Abstract
Background
Influenza is an infectious virus affecting both humans and animals. In humans, symptoms present as fever, cough, sore throat, runny nose, headache, muscle and joint pain, and malaise. The epidemiological profile of influenza is influenced by multiple factors, including transmissibility of the virus and the susceptibility of the population. Annually, influenza is estimated to infect 5% to 10% of adults, with higher rates in winter seasons in countries with seasonal variation. Exercise could be an intervention to enhance immune response and limit influenza incidence and its related complications.
Objectives
To assess the efficacy and safety of short and long‐term exercise prior to influenza vaccination in enhancing influenza prevention in adults.
Search methods
We searched CENTRAL (2015, Issue 11), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (1966 to 3 November 2015), Embase (1974 to 3 November 2015), CINAHL (1981 to 3 November 2015), LILACS (Latin American and Caribbean Health Sciences, 1982 to 3 November 2015), PEDro (1980 to 3 November 2015), SPORTDiscus (1985 to 3 November 2015), the WHO International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov (November 2015).
Selection criteria
Randomised controlled trials (RCTs) of short‐ and long‐term exercise prior to influenza vaccination for the general adult population were eligible for inclusion.
Data collection and analysis
Two review authors independently extracted and checked data from the included trials using a standard form. We used the random‐effects model due to differences in the type, duration, intensity and frequency of exercise in the analysis.
Main results
We included six trials published between 2007 and 2014 that randomised 599 adult participants. Study size ranged from 46 to 158 participants. Participants were aged between 18 years and 80 years; we could not derive gender proportions, as participants' sex was not reported in all studies. One study was available in abstract form only.
We did not find a significant difference in outcomes between people who exercised and those who did not exercise before receiving influenza vaccination.
Pre‐vaccination exercises included endurance activities such as walking or using a treadmill, and resistance activities included biceps curls and lateral raises. Five of the studies provided one session of exercise between 25 and 50 minutes. In five studies, exercise was undertaken on the same day as the vaccination. One study provided exercise over a period of eight weeks before vaccination, with one 2½ hour supervised session, plus daily home exercise practice of 45 minutes. Exercise intensity ranged from 55% to 85% of maximal heart rate. Control group participants undertook a range of activities, including quiet rest, sitting, reading, meditation or unspecified activity.
One study reported numbers of people who contracted influenza; no significant difference was reported between exercise and no‐exercise participants. None of the included studies reported complications related to influenza illness. Only one study, which we assessed as providing low‐quality evidence, reported numbers of people who experienced adverse events. This study reported no significant difference in outcomes between people who exercised and those who did not. No studies reported numbers of working days or days lost related to influenza illness. Only two studies reported participant‐centred outcomes.
Overall, study quality was unclear; we assessed five of the six included studies to have at least four unclear 'Risk of bias' domains (allocation concealment, blinding of outcome assessment, selective reporting and other bias). Insufficient reporting in four studies about selective reporting did not provide enough information to enable judgement; only two studies were included in trials registers.
Authors' conclusions
From the available evidence, we found that exercising before influenza vaccination is neither beneficial nor harmful. However, study data were limited and of low quality. Small sample sizes, study design limitations, exercise types, and focus on biochemical rather than participant‐centred outcomes strongly influenced our findings.
Plain language summary
Can exercising before vaccination reduce numbers of adults who get flu or develop complications?
Review question We looked at whether exercise before influenza (flu) vaccination can decrease numbers of adults who develop flu and reduce complications.
Background
Exercise can change immune response. Flu is an infectious virus estimated to affect three to five million people worldwide each year. Seasonal flu vaccination, which acts on the immune system to protect people from infection, is common in many countries.
Search date
We searched the literature up to November 2015.
Study characteristics We looked at six studies that involved 599 people aged between 18 and 80 years that assessed exercise before flu vaccination. Exercises included walking or using a treadmill (endurance) and biceps curls and lateral raises (resistance) activities that ranged from 25 to 50 minutes per session. People in five studies did one session of exercise on the day of vaccination; in one study people exercised eight weeks before vaccination. Exercise was supervised in three studies. People not undertaking exercise (control group) were assessed after periods of quiet rest.
Study funding sources
Three studies did not report study funding sources; one received support from a drug company that donated flu vaccine, one from a professional society, and another from government agencies.
Key results We found no differences in numbers of people who caught flu or developed complications between people who exercised and those who did not before flu vaccination. Only one study reported how many people developed flu after exercise and vaccination. No studies reported complications related to flu; only one reported adverse events. None reported numbers of working or other days lost due to flu. No beneficial differences were reported between exercise and no‐exercise groups before vaccination.
Small numbers of people who were involved in the studies, limitations in study design, and different exercise types meant we were unable to draw robust conclusions about any benefits of exercise before vaccination. There appears to be no benefit or harm from exercising before receiving flu vaccination.
Quality of evidence Evidence quality was very low or low. More robust study designs that include enough people to enable assessment and analysis of findings may help to determine if exercise before vaccination can reduce numbers of people who develop flu or complications.
Author's conclusions
We found that exercising before influenza vaccination is neither beneficial nor harmful. Small number of people in each included study, many types of exercises, and focus on blood examination instead of participant‐centred outcomes strongly influenced our findings.
Summary of findings
Summary of findings for the main comparison. Resistance and endurance exercise prior to influenza vaccination versus no exercise prior to influenza vaccination.
| Exercise prior to influenza vaccination for limiting influenza incidence and its related complications in adults | ||||||
| Patient or population: Adults and elderly Setting: Study laboratory Intervention: Resistance and endurance exercise Comparison: no exercise | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk of contracting influenza ‐ no exercise before vaccination | Risk of contracting influenza ‐ exercise before vaccination | |||||
|
Incidence of influenza Self‐reported influenza‐like illness follow‐up: adjusted for 1 year |
Median risk in study population | RR 0.22 (0.01 to 4.40) | 98 (1 study) | ⊕⊕⊝⊝ very low1,2,3 |
||
| 39 per 1000 | 9 per 1000 (0 to 172) | |||||
| Complications related to influenza illness | Not pooled | Not pooled | Not pooled | (no studies) | No events in either arm | |
| Adverse events Hypersensitivity reaction to vaccination | Median risk in study population | Not estimable | 120 (1 study) | ⊕⊕⊝⊝ low¹ |
No events in either arm | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Numbers of working days or days lost related to influenza illness | Not pooled | Not pooled | Not pooled | (no studies) | No events in either arm | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Limitations in the design and implementation. Risk of selection bias and lack of blinding; allocation concealment not reported. 2Imprecision ‐ wide confidence intervals. 3Indirectness ‐ self‐reported influenza‐like illness.
Background
Description of the condition
Influenza is an infectious virus affecting both humans and animals. In humans, symptoms present as fever, cough, sore throat, runny nose, headache, muscle and joint pain, and malaise (CDC 2009; WHO 2011). There are three types of influenza: strains: A, B and C. Influenza A has three subtypes of haemagglutinins (H1, H2 and H3) and two subtypes of neuraminidases (N1 and N2). Influenza B and C are not subdivided. Influenza A has the ability to make periodic changes to its strain over time, making efficacious prevention by vaccination a challenge (Demicheli 2014). The epidemiological profile of influenza is influenced by several factors, including transmissibility of the virus and the susceptibility of the population (CDC 2009; Nair 2011). Influenza is estimated to infect 5% to 10% of adults, with higher rates in winter seasons in countries with seasonal variation, each year (WHO 2012).
Influenza incidence varies according to age group, with greater risk observed in children under five years old and adults aged 65 years or over. Priority groups for influenza vaccination are people with chronic medical conditions and pregnant women, because these conditions predispose people to influenza‐related illness and hospitalisation (CDC 2010; Molinari 2007).
Influenza is estimated to affect three to five million people worldwide annually. Economic impact, estimated using a probabilistic model based on publicly available epidemiological data, is a mean of USD 87.1 billion per year (Molinari 2007). Costs are predominantly due to medical care, loss of productivity, life‐years lost, and death (CDC 2010; WHO 2012).
Description of the intervention
Regular exercise has a positive impact on health and is considered a core behavioural strategy for health promotion (Booth 2012). For this review we defined exercise as "a planned and structured programme of motor actions to improve or maintain components of physical fitness" (Carpersen 1985). Exercise, like administration of drug therapies, includes components such as frequency, time, type and intensity (Lobelo 2014). Different types of exercise provoke different physiological responses (Burton 2004; Edwards 2006).
How the intervention might work
Moderate exercise appears to enhance immune function (Edwards 2007) through the induction of a pro‐inflammatory environment in skeletal muscle, particularly during the eccentric phase of muscle contraction (Peake 2005). Proposed benefits include increased leukocyte numbers, particularly monocytes and dendritic cells (Tossige‐Gomes 2014), enabling enhanced cell migration to the antigen exposure site (Krüger 2008). Immune transport is further aided by augmentation of lymph drainage resulting from muscle contraction (Schillinger 2006). In contrast, intense exercise may suppress the immune system, causing increased susceptibility to infection for the first 24 hours after exercise through a negative effect on neutrophil phagocytic function and a reduction in natural killer cell and lymphocyte numbers (Kakanis 2010). Moderate‐intensity exercise before intramuscular vaccine administration may therefore improve the initial immune response to vaccination in comparison to preceding rest or vigorous muscle activity (Pascoe 2014).
Why it is important to do this review
Despite evidence from Cochrane Reviews showing no impact on hospitalisation or complication rates (Demicheli 2014; Jefferson 2010; Thomas 2013), influenza vaccination is ingrained in many public health agendas. These reviews demonstrated a moderate effect from vaccination on symptom reduction and in decreasing numbers of working days lost through ill health (Demicheli 2014; Jefferson 2010; Thomas 2013). We aimed to establish if exercise before vaccination can enhance efficacy by reducing numbers of sick days and symptoms caused by influenza, and build upon previous Cochrane Reviews about influenza vaccination. Numbers of RCTs supporting exercise before influenza vaccination are growing (Campbell 2010; Edwards 2006; Edwards 2007; Edwards 2010; Kohut 2002; Kohut 2004; Ranadive 2014; Woods 2009).
Objectives
To assess the efficacy and safety of short‐ and long‐term exercise prior to influenza vaccination to enhance influenza prevention in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) investigating exercise before influenza vaccination for the general population. Cross‐over and cluster‐RCTs were also eligible for inclusion.
Types of participants
We included participants randomised to exercise or no exercise before receiving influenza vaccination. We included no studies involving children aged up to 18 years.
Types of interventions
We included studies that compared exercise before influenza vaccination with participants who did not exercise before influenza vaccination. Exercise could be single or multiple sessions, resistance or aerobic exercise, specific muscle group or whole‐body exercise.
Types of outcome measures
Primary outcomes
Incidence of influenza (as reported by primary studies).
Complications related to influenza illness.
Adverse effects of vaccination.
Secondary outcomes
Number of working days or days lost related to influenza illness.
Blood parameters (IgG, IgM, IL‐6, etc.).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2015, Issue 11), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (1966 to 3 November 2015), Embase (1974 to 3 November 2015), CINAHL (1981 to 3 November 2015), LILACS (Latin American and Caribbean Health Sciences, 1982 to 3 November 2015), PEDro (Physiotherapy Evidence Database, 1980 to 3 November 2015) and SPORTDiscus (1985 to 3 November 2015).
We present MEDLINE and Embase search strategies in Appendix 1 and Appendix 2. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE (Lefebvre 2011). We adapted the search strategy to search the other databases. We did not use language or publication restrictions. In case of articles in other languages, we will use academic networks and Cochrane resources to translate the critical parts to enable screening of abstracts and, if necessary, critical parts of the full paper.
Searching other resources
We searched the World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch/) and ClinicalTrials.gov for completed and ongoing studies to 3 November 2015. We checked the reference lists of all primary studies and review articles for additional references. We did not email experts in the field about other published and unpublished studies that may be eligible for inclusion.
Data collection and analysis
Selection of studies
Two review authors independently screened all titles and abstracts identified from searches to determine which met the inclusion criteria. We retrieved potentially relevant papers in full text. The same review authors independently screened full‐text articles for inclusion or exclusion, with discrepancies resolved by discussion and by consulting a third review author where necessary to reach consensus. We planned to present reports of included studies. All excluded studies, with reasons for exclusion, are presented in Characteristics of excluded studies. The study screening and selection process is presented in Figure 1.
