Abstract
A double-strand break (DSB) in the mammalian genome has been shown to be a very potent signal for the cell to activate repair processes. Two different types of repair have been identified in mammalian cells. Broken ends can be rejoined with or without loss or addition of DNA or, alternatively, a homologous template can be used to repair the break. For most genomic sequences the latter event would involve allelic sequences present on the sister chromatid or homologous chromosome. However, since more than 30% of our genome consists of repetitive sequences, these would have the option of using nonallelic sequences for homologous repair. This could have an impact on the evolution of these sequences and of the genome itself. We have designed an assay to look at the repair of DSBs in LINE-1 (L1) elements which number 105 copies distributed throughout the genome of all mammals. We introduced into the genome of mouse epithelial cells an L1 element with an I-SceI endonuclease site. We induced DSBs at the I-SceI site and determined their mechanism of repair. We found that in over 95% of cases, the DSBs were repaired by an end-joining process. However, in almost 1% of cases, we found strong evidence for repair involving gene conversion with various endogenous L1 elements, with some being used preferentially. In particular, the TF family and the L1Md-A2 subfamily, which are the most active in retrotransposition, appeared to be contributing the most in this process. The degree of homology did not seem to be a determining factor in the selection of the endogenous elements used for repair but may be based instead on accessibility. Considering their abundance and dispersion, gene conversion between repetitive elements may be occurring frequently enough to be playing a role in their evolution.
DNA double-strand breaks (DSBs) represent a threat to genomic integrity that have to be repaired efficiently or they will lead to serious consequences, such as programmed cell death (45). In mammalian cells, a single DSB can cause a cell cycle arrest (17). Mammalian DSB repair is a complex process that involves multiple proteins that vary depending on the cell cycle phase (16). Two main repair pathways are utilized (22). The first one involves the rejoining of the broken ends with or without the loss and/or addition of DNA. The second one involves homologous recombination with an intact copy of the broken DNA segment acting as a template. In most cases the homologous template could be the allelic copy present on the sister chromatid or homologous chromosome. However, since 30% of the genome is made of highly repetitive sequences, this raises the possibility that nonallelic homologous templates could be used for the repair of a DSB. Indeed, several groups have shown that a DSB occurring in a chromosomal construct containing tandemly repeated sequences could be repaired by homologous recombination at a frequency of as high as 30% (7, 12, 21, 22, 39, 44). Also, it was shown that homologous sequences located at a nonallelic position on a distinct chromosome could be used for DSB repair, albeit at a much lower frequency (34).
We wanted to determine how a DSB in highly repetitive sequences would be repaired. In particular, we were interested in the repair of a DSB occurring in LINE-1 (L1) elements. L1 elements are present in all mammals at around 105 copies dispersed throughout the genome and are more conserved within a given species than between closely related species (18). This concerted evolution of L1 elements could be due in part to homologous recombination between nonallelic L1 elements. Indeed, Saxton and Martin (40) suggested that recombination between particular L1 subtypes in mouse could have created the TF family. There is some evidence that neighboring L1 elements can be involved in homologous recombination leading to chromosomal deletions (9, 43). Also, recombination between L1 elements would be partly responsible for the present hominid Y chromosome structure (42). However, it could not be determined if DSB repair was implicated in these processes.
In order to study DSB repair of L1 elements, we devised an assay based on the I-SceI system (19, 36). We integrated at several distinct sites in the genome of a mouse epithelial cell line an L1 element containing an I-SceI site and then introduced a DSB at that site by the transfection in these cells of a vector expressing the I-SceI endonuclease. The results were that, over 95% of the time, the DSBs in the L1 sequences were repaired by an end-joining process. However, in almost 1% of the cases we found strong evidence for homologous recombination repair involving gene conversion with endogenous L1 elements. Several distinct endogenous L1 elements were used for homologous recombination repair, some preferentially. In particular, the TF family and L1Md-A2 subfamily, which are currently the most active in retrotransposition, appeared to be contributing most of the elements in these gene conversion events.
MATERIALS AND METHODS
Cells and growth conditions.
Nod-2 are nonciliated epithelial cells of the bronchioles derived from a pulmonary adenocarcinoma (nodule) of an FVB transgenic mouse that harbors a polyomavirus large-T-antigen (T-Ag) transgene (20). Nod-2 cells are probably derived from precursor cells within the basal bronchiolar epithelium. Cells were maintained in Dulbecco modified Eagle medium (DMEM) medium supplemented with 10% fetal bovine serum plus 50 μg of gentamicin per ml and kept in a 5% CO2 atmosphere at 37°C. When used, hygromycin was added at a final concentration of 150 μg/ml.
Vector construction.
pASB-SceI is derived from pASB-HindIII which contains the 3-kb KpnI-EaeI fragment of the BALB/c L1Md-A2 (4). An I-SceI restriction site was introduced at the unique HindIII site by using an oligodimer (see Fig. 1b).
FIG. 1.
Structure of L1Md-A2 (23) (a) and the pASB-SceI vector used in our assay (b), which contains the 3-kb KpnI-EaeI fragment of L1Md-A2. A linker containing the I-SceI restriction site was introduced at the HindIII site: H′ designates the partial HindIII sites flanking the I-SceI cassette, and the I-SceI cut site is shown. oriPy, polyomavirus origin that was present in the initial pASB-HindIII vector (4). Position of PCR primers (1 to 4) and sequencing primers (5 to 8) in pASB-SceI are as follows: 1 (5850 to 5872), 2 (3090 to 3070), 3 (5887 to 5916), 4 (3043 to 3014), 5 (1500 to 1481), 6 (1107 to 1087), 7 (872 to 850), and 8 (562 to 542).
