Abstract
Background
This review is one of a suite of six Cochrane reviews looking at the primary medical management options for patients with chronic rhinosinusitis.
Chronic rhinosinusitis is a common condition involving inflammation of the lining of the nose and paranasal sinuses. It is characterised by nasal blockage and nasal discharge, facial pressure/pain and loss of sense of smell. The condition can occur with or without nasal polyps. Oral corticosteroids are used to control the inflammatory response and improve symptoms.
Objectives
To assess the effects of oral corticosteroids compared with placebo/no intervention or other pharmacological interventions (intranasal corticosteroids, antibiotics, antifungals) for chronic rhinosinusitis.
Search methods
The Cochrane ENT Information Specialist searched the ENT Trials Register; Central Register of Controlled Trials (CENTRAL 2015, Issue 7); MEDLINE; EMBASE; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 11 August 2015.
Selection criteria
Randomised controlled trials (RCTs) comparing a short course (up to 21 days) of oral corticosteroids with placebo or no treatment or compared with other pharmacological interventions.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. Our primary outcomes were disease‐specific health‐related quality of life (HRQL), patient‐reported disease severity, and the adverse event of mood or behavioural disturbances. Secondary outcomes included general HRQL, endoscopic nasal polyp score, computerised tomography (CT) scan score and the adverse events of insomnia, gastrointestinal disturbances and osteoporosis. We used GRADE to assess the quality of the evidence for each outcome; this is indicated in italics.
Main results
We included eight RCTs (474 randomised participants), which compared oral corticosteroids with placebo or no intervention. All trials only recruited adults with chronic rhinosinusitis with nasal polyps. All trials reported outcomes at two to three weeks, at the end of the short‐course oral steroid treatment period. Three trials additionally reported outcomes at three to six months. Two of these studies prescribed intranasal steroids to patients in both arms of the trial at the end of the oral steroid treatment period.
Oral steroids versus placebo or no intervention
Disease‐specific health‐related quality of life was reported by one study. This study reported improved quality of life after treatment (two to three weeks) in the group receiving oral steroids compared with the group who received placebo (standardised mean difference (SMD) ‐1.24, 95% confidence interval (CI) ‐1.92 to ‐0.56, 40 participants, modified RSOM‐31), which corresponds to a large effect size. We assessed the evidence to be low quality (we are uncertain about the effect estimate; the true effect may be substantially different from the estimate of the effect).
Disease severity as measured by patient‐reported symptom scores was reported by two studies, which allowed the four key symptoms used to define chronic rhinosinusitis (nasal blockage, nasal discharge, facial pressure, hyposmia) to be combined into one score. The results at the end of treatment (two to three weeks) showed an improvement in patients receiving oral steroids compared to placebo, both when presented as a mean final value (SMD ‐2.84, 95% CI ‐4.09 to ‐1.59, 22 participants) and as a change from baseline (SMD ‐2.28, 95% CI ‐2.76 to ‐1.80, 114 participants). These correspond to large effect sizes but we assessed the evidence to be low quality.
One study (114 participants) followed patients for 10 weeks after the two‐week treatment period. All patients in both arms received intranasal steroids at the end of the oral steroid treatment period. The results showed that the initial results after treatment were not sustained (SMD ‐0.22, 95% CI ‐0.59 to 0.15, 114 participants, percentage improvement from baseline). This corresponds to a small effect size and we assessed the evidence to be low quality.
There was an increase in adverse events in people receiving orals steroids compared with placebo for gastrointestinal disturbances (risk ratio (RR) 3.45, 95% CI 1.11 to 10.78; 187 participants; three studies) and insomnia (RR 3.63, 95% CI 1.10 to 11.95; 187 participants; three studies). There was no significant impact of oral steroids on mood disturbances at the dosage used in the included study (risk ratio (RR) 2.50, 95% CI 0.55 to 11.41; 40 participants; one study). We assessed the evidence to be low quality due to the lack of definitions of the adverse events and the small number of events or sample size, or both).
Other comparisons
No studies that compared short‐course oral steroids with other treatment for chronic rhinosinusitis met the inclusion criteria.
Authors' conclusions
At the end of the treatment course (two to three weeks) there is an improvement in health‐related quality of life and symptom severity in patients with chronic rhinosinusitis with nasal polyps taking oral corticosteroids compared with placebo or no treatment. The quality of the evidence supporting this finding is low. At three to six months after the end of the oral steroid treatment period, there is little or no improvement in health‐related quality of life or symptom severity for patients taking an initial course of oral steroids compared with placebo or no treatment.
The data on the adverse effects associated with short courses of oral corticosteroids indicate that there may be an increase in insomnia and gastrointestinal disturbances but it is not clear whether there is an increase in mood disturbances. All of the adverse events results are based on low quality evidence.
More research in this area, particularly research evaluating patients with chronic rhinosinusitis without nasal polyps, longer‐term outcomes and adverse effects, is required.
There is no evidence for oral steroids compared with other treatments.
Plain language summary
Short‐term oral corticosteroids compared with no treatment or other treatments for chronic rhinosinusitis
Review question
We reviewed the evidence for the benefits and harms of a short course (typically up to 21 days) of corticosteroid given by mouth to people with chronic rhinosinusitis compared with giving a placebo or no treatment, or another type of treatment.
Background
Chronic rhinosinusitis is a common condition that is defined as inflammation of the nose and paranasal sinuses (a group of air‐filled spaces behind the nose, eyes and cheeks). Patients with chronic rhinosinusitis experience at least two or more of the following symptoms for at least 12 weeks: blocked nose, discharge from their nose or runny nose, pain or pressure in their face and/or a reduced sense of smell (hyposmia). Some people will also have nasal polyps, which are grape‐like swellings of the normal nasal lining inside the nasal passage and sinuses.
Short courses of oral corticosteroids are a widely used treatment for chronic rhinosinusitis. They work by controlling the inflammatory response and when polyps are present they rapidly reduce the size of the polyps to improve symptoms. The adverse effects of corticosteroids can include insomnia, mood changes and gastrointestinal changes (such as stomach pain, heartburn, diarrhoea, constipation, nausea and vomiting). When given over the longer term, or through many repeated short courses, it is also possible to develop osteoporosis (fragile bones).
Study characteristics
This review includes evidence up to 11 August 2015. We included eight randomised controlled trials with a total of 474 participants. All of the patients were adults who had chronic rhinosinusitis with nasal polyps. All of the studies followed patients until the end of treatment (two to three weeks) and three studies (210 participants) followed up people for three to six months after the initial treatment had ended. Five of the eight reports mentioned how the trial was funded. None of the funding sources were pharmaceutical companies.
Key results
At the end of a two‐ or three‐week treatment course, people who took oral steroids may have had a better quality of life, less severe symptoms and smaller nasal polyps than people who had placebo or did not receive any treatment. After three to six months, there was little or no difference in quality of life, symptom severity or nasal polyps between the people who had oral steroids and the people who had placebo or no intervention.
The people who took oral steroids may have had more gastrointestinal disturbances and insomnia than the people who had placebo or no intervention. It is not clear if the people who took oral steroids had more mood disturbances than the people who had placebo or no intervention.
Quality of the evidence
We judged the quality of the evidence for oral steroids plus intranasal steroids for adults with nasal polyps to be low (further research is very likely to have an important impact on our confidence in the effect estimate and is likely to change the estimate), as the some of the results are only from one or two studies, which do not have a lot of participants. Most of the trials do not have a high risk of bias, but only people with nasal polyps were included in the review.
Summary of findings
Summary of findings for the main comparison. Short‐course oral corticosteroids compared with placebo/no treatment for chronic rhinosinusitis.
| Short‐course oral corticosteroids compared with placebo/no treatment for chronic rhinosinusitis | ||||||
| Patient or population: chronic rhinosinusitis with nasal polyps Intervention: short‐course oral corticosteroids Comparison: placebo/no treatment | ||||||
|
Outcomes № of participants (studies) |
Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Without oral steroids | With oral steroids | Difference | ||||
| Disease‐specific health‐related quality of life measured by
severity score of RSOM‐31 (unclear range) Follow‐up: 2 weeks № of participants: 40 (1 RCT) |
— | Not estimable | — | The mean disease‐specific health‐related quality of life in the intervention group was 1.24 standard deviations lower (1.92 lower to 0.56 lower) | ⊕⊕⊝⊝ LOW 1 | A lower score indicates reduced impairment. Treatment effect in favour of short‐course oral steroids.
|
Disease severity, as measured by patient‐reported symptom score,
measured by combining 4 individual symptoms
|
— | — | — |
|
⊕⊕⊝⊝
LOW 3 ⊕⊕⊝⊝ LOW 9 ⊕⊕⊝⊝ LOW 10 |
A lower score indicates milder symptoms in favour of short‐course oral steroids.
|
| Adverse events: significant mood disturbance
Follow‐up: 2 weeks № of participants: 40 (1 RCT) |
RR 2.50 (0.55 to 11.41) | Study population | ⊕⊕⊝⊝ LOW 4 | It is uncertain whether there were more mood disturbance adverse events in the oral corticosteroids group. | ||
| 100 per 1000 | 250 per 1000 (55 to 1000) |
150 more per 1000 (45 fewer to 1041 more) |
||||
| Health‐related quality of life, using generic quality of life scores | This outcome was not reported in any of the studies | |||||
| Adverse events: gastrointestinal disturbance Follow‐up: 3 months № of participants:187 (3 RCTs) |
RR 3.45 (1.11 to 10.78) | Study population | ⊕⊕⊝⊝ LOW 5 | There were more gastrointestinal disturbance adverse events in the oral corticosteroids group. | ||
| 47 per 1000 | 160 per 1000 (52 to 501) |
114 more per 1000 (5 more to 455 more) |
||||
| Adverse events: insomnia Follow‐up: 3 months № of participants:187 (3 RCTs) |
RR 3.63 (1.10 to 11.95) | Study population | ⊕⊕⊝⊝ LOW 6 | There were more insomnia adverse events in the oral corticosteroids group. | ||
| 23 per 1000 | 84 per 1000 (26 to 278) |
61 more per 1000 (2 more to 255 more) |
||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio; RSOM‐31: Rhinosinusitis Outcome Measures‐31; SMD: standard mean difference | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded to low quality due to limitations in study methodology and imprecision. Only the disease severity scale of the RSOM‐31 was used (unknown validity of this subscale and the range of scores is unclear). One small study (n = 40), which lacked information about the method of randomisation and allocation concealment. There is a also concern that the magnitude of improvement is not sustained; one study that used a non‐validated instrument reported smaller benefit at three to six months than at two to three weeks for health‐related quality of life.
2The individual symptoms measured were: nasal obstruction, nasal discharge, sense of smell and pressure over the sinuses. Scores for the individual symptoms (0 to 10 visual analogue scale (VAS)) were summed to find the total score.The effect size could be underestimated with this method.
3Downgraded to low quality due to imprecision. Results are from one very small study (n = 22) and the results were only measured at the end of treatment (17 days). There is a concern that the magnitude of improvement is not sustained. The outcome was not measured using a validated tool.
4Downgraded to low quality due to limitations in study methodology and imprecision. One small study (n = 40), which lacked information about the method of randomisation and allocation concealment. The definition of 'mood disturbance' is not provided in the paper. The results have large confidence intervals.
5Downgraded to low quality due to inconsistency and imprecision. The terminology between the papers for this outcome differed from "diarrhoea/GI disturbance" to "gastrointestinal disturbance" to "reflux and/or gastric pain". A low number of events were reported resulting in large confidence intervals.
6Downgraded to low quality due to inconsistency and imprecision. The definition of 'insomnia' is not provided in the papers. A low number of events were reported resulting in large confidence intervals.
7The individual symptoms measured were: blocked nose, rhinorrhoea, hyposmia and sinonasal pain. The results were measured as individual symptoms on a seven‐point Likert scale (0 = no symptoms) and presented as percentage change from baseline for each symptom, which was averaged across the four symptoms to create an average change from baseline. The effect size could be underestimated with this method.
8All patients in both groups received intranasal steroids at the end of the treatment period until the end of follow‐up (12 weeks).
9Downgraded to low quality due to limitations in study methodology and imprecision. Results are from one small study (n = 117) with unclear randomisation and allocation concealment. The results were measured at the end of treatment (two weeks). There is a concern that the results are not sustained. The outcome was not measured using a validated tool.
10Downgraded to low quality due to limitations in study methodology and imprecision. Results are from one small study (n = 117) with unclear randomisation and allocation concealment. There is a small effect size with large confidence intervals. The outcome was not measured using a validated tool.
Background
Description of the condition
Chronic rhinosinusitis is defined as inflammation of the nose and paranasal sinuses characterised by two or more symptoms, one of which must be nasal blockage/obstruction/congestion or nasal discharge (anterior/posterior nasal drip). The other possible symptoms include facial pain/pressure, reduction or loss of sense of smell (in adults) or cough (in children). Symptoms must have continued for at least 12 weeks. In addition, people must have either mucosal changes within the ostiomeatal complex and/or sinuses as evidenced by a computerised tomography (CT) scan and/or endoscopic signs of at least one of the following: nasal polyps, mucopurulent discharge primarily from middle meatus or oedema/mucosal obstruction primarily in the middle meatus (EPOS 2012).
Chronic rhinosinusitis represents a common source of ill health; 11% of UK adults reported chronic rhinosinusitis symptoms in a worldwide population study (Hastan 2011). Symptoms, including nasal obstruction, nasal discharge, facial pain, anosmia and sleep disturbance, have a major impact on quality of life, reportedly greater in several domains of the SF‐36 than angina or chronic respiratory disease (Gliklich 1995). Acute exacerbations, inadequate symptom control and respiratory disease exacerbation are common. Complications are rare, but may include visual impairment and intracranial infection.
Two major phenotypes of chronic rhinosinusitis have been identified based on the presence or absence of nasal polyps on examination. Nasal polyps are tumour‐like hyperplastic swellings of the nasal mucosa, most commonly originating from within the ostiomeatal complex (Larsen 2004). Chronic rhinosinusitis with nasal polyps (CRSwNP) is diagnosed when polyps are seen (on direct or endoscopic examination) bilaterally in the middle meatus. The acronym CRSsNP is used for the condition in which no polyps are present.
Although the aetiology of chronic rhinosinusitis is not fully understood, it may involve abnormalities in the host response to irritants, commensal and pathogenic organisms and allergens, obstruction of sinus drainage pathways, abnormalities of normal mucociliary function, loss of the normal mucosal barrier or infection. Two typical profiles may be observed with respect to inflammatory mediators; in eosinophilic chronic rhinosinusitis, which is typically associated with nasal polyps, high levels of eosinophils, immunoglobulin E (IgE) and interleukin (IL)‐5 may be found, while in neutrophilic chronic rhinosinusitis, more often associated with chronic rhinosinusitis without polyps, neutrophils predominate, with elevated interferon (IFN) gamma, IL‐8 and tumour necrosis factor (TNF) (EPOS 2012).
While treatment decisions should be made based on an understanding of the patient's chronic rhinosinusitis phenotype and likely aetiology, in practice treatment may be initiated without knowledge of the polyp status, particularly in primary care. This review (and most of its companion reviews) consider patients with and without polyps together in the initial evaluation of treatment effects. However, subgroup analyses explore potential differences between them (see below).
The most commonly used interventions for chronic rhinosinusitis are used either topically (sprayed into the nose) or systemically (by mouth) and include steroids, antibiotics and saline.
Description of the intervention
Short courses of oral steroids are widely used in medicine for a variety of inflammatory conditions. In patients with chronic rhinosinusitis they are often used with a view to gaining a rapid improvement in symptoms and to allow improved access for topically applied agents. They are typically given over a seven‐ to 21‐day period and may be at a fixed dose or incorporate a reducing dose over the course. This strategy is thought to reduce the risk of adverse effects (Mygind 1996). A wide spectrum of adverse events are reported with systemic steroid usage (see Table 2); however, data on the incidence in association with chronic rhinosinusitis are lacking. While it is possible to extrapolate findings from trials in other diseases, there is a risk that the incidence is disease‐specific; for example, a high incidence of avascular necrosis is seen with high‐dose steroid use in systemic lupus erythematosus, which is in part attributed to the underlying disease process and severity as well as the higher dosages prescribed in severe disease (Da Silva 2006).
1. Summary of the most commonly reported side effects of systemic steroids.
| System | Adverse events | Notes |
| Musculoskeletal | Osteoporosis | Largely limited to long‐term use Significantly increased risk of fractures with prolonged use |
| Osteonecrosis | Rare, appears to be dose‐dependent | |
| Endocrine | Hyperglycaemia | Common; dose‐dependent, usually reversible |
| Cardiovascular | Hypertension | Common; dose‐dependent, usually reversible |
| Dermatological | Striae, bruising | Dose‐dependent; occurs after > 1 month usage |
| Ophthalmological | Cataracts | Irreversible; largely related to long‐term usage |
| Glaucoma | High risk with pre‐existing disease | |
| Gastrointestinal tract | Peptic ulceration | Increased risk largely due to concomitant NSAIDs |
| Psychological | Psychosis | Common; increased risk with dosages > 40 mg/day |
References: Da Silva 2006; Naber 1996; Stanbury 1998
NSAIDs: non‐steroidal anti‐inflammatory drugs
Adverse effects associated with short‐term oral steroid use are said to include gastrointestinal disturbances, insomnia and altered mental states. However, there are few or no published data on the frequency of these effects when short‐term courses are prescribed. Adverse effects associated with long‐term use of oral steroids are also listed in Table 2.
How the intervention might work
Short courses of oral steroids are most often used in patients with chronic rhinosinusitis with nasal polyps. The intention is to reduce the inflammation in order to produce a rapid reduction in the size of the polyps, to improve symptoms and allow better penetration of topical treatments into the nasal cavity. They may be used in a similar way for patients with chronic rhinosinusitis without polyps, who have severe nasal obstruction or complete anosmia (loss of sense of smell). The initial effect of treatment is expected to be immediate. Any observed improvement may continue, especially if one effect of the intervention is to improve the bio‐availability of an adjunct treatment.
There is, however, a lack of evidence regarding the optimal treatment regimen of oral steroids with respect to indication, dose and duration. The optimum usage of steroids is clinically important as it may reduce the need for surgery by providing good symptomatic control.
Why it is important to do this review
Short courses of oral steroids are widely used either alone or as a form of add‐on therapy in patients with chronic rhinosinusitis. This review and a closely related new review of 'Short‐course oral steroids as an adjunct therapy for chronic rhinosinusitis', Head 2016a, update and expand a previous Cochrane review that looked at this treatment in patients with polyps (Martinez‐Devesa 2011). This review seeks to establish the effectiveness of oral steroids (compared to no treatment or placebo) and their relative effectiveness compared to other commonly used agents for chronic rhinosinusitis (such as intranasal corticosteroids). In contrast, the companion review tries to establish the additional benefits (and harms) of steroids when added on to existing therapies for chronic rhinosinusitis.
This review is one of a suite of Cochrane reviews looking at common management options for patients with chronic rhinosinusitis (Chong 2016a; Chong 2016b; Chong 2016c; Head 2016b; Head 2016a), and we use the same outcome measures across the reviews. We have not included studies designed to evaluate interventions in the immediate peri‐surgical period, which are focused on assessing the impact of the intervention on the surgical procedure or on modifying the post‐surgical results (preventing relapse).
Objectives
To assess the effects of oral corticosteroids compared with placebo/no intervention or other pharmacological interventions (intranasal corticosteroids, antibiotics, antifungals) for chronic rhinosinusitis.
Methods
Criteria for considering studies for this review
Types of studies
We included studies with the following design characteristics:
randomised controlled trials, including cluster‐randomised trials and quasi‐randomised trials (cross‐over trials were only to be included if the data from the first phase were available); and
patients were followed up for at least two weeks.
We excluded studies with the following design characteristics:
randomised patients by side of nose (within‐patient controlled) because it is difficult to ensure that the effects of any of the interventions considered can be localised; or
perioperative studies, where the sole purpose of the study was to investigate the effect of the intervention on surgical outcome.
Types of participants
Patients with chronic rhinosinusitis, whether with polyps or without polyps.
We excluded studies that included a majority of patients with:
cystic fibrosis;
allergic fungal sinusitis/eosinophilic fungal/mucinous rhinosinusitis;
aspirin‐exacerbated respiratory disease;
antrochoanal polyps (benign polyps originating from the mucosa of the maxillary sinus);
malignant polyps;
primary ciliary dyskinesia;
gross immunodeficiency (congenital or acquired);
a history of surgery for nasal polyps within six weeks of entry to the study.
Types of interventions
We included all short (see below) courses of oral steroids, regardless of dose. This included:
prednisone;
prednisolone;
methylprednisolone;
hydrocortisone;
cortisone acetate.
Short courses of oral steroids are defined as lasting up to, but not exceeding, 21 days.
The main comparators were: placebo or no intervention.
The main comparison pairs were:
oral steroids versus placebo or no treatment;
oral steroids followed by intranasal corticosteroids versus placebo or no treatment followed by intranasal corticosteroids.
Other possible comparison pairs included:
oral steroids versus intranasal corticosteroids;
oral steroids versus antibiotics;
oral steroids versus antifungals.
This review is part of a larger series of six reviews of the treatment of chronic rhinosinusitis.
Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis (Chong 2016b).
Different types of intranasal steroids for chronic rhinosinusitis (Chong 2016a). This review compares different classes, doses and delivery methods of intranasal corticosteroids for chronic rhinosinusitis.
Short‐course oral steroids alone for chronic rhinosinusitis (this review). This review compares short‐course oral steroids alone with placebo or no intervention, or against other pharmacological interventions such as antibiotics or nasal saline irrigation.
Short‐course oral steroids as an adjunct therapy for chronic rhinosinusitis (Head 2016a). This review compares oral steroids where they have been used as add‐on therapy to other treatments for chronic rhinosinusitis (such as intranasal corticosteroids, antibiotics or saline solution).
Saline irrigation for chronic rhinosinusitis (Chong 2016c). This review compares nasal saline irrigation for chronic rhinosinusitis with both placebo/no intervention and with intranasal corticosteroids or antibiotics.