Data extraction and management
Two review authors independently extracted data from the included studies using a standard data extraction form. We resolved any discrepancies by discussion until we reached consensus, or through consultation with a third review author where necessary. Two review authors extracted data for blood parameters from figures in four studies (Edwards 2007; Edwards 2010; Long 2012; Ranadive 2014) to measure the mean and standard errors for analysis and comparison (Silva 2012).
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each included study using the 'Risk of bias' tool published in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). A third review author resolved any disagreements by discussion. We assessed risk of bias according to the following domains.
Random sequence generation (selection bias). We classified studies as low risk of bias if they properly described how the random sequence was generated. We classified them as unclear risk of bias if they did not provide enough information to be judged, and as high risk of bias if the randomisation was conducted in a predictable way.
Allocation concealment (selection bias). We classified the studies as low risk of bias if they properly described how the allocation was concealed. We classified them as unclear risk of bias if they did not provide enough information to be judged, and as high risk of bias if the allocation was predictable.
Blinding of participants and personnel (performance bias). Due to the characteristics of the intervention (exercise), the participants could not be blinded.
Blinding of outcome assessment (detection bias): Due to the characteristics of the intervention (exercise), the participants could not be blinded; however, they could be blinded to the injection, control group with no vaccine inside.
Incomplete outcome data (attrition bias). We classified the studies as low risk of bias if they properly described data losses or incomplete data and provided reasons. We classified them as unclear risk of bias if they did not provide enough information to be judged. If the studies excluded or omitted analyses of data outcomes we considered them as high risk of bias.
Selective reporting (reporting bias). We classified the studies as low risk of bias if they properly described what they planned to do in their project and published all the outcomes planned. We classified them as unclear risk of bias if they did not provide enough information to be judged. If the studies reported only statistically significant findings rather than show the complete results we considered them high risk of bias.
Other bias (other potential sources of bias) related to particular trial design (cross‐over and cluster‐randomised) or specific circumstances such as adjustment of prognostic factors (e.g. baseline exercise habits, physical fitness).
Measures of treatment effect
Types of measurement of treatment effects that were analysed included:
Dichotomous data: we used risk ratio (RR) for the binary outcomes: complications arising from influenza‐related illness, adverse effects of vaccination, and adherence to the group intervention.
Continuous data: we combined results using the mean difference (MD) for measures that used the same scale, or the standardised mean difference (SMD) where different scales had been used to evaluate the same outcome for blood parameters (antibody titres).
Rate: we did not use rate ratio to compare rates between groups for number of influenza episodes, due to lack of reported studies.
Unit of analysis issues
We considered individual participants as the unit of analysis. Among the six included studies, we included one cross‐over RCT and considered only the phase before crossing over interventions. If cluster‐RCTs had been included, we would have made the necessary statistical adjustments for clustering participants.
Dealing with missing data
We did not attempt to contact study authors because we found no inconsistencies between randomised and analysed study participants.
Assessment of heterogeneity
We assessed the inconsistencies between studies using the I² statistic and described the percentage of variability in effect. We considered heterogeneity substantial if I² statistic was over 50%.
Assessment of reporting biases
We identified no mismatches between study protocols and reports. We had planned to assess the impact of including such studies by conducting a sensitivity analysis. We did not perform a funnel plot asymmetry test, as we only included six trials.
Data synthesis
Meta‐analysis of the included RCTs was possible only for participants' blood parameters. We used a random‐effects model, independently of the heterogeneity identified. When it was not possible to pool data, we present narrative analyses of individual studies.
GRADE and 'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: incidence of influenza, complications related to influenza illness, adverse events, and numbers of working days or days lost related to influenza illness. We used the five GRADE considerations (Atkins 2004): study limitations, consistency of effect, imprecision, indirectness and publication bias, to assess the quality of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), using GRADEproGDT software (GRADEproGDT 2015). We justified all decisions to down‐ or upgrade the quality of studies using footnotes, and we made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We conducted subset analyses for blood parameters antibody titre:
follow‐up over time;
timing of vaccination; and
type of exercise.
Sensitivity analysis
We pooled studies to verify if the impact of risk of bias affected the overall treatment effect of exercising. We also assessed which studies contributed to heterogeneity.
Results
Description of studies
Results of the search
Our search identified 485 records from databases, handsearches of reference lists, grey literature and trial register search results. We removed 68 duplicate records (n = 317 records). Two review authors then assessed titles and abstracts and deleted 305 records that did not match inclusion criteria. We obtained full‐text copies of the remaining 12 potentially relevant references. Of these, six studies did not meet inclusion criteria. See Figure 1.
1.
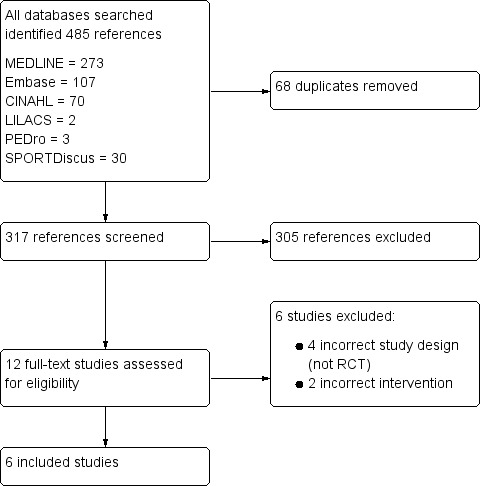
Study flow diagram
Included studies
We included six trials that randomised 599 adult participants, published between 2007 and 2014 in English language (Edwards 2007; Edwards 2010; Edwards 2013; Hayney 2014; Long 2012; Ranadive 2014). Edwards 2013 was available as an abstract only. (See Included studies; Table 2; Table 3).
1. Included study participant characteristics at baseline.
| Study name/location | Participants randomised/withdrew | % women | Age | Participant characteristics |
| Edwards 2007UK | 60/3 | 51% | 20.35 ± 2.09 years | Physically active students |
| Edwards 2010UK | 160/2 | 51% | 20.5 ± 1.6 years | Mean BMI = 21.6 ± 2.6 kg/m² |
| Edwards 2013UK (Abstract only) | 46/0 | 50% | 73 ± 7 years | Mean BMI = 27.20 ± 5 kg/m² |
| Hayney 2014USA | 154/5 | 81.8% | 50 years and over | Healthy, sedentary |
| Long 2012UK | 122/2 | NR | 18 to 30 years cohort; 50 to 64 years cohort | Mean BMI = 24.50 ± 4.23 kg/m² Mean BMI = 25.97 ± 4.12 kg/m² |
| Ranadive 2014USA | 59/4 | 54% | 66.5 ± 0.85 | Mean BMI = 25.55 ± 0.9 kg/m² |
Abbreviations: BMI ‐ body mass index; NR ‐ not reported
2. Characteristics of exercise intervention and timing of influenza vaccination.
| ID Study location/s | Exercise type | Frequency | Intensity | Duration | Supervision | Control group | Timing of Influenza vaccination | Participant follow‐up | Outcomes in meta‐analysis |
| Edwards 2007UK | Biceps curl and lateral raise | 1 session | 85% of 1 RM | 25 min (6 sets of 7 repetitions and 2 sets of 4 repetitions) | Supervised | Quiet resting | Exercise immediately before vaccination | 6, 8, 20 weeks post‐vaccination | Adverse effects; blood parameters |
| Edwards 2010UK | Non‐dominant arm biceps curl and lateral raise | 1 session | 60% of 1 RM; 85% of 1 RM; 110% of 1 RM | 25 min (10 sets of 5 repetitions) | Supervised | Quiet resting | Exercise immediately before vaccination | 4 weeks post‐vaccination | Adverse effects; blood parameters |
| Edwards 2013UK | Resistance upper and lower body | 1 session | 60% of 1 RM | 50 minutes | NR | Quiet reading | Exercise immediately before vaccination | 4 weeks post‐vaccination | None |
|
Hayney 2014 USA |
Bicycle, treadmill and brisk walk |
1 week session (2½ hours) + 45 minutes daily home practice |
12 to 16 Borg scale |
8 weeks | 1 week session supervised by exercise physiologist |
Meditation and no exercise |
6 months after exercise | 12 weeks post‐vaccination | Incidence of influenza; number of working days lost; blood parameters |
| Long 2012UK | Walk | 1 session | 55% MHR | 45 minutes | No supervision | Quiet sitting | Exercise immediately before vaccination | 4 weeks post‐vaccination | Adverse effects; blood parameters |
| Ranadive 2014USA | Aerobic exercise | 1 session | 55% to 65% MHR | 40 minutes | NR | NR | Exercise immediately before vaccination | 4 weeks post‐vaccination | Blood parameters |
Abbreviations: HRR ‐ heart rate reserve; ID ‐ identification; MHR ‐ maximum heart rate; NR ‐ not reported; RM ‐ repetition maximum
Design
Five included studies were parallel RCTs (Edwards 2007; Edwards 2010; Edwards 2013; Hayney 2014; Long 2012); Ranadive 2014 was a randomised, cross‐over design.
Sample sizes
The included studies randomised 599 adult participants. Sample size ranged from 46 participants (Edwards 2013) to 158 participants (Edwards 2010).
Setting
All included trials were single‐centre studies: two were conducted in the USA (Hayney 2014; Ranadive 2014) and four in the UK (Edwards 2007; Edwards 2010; Edwards 2013; Long 2012).
Participants
Participants were aged from 18 years to 80 years. Proportions of women ranged from 50% to 82%. One study did not report participants' gender (Long 2012).
Interventions
Pre‐vaccination exercises included endurance activities such as walking or using a treadmill, and resistance activities including biceps curls and lateral raises. Five studies included one exercise session of between 25 minutes and 50 minutes on the same day as the vaccination (Edwards 2007; Edwards 2010; Edwards 2013; Long 2012; Ranadive 2014). Hayney 2014 provided exercise over a period of eight weeks before vaccination, with one 2½‐hour supervised session, plus daily home exercise practice of 45 minutes. Exercise intensity ranged from 55% to 85% of maximal heart rate. Control group participants undertook a range of activities including quiet rest, sitting, reading or no exercise.
Outcomes
Hayney 2014 reported numbers of people who contracted influenza; no significant difference was reported between exercise and no‐exercise participants. None of the included studies reported complications related to influenza illness.
Long 2012 reported numbers of people who experienced adverse events. This study reported no significant difference in outcomes between people who exercised and those who did not.
No studies reported numbers of working days or days lost related to influenza illness.
Excluded studies
We excluded six studies. Of these, four were not RCTs, and two did not match our inclusion criteria because control group participants also undertook exercise (see Characteristics of excluded studies).
Risk of bias in included studies
Figure 2 and Figure 3 are graphic presentations of risk of bias' assessments. The overall quality of the studies was unclear, since five of the six included studies had at least four unclear risk of bias domains (allocation concealment, blinding of outcome assessment, selective reporting and other bias). Insufficient information in four studies about selective reporting did not allow judgement (Edwards 2007; Edwards 2010; Edwards 2013; Ranadive 2014), as only two studies were included in trials registers (Hayney 2014; Long 2012).
2.
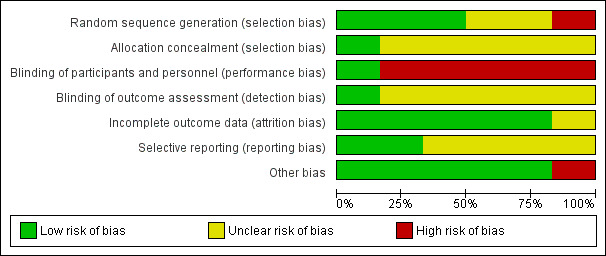
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
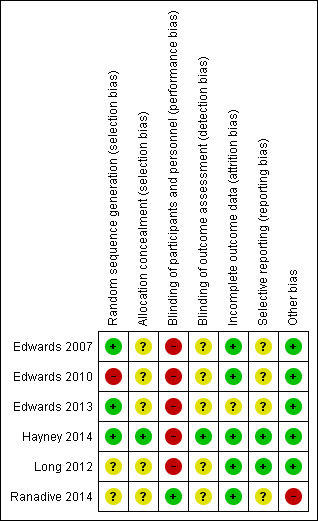
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Three studies appropriately described randomisation procedures (Edwards 2007; Edwards 2013; Hayney 2014), two studies did not provide sufficient information to enable assessment (Long 2012;Ranadive 2014), and one study was classified as high risk of bias: "Participants were pseudo‐randomised, maintaining an even sex distribution" (Edwards 2010). For allocation concealment, we classified five studies as unclear risk of bias since they did not provide sufficient information to enable assessment. We assessed only one study at low risk of bias, because investigators used codes provided in concealed envelopes, which were opened after consent to indicate allocation (Hayney 2014).