Transfection.
A total of 2 × 106 nod-2 cells were transfected in DMEM by electroporation with 10 μg of NarI-digested pASB-SceI and 1 μg of BamHI-linearized hygromycin vector, p3′SS (Stratagene). The electroporation conditions used were as follows: 0.4-cm gap chamber, 960 μF, and 270 V. Hygromycin was added 48 h after the electroporation, and Hygr colonies were picked 3 weeks later.
Transient I-SceI expression.
pCBASce (34) is an expression vector that contains the coding sequences of the I-SceI endonuclease from Saccharomyces cerevisiae, under the control of the β-actin gene promoter. A total of 4 × 106 cells in DMEM of the different pASB-SceI containing clones were electroporated (see conditions in the previous section) with 100 μg of uncut pCBASce vector or without vector as a control. Viable cells were harvested 72 h later for genomic DNA extraction and analysis.
DNA analysis.
Southern analysis was performed by using 13 μg of digested genomic DNA according to Ausubel et al. (3). The NarI-KpnI polyomavirus origin fragment of pASB-SceI was used as a probe. All of the restriction enzymes used were purchased at New England Biolabs except Meganuclease I-SceI (Boehringer Mannheim). For all PCR amplifications, the Expand High-Fidelity PCR System (Boehringer Mannheim) was used. The primers used for PCR were as follows: 1, 5′-CAGAGGAGGTGTATGGGTTTGTC-3′; 2, 5′-CGAGTCAGTGAGCGAGGAAGC-3′; 3, 5′-GTTTGTAAGTCGAACAGCGGGGGCTATATG-3′; and 4, 5′-TCTCCCCGCGCGTTGGCCGATTCATTAATG-3′. PCR1 (primers 1 and 2) amplification was performed on 150 ng of digested genomic DNA as follows: 94°C for 2 min; 10 cycles of 94°C for 1 min, 62°C for 1 min, and 68°C for 2 min; and 20 cycles of 94°C for 1 min, 62°C for 1 min, and 68°C for 2 min (plus a 20-s/cycle). For PCR2 (primers 3 and 4), the same procedure was followed except that the annealing temperature was changed from 62 to 70°C. For DNA sequencing of PCR products, PCR fragments were cloned (KpnI-SacI) or subcloned (KpnI-HindIII) into pBluescript SK(+) (Stratagene). Sequencing was done by the dideoxy chain termination reaction (38) by using Sequenase (Amersham). Sequence data were compiled and analyzed by using Genetic Computer Group software.
RESULTS
Experimental design.
The strategy that we used was to introduce in the genome of a mouse cell line, an L1 fragment in which we had replaced a highly conserved restriction site by an I-SceI site. We then created a DSB at the I-SceI site in vivo and scored for repair events by the loss of the I-SceI. To enrich for homologous recombination repair events, we selected for the reacquisition of the highly conserved restriction site.
The vector we used has been described previously (4). It contains a 3-kb fragment from the 3′ end of the well-characterized L1Md-A2 element (Fig. 1a). We chose the 3′-end fragment since most L1 elements of the mouse genome are truncated at their 5′ end (47). Certain restriction sites are highly conserved in mouse L1 elements, and this is the case for the HindIII site present in the L1 fragment we used, which is present in more than 50% of the mouse L1 elements (4, 8) (GenBank-EMBL L1 sequences). We introduced in the HindIII site an I-SceI cassette to create the vector pASB-SceI (Fig. 1b).
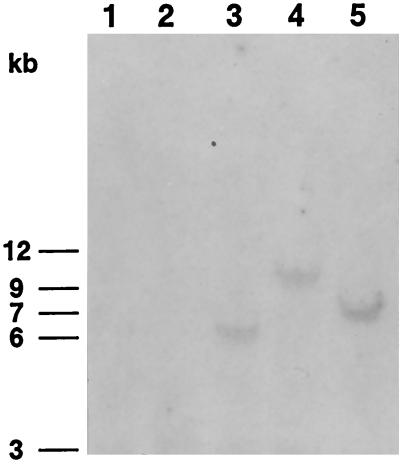
pASB-SceI was transfected in a mouse epithelial cell line (nod-2 [20]), along with the hygromycin selection vector p3′SS. Three independent clones were selected (clones 19, 22, and 25); each had integrated into their genome a single complete copy of the NarI-digested pASB-SceI vector, as indicated by the presence of a single complete insert seen by Southern analysis (Fig. 2). We then created a DSB in the integrated L1 sequence in clones 19, 22, and 25 by transfecting an I-SceI expression vector (pCBASce [34]).
FIG. 2.
Southern blot analysis of the PstI-digested genomic DNA isolated from clone 19 (lane 3), clone 22 (lane 4), and clone 25 (lane 5) derived from nod-2 cells transfected with pASB-SceI and p3′SS, and from parental nod-2 cells (lane 1) and nod-2 transfected with the selection vector p3′SS alone (lane 2). The NarI-KpnI polyomavirus origin fragment of pASB-SceI was used as a probe (Fig. 1b).
