risk populations.
Additional files:
Additional file 1: STROBE checklist.
Additional file 2: Open peer review reports 1 (85.8KB, pdf) and 2 (87.8KB, pdf) .
Footnotes
Conflicts of interest: The authors declare that they have no competing interests.
Financial support: This study was supported by a grant from the Clinical Research Project of Affiliated Hospital of Guangdong Medical University of China, Nos. LCYJ2018A00 (to ZL) and LCYJ2019C006 (to YSC); the Natural Science Foundation of Guangdong Province of China, No. 2020A151501284 (to ZL); the Science and Technology Planning Project of Zhanjiang of China, No. 2018A01021 (to ZL); and a grant from the Characteristic Innovation Projects of Colleges and Universities in Guangdong Province of China, No. 2019KTSCX045 (to ZL). The funding bodies played no role in the study design, collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Institutional review board statement: The present study is a secondary analysis using the CLHLS data, and the ethical approval is waived by an IRB (IRB00001052–13074) to the CLHLS study that was approved by the Research Ethics Committees of Duke University and Peking University.
Declaration of patient consent: The present study is a secondary analysis using the CLHLS data, and the CLHLS study was approved by the Research Ethics Committees of Duke University and Peking University. Thus, consent to participate from participants is deemed unnecessary for this study. The data were anonymized before its use.
Reporting statement: The study followed the STrengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement
Biostatistics statement: The statistical methods of this study were reviewed by the epidemiologist of Affiliated Hospital of Guangdong Medical University, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: The CLHLS questionnaire can be obtained from https://sites.duke.edu/centerforaging/programs/chinese-longitudinal-healthy-longevity-survey-clhls/survey-documentation/questionnaires/. The complete data set of CLHLS can be obtained from its official website. If there are reasonable requirements, the codes used for data extraction, data coding and statistical analysis in this study can be obtained from the corresponding author.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Vanessa Castelli, University of L’Aquila, Italy; Jonathan M. Borkum, University of Maine, USA.
Funding: This study was supported by a grant from the Clinical Research Project of Affiliated Hospital of Guangdong Medical University of China, Nos. LCYJ2018A00 (to ZL) and LCYJ2019C006 (to YSC); the Natural Science Foundation of Guangdong Province of China, No. 2020A151501284 (to ZL); the Science and Technology Planning Project of Zhanjiang of China, No. 2018A01021 (to ZL); and a grant from the Characteristic Innovation Projects of Colleges and Universities in Guangdong Province of China, No. 2019KTSCX045 (to ZL).
P-Reviewers: Castelli V, Borkun JM; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editor: Song LP; T-Editor: Jia Y
References
- 1.Austin PC. Propensity-score matching in the cardiovascular surgery literature from 2004 to 2006: a systematic review and suggestions for improvement. J Thorac Cardiovasc Surg. 2007;134:1128–1135. doi: 10.1016/j.jtcvs.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 2.Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018;53:1112–1117. doi: 10.1093/ejcts/ezy167. [DOI] [PubMed] [Google Scholar]
- 3.Capistrant BD, Wang Q, Liu SY, Glymour MM. Stroke-associated differences in rates of activity of daily living loss emerge years before stroke onset. J Am Geriatr Soc. 2013;61:931–938. doi: 10.1111/jgs.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke PJ, Blount V, Colantonio A. Cognitive impairment predicts fatal incident stroke: findings from a national sample of older adults. J Am Geriatr Soc. 2011;59:1490–1496. doi: 10.1111/j.1532-5415.2011.03494.x. [DOI] [PubMed] [Google Scholar]