Systemic and topical antibiotics for chronic rhinosinusitis (Head 2016b). This review compares both topical and systemic antibiotics with placebo/no treatment, two different antibiotics with each other and antibiotics with intranasal corticosteroids.
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
Both short‐term (at the end of treatment) and long‐term effects are important therefore we evaluated outcomes at the end of treatment or within three weeks thereof in addition to three to six months, six to 12 months and more than 12 months. For adverse events, we analysed data from the longest time periods.
Primary outcomes
Health‐related quality of life, using disease‐specific health‐related quality of life scores, such as the Sino‐Nasal Outcome Test‐22 (SNOT‐22), Rhinosinusitis Outcome Measures‐31 (RSOM‐31) and SNOT‐20.
Disease severity, as measured by patient‐reported symptom score (such as the Chronic Sinusitis Survey (CSS) questionnaire and visual analogue scales). In the absence of validated symptom score data, patient‐reported individual symptom scores were reported for the following symptoms: nasal obstruction/blockage/congestion, nasal discharge (rhinorrhoea), facial pressure/pain, loss of sense of smell (adults), cough (children).
Significant adverse effect: mood or behavioural disturbances.
Secondary outcomes
Health‐related quality of life, using generic quality of life scores, such as the SF‐36, EQ‐5D and other well‐validated instruments.
Other adverse effects: gastrointestinal disturbances.
Other adverse effects: insomnia.
Other adverse effects: osteoporosis.
Endoscopic score (depending on population, either nasal polyps size score or endoscopy score, e.g. Lund‐Mackay/Lund‐Kennedy).
Computerised tomography (CT) scan score (e.g. Lund‐Mackay).
The adverse events that we collected from studies including one of the various comparators listed above were the same as those collected in the companion reviews assessing the effects of these interventions as primary treatments.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 11 August 2015.
Electronic searches
The Information Specialist searched:
the Cochrane Register of Studies ENT Trials Register (searched 11 August 2015);
the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 7);
-
Ovid MEDLINE (1946 to July week 5 2015);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (searched 11 August 2015);
PubMed (as a top up to searches in Ovid MEDLINE) (searched 11 August 2015);
Ovid EMBASE (1974 to 2015 week 32);
ClinicalTrials.gov, www.clinicaltrials.gov (search via the Cochrane Register of Studies) (searched 11 August 2015);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (searched 11 August 2015);
Google Scholar (searched 11 August 2015).
The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched PubMed, The Cochrane Library and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials.
Data collection and analysis
Selection of studies
Two review authors independently screened all titles and abstracts of the studies obtained from the database searches to identify potentially relevant studies. Two review authors evaluated the full text of each potentially relevant study to determine whether it met the inclusion and exclusion criteria for this review.
We resolved any differences by discussion and consensus, with the involvement of a third author for clinical and methodological input where necessary.
Data extraction and management
Two review authors independently extracted data from each study using a standardised data collection form (see Appendix 2). Whenever a study had more than one publication, we retrieved all publications to ensure complete extraction of data. Where there were discrepancies in the data extracted by different review authors, we checked these against the original reports and resolved differences by discussion and consensus, with the involvement of a third author or a methodologist where appropriate. We contacted the original study authors for clarification or for missing data whenever possible. If differences were found between publications of a study, we contacted the original authors for clarification. We used data from the main paper(s) if no further information was found.
We included key characteristics of the studies, such as study design, setting, sample size, population and how outcomes were defined or collected in the studies. In addition, we also collected baseline information on prognostic factors or effect modifiers. For this review, this included:
presence or absence of nasal polyps;
baseline nasal polyp score (where appropriate);
whether the patient has had previous sinus surgery;
number of previous courses of oral steroids.
For the outcomes of interest to the review, we extracted the findings of the studies on an available case analysis basis; i.e. we included data from all patients available at the time points based on the treatment randomised whenever possible, irrespective of compliance or whether patients had received the treatment as planned.
In addition to extracting pre‐specified information about study characteristics and aspects of methodology relevant to risk of bias, we extracted the following summary statistics for each trial and each outcome:
For continuous data: the mean values, standard deviations and number of patients for each treatment group. Where endpoint data were not available, we extracted the values for change from baseline. We analysed data from measurement scales such as SNOT‐22 and EQ‐5D as continuous data.
For binary data: the numbers of participants experiencing an event and the number of patients assessed at the time point.
For ordinal scale data: if the data appeared to be approximately normally distributed or if the analysis that the investigators performed suggested parametric tests were appropriate, then we treated the outcome measures as continuous data. Alternatively, if data were available, we converted into binary data.
We prespecified the time points of interest for the outcomes in this review. While studies may report data at multiple time points, we only extracted the longest available data within the time points of interest. For example, for 'medium‐term' follow‐up periods, our time point was defined as 'three to six months' post‐randomisation. If a study had reported data at three, four and six months, we only extracted and analysed the data for the six‐month follow‐up.
Extracting data from figures
Where values for primary or secondary outcomes were shown as figures within the paper we contacted the study authors to try to obtain the raw values. When the raw values were not provided we extracted information from the graphs using an online data extraction tool (http://arohatgi.info/WebPlotDigitizer/app/), using the best quality version of the relevant figures available.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of each included study. We followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), and we used the Cochrane 'Risk of bias' tool. With this tool we assessed the risk of bias as 'low', 'high' or 'unclear' for each of the following six domains:
sequence generation;
allocation concealment;
blinding of participants, personnel and outcome assessment;
incomplete outcome data;
selective reporting;
other sources of bias.
Measures of treatment effect
We summarised the effects of dichotomous outcomes (e.g. proportion of patients with symptom resolution) as risk ratios (RR) with CIs. For the key outcomes that we presented in the 'Summary of findings' table, we also expressed the results as absolute numbers based on the pooled results and compared to the assumed risk. We had also planned to calculate the number needed to treat to benefit (NNTB) using the pooled results where dichotomous efficacy outcomes were available. The assumed baseline risk is typically either (a) the median of the risks of the control groups in the included studies, this being used to represent a 'medium risk population' or, alternatively, (b) the average risk of the control groups in the included studies is used as the 'study population' (Handbook 2011). If a large number of studies were available, and where appropriate, we had planned also to present additional data based on the assumed baseline risk in (c) a low‐risk population and (d) a high‐risk population.
For continuous outcomes, we expressed treatment effects as a mean difference (MD) with standard deviation (SD) or as standardised mean difference (SMD) if different scales were used to measure the same outcome. We provided a clinical interpretation of the SMD values.
Unit of analysis issues
This review did not use data from phase II of cross‐over studies or from studies where the patient was not the unit of randomisation, i.e. studies where the side (right versus left) was randomised.
If we had found cluster‐randomised trials, we would have analysed these according to the methods in section 16.3.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
Dealing with missing data
We tried to contact study authors via email whenever the outcome of interest was not reported, if the methods of the study suggested that the outcome had been measured. We did the same if not all data required for meta‐analysis were reported, unless the missing data were standard deviations. If standard deviation data were not available, we approximated these using the standard estimation methods from P values, standard errors or 95% CIs if these were reported, as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). Where it was impossible to estimate these, we contacted the study authors.
Apart from imputations for missing standard deviations, we conducted no other imputations. However, we completed calculations relating to disease severity (measured by patient‐reported symptom scores) as most of the data measured individual symptoms rather than using validated instruments (see 'Imputing total symptom scores' below). We extracted and analysed data for all outcomes using the available case analysis method.
Imputing total symptom scores
Where a paper did not present information for the total disease severity in terms of patient‐reported symptom scores but did present data for the results of individual symptoms, we used the symptoms covering the important domains of the EPOS chronic rhinosinusitis diagnosis criteria, EPOS 2012, to calculate a total symptom score. The EPOS 2012 criteria for chronic rhinosinusitis require at least two symptoms. One of the symptoms must be either nasal blockage or nasal discharge; other symptoms can include facial pressure/pain, loss of sense of smell (for adults) or cough (for children). Where mean final values or changes from baseline were presented in the paper for the individual symptoms we summed these to calculate a 'total symptom score'. We calculated standard deviations for the total symptom score as if the symptoms were independent, random variables that were normally distributed. We acknowledge that there is likely to be a degree of correlation between the individual symptoms, however we used this process because the magnitude of correlation between the individual symptoms is not currently well understood (no evidence found). If the correlation is high, the summation of variables as discrete variables is likely to give a conservative estimate of the total variance of the summed final score. If the correlation is low, this method of calculation will underestimate the standard deviation of the total score. However, the average patient‐reported symptom scores have a correlation coefficient of about 0.5; if this is also applicable to chronic rhinosinusitis symptoms, the method used should have minimal impact (Balk 2012). As this method of calculation does not take into account weighting of different symptoms (no evidence found), we downgraded all the disease severity outcomes for lack of use of validated scales whenever this occurred.
Assessment of heterogeneity
We assessed clinical heterogeneity (which may be present even in the absence of statistical heterogeneity) by examining the included trials for potential differences between studies in the types of participants recruited, interventions or controls used and the outcomes measured.
We assessed statistical heterogeneity by visually inspecting the forest plots and by considering the Chi² test (with a significance level set at P value < 0.10) and the I² statistic, which calculates the percentage of variability that is due to heterogeneity rather than chance, with I² values over 50% suggesting substantial heterogeneity (Handbook 2011).
Assessment of reporting biases
We assessed reporting bias as between‐study publication bias and within‐study outcome reporting bias.
Outcome reporting bias (within‐study reporting bias)
We assessed within‐study reporting bias by comparing the outcomes reported in the published report against the study protocol, whenever this could be obtained. If the protocol was not available, we compared the outcomes reported to those listed in the methods section. If results were mentioned but not reported adequately in a way that allowed analysis (e.g. the report only mentions whether the results were statistically significant or not), bias in a meta‐analysis is likely to occur. We therefore sought further information from the study authors. If no further information could be found, we noted this as being a 'high' risk of bias. Quite often there was insufficient information to judge the risk of bias; we noted this as an 'unclear' risk of bias (Handbook 2011).
Publication bias (between‐study reporting bias)
We planned to assess funnel plots if sufficient trials (more than 10) were available for an outcome. If we observed asymmetry of the funnel plot, we would have conducted more formal investigation using the methods proposed by Egger 1997.
Data synthesis
We conducted all meta‐analyses using Review Manager 5.3 (RevMan 2014). For dichotomous data, we planned to analyse treatment differences as a risk ratio (RR) calculated using the Mantel‐Haenszel methods. We analysed time‐to‐event data using the generic inverse variance method.
For continuous outcomes, if all the data were from the same scale, we planned to pool mean values obtained at follow‐up with change outcomes and report this as a MD. However, if the SMD had to be used as an effect measure, we did not pool change and endpoint data.
We used a fixed‐effect model for data analysis, unless statistical heterogeneity was substantial (> 50%). When statistical heterogeneity is low, random‐effects versus fixed‐effect methods yield trivial differences in treatment effects. However, when statistical heterogeneity is high, the random‐effects method provides a more conservative estimate of the difference. If statistical heterogeneity was high, we conducted analysis using a random‐effects model, if the source of heterogeneity was unexplained.
Subgroup analysis and investigation of heterogeneity
We conducted some subgroup analyses regardless of whether statistical heterogeneity was observed, as these are widely suspected to be potential effect modifiers. For this review, this included:
phenotype of patients: whether patients had chronic rhinosinusitis without nasal polyps, chronic rhinosinusitis with nasal polyps, were a mixed group or the status of polyps is not known or not reported. We undertook the subgroup analysis because although there appears to be a considerable overlap between the two forms of chronic rhinosinusitis with regards to inflammatory profile, clinical presentation and effect of treatment (Cho 2012; DeMarcantonio 2011; Ebbens 2010; Fokkens 2007; Ragab 2004; Ragab 2010; van Drunen 2009), there is some evidence pointing to differences in the respective inflammatory profiles (Kern 2008; Keswani 2012; Tan 2011; Tomassen 2011; Zhang 2008; Zhang 2009), and potentially even differences in treatment outcome (Ebbens 2011).
We presented the main analyses of this review according to the subgroups of phenotypes of chronic rhinosinusitis. We presented all other subgroup analysis results in tables.
When studies had a mixed group of patients, we analysed the study as one of the subgroups (rather than as a mixed group) if more than 80% of patients belonged to one category. For example, if 81% of patients had chronic rhinosinusitis without nasal polyps, we analysed the study as that subgroup.
In addition to the subgroups above, we conducted the following subgroup analyses in the presence of statistical heterogeneity:
patient age (children versus adults);
dose;
duration of treatment.
Sensitivity analysis
We carried out sensitivity analyses to determine whether the findings are robust to the decisions made in the course of identifying, screening and analysing the trials. We planned to conduct sensitivity analysis for the following factors, whenever possible:
impact of model chosen: fixed‐effect versus random‐effects model;
risk of bias of included studies: excluding studies with high risk of bias (we defined these as studies that have a high risk of allocation concealment bias and a high risk of attrition bias (overall loss to follow‐up of 20%, differential follow‐up observed);
how outcomes were measured: we investigated the impact of including data where the validity of the measurement was unclear.
If any of these investigations found a difference in the size of the effect or heterogeneity, we mentioned this in the Effects of interventions section.
GRADE and 'Summary of findings' table
We used the GRADE approach to rate the overall quality of evidence for each outcome using the GDT tool (http://www.guidelinedevelopment.org/) for the main comparison pairs listed in the Types of interventions section. The quality of evidence reflects the extent to which we are confident that an estimate of effect is correct and we applied this in the interpretation of results. There are four possible ratings: 'high', 'moderate', 'low' and 'very low'. A rating of 'high' quality evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of 'very low' quality implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high quality. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
study limitations (risk of bias);
inconsistency;
indirectness of evidence;
imprecision;
publication bias.
The 'Summary of findings' table presents only the six top priority outcomes (disease‐specific health‐related quality of life, disease severity score, generic quality of life and three adverse effects: mood disturbances, gastrointestinal disturbance and insomnia). We did not include the outcomes of endoscopic score or CT scan score, or the adverse effect of osteoporosis in the 'Summary of findings' table. Similarly, we did not present the results for the individual symptoms in the 'Summary of findings' table.
Results
Description of studies
Results of the search
The searches retrieved a total of 2470 references after removal of duplicates. We screened titles and abstracts and subsequently removed 2424 studies. We assessed 46 full texts for eligibility. We excluded 30 studies, with reasons. Thirteen papers are included (eight studies). We identified three ongoing studies. There are no studies awaiting assessment.
A flow chart of study retrieval and selection is provided in Figure 1.
1.
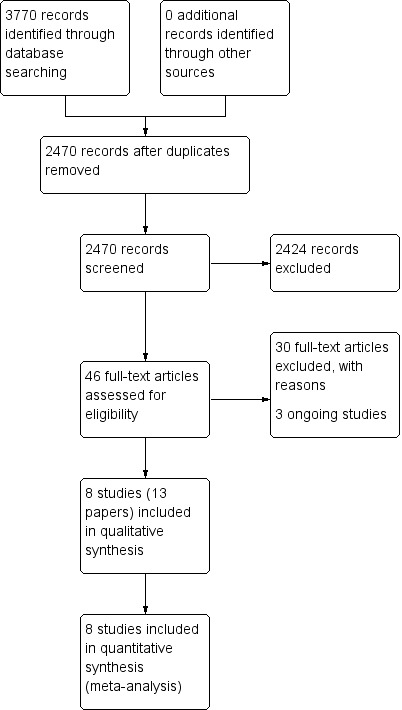
Process for sifting search results and selecting studies for inclusion.
Included studies
We included eight published studies (13 papers) in the review (Alobid 2014; Benitez 2006; Ecevit 2015; Hissaria 2006; Kapucu 2012; Kirtsreesakul 2012; Vaidyanathan 2011; Van Zele 2010). See Characteristics of included studies.
There were five papers from one group in Spain (Alobid 2014; Benitez 2006). After contacting the lead author of these papers we clarified that there were two separate trials reported within the different papers. We included the results from only two of these papers: Alobid 2014 for the more recent trial and Benitez 2006 for the earlier trial. The additional papers either present identical groups of patients, or results for subsets of patients.
The aim of the Ecevit 2015 study was to look at the impact of short‐course oral steroids on surgical outcomes. However, disease severity was reported after oral steroid treatment had completed but prior to surgery taking place and so we included the study in this review.
Two of the trials had more than two study arms (Kapucu 2012; Van Zele 2010). Kapucu 2012 was a four‐arm study that compared a short‐course oral steroid, an intra‐polyp steroid injection, intranasal steroid treatment (triamcinolone acetonide spray 55 μg, two times daily with two puffs in both nostril cavities) and a control group who were not given any medication. The oral steroid and the control group are included in this review. Van Zele 2010 was a three‐arm study comparing oral corticosteroids (methylprednisolone), placebo and antibiotics (doxycycline). Only the arm comparing oral steroids with placebo is included in this review although the results for the comparisons that include antibiotics are reported in Head 2016b.
Design
All eight included studies are parallel‐group, randomised controlled trials (Alobid 2014; Benitez 2006; Ecevit 2015; Hissaria 2006; Kapucu 2012; Kirtsreesakul 2012; Vaidyanathan 2011; Van Zele 2010). Three studies were unblinded and no steroid treatment was provided in the control arm (Alobid 2014; Benitez 2006; Kapucu 2012). Five studies stated that participants and healthcare professionals were blind to the treatment group (Ecevit 2015; Hissaria 2006; Kirtsreesakul 2012; Vaidyanathan 2011; Van Zele 2010). A further discussion of blinding is made in the section Blinding (performance bias and detection bias).
Setting
Four studies were conducted in ENT departments within hospitals (Alobid 2014; Benitez 2006; Ecevit 2015, Van Zele 2010), two in allergy outpatient clinics (Hissaria 2006; Kirtsreesakul 2012), and one in a speciality clinic (Vaidyanathan 2011). The setting of one study was unclear (Kapucu 2012).
Van Zele 2010 was a multicentre trial conducted on five sites in four countries (Belgium, Germany, Holland and Australia). Five studies were single‐centre: two in the same unit in Spain (Alobid 2014; Benitez 2006), one in Turkey (Ecevit 2015), one in Thailand (Kirtsreesakul 2012), and one in Scotland (Vaidyanathan 2011). The number of sites involved in the other studies are not known although one was from Australia (Hissaria 2006), and the other was Turkey (Kapucu 2012).
Participants and sample size
All of the published trials only included adults diagnosed with chronic rhinosinusitis with nasal polyps. There were 474 participants included in the comparison of oral steroids with placebo or no intervention.
The diagnostic criteria for inclusion into the trials varied by study. Three studies did not refer to a minimum grade of nasal polyps for inclusion (Hissaria 2006; Kapucu 2012; Kirtsreesakul 2012), although Hissaria 2006 recruited only "symptomatic polyp patients". Two studies included patients with moderate‐to‐severe bilateral polyps (Alobid 2014; Vaidyanathan 2011). Alobid 2014 based their inclusion on the EPOS 2012 criteria (Appendix 3) and Vaidyanathan 2011 was based on the European Position Paper on Rhinosinusitis and Nasal Polyps 2007.
Three papers included a more severely affected or recalcitrant population (Benitez 2006; Ecevit 2015; Van Zele 2010). Benitez 2006 only included people with "severe" nasal polyps (mean score: 2.7 out of a possible 3 using the Lildholdt score), whereas it was required in the participants in Van Zele 2010 that either the nasal polyps had recurred after surgical resection or were bilateral and grades 3 or 4 in both nares using their five‐point nasal polyp scoring scale (Appendix 4). In Ecevit 2015, the inclusion criteria were patients with moderate or severe nasal polyps who had not responded to a six‐week course of fluticasone nasal drops (200 µg/day). Out of 124 people treated with fluticasone, 23 met the inclusion criteria and were randomised to oral steroids or placebo.
Across all the included studies 67% of participants were male, in keeping with the male preponderance seen in a recent epidemiological study (Hopkins 2016; Philpott 2015). However, the mean age of participants was 46 years, which is a decade lower than the above referenced study; in fact it is notable that the mean age in the control arm of Ecevit 2015 was 26.6 years (although this may have been a reporting error) and the mean age for both arms in Kapucu 2012 was 32.2 years. These participant groups may therefore not be fully representative of the overall chronic rhinosinusitis population.
Interventions and comparisons
All of the eight included studies provided results for a short course of treatment (14 to 21 days) with oral steroids compared with placebo or no treatment (Alobid 2014; Benitez 2006; Ecevit 2015; Hissaria 2006; Kapucu 2012; Kirtsreesakul 2012; Vaidyanathan 2011; Van Zele 2010).
Four trials had washout periods prior to starting the trial (Alobid 2014; Benitez 2006; Kirtsreesakul 2012; Vaidyanathan 2011), in which the patients received no steroids in the two weeks (Vaidyanathan 2011), or four weeks prior to starting oral steroids (Alobid 2014; Benitez 2006; Kirtsreesakul 2012).
Three different oral steroids were given within the trials: prednisone (Alobid 2014; Benitez 2006), prednisolone (Ecevit 2015; Hissaria 2006; Kirtsreesakul 2012; Vaidyanathan 2011), and methylprednisolone (Kapucu 2012; Van Zele 2010). Both studies using prednisone started at 30 mg and reduced the dose over the 14‐day treatment course (reduced by 5 mg every two days) (Alobid 2014; Benitez 2006). For prednisolone, Ecevit 2015 gave a starting dose of 60 mg/day and then reduced this over the 17‐day treatment course. The other three studies gave a 14‐day course but with no reduction: Hissaria 2006 and Kirtsreesakul 2012 gave 50 mg/day whilst Vaidyanathan 2011 gave a lower dose of 25 mg/day. Both studies using methylprednisolone reduced the dose over the trial period. Kapucu 2012 gave oral methylprednisolone at a varying dose depending on the weight of the patient (1 mg/kg/day for three days then reduced by 8 mg/three days). The study did not give details of the average duration of treatment. Van Zele 2010 gave 32 mg/day on days one to five, 16 mg/day on days 6 to 10 and 8 mg/day on days 11 to 20.