To analyze the repair events, the genomic DNA was first digested with PstI to break down the genome and I-SceI to cleave the integrated L1 sequences which had not lost the I-SceI site. Integrated L1 sequences which had lost the I-SceI site were then amplified by PCR with primers 1 and 2 (Fig. 1b). The resulting PCR products were digested with KpnI and SacI, cloned, and sequenced by using primer 5. To enrich for homologous recombination events involving the reacquisition of the HindIII site, after the first PCR the procedure was repeated (I-SceI digestion and PCR amplification) with internal primers 3 and 4. The resulting products were digested with KpnI-HindIII, cloned, and sequenced from the HindIII site, with primers flanking the cloning site and primers 6, 7, and 8 if necessary.
Nature of the repair events.
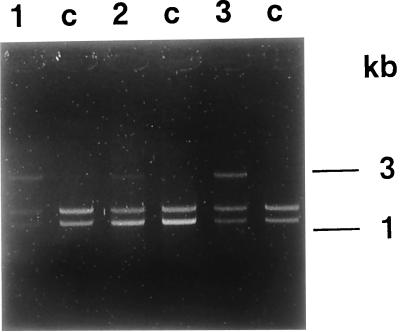
The I-SceI cleavage efficiency was determined for each of the six independent pCBASce transfection assays performed and was found to vary depending on the clone used. After each transfection with the pCBASce vector, the genomic DNA was extracted and digested with PstI, amplified by PCR with primers 1 and 2 (Fig. 1b), digested with I-SceI, and analyzed on a gel. The relative proportion of digested to nondigested DNA was quantified by densitometry. For clones 19 (three assays) and 25 (one assay), ca. 35% of the PCR products were resistant to I-SceI digestion, while for clone 22 (two assays), only ca. 20% were resistant to I-SceI digestion (Fig. 3). Thus, for clones 19 and 25, 35% of the integrated L1 sequences were involved in a repair event, while it appears that for clone 22 only 20% were involved in a repair event. It should be noted that it is not possible with this assay to determine if repair events occurred that would have regenerated the I-SceI site.
FIG. 3.
I-SceI digestion of PCR1 products amplified from PstI-digested genomic DNA isolated from the pCBASce-transfected clones 19 (lane 1), 22 (lane 2), and 25 (lane 3). Lanes c correspond to the same clones subjected to electroporation but in the absence of pCBASce. This control indicates that the genomic DNA from each clone could be digested by I-SceI. The I-SceI digestion of the PCR1 product of 3 kb should yield bands of 1.7 and 1.3 kb. In clones 19 (lane 1) and 25 (lane 3), ca. 35% of the PCR product is resistant to I-SceI digestion. In the case of clone 22 (lane 2), only 20% of the PCR product is resistant to I-SceI digestion. The numbers at the side of the figure indicate the molecular weight.
The largest number of repair events were analyzed from clone 19. Of 111 repair events defined by the loss of the I-SceI site, 4 had deletions larger than 1 kb and 81 had small deletions (on average, 10 bp), including 6 with additions of small DNA fragments. Another 20 repair events had additions of small DNA fragments without deletion. Thus, at least 105 of the 111 repair events (95%) were the result of end joining. The remaining six repair events involved the acquisition of an HindIII site replacing the I-SceI inserted sequences. To determine if this was reproducible, PCR products from other assays with clones 19, 22, and 25 were tested for the acquisition of the HindIII site. As in the initial experiment, HindIII-acquiring events were also found to represent fewer than 5% of the events in each case, as evaluated by restriction enzyme analysis. The HindIII-acquiring events were due to DSB repair, since we were not able to isolate HindIII-acquiring events in controls not transfected with pCBASce.
To enrich for homologous recombination events, 304 HindIII-acquiring events were cloned and sequenced from six independent pCBASce electroporation assays. From these, 20 had acquired an HindIII site as a result of a fortuitous deletion of vector sequences around the I-SceI site that generated a de novo HindIII site or as a result of the acquisition at the I-SceI site of a pCBASce fragment that contained an HindIII site. Of the remaining 284 HindIII-acquiring events, the vast majority (252) had sequences identical to that of the L1 sequences present in our vector (or with 1 or 2 random base changes per 300 bp; see below). Since, on average, endogenous mouse L1 elements differ in their sequences from one another by 4% (24) and since a number of polymorphic bases are located near the HindIII site (Table 1), a gene conversion event might be expected to generally introduce other base changes beside the acquisition of the HindIII site. Instead, these were most probably generated by end-to-end joining at the two half HindIII sites bordering the I-SceI cassette (see H′; figure 1b) rather than gene conversion events. In this case, end-joining would occur at the 4-bp H′ repeat, perhaps by a single-strand annealing type of mechanism that uses such microhomologies. Indeed, in our analysis of the 111 PCR products described above, we found single-strand annealing-type events at repeats of 2 to 4 bases at about the same frequency (8 of 111) as the HindIII-acquiring events (6 of 111). Previous studies had already shown that repair events involving strand annealing of a few bases are a common end-joining repair process (11, 28, 29, 31, 35), including those occurring at short repeats flanking an I-SceI site (37).
TABLE 1.