- 5.Colantonio A, Kasl SV, Ostfeld AM. Level of function predicts first stroke in the elderly. Stroke. 1992;23:1355–1357. doi: 10.1161/01.str.23.9.1355. [DOI] [PubMed] [Google Scholar]
- 6.Deb S, Austin PC, Tu JV, Ko DT, Mazer CD, Kiss A, Fremes SE. A review of propensity-score methods and their use in cardiovascular research. Can J Cardiol. 2016;32:259–265. doi: 10.1016/j.cjca.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Deng Y, Gao Q, Yang D, Hua H, Wang N, Ou F, Liu R, Wu B, Liu Y. Association between biomass fuel use and risk of hypertension among Chinese older people: a cohort study. Environ Int. 2020;138:105620. doi: 10.1016/j.envint.2020.105620. [DOI] [PubMed] [Google Scholar]
- 8.Diener HC, Hankey GJ. Primary and secondary prevention of ischemic stroke and cerebral hemorrhage: JACC Focus Seminar. J Am Coll Cardiol. 2020;75:1804–1818. doi: 10.1016/j.jacc.2019.12.072. [DOI] [PubMed] [Google Scholar]
- 9.Edemekong PF, Bomgaars DL, Sukumaran S, Levy SB. Activities of Daily Living. Treasure Island (FL): StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 10.Fang Q, Lai X, Yang L, Wang Z, Zhan Y, Zhou L, Xiao Y, Wang H, Li D, Zhang K, Zhou T, Yang H, Guo H, He MA, Kong W, Wu T, Zhang X. Hearing loss is associated with increased stroke risk in the Dongfeng-Tongji Cohort. Atherosclerosis. 2019;285:10–16. doi: 10.1016/j.atherosclerosis.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Ferri CP, Schoenborn C, Kalra L, Acosta D, Guerra M, Huang Y, Jacob KS, Llibre Rodriguez JJ, Salas A, Sosa AL, Williams JD, Liu Z, Moriyama T, Valhuerdi A, Prince MJ. Prevalence of stroke and related burden among older people living in Latin America, India and China. J Neurol Neurosurg Psychiatry. 2011;82:1074–1082. doi: 10.1136/jnnp.2010.234153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fong JH. Disability incidence and functional decline among older adults with major chronic diseases. BMC Geriatr. 2019;19:323. doi: 10.1186/s12877-019-1348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuller-Thomson E, Yu B, Nuru-Jeter A, Guralnik JM, Minkler M. Basic ADL disability and functional limitation rates among older AMERICANS from 2000-2005: the end of the decline? J Gerontol A Biol Sci Med Sci. 2009;64:1333–1336. doi: 10.1093/gerona/glp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry-Sánchez JT, Kurichi JE, Xie D, Pan Q, Stineman MG. Do elderly people at more severe activity of daily living limitation stages fall more? Am J Phys Med Rehabil. 2012;91:601–610. doi: 10.1097/PHM.0b013e31825596af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heshmatollah A, Mutlu U, Koudstaal PJ, Ikram MA, Ikram MK. Cognitive and physical impairment and the risk of stroke - A prospective cohort study. Sci Rep. 2020;10:6274. doi: 10.1038/s41598-020-63295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou C, Ping Z, Yang K, Chen S, Liu X, Li H, Liu M, Ma Y, Van Halm-Lutterodt N, Tao L, Luo Y, Yang X, Wang W, Li X, Guo X. Trends of activities of daily living disability situation and association with chronic conditions among elderly aged 80 years and over in China. J Nutr Health Aging. 2018;22:439–445. doi: 10.1007/s12603-017-0947-7. [DOI] [PubMed] [Google Scholar]