The comparator in three studies was no steroid treatment (no placebo) (Alobid 2014; Benitez 2006; Kapucu 2012), placebo tablets in four studies (Ecevit 2015; Hissaria 2006; Kirtsreesakul 2012; Vaidyanathan 2011), and placebo capsules in one study (Van Zele 2010).
No information on any concurrent treatment was given in four studies (Alobid 2014; Benitez 2006; Ecevit 2015; Kapucu 2012). Other medications were not permitted during the oral steroid treatment stage in a further three (Kirtsreesakul 2012; Vaidyanathan 2011; Van Zele 2010). Only Hissaria 2006 identified that participants were allowed to continue the use of regular antihistamines (33% (13/40)), topical corticosteroids (55% (22/40)), or both (it is unclear how many patients used both treatments).
Three studies followed up patients beyond the end of the oral steroid treatment phase (Kirtsreesakul 2012; Vaidyanathan 2011; Van Zele 2010). Intranasal steroids were routinely prescribed to patients in both treatment arms at the end of the oral steroid treatment in two studies (Kirtsreesakul 2012; Vaidyanathan 2011). In Vaidyanathan 2011, all participants received fluticasone propionate nasal drops for eight weeks and then fluticasone propionate nasal spray for a further 18 weeks, making a total treatment time of 28 weeks (two weeks oral steroids or placebo followed by 26 weeks of intranasal steroids). In Kirtsreesakul 2012, all patients received mometasone furoate nasal spray for a further 10 weeks after initial treatment. Although patients were followed up at 12 weeks in Van Zele 2010, intranasal steroids were not routinely prescribed and were only permitted as rescue medication two months after dosing with the study medication.
Overall, the choice of oral corticosteroids used and the variety of differing regimens reflect the variety seen in mainstream clinical practice. Use of topical corticosteroids after the oral dose was included as a definitive part of the patient pathway in two studies, which reflects current practice.
Outcomes
One study did not report any of the primary or secondary outcomes as defined in the methods section of this review (Kapucu 2012).
Disease‐specific health‐related quality of life (HRQL)
This was measured in two studies using different measurement instruments (Hissaria 2006; Vaidyanathan 2011). Hissaria 2006 used the RSOM‐31 questionnaire to measure HRQL after treatment (14 days), but modified the scoring system, using only the severity parameter but not the importance parameter. Vaidyanathan 2011 used the Jupiter mini‐Rhinoconjunctivitis Quality of Life questionnaire (RQLQ) both immediately after treatment (14 days) and at 26 weeks after treatment. This scale is validated for patients with seasonal or perennial rhinoconjunctivitis but the validity of this instrument is not known in chronic rhinosinusitis patients and the scale is not clear within the paper.
Disease severity, as reported using patient‐reported outcomes
Five studies provided information on patient‐reported disease severity at the end of treatment in terms of a combined score or individual symptom scores, which could be combined into a single score (Ecevit 2015; Hissaria 2006; Kirtsreesakul 2012; Vaidyanathan 2011; Van Zele 2010). The symptoms measured, the scales of measurement used and the way in which data were reported varied greatly between studies. See Effects of interventions. Three of the studies provided medium‐term data on patient‐reported symptoms after a follow‐up period of 10 to 12 weeks (Kirtsreesakul 2012; Van Zele 2010), and 26 weeks (Vaidyanathan 2011).
Endoscopic score
Nasal polyp size was reported at the end of treatment in seven studies (Alobid 2014; Benitez 2006; Ecevit 2015; Hissaria 2006; Kirtsreesakul 2012; Vaidyanathan 2011; Van Zele 2010), and after a three‐ to six‐month follow‐up in three studies (Kirtsreesakul 2012; Vaidyanathan 2011; Van Zele 2010). Hissaria 2006 reported the estimated percentage reduction in polyp size using pairs of photographs taken pre‐ and post‐treatment. Five studies measured nasal polyps on a 0‐ to 3‐point scale although the definitions vary between categories (Alobid 2014; Benitez 2006; Ecevit 2015; Kirtsreesakul 2012; Vaidyanathan 2011), and Van Zele 2010 used a 0‐ to 4‐point scale. The scales used are summarised in Appendix 4. There was a lack of information in the papers about the methods used (e.g. was the value recorded the worst affected nostril or an average of the two nostrils?), about the validation of the scales used and about any precautions taken or calculation made to ensure consistency between investigators.
Adverse events
Two studies made no mention of whether adverse events were sought or identified in their papers (Alobid 2014; Benitez 2006). Two studies reported that no adverse effects were observed: Ecevit 2015 stated that "Adverse effects were not observed in either group", whilst Kapucu 2012 stated that "No systemic or local side effects of steroid treatment were seen in any patients". Vaidyanathan 2011 presented information about adverse events well, but did not report any of the specific adverse effects of oral steroids outcomes as pre‐defined by this review, although adverse events for intranasal steroid use after oral steroid treatment had finished were reported. The remaining three studies provided clear information about at least one of the adverse effects of interest (Hissaria 2006; Kirtsreesakul 2012; Van Zele 2010).
Excluded studies
We excluded 30 studies after reviewing the full paper. Further details of the reasons for exclusion are summarised in Characteristics of excluded studies. We identified 19 of these from the excluded papers list in previous version of the Cochrane review (Martinez‐Devesa 2011), and we found the reasons for exclusion from the previous review to still be valid under the updated inclusion criteria developed for this review (Alobid 2005; Blomqvist 2001; Blomqvist 2009; Bonfils 1998; Bonfils 2003; Bonfils 2006; Chi Chan 1996; Damm 1999; Hessler 2007; Jankowski 2003a; Jankowski 2003b; Kroflic 2006; Lildholdt 1988; Lildholdt 1989; Nores 2003; Ragab 2006; Rasp 2000; Sieskiewicz 2006; Stevens 2001).
Two papers reported RCTs comparing oral steroid treatment with placebo or no treatment, but all study participants also received concurrent treatment with antibiotics (Ozturk 2011), or intranasal steroids (Bülbül 2013). These studies are included in the Cochrane review of short‐course oral steroids as an adjunct for chronic rhinosinusitis (Head 2016a). In addition, we identified one protocol for an ongoing RCT, which will aim to compare a short course of oral steroids then intranasal steroids with intranasal steroids alone. All patients in both arms will also receive antibiotics (NCT01676415). Further details for this study can be found in the Cochrane review 'Short‐course oral steroids as an adjunct therapy for chronic rhinosinusitis' (Head 2016a).
Of the remaining seven papers, Rupa 2010 included a population of people with allergic fungal rhinosinusitis, which was out of scope for this review. One study compared intranasal steroids with oral steroids but intranasal steroid treatment was only given for 16 days (Reychler 2015). Six were either non‐randomised studies or commentaries on existing, included RCTs (Grammer 2013; Rasp 1997; Remer 2005; Sousa 2009; Tuncer 2003; van Camp 1994).
Ongoing studies
We identified three ongoing studies (Chi 2011; NCT00841802; NCT02367118). All studies are investigating oral steroids compared with either placebo or no treatment.Chi 2011 aims to compare oral prednisone with placebo treatment for 20 days in patients with nasal polyps. The trial was registered in 2011 but no further information was available despite attempts to contact the author. NCT00841802 compares oral prednisone for 21 days with placebo treatment in patients without nasal polyps. We contacted the study authors and confirmed that the study was currently recruiting participants but no results were currently available. The other ongoing study, NCT02367118, aims to compare a five‐day course oral prednisone with no intervention, prior to surgery. The study includes a mixture of patients with chronic rhinosinusitis with and without nasal polyps and the authors confirmed that they should be completing the study shortly, however no results were available in time for this review. See Characteristics of ongoing studies.
Risk of bias in included studies
The included studies were all randomised and controlled. Details of the risk of bias for each study can be found in Figure 2. A 'Risk of bias' graph shows our judgements about each risk of bias item presented as percentages across all included studies (Figure 3). In general the reporting of the trials was not of a high quality.
2.
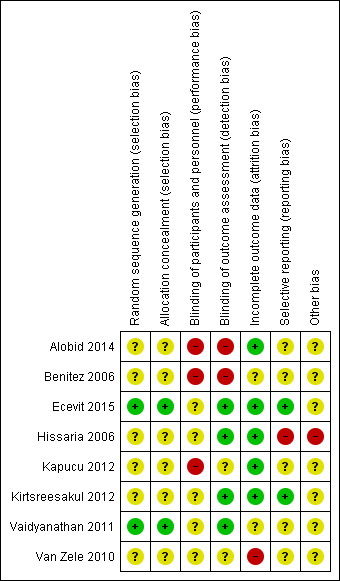
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.
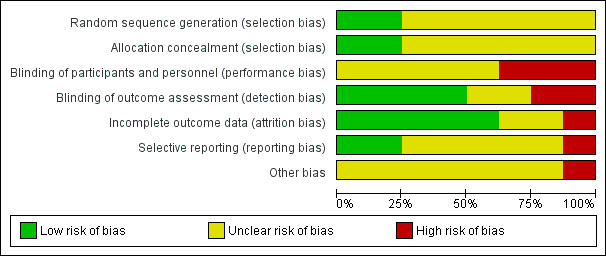
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation
Six of the included studies reported that the participants had been randomised to treatment groups but provided no further information on the methods of sequence generation (Alobid 2014; Benitez 2006; Hissaria 2006; Kapucu 2012; Kirtsreesakul 2012; Van Zele 2010). The ratios for randomising into the separate groups were provided for three of the studies: Alobid 2014 and Benitez 2006 randomised participants at a ratio of 3:1 into the intervention and control arms respectively, whereas Kirtsreesakul 2012 randomised at a ratio of 3:2 into the treatment and control arms respectively.
We assessed both of the remaining two studies to be at low risk of bias with respect to randomisation. Ecevit 2015 randomised participants in blocks of eight, whereas Vaidyanathan 2011 used a computer‐generated random allocation sequence to randomise the trial, using block randomisation with a block size of four.
Allocation concealment
Six studies did not provide any information about allocation concealment (Alobid 2014; Benitez 2006; Hissaria 2006; Kapucu 2012; Kirtsreesakul 2012; Van Zele 2010). For three trials the risk of bias is increased as there was no blinding (Alobid 2014; Benitez 2006; Kapucu 2012).
We assessed two studies to be at low risk of allocation concealment bias (Ecevit 2015; Vaidyanathan 2011).
Baseline characteristics
In three studies the baseline characteristics are poorly reported (Alobid 2014; Benitez 2006; Kapucu 2012). The majority of the information in Alobid 2014 relates to the overall cohort and baseline characteristics for each group are not provided for age or gender. Similarly, in Benitez 2006 some characteristics are only presented for the cohort as a whole (e.g. gender, aspirin sensitivity and comorbidity of asthma). In Kapucu 2012, there is a lack of information about the included population prior to treatment.
In addition, some of the studies do not report key information for key potential effect modifiers that would be expected to be reported. Four studies do not provide information about the severity of the nasal polyps in the different groups at the start of the trial (Alobid 2014; Benitez 2006; Kapucu 2012; Kirtsreesakul 2012). Similarly, information about any previous surgery is not presented in three papers (Benitez 2006; Ecevit 2015; Kirtsreesakul 2012).
In Van Zele 2010 there was an imbalance in the number of aspirin‐intolerant patients in the baseline characteristics (oral steroids: 14.3%; placebo: 26.3%; antibiotics: 7.1%).
Blinding
The participants and healthcare professionals in three studies were not blinded to the treatment group (Alobid 2014; Benitez 2006; Kapucu 2012). Since the main outcomes of interest in the review are patient‐reported, we considered the risk of bias for outcome assessments to be high.
Van Zele 2010 states that the study was "double blinded" but provides no information about the dosing schedule of the three arms within the trial (oral steroids, placebo and antibiotics) and what precautions were taken to prevent the participants and healthcare professionals from identifying the treatment arm to which they had been allocated. There was no information about blinding of outcome assessment in the paper.
The remaining four studies were all reported to be blinded and provide good explanations of the methods used to prevent bias from knowing the treatment arm to which participants had been allocated (Ecevit 2015; Hissaria 2006; Kirtsreesakul 2012; Vaidyanathan 2011). However, none of the studies make any comment on the taste of the placebo tablet. Oral steroids are known to have a distinctive bitter taste, which may be recognisable to patients who have previously received steroids, thus compromising the blinding. It is unclear whether the taste of the interventions was matched in these four studies and so we downgraded the risk of bias to 'unclear'.
Incomplete outcome data
We assessed five studies to be at a low risk of attrition bias (Alobid 2014; Ecevit 2015; Hissaria 2006; Kapucu 2012; Kirtsreesakul 2012). One of these studies reported that all the patients completed the trial and were included in the outcomes (Kapucu 2012). Four other studies reported drop‐out rates of less than 5% (Alobid 2014; Hissaria 2006; Ecevit 2015; Kirtsreesakul 2012).
In Benitez 2006, there was no mention of anyone who dropped out of the trial or had to discontinue for any reason. However, it was not stated within the paper how many patients were analysed for each outcome and so we assessed the risk of bias for incomplete outcome data to be unclear. We also assessed Vaidyanathan 2011 to have an unclear risk of attrition bias; the study had a relatively large drop‐out rate (9/60 (15%)), although the reasons for these drop‐outs are well described. The results table gives different numbers of participants included in each analysis, which are closer to the number of patients available rather than patients randomised and it is unclear why there is a discrepancy.
We assessed the risk of attrition bias in Van Zele 2010 as high. Seven of the initial 47 patients dropped out of the study (14.9%) and an intention‐to‐treat analysis was conducted with the last value carried forward. However, all of the patients who dropped out were from the placebo group: 7/19 (36.8%). The report implies that they all dropped out after the treatment stage during follow‐up. This may have had an effect on the overall results and no sensitivity analysis appears to have been completed to identify the impact.
Selective reporting
We assessed Ecevit 2015 and Kirtsreesakul 2012 to be at a low risk of selective reporting bias.
We assessed five studies to have an unclear risk of selective reporting bias (Alobid 2014; Benitez 2006; Kapucu 2012; Vaidyanathan 2011; Van Zele 2010). Despite meeting the inclusion criteria Kapucu 2012 did not report any of the primary or secondary outcomes specified in this review. Two studies reported primary or secondary outcomes (or both) but did not report any information about whether adverse effects were experienced by any participant within the study (Alobid 2014; Benitez 2006). Vaidyanathan 2011 did not report the methods for collecting data for adverse events (other than biological assays). In Van Zele 2010, all outcomes in the methods section have been reported in the full paper, although many of them have been presented graphically, without providing values at key time periods. The data were not reported in a way that is sufficient to be included in the meta‐analysis of this review. We contacted the study authors but further information was not provided.
We assessed Hissaria 2006 to be at high risk of reporting bias; the nasoendoscopy findings were reported inconsistently within the paper using differing criteria that had not been pre‐specified in the methods section. We were concerned that the cut‐off points for reporting could have been chosen after the results were available to make the results look more favourable.
Protocols could be identified for two of the included studies (Vaidyanathan 2011; Van Zele 2010). For Vaidyanathan 2011, no differences were identified between the outcomes at the protocol stage and those reported in the paper. For Van Zele 2010, it was difficult to judge whether there were differences between the protocol and the full paper as the protocol was not very detailed. We noted that the number of participants that the study aimed to recruit was different from the number actually recruited (120 and 48 respectively).
Other potential sources of bias
Use of validated outcome measures
The validation of outcomes was one area that we identified at the start of the review as an aspect that could lead to potential bias. If an instrument is insensitive to measuring differences, this biases the results to no difference. Six of the eight studies did not provide information about the validation of any of the outcomes relevant to this review (Alobid 2014; Benitez 2006; Ecevit 2015; Kapucu 2012; Kirtsreesakul 2012; Van Zele 2010). Furthermore, Van Zele 2010 also failed to provide information about the scale used for measuring symptoms.
Vaidyanathan 2011 reported validation of the health‐related quality of life measure (mini‐RQLQ), although on further investigation it appears that the validation was not completed in a chronic rhinosinusitis population. The validation of other outcomes was not mentioned. Hissaria 2006 provided references to the validation of the health‐related quality of life outcomes (RSOM‐31), although they use a modified version and no information on how this modification impacts the validation was made. For nasoendoscopy outcomes, the procedure to ensure reliability of measurements was well presented.
Funding and conflicts of interest in trials
Three studies did not report information about funding of the trials, or reported that no funding was provided (Ecevit 2015; Hissaria 2006; Kapucu 2012). The remaining five studies reported funding sources (Alobid 2014; Benitez 2006; Kirtsreesakul 2012; Vaidyanathan 2011; Van Zele 2010). None of the studies were explicitly funded by pharmaceutical companies with most of the funding appearing to be from governmental or university grants.
Two studies did not provide information on any of the authors' potential conflicts of interest (Alobid 2014; Benitez 2006), and four studies reported that the authors did not have any conflicts of interest (Ecevit 2015; Kapucu 2012; Kirtsreesakul 2012; Vaidyanathan 2011).
Two studies noted that one or more authors had a potential conflict of interest (Hissaria 2006; Van Zele 2010). Hissaria 2006 reported one of the authors as receiving royalties from a medical device company. Van Zele 2010 reported that one author had received royalties from a medical device company and was a consultant for another company (NeilMed). This author along with two other authors received research grants from external bodies (Garnett Passe and Rodney Williams Foundation, GlaxoSmithKline, Stallergenes, European Union).
Effects of interventions
See: Table 1
See also Table 1.
We analysed the pre‐specified primary and secondary outcomes. We included eight trials comprising 474 participants comparing oral steroids with placebo in this review (Alobid 2014; Benitez 2006; Ecevit 2015; Hissaria 2006; Kapucu 2012; Kirtsreesakul 2012; Vaidyanathan 2011; Van Zele 2010), although one of these studies did not include any of the pre‐specified primary or secondary outcomes and so is not included in the results (Kapucu 2012). All of the studies followed up patients until the end of treatment (14 to 21 days).
Three studies (224 participants) also followed up patients in both arms for a further 10 to 26 weeks after treatment (Kirtsreesakul 2012; Vaidyanathan 2011; Van Zele 2010). Kirtsreesakul 2012 and Vaidyanathan 2011 (177 participants) gave patients from both arms of the study intranasal corticosteroids after the end of oral steroid treatment and followed up patients for a further 10 to 26 weeks. Van Zele 2010 (47 participants) did not routinely allow intranasal corticosteroids after treatment with oral steroids but followed up patients for 10 weeks after oral steroid treatment had finished. The treatment of both arms with intranasal steroids, as represented in Kirtsreesakul 2012 and Vaidyanathan 2011, more accurately reflects current clinical practice than not providing any treatment. However, the results for all three of the longer‐term trials are also analysed together and are presented below as three‐ to six‐month results.
Where the range of scales and values for minimal important differences were unclear, we used the standardised mean difference (SMD) as a guide to estimate the effect sizes. As suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), we used standard rules of thumb in the interpretation of effect sizes (SMD, or Cohen's effect size of < 0.41 = small, 0.40 to 0.70 = moderate, > 0.70 = large) (Cohen 1988).
Primary efficacy outcomes
1. Health‐related quality of life, using disease‐specific health‐related quality of life scores
After treatment (two to three weeks)
Two studies (98 participants) measured 'health‐related quality of life' using a disease‐specific instrument (Hissaria 2006; Vaidyanathan 2011). However, these are not fully validated instruments for patients with chronic rhinosinusitis:
Hissaria 2006 used the RSOM‐31 questionnaire (a validated instrument) but modified the scoring system, using only the severity parameter but not the importance parameter. The study does not report the possible range of values that could be obtained.
Vaidyanathan 2011 used the mini‐Rhinoconjunctivitis Quality of Life Questionnaire (mRQLQ). This questionnaire was developed to measure the quality of life for people with seasonal or perennial rhinoconjunctivitis, and the validity for chronic rhinosinusitis patients is unknown. It has at least three to four items (out of 14) that are related to allergy but not applicable to patients with chronic rhinosinusitis. In addition, it does not include any items on sinonasal or facial pain and sense of smell, which are symptoms included in the EPOS 2012 diagnostic criteria.
Therefore, we have not pooled the results of these studies but they are plotted in Figure 4. The standardised mean difference (SMD) observed in Hissaria 2006 was ‐1.24 (95% confidence interval (CI) ‐1.92 to ‐0.56; 40 participants), whereas the SMD in Vaidyanathan 2011 was ‐0.79 (95% CI ‐1.32 to ‐0.25; 58 participants) (Analysis 1.1). We considered both of these results to be large effect sizes.
4.
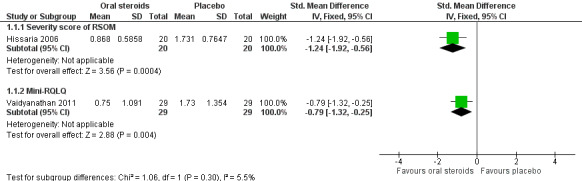
Forest plot of comparison: 1 Oral steroids versus no treatment/placebo, outcome: 1.1 Disease‐specific health‐related quality of life ‐ no pooling (2 to 3 weeks).
1.1. Analysis.
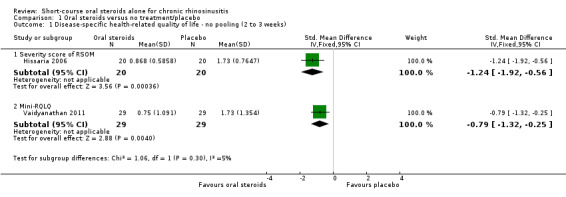
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 1 Disease‐specific health‐related quality of life ‐ no pooling (2 to 3 weeks).
Medium‐term (three to six months)
Vaidyanathan 2011 also presented results for the mini‐RQLQ data at 26 weeks. The scale is not clear within the paper. The SMD was ‐0.59 (95% CI ‐1.16 to ‐0.02; 50 participants; one study) (Analysis 1.2). We considered this result to be a moderate effect size.
1.2. Analysis.
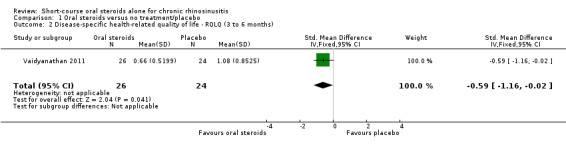
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 2 Disease‐specific health‐related quality of life ‐ RQLQ (3 to 6 months).