List of the 32 gene conversion events of clones 19, 22, and 25a
| Gene conversion event(s) | Sequence change from pASB-SceI at position (base):
|
No. of base changes with pASB-SceI | Minimum conversion tract length | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1064 (A) | 1196 (T) | 1210 (G) | 1231 (T) | 1232 (G) | 1233 (T) | 1252 (T) | 1262 (A) | 1266 (G) | 1283 (A) | 1288 (T) | 1292 (G) | 1294 (C) | 1295 (G) | 1301 (A) | 1305 (T) | 1309 (A) | |||
| 19-A.01, 19-3.02 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | T | 1 | 13 |
| 19-5.09 | — | — | — | — | — | — | — | — | — | — | — | — | T | — | — | — | T | 2 | 28 |
| 19-9.01 | — | — | — | — | — | — | — | — | — | — | C | — | T | — | — | — | T | 4 | 34 |
| 19-1.02, 19-2.08, 19-4.06, 19-8.01 | — | — | — | — | — | — | — | — | C | — | C | — | T | — | — | — | T | 4 | 56 |
| 19-7.08 | — | — | — | — | — | — | G | — | C | — | C | — | T | — | — | — | T | 5 | 70 |
| 19-0.01 | — | — | A | C | A | — | G | — | C | — | C | — | T | — | — | — | T | 24 | 783 |
| 19-α.18 | — | — | — | — | — | — | — | — | — | — | — | 0 | — | — | G | — | — | 3 | 37 |
| 19-6.05 | — | — | — | — | — | — | — | G | — | — | — | — | — | — | — | — | — | 3 | 122 |
| 19-6.06 | — | — | — | — | — | — | — | G | — | G | — | — | — | — | — | — | — | 2 | 60 |
| 19-2.21 | — | — | — | — | — | — | — | — | — | G | — | — | — | — | — | — | — | 2 | 140 |
| 19-1.03 | — | — | — | — | — | — | — | — | — | G | — | — | — | — | — | — | — | 1 | 39 |
| 19-6.01 | — | C | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 2 | 154 |
| 19-2.17 | — | C | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | 126 |
| 19-E.05, 19-B.07 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | C | — | 1 | 17 |
| 19-A.04, 19-α.06 | — | — | — | — | — | — | — | — | — | — | — | — | — | A | — | — | — | 1 | 27 |
| 22-1.04 | — | — | — | — | — | — | — | — | — | — | — | 0 | — | — | G | — | — | 3 | 57 |
| 22-4.03 | G | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 2 | 258 |
| 22-9.06 | G | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 2 | 258 |
| 22-2.02 | — | — | — | C | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | 91 |
| 22-H.03 | — | — | A | C | A | — | — | — | C | — | C | — | T | — | — | — | T | 9 | 118 |
| 25-1.07, 25-5.03 | — | — | — | — | — | C | — | — | — | — | — | — | — | — | — | — | — | 1 | 89 |
| 25-4.08 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 3 | 246 |
| 25-4.05 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | T | 2 | 133 |
| 25-2.19 | — | — | — | — | — | — | — | — | — | — | C | — | T | — | — | — | T | 4 | 87 |
| 25-3.01 | — | — | — | — | — | — | — | — | C | — | C | — | T | — | — | — | T | 5 | 135 |
The base changes with the pASB-SceI LINE (L1Md-A2) sequence observed in at least two distinct gene conversion events are indicated. The positioning of the base changes refers to Fig. 1b. The “0” indicates that there is no base at the corresponding position. For each gene conversion event, the first number identifies the clone from which it was isolated; the second number (first pCBASce electroporation) or letter (second electroporation) or symbol (third electroporation experiment) identifies the individual independent PCR, and the third number identifies the specific event. The length of the conversion tract is delineated by the farthest base change from the HindIII site (positions 1316 to 1321). —, No change.
The remaining 32 HindIII-acquiring events appeared to be bona fide gene conversion events with endogenous L1 elements, based on at least two of the following criteria: (i) they had at least one base change beside the acquisition of the HindIII site; (ii) specific base changes were repeated in different HindIII-acquiring events originating from different PCR amplifications; (iii) the base changes could be regrouped in conversion tracts of different lengths; (iv) the base changes were skewered toward the HindIII site; (v) the base changes corresponded to diagnostic bases of various L1 subfamilies.
Since base changes could be produced as an artifact of PCR, we did a control experiment to evaluate the number of base changes that could be attributed to PCR. We spiked nod-2 genomic DNA with 1 copy per genome of the pASB-SceI plasmid and followed the experimental procedure described above. Of 5,225 bp sequenced from 20 different amplified pASB-SceI molecules, we found altogether seven base changes located at random positions. Thus, we excluded from the conversion events infrequent HindIII-acquiring events that had only one or two base changes that did not repeat themselves in an independent PCR amplification.
Taken together, these results indicate that 5% (6 of 111) of the repair events were HindIII-acquiring events. From these, 11% (32 of 284) could be identified as bona fide homologous recombination repair events, giving an overall homologous recombination frequency of almost 0.6%. It is possible that we are underestimating the frequency of gene conversion events, since those occurring with endogenous L1 elements without an HindIII site would not be part of the HindIII-acquiring events. However, these are apparently infrequent, since none were seen in the 111 events analyzed. Also, events with extremely short conversion tracts, i.e., those that did not extend past the HindIII site to the first mismatched base between the endogenous L1 element and the pASB-SceI L1 sequence, would not have been scored as a conversion event. Short conversion tracts have been observed in other studies involving the repair of chromosomal DSBs (13, 44). Nevertheless, the proximity and abundance of polymorphic bases near the HindIII site should have permitted the detection of most events (Table 2).