Blinding
We considered five studies at high risk of bias for blinding participants and personnel, since the nature of the study interventions (exercise) meant that blinding of participants was not possible. However, Ranadive 2014 was considered low risk of bias because personnel were blinded by using sham vaccination. Considering detection bias and blinding of outcome assessment, five studies did not provide sufficient information to enable assessment; Hayney 2014 blinded laboratory staff performing the haemagglutination inhibition assay (HIA) to participant status. Only one study used a placebo vaccination.
Incomplete outcome data
We classified five studies (Edwards 2007; Edwards 2010; Hayney 2014; Long 2012; Ranadive 2014) at low risk of bias, since losses to follow‐up were less than 10% for the antibody titre analysed in each study; these studies also provided adequate data analysis in relation to losses to follow‐up. We rated one study at unclear risk of bias because of insufficient information to enable judgement about exclusions, and no reporting of missing data. This study was available in abstract form only (Edwards 2013).
Selective reporting
Only two studies registered their trials before conducting the study. We found no differences between the protocols and the study reports, and classified both at low risk of bias (Hayney 2014; Long 2012). We classified the remaining four included studies at unclear risk of bias, because of insufficient information to enable outcome assessment (antibody titre) from different timepoints or analysis across study follow‐up.
Other potential sources of bias
We considered one study to be at high risk of bias due to baseline imbalance of older people, either sedentary or physically active, since the authors did not control for physical activity level in the analysis (Ranadive 2014).
Three studies did not report study funding sources; one received support from a drug company that donated flu vaccine, one from a professional society, and another from government agencies.
Effects of interventions
See: Table 1
Incidence of influenza
Hayney 2014 reported no significant difference between exercise and non‐exercise groups in relation to influenza incidence (risk ratio (RR) 0.22, 95% confidence interval (CI) 0.01 to 4.40, participants = 98). We rated the evidence quality as very low (Table 1).
Complications related to influenza illness
None of the included studies reported data about complications relating to influenza.
Adverse events associated with vaccination
Long 2012 (120 participants) reported adverse events associated with vaccination; none were reported in the influenza vaccination and non‐vaccination groups. We assessed evidence quality as low (Table 1).
Numbers of working days or days lost related to influenza illness
None of the included studies reported data on numbers of working days or days lost related to influenza illness.
Blood parameters
Five studies reported blood parameters (Edwards 2007; Edwards 2010; Hayney 2014; Long 2012; Ranadive 2014). We conducted subset analyses for antibody titres of viral strains A and B for follow‐up over time (Analysis 1.1), vaccination timing (Analysis 1.2) and exercise type (Analysis 1.3). We assessed the evidence quality as low due to lack of information about study design and implementation (risk of selection bias and lack of blinding, allocation concealment) not being adequately reported in most studies; we also found imprecision, indicated by wide confidence intervals.
1.1. Analysis.
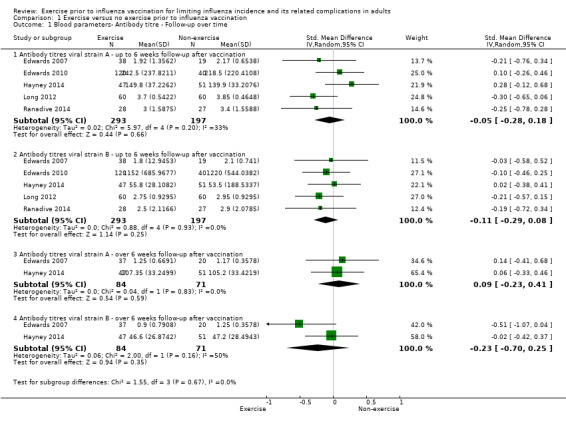
Comparison 1 Exercise versus no exercise prior to influenza vaccination, Outcome 1 Blood parameters‐ Antibody titre ‐ Follow‐up over time.
1.2. Analysis.
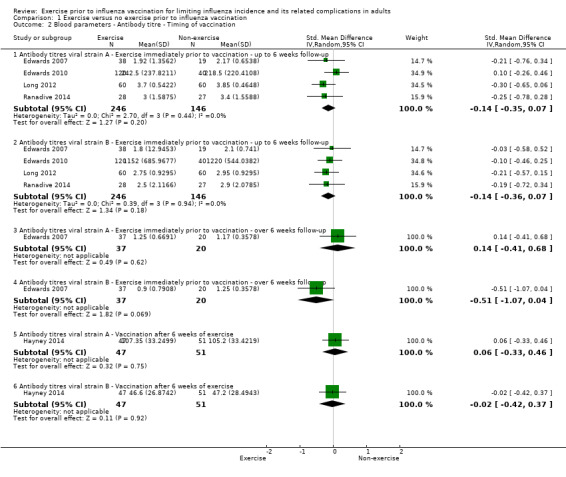
Comparison 1 Exercise versus no exercise prior to influenza vaccination, Outcome 2 Blood parameters ‐ Antibody titre ‐ Timing of vaccination.
1.3. Analysis.
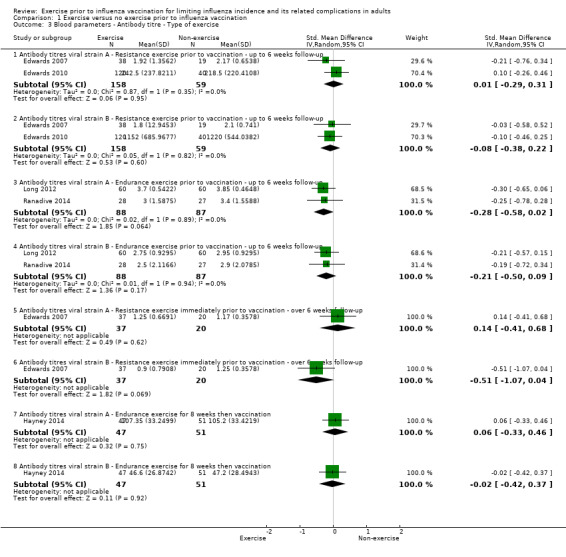
Comparison 1 Exercise versus no exercise prior to influenza vaccination, Outcome 3 Blood parameters ‐ Antibody titre ‐ Type of exercise.
Antibody titre ‐ follow‐up over time
Up to six weeks follow‐up after vaccination
We found no significant difference between exercise and no‐exercise study arm participants (five studies, 490 participants; strain A: standardised mean difference (SMD) ‐0.05, 95% CI ‐0.28 to 0.18, Analysis 1.1.1; Chi² test 5.97; df = 4; P = 0.20; I² statistic 33%. Strain B: SMD ‐0.11, 95% CI ‐0.29 to 0.08, Analysis 1.1.2; Tau² = 0.00, Chi² test 0.88, df = 4, P = 0.93; I² statistic 0%).
Over 6 weeks follow‐up after vaccination
We found no significant difference between exercise and no‐exercise participants (two studies, 155 participants; strain A: SMD 0.09, 95% CI ‐0.23 to 0.41, Analysis 1.1.3; Tau² = 0.00; Chi² test 0.04, df = 1, P = 0.83, I² statistic 0%. strain B: SMD ‐0.23, 95% CI ‐0.70 to 0.25, Analysis 1.1.4; Tau² = 0.06; Chi² test 2.00, df = 1, P = 0.16, I² statistic 50%).
Antibody titre ‐ vaccination timing
Five studies contributed data to this outcome. We analysed data in six subsets (Analysis 1.2).
Antibody titres viral strains A and B ‐ Exercise immediately prior to vaccination ‐ up to six weeks follow‐up
We found no significant difference between exercise and no‐exercise study arm participants (four studies, 392 participants; strain A: SMD ‐0.14, 95% CI ‐0.35 to 0.07; Analysis 1.2.1; Tau² = 0.00; Chi² test 2.70, df = 3, P = 0.44, I² statistic 0%. Strain B: SMD ‐0.14, 95% CI ‐0.36 to 0.07; Analysis 1.2.2; Tau² = 0.00; Chi² test 0.39, df = 3, P = 0.94, I² statistic 0%).
Antibody titres viral strains A and B ‐ Exercise immediately prior to vaccination ‐ over six weeks follow‐up
We found no significant difference between exercise and no‐exercise study arm participants (one study, 57 participants; strain A: SMD 0.14, 95% CI ‐0.41 to 0.68; Analysis 1.2.3. Strain B: SMD ‐0.51, 95% CI ‐1.07 to 0.04; Analysis 1.2.4).
Antibody titres viral strains A and B ‐ Vaccination after six weeks of exercise
We found no significant difference between exercise and no‐exercise study arm participants (one study, 98 participants; strain A: SMD 0.06, 95% CI ‐0.33 to 0.46; Analysis 1.2.5. Strain B: SMD ‐0.02, 95% CI ‐0.42 to 0.37; Analysis 1.2.6).
Antibody titre ‐ exercise type
We identified five relevant studies and categorised data into eight subsets (Analysis 1.3).
Antibody titres viral strains A and B ‐ Resistance exercise prior to vaccination ‐ up to six weeks follow‐up
We found no significant difference between exercise and no‐exercise study arm participants (two studies, 217 participants; strain A: SMD 0.01, 95% CI ‐0.29 to 0.31, Analysis 1.3.1; Tau² = 0.00; Chi² test 0.87, df = 1, P = 0.35, I² statistic 0%. Strain B: SMD ‐0.08, 95% CI ‐0.38 to 0.22, Analysis 1.3.2; Tau² = 0.00; Chi² test 0.05, df = 1, P value 0.82, I² statistic 0%).
Antibody titres viral strains A and B ‐ Endurance exercise prior to vaccination ‐ up to six weeks follow‐up
We found no significant difference between exercise and no‐exercise study arm participants (two studies, 175 participants; strain A: SMD ‐0.28, 95% CI ‐0.58 to 0.02, Analysis 1.3.3; Tau² = 0.00; Chi² test 0.02, df = 1, P = 0.89, I² statistic 0%. Strain B: SMD ‐0.21, 95% CI ‐0.50 to 0.09, Analysis 1.3.4; Tau² = 0.00; Chi² test 0.01, df = 1, P = 0.94, I² statistic 0%).
Antibody titres viral strains A and B ‐ Resistance exercise immediately prior to vaccination ‐ over six weeks follow‐up
We found no significant difference between exercise and no‐exercise study arm participants (one study, 57 participants; strain A: SMD 0.14, 95% CI ‐0.41 to 0.68, Analysis 1.3.5. Strain B: SMD ‐0.51, 95% CI ‐1.07 to 0.04, Analysis 1.3.6).
Antibody titres viral strains A and B ‐ Endurance exercise for eight weeks then vaccination
We found no significant difference between exercise and no‐exercise study arm participants (one study, 98 participants; strain A: SMD 0.06, 95% CI ‐0.33 to 0.46, Analysis 1.3.7. Strain B: SMD ‐0.02, 95% CI ‐0.42 to 0.37, Analysis 1.3.8).
Discussion
Summary of main results
We aimed to assess the efficacy and safety of short‐ and long‐term exercise prior to influenza vaccination to enhance influenza prevention in adults.
We did not find significant differences for any outcomes between people who exercised and those who did not before influenza vaccination. Only one study reported influenza incidence; none investigated complications related to influenza illness; and one study reported adverse events. No included studies investigated numbers of working days or days lost related to influenza; five studies reported blood parameter data. We reported data for several subsets: antibody titre follow‐up over time and according to exercise type. We pooled effects of antibody titre post‐intervention. Only two studies reported participant‐centred outcomes; all studies planned to investigate blood parameters to identify immunological reasoning to support the use of exercise prior to influenza vaccination. Overall, we assessed study quality as low.
We analysed findings from six RCTs that compared exercise versus no exercise before and immediately before influenza vaccination and found:
Influenza incidence was slightly lower in the exercise group; however, data came from only one study assessed as providing very low‐quality evidence.
No adverse events were reported for either exercise or no‐exercise participant groups; however, data came from only one study assessed as providing low‐quality evidence.
The standardised mean difference (SMD) in the intervention group for blood parameters was 0.05 units lower (range ‐0.28 to +0.18) in serum antibody titres of viral strain A. Data for this outcome came from five studies.
The SMD in the intervention group for blood parameters was 0.11 units lower (range ‐0.29 to +0.08) in serum antibody titres of viral strain B. Data for this outcome came from five studies.
Overall completeness and applicability of evidence
We found that exercise before influenza vaccination did not improve protection provided by influenza vaccination. We assessed the current evidence as low quality, due to imprecision and high risk of bias. Given the small body of evidence, and the relative novelty of the concept that exercise before vaccination could influence outcomes, it may be too soon to draw further conclusions. Different types of exercise and intensity of physical effort were not widely considered by the included studies and may confer different physiological adaptations, either beneficial or harmful.