2. Disease severity, as measured by patient‐reported symptom score
None of the papers provided results for a patient‐reported total symptom score validated in a chronic rhinosinusitis population. Where available we combined the results for the individual symptoms into a total score according to the methods section (see Dealing with missing data). In order to be included in the analysis the results needed to provide enough data to meet the EPOS 2012 diagnostic criteria (Appendix 3), which requires at least two symptoms to be present, one of which must be nasal blockage/obstruction/congestion or nasal discharge (anterior/posterior nasal drip), with the other possible symptoms being facial pressure/pain, loss of sense of smell (adults) or cough (children).
Four studies (232 participants) reported this outcome (Ecevit 2015; Hissaria 2006; Kirtsreesakul 2012; Vaidyanathan 2011), but all used different measurements and presented results in different ways.
Ecevit 2015 (22 participants) asked patients to report individual symptoms on a visual analogue scale (VAS) (0 to 10; 0 = no symptoms). Scores were given for the four symptoms included in the EPOS 2012 definition (nasal obstruction, nasal discharge, sense of smell and pressure over the sinuses), which were combined to give a total mean final score with a possible range of 0 to 40.
Hissaria 2006 (40 participants) measured symptoms using the nasal subscale of the RSOM‐31, which measures six symptoms: congestion, rhinorrhoea, sneezing, hyposmia, postnasal discharge and thick nasal debris, all scored on a one‐ to five‐point VAS (1 = least severe, 5 = most severe). To obtain a total value, the authors averaged the scores across all domains and presented them graphically in the paper. The combined results should, therefore, have been on a scale of one to five but the results clearly show that one of the data points on the graph is less than 1. We contacted the study authors to provide further information but they did not respond. The nasal subscale of the RSOM‐31 represents three of the symptoms of the EPOS 2012 criteria. Facial pain/pressure was not reported.
Kirtsreesakul 2012 (114 participants) asked patients to report individual symptoms on a seven‐point Likert scale (0 to 6; 0 = no symptoms, 6 = most severe). The results for each symptom were presented graphically in the paper as percentage change from baseline. The results for the four symptoms representing the EPOS 2012 criteria (blocked nose, rhinorrhoea, hyposmia and sinonasal pain) were averaged to create an average change from baseline score.
Vaidyanathan 2011 (57 participants) used the "total nasal symptoms score", which is calculated from the sum of scores for nasal discharge, nasal blockage, nasal itch and sneezing, each measured on a 0‐ to 3‐point scale (total range 0 to 12; 0 = least affected, 12 = most affected). The results include two of the symptoms listed in the EPOS 2012 criteria (nasal blockage and nasal discharge). Data for facial pain/pressure or loss of sense of smell were not recorded. The validation status of the scale is unknown and it is likely to be more specific for rhinitis symptoms.
The results were presented in two different ways within the papers, either as 'mean final value' (Ecevit 2015; Hissaria 2006; Vaidyanathan 2011), or as 'change from baseline' (Kirtsreesakul 2012). Results were only presented after treatment (at two to three weeks) in both studies (Ecevit 2015; Hissaria 2006), and also as medium‐term results (three to six months after treatment) in two studies (Kirtsreesakul 2012; Vaidyanathan 2011).
We considered whether the results using different scales could be pooled, but due to the differences in the individual symptoms included in the scale, we did not consider pooling to be appropriate. We plotted all results separately using SMD on the same forest plot but did not present any totals (see Figure 5; Analysis 1.3).
5.
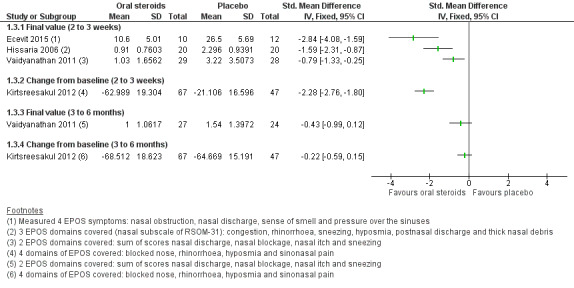
Forest plot of comparison: 1 Oral steroids versus no treatment/placebo, outcome: 1.3 Disease severity (patient‐reported total symptom score).
1.3. Analysis.
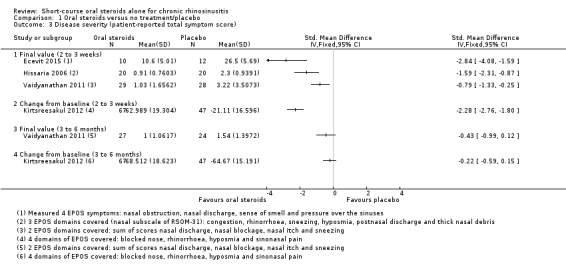
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 3 Disease severity (patient‐reported total symptom score).
After treatment (two to three weeks)
Mean final values
Three studies, with a total of 119 participants, presented results as mean final values immediately after treatment (14 to 17 days) (Ecevit 2015; Hissaria 2006; Vaidyanathan 2011). All of the results showed an improvement in the combined symptoms severity score for oral steroids compared with placebo at the end of treatment, with the largest effect being observed in Ecevit 2015 (SMD ‐2.84, 95% CI ‐4.08 to ‐1.59, 22 participants), followed by Hissaria 2006 (SMD ‐1.59, 95% CI ‐2.31 to ‐0.87, 40 participants) and Vaidyanathan 2011 (SMD ‐0.79, 95% CI ‐1.33 to ‐0.25, 57 participants) (Analysis 1.3). All of these SMD values corresponded to large effect sizes.
We observed a variation in the results for symptom severity between the trials. This may have been due to:
differences in the outcome measurement being used in each scale ‐ different individual symptoms were measured in each case and the validity and sensitivity of the scales to measure this outcome are unknown;
differences in the included populations within the studies ‐ participants in Ecevit 2015 were more severely affected at baseline according to the diagnostic criteria;
differences in the interventions provided ‐ Ecevit 2015 provided oral steroids at a higher dose than Hissaria 2006 or Vaidyanathan 2011.
Change from baseline
Kirtsreesakul 2012 (114 participants) showed a larger average percentage improvement in total symptom score for the oral steroids group compared with placebo at the end of treatment (14 days) (SMD ‐2.28, 95% CI ‐2.76 to ‐1.80) (Analysis 1.3). This corresponds to a large effect size.
Medium‐term (three to six months)
Mean final value
Vaidyanathan 2011 (51 participants) provided results at 26 weeks after the end of oral steroid treatment, where both arms of the trial had been given intranasal steroids after 21 days (when the short course of oral steroids ended). The result for total nasal symptom score was SMD ‐0.43 (95% CI ‐0.99 to 0.12) (Analysis 1.3). This corresponds to a moderate effect size.
Change from baseline
Kirtsreesakul 2012 (114 participants) provided data that allowed the calculation of three‐ to six‐month results for percentage change in total symptoms score from baseline, 10 weeks after completing the oral steroid treatment, where both arms of the trial had been given intranasal steroid therapy from two weeks (i.e. at the end of the oral steroid treatment period). The result for percentage change from baseline was SMD ‐0.22 (95% CI ‐0.59 to 0.15) (Analysis 1.3). This corresponds to a small effect size.
Individual symptom scores
Data for patient‐reported individual symptoms were presented in three papers (Ecevit 2015; Kirtsreesakul 2012; Vaidyanathan 2011). These papers used different measurement scales for the rating of symptoms.
Ecevit 2015 (22 participants) asked participants to rank symptoms on a visual analogue scale of 0 to 10 (0 = no complaint, 10 = most annoying). The paper presented the final mean values.
Kirtsreesakul 2012 (114 participants) asked participants to rate symptoms using a seven‐point Likert scale (0 to 6, 0 = no symptoms, 6 = severe symptoms). The paper presented results graphically in figures for percentage improvement from baseline for each symptom. We extracted the data from graphs. Data were presented for after treatment (two weeks) and also at 12 weeks after both groups had received intranasal steroids.
In Vaidyanathan 2011 the only individual symptom for which data were extractable was hyposmia, which was measured by patients on a 0 to 100 mm hyposmia visual analogue scale. It is not clearly described within the paper but it is inferred from the discussion that a higher score relates to greater severity of smell loss.
Although Alobid 2014 measured loss of sense of smell using the Barcelona Smell Test‐24 score, the results were presented for all patients, control group, patients with asthma and patients without asthma and so it was not possible to include these results.
Nasal obstruction/congestion/blockage
Final value
One study (22 participants) presented data for the mean final value of the nasal obstruction symptom score (measured on a 0 to 10 VAS, 0 = no nasal blockage) for oral steroids compared with placebo at the end of the 17‐day treatment course (mean difference (MD) ‐4.50, 95% CI ‐6.42 to ‐2.58) (Ecevit 2015) (Analysis 1.4).
1.4. Analysis.
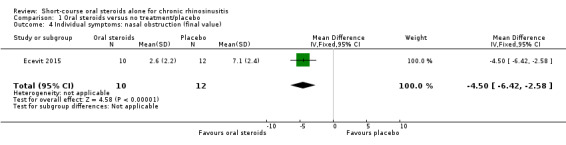
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 4 Individual symptoms: nasal obstruction (final value).
Change from baseline
One study (114 participants) presented data for percentage change in nasal blockage (measured on a seven‐point Likert scale, 0 = no symptoms) for oral steroid treatment compared with placebo after a 14‐day treatment course (MD ‐38.02, 95% CI ‐48.16 to ‐27.88; 114 participants) and at three months after oral steroid treatment had finished when all patients in both study arms had received intranasal steroids for 10 weeks (MD 0.90, 95% CI ‐8.97 to 10.77) (Kirtsreesakul 2012) (Analysis 1.5).
1.5. Analysis.
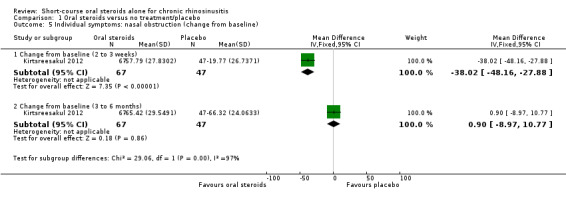
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 5 Individual symptoms: nasal obstruction (change from baseline).
Nasal discharge
Final value
One study (22 participants) presented data for the mean final value of nasal discharge symptom score (measured on a 0 to 10 VAS, 0 = no nasal discharge) for oral steroids compared with placebo at the end of a 17‐day treatment course (MD ‐4.70, 95% CI ‐6.79 to ‐2.61) (Ecevit 2015) (Analysis 1.6).
1.6. Analysis.
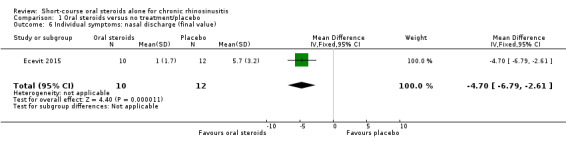
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 6 Individual symptoms: nasal discharge (final value).
Change from baseline
One study (114 participants) presented data for percentage change in rhinorrhoea (measured on a seven‐point Likert scale, 0 = no symptoms) for oral steroid treatment compared with placebo after a 14‐day treatment course (MD ‐55.57, 95% CI ‐69.23 to ‐41.91) and at three months after oral steroid treatment had finished when all patients in both study arms had received intranasal steroids for 10 weeks (MD ‐1.83, 95% CI ‐13.46 to 9.81; 114 participants) (Kirtsreesakul 2012) (Analysis 1.7). Rhinorrhoea was used in preference to the individual symptom of postnasal drip, which was also reported in the paper.
1.7. Analysis.
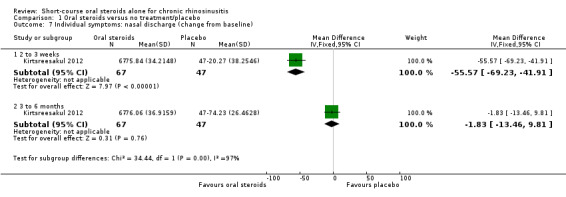
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 7 Individual symptoms: nasal discharge (change from baseline).
Facial pain/pressure
Final value
One study (22 participants) presented data for the mean final value of facial pressure symptom score (measured on a 0 to 10 VAS, 0 = no facial pressure) for oral steroids compared with placebo at the end of a 17‐day treatment course (MD ‐3.70, 95% CI ‐6.02 to ‐1.38) (Ecevit 2015) (Analysis 1.8). The symptom of facial pressure was used in preference to the individual symptom of headache.
1.8. Analysis.
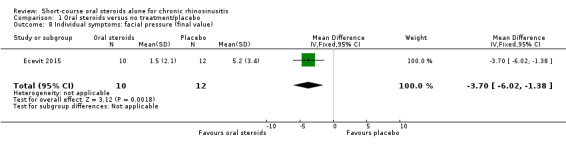
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 8 Individual symptoms: facial pressure (final value).
Change from baseline
One study (114 participants) presented data for percentage change in sinonasal pain (measured on a seven‐point Likert scale, 0 = no symptoms) for oral steroid treatment compared with placebo after a 14‐day treatment course (MD ‐30.66, 95% CI ‐46.28 to ‐15.04) and at three months after oral steroid treatment had finished when all patients in both study arms had received intranasal steroids for 10 weeks (MD 0.60, 95% CI ‐12.56 to 13.76) (Kirtsreesakul 2012) (Analysis 1.9). Rhinorrhoea was used in preference to the individual symptom of postnasal drip, which was also reported in the paper.
1.9. Analysis.
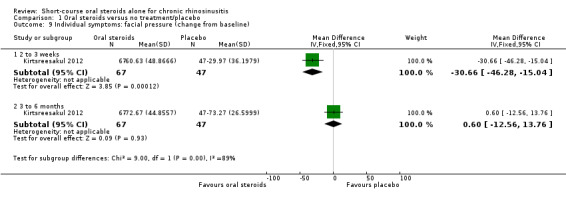
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 9 Individual symptoms: facial pressure (change from baseline).
Loss of sense of smell
Final value
Two studies (80 participants) presented data for the mean final value of loss of sense of smell (Ecevit 2015; Vaidyanathan 2011). Ecevit 2015 measured smell on a 0 to 10 scale (0 = no loss of sense of smell) and Vaidyanathan 2011 measured this on a 0 to 100 mm hyposmia VAS (0 = no loss of sense of smell), which we subsequently scaled to represent a 0 to 10 scale. The result for oral steroids compared with placebo at the end of treatment (14 to 17 days) was MD ‐2.79 (95% CI ‐4.11 to ‐1.47). Vaidyanathan 2011 also presented results 26 weeks after the end of treatment when patients in both arms had received intranasal steroids (MD ‐1.20, 95% CI ‐2.68 to 0.28) (Analysis 1.10).
1.10. Analysis.
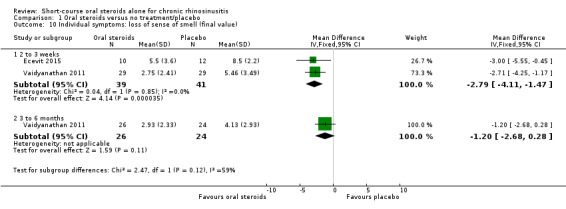
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 10 Individual symptoms: loss of sense of smell (final value).
Change from baseline
One study (114 participants) presented data for percentage change in hyposmia (measured on a seven‐point Likert scale, 0 = no symptoms) for oral steroid treatment compared with placebo after a 14‐day treatment course (MD ‐44.35, 95% CI ‐57.31 to ‐31.39) and at three months after oral steroid treatment had finished when all patients in both study arms had received intranasal steroids for 10 weeks (MD ‐15.05, 95% CI ‐29.69 to ‐0.41) (Kirtsreesakul 2012) (Analysis 1.11).
1.11. Analysis.
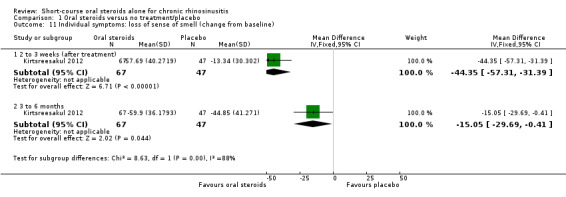
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 11 Individual symptoms: loss of sense of smell (change from baseline).
None of the results for individual symptoms are presented in the GRADE 'Summary of findings' table as we considered it to be re‐presenting information that was already included in the disease severity score and, as such, not considered to be a priority outcome.
3. Significant adverse effect: mood or behavioural disturbances
One study (40 participants) reported mood disturbances as an adverse event (Hissaria 2006). This study found that there were no differences between the oral steroid and the placebo group after the two‐week treatment course (5/20 oral steroids, 0/20 placebo) (risk ratio (RR) 2.50, 95% CI 0.55 to 11.41) (Analysis 1.12).
1.12. Analysis.
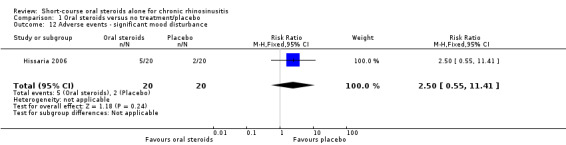
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 12 Adverse events ‐ significant mood disturbance.
Secondary efficacy outcomes
1. Health‐related quality of life, using generic quality of life scores, such as the SF‐36, EQ‐5D and other well‐validated instruments
None of the studies reported this as an outcome.
2. Other adverse effects: gastrointestinal disturbances
We analysed together the short‐term 'after treatment' results (two to three weeks) and the medium‐term 'three to six months' results for gastrointestinal disturbances. Three studies (187 participants) reported gastrointestinal disturbances as an adverse event (Hissaria 2006; Kirtsreesakul 2012; Van Zele 2010). Hissaria 2006 reported adverse events after the treatment course had ended (two weeks), whereas Kirtsreesakul 2012 and Van Zele 2010 reported adverse events at 12 weeks. We meta‐analysed the results and there was an increase in gastrointestinal disturbance in the oral steroid group compared with placebo (15/101, 4/86) (RR 3.45, 95% CI 1.11 to 10.78) (Analysis 1.13).
1.13. Analysis.
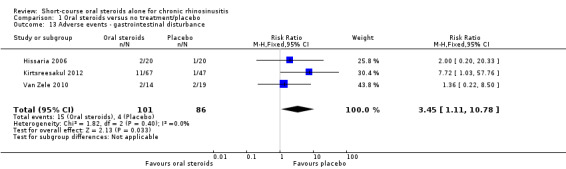
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 13 Adverse events ‐ gastrointestinal disturbance.
3. Other adverse effects: insomnia
Three studies (187 participants) reported insomnia as an adverse event (Hissaria 2006; Kirtsreesakul 2012; Van Zele 2010). Hissaria 2006 reported adverse events after the treatment course had ended (two weeks), whereas Kirtsreesakul 2012 and Van Zele 2010 reported adverse events at 12 weeks. We meta‐analysed the results and there was an increase in insomnia in the oral steroid group compared with placebo (10/101, 2/86) (RR 3.63, 95% CI 1.10 to 11.95) (Analysis 1.14).
1.14. Analysis.
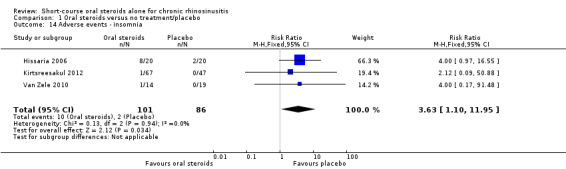
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 14 Adverse events ‐ insomnia.
4. Adverse effects: osteoporosis
None of the studies reported this as an outcome.
5. Endoscopic score (polyps size for chronic rhinosinusitis with polyps population, or overall endoscopy score for chronic rhinosinusitis without polyps population)
Five studies measured nasal polyps on a zero‐ to three‐point scale, although the wording used to describe each of the categories is not consistent between the studies (Appendix 4) (Alobid 2014; Benitez 2006; Ecevit 2015; Kirtsreesakul 2012; Vaidyanathan 2011). After reviewing the scales we agreed to analyse the results of the studies together as we thought the definitions between the categories to be roughly equivalent.
After treatment (two to three weeks)
Final value
Four studies (253 patients) reported the total nasal polyp score at the end of treatment (Alobid 2014;Benitez 2006; Ecevit 2015; Vaidyanathan 2011). All of the studies used the four‐point (0 to 3) scale for the measurement of polyps severity. Vaidyanathan 2011 summed the scores for each nostril together to give a total scale of 0 to 6. We have divided these results by two to provide the average polyp score for both nostrils on a scale of 0 to 3, to be consistent with the other studies. It is unclear in the other three papers whether the result refers to the polyp grade in the worst affected nostril or an average of the polyp grade by nostril (Alobid 2014; Benitez 2006; Ecevit 2015). The results showed that there was a reduction in nasal polyp score for oral steroids compared with placebo/no treatment at two weeks (MD ‐0.76, 95% CI ‐0.92 to ‐0.61, 253 participants) (Analysis 1.15). This observed mean difference corresponds to large effect size (SMD of ‐1.21).
1.15. Analysis.

Comparison 1 Oral steroids versus no treatment/placebo, Outcome 15 Endoscopy score ‐ nasal polyps (final value).
There is moderate heterogeneity within the mean final nasal polyp size results as presented above (heterogeneity: Chi² = 7.20, df = 3 (P value = 0.07); I² = 58%). When Vaidyanathan 2011 is removed from the analysis, the heterogeneity is reduced. This may have been due to differences in the methods of assessing the nasal polyps.
Change from baseline
Van Zele 2010 measured the nasal polyps score on a five‐point scale (0 to 4), which was used for each nostril and then summed to get an overall nasal polyp score. The paper presented the results graphically as change from baseline polyps score, however data on the variance of the point estimates were not available for this study and it was not possible to impute them from other studies due to differences in the scale, so it was not included in the meta‐analysis.