TABLE 2.
Categorization of the 32 gene conversion events with respect to the predominant L1 polymorphic bases from the HindIII sitea
| Family (subfamily) pattern and event(s) | Sequence change at position:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1048 | 1051 | 1119 | 1180 | 1210 | 1231 | 1232 | 1252 | 1266 | 1288 | 1294 | 1309 | |
| L1Md-A2 subfamily | A | T | T | C | G | T | G | T | G | T | C | A |
| 22-9.06, 22-4.03, 25-4.08 | — | — | T | C | G | T | G | T | G | T | C | A |
| 19-6.01 | — | — | — | C | G | T | G | T | G | T | C | A |
| 19-6.05, 19-2.21, 19-2.17 | — | — | — | — | G | T | G | T | G | T | C | A |
| 25-1.07, 25-5.03 | — | — | — | — | — | — | — | T | G | T | C | A |
| 19-6.06, 22-1.04 | — | — | — | — | — | — | — | — | G | T | C | A |
| 19-α.18, 19-1.03 | — | — | — | — | — | — | — | — | — | T | C | A |
| 19-E.05, 19-B.07, 19-A.04, 19-α.06 | — | — | — | — | — | — | — | — | — | — | — | A |
| TF family | g | c | c | t | a | c | a | g | c | c | t | t |
| 19-0.01 | g | c | c | t | a | c | a | g | c | c | t | t |
| 19-7.08 | — | — | — | — | — | — | — | g | c | c | t | t |
| 19-1.02, 19-2.08, 19-4.06, 19-8.01 | — | — | — | — | — | — | — | — | c | c | t | t |
| 19-9.01 | — | — | — | — | — | — | — | — | — | c | t | t |
| 19-5.09 | — | — | — | — | — | — | — | — | — | — | t | t |
| 19-A.01, 19-3.02 | — | — | — | — | — | — | — | — | — | — | — | t |
| Mixed L1Md-A2/TF | ||||||||||||
| 22-0.02 | — | — | — | — | — | c | G | T | G | T | C | A |
| 25-4.05 | — | — | — | — | G | T | G | T | G | T | C | t |
| 25-2.19 | — | — | — | — | — | — | — | T | G | c | t | t |
| 25-3.01 | — | — | — | — | G | T | G | T | c | c | t | t |
| 22-H.03 | — | — | — | — | a | c | a | T | c | c | t | t |
For the 12 most polymorphic L1 bases, the bases are specified for the L1Md-A2 subfamily (in capital letters) and TF family (in lowercase letters) and the 32 gene conversion events for their respective conversion tracts (Table 1). Highly polymorphic L1 bases were determined from an alignment of 20 GenBank-EMBL HindIII-containing mouse L1 elements (accession numbers AC002315, AC002406, AC003019, AE000663, AF016099, AF021335, AF037352, D84391, K00582, K00587, K00588, M13002, M11515, M23236, M29324, M29325, M68842, MMMHC29N7, U15647, and X14061). —, No change.
Gene conversion events with endogenous L1 elements.
The conversion tracts of the 32 gene conversion events that were identified in this study are presented in Table 1. The extent of conversion from the HindIII site that could be scored by the presence of base changes was as short as 13 bp or as long as 780 bp, with the majority being shorter than 100 bp. The conversion tracts occurred between L1 sequences that were as much as 10% heterologous, with most being less than 4%.
For each clone, several different patterns of gene conversion were seen that involved distinct endogenous L1 partners (11 of 21 for clone 19, 5 of 5 for clone 22, and 5 of 6 for clone 25). In at least one instance, it appeared that several independent gene conversion events involved the same endogenous L1 partner, resulting in a set of nested conversion tracts (the first 10 gene conversion events for clone 19). Interestingly, the longest conversion tract of this cohort (783 bp, event 19-0.01), which has incorporated 24 base changes compared to pASB-SceI, is 100% identical to the sequence of a L1 element that has recently been reported as actively being involved in retrotransposition events (L1spa, AF016099 GenBank/EMBL accession number) (27).
Because their sequences are identical and they come from the same pCBASce electroporation assay (but not the same PCR amplification), gene conversion events 19-1.02, 19-2.08, 19.4.06, and 19-8.01 could represent the same clonal event, as could be the case for events 19-E.05 and 19-B.07 or events 25-1.07 and 25-5.03.
Comparative analysis of mouse L1 elements indicate that they can be divided into at least three groups or families of more-related sequences that have been designated the F, A, and TF families (10). The F family would be the oldest and most diverged one, while the A and TF families would be more recent, with a higher degree of homology among their members (97 and 99.8%, respectively). The TF family would possibly be the most active in retrotransposition (27). The L1 sequences present in pASB-SceI are derived from L1Md-A2 (23), which is part of an A subfamily in which members share 99.5% of homology and are also active in retrotransposition (41). The TF family and the L1Md-A2 subfamily have been estimated to contain the same number of full-length members, i.e., 2,000 to 3,000 per diploid genome (40). The L1 segments that were sequenced in this study include a number of diagnostic bases that are identified with the L1Md-A2 subfamily or TF family. These are indicated in Table 2. Analysis of the gene conversion tracts we observed indicated that the majority of events can be classified as possibly having involved a L1Md-A2 subfamily partner (17 events) or a TF partner (10 events). Five events have a mixed TF and A subfamily conversion tract that could have arisen from either mismatch repair or recombination with a mosaic TF/A L1 partner (40).