It is also important to highlight that study data were limited in terms of numbers of outcomes reported; most did not assess participant‐centred outcomes. Most studies investigated immunological outcomes to assess the hypothesis that exercise can improve efficacy and reduce complications related to influenza vaccination.
The applicability of evidence to clinical practice remains uncertain.
Quality of the evidence
The overall quality of evidence was low. We based this assessment on lack of blinding and risk of selection bias (allocation concealment not reported in the studies), and imprecision, with small sample sizes and wide confidence intervals. We found a high risk of bias for blinding of participants and personnel, and reporting deficits in at least four domains for five of the six included studies.
Randomisation sequence generation was not clearly described in Long 2012 and Edwards 2013; these studies applied pseudo‐randomisation.
The nature of the exercise interventions investigated meant that it was not possible to blind participants. Sham vaccination was used in Ranadive 2014. While outcome assessors could be blinded, only Hayney 2014 provided sufficient information to confirm that blinding was carried out. Attrition bias was not a concern in most studies; only Edwards 2013 was unclear regarding this domain, although only Long 2012 and Ranadive 2014 provided appropriate methods (e.g. intention‐to‐treat analysis) for dealing with missing data and dropouts. Four studies were not listed in trials registers (Edwards 2007; Edwards 2010; Edwards 2013; Ranadive 2014).
Potential biases in the review process
We followed recommendations made for study selection, data collection, and data analysis provided in the Cochrane Handbook for Systematic Reviews of Interventions in this review (Higgins 2011b). The strengths of the review process were numbers of databases searched and search sensitivity to detect all potentially relevant studies.
Review limitations were that too few studies were included to assess for publication bias by constructing funnel plots; poor study reporting meant that we could not assess some 'Risk of bias' domains; and we found an absence of participant‐centred outcome data in the included RCTs.
Agreements and disagreements with other studies or reviews
Pascoe 2014 conducted a systematic review about vaccination programmes for influenza, meningococcus, tetanus, keyhole limpet haemocyanin (KLH) and pneumococcus to observe the effects of exercise on immune response prior to vaccination. The authors included 20 studies that applied RCT, cross‐sectional and observational study designs. The evidence presented by Pascoe 2014 showed that exercise significantly improved immune response to vaccination. The authors used the "Assessing methodological quality of systematic review reviews (AMSTAR)" instrument to assess data quality (good quality, 8 out of a possible 11 points). However, there were some limitations in Pascoe 2014: the quality of the included studies was not assessed and documented; the scientific quality of the included studies was not appropriately applied in formulating conclusions; results were not pooled for assessment.
Our review focused on exercise before influenza vaccination, and we have improved on the work presented by Pascoe 2014. We reported synthesis and assessment of the evidence, documented assessment of study quality, and provided conclusions based on our findings.
We found no differences in benefits or harms from exercise before influenza vaccination. A possible reason could be that the included studies did not adjust for baseline characteristics according to the physical activity levels of each participant, so that the dose‐response of exercising before influenza vaccination could have been diluted in the results.
Analysis of the published evidence about exercise before influenza vaccination demonstrated that exercise in its many types and intensities did not provide either additional benefit or harm before influenza vaccination.
Authors' conclusions
Implications for practice.
We were unable to determine if exercise before influenza vaccination is effective for reducing influenza incidence, complications related to influenza illness, adverse events or blood parameter outcomes. We rated the quality of the evidence as low or very low; there is significant uncertainty around benefits or harms of exercise before influenza vaccination.
Implications for research.
The biological plausibility of how exercise functions influence health outcomes remains unclear, and exercise mechanisms should be further explored. Exercise type, intensity, duration and frequency are challenging to estimate to derive beneficial levels before influenza vaccination. We suggest that researchers designing future studies should investigate participant‐focused outcomes such as influenza incidence, complications related to influenza illness, and adverse events. It is also essential to adequately describe and report study design, results and outcomes that are relevant to public health objectives and agendas.
Acknowledgements
We wish to thank Clare Dooley and Liz Dooley from Cochrane Acute Respiratiry Infections Group for their comments and discussing the relevance of the idea for this review. We also thank the following people for commenting on the draft protocol: Ann Fonfa, Theresa Wrangham, Helena Liira, Neil Walsh, Stéphane Bermon, Mark Jones and Mieke van Driel. We also thank the following people for commenting on the draft of the full review: Sheila Page, Theresa Wrangham, Terry Neeman, Stéphane Bermon.
Appendices
Appendix 1. MEDLINE (OVID)
1 exp INFLUENZA/ 2 influenza.mp. 3 or/1‐2 4 exp VACCINES/ 5 exp VACCINATION/ 6 (immuniz$ or immunis$).mp. 7 vaccin$.mp. 8 or/4‐7 9 3 and 8 10 exp Influenza Vaccine/ 11 (influenz$ adj (vaccin$ or immun$)).mp. 12 or/10‐11 13 9 or 12 14 exp Exercise/ 15 exp Exercise Movement Techniques/ 16 exp Exercise Therapy/ 17 Physical Fitness/ 18 physical endurance/ or exercise tolerance/ 19 Physical Exertion/ 20 exp Sports/ 21 Dancing/ 22 (exercis* or sport* or fitness* or gym* or aerobic*).tw. 23 ((weight* or strength* or enduranc* or circuit*) adj5 (program* or train* or session*)).tw. 24 (physical* adj5 (fit* or activ* or movement* or train* or condition* or program*)).tw. 25 (activ* adj2 life*).tw. 26 (run* or walk* or jog* or sprint* or treadmill* or row* or swim* or bicycl* or cycl* or danc* or yoga or tai chi or tai ji or qigong or qi gong).tw. 27 or/14‐26 28 13 and 27
Appendix 2. Embase search strategy
#23 #11 AND #22 #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #21 run*:ab,ti OR walk*:ab,ti OR jog*:ab,ti OR sprint*:ab,ti OR treadmill*:ab,ti OR row*:ab,ti OR swim*:ab,ti OR bicycl*:ab,ti OR cycl*:ab,ti OR danc*:ab,ti OR yoga:ab,ti OR 'tai chi':ab,ti OR 'tai ji':ab,ti OR qigong:ab,ti OR 'qi gong':ab,ti AND [embase]/lim #20 (activ* NEAR/2 life*):ab,ti AND [embase]/lim #19 (physical* NEAR/5 (fit* OR activ* OR movement* OR train* OR condition* OR program*)):ab,ti AND [embase]/lim #18 ((weight* OR strength* OR enduranc* OR circuit*) NEAR/5 (program* OR train* OR session*)):ab,ti AND [embase]/lim #17exercis*:ab,ti OR sport*:ab,ti OR fitness*:ab,ti OR gym*:ab,ti OR aerobic*:ab,ti AND [embase]/lim #16 'sport'/exp AND [embase]/lim #15 'training'/de OR 'endurance'/de OR 'exercise tolerance'/de OR 'physical capacity'/de AND [embase]/lim #14 'physical activity'/exp OR 'physical activity, capacity and performance'/de AND [embase]/lim #13 'kinesiotherapy'/exp AND [embase]/lim #12 'exercise'/exp AND [embase]/lim #11 #1 OR #10 #10 #5 AND #9 #9 #6 OR #7 OR #8 #8 immuniz*:ab,ti OR immunis*:ab,ti #7 ’immunization’/exp #6 ’vaccine’/exp OR ’vaccination’/de #5 #2 OR #3 OR #4 #4 ’influenza virus a’/exp OR ’influenza virus b’/de #3 influenza*:ab,ti OR flu:ab,ti #2 ’influenza’/exp #1 ’influenza vaccine’/de
Appendix 3. CINAHL search strategy
S38 S28 AND S37 S37 S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 S36 (MH "Quantitative Studies") S35 TI placebo* OR AB placebo* S34 (MH "Placebos") S33 TI random* OR AB random* S32 TI ((singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*)) OR AB ((singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*)) S31 TI clinic* W1 trial* OR AB clinic* W1 trial* S30 PT clinical trial S29 (MH "Clinical Trials+") S28 S14 AND S27 S27 S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 S26 TI (run* or walk* or jog* or sprint* or treadmill* or row* or swim* or bicycl* or cycl* or danc* or yoga or tai chi or tai ji or qigong or qi gong) OR AB (run* or walk* or jog* or sprint* or treadmill* or row* or swim* or bicycl* or cycl* or danc* or yoga or tai chi or tai ji or qigong or qi gong) S25 TI activ* N5 life* OR AB activ* N5 life* S24 TI (physical* N5 (fit* or activ* or movement* or train* or condition* or program*)) OR AB (physical* N5 (fit* or activ* or movement* or train* or condition* or program*)) S23 TI ( (weight* or strength* or enduranc* or circuit*) N5 (program* or train* or session*)) OR AB ((weight* or strength* or enduranc* or circuit*) N5 (program* or train* or session*)) S22 TI (exercis* or sport* or fitness* or gym* or aerobic*) OR AB (exercis* or sport* or fitness* or gym* or aerobic*) S21 (MH "Dancing+") S20 (MH "Sports+") S19 (MH "Exertion") OR (MH "Exercise Intensity") S18 (MH "Physical Endurance+") S17 (MH "Physical Fitness+") S16 (MH "Therapeutic Exercise+") S15 (MH "Exercise+") S14 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 S13 TI (ari or urti or lrti) OR AB (ari or urti or lrti) S12 TI (bronchit* or pneumon* or bronchopneumon* or pleuropneumon*) OR AB (bronchit* or pneumon* or bronchopneumon* or pleuropneumon*) S11 TI (sinusit* or rhinosinusit* or nasosinusit*) OR AB (sinusit* or rhinosinusit* or nasosinusit*) S10 TI cough* OR AB cough* S9 (MH "Cough") S8 TI (nasopharyngit* or rhinopharyngit*) OR AB (nasopharyngit* or rhinopharyngit*) S7 TI (throat* N3 (infect* or inflam*)) OR AB (throat* N3 (infect* or inflam*)) S6 TI (pharyngit* or laryngit* or tonsillit* or sore throat*) OR AB (pharyngit* or laryngit* or tonsillit* or sore thoat*) S5 TI (influenza* or flu or ili) OR AB (influenza* or flu or ili) S4 TI ((acute or viral or virus* or bacter*) N2 rhinit*) OR AB ((acute or viral or virus* or bacter*) N2 rhinit*) S3 TI (common cold* or colds or coryza) OR AB (common cold* or colds or coryza) S2 TI (respiratory N2 (infect* or illness or symptom* or acute or virus*)) OR AB (respiratory N2 (infect* or illness or symptom* or acute or virus*)) S1 (MH "Respiratory Tract Infections") OR (MH "Bronchitis") OR (MH "Bronchitis, Acute") OR (MH "Common Cold") OR (MH "Influenza") OR (MH "Influenza, Human") OR (MH "Influenza, Seasonal") OR (MH "Laryngitis") OR (MH "Pharyngitis") OR (MH "Pneumonia+") OR (MH "Sinusitis+") OR (MH "Tonsillitis")