Two studies (146 patients) reported the percentage improvement from baseline values at the end of treatment (Hissaria 2006; Kirtsreesakul 2012). Kirtsreesakul 2012 measured the polyps grade using a 0 to 3 scale, whereas Hissaria 2006 reported the estimated percentage reduction in polyp size using pairs of photographs taken pre‐ and post‐treatment. As these ways of measuring polyps were different, we analysed the data using standardised mean difference to report the results. The results showed that there was a larger change from baseline in the size of nasal polyps in the oral steroids group compared with the control group after treatment (two to three weeks) (SMD ‐1.77, 95% CI ‐2.16 to ‐1.38). This corresponds to a large effect size (Analysis 1.16).
1.16. Analysis.
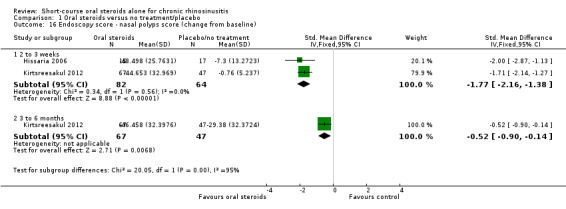
Comparison 1 Oral steroids versus no treatment/placebo, Outcome 16 Endoscopy score ‐ nasal polyps score (change from baseline).
Medium‐term (three to six months)
Final value
One study (50 patients) provided results for final mean value of nasal polyps score for oral steroids compared with placebo, 26 weeks after the initial treatment period with both study arms receiving treatment with intranasal steroids (Vaidyanathan 2011). The mean difference in nasal polyps at 26 weeks was ‐0.25 (95% CI ‐0.62 to 0.12, 50 participants) on a 0‐ to 3‐point scale (Analysis 1.15). The observed mean difference corresponds to a small effect size (SMD of 0.36).
Change from baseline
One study measured results at three months from the start of the trial when all patients in both study arms had received intranasal steroids for 10 weeks (Kirtsreesakul 2012). The results for the oral steroid arm compared to the placebo arm were SMD ‐0.52 (95% CI ‐0.90 to ‐0.14) (Analysis 1.16). This corresponds to a moderate effect size.
The results for endoscopic score are not presented in the 'Summary of findings' table as we did not consider it to be a priority outcome.
6. Computerised tomography (CT) scan score
None of the studies reported this as an outcome.
Discussion
Summary of main results
This review includes eight trials comparing the effectiveness of short‐course oral steroids with placebo or no treatment in adults with chronic rhinosinusitis with nasal polyps (Table 1).
There was low quality evidence of an improvement in disease‐specific health‐related quality of life after treatment (two to three weeks) with oral corticosteroids compared with placebo or no treatment. There is a concern that the magnitude of the improvement is not sustained.
There was low quality evidence of an improvement in disease severity (lower symptom score) in the oral steroids group compared with the control group at the end of the steroid treatment (two to three weeks). At three months, when all patients had received intranasal steroids after the treatment period had ended, there was low quality evidence that there was no difference in the change from baseline in symptom severity between the oral steroids and control groups.
There was low quality evidence of an increase in insomnia and gastrointestinal disturbances in the oral steroids group compared with the control group. It is unclear whether there is a difference between the intervention and control groups for mood disturbances (low quality evidence). None of the studies provided data for osteoporosis.
Immediately after treatment (two to three weeks), there was evidence (high risk of bias) of an improvement in nasal polyp score for the oral steroids group compared with the control group. Results at three to six months after the end of oral steroid treatment indicate that the magnitude of the difference between the groups may not be sustained (high risk of bias).
No studies reported generic health‐related quality of life or CT scan score as outcomes.
Overall completeness and applicability of evidence
All of the included studies only included adults with nasal polyps (Alobid 2014; Benitez 2006; Ecevit 2015; Hissaria 2006; Kapucu 2012; Kirtsreesakul 2012; Vaidyanathan 2011; Van Zele 2010). This limits the applicability of the evidence to both adults without nasal polyps and the paediatric population with chronic rhinosinusitis. Two of the three ongoing studies that we identified include patients with chronic rhinosinusitis who do not have nasal polyps (NCT00841802; NCT02367118). NCT00841802 only includes patients with chronic rhinosinusitis without nasal polyps, whereas the other study, NCT02367118, includes patients with and without nasal polyps,. Evidence for oral steroids in patients with chronic rhinosinusitis without polyps may therefore be available for the next update of this review.
The doses of oral steroids used in the trials differed. Using a steroid equivalence converter (http://www.medcalc.com/steroid.html) for the three oral steroid drugs in the included trials (prednisone, prednisolone and methylprednisolone) to convert all doses into a reference drug of prednisolone, the dose of drug ranged from 25 mg/day (Vaidyanathan 2011) to 60 mg/day (Ecevit 2015). In the analysis, we analysed all of the doses together. This excludes the Kapucu 2012 study, for which no outcomes are available, which calculated the dosage based on the weight of participants at 1 mg/kg/day methylprednisolone; for a weight of 84 kg (average weight of a UK man) this would be equivalent to 105 mg/day of prednisolone.
The primary outcomes were often either not reported or very poorly reported within the papers. There was considerable inconsistency between the papers with regards to the instruments used and how the results were reported (mean final value or change from baseline), making the analysis of results difficult.
There was a lack of consistent information on disease‐related symptom severity. There were no studies that used a validated tool to report this as an outcome. Of the four studies that attempted to reported this outcome (Ecevit 2015; Hissaria 2006; Kirtsreesakul 2012; Vaidyanathan 2011), only two, Ecevit 2015 and Kirtsreesakul 2012, presented data for all four of the symptoms required to diagnose chronic rhinosinusitis using the EPOS 2012 criteria (Appendix 3). The lack of reporting of symptoms, particularly of facial pressure/pain and hyposmia, may have been due to all of the study populations being composed entirely of people with nasal polyps. The studies usually looked for changes in symptoms associated with nasal polyps (such as nasal discharge and obstruction) rather than more general chronic rhinosinusitis symptoms. The facial pain/pressure symptom was not often studied.
The two studies that reported a 'symptom severity score' without providing information on the individual elements included used scales that included items that may have been more relevant to rhinitis outcomes (Hissaria 2006; Vaidyanathan 2011), which limits the applicability of these results to the chronic rhinosinusitis population. Vaidyanathan 2011 used 'total nasal symptoms' to report symptom severity, which covers five domains: two are relevant to chronic rhinosinusitis symptoms (nasal congestion and runny nose), two domains are more specific to rhinitis symptoms (nasal itching and sneezing) and one domain relates to the impact of the symptoms on sleeping. Similarly, Hissaria 2006 used the nasal subscale of the RSOM‐31 to look at 'symptom severity'. This subscale measures congestion, rhinorrhoea, sneezing, hyposmia, postnasal discharge and thick nasal debris, of which some elements are more specific to rhinitis symptoms. These studies may change the magnitude of the effect.
The methods used for assessing polyps were poorly reported in the papers and the assessment of polyp bulk endoscopically was the subject of several scoring systems (Appendix 4). Unless examinations are videoed and assessed centrally, these are somewhat subjective, even if previously validated, and should be seen as a guide to responsiveness rather than an absolute measure, as these studies did not report intra‐ and inter‐reporter reliability. There could be potential for bias in the way the scores have been reported, particularly in studies without blinding.
The information about adverse events was incomplete. Only three of eight studies included clear information about adverse events (Hissaria 2006; Vaidyanathan 2011; Van Zele 2010). This is a trend repeated in other conditions and reliable data for adverse events associated with short‐term steroid use have not been well recorded in the literature (Burton 2008). Additionally, there was a lack of long‐term data from the trials, which could identify longer‐term effects of oral steroids such as osteoporosis.
There was also a lack of information on other longer‐term outcomes. Five of the eight studies only reported the results for both arms of the trials at the end of treatment (Alobid 2014; Benitez 2006; Ecevit 2015; Hissaria 2006; Kapucu 2012). Of the three studies that provided longer‐term results, two reported outcomes at three months (Kirtsreesakul 2012; Van Zele 2010), and one provided six‐month results (Vaidyanathan 2011).
Quality of the evidence
The quality of the evidence for all of the outcomes assessed was low, including for the adverse events outcomes that were reported.
The quality of the evidence was affected by a number of issues: methodological limitations, length of follow‐up, validation of outcome instruments and the size of the studies.
The studies were generally poorly reported and information about randomisation, allocation concealment and blinding was unclear in the majority. Five of the trials only followed up patients until the end of oral steroid treatment (two to three weeks), which limits the applicability of the evidence for the longer‐term outcomes. Where longer‐term outcomes were presented (three to six months) it was often found that the initial results were not sustained.
Another issue is the lack of use of validated instruments. Many studies did not use validated patient‐reported outcome measures and some used instruments that were validated for other populations (e.g. rhinitis patients). A 'validated instrument' is no longer valid when used outside the population it was intended for, as the items being used may no longer be relevant and important areas for chronic rhinosinusitis patients may not be covered. We also downgraded the disease severity outcome whenever an overall validated disease severity score was not reported and imputations had to be made to calculate total symptom scores (see Dealing with missing data).
The size of the studies included in this review was generally small with an average sample size of 60 participants (30 in each arm). This limits how much confidence can be placed in the results.
Potential biases in the review process
What defined a 'short course' of oral steroids was an issue that we discussed at great length during the development of the protocol for this review (Chong 2015). We finally agreed that up to a 21‐day course should be considered to be a short course, but there were some opinions that the maximum duration should be 14 days. Limiting the evidence to 14 days would have excluded two studies (Ecevit 2015; Van Zele 2010), and possibly a further study where the duration is unclear and based on weight rather than time (Kapucu 2012). As there were only a small number of papers for each outcome it is not possible to evaluate the impact of this decision.
The validation of outcome measures was a potential bias that we identified at the protocol stage as something that could affect the validity of the results. Many of the studies did not use patient‐reported symptom scoring scales that have been appropriately validated. The lack of validated scores means that we often have to make judgements based on the face validity of the scale, rather than having reliable validity data. For example, in Vaidyanathan 2011, health‐related quality of life was measured using a measure that was validated in people with seasonal or perennial allergic rhinitis but not in people with chronic rhinosinusitis, the mini‐Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ). This instrument has 14 questions covering five domains: activity limitation, practical problems, nose symptoms, eye symptoms and non‐nose/eye symptoms, with a focus of eye and nose symptoms related to allergy. Similarly, many of other studies also used instruments that were not validated in people with chronic rhinosinusitis to measure symptom severity.
The lack of use of validated instruments to measure patient‐important outcomes, such as the impact on quality of life and disease severity, is the probably the single most important issue that hampers the ability to meta‐analyse results or to compare results between studies. Validated disease‐specific questionnaires exist and future trials would benefit from including these as primary outcome measures. Recent preliminary work in the UK has underlined this and identified the need to establish a core outcome set for rhinosinusitis (Hopkins 2016).
As there was a lack of outcomes reported using validated measures, in order to enable some comparison between studies and reviews (Chong 2016a; Chong 2016b; Chong 2016c; Head 2016a; Head 2016b), we took the decision to combine the scores for individual symptoms to create a total symptoms score. The methods and limitations are described in the Methods section (Dealing with missing data). The symptoms included were based on the EPOS 2012 diagnostic criteria. However, this score was not a validated measure and as there is no evidence on the correlation coefficient between symptoms, the calculation could not account for it. This may have had an effect on the magnitude of the effect size when interpreted as a standardised mean difference due to the potentially higher or lower standard deviations but not in mean differences (MD) observed. To account for the lack of validated scales used and the lack of validated methods to sum the scores, we downgraded all the disease severity outcomes for lack of use of validated scales whenever this occurred.
Another potential bias in the review process was that many of the data for the included studies were presented in the papers in graphs or charts. Where this was the case, we contacted the study authors to try to obtain more precise data, however none of the authors provided additional data. We therefore extracted the data from the paper using an online programme (http://arohatgi.info/WebPlotDigitizer/app/). There will inevitably be a degree of error in using these data, both from inaccuracies during the printing process and the process used to collect the data. We carefully considered the amount of any additional transformation of the data where they had been interpreted from graphs (such as combining individual symptoms into total scores) to try to minimise additional errors.
Agreements and disagreements with other studies or reviews
The current review updates a previous Cochrane review (Martinez‐Devesa 2011), and it increases the scope of the review to include patients both with and without nasal polyps.
The previous Cochrane review included three studies (Alobid 2006 ‐ reported in this review as Benitez 2006; Hissaria 2006; Van Zele 2010), and referenced one ongoing study. We included all four of these studies in this updated review (including the ongoing study, which has now been published in full ‐ Vaidyanathan 2011). After communication with the study author we determined that Alobid 2006 partially reported on participants in Benitez 2006 and the author recommended that Benitez 2006 was the best paper to use. The previous review concluded that "the limited number of trials of moderate to poor methodological quality showed a short‐term improvement with a short (two to four‐week) and variable dose course of oral steroids in the treatment of nasal polyps." Furthermore, they highlighted the lack of long‐term data preventing any conclusions about a sustained effect of this treatment in the management of nasal polyps. The current review presents additional evidence for the short‐term effects, although the conclusion that there is a short‐term improvement after a short course of oral steroids is still valid. In addition, this review adds three studies that reported results at three to six months (Kirtsreesakul 2012; Vaidyanathan 2011; Van Zele 2010). The results at three to six months show that the magnitude of improvement is not sustained after the oral steroid treatment period.
We changed the inclusion criteria with regards to previous surgery from the previous review to include studies where patients had surgery within six weeks of the start of the trial, compared with three months for the previous review. This did not increase the number of included studies. Some of the studies specifically investigated the use of oral steroids pre‐operatively to improve surgical field conditions during surgery (Ecevit 2015; Sieskiewicz 2006). We included these studies if the treatment duration was equivalent to the other studies included and any of the pre‐specified outcomes were measured prior to surgery (Ecevit 2015). For one study the treatment course was only five days and the outcomes were specifically related to surgical field conditions, and so we excluded this study (Sieskiewicz 2006).
The EPOS 2012 document splits the chronic rhinosinusitis population into those with and without nasal polyps. For the population without nasal polyps we identified only one case series with information about adverse events extrapolated from the chronic rhinosinusitis with nasal polyps population. The recommendation, based on very weak evidence, within the EPOS document for chronic rhinosinusitis without nasal polyps (CRSsNP) was that "systemic corticosteroids benefit CRSsNP". For the chronic rhinosinusitis with nasal polyps (CRSwNP) population, the EPOS document uses the three studies included in the previous Cochrane review (Alobid 2006 ‐ reported in this study as Benitez 2006; Hissaria 2006; Van Zele 2010) and adds an additional three studies/four papers (Benitez 2006; Vaidyanathan 2011; Martinez‐Anton 2008 ‐ reported in this review as Benitez 2006; Rupa 2010). Vaidyanathan 2011 was also included in this review. After communication with the study author we confirmed that Benitez 2006, Alobid 2006 and Martinez‐Anton 2008 all contained subsets of participants from the same trial and therefore some patients were presented in the analysis of all three trials. The author also suggested that Benitez 2006 was the best paper to use as the most complete information for the trial and this is what is included in this Cochrane review. We excluded Rupa 2010 from this review as it reported on a population that was out of scope for the review (allergic fungal rhinosinusitis). The EPOS 2012 document concludes, based on strong evidence, that "Systemic corticosteroids benefit CRSwNP but the effects are time limited post therapy".
As part of this suite of Cochrane reviews on the interventions for chronic rhinosinusitis, a review on the use of short‐course oral steroids as an adjunct has been published (Head 2016a). Two studies identified in that review used oral steroids as an adjunct treatment to either intranasal steroids (Bülbül 2013), or antibiotics (Ozturk 2011). The trials were small, low quality and did not report many of the pre‐specified outcomes. There was not enough evidence to support or oppose the use of oral steroids as an adjunct to other treatments for chronic rhinosinusitis.
As the included studies did not report the incidence of adverse events and the risk of side effects may vary according to the condition that they are used to treat, it is important to consider data from similar conditions where possible. A recent review of systemic steroids in acute rhinosinusitis identified five trials including 1193 participants, receiving either oral steroids (prednisolone at dosages ranging from 24 mg to 80 mg for three to seven days) or placebo, where adverse events were reported (Venekamp 2014; Venekamp 2015). There was no difference between the active or control arms in terms of the risk of adverse events, with respect to mild or severe events, or the risk of discontinuation of treatment.
Authors' conclusions
Implications for practice.
The results of this review, current to August 2015, show that there is low quality evidence (we are uncertain about the estimates) to suggest that, for people with chronic rhinosinusitis with nasal polyps, adding oral corticosteroids is beneficial in reducing the size of the polyps and probably also in reducing symptom severity when compared to placebo. The quality of the evidence for adverse effects was very low; some studies did not report these well and there were no data on important longer‐term effects. The results for longer‐term outcomes, which are important to determine whether there is sustained benefit, suggest that the difference between the groups becomes smaller, but the evidence is inconclusive due its low quality. No evidence was found for people with chronic rhinosinusitis without nasal polyps.
Short‐course oral corticosteroids alone for chronic rhinosinusitis have potential short‐term benefits with tolerated side effects. However, the beneficial effect of a short course of treatment is unlikely to persist, hence the need for additional, ongoing topical treatment. Occasional, intermittent courses of oral corticosteroids may have a place within a long‐term treatment strategy. Clear guidance on how short courses of oral corticosteroids can be used alone or as an adjunct to long‐term topical treatment should be provided for use in both primary and secondary care.
Implications for research.
There is clearly room for more trials with adequate outcomes for the population being addressed to further underline the role of short‐term oral steroids, including trials that assess the choice of agent and dosing/duration. There are two scenarios where further research would be very valuable:
Upon entry to secondary care. When patients are initially referred to secondary care, they are likely to have symptoms that have not responded well to the treatment normally prescribed in primary care (intranasal steroids and nasal irrigation). The aim of research would be to determine whether a short course of oral steroid treatment would allow the patient to regain control of their symptoms and whether oral steroids may reduce the need for surgical intervention.
As a rescue medication, when patients who are on existing topical treatments have an exacerbation of symptoms related to chronic rhinosinusitis. The aim of the research would be to determine whether a short course of oral steroids would allow the quick relief of symptoms.
Future research should recruit patients with chronic rhinosinusitis diagnosed using the EPOS 2012 criteria and include both patients with and without nasal polyps (stratified randomisation by subgroup). Oral steroids should be given for between one and three weeks at an appropriate dose. The primary outcomes should be relevant to patients and any disease‐specific instruments should be validated in people with chronic rhinosinusitis. Endoscopic evaluation should not be chosen as a primary outcome because the correlation between endoscopic results and patient symptoms is unclear. Adverse events should be defined in the protocol and measured during treatment and in the follow‐up period.
In addition to measuring outcomes at the end of oral corticosteroid treatment, future trials should follow up patients and measure outcomes for at least six months.
This review is one of a suite of reviews of medical treatments for chronic rhinosinusitis, each of which features its own research recommendations. Across all reviews, key features of future research are as follows:
Trials should be adequately powered and imbalances in prognostic factors (for example, prior sinus surgery) must be accounted for in the statistical analysis.
Study participants should be diagnosed with chronic rhinosinusitis using the EPOS 2012 criteria and should primarily be recruited based on their symptoms. Different patient phenotypes (that is, those with and without nasal polyps) should be recognised and trials should use stratified randomisation within these subgroups or focus on one or other of the phenotypes.
Studies should focus on outcomes that are important to patients and use validated instruments to measure these. Validated chronic rhinosinusitis‐specific health‐related quality of life questionnaires exist, for example the Sino‐Nasal Outcome Test‐22 (SNOT‐22). Patients may find dichotomised outcomes easiest to interpret; for example the percentage of patients achieving a minimal clinically important difference (MCID) or improvement for that outcome. Such MCIDs or cut‐off points should be included in the study protocol and clearly outlined in the methods section.
Trials and other high‐quality studies should use consistent outcomes and adhere to reporting guidelines, such as CONSORT, so that results can be compared across future trials. The development of a standardised set of outcomes, or core outcome set, for chronic rhinosinusitis, agreed by researchers, clinicians and patients, will facilitate this process.
Acknowledgements
This project is one of a suite of reviews on the medical treatment of chronic rhinosinusitis, funded by the National Institute for Health Research (award reference 14/174/03).
This project was also supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We would like to express our thanks to the external peer reviewer, Professor Wytske Fokkens, the consumer referee Joan Blakley and the Cochrane ENT editors for their detailed and insightful comments, which helped to strengthen this review. Thank you also to acting Co‐ordinating Editor, Professor Richard Harvey, for his oversight of this publication.
We are indebted to Samuel MacKeith for translation help for this review.
The authors are grateful for the assistance provided by Jenny Bellorini and Samantha Faulkner, with editorial support and searching for studies.