Gene conversion event 22-H.03 has an interesting structure (Fig. 4a) that suggests a one-sided invasion homologous recombination event. This event has an 118-bp L1 duplication containing 9-bp changes predominantly of the TF pattern, and a deletion of 12 bases from the I-SceI cassette. Figure 4b illustrates the different steps that have possibly been involved in the genesis of event 22-H.03, based on the one-sided homologous recombination model (4, 5). The steps are invasion of the L1 donor sequence by only one side of the double-strand break, followed by DNA synthesis and then release of the invading end, and end-to-end joining with the other side of the DSB. This had also been observed in earlier studies on homologous recombination between extrachromosomal and endogenous chromosomal L1 elements in mouse cells (4, 6).
FIG. 4.
Proposed mechanism (b) for the production of gene conversion event 22-H.03 (a).
DISCUSSION
In the present study, we have shown that a DSB in LINE elements could be repaired by homologous recombination with nonallelic endogenous L1 elements. To our knowledge, this is the first demonstration of homologous repair of a chromosomal DSB by endogenous genomic repeat sequences in mammal cells. However, repair of a DSB in Ty elements by nonallelic Ty chromosomal sequences has been reported in yeast cells (30). We found that the dominant repair mechanism leading to I-SceI site loss was by far end joining rather than gene conversion with an endogenous L1 element. This contrasts with yeast, in which nonallelic repetitive elements are readily used (30). Nevertheless, considering the large number of repetitive elements, a frequency of almost 1% DSB repair by homologous recombination with nonallelic L1 elements could be enough to have played a role in the evolution of repetitive elements. In this regard, it is interesting that the selection of partners was not based primarily on the degree of homology, since partners with higher (L1Md-A2 subfamily) and lower (TF family) homology appeared to have participated equally in the process. A lack of preference for highly homologous partners had also been seen for homologous recombination between extrachromosomal and chromosomal L1 elements (6, 33). This could explain the genesis of mosaic lineages in L1 elements (40).
The choice of partners for DSB repair by homologous recombination could be more a question of accessibility than homology. We do not know the relative location of the endogenous L1 partners relative to our pASB-SceI L1 element. It could have been intrachromosomal proximal or distal, or interchromosomal, or even extrachromosomal. This last possibility seems less likely since what appears to be the same partner was in some cases involved repeatedly in the process, resulting in a set of encompassing conversion tracts (see clone 19, Table 1). In any case, several partners were clearly accessible for DSB repair.
It would seem that the most likely endogenous L1 partners were members of the L1-MdA2 subfamily or the TF family. These represent only a fraction of the total L1 elements of the mouse genome. The full-length members in these two groups have been estimated at 2,000 to 3,000 for each (40). However, they are by far the most active in retrotransposition (10, 27, 40, 41). It is tempting to speculate that this could somehow influence their accessibility. In particular, we observed for one endogenous L1 partner a 100% homology over 800 bp with a TF element, L1spa, that has been shown to be currently involved in retrotransposition (27).
In one case, we observed that the DSB repair involved a gene conversion event with a structure compatible with a one-sided invasion (OSI) mechanism, as we had described previously (4). OSI appears to be a universal homologous recombination mechanism seen in mammals (1, 4, 14, 26, 34), yeasts (15), plants (32), insects (46), and birds (25). It can lead to the transfer of several kilobases of nonhomologous sequences between nonallelic homologous sites (2, 4, 15). Thus, OSI between L1 elements could have had an impact on the evolution of the mammalian genome, permitting the transfer of unique sequences from one location to another via repetitive elements that would provide numerous entry points. In this regard, it is interesting to note that we have readily detected homologous recombination between nonallelic L1 elements in germ cells of transgenic mice (A. Tremblay et al., unpublished data).
ACKNOWLEDGMENTS
We gratefully acknowledge Dominic Corbeil, Hélène D'anjou, Julie Guay, Nadine Gusew, Benoit Houle, Roxane Lussier, and Hugo Würtele for their technical assistance. We also thank Anne-Marie Mes-Masson for her interest in this work and for providing the nod-2 cells.
This study has been supported by a grant from the Medical Research Council (MRC) of Canada. A.T. is a recipient of an MRC fellowship.