Appendix 4. LILACS search strategy
MH:"Respiratory Tract Infections" OR "Upper Respiratory Tract Infections" OR MH:C01.539.739$ OR MH:C08.730$ OR MH:Bronchitis OR MH:C08.127.446$ OR MH:C08.381.495.146 OR MH:C08.730.099 OR MH:"Common Cold" OR MH:C02.782.687.207$ OR MH:C08.730.162$ OR "Cold, Common" OR "Coryza, Acute" OR MH:"Influenza, Human" OR MH:C02.782.620.365$ OR MH:C08.730.310$ OR Grippe OR "Human Flu" OR "Human Influenza" OR "Influenza in Humans" OR MH:Laryngitis OR MH:C08.360.535 OR MH:C08.730.368 OR MH:C09.400.535 OR MH:Pharyngitis OR MH:C07.550.781$ OR MH:C08.730.561$ OR MH:C09.775.649$ OR "Sore Throat" OR MH:C08.381.677$ OR MH:08.730.610$ OR "Experimental Lung Inflammation" OR "Lobar Pneumonia" OR "Lung Inflammation" OR "Pulmonary Inflammation" OR MH:Sinusitis OR MH:C08.460.692.752$ OR MH:C08.730.749$ OR MH:C09.603.692.752$ OR MH:Nasopharyngitis OR MH:C07.550.350.700$ OR MH:C07.550.781.500$ OR MH:C08.730.561.500$ OR MH:C09.775.350.700$ OR MH:C09.775.649.500$ OR MH:Cough OR MH:Tos OR MH:Tosse OR MH:C08.618.248$ MH:C23.888.852.293$ OR MH:Bronchitis OR MH:Bronquitis OR MH:Bronquite OR MH:C08.127.446$ OR MH:C08.381.495.146$ OR MH:C08.730.099$ OR MH:Bronconeumonía OR MH:C08.127.509$ OR MH:C08.381.677.127$ OR MH:C08.730.610.127$ OR MH:Pleuropneumonia OR MH:C08.381.677.473$ OR MH:C08.528.735.473$ OR MH:C08.730.582.473$ OR MH:C08.730.610.473$ AND MH:Exercise OR "Aerobic Exercise" OR "Exercise, Aerobic" OR "Exercise, Isometric" OR "Exercise, Physical" OR "Isometric Exercise" OR MH:G11.427.590.530.698.277$ OR MH:I03.350$ OR MH:"Exercise Movement Techniques" OR MH:"Exercise Therapy" OR OR MH:E02.779.483$ OR MH:E02.831.387$ OR MH:"Physical Fitness" OR "Physical Conditioning, Human" OR MH:I03.621$ OR MH:N01.400.545$ OR MH:"Physical Endurance" OR MH:G11.427.680$ OR MH:I03.450.642.845.054.600$ OR MH:"Exercise Tolerance" OR MH:G11.427.680.270$ OR MH: "Physical Exertion" OR "Physical Effort" OR MH:G11.427.590.780$ OR MH:Sports OR Athletics OR MH:I03.450.642.845$ OR MH:Dancing OR MH:I03.450.642.287$ OR MH:Gymnastics OR Calisthenics OR MH:I02.233.543.454$ OR MH:I03.450.642.845.417$ OR MH:"Weight Lifting" OR MH:I03.450.642.845.950$ OR MH:"Muscle Strength" OR MH:E01.370.600.425$ OR MH:G11.427.560$ OR MH: "Physical Education and Training" OR MH:I02.233.543$ OR MH:Running OR MH:G11.427.590.530.568.610$ OR MH:G11.427.590.530.698.277.750$ OR MH:I03.450.642.845.610$ OR MH:Jogging OR MH:G11.427.590.530.568.610.320$ OR MH:G11.427.590.530.698.277.750.320$ OR MH:I03.450.642.845.610.320$ OR MH: "Exercise Test" OR Bicycle OR "Ergometry Test" OR "Arm Ergometry Test" OR "Step Test" OR "Stress Test" OR "Treadmill Test" OR MH:E01.370.370.380.250$ OR MH:E01.370.386.700.250$ OR MH:E05.333.250$ OR "Prueba Ergométrica de Bicicleta" OR "Test Ergométrico de Bicicleta" OR MH:Swimming OR Natación OR Natação OR MH:G11.427.590.530.568.800$ OR MH:G11.427.590.530.698.277.875$ OR MH:I03.450.642.845.869$ OR MH:Bicycling OR MH:I03.450.642.845.140$ OR MH:Yoga OR MH:E02.190.525.937$ OR MH:E02.190.901.984$ OR MH:E02.779.474.937$ OR MH:K01.844.799.867$ OR MH:"Tai Ji" OR MH:E02.190.525.890$ OR MH:E02.779.474.913$ OR MH:I03.450.642.845.560.500$ OR MH:"Breathing Exercises" OR "Ch'i Kung" OR "Qi Gong" OR Qigong OR "Respiratory Muscle Training" OR MH:E02.190.525.186$ OR MH:E02.779.474.124$ OR "Ch'i Kung" OR "Qi Gong" OR Qigong
Appendix 5. PEDro search strategy
exercise AND vaccination
Appendix 6. SPORTDiscus search strategy
S40 S33 AND S39 S39 S34 OR S35 OR S36 OR S37 OR S38 OR S39 S38 TI ( crossover* or cross over* ) OR AB ( crossover* or cross over* ) S37 TI ( (singl* or doubl*) W1 (blind* or mask*) ) OR AB ( (singl* or doubl*) W1 (blind* or mask*) ) S36 TI trial S35 TI clinic* W1 trial* OR AB clinic* W1 trial* S34 TI placebo* OR AB placebo* S33 TI random* OR AB random* S32 S19 AND S31 S31 S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 S30 TI ( run* or walk* or jog* or sprint* or treadmill* or row* or swim* or bicycl* or cycl* or danc* or yoga or tai chi or tai ji or qigong or qi gong ) OR AB ( run* or walk* or jog* or sprint* or treadmill* or row* or swim* or bicycl* or cycl* or danc* or yoga or tai chi or tai ji or qigong or qi gong ) S39 TI activ* N2 life* OR AB activ* N2 life* S28 TI ( physical* N5 (fit* or activ* or movement* or train* or condition* or program*) ) OR AB ( physical* N5 (fit* or activ* or movement* or train* or condition* or program*) ) S27 TI ( (weight* or strength* or enduranc* or circuit*) N5 (program* or train* or session*) ) OR AB ( (weight* or strength* or enduranc* or circuit*) N5 (program* or train* or session*) ) S26 TI ( exercis* or sport* or fitness* or gym* or aerobic* ) OR AB ( exercis* or sport* or fitness* or gym* or aerobic* ) S25 DE "DANCE" OR DE "AERIAL dance" OR DE "AEROBIC dancing" OR DE "AFRICAN American dance" OR DE "BALLET" OR DE "BALLROOM dancing" OR DE "BELLY dance" OR DE "BREAK dancing" OR DE "CHA‐cha (Dance)" OR DE "COUNTRY dancing" OR DE "DANCE for people with disabilities" OR DE "FLAMENCO" OR DE "FOLK dancing" OR DE "FREE skating" OR DE "HIP‐hop dance" OR DE "ICE dancing" OR DE "JAZZ dance" OR DE "LINE dancing" OR DE "LION dance" OR DE "MODERN dance" OR DE "MOVEMENT notation" OR DE "ORIGINAL set pattern dance (Skating)" OR DE "POLE dancing" OR DE "ROUND dancing" OR DE "SALSA (Dance)" OR DE "SHISHIMAI (Dance)" OR DE "SQUARE dancing" OR DE "STEP dancing" OR DE "TANGO (Dance)" OR DE "TAP dancing" S24 DE "PHYSICAL training & conditioning" OR DE "ACROBATICS ‐‐ Training" OR DE "ALTITUDE training" OR DE "ANAEROBIC training" OR DE "ARCHERY ‐‐ Training" OR DE "BADMINTON (Game) ‐‐ Training" OR DE "BASE training (Exercise)" OR DE "BASEBALL ‐‐ Training" OR DE "BASKETBALL ‐‐ Training" OR DE "BICYCLE racing ‐‐ Training" OR DE "BODYBUILDING ‐‐ Training" OR DE "BOWLING ‐‐ Training" OR DE "BOXING ‐‐ Training" OR DE "BULLFIGHT training & conditioning" OR DE "BUNGEE jumping training & conditioning" OR DE "CANOES & canoeing ‐‐ Training" OR DE "CAVING training & conditioning" OR DE "COMPOUND exercises" OR DE "CONTRAST training (Physical training & conditioning)" OR DE "COXSWAINING ‐‐ Training" OR DE "CRICKET training & conditioning" OR DE "CROSS‐training (Sports)" OR DE "CYCLING ‐‐ Training" OR DE "DANCE training & conditioning" OR DE "DEEP diving training & conditioning" OR DE "DIVING ‐‐ Training" OR DE "DOGSLEDDING training & conditioning" OR DE "ENDURANCE sports ‐‐ Training" OR DE "FENCING ‐‐ Training" OR DE "FIELD hockey training & conditioning" OR DE "FOOTBALL ‐‐ Training" OR DE "FUNCTIONAL training" OR DE "GLIDING & soaring training & conditioning" OR DE "GOLF ‐‐ Training" OR DE "GYMNASTICS ‐‐ Training" OR DE "HANDBALL training & conditioning" OR DE "HIKING training & conditioning" OR DE "HOCKEY ‐‐ Training" OR DE "HUNTING training & conditioning" OR DE "INTERVAL training" OR DE "ISOLATION exercises" OR DE "KAYAKING ‐‐ Training" OR DE "KNIFE fighting ‐‐ Training" OR DE "KORFBALL ‐‐ Training" OR DE "LACROSSE training & conditioning" OR DE "LONG slow distance training" OR DE "MARTIAL arts ‐‐ Training" OR DE "MOTORSPORTS training & conditioning" OR DE "MOUNTAINEERING ‐‐ Training" OR DE "NUNCHAKU ‐‐ Training" OR DE "ORIENTEERING ‐‐ Training" OR DE "OVERTRAINING" OR DE "PACE training" OR DE "PARACHUTING training & conditioning" OR DE "PARAKITING training & conditioning" OR DE "PERIODIZATION training" OR DE "PERSONAL training" OR DE "POLO training & conditioning" OR DE "PRACTICE (Sports)" OR DE "PRESEASON (Sports)" OR DE "RACQUETBALL ‐‐ Training" OR DE "RECOVERY training" OR DE "RELAY racing ‐‐ Training" OR DE "REPETITION training" OR DE "RESISTANCE training (Physical training & conditioning)" OR DE "ROCK climbing ‐‐ Training" OR DE "RODEO training & conditioning" OR DE "ROLLER skating training & conditioning" OR DE "ROWING ‐‐ Training" OR DE "RUGBY football ‐‐ Training" OR DE "RUNNING ‐‐ Training" OR DE "SHOT putting ‐‐ Training" OR DE "SKATING ‐‐ Training" OR DE "SKIS & skiing ‐‐ Training" OR DE "SKYDIVING training & conditioning" OR DE "SOCCER ‐‐ Training" OR DE "SOFTBALL ‐‐ Training" OR DE "SPEED endurance training" OR DE "SQUASH (Game) ‐‐ Training" OR DE "STRENGTH training" OR DE "SURFING ‐‐ Training" OR DE "SWIMMING ‐‐ Training" OR DE "TABLE tennis training & conditioning" OR DE "TEAM handball ‐‐ Training" OR DE "TENNIS ‐‐ Training" OR DE "TRACK & field ‐‐ Training" OR DE "TRIATHLON ‐‐ Training" OR DE "TUG of war (Game) ‐‐ Training" OR DE "VAULTING (Horsemanship) ‐‐ Training" OR DE "VOLLEYBALL ‐‐ Training" OR DE "WATER polo ‐‐ Training" OR DE "WEIGHT training" OR DE "WHEELCHAIR sports ‐‐ Training" OR DE "WINTER sports training & conditioning" OR DE "WRESTLING ‐‐ Training" OR DE "YOGA training & conditioning" S23 DE "BALL games" OR DE "ANETSO" OR DE "BALL hockey" OR DE "BALLE au tamis (Game)" OR DE "BASEBALL" OR DE "BASKETBALL" OR DE "BATTLE ball" OR DE "BICYCLE polo" OR DE "BILLIARDS" OR DE "BOWLING games" OR DE "BROOMBALL" OR DE "CAMOGIE (Game)" OR DE "CRICKET (Sport)" OR DE "CROQUET" OR DE "DODGEBALL" OR DE "FIELD hockey" OR DE "FLICKERBALL" OR DE "FOOTBALL" OR DE "GOAL ball" OR DE "GOLF" OR DE "GOLF croquet" OR DE "HANDBALL" OR DE "HURLING (Game)" OR DE "INDOOR hockey" OR DE "JAPANESE polo" OR DE "JIAN zi (Game)" OR DE "KANG (Game)" OR DE "KICKBALL" OR DE "LACROSSE" OR DE "LAPTA (Game)" OR DE "LAWN tempest (Game)" OR DE "MINTON (Game)" OR DE "PARLOR football" OR DE "PARLOR tennis" OR DE "PICKLE ball" OR DE "PICKLEBALL (Game)" OR DE "PIZE‐ball" OR DE "POLO" OR DE "POLOCROSSE" OR DE "PUSH ball" OR DE "QUIDDITCH (Game)" OR DE "RACQUETBALL" OR DE "RAGA (Game)" OR DE "ROLL ball" OR DE "ROUNDERS" OR DE "RUGBALL" OR DE "SCHLAGBALL" OR DE "SHINTY (Game)" OR DE "SOCCER" OR DE "SOFTBALL" OR DE "SPEED‐a‐way (Game)" OR DE "SPEEDBALL" OR DE "STICKBALL (Game)" OR DE "STOOLBALL" OR DE "TABLE tennis" OR DE "TCHOUKBALL" OR DE "TENNIS" OR DE "TETHERBALL" OR DE "TRAPBALL" OR DE "VOLLEYBALL" OR DE "WALLYBALL" OR DE "WATER polo" OR DE "WICKET" OR DE "WIFFLE ball" S22 DE "EXERCISE tolerance" S21 DE "PHYSICAL fitness" OR DE "ANAEROBIC exercises" OR DE "ASTROLOGY & physical fitness" OR DE "BODYBUILDING" OR DE "CARDIOVASCULAR fitness" OR DE "CIRCUIT training" OR DE "COMPOUND exercises" OR DE "ISOLATION exercises" OR DE "LIANGONG" OR DE "MUSCLE