Appendices
Appendix 1. Search strategies
| CENTRAL | Ovid MEDLINE |
| #1 MeSH descriptor: [Sinusitis] explode all trees #2 MeSH descriptor: [Rhinitis] this term only #3 MeSH descriptor: [Rhinitis, Atrophic] this term only #4 MeSH descriptor: [Rhinitis, Vasomotor] this term only #5 MeSH descriptor: [Paranasal Sinus Diseases] this term only #6 MeSH descriptor: [Paranasal Sinuses] explode all trees #7 rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis #8 kartagener* near syndrome* #9 inflamm* near sinus* #10 (maxilla* or frontal*) near sinus* #11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 #12 MeSH descriptor: [Chronic Disease] explode all trees #13 MeSH descriptor: [Recurrence] explode all trees #14 chronic or persis* or recurrent* #15 #12 or #13 or #14 #16 #11 and #15 #17 CRSsNP #18 (sinusitis or rhinitis) near (chronic or persis* or recurrent*) #19 #16 or #17 or #18 #20 MeSH descriptor: [Nasal Polyps] explode all trees #21 MeSH descriptor: [Nose] explode all trees #22 MeSH descriptor: [Nose Diseases] explode all trees #23 #21 or #22 #24 MeSH descriptor: [Polyps] explode all trees #25 #23 and #24 #26 (nose or nasal or rhino* or rhinitis or sinus* or sinonasal) near (papilloma* or polyp*) #27 rhinopolyp* or CRSwNP #28 #19 or #20 or #25 or #26 or #27 #29 MeSH descriptor: [Steroids] explode all trees #30 MeSH descriptor: [Adrenal Cortex Hormones] explode all trees #31 MeSH descriptor: [Glucocorticoids] explode all trees #32 MeSH descriptor: [Anti‐Inflammatory Agents] explode all trees #33 MeSH descriptor: [Anti‐Inflammatory Agents, Non‐Steroidal] explode all trees #34 #32 not #33 #35 steroid* or glucocorticoid* or corticosteroid* or glucosteroid* or cyclocosteroid* #36 beclomethasone or beclometasone or beclamet or beclocort or becotide #37 betamethasone or betadexamethasone or flubenisolone or celeston* or cellestoderm or betnelan or oradexon #38 dexamethasoneor dexameth or dexone or dexametasone or decadron or dexasone or hexadecadron or hexadrol or methylfluorprednisolone or millicorten #39 flunisolide or fluticasone or hydrocortisone or cortisol or cortifair or cortril or hyrocortone or cortef or epicortisol or efcortesol or Cortisone #40 methylprednisolone or medrol or metripred or urbason #41 mometasone or prednisolone or precortisyl or deltacortril or deltastab or prednesol or deltasone or prednisone or cortan or liquid next pred or meticorten #42 paramethasone or triamcinolone or aristocort or volon or atolone or kenacort or orasone or panasol or prednicen #43 corticoid* or betamethason* or betamethasone or hydrocortison* or celesto* or dexamethason* or hexadecadrol or budesonid* or horacort or pulmicort or rhinocort or methylfluorprednisolone or flunisolid* or nasalide or fluticason* or flonase or flounce or mometason* or nasonex or triamclinolon* or nasacort or tri next nasal or aristocort or Ciclesonide #44 #29 or #30 or #31 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 #45 #28 and #44 |
1 exp Sinusitis/ 2 paranasal sinus diseases/ or rhinitis/ or rhinitis, atrophic/ or rhinitis, vasomotor/ 3 exp Paranasal Sinuses/ 4 (rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis).ab,ti. 5 (kartagener* adj3 syndrome*).ab,ti. 6 (inflamm* adj5 sinus*).ab,ti. 7 ((maxilla* or frontal*) adj3 sinus*).ab,ti. 8 1 or 2 or 3 or 4 or 5 or 6 or 7 9 exp chronic disease/ 10 exp Recurrence/ 11 (chronic or persis* or recurrent*).ab,ti. 12 9 or 10 or 11 13 8 and 12 14 CRSsNP.ab,ti. 15 ((sinusitis or rhinitis) adj3 (chronic or persis* or recurrent*)).ab,ti. 16 13 or 14 or 15 17 exp Nasal Polyps/ 18 exp Nose/ or exp Nose Diseases/ 19 exp Polyps/ 20 18 and 19 21 ((nose or nasal or rhino* or rhinitis or sinus* or sinonasal) adj3 (papilloma* or polyp*)).ab,ti. 22 (rhinopolyp* or CRSwNP).ab,ti. 23 16 or 17 or 20 or 21 or 22 24 exp Steroids/ 25 exp Adrenal Cortex Hormones/ 26 exp Glucocorticoids/ 27 exp Anti‐Inflammatory Agents/ 28 exp Anti‐Inflammatory Agents, Non‐Steroidal/ 29 27 not 28 30 (steroid* or glucocorticoid* or corticosteroid* or glucosteroid* or cyclocosteroid* orbeclomethasone or beclometasone or beclamet or beclocort or becotide or betamethasone or betadexamethasone or flubenisolone or celeston* or cellestoderm or betnelan or oradexon or dexamethasone or dexameth or dexone or dexametasone or decadron or dexasone or hexadecadron or hexadrol or methylfluorprednisolone or millicorten or flunisolide or fluticasone or hydrocortisone or cortisol or cortifair or cortril or hyrocortone or cortef or epicortisol or efcortesol or Cortisone or methylprednisolone or medrol or metripred or urbason or mometasone or prednisolone or precortisyl or deltacortril or deltastab or prednesol or deltasone or prednisone or cortan or liquid next pred or meticorten or paramethasone or triamcinolone or aristocort or volon or atolone or kenacort or orasone or panasol or prednicen).ab,ti. 31 (corticoid* or betamethason* or betamethasone or hydrocortison* or celesto* or dexamethason* or hexadecadrol or budesonid* or horacort or pulmicort or rhinocort or methylfluorprednisolone or flunisolid* or nasalide or fluticason* or flonase or flounce or mometason* or nasonex or triamclinolon* or nasacort or (tri adj3 nasal) or aristocort or Ciclesonide).ab,ti. 32 24 or 25 or 26 or 29 or 30 or 31 33 23 and 32 |
| Ovid EMBASE | Trial registries (via CRS) |
| 1 exp sinusitis/ or paranasal sinus disease/ 2 atrophic rhinitis/ or chronic rhinitis/ or rhinosinusitis/ or vasomotor rhinitis/ 3 exp paranasal sinus/ 4 (rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis).tw. 5 (kartagener* adj3 syndrome*).tw. 6 (inflamm* adj5 sinus*).tw. 7 ((maxilla* or frontal*) adj3 sinus*).tw. 8 1 or 2 or 3 or 4 or 5 or 6 or 7 9 exp chronic disease/ 10 exp recurrent disease/ 11 (chronic or persis* or recurrent*).tw. 12 9 or 10 or 11 13 8 and 12 14 CRSsNP.tw. 15 ((sinusitis or rhinitis) adj3 (chronic or persis* or recurrent*)).tw. 16 13 or 14 or 15 17 exp nose polyp/ 18 exp nose disease/ or exp nose/ 19 exp polyp/ 20 18 and 19 21 ((nose or nasal or rhino* or rhinitis or sinus* or sinonasal) adj3 (papilloma* or polyp*)).tw. 22 (rhinopolyp* or CRSwNP).tw. 23 16 or 17 or 20 or 21 or 22 24 exp *corticosteroid/ 25 exp steroid/ 26 exp antiinflammatory agent/ 27 exp nonsteroid antiinflammatory agent/ 28 26 not 27 29 (steroid* or glucocorticoid* or corticosteroid* or glucosteroid* or cyclocosteroid* or beclomethasone or beclometasone or beclamet or beclocort or becotide or betamethasone or betadexamethasone or flubenisolone or celeston* or cellestoderm or betnelan or oradexon or dexamethasone or dexameth or dexone or dexametasone or decadron or dexasone or hexadecadron or hexadrol or methylfluorprednisolone or millicorten or flunisolide or fluticasone or hydrocortisone or cortisol or cortifair or cortril or hyrocortone or cortef or epicortisol or efcortesol or Cortisone or methylprednisolone or medrol or metripred or urbason or mometasone or prednisolone or precortisyl or deltacortril or deltastab or prednesol or deltasone or prednisone or cortan or liquid next pred or meticorten or paramethasone or triamcinolone or aristocort or volon or atolone or kenacort or orasone or panasol or prednicen).tw. 30 24 or 28 or 29 31 23 and 30 |
ClinicalTrials.gov Condition: rhinitis OR sinusitis OR rhinosinusitis OR (nose AND polyp*) OR (nasal AND polyp*) OR CRSsNP OR CRSwNP OR CRS ICTRP Title: rhinitis OR sinusitis OR rhinosinusitis OR CRSsNP OR CRSwNP OR CR OR All: (nose AND polyp*) OR (nasal AND polyp*) NB These searches were run from 1 March 2015 to 11 August 2015, when these terms were last searched to populate the Cochrane ENT trials register in CRS |
Appendix 2. Data extraction form
| REF ID: | Study title: |
| Date of extraction: | Extracted by: |
| General comments/notes (internal for discussion): |
| Flow chart of trial | ||
| Group A (Intervention) | Group B (Comparison) | |
| No. of people screened | ||
| No. of participants randomised ‐ all | ||
| No. randomised to each group | ||
| No. receiving treatment as allocated | ||
| No. not receiving treatment as allocated ‐ Reason 1 ‐ Reason 2 |
||
| No. dropped out (no follow‐up data for any outcome available) |
||
| No. excluded from analysis1 (for all outcomes) ‐ Reason 1 ‐ Reason 2 |
||
| 1This should be the people who received the treatment and were therefore not considered 'drop‐outs' but were excluded from all analyses (e.g. because the data could not be interpreted or the outcome was not recorded for some reason). | ||
| Information to go into 'Characteristics of included studies' table | |
| Methods | X arm, double/single/non‐blinded, [multicentre] parallel‐group/cross‐over/cluster‐RCT, with x duration of treatment and x duration of follow‐up |
| Participants |
Location: country, no of sites etc. Setting of recruitment and treatment: Sample size:
Participant (baseline) characteristics:
Inclusion criteria:[state diagnostic criteria used for CRS, polyps score if available] Exclusion criteria: |
| Interventions |
Intervention (n = x): drug name, method of administration, dose per day/frequency of administration, duration of treatment Comparator group (n = y): Use of additional interventions (common to both treatment arms): |
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
|
| Funding sources | 'No information provided'/'None declared'/State source of funding |
| Declarations of interest | 'No information provided'/'None declared'/State conflict |
| Notes | |
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Quote: "…" Comment: |
|
| Allocation concealment (selection bias) | Quote: "…" Comment: |
|
| Blinding of participants and personnel (performance bias) | Quote: "…" Comment: |
|
| Blinding of outcome assessment (detection bias) | Quote: "…" Comment: |
|
| Incomplete outcome data (attrition bias) | Quote: "…" Comment: |
|
| Selective reporting (reporting bias) | Quote: "…" Comment: |
|
| Other bias (see section 8.15) Insensitive/non‐validated instrument? |
Quote: "…" Comment: |
|
| Other bias (see section 8.15) | Quote: "…" Comment: |
| Findings of study: continuous outcomes | |||||||
| Results (continuous data table) | |||||||
| Outcome | Group A | Group B | Other summary stats/Notes | ||||
| Mean | SD | N | Mean | SD | N | Mean difference (95% CI), P values etc. | |
| Disease‐specific HRQL (instrument name/range) Time point: |
|||||||
| Generic HRQL (instrument name/range) Time point: |
|||||||
| Symptom score (overall) (instrument name/range) Time point: |
|||||||
|
Added total ‐ if scores reported separately for each symptom (range) Time point: |
|||||||
| Nasal blockage/obstruction/congestion (instrument name/range) |
|||||||
| Nasal discharge (instrument name/range) |
|||||||
| Facial pain/pressure (instrument name/range) |
|||||||
| Smell (reduction) (instrument name/range) |
|||||||
| Headache (instrument name/range) |
|||||||
| Cough (in children) (instrument name/range) |
|||||||
| Polyp size (instrument name/range) |
|||||||
| CT score (instrument name/range) |
|||||||
| Comments: | |||||||
| Results (dichotomous data table) | ||||||
| Outcome | Applicable review/intervention | Group A | Group B | Other summary stats/notes | ||
| No. of people with events | No. of people analysed | No. of people with events | No. of people analysed | P values, RR (95% CI), OR (95% CI) | ||
| Epistaxis/nose bleed | INCS Saline irrigation |
|||||
| Local irritation (sore throat, oral thrush, discomfort) | INCS Saline irrigation |
|||||
| Osteoporosis (minimum 6 months) | INCS | |||||
| Stunted growth (children, minimum 6 months) | INCS | Can also be measured as average height | ||||
| Mood disturbances | OCS | |||||
| Gastrointestinal disturbances (diarrhoea, nausea, vomiting, stomach irritation) |
OCS Antibiotics |
|||||
| Insomnia | OCS | |||||
| Osteoporosis (minimum 6 months) | INCS OCS |
|||||
| Discomfort | Saline irrigation | |||||
| Suspected allergic reaction (rash or skin irritation) | Antibiotics | |||||
| Anaphylaxis or other serious allergic reactions such as Stevens‐Johnson | Antibiotics | |||||
| Comments: | ||||||
Appendix 3. EPOS 2012 diagnostic criteria
Clinical definition of rhinosinusitis in adults
Chronic rhinosinusitis, with or without nasal polyps, in adults is defined as:
inflammation of the nose and the paranasal sinuses characterised by two or more symptoms, one of which should be either nasal blockage/obstruction/congestion or nasal discharge (anterior/posterior nasal drip);
± facial pain/pressure;
± reduction or loss of sense of smell.
for ≥12 weeks.
This should be supported by demonstrable disease.
Either endoscopic signs of:
nasal polyps; and/or
mucopurulent discharge primarily from middle meatus; and/or
oedema/mucosal obstruction primarily in middle meatus;
and/or
-
CT changes:
mucosal changes within the ostiomeatal complex and/or sinuses.
Clinical definition of rhinosinusitis in children
Paediatric rhinosinusitis is defined as: inflammation of the nose and the paranasal sinuses characterised by two or more symptoms, one of which should be either nasal blockage/obstruction/congestion or nasal discharge (anterior/ posterior nasal drip):
± facial pain/pressure;
± cough;
and either endoscopic signs of:
nasal polyps; and/or
mucopurulent discharge primarily from middle meatus; and/or
oedema/mucosal obstruction primarily in middle meatus;
and/or
-
CT changes:
mucosal changes within the ostiomeatal complex and/ or sinuses.
Appendix 4. Nasal polyp grading scales
| Grade | Ecevit 2015 | Lildholdt scale: Alobid 2014; Benitez 2006; Vaidyanathan 2011 | Van Zele 2010 | Kirtsreesakul 2012 |
| 0 | No polyps | No nasal polyps | No polyps | No polyps |
| 1 | Mild polyposis (small polyps not reaching the upper edge of the inferior turbinate) | Small polyps confined to the middle meatus | Small polyps in the middle meatus not reaching below the inferior border of the middle concha | Mild polyposis (small polyps, extending downward from the upper nasal cavity but not below the upper edge of the inferior turbinate, causing only slight obstruction) |
| 2 | Moderate polyposis (medium polyps between the upper and lower edges of the inferior turbinate) | Moderate sized polyps not crossing the lower edge of the inferior turbinate | Polyps reaching below the lower border of the middle turbinate | Moderate polyposis (medium sized polyps, extending downward from the upper nasal cavity and reaching between the upper and lower edges of the inferior turbinate, causing troublesome obstruction) |
| 3 | Severe polyposis (large polyps reaching the lower edge of the inferior turbinate, polyps from posterior ethmoidal sinuses, or both) | Large polyps crossing the lower edge of the inferior turbinate | Large polyps reaching the lower border of the inferior turbinate or polyps medial to the middle concha | Severe polyposis (large‐sized polyps, extending downward from the upper nasal cavity and reaching below the lower edge of the inferior turbinate, causing total or almost total obstruction) |
| 4 | — | — | Large polyps causing almost complete congestion/obstruction of the inferior meatus | — |
Data and analyses
Comparison 1. Oral steroids versus no treatment/placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease‐specific health‐related quality of life ‐ no pooling (2 to 3 weeks) | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Severity score of RSOM | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.24 [‐1.92, ‐0.56] |
| 1.2 Mini‐RQLQ | 1 | 58 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.79 [‐1.32, ‐0.25] |
| 2 Disease‐specific health‐related quality of life ‐ RQLQ (3 to 6 months) | 1 | 50 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐1.16, ‐0.02] |
| 3 Disease severity (patient‐reported total symptom score) | 4 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Final value (2 to 3 weeks) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Change from baseline (2 to 3 weeks) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Final value (3 to 6 months) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Change from baseline (3 to 6 months) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Individual symptoms: nasal obstruction (final value) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐4.5 [‐6.42, ‐2.58] |
| 5 Individual symptoms: nasal obstruction (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Change from baseline (2 to 3 weeks) | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐38.02 [‐48.16, ‐27.88] |
| 5.2 Change from baseline (3 to 6 months) | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐8.97, 10.77] |
| 6 Individual symptoms: nasal discharge (final value) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐4.7 [‐6.79, ‐2.61] |
| 7 Individual symptoms: nasal discharge (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 2 to 3 weeks | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐55.57 [‐69.23, ‐41.91] |
| 7.2 3 to 6 months | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐1.83 [‐13.46, 9.81] |
| 8 Individual symptoms: facial pressure (final value) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐3.7 [‐6.02, ‐1.38] |
| 9 Individual symptoms: facial pressure (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 2 to 3 weeks | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐30.66 [‐46.28, ‐15.04] |
| 9.2 3 to 6 months | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐12.56, 13.76] |
| 10 Individual symptoms: loss of sense of smell (final value) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 2 to 3 weeks | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐2.79 [‐4.11, ‐1.47] |
| 10.2 3 to 6 months | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.68, 0.28] |
| 11 Individual symptoms: loss of sense of smell (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 2 to 3 weeks (after treatment) | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐44.35 [‐57.31, ‐31.39] |
| 11.2 3 to 6 months | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐15.05 [‐29.69, ‐0.41] |
| 12 Adverse events ‐ significant mood disturbance | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.55, 11.41] |
| 13 Adverse events ‐ gastrointestinal disturbance | 3 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.45 [1.11, 10.78] |
| 14 Adverse events ‐ insomnia | 3 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.63 [1.10, 11.95] |
| 15 Endoscopy score ‐ nasal polyps (final value) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1 2 to 3 weeks | 4 | 253 | Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐0.92, ‐0.61] |
| 15.2 3 to 6 months | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.62, 0.12] |
| 16 Endoscopy score ‐ nasal polyps score (change from baseline) | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.1 2 to 3 weeks | 2 | 146 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.77 [‐2.16, ‐1.38] |
| 16.2 3 to 6 months | 1 | 114 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.90, ‐0.14] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alobid 2014.
| Methods | 2‐arm, non‐blinded, parallel‐group RCT, with a 2‐week duration of treatment and follow‐up | |
| Participants |
Location: Spain, 1 site Setting of recruitment and treatment: rhinology and smell clinic, department of otorhinolaryngology, hospital clinic, Barcelona Sample size: Number randomised: 92 (it is unclear if these were all randomised or if the 3 drop‐outs occurred prior to randomisation) Number completed: 67 in intervention group, 22 in comparison group Participant (baseline) characteristics:
Other important effect modifiers:
Inclusion criteria: diagnosis of bilateral nasal polyps was based on the EPOS criteria: "Presence of two or more nasal symptoms, one of which should be either nasal blockage or nasal discharge, and/or the reduction/loss of sense of smell, and/or facial pain for more than 12 weeks, and/or the presence of nasal polyps by nasal endoscopy or mucosal changes within the ostiomeatal complex, and/or paranasal sinuses by computed tomography (CT) scan" Exclusion criteria: none listed |
|
| Interventions |
Intervention (n = 67): oral prednisone (30 mg daily for 4 days followed by a 2‐day reduction of 5 mg) and intranasal budesonide 800 μg daily (400 μg twice daily) for 2 weeks Comparator group (n = 22): no corticosteroid treatment for 2 weeks Use of additional interventions (common to both treatment arm): both groups had a 4‐week washout period for intranasal and oral steroids. No other adjunct treatment is listed. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes
Secondary outcomes
Other outcomes reported by the study:
|
|
| Funding sources | "This article was partially sponsored by a research project from Fondo de Investigacion Sanitaria (FIS 99–0133), Instituto de Salud Carlos III." There was no information regarding the funding of the original study. | |
| Declarations of interest | No information is provided in the paper | |
| Notes | In the group receiving oral steroids, the treatment period was followed up by a 10‐week course of INCS. The "no steroids treatment" group were not followed up after 2 weeks and so these results have not been presented. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…randomised…" Comment: no information about the randomisation procedures for the trial |
| Allocation concealment (selection bias) | Unclear risk | Comment: there is no information about the allocation concealment. Given the trial was not blinded, there is reason to believe that the study personnel may have been able to influence the groups into which participants were allocated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: the trial does not appear to be blinded to patients or study personnel. The comparison group had no steroid treatment and did not receive placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: the main outcomes were subjective outcomes rated by patients. There was no mention of blinding of outcome assessors (for endoscopy) included in the paper. Since the control arm received no treatment, the risk of bias is high. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: paper indicates that 92 patients were recruited but only 89 patients are accounted for in the analysis. The 3 drop‐outs are identified but there is no indication if they dropped out prior to randomisation or after randomisation. The impact of the 3 drop‐outs (3%) is likely to be small. |
| Selective reporting (reporting bias) | Unclear risk | Comment: adverse event data are not presented in the paper. No protocol for the trial could be found. |
| Other bias | Unclear risk | Comment: only details of the validation of the smell test used in the study (BAST‐24) were provided. No details of validation for other outcomes (e.g. 0‐ to 3‐point Likert scale used to assess nasal congestion). |
Benitez 2006.
| Methods | 2‐arm, non‐blinded, parallel‐group RCT, with 2‐week duration of treatment and follow‐up | |
| Participants |
Location: Spain, 1 site Setting of recruitment and treatment: rhinology unit, ENT department, hospital clinic, Barcelona Sample size: 84 Number randomised: 63 in intervention group, 21 in comparison group Number completed: 63 in intervention group, 21 in comparison group Participant (baseline) characteristics:
Other important effect modifiers:
Inclusion criteria: diagnosis of severe nasal polyps based on nasal endoscopic examination (mean score of 2.7 over 3 using the Lildholdt score) Nasal polyps score: 0 – no polyps; 1 – mild polyposis; 2 – moderate polyposis; 3 – severe polyposis Exclusion criteria: patients with a steroid contraindication |
|
| Interventions |
Intervention (n = 63): oral prednisone, 30 mg daily for 4 days followed by 2‐day reduction of 5 mg, total duration 14 days Comparator group (n = 21): no steroid treatment Use of additional interventions (common to both treatment arms): asthmatic patients did not modify their treatment during the study. No patients were receiving treatment with leukotriene antagonists. (Note: both groups had a 4‐week washout period for intranasal and oral steroids) |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes
Other outcomes reported by the study:·
|
|
| Funding sources | "Generalitat de Catalunya (2001SGR00384), Red RESPIRA (FIS, V‐2003‐REDC11D‐0) and Fondo de Investigaciones Sanitarias (99‐3121m PI020329)" | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: study design details that the study was "randomised" but no further details were provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details about allocation concealment were provided in the paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: the control group received no steroid treatment or placebo. No mention of blinding of participants or personnel was included in the paper. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: the main outcomes were subjective outcomes rated by patients. There was no mention of blinding of outcome assessors (for endoscopy) included in the paper. Since the control arm received no treatment, the risk of bias is high. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there was no mention of anyone who dropped out of the trial or had to discontinue for any reason. However, they also did not state how many patients were analysed for each outcome. |
| Selective reporting (reporting bias) | Unclear risk | Comment: adverse event data are not presented in the paper. No protocol for the trial could be found. |
| Other bias | Unclear risk | Comment: no information is provided about the validation of the symptom scores used |
Ecevit 2015.