REFERENCES
- 1.Adair G M, Nairn R S, Wilson J H, Seidman M M, Brotherman K A, Mackinnon C, Scheerer J B. Targeted homologous recombination at the adenine phosphoribosyltransferase locus in Chinese hamster cells. Proc Natl Acad Sci USA. 1989;86:4574–4578. doi: 10.1073/pnas.86.12.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aratani Y, Okazaki R, Koyama H. End extension repair of introduced targeting vectors mediated by homologous recombination in mammalian cells. Nucleic Acids Res. 1992;20:4795–4801. doi: 10.1093/nar/20.18.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent K, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1992. [Google Scholar]
- 4.Belmaaza A, Wallenburg J C, Brouillette S, Gusew N, Chartrand P. Genetic exchange between endogenous and exogenous LINE-1 repetitive elements in mouse cells. Nucleic Acids Res. 1990;18:6385–6391. doi: 10.1093/nar/18.21.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belmaaza A, Chartrand P. One-sided invasion events in homologous recombination at double-strand breaks. Mutat Res. 1994;314:199–208. doi: 10.1016/0921-8777(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 6.Belmaaza A, Milot E, Villemure J-F, Chartrand P. Interference of DNA sequence divergence with precise recombinational DNA repair in mammalian cells. EMBO J. 1994;13:5355–5360. doi: 10.1002/j.1460-2075.1994.tb06870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenneman M, Gimble F S, Wilson J H. Stimulation of intrachromosomal homologous recombination in human cells by electroporation with site-specific endonucleases. Proc Natl Acad Sci USA. 1996;93:3608–3612. doi: 10.1073/pnas.93.8.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown S D M, Dover G. Organization and evolutionary progress of a dispersed repetitive family of sequences in widely separated rodent genomes. J Mol Biol. 1981;150:441–466. doi: 10.1016/0022-2836(81)90374-0. [DOI] [PubMed] [Google Scholar]
- 9.Burwinkel B, Kilimann M W. Unequal homologous recombination between LINE-1 elements as a mutational mechanism in human. J Mol Biol. 1998;277:513–517. doi: 10.1006/jmbi.1998.1641. [DOI] [PubMed] [Google Scholar]
- 10.DeBerardinis R J, Goodier J L, Ostertag E M, Kazazian H H., Jr Rapid amplification of a retrotransposon subfamily is evolving in the mouse genome. Nat Genet. 1998;20:288–290. doi: 10.1038/3104. [DOI] [PubMed] [Google Scholar]
- 11.Derbyshire M K, Epstein L H, Young C S H, Munz P L, Fishel R. Nonhomologous recombination in human cells. Mol Cell Biol. 1994;14:156–169. doi: 10.1128/mcb.14.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donoho G, Jasin M, Berg P. Analysis of gene targeting and intrachromosomal homologous recombination stimulated by genomic double-strand breaks in mouse embryonic stem cells. Mol Cell Biol. 1998;18:4070–4078. doi: 10.1128/mcb.18.7.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott B, Richardson C, Winderbaum J, Nickoloff J A, Jasin M. Gene conversion tracts from double-strand break repair in mammalian cells. Mol Cell Biol. 1998;18:93–101. doi: 10.1128/mcb.18.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis J, Bernstein A. Gene targeting with retroviral vectors, recombination by gene conversion into regions of nonhomology. Mol Cell Biol. 1989;9:1621–1627. doi: 10.1128/mcb.9.4.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haber J E. Mating-type gene switching in Saccharomyces cerevisiae. Trends Genet. 1992;8:446–452. doi: 10.1016/0168-9525(92)90329-3. [DOI] [PubMed] [Google Scholar]
- 16.Hendrickson E A. Insights from model systems: cell-cycle regulation of mammalian DNA double-strand-break repair. Am J Hum Genet. 1997;61:795–800. doi: 10.1086/514895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang L-C, Clarkin K C, Wahl G M. Sensitivity and selectivity of the DNA damage sensor responsible for activating p53-dependent G1 arrest. Proc Natl Acad Sci USA. 1996;93:4827–4832. doi: 10.1073/pnas.93.10.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutchison C A, III, Hardies S C, Loeb D D, Shehee W R, Edgell M H. LINEs and related retroposons: long interspersed repeated sequences in the eucaryotic genome. In: Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C.: American Society for Microbiology; 1989. pp. 593–617. [Google Scholar]
- 19.Jasin M. Genetic manipulation of genomes with rare-cutting endonucleases. Trends Genet. 1996;12:224–228. doi: 10.1016/0168-9525(96)10019-6. [DOI] [PubMed] [Google Scholar]
- 20.Lebel M, Webster M, Muller W J, Royal A, Gauthier J, Mes-Masson A-M. Transgenic mice bearing the polyomavirus large T antigen directed by 2,1 kb of the keratin 19 promoter develop bronchiolar papillary tumors with progression to lung adenocarcinomas. Cell Growth Differ. 1995;6:1591–1600. [PubMed] [Google Scholar]
- 21.Liang F, Romanienko P J, Weaver D T, Jeggo P A, Jasin M. Chromosomal double-strand break repair in Ku80-deficient cells. Proc Natl Acad Sci USA. 1996;93:8929–8933. doi: 10.1073/pnas.93.17.8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang F, Han M, Romanienko P J, Jasin M. Homology-directed repair is a major double-strand break repair pathway in mammalian cells. Proc Natl Acad Sci USA. 1998;95:5172–5177. doi: 10.1073/pnas.95.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loeb D D, Padgett R W, Hardies S C, Shehee W R, Comer M B, Edgell M H, Hutchison C A., III The sequence of a large L1md element reveals a tandemly repeated 5′ end and several features found in retrotransposons. Mol Cell Biol. 1986;6:168–182. doi: 10.1128/mcb.6.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin S L, Voliva C F, Hardies S C, Edgell M H, Hutchison C A., III Tempo and mode of concerted evolution in the L1 repeat family of mice. Mol Biol Evol. 1985;2:127–140. doi: 10.1093/oxfordjournals.molbev.a040340. [DOI] [PubMed] [Google Scholar]