strength" OR DE "PERIODIZATION training" OR DE "PHYSICAL fitness ‐‐ Genetic aspects" OR DE "PHYSICAL fitness for children" OR DE "PHYSICAL fitness for girls" OR DE "PHYSICAL fitness for men" OR DE "PHYSICAL fitness for older people" OR DE "PHYSICAL fitness for people with disabilities" OR DE "PHYSICAL fitness for women" OR DE "PHYSICAL fitness for youth" OR DE "SPORT for All" S20 DE "EXERCISE" OR DE "ABDOMINAL exercises" OR DE "AEROBIC exercises" OR DE "ANAEROBIC exercises" OR DE "AQUATIC exercises" OR DE "ARM exercises" OR DE "BACK exercises" OR DE "BREATHING exercises" OR DE "BREEMA" OR DE "BUTTOCKS exercises" OR DE "CALISTHENICS" OR DE "CHAIR exercises" OR DE "CHEST exercises" OR DE "CIRCUIT training" OR DE "COMPOUND exercises" OR DE "DO‐in" OR DE "EXERCISE ‐‐ Immunological aspects" OR DE "EXERCISE adherence" OR DE "EXERCISE for children" OR DE "EXERCISE for girls" OR DE "EXERCISE for men" OR DE "EXERCISE for middle‐aged persons" OR DE "EXERCISE for older people" OR DE "EXERCISE for people with disabilities" OR DE "EXERCISE for women" OR DE "EXERCISE for youth" OR DE "EXERCISE therapy" OR DE "EXERCISE video games" OR DE "FACIAL exercises" OR DE "FALUN gong exercises" OR DE "FOOT exercises" OR DE "GYMNASTICS" OR DE "HAND exercises" OR DE "HATHA yoga" OR DE "HIP exercises" OR DE "ISOKINETIC exercise" OR DE "ISOLATION exercises" OR DE "ISOMETRIC exercise" OR DE "ISOTONIC exercise" OR DE "KNEE exercises" OR DE "LEG exercises" OR DE "LIANGONG" OR DE "METABOLIC equivalent" OR DE "MULAN quan" OR DE "MUSCLE strength" OR DE "PILATES method" OR DE "PLYOMETRICS" OR DE "QI gong" OR DE "REDUCING exercises" OR DE "RUNNING" OR DE "RUNNING ‐‐ Social aspects" OR DE "SCHOOLS ‐‐ Exercises & recreations" OR DE "SEXUAL exercises" OR DE "SHOULDER exercises" OR DE "STRENGTH training" OR DE "STRESS management exercises" OR DE "STRETCHING exercises" OR DE "TAI chi" OR DE "TREADMILL exercise" OR DE "WHEELCHAIR workouts" OR DE "YOGA" S19 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 S18 TI ( ari or urti or lrti ) OR AB ( ari or urti or lrti ) S17 TI ( bronchit* or pneumon* or bronchopneumon* or pleuropneumon* ) OR AB ( bronchit* or pneumon* or bronchopneumon* or pleuropneumon* ) S16 TI ( sinusit* or rhinosinusit* or nasosinusit* ) OR AB ( sinusit* or rhinosinusit* or nasosinusit* ) S15 TI cough* OR AB cough* S14 TI ( nasopharyngit* or rhinopharyngit* ) OR AB ( nasopharyngit* or rhinopharyngit* ) S13 TI ( throat* N3 (infect* or inflam*) ) OR AB ( throat* N3 (infect* or inflam*) ) S12 TI ( pharyngit* or laryngit* or tonsillit* or sore throat* ) OR AB ( pharyngit* or laryngit* or tonsillit* or sore thoat* ) S11 TI ( influenza* or flu or ili ) OR AB ( influenza* or flu or ili ) S10 TI ( (acute or viral or virus* or bacter*) N2 rhinit* ) OR AB ( (acute or viral or virus* or bacter*) N2 rhinit* ) S9 TI ( common cold* or colds or coryza ) OR AB ( common cold* or colds or coryza ) S8 TI ( respiratory N2 (infect* or illness or symptom* or acute or virus*) ) OR AB ( respiratory N2 (infect* or illness or symptom* or acute or virus*) ) S7 DE "BRONCHITIS" S6 DE "COUGH" S5 DE "PNEUMONIA" S4 DE "SINUSITIS" S3 DE "INFLUENZA" S2 DE "COLD (Disease)" S1 DE "RESPIRATORY infections"
Data and analyses
Comparison 1. Exercise versus no exercise prior to influenza vaccination.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood parameters‐ Antibody titre ‐ Follow‐up over time | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Antibody titres viral strain A ‐ up to 6 weeks follow‐up after vaccination | 5 | 490 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.28, 0.18] |
| 1.2 Antibody titres viral strain B ‐ up to 6 weeks follow‐up after vaccination | 5 | 490 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.29, 0.08] |
| 1.3 Antibody titres viral strain A ‐ over 6 weeks follow‐up after vaccination | 2 | 155 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.23, 0.41] |
| 1.4 Antibody titres viral strain B ‐ over 6 weeks follow‐up after vaccination | 2 | 155 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.70, 0.25] |
| 2 Blood parameters ‐ Antibody titre ‐ Timing of vaccination | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Antibody titres viral strain A ‐ Exercise immediately prior to vaccination ‐ up to 6 weeks follow‐up | 4 | 392 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.35, 0.07] |
| 2.2 Antibody titres viral strain B ‐ Exercise immediately prior to vaccination ‐ up to 6 weeks follow‐up | 4 | 392 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.36, 0.07] |
| 2.3 Antibody titres viral strain A ‐ Exercise immediately prior to vaccination ‐ over 6 weeks follow‐up | 1 | 57 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.41, 0.68] |
| 2.4 Antibody titres viral strain B ‐ Exercise immediately prior to vaccination ‐ over 6 weeks follow‐up | 1 | 57 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.07, 0.04] |
| 2.5 Antibody titres viral strain A ‐ Vaccination after 6 weeks of exercise | 1 | 98 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.33, 0.46] |
| 2.6 Antibody titres viral strain B ‐ Vaccination after 6 weeks of exercise | 1 | 98 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.42, 0.37] |
| 3 Blood parameters ‐ Antibody titre ‐ Type of exercise | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Antibody titres viral strain A ‐ Resistance exercise prior to vaccination ‐ up to 6 weeks follow‐up | 2 | 217 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.29, 0.31] |
| 3.2 Antibody titres viral strain B ‐ Resistance exercise prior to vaccination ‐ up to 6 weeks follow‐up | 2 | 217 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.38, 0.22] |
| 3.3 Antibody titres viral strain A ‐ Endurance exercise prior to vaccination ‐ up to 6 weeks follow‐up | 2 | 175 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.58, 0.02] |
| 3.4 Antibody titres viral strain B ‐ Endurance exercise prior to vaccination ‐ up to 6 weeks follow‐up | 2 | 175 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.50, 0.09] |
| 3.5 Antibody titres viral strain A ‐ Resistance exercise immediately prior to vaccination ‐ over 6 weeks follow‐up | 1 | 57 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.41, 0.68] |
| 3.6 Antibody titres viral strain B ‐ Resistance exercise immediately prior to vaccination ‐ over 6 weeks follow‐up | 1 | 57 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.07, 0.04] |
| 3.7 Antibody titres viral strain A ‐ Endurance exercise for 8 weeks then vaccination | 1 | 98 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.33, 0.46] |
| 3.8 Antibody titres viral strain B ‐ Endurance exercise for 8 weeks then vaccination | 1 | 98 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.42, 0.37] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Edwards 2007.
| Methods |
AIMS AND OBJECTIVES To compare the effects of eccentric exercise on the immune response in men and women STUDY DESIGN Parallel RCT DATES OF STUDY IMPLEMENTATION from December 2004 to an unspecified date STUDY DURATION 20 weeks SETTING, NUMBER OF CENTRES University of Birmingham, UK; single centre |
|
| Participants |
INCLUSION CRITERIA Physically active students EXCLUSION CRITERIA "None of the participants had received the influenza vaccine in the past year, or reported influenza in the winter prior to participation. None of the participants smoked, had a history of immune or cardiovascular disease, were suffering from an acute infection or illness, were pregnant, were currently taking medication (except birth control), or had a history of vaccine‐related allergies or side‐effects. All participants were instructed to abstain from vigorous exercise for at least 24 hours, alcohol for at least 12 hours, caffeine for at least 2 hours, and food for at least 1 hour prior to testing. In addition, none of the participants had performed any regular resistance training in the past 6 months." RECRUITMENT N SCREENED 60 students N RANDOMISED 60 students N COMPLETED 57 students N INTERVENTION GROUP 40 students N CONTROL GROUP 20 students GENDER 29 men; 31 women AGE Men = 20.1 years (SD 1.64 years); Women = 20.6 years (SD 2.55 years) |
|
| Interventions |
DESCRIPTION OF INTERVENTION "Eccentric portions of the biceps curl and lateral raise exercises, contracting the biceps brachii and deltoid muscles of the non‐dominant arm. The weights used were 85% of each participant’s single repetition concentric maxima which had been determined 1 week earlier. All participants performed 50 repetitions of each contraction (6 sets of 7 repetitions followed by 2 sets of 4 repetitions). They completed a set of lateral raises, rested 30 seconds, completed a set of biceps curls, and rested 60 seconds before the next set of lateral raises. The task lasted approximately 25 min." DELIVERY An investigator tracked each movement COMPARATOR GROUP Remained resting quietly, reading for 25 minutes CO‐INTERVENTIONS None |
|
| Outcomes |
PRESPECIFIED OUTCOME MEASURES Blood samples, haemagglutination inhibition test, cell‐mediated antigen‐specific immunity, limb circumference and pain, stress DETAILS OF ALL GROUPS Exercise group: allocated n = 40, analysed at 6 weeks n = 38, analysed at 8 weeks n = 35, analysed at 20 weeks n = 37 Control group: allocated n = 20, analysed at 6 weeks n = 19, analysed at 8 weeks n = 19, analysed at 20 weeks n = 20 FOLLOW‐UP PERIOD 6 week, 8 week and 20 week points |
|
| Notes |
TRIAL REGISTRATION Not registered ETHICS All participants gave written informed consent and the study was approved by the South Birmingham Local Research Ethics Committee FUNDING Influenza vaccines donated by GlaxoSmithKline, UK CONFLICTS OF INTEREST Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were allocated on a 2:1 ratio to either the exercise (N = 40) or control (N = 20) groups, based on a pre‐determined restricted random assignment to force an unequal sample size schedule" |
| Allocation concealment (selection bias) | Unclear risk | No information provided. We assumed that participants and investigators could not foresee assignment prior to allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blind to exercise or control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7.5% dropout in the exercise group. No intention‐to‐treat used |
| Selective reporting (reporting bias) | Unclear risk | Outcomes proposed in the Methods are reported in the results. However, study protocol was not registered |
| Other bias | Low risk | None found |
Edwards 2010.