| Methods | 2‐arm, double‐blind, parallel‐group RCT, with a 17‐day duration of oral steroid treatment | |
| Participants |
Location: Turkey, 1 site Setting of recruitment and treatment: Department of Otorhinolaryngology ‐ Head and Neck Surgery, Dokuz Eylul University Hospital Sample size: 23 Number randomised: 11 in intervention group, 12 in comparison group Number completed: 10 in intervention group, 12 in comparison group Participant (baseline) characteristics:
Inclusion criteria: nasal polyps, age between 18 and 65 years, endoscopic stage II or III nasal polyposis patients Nasal polyps score: graded according to the following criteria: 0: no polyp; 1: mild polyposis (small polyps not reaching the upper edge of the inferior turbinate); 2: moderate polyposis (medium polyps between the upper and lower edges of the inferior turbinate); 3: severe polyposis (large polyps reaching the lower edge of the inferior turbinate, polyps from posterior ethmoidal sinuses, or both) Exclusion criteria: hypertension, type I or II diabetes mellitus, the signs of systemic infection, pregnancy or lactation, any type of M. tuberculosis infection, peptic ulcer, viral infection (measles, chicken pox or ocular herpes), myasthenia gravis, stage I nasal polyposis, aspirin intolerance, previous major head trauma |
|
| Interventions |
Intervention (n = 11): prednisolone, oral, 60 mg/day (6 tablets per day) for 7 days, then reduced to 10 mg (1 tablet) taken every other day, stopping on day 17 Comparator group (n = 12): placebo, 6 tablets per day for 7 days, then reduced to 1 tablet every other day, stopping on day 17 Use of additional interventions (common to both treatment arms): none listed |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes
Other outcomes reported by the study:
|
|
| Funding sources | "The authors have no funding, financial relationships, or conflicts of interest to disclose" | |
| Declarations of interest | "The authors have no funding, financial relationships, or conflicts of interest to disclose" | |
| Notes | The patients who met the inclusion criteria gave informed consent and were prescribed fluticasone nasal drops 1 x/day, 200 mg, for 6 weeks. Patients who did not respond to this medical treatment were evaluated for the study. All patients in the trial underwent surgery between the 15th and 17th day. Outcomes presented for this review are the pre‐operative results. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Every eight boxes, including four study drug boxes and four placebo drug boxes, respectively, were assembled as a group by the pharmacy department." Comment: randomisation in blocks of 8 (pg 2042, col 1, para 6) |
| Allocation concealment (selection bias) | Low risk | Quote: "…The hospital pharmacy department performed the drug/placebo randomization, and the identity of the contents in the boxes was not disclosed to any clinicians interacting with patients throughout the study" Comment: pg 2042, col 1, para 6 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "…the identity of the contents in the boxes was not disclosed to any clinicians interacting with patients throughout the study" "The surgeon was blinded to the patient treatment group" Comment: pg 2042, col 1, para 6/7. It is unclear whether the placebo tablets provide adequate masking in terms of taste, since prednisolone is bitter and may be recognisable to patients. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: from the flowchart on pg 2042 it appears that the codes were broken after all of the data had been collected |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only 1 patient did not complete the study (4.3%). The drop‐out reason was that the patient was not given the study medication after surgery. No intention‐to‐treat analysis was completed. |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes appear to be reported well in the paper. No protocol for the trial could be found. |
| Other bias | Unclear risk | No information about the validation of any outcomes The mean age of participants in the oral steroid group was 45.6 ± 11.5 and the mean age in the control group was reported as 26.6 ± 43.6. This may be a reporting error since the range in the control group is 26 to 58 years. |
Hissaria 2006.
| Methods | 2‐arm, double‐blinded, parallel‐group RCT, with 14 days duration of treatment follow‐up | |
| Participants |
Location: Australia, unclear number of sites Setting of recruitment and treatment: allergy outpatient clinics Sample size: Number randomised: 20 in intervention group, 21 in comparison group Number completed: 20 in intervention group, 20 in comparison group Participant (baseline) characteristics:
Other important effect modifiers:
Inclusion criteria: aged 18 to 65 years drawn mainly from allergy outpatient clinics who had symptomatic polyp disease diagnosed on nasoendoscopy Exclusion criteria: previous use of oral steroids, unstable asthma, recent sinus surgery, acute infection within 1 month of recruitment, polyps caused by cystic fibrosis or mucociliary disorders, diabetes mellitus, cataract, glaucoma, fungal sinusitis, contraindications for MRI scanning, or any other significant comorbid condition that contraindicated the use of systemic corticosteroids |
|
| Interventions |
Intervention (n = 20): prednisolone, 50 mg/day for 14 days Comparator group (n = 21): placebo, for 14 days Use of additional interventions (common to both treatment arms): participants were allowed to continue the use of regular antihistamines (13/40), topical corticosteroids (22/40), or both |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes
Secondary outcomes:
Other outcomes reported by the study:
|
|
| Funding sources | No information provided | |
| Declarations of interest | "P. Wormwald receives royalties from Medtronic Xomed for instruments designed. The rest of the authors have declared that they have no conflict of interest." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "… randomized by the hospital pharmacy to receive the study medication ..." Comment: pg 129, col 1, para 2 No information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "These patients… were blinded to their treatment status"; "study personnel were not informed of the patient’s treatment status until all assessments were completed" Comment: pg 129, col 1, para 2. It is unclear whether the placebo tablets provide adequate masking in terms of taste, since prednisolone is bitter and may be recognisable to patients. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "study personnel were not informed of the patient's treatment status until all assessments were completed" Comment: pg 129, col 1, para 2 Blinding of nasoendoscopy scans (pg 129, col 2, para 3) were well documented when describing the outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 1 patient did not complete the study and their data were not included in the analysis |
| Selective reporting (reporting bias) | High risk | Comment: nasoendoscopy findings were reported inconsistently within the paper using differing criteria which had not been pre‐specified in the methods section. There was concern that the cut‐off points for reporting could have been chosen after the results were available. In addition, some of the scales used are unclear. No information is provided for the modified RSOM‐31 instrument or the RSOM‐31 nasal subscale. No protocol for the trial could be found |
| Other bias | High risk | The RSOM‐31 was modified, without further evidence of validation, and it is unclear whether the methods for measuring change in polyps size were validated |
Kapucu 2012.
| Methods | 4‐arm, unblinded, parallel‐group RCT, with unclear duration of treatment and 7‐day duration of follow‐up | |
| Participants |
Location: Turkey, unclear number of sites (probably 1) Setting of recruitment and treatment: Department of Otorhinolaryngology, GATA medical faculty, Ankara Sample size:
Participant (baseline) characteristics: (based on all 4 groups)
Inclusion criteria: Nasal polyps diagnosed by computed tomography Exclusion criteria: past surgeries for nasal polyps, any glucocorticoid usage for any reason within 1 month, nasal polyp that was not eosinophilic nasal polyp according to the pathology study, fungal chronic sinusitis, age younger than 15 years, Churg‐Strauss syndrome, immunodeficiency, Kartagener's syndrome, Young's syndrome, cystic fibrosis, antrochoanal polyp and unilateral nasal polyp. Additional exclusion criteria were any contraindications for steroid treatment (such as glaucoma, peptic ulcer, acute psychosis, herpetic keratitis, chronic infections, severe osteoporosis, severe hypertension, uncontrolled diabetes mellitus, thromboembolic predisposition, newly formed bowel anastomosis, diverticulitis and Cushing's syndrome) |
|
| Interventions |
Intervention (n = 12): oral methylprednisolone (Prednol 16 mg tablet, Prednol 4 mg tablet; Mustafa Nevzat Pharmaceutical, Istanbul, Turkey), 1 mg/kg/day. The dose was applied for 3 days and tapered gradually, with a reduction rate of 8 mg/3 days. The duration of drug use varied for each patient changing according to his or her weight. Comparator group (n = 12): no medication was given Use of additional interventions (common to both treatment arm): no information |
|
| Outcomes | No primary, secondary or adverse events were reported Other outcomes reported by the study:
|
|
| Funding sources | "None" | |
| Declarations of interest | Competing interests "None"; sponsorships "None" | |
| Notes | 2 of the 4 groups within the study were not recorded in this data extraction. The interventions in the 2 additional groups were: intra‐polyp injection and INCS alone (for 30 days). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…randomly assigned… " Comment: pg 564, col 1, para 3 |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no medication was given to the comparator group, implying there was no placebo used. It is assumed that the study is not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information, but the study only measures objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no information. The paper implies that all patients completed treatment and were recorded in the outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no outcomes directly relevant to patients were reported. No protocol for the trial could be found. |
| Other bias | Unclear risk | Comment: no information about the validation of outcomes |
Kirtsreesakul 2012.
| Methods | 2‐arm, double‐blind, parallel‐group RCT, with 14 days oral steroid treatment duration and 12‐week follow‐up period | |
| Participants |
Location: 1 site, Thailand Setting of recruitment and treatment: Allergy and Rhinology Clinic, Prince of Songkla University Sample size: 117 Number randomised: 69 in intervention group, 48 in comparison group Number completed: 67 in intervention group, 47 in comparison group Participant (baseline) characteristics:
Inclusion criteria: patients with benign bilateral nasal polyps diagnosed clinically and confirmed by nasal endoscopy Nasal polyps score: graded on a 4‐point scale (0 to 3): 0 ‐ no polyps, 1 – mild polyposis (small polyps, extending downward from the upper nasal cavity but not below the upper edge of the inferior turbinate, causing only slight obstruction), 2 – moderate polyposis (medium‐sized polyps, extending downward from the upper nasal cavity and reaching between the upper and lower edges of the inferior turbinate, causing troublesome obstruction), 3 – severe polyposis (large‐sized polyps, extending downward from the upper nasal cavity and reaching below the lower edge of the inferior turbinate, causing total or almost total obstruction). The total nasal polyps score was calculated as the sum of the polyps scores for each nostril. Exclusion criteria: patients with symptoms or physical signs suggestive of renal disease, hepatic disease, diabetes mellitus, cataract, glaucoma, cardiovascular disease, unstable asthma, cystic fibrosis, mucociliary disorders, immunocompromise, severe septal deviation or acute infection within the previous 2 months. Patients who had used nasal, inhaled or systemic steroids within 2 months; an antihistamine within 2 to 7 days; and/or a decongestant within 2 days or had had previous sinonasal surgery were also excluded. |
|
| Interventions |
Intervention (n = 67): oral prednisolone 50 mg daily for 14 days Comparator group (n = 47): placebo tablet daily for 14 days Use of additional interventions (common to both treatment arms): at the end of the "test treatment" stage all patients were then treated with administration of mometasone furoate nasal spray (MFNS) at 200 µg twice daily for 10 weeks Medications for rhinitis or allergy or nasal saline irrigation were not allowed during their participation in the study |
|
| Outcomes | Primary outcomes
Secondary outcomes
Other outcomes reported by the study:
|
|
| Funding sources | "Funded by the Faculty of Medicine, Prince of Songkla University" | |
| Declarations of interest | "The authors have no conflicts of interest to declare pertaining to this article" | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned at a 3:2 ratio" Comment: pg 456, col 1, last para. There is no information regarding the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The study personnel were not informed of the treatment modality of the patients until all assessments were completed" Comment: no details on how the patients were allocated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "... prednisolone or placebo", "… were blinded to their treatment regimen." (pg 455, col 2, last para) "The study personnel were not informed of the treatment modality of the patients until all assessments were completed" (pg 456, col 2, para 1) Comment: it is unclear whether the placebo tablets provide adequate masking in terms of taste, since prednisolone is bitter and may be recognisable to patients |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The study personnel were not informed of the treatment modality of the patients until all assessments were completed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: low numbers of patients dropped out – unlikely to affect the overall result |
| Selective reporting (reporting bias) | Low risk | Comment: appears that all of the outcomes listed were adequately reported in the paper. No protocol for the trial could be found. |
| Other bias | Unclear risk | Comment: no information about the validation of the "total nasal symptom score" |
Vaidyanathan 2011.
| Methods | 2‐arm, double‐blind, parallel‐group RCT, with 2‐week duration of oral steroid treatment, followed by a 6‐month duration of intranasal steroid treatment and duration of follow‐up | |
| Participants |
Location: Scotland, 1 site Setting of recruitment and treatment: speciality referral clinic Sample size: Number randomised: 30 in intervention group, 30 in comparison group Number completed: 27 in intervention group, 24 in comparison group Participant (baseline) characteristics:
Other important effect modifiers
Inclusion criteria: diagnosis of CRS with nasal polyposis made on the basis of the EPOS 2007 criteria Inclusion criteria were the presence on nasoendoscopy of bilateral moderate‐sized to large nasal polyps (grade > 1) according to the Lildholdt scale and at least 2 of anterior or posterior nasal discharge, nasal obstruction or decreased sense of smell for more than 12 weeks Lildholdt nasal polyps scale: 0, no nasal polyps; 1, small polyps confined to the middle meatus; 2, moderate sized polyps not crossing the lower edge of the inferior turbinate; 3, large polyps crossing the lower edge of the inferior turbinate Exclusion criteria: exclusion criteria included treatment with an oral corticosteroid in the past 3 months, sinus surgery in the past year, recent upper respiratory tract infection, mechanical nasal airway obstruction of more than 50% due to septal deviation, or pregnancy or lactation |
|
| Interventions |
Intervention (n = 30): prednisolone tablets, 25 mg/day, 2 weeks Comparator group (n = 30): placebo tablets, daily, 2 weeks Use of additional interventions (common to both treatment arm): All patients underwent a 2‐week 'run‐in' period prior to the trial during which therapy for CRS with nasal polyps was stopped. After the 2‐week oral steroid treatment period both study arms received fluticasone propionate nasal drops, 400 µg twice daily, for 8 weeks then fluticasone propionate nasal spray, 200 µg twice daily for a further 18 weeks No other rhinitis medications were permitted, including antihistamines, leukotriene receptor antagonists, intranasal corticosteroids or nasal decongestants. No antibiotics were permitted during the study. |
|
| Outcomes | Primary outcomes
Secondary outcomes
Other outcomes reported by the study:
|
|
| Funding sources | "Chief Scientist Office, Scotland; National Health Service Tayside Small Grants Scheme; and an Anonymous Trust grant from University of Dundee." | |
| Declarations of interest | The link from the paper to the website does not appear to list any declarations of interests | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent, off‐site clinical trials pharmacist (Pharmacy Production Unit, Western Infirmary, Glasgow, United Kingdom) used a computer‐generated random allocation sequence to randomize the trial, using block randomization with a block size of 4." Comment: pg 294, col 2, para 5 |
| Allocation concealment (selection bias) | Low risk | Quote: "Tablets were distributed in sealed opaque envelopes at the research unit, in sequential order, by a laboratory technician who was not directly involved with the study." Comment: pg 294, col 2, para 5 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The same pharmacist masked and blinded the 25‐mg prednisolone tablet and an identical placebo tablet to double‐blind the study from the investigator and participants." (pg 294, col 2, para 5) "Three patients in the prednisolone group and 4 in the placebo group had previously received oral steroids" (pg 297, col 2) Comment: it is unclear whether the placebo tablets provide adequate masking in terms of taste, since prednisolone is bitter and may be recognisable to patients |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Standard video sequences were stored on a computer and viewed by 2 independent observers, who were blinded to patient, treatment, and sequence. Disagreements were resolved by discussion." Comment: pg 295, col 2, para 1 Patients and the main outcome assessors should remain adequately blinded throughout. The other outcomes were assessed by 2 blinded outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "… We included all patients who received the allocated intervention in the analysis" Comment: pg 295, col 2, para 3 5 patients discontinued after the 2‐week treatment with oral corticosteroids; 4 of these were in the oral steroids group. Another 2 dropped out from each group by the 3‐month follow‐up (total 9/60, 15% overall; 10% in treatment group, 20% in control group) The results table gives different numbers of participants included in each analysis, which are closer to the number of patients available, rather than patients randomised. It is unclear why there is a discrepancy. |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes were reported in the results as described in the methods section. The methods for collecting data for adverse events (other than biological assays) were not reported. The paper reports that no oral steroid‐specific adverse events were reported, but it is unclear whether the patients were specifically asked. The protocol document was available (NCT00788749) and the outcomes appear to be consistent between the protocol and the paper. |
| Other bias | Unclear risk | Comment: there is no information about the total symptom score (e.g. validation) Study used the RQLQ (Rhinoconjunctivitis Quality of Life Questionnaire), which is validated for patients with allergy. Many of the items are not relevant for CRS patients, while items related to smell and sinonasal pain were not included. |
Van Zele 2010.
| Methods | 3‐arm, double‐blind, multicentre, parallel‐group RCT, with 20 days duration of treatment and 12 weeks duration of follow‐up | |
| Participants |
Location: 5 sites in Belgium, Germany, Holland and Australia Setting of recruitment and treatment: not given Sample size: 47 Number randomised: 14 in oral steroids, 19 in placebo Number completed: 14 in oral steroids, 12 in placebo Participant (baseline) characteristics:
Other important effect modifiers:
Inclusion criteria: Participants had to be at least 18 years with a diagnosis of bilateral nasal polyps at screening and baseline that have recurred after surgical resection or nasal polyps that are grades 3 or 4 in both nares using the polyp scoring system. Women of childbearing potential had to use a medically acceptable form of birth control as defined by the study. Male participants had to agree to use an adequate form of birth control for the duration of the study as defined by the study. Participants with concurrent asthma had to be maintained on no more than 1000 µg/day beclomethasone dipropionate or the equivalent. Nasal polyp score: 0 ‐ no polyp; 1 ‐ small polyps in the middle meatus not reaching below the inferior border of the middle concha; 2 ‐ polyps reaching below the lower border of the middle turbinate; 3 ‐ large polyps reaching the lower border of the inferior turbinate or polyps medial to the middle concha, 4 ‐ large polyps causing almost complete congestion/obstruction of the inferior meatus Exclusion criteria: the following are exclusion criteria for the study: pregnancy, breast‐feeding or premenarcheal; oral corticosteroids within the 3 months before screening; systemic fungoid infections; known allergic reaction on methylprednisolone or tetracyclines; hypertension; diabetes (type 1 and 2); glaucoma; tuberculosis; herpes infection; zona ophthalmica; antineutrophil cytoplasmic antibodies such as Wegener granulomatosis, Churg‐Strauss syndrome and microscopic polyangiitis Participants with acute sinusitis or concurrent nasal infection or participants who have had a nasal or upper respiratory tract infection within 2 weeks of the screening visit; cystic fibrosis, primary ciliary dysfunction or Kartagener syndrome by history; those diagnosed with a parasitic infection; HIV‐positive or positive to hepatitis B surface antigen or C antibodies. Participants must not have had an acute asthmatic attack requiring admission to a hospital (excluding emergency department visits that resulted in direct discharge without hospitalisation) within the 4 weeks before screening Participants must not have received immunotherapy within the previous 3 months |
|
| Interventions |
Intervention (n = 14): oral methylprednisolone (32 mg/day on days 1 to 5; 16 mg/day on days 6 to 10; 8 mg/day on days 11 to 20) Control (n = 19): placebo, unlabelled lactose capsules, 20 days Use of additional interventions (common to all treatment arm): Systemic or local corticosteroids or antibiotics were not allowed; if necessary nasal corticosteroids were permitted as rescue medication 2 months after dosing with the study medication |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
|
|
| Funding sources | "Supported by a grant from the Flemish Scientific Research Board, FWO Nr. A12/5‐HBKH 3 (holder of a Fundamenteel Klinisch Mandaat), by a postdoctoral grant from the Research Foundation Flanders (FWO), and by postdoctoral mandate from the Research Foundation Flanders (FWO)." | |
| Declarations of interest | "Disclosure of potential conflict of interest: P. J. Wormald has received royalties from Medtronic ENT, is a consultant for NeilMed, and has received research support from the Garnett Passe and Rodney Williams Foundation. W. Fokkens has received research support from GlaxoSmithKline and Stallergenes. A. Beule has received research support from the European Union. The rest of the authors have declared that they have no conflict of interest." | |
| Notes | 3rd arm of the study was antibiotics (doxycycline). Results for this arm of the study are included in Head 2016b. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were randomly assigned to 3 groups by individuals not involved in the study." Comment: pg 1070, col 1, para 3. No information was provided about how the sequence was generated. The number randomised was small and there is a risk that it was not balanced (14 versus 19) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "… patients were randomly assigned to 3 groups by individuals not involved in the study" Comment: pg 1070, col 1, para 3 There is no information about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "… double‐blind… " "... Placebo (lactose) in unlabelled capsules" Comment: pg 1069, abstract: methods, pg 1070 methods Details of blinding not clear within the paper and it does not detail whether the placebo (and antibiotic) medications were given on the same dosing schedule with medication in an identical form |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Study participants and personnel were blind during the duration of the study. Randomisation codes were revealed to researchers after recruitment,data collection, and data entry" Comment: details of blinding not clear within the paper and it is not clear whether the oral steroids and antibiotic medications were given on the same dosing schedule and were an identical form, which could compromise blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: only 7/47 patients dropped out of the study (14.9%) but all were from the placebo group 7/19 (36.8%). This is an imbalance in drop‐out rate and the reasons for drop‐out include "unsatisfactory therapeutic effects", "withdrawal of consent" and "serious adverse events (asthma attack)". Patients who dropped out were still included in the analysis using the last observed carried forward. This may have had an effect on the overall results and no sensitivity analysis appears to have been completed to identify the impact. |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes in the methods section were reported in the full paper, although many of them were presented graphically, without providing values at key time periods. The data were not reported in a way that is sufficient to be included in the meta‐analysis of the review. The protocol document was available (NCT00480298) and the outcomes appear to be consistent between the protocol and the paper |
| Other bias | Unclear risk | Comment: details of the scales used to measure symptoms were not provided in the paper and there is no information on validation of the outcomes There was an imbalance in the number of participants with "allergy" (oral steroids: 35.7; placebo: 57.9%; antibiotics: 14.3%) and "aspirin intolerant" in the baseline characteristics (oral steroids: 14.3%; placebo: 26.3%; antibiotics: 7.1%). This was not a statistical difference between the groups due to the study size being small. A sensitivity analysis was completed by the study authors to determine if this affected the results. |
CRS: chronic rhinosinusitis CT: computerised tomography ENT: ear, nose and throat EPOS: European Position Paper on Rhinosinusitis and Nasal Polyps 2012 F: female INCS: intranasal corticosteroids M: male MFNS: mometasone furoate nasal spray MRI: magnetic resonance imaging RCT: randomised controlled trial RSOM‐31: Rhinosinusitis Outcome Measures‐31 SD: standard deviation SEM: standard error of the mean
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alobid 2005 | INTERVENTION: oral steroids versus surgery |
| Blomqvist 2001 | INTERVENTION: surgery |
| Blomqvist 2009 | INTERVENTION: combined medical and surgical treatment |
| Bonfils 1998 | STUDY DESIGN: not randomised |
| Bonfils 2003 | STUDY DESIGN: not randomised |
| Bonfils 2006 | STUDY DESIGN: not randomised |
| Bülbül 2013 | INTERVENTION: both arms of the study received intranasal steroids |
| Chi Chan 1996 | STUDY DESIGN: not randomised |
| Damm 1999 | INTERVENTION: oral steroid (12 days) + INCS versus oral steroid (20 days) + INCS |
| Grammer 2013 | STUDY DESIGN: review of previous oral steroids trials |
| Hessler 2007 | STUDY DESIGN: not randomised |
| Jankowski 2003a | STUDY DESIGN: not randomised |
| Jankowski 2003b | STUDY DESIGN: not randomised |
| Kroflic 2006 | INTERVENTION: endoscopic polypectomy with ethmoidectomy |
| Lildholdt 1988 | INTERVENTION: surgical removal versus systemic corticosteroids |
| Lildholdt 1989 | INTERVENTION: surgical polypectomy followed by continuous topical steroid treatment versus a single dose of depot steroid |
| NCT01676415 | INTERVENTION: oral steroid versus intranasal steroids; both arms of the study received antibiotics Ongoing study |
| Nores 2003 | STUDY DESIGN: not randomised |
| Ozturk 2011 | INTERVENTION: oral steroids versus placebo; all patients in both arms received antibiotics |
| Ragab 2006 | INTERVENTION: medical versus surgical treatment |
| Rasp 1997 | STUDY DESIGN: not randomised |
| Rasp 2000 | STUDY DESIGN: not randomised |
| Remer 2005 | STUDY DESIGN: not randomised |
| Reychler 2015 | INTERVENTION: oral steroid versus INCS; duration of follow‐up less than 3 months |
| Rupa 2010 | POPULATION: allergic fungal sinusitis |
| Sieskiewicz 2006 | STUDY DESIGN: surgical outcomes paper |
| Sousa 2009 | STUDY DESIGN: not randomised |
| Stevens 2001 | STUDY DESIGN: not randomised |
| Tuncer 2003 | STUDY DESIGN: not randomised |
| van Camp 1994 | STUDY DESIGN: not randomised |
INCS: intranasal corticosteroids
Characteristics of ongoing studies [ordered by study ID]
Chi 2011.