- 25.McCormack W T, Thompson C B. Chicken IgL variable region gene conversions display pseudogene donor preference and 5′ to 3′ polarity. Genes Dev. 1990;4:548–558. doi: 10.1101/gad.4.4.548. [DOI] [PubMed] [Google Scholar]
- 26.Mudgett J S, Taylor W D. Recombination between irradiated shuttle vector DNA and chromosomal DNA in African green monkey kidney cells. Mol Cell Biol. 1990;10:37–46. doi: 10.1128/mcb.10.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nass T P, Deberardinis R J, Moran J V, Ostertag E M, Kingsmore S F, Seldin M F, Hayashizaki Y, Martin S L, Kazazian H H. An actively retrotransposing, novel subfamily of mouse L1 elements. EMBO J. 1998;17:590–597. doi: 10.1093/emboj/17.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicolas A P, Young C S H. Characterization of DNA end joining in a mammalian cell nuclear extract: junction formation is accompanied by nucleotide loss, which is limited and uniform but not site specific. Mol Cell Biol. 1994;14:170–180. doi: 10.1128/mcb.14.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.North P, Ganesh A, Thacker J. The rejoining of double-strand breaks in DNA by human cell extracts. Nucleic Acids Res. 1990;18:6205–6210. doi: 10.1093/nar/18.21.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parket A, Inbar O, Kupiec M. Recombination of Ty elements in yeast can be induced by a double-strand break. Genetics. 1995;140:67–77. doi: 10.1093/genetics/140.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfeiffer P, Vielmetter W. Joining of nonhomologous DNA double strand breaks in vitro. Nucleic Acis Res. 1988;16:907–924. doi: 10.1093/nar/16.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puchta H, Dujon B, Hohn B. Two different but related mechanisms are used in plants for the repair of genomic double-strand breaks by homologous recombination. Proc Natl Acad Sci USA. 1996;93:5055–5060. doi: 10.1073/pnas.93.10.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richard M, Belmaaza A, Gusew N, Wallenburg J C, Chartrand P. Integration of a vector containing a repetitive LINE-1 element in the human genome. Mol Cell Biol. 1994;14:6689–6695. doi: 10.1128/mcb.14.10.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson C, Moynahan M E, Jasin M. Double-strand break repair by interchromosomal recombination: suppression of chromosomal translocations. Genes Dev. 1998;12:3831–3842. doi: 10.1101/gad.12.24.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roth D B, Wilson J H. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol Cell Biol. 1986;6:4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rouet P, Smith F, Jasin M. Expression of a site-specific endonuclease stimulates homologous recombination in mammalian cells. Proc Natl Acad Sci USA. 1994;91:6064–6068. doi: 10.1073/pnas.91.13.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rouet P, Smith F, Jasin M. Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol Cell Biol. 1994;14:8096–8106. doi: 10.1128/mcb.14.12.8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sargent R G, Brenneman M A, Wilson J H. Repair of site-specific double-strand breaks in a mammalian chromosome by homologous and illegitimate recombination. Mol Cell Biol. 1997;17:267–277. doi: 10.1128/mcb.17.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saxton J A, Martin S L. Recombination between subtypes creates a mosaic lineage of LINE-1 that is expressed and actively retrotransposing in the mouse genome. J Mol Biol. 1998;280:611–622. doi: 10.1006/jmbi.1998.1899. [DOI] [PubMed] [Google Scholar]
- 41.Schichman S A, Severynse D M, Edgell M H, Hutchinson C A., III Strand-specific LINE-1 transcription in mouse F9 cells originates from the youngest phylogenetic subgroup of LINE-1 elements. J Mol Biol. 1992;224:559–574. doi: 10.1016/0022-2836(92)90544-t. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz A, Chan D C, Brown L G, Alagappan R, Pettay D, Disteche C, McGillivray B, de la Chapelle A, Page D C. Reconstructing hominid Y evolution: X-homologous block, created by X-Y transposition, was disrupted by Yp inversion through LINE-LINE recombination. Hum Mol Genet. 1998;7:1–11. doi: 10.1093/hmg/7.1.1. [DOI] [PubMed] [Google Scholar]
- 43.Segal Y, Peissel B, Renieri A, de Marchi M, Ballabio A, Pei Y, Zhou J. LINE-1 elements at the sites of molecular rearrangements in Alport syndrome-diffuse leiomyomatosis. Am J Hum Genet. 1999;64:62–69. doi: 10.1086/302213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taghian D G, Nickoloff J A. Chromosomal double-strand breaks induce gene conversion at high frequency in mammalian cells. Mol Cell Biol. 1997;17:6386–6393. doi: 10.1128/mcb.17.11.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vamvakas S, Vock E H, Lutz W K. On the role of DNA double-strand breaks in toxicity and carcinogenesis. Crit Rev Toxicol. 1997;27:155–174. doi: 10.3109/10408449709021617. [DOI] [PubMed] [Google Scholar]
- 46.Van der Ploeg L H T, Gottesdien K, Lee M G-S. Antigenic variation in the African trypanosome. Trends Genet. 1992;8:452–456. doi: 10.1016/0168-9525(92)90330-7. [DOI] [PubMed] [Google Scholar]
- 47.Voliva C F, Jahn C L, Comer M B, Hutchison III C A, Edgell M H. The L1Md long interspersed repeat family in the mouse: almost all examples are truncated at one end. Nucleic Acids Res. 1983;11:8847–8859. doi: 10.1093/nar/11.24.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]