- 18.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Huang J, Wang Y, Yin P, Wang L, Liu Y, Pan X, Zhou M, Li G. Years of life lost from ischaemic and haemorrhagic stroke related to ambient nitrogen dioxide exposure: A multicity study in China. Ecotoxicol Environ Saf. 2020;203:111018. doi: 10.1016/j.ecoenv.2020.111018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liccardo D, Cannavo A, Spagnuolo G, Ferrara N, Cittadini A, Rengo C, Rengo G. Periodontal disease: a risk factor for diabetes and cardiovascular disease. Int J Mol Sci. 2019;20:1414. doi: 10.3390/ijms20061414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malhotra C, Chan A, Malhotra R, Ostbye T. Prevalence, correlates and perceived causes of limitations in activities of daily living among older Singaporeans. Aging Clin Exp Res. 2012;24:56–61. doi: 10.1007/BF03325354. [DOI] [PubMed] [Google Scholar]
- 22.National Bureau of Statistics. Statistical Communiqué of the People’s Republic of China on the 2011 National Economic and Social Development. China Popul Today. 2012;29:22–39. [Google Scholar]
- 23.O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao-Melacini P, Zhang X, Pais P, Agapay S, Lopez-Jaramillo P, Damasceno A, Langhorne P, McQueen MJ, Rosengren A, Dehghan M, Hankey GJ, Dans AL, Elsayed A, Avezum A, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761–775. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 24.Pollard TJ, Johnson AEW, Raffa JD, Mark RG. tableone: An open source Python package for producing summary statistics for research papers. JAMIA Open. 2018;1:26–31. doi: 10.1093/jamiaopen/ooy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seabold S, Perktold J. Proceedings of the 9th Python in Science Conference (SciPy 2010). Austin. 2010. Statsmodels: econometric and statistical modeling with python. [Google Scholar]
- 27.Sousa RM, Ferri CP, Acosta D, Albanese E, Guerra M, Huang Y, Jacob KS, Jotheeswaran AT, Rodriguez JJ, Pichardo GR, Rodriguez MC, Salas A, Sosa AL, Williams J, Zuniga T, Prince M. Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: a 10/66 Dementia Research Group population-based survey. Lancet. 2009;374:1821–1830. doi: 10.1016/S0140-6736(09)61829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turin TC, Okamura T, Afzal AR, Rumana N, Watanabe M, Higashiyama A, Nakao Y, Nakai M, Takegami M, Nishimura K, Kokubo Y, Okayama A, Miyamoto Y. Hypertension and lifetime risk of stroke. J Hypertens. 2016;34:116–122. doi: 10.1097/HJH.0000000000000753. [DOI] [PubMed] [Google Scholar]
- 29.Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22:613–626. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- 30.Wada T, Ishine M, Sakagami T, Okumiya K, Fujisawa M, Murakami S, Otsuka K, Yano S, Kita T, Matsubayashi K. Depression in Japanese community-dwelling elderly--prevalence and association with ADL and QOL. Arch Gerontol Geriatr. 2004;39:15–23. doi: 10.1016/j.archger.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Wang W, Hu SS, Kong LZ, Gao RL, Zhu ML, Wang WY, Wu ZS, Chen WW, Yang JG, Ma LY, Liu MB. Summary of report on cardiovascular diseases in China, 2012. Biomed Environ Sci. 2014;27:552–558. doi: 10.3967/bes2014.085. [DOI] [PubMed] [Google Scholar]
- 32.Wen M, Gu D. The effects of childhood, adult , and community socioeconomic conditions on health and mortality among older adults in China. Demography. 2011;48:153–181. doi: 10.1007/s13524-010-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodward M, Lam TH, Barzi F, Patel A, Gu D, Rodgers A, Suh I. Smoking, quitting, and the risk of cardiovascular disease among women and men in the Asia-Pacific region. Int J Epidemiol. 2005;34:1036–1045. doi: 10.1093/ije/dyi104. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Li Q, Han X, Wang S, Li P, Ding Y, Zhang T, Zhao J, Chen Y, Liu J, Li J, Tan X, Liu W, Zhang R, Cao G. Associations of socioeconomic factors with cause-specific Mortality and burden of cardiovascular diseases: findings from the vital registration in urban Shanghai, China , during 1974-2015. BMC Public Health. 2020;20:1291. doi: 10.1186/s12889-020-09390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