| Methods |
AIMS AND OBJECTIVES "To investigate whether exercise augmented the immune response to a reduced dose vaccine, and whether the intensity of exercise influenced the efficacy of the intervention." "To compare the efficacy of a relatively low (60% of concentric one repetition maximum (1 RM)), medium (85% 1 RM), and high (110% 1 RM) intensity eccentric exercise intervention" STUDY DESIGN RCT DATES OF STUDY IMPLEMENTATION Not known STUDY DURATION 28 days SETTING, NUMBER OF CENTRES University of Birmingham, UK. Single centre |
|
| Participants |
INCLUSION CRITERIA Not reported EXCLUSION CRITERIA "Smoking, a history of immune or cardiovascular disease, current acute infection or illness, pregnancy or suspected pregnancy, and a history of vaccine‐related allergies or side effects. Use of prescription medication within 1 month of participation was also an important exclusion criterion; but females taking the contraceptive pill were not excluded. In addition, none of the participants reported having performed any resistance training in the 6 months prior to testing." RECRUITMENT N SCREENED 160 participants N RANDOMISED 158 participants N COMPLETED 158 participants INTERVENTION A 40 studentsINTERVENTION B 40 studentsINTERVENTION C 40 studentsCONTROL 40 students GENDER (completed study) 78 men; 80 women AGE 20.5 ± 1.6 years |
|
| Interventions |
DESCRIPTION OF INTERVENTION "Eccentric portions of the biceps curl and lateral raise exercises, contracting the biceps brachii and deltoid muscles of the non‐dominant arm. The weights used were 85% of each participant’s single repetition concentric maxima which had been determined 1 week earlier. All participants performed 50 repetitions of each contraction (6 sets of 7 repetitions followed by 2 sets of 4 repetitions). They completed a set of lateral raises, rested 30 seconds, completed a set of biceps curls, and rested 60 seconds before the next set of lateral raises. The task lasted approximately 25 minutes." DELIVERY An investigator tracked each movement CONTROL GROUP Quiet rest for 25 minutes CO‐INTERVENTIONS None |
|
| Outcomes |
PRESPECIFIED OUTCOME MEASURES Blood samples, haemagglutination inhibition test, cell‐mediated antigen‐specific immunity, limb circumference, pain, stress, CK and IL‐6 GROUP ALLOCATION Exercise group 60% of the concentric one repetition maxima (1RM): allocated n = 40, analysed at 28 days n = 40 Exercise group 85% of the concentric one repetition maxima (1RM): allocated n = 40, analysed at 28 days n = 40 Exercise group 110% of the concentric one repetition maxima (1RM): allocated n = 40, analysed at 28 days n = 39 Control group: allocated n = 40, analysed at 28 days n = 39, analysed at 8 weeks n = 19, analysed at 20 week n = 20 FOLLOW‐UP PERIOD 28 days |
|
| Notes |
TRIAL REGISTRATION No registry ETHICS Participants provided written informed consent; study protocol approved by the Black Country Local Research Ethics Committee FUNDING Not reported CONFLICTS OF INTEREST Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Participants were pseudo‐randomised, maintaining an even sex distribution" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blind to exercise or control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1% dropout |
| Selective reporting (reporting bias) | Unclear risk | The outcomes proposed in the Methods were reported in the Results. However, study protocol was not registered |
| Other bias | Low risk | None found |
Edwards 2013.
| Methods |
CONFERENCE ABSTRACT AIMS AND OBJECTIVES "To investigate the effects of exercise immediately prior to influenza vaccination on immune responses" STUDY DESIGN Parallel RCT DATES OF STUDY IMPLEMENTATION Not known STUDY DURATION 1 month SETTING, NUMBER OF CENTRES Not reported |
|
| Participants |
INCLUSION CRITERIA Not reported EXCLUSION CRITERIA Not reported RECRUITMENT N SCREENED 46 participants N RANDOMISED 46 participants N COMPLETED 46 participants GENDER 23 men; 23 women AGE Mean age = 73 ± 7 years |
|
| Interventions |
DESCRIPTION OF INTERVENTION The exercise group performed 50 mins of resistance exercises using upper and lower body muscle groups at an intensity of 60% of predetermined 1 repetition maximum (1RM) DELIVERY Not reported COMPARATOR GROUP Quiet reading CO‐INTERVENTIONS None |
|
| Outcomes |
PRESPECIFIED OUTCOME MEASURES Changes in inflammatory markers GROUP ALLOCATION Exercise group: allocated n = 23, analysed at 1 month not reported Control group: allocated n = 23, analysed at 1 month not reported FOLLOW‐UP PERIOD 1 month |
|
| Notes |
TRIAL REGISTRATION Not reported ETHICS Not reported FUNDING Not reported CONFLICTS OF INTEREST Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned to complete a control (quiet reading) or exercise task..." |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blind to exercise or control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was registered |
| Other bias | Low risk | None found |
Hayney 2014.
| Methods |
AIMS AND OBJECTIVES To compare influenza vaccine responses due to meditation or exercise training with controls STUDY DESIGN Parallel RCT DATES OF STUDY IMPLEMENTATION June 2012 to June 2016 STUDY DURATION 20 weeks SETTING, NUMBER OF CENTRES University of Wisconsin Health Sports Medicine Center; single centre |
|
| Participants |
INCLUSION CRITERIA Participants aged 50 years or older, willing to undertake any of the 3 randomisation outcomes, and reporting either 2 or more colds in the last 12 months or an average of 1 or more colds per year EXCLUSION CRITERIA "...previous training or current practice of meditation, moderate exercise at least 2 times a week or vigorous exercise at least 1 time a week, and a score of less than 24 points on the Folstein Mini‐Mental State Examination or more than 14 points on the 9‐item Patient Health Questionnaire (PHQ‐9) depression screen; immunodeficiency, autoimmune, or malignant disease; or prior allergic reaction to influenza vaccine or egg allergy." RECRUITMENT N RANDOMISED 154 participants N COMPLETED 149 participants GENDER 21 men, 133 women AGE Mean age 59.26 ± 8.87 years |
|
| Interventions |
DESCRIPTION OF INTERVENTION "The exercise program was designed and led by exercise physiologist at was matched to the meditation program in terms of duration (8 weeks), contact time (weekly 2.5 hour group sessions), home practice (45 min per day), and location. Borg’s Rating of Perceived Exertion was used to guide participants toward moderate intensity, sustained exercise, with a target rating of 12 to 16 points on the 6‐to‐20 point scale." DELIVERY Senior exercise physiology staff CONTROL GROUP Wait‐list control |
|
| Outcomes |
PRESPECIFIED OUTCOME MEASURES Influenza antibody responses post‐immunisation; Seroprotection at least 160 HA U and measures of well‐being GROUP ALLOCATION Exercise: 47 participants; Meditation: 51 participants; Control: 51 participants FOLLOW‐UP PERIOD 3 months |
|
| Notes |
TRIAL REGISTRATION NCT01057771 ETHICS Not reported FUNDING Supported by a grant from the National Institutes of Health (NIH), National Center for Complementary and Alternative Medicine (1R01AT004313); and by a grant UL1RR025011 from the Clinical and Translational Science Award (CTSA) Program of the National Center for Research Resources, National Institutes of Health. Aleksandra Zgierska is supported by grant K23 AA017508 from National Institute on Alcohol Abuse and Alcoholism at NIH CONFLICTS OF INTEREST Bruce Barrett was previously supported by career development grants from the National Center for Complementary and Alternative Medicine (K23AT00051) and from the Robert Wood Johnson Foundation, both of which were essential for developing the methodology and infrastructure for recruiting and monitoring research subjects, and for assessing their acute respiratory infections |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "SAS software was used to generate 165 unique identification numbers in balanced blocks of 3" |
| Allocation concealment (selection bias) | Low risk | "Codes were concealed envelopes, which were opened after consent to indicate allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blind to exercise or control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The laboratory staff performing the HIA used the standard micro titre techniques and was blinded to participant status" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3% dropout |
| Selective reporting (reporting bias) | Low risk | Outcomes proposed in the protocol were included in published results |
| Other bias | Low risk | None found |
Long 2012.
| Methods |
AIMS AND OBJECTIVES To compare the responses of a young and older participant cohort to determine if the efficacy of this intervention differs between the age groups STUDY DESIGN Parallel RCT DATES OF STUDY IMPLEMENTATION Not known STUDY DURATION 1 month SETTING, NUMBER OF CENTRES University of Birmingham, UK and the West Midlands area. Centre/s not specified |
|
| Participants |
INCLUSION CRITERIA "Either 18–30 or 50–64 years old; not highly endurance trained; no vaccination against influenza in the previous year or pneumonia in the last 10 years; no allergy to egg or chicken proteins; no latex allergy; no history of cancer, inflammatory disease or cardiovascular disease; no chronic obstructive pulmonary disorder, diabetes mellitus, asthma, congestive heart failure, Guillain Barre syndrome or psychiatric disorder; no females who were pregnant or breastfeeding; no current infection; and no medication or medical condition which may influence immune function" EXCLUSION CRITERIA Not reported RECRUITMENT West Midlands area via local newspapers, flyers, email and Internet advertisements, local community group visits, and by word of mouth N SCREENED 161 participants N RANDOMISED 122 participants N COMPLETED 120 participants GENDER Not reported AGE 18 to 30 years cohort; 50 to 64 years cohort |
|
| Interventions |
SETTING OF INTERVENTION Participants attended the laboratory on 2 separate occasions, 1 month apart DESCRIPTION OF INTERVENTION "Participants in the intervention group wore a heart rate monitor throughout the walk; average heart rate was used as a marker of physical activity intensity. After completing a warm‐up lap and a series of lower body isometric muscle stretches, participants were instructed to walk at a brisk pace for 45 min around a specified route while maintaining their heart rate at or above 55% of their heart rate maximum, calculated as 220 minus age. The walking route included sections of uphill and downhill walking to elicit both concentric and eccentric contractions of the muscles. After the intervention, participants carried out a cool down involving isometric muscle stretches." DELIVERY Not reported CONTROL GROUP Sat quietly in the laboratory for 45 minutes prior to receiving the vaccinations CO‐INTERVENTIONS None |
|
| Outcomes |
PRESPECIFIED OUTCOME MEASURES Health behaviours, IgG and IgM pneumococcal, influenza antibody titres, cardiac responses to intervention GROUP ALLOCATIONS Exercise group: allocated n = 60, analysed at 4 weeks n = 60; Control group: allocated n = 60, analysed at 4 weeks n = 60 FOLLOW‐UP PERIOD 4 weeks |
|
| Notes |
TRIAL REGISTRATION Not reported ETHICS All participants gave written informed consent and the study was approved by the South Birmingham Local Research Ethics Committee FUNDING Not reported CONFLICTS OF INTEREST No conflicts of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised into two groups, with an equal number of males and females in each group" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blind to exercise or control |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1% dropout rate |
| Selective reporting (reporting bias) | Low risk | The outcomes proposed in the Methods are reported in the Results. However, study protocol was not registered |
| Other bias | Low risk | None found |
Ranadive 2014.
| Methods |
AIMS AND OBJECTIVES "To evaluate the effect of acute moderate aerobic exercise immediately before administration of the influenza vaccine on antibody titres and seroprotective responses in older adults. To investigate whether acute exercise immediately before influenza vaccination produces different effects on antibody response in older men versus older women." STUDY DESIGN Randomised, counterbalanced, cross‐over design DATES OF STUDY IMPLEMENTATION Not known STUDY DURATION 6 weeks SETTING, NUMBER OF CENTRES University of Illinois at Urbana‐Champaign |
|
| Participants |
INCLUSION CRITERIA "All subjects were free from cardiovascular or respiratory disease, and none smoked. A stress test was performed on visit 1 to exclude any participant with evidence of stress‐induced ischaemia" EXCLUSION CRITERIA "Participants were excluded if they had metabolic disease (diabetes mellitus), inflammatory diseases (rheumatoid arthritis and systemic lupus erythematosus), or bleeding disorder; were taking medications known to affect inflammation (aspirin); and had a history of smoking (any form). Participants who had suffered from common cold or influenza and bacterial or viral infection or upper respiratory tract infection 2 months preceding testing were also excluded. Participants who had already received influenza vaccination for the season or who were taking thyroid medication or allergy medication on a regular basis were excluded as well. Participants were also excluded if they were taking over‐the‐counter pain/anti‐inflammatory medication during the course of the study." RECRUITMENT All participants were recruited from the local community and provided written informed consent before participation N SCREENED 59 participants N RANDOMISED 59 participants N COMPLETED 55 participants GENDER (completed) 23 men, 32 women AGE 66.5 ± 0.8 years |
|
| Interventions |
SETTING Temperature‐controlled room DESCRIPTION OF INTERVENTION Exercise group performed a 40‐min moderate‐intensity aerobic exercise bout at an intensity of 55% to 65% of their maximum HR immediately preceding the vaccination and sham injection DELIVERY Not reported COMPARATOR GROUP Non‐exercise group did not perform physical activity preceding vaccination; received sham injection CO‐INTERVENTIONS None |
|
| Outcomes |
PRESPECIFIED OUTCOME MEASURES Influenza antibody titres, anthropometrics, brachial blood pressure, hemagglutination inhibition titre, inflammatory markers GROUP ALLOCATIONS Exercise group: allocated n = 30, analysed at 4 weeks n = 29; Control group: allocated n = 29, analysed at 4 weeks n = 27 FOLLOW‐UP PERIOD 4 weeks |
|
| Notes |
TRIAL REGISTRATION Not registered ETHICS Approved by the Institutional Review Board of the University of Illinois at Urbana‐Champaign FUNDING Authors thanked the American Heart Association for the 2‐yr pre‐doctoral fellowship (no. 10PRE3500083) CONFLICTS OF INTEREST The authors declared no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomised to an acute exercise or to a non‐exercise group". Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The participant and the researchers were blinded to when they received the vaccine or sham injection" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7% dropout rate |
| Selective reporting (reporting bias) | Unclear risk | The outcomes proposed in the Methods are reported in the Results. However, no study protocol was registered |
| Other bias | High risk | "...the cohort included older individuals who are sedentary as well as physically active and did not control it" |
Abbreviations: HIA ‐ hemagglutination inhibition assay; HR ‐ heart rate; IgG ‐ immunoglobulin G; IgM ‐ immunoglobulin M; IL ‐ interleukin; RM ‐ repetition maximum; SD ‐ standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Hara 2002 | Not RCT. Study analysed physical activity level as an outcome |
| Kohut 2002 | Not RCT. Study included a control group but randomisation was not conducted |
| Kohut 2004 | RCT. Control group had participants exercising (low intensity) and participants not exercising together in the control group |
| Whitham 2003 | Not RCT. Heavy versus light exercise training groups. No non‐exercise group |
| Woods 2009 | RCT. Control group participants engaged in low intensity exercise |
| Yang 2007 | Not RCT. Study included a control group but randomisation was not conducted |
Differences between protocol and review
We added subset analysis to assess vaccination timing because we felt it was important to distinguish if vaccination was administered immediately after exercise or some time before vaccination. We deleted the comparison 'Exercise with influenza vaccination versus exercise with placebo vaccination' from the review, since this outcome was not addressed in studies.
We deleted a secondary outcome proposed in our protocol: 'Exercise compliance rates of participants'. We made this exclusion because it was not an outcome of the intervention.
Contributions of authors
Antonio Jose Grande: co‐ordinated retrieval of papers and wrote the background, methods, results, discussion and conclusion. Hamish Reid: participated in retrieval of papers, quality assessment and co‐wrote the background, methods, results, discussion and conclusion. Emma Thomas: participated in retrieval of papers, quality assessment and co‐wrote the background, methods, results, discussion and conclusion. David Nunan: co‐wrote the background, methods, results, discussion and conclusion. Charles Foster: co‐wrote the background, methods, results, discussion and conclusion.
Sources of support
Internal sources
CF is funded by BHF core grant 021/P&C/core/2010/HPRG, UK.
AJG is a recipient of a Postdoctoral Scholarship from Science Without Borders ‐ Cnpq, Brazil.
DN has received funding from the NIHR School of Primary Care Research, UK.
External sources
No sources of support supplied
Declarations of interest
Antonio Jose Grande: none known Hamish Reid: none known Emma E Thomas: none known David Nunan: I have received funding from the NIHR School for Primary Care Research for a project related to physical activity Charles Foster: none known.
New
References
References to studies included in this review
Edwards 2007 {published data only}
- Edwards K, Burns V, Allen L, McPhee J, Bosch J, Carroll D, et al. Eccentric exercise as an adjuvant to influenza vaccination in humans. Brain, Behavior, and Immunity 2007;21(2):209‐17. [DOI] [PubMed] [Google Scholar]
Edwards 2010 {published data only}
- Campbell JP, Edwards KM, Ring C, Drayson MT, Bosch JA, Inskip A, et al. The effects of vaccine timing on the efficacy of an acute eccentric exercise intervention on the immune response to an influenza vaccine in young adults. Brain, Behavior and Immunity 2010;24(2):236‐42. [DOI] [PubMed] [Google Scholar]
- Edwards K, Campbell J, Ring C, Drayson M, Bosch J, Downes C, et al. Exercise intensity does not influence the efficacy of eccentric exercise as a behavioural adjuvant to vaccination. Brain, Behavior and Immunity 2010;24(4):623‐30. [DOI] [PubMed] [Google Scholar]
Edwards 2013 {published data only}
- Edwards K, Pascoe A, Fiatrone Singh MA, Kok J, Dwyer D, Booy R, et al. Exercise as an adjuvant for influenza vaccination in older adults. 71st Annual Scientific Meeting of the American Psychosomatic Society. March 13 to 16, 2013. Meeting abstracts. McLean, VA, USA: American Psychosomatic Society, 2013:Abstract 535.
Hayney 2014 {published data only}
- Hayney M, Coe C, Muller D, Obasi C, Backonja U, Ewers T, et al. Age and psychological influences on immune responses to trivalent inactivated influenza vaccine in the meditation or exercise for preventing acute respiratory infection (MEPARI) trial. Human Vaccines and Immunotherapeutics 2014;10(1):2759‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Long 2012 {published data only}
- Long J, Ring C, Drayson M, Bosch J, Campbell J, Bhabra J, et al. Vaccination response following aerobic exercise: Can a brisk walk enhance antibody response to pneumococcal and influenza vaccinations?. Brain, Behavior, and Immunity 2012;26(4):680‐7. [DOI] [PubMed] [Google Scholar]
Ranadive 2014 {published data only}
- Ranadive S, Cook M, Kappus R, Yan H, Lane A, Woods J, et al. Effect of acute aerobic exercise on vaccine efficacy in older adults. Medicine and Science in Sports and Exercise 2014;46(3):455‐61. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Hara 2002 {published data only}
- Hara Y, Hagihara A, Ikematu H, Nobutomo K. Efficacy of influenza vaccine among elderly patients by physical activity status. Environmental Health and Preventive Medicine 2002;7(5):183‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kohut 2002 {published data only}
- Kohut M, Cooper M, Nickolaus M, Russell D, Cunnick J. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. Journal of Gerontology 2002;57(9):557‐62. [DOI] [PubMed] [Google Scholar]
Kohut 2004 {published data only}
- Kohut M, Arntson B, Lee W, Rozeboom K, Yoon K, Cunnick J, et al. Moderate exercise improves antibody response to influenza immunization in older adults. Vaccine 2004;22(17):2298‐3006. [DOI] [PubMed] [Google Scholar]
- Kohut M, Lee W, Martin A, Arnston B, Russell D, Ekkekakis P, et al. The exercise‐induced enhancement of influenza immunity is mediated in part by improvements in psychosocial factors in older adults. Brain, Behavior and Immunity 2005;19(4):357‐66. [DOI] [PubMed] [Google Scholar]
Whitham 2003 {published data only}
- Whitham M, Blannin A. The effect of exercise training on the kinetics of the antibody response to influenza vaccination. Journal of Sports Sciences 2003;21(12):991‐1000. [DOI] [PubMed] [Google Scholar]
Woods 2009 {published data only}
- Woods J, Keylock K, Lowder T, Vieira V, Zelkovich W, Dumich S, et al. Cardiovascular exercise training extends influenza vaccine seroprotection in sedentary older adults. Journal of the American Geriatrics Society 2009;57(12):2183‐91. [DOI] [PubMed] [Google Scholar]
Yang 2007 {published data only}
- Yang Y, Verkuilen J, Rosengren K, Mariani R, Reed M, Grubisich S, et al. Effects of a Taiji and Qigong intervention on the antibody response to influenza vaccine in older adults. American Journal of Chinese Medicine 2007;35(4):597‐607. [DOI] [PubMed] [Google Scholar]
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. British Medical Journal 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Booth 2012
- Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Comprehensive Physiology 2012;2(2):1143‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burton 2004
- Burton DA, Stokes K, Hall GM. Physiological effects of exercise. Continuing Education in Anaesthesia, Critical Care & Pain 2004;4(6):185‐8. [Google Scholar]
Campbell 2010
- Campbell JP, Edwards KM, Ring C, Drayson MT, Bosch JA, Inskip A, et al. The effects of vaccine timing on the efficacy of an acute eccentric exercise intervention on the immune response to an influenza vaccine in young adults. Brain, Behavior, and Immunity 2010;24(2):236‐42. [DOI] [PubMed] [Google Scholar]
Carpersen 1985
- Carpersen JC, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health‐related research. Public Health Reports 1985;100(2):126‐31. [PMC free article] [PubMed] [Google Scholar]
CDC 2009
- Centers for Disease Control and Prevention. Types of influenza viruses. www.cdc.gov/flu/about/viruses/types.htm 2009 (accessed 11 March 2015).
CDC 2010
- Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP). www.cdc.gov/mmwr/pdf/rr/rr59e0729.pdf 2010 (accessed 23 July 2015).
Demicheli 2014
- Demicheli V, Jefferson T, Al‐Ansary LA, Ferroni E, Rivetti A, Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD001269.pub5] [DOI] [PubMed] [Google Scholar]
Edwards 2006
- Edwards KM, Burns VE, Reynolds T, Carroll D, Drayson M, Ring C. Acute stress exposure prior to influenza vaccination enhances antibody response in women. Brain, Behavior, and Immunity 2006;20(2):159‐68. [DOI] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- GRADEproGDT. GRADEpro Guideline Development Tool [www.guidelinedevelopment.org]. Hamilton: McMaster University (developed by Evidence Prime, Inc.), 2015.
Higgins 2011a
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jefferson 2010
- Jefferson T, Pietrantonj C, Al‐Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD004876.pub3] [DOI] [PubMed] [Google Scholar]
Kakanis 2010
- Kakanis MW, Peake J, Brenu EW, Simmonds M, Gray B, Hooper SL, et al. The open window of susceptibility to infection after acute exercise in healthy young male elite athletes. Exercise Immunology Review 2010;16:119‐37. [PubMed] [Google Scholar]
Krüger 2008
- Krüger K, Lechtermann A, Fobker M, Völker K, Mooren FC. Exercise‐induced redistribution of T lymphocytes is regulated by adrenergic mechanisms. Brain, Behavior, and Immunity 2008;22(3):324‐38. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lobelo 2014
- Lobelo F, Stoutenberg M, Hutber A. The Exercise is Medicine Global Health Initiative: a 2014 update. British Journal of Sports Medicine 2014;48(22):1627‐33. [DOI] [PubMed] [Google Scholar]
Molinari 2007
- Molinari NA, Ortega‐Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007;25(27):5086‐96. [DOI] [PubMed] [Google Scholar]
Nair 2011
- Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta‐analysis. Lancet 2011;378(9807):1917‐30. [DOI] [PubMed] [Google Scholar]
Pascoe 2014
- Pascoe AR, Fiatarone Singh MA, Edwards KM. The effects of exercise on vaccination responses: a review of chronic and acute exercise interventions in humans. Brain, Behavior, and Immunity 2014;39:33‐41. [DOI] [PubMed] [Google Scholar]
Peake 2005
- Peake J, Nosaka K, Suzuki K. Characterization of inflammatory responses to eccentric exercise in humans. Exercise Immunology Review 2005;11:64‐85. [PubMed] [Google Scholar]
Schillinger 2006
- Schillinger A, Koenig D, Haefele C, Vogt S, Heinrich L, Aust A, et al. Effect of manual lymph drainage on the course of serum levels of muscle enzymes after treadmill exercise. American Journal of Physical Medicine & Rehabilitation 2006;85(6):516‐20. [DOI] [PubMed] [Google Scholar]
Silva 2012
- Silva V, Carvalho APVD, Grande AJ, Martimbianco ALC, Riera R, Atallah AN. Can data extraction from figures perform a meta‐analysis?. Cochrane Colloquium. 2012.
Thomas 2013
- Thomas RE, Jefferson T, Lasserson TJ. Influenza vaccination for healthcare workers who care for people aged 60 or older living in long‐term care institutions. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD005187.pub4] [DOI] [PubMed] [Google Scholar]
Tossige‐Gomes 2014
- Tossige‐Gomes R, Ottone VO, Oliveira PN, Viana DJS, Araújo TL, Gripp FJ, et al. Leukocytosis, muscle damage and increased lymphocyte proliferative response after an adventure sprint race. Brazilian Journal of Medical and Biological Research 2014;47(6):492‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. WHO Global Influenza Surveillance Network: Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza. WHO Press, 2011. [Google Scholar]
WHO 2012
- World Health Organization. WHO position paper. Weekly Epidemiological Record 2012;87(47):461‐76. [Google Scholar]
References to other published versions of this review
Grande 2015
- Grande A, Nunan D, Reid H, Thomas EE, Foster C. Exercise prior to influenza vaccination for limiting influenza incidence and its related complications in adults. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD011857] [DOI] [PMC free article] [PubMed] [Google Scholar]