| Trial name or title | ChiCTRTRC11001323: Research on clinical efficacy of oral glucocorticoid in the treatment of eosinophilic nasal polyps and non‐eosinophilic nasal polyps |
| Methods | Randomised, parallel‐group, controlled trial |
| Participants | Chronic sinusitis with nasal polyps |
| Interventions | Oral prednisolone tablets versus placebo |
| Outcomes | VAS score, Lanza‐Kennedy nasal endoscopy score |
| Starting date | January 2011 |
| Contact information | Ming Zeng, Department of Otorhinolaryngology Head and Neck Surgery, Tongji Hospital of Tongji Medical College of
Huazhong University of Science and Technology, 430030 Email: zmsx77@163.com |
| Notes | No response from study authors |
NCT00841802.
| Trial name or title | NCT00841802: Chronic rhinosinusitis with or without nasal polyps steroid study |
| Methods | Open‐label, parallel‐group randomised controlled trial |
| Participants | Diagnosis of chronic rhinosinusitis with or without nasal polyps and undergoing sinonasal surgery for this condition No diagnosis of CRS and NP and undergoing nasal surgery (septoplasty/rhinoplasty, nasal fracture repair, etc.) |
| Interventions | Prednisone versus no intervention |
| Outcomes | Alterations of inflammatory cells, levels of key antibodies and cytokines, and expression of key epithelial genes |
| Starting date | February 2009 |
| Contact information | Robert P Schleimer, PhD; email: rpschleimer@northwestern.edu Kathleen E Harris, BS; email: keharris@ northwestern.edu |
| Notes | Authors responded to enquiry to say that the study is still in the process of being completed |
NCT02367118.
| Trial name or title | NCT02367118: Prednisone in chronic rhinosinusitis without nasal polyps |
| Methods | Double‐blind, parallel‐group randomised controlled trial |
| Participants | Diagnosis of CRSsNP as recommended by European Position Paper on Rhinosinusitis and Nasal Polyps 2012 |
| Interventions | Prednisone (30 mg for 7 days then 15 mg for 7 days then 5 mg for 7 days) versus placebo (21 days) |
| Outcomes | Changes in symptoms as measured by SNOT‐22 questionnaire and visual analogue scale at 6 months Change in olfactory function as measured by "Sniffin' Sticks 12 tests" at 6 months Change in nasal patency as measured by acoustic rhinometry and rhinomanometry at 6 months Changes in nasal endoscopy findings as measured by Lund‐Kennedy score at 6 months |
| Starting date | June 2015 |
| Contact information | Constanza J Valdes, MD; email: cjvaldes@gmail.com Marcela A Veloz, MD; email: marceveloz@gmail.com |
| Notes | Authors responded to our enquiry to say that the study is still in the process of being completed |
CRS: chronic rhinosinusitis CRSsNP: chronic rhinosinusitis without nasal polyps NP: nasal polyps SNOT‐22: Sino‐Nasal Outcome Test‐22 VAS: visual analogue scale
Differences between protocol and review
As part of the discussions about the use of a total symptom score we noted that many papers within the suite of reviews did not present information for all four elements of the EPOS criteria for defining chronic rhinosinusitis (EPOS 2012). In particular, many studies that only included patients with nasal polyps did not present information on facial pressure or pain. We made the decision that where individual symptoms were recorded, they should be presented within the outcome of disease severity symptom score within the paper as this information would be useful for the reader.
Contributions of authors
Karen Head: reviewed and edited the protocol. Abstract screening, full paper review, data extraction, data analysis, drafting and writing the report.
Lee Yee Chong: scoped, designed and wrote the protocol. Abstract screening, full paper review, data extraction, data analysis and editing the report.
Claire Hopkins: clinical guidance at all stages of project scoping and protocol development. Clinical input into data analysis, reviewing and editing the report.
Carl Philpott: clinical guidance at all stages of project scoping and protocol development. Clinical input into data analysis, reviewing and editing the report.
Martin J Burton: helped to draft the protocol; clinical guidance at all stages of project scoping and protocol development. Clinical input into data analysis, reviewing and editing the report.
Anne GM Schilder: clinical input into data analysis, reviewing and editing the report.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
Funding to complete a suite of reviews on medical interventions for chronic rhinosinusitis in 2015/2016 (award reference 14/174/03), in addition to infrastructure funding for Cochrane ENT
Declarations of interest
Lee Yee Chong: none known.
Karen Head: none known.
Claire Hopkins: I have received financial support from several companies involved in producing instruments for sinus surgery: Acclarent, Sinusys, Cryolife and Medtronic.
Carl Philpott: I have previously received consultancy fees from the companies Acclarent, Navigant, Aerin Medical and Entellus.
Martin J Burton: Professor Martin Burton is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
Anne GM Schilder: Professor Anne Schilder is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review. Her evidENT team at UCL is supported by her NIHR Research Professorship award with the remit to develop a UK infrastructure and programme of clinical research in ENT, Hearing and Balance. Her institution has received a grant from GSK for a study on the microbiology of acute tympanostomy tube otorrhoea.
New
References
References to studies included in this review
Alobid 2014 {published data only}
- Alobid I, Benitez P, Valero A, Munoz R, Langdon C. Oral and intranasal steroid treatments improve nasal patency and paradoxically increase nasal nitric oxide in patients with severe nasal polyposis. Rhinology 2012;2:171‐7. [DOI] [PubMed] [Google Scholar]
- Alobid I, Benítez P, Cardelús S, Borja Callejas F, Lehrer‐Coriat E, Pujols L, et al. Oral plus nasal corticosteroids improve smell, nasal congestion, and inflammation in sino‐nasal polyposis. Laryngoscope 2014;124(1):50‐6. [DOI] [PubMed] [Google Scholar]
Benitez 2006 {published data only}
- Alobid I, Benitez P, Pujols L, Maldonado M, Bernal‐Sprekelsen M, Morello A, et al. Severe nasal polyposis and its impact on quality of life. The effect of a short course of oral steroids followed by long‐term intranasal steroid treatment. Rhinology 2006;44(1):8‐13. [PubMed] [Google Scholar]
- Benitez P, Alobid I, Haro J, Berenguer J, Bernal‐Sprekelsen, Pujols L, et al. A short course of oral prednisone followed by intranasal budesonide is an effective treatment of severe nasal polyps. Laryngoscope 2006;116(5):770‐5. [DOI] [PubMed] [Google Scholar]
- Martínez‐Antón A, Bolós C, Alobid I, Benítez P, Roca‐Ferrer J, Picado C, et al. Corticosteroid therapy increases membrane‐tethered while decreases secreted mucin expression in nasal polyps. Allergy: European Journal of Allergy and Clinical Immunology 2008;63(10):1368‐76. [DOI] [PubMed] [Google Scholar]
Ecevit 2015 {published and unpublished data}
- Ecevit MC, Erdag TK, Dogan E, Sutay S. Effect of steroids for nasal polyposis surgery: a placebo‐controlled, randomized, double‐blind study. Laryngoscope 2015;125(9):2041‐5. [DOI: 10.1002/lary.25352] [DOI] [PubMed] [Google Scholar]
Hissaria 2006 {published data only}
- Hissaria P, Smith W, Wormald PJ, Taylor J, Vadas M, Gillis D, et al. Short course of systemic corticosteroids in sinonasal polyposis: a double‐blind, randomized, placebo‐controlled trial with evaluation of outcome measures. Journal of Allergy and Clinical Immunology 2006;118(1):128‐33. [DOI] [PubMed] [Google Scholar]
Kapucu 2012 {published data only}
- Kapucu B, Cekin E, Erkul BE, Cincik H, Gungor A, Berber U. The effects of systemic, topical, and intralesional steroid treatments on apoptosis level of nasal polyps. Otolaryngology ‐ Head and Neck Surgery 2012;147(3):563‐7. [DOI] [PubMed] [Google Scholar]
Kirtsreesakul 2012 {published data only}
- Kirtsreesakul V, Wongsritrang K, Ruttanaphol S. Clinical efficacy of a short course of systemic steroids in nasal polyposis. Rhinology 2011;49(5):525‐32. [DOI] [PubMed] [Google Scholar]
- Kirtsreesakul V, Wongsritrang K, Ruttanaphol S. Does oral prednisolone increase the efficacy of subsequent nasal steroids in treating nasal polyposis?. American Journal of Rhinology & Allergy 2012;26(6):455‐62. [DOI: 10.2500/ajra.2012.26.3820] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vaidyanathan 2011 {published data only}
- Vaidyanathan S, Barnes M, Williamson P, Hopkinson P, Donnan PT, Lipworth B. Treatment of chronic rhinosinusitis with nasal polyposis with oral steroids followed by topical steroids: a randomized trial. Annals of Internal Medicine 2011;154:293‐302. [DOI] [PubMed] [Google Scholar]
Van Zele 2010 {published data only (unpublished sought but not used)}
- Zele T, Gevaert P, Holtappels G. Treatment of nasal polyposis with oral methylprednisolone: a double‐blind, randomized, placebo‐controlled trial with evaluation of clinical and biological activity. Journal of Allergy and Clinical Immunology 2008;121(2 Suppl 1):S265. [Google Scholar]
- Zele T, Gevaert P, Holtappels G, Beule A, Wormald PJ, Mayr S, et al. Oral steroids and doxycycline: two different approaches to treat nasal polyps. Journal of Allergy and Clinical Immunology 2010;125(5):1069‐76.e4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alobid 2005 {published data only}
- Alobid I, Benítez P, Bernal‐Sprekelsen M, Roca J, Alonso J, Picado C, et al. Nasal polyposis and its impact on quality of life: comparison between the effects of medical and surgical treatments. Allergy 2005;60(4):452‐8. [DOI] [PubMed] [Google Scholar]
Blomqvist 2001 {published data only}
- Blomqvist EH, Lundblad L, Anggard A, Haraldsson P‐O, Stjarne P. A randomized controlled study evaluating medical treatment versus surgical treatment in addition to medical treatment of nasal polyposis. Journal of Allergy and Clinical Immunology 2001;107(2):224‐8. [DOI] [PubMed] [Google Scholar]
Blomqvist 2009 {published data only}
- Blomqvist EH, Lundblad L, Bergstedt H, Stjarne P. A randomized prospective study comparing medical and medical‐surgical treatment of nasal polyposis by CT. Acta Oto‐Laryngologica 2009;129(5):545‐9. [DOI] [PubMed] [Google Scholar]
Bonfils 1998 {published data only}
- Bonfils P. Medical treatment of paranasal sinus polyposis: a prospective study in 181 patients [Le traitement medical de la polypose naso‐sinusienne: etude prospective sur une serie de 181 patients]. Annales d'Oto‐Laryngologie et de Chirurgie Cervico Faciale 1998;115(4):202‐14. [PubMed] [Google Scholar]
Bonfils 2003 {published data only}
- Bonfils P, Nores J‐M, Halimi P, Avan P. Medical treatment of stage I nasal polyposis over a 3‐year follow‐up period. ORL; Journal of Oto‐Rhino‐Laryngology and Its Related Specialties 2004;66(1):27‐34. [DOI] [PubMed] [Google Scholar]
Bonfils 2006 {published data only}
- Bonfils P, Halimi P, Malinvaud D. Adrenal suppression and osteoporosis after treatment of nasal polyposis. Acta Oto‐Laryngologica 2006;126(11):1195‐200. [DOI] [PubMed] [Google Scholar]
Bülbül 2013 {published data only}
- Bülbül T, Bülbül OG, Güçlü O, Bilsel AS, Gürsan SÖ. Effect of glucocorticoids on nasal polyposis, with detection of inflammatory response by measurement of nitric oxide levels in nasal polyp tissue. Journal of Laryngology and Otology 2013;127(6):584‐9. [DOI] [PubMed] [Google Scholar]
Chi Chan 1996 {published data only}
- Chi Chan A, Couto y Arcos F, Martin Biasotti F, Bross Soriano D, Vazquez Valle MDC, Gonzalez Olvera S. Oral steroids as preoperative medication in nasal polyposis [Esteroides orales en la preparacion preoperatoria de poliposis nasal]. Anales de Otorrinolaringologia Mexicana 1996;41(3):155‐60. [Google Scholar]
Damm 1999 {published data only}
- Damm M, Jungehulsing M, Eckel HE, Schmidt M, Theissen P. Effects of systemic steroid treatment in chronic polypoid rhinosinusitis evaluated with magnetic resonance imaging. Otolaryngology ‐ Head and Neck Surgery 1999;120(4):517‐23. [DOI] [PubMed] [Google Scholar]
Grammer 2013 {published data only}
- Grammer LC. Doxycycline or oral corticosteroids for nasal polyps. Journal of Allergy and Clinical Immunology. In Practice 2013;1(5):541‐2. [DOI] [PubMed] [Google Scholar]
Hessler 2007 {published data only}
- Hessler JL, Piccirillo JF, Fang D, Vlahiotis A, Banerji A, Levitt RG, et al. Clinical outcomes of chronic rhinosinusitis in response to medical therapy: results of a prospective study. American Journal of Rhinology 2007;21(1):10‐8. [DOI] [PubMed] [Google Scholar]
Jankowski 2003a {published data only}
- Jankowski R, Bodino C. Evolution of symptoms associated to nasal polyposis following oral steroid treatment and nasalisation of the ethmoid ‐ radical ethmoidectomy is functional surgery for NPS. Rhinology 2003;41(4):211‐9. [PubMed] [Google Scholar]
Jankowski 2003b {published data only}
- Jankowski R, Bodino C. Olfaction in patients with nasal polyposis: effects of systemic steroids and radical ethmoidectomy with middle turbinate resection (nasalisation). Rhinology 2003;41(4):220‐30. [PubMed] [Google Scholar]
Kroflic 2006 {published data only}
- Kroflic B, Baudoin T, Kalogjera L. Topical furosemide versus oral steroid in preoperative management of nasal polyposis. European Archives of Oto‐Rhino‐Laryngology 2006;263(8):767‐71. [DOI] [PubMed] [Google Scholar]
Lildholdt 1988 {published data only}
- Lildholdt T, Fogstrup J, Gammelgaard N, Kortholm B, Ulsoe C. Surgical versus medical treatment of nasal polyps. Acta Oto‐Laryngologica 1988;105(1‐2):140‐3. [DOI] [PubMed] [Google Scholar]
Lildholdt 1989 {published data only}
- Lildholdt T. Surgical versus medical treatment of nasal polyps. Rhinology. Supplement 1989;8:31‐3. [PubMed] [Google Scholar]
NCT01676415 {published data only}
- Northwestern University Feinberg School of Medicine. Corticosteroid therapy for chronic rhinosinusitis without nasal polyps (CRSsNP). http://clinicaltrials.gov/ct2/show/NCT01676415. [DOI: ]
Nores 2003 {published data only}
- Nores J‐M, Avan P, Bonfils P. Medical management of nasal polyposis: a study in a series of 152 consecutive patients. Rhinology 2003;41(2):97‐102. [PubMed] [Google Scholar]
Ozturk 2011 {published data only}
- Ozturk F, Bakirtas A, Ileri F, Turktas I. Efficacy and tolerability of systemic methylprednisolone in children and adolescents with chronic rhinosinusitis: a double‐blind, placebo‐controlled randomized trial. Journal of Allergy and Clinical Immunology 2011;128(2):348‐52. [DOI] [PubMed] [Google Scholar]
Ragab 2006 {published data only}
- Ragad S, Scadding GK, Lund VJ, Saleh H. Treatment of chronic rhinosinusitis and its effects on asthma. European Respiratory Journal 2006;28(1):68‐74. [DOI] [PubMed] [Google Scholar]
Rasp 1997 {published data only}
- Rasp G, Bujia J. Treatment of nasal polyposis with systemic and local corticoids. Acta Otorrinolaringologica Espanola 1997;48(1):37‐40. [PubMed] [Google Scholar]
Rasp 2000 {published data only}
- Rasp G, Kramer MF, Ostertag P, Kastenbauer E. A new system for the classification of ethmoid polyposis. Effect of combined local and systemic steroid therapy. Laryngo‐Rhino‐Otologie 2000;79(5):266‐72. [DOI] [PubMed] [Google Scholar]
Remer 2005 {published data only}
- Remer M, Polberg K, Obszańska B, Klatka J. Chronic sinusitis therapy with antibiotics (axetyl cefuroxym, clarithromycin) and steroid (prednisone) [Leczenie zapalenia zatok z zastosowaniem antybiotykow (aksetyl cefuroksymu, klarytromycyna) w polaczeniu ze sterydem stosowanym doustnie (prednison)]. Polski Merkuriusz Lekarski 2005;19(111):343‐4. [PubMed] [Google Scholar]
Reychler 2015 {published data only}
- Reychler G, Colbrant C, Huart C, Guellec S, Vecellio L, Liistro G, et al. Effect of three‐drug delivery modalities on olfactory function in chronic sinusitis. Laryngoscope 2015;125(3):549‐55. [DOI] [PubMed] [Google Scholar]
Rupa 2010 {published data only}
- Rupa V, Jacob M, Mathews MS, Seshadri MS. A prospective, randomised, placebo‐controlled trial of postoperative oral steroid in allergic fungal sinusitis. European Archives of Oto‐rhino‐laryngology 2010;267(2):233‐8. [DOI: 10.1007/s00405-009-1075-8] [DOI] [PubMed] [Google Scholar]
Sieskiewicz 2006 {published data only}
- Sieskiewicz A, Olszewska E, Rogowski M, Grycz E. Preoperative corticosteroid oral therapy and intraoperative bleeding during functional endoscopic sinus surgery in patients with severe nasal polyposis: a preliminary investigation. Annals of Otology, Rhinology and Laryngology 2006;115(7):490‐4. [DOI] [PubMed] [Google Scholar]
Sousa 2009 {published data only}
- Sousa MC, Becker HM, Becker CG, Castro MM, Sousa NJ, Guimarães RE. Reproducibility of the three‐dimensional endoscopic staging system for nasal polyposis. Brazilian Journal of Otorhinolaryngology 2009;75(6):814‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevens 2001 {published data only}
- Stevens MH. Steroid‐dependent anosmia. Laryngoscope 2001;111(2):200‐3. [DOI] [PubMed] [Google Scholar]
Tuncer 2003 {published data only}
- Tuncer U, Soylu L, Aydogan B, Karakus F, Akcali C. The effectiveness of steroid treatment in nasal polyposis. Auris Nasus Larynx 2003;30(3):263‐8. [DOI] [PubMed] [Google Scholar]
van Camp 1994 {published data only}
- Camp C, Clement PA. Results of oral steroid treatment in nasal polyposis. Rhinology 1994;32(1):5‐9. [PubMed] [Google Scholar]
References to ongoing studies
Chi 2011 {published data only}
- ChiCTR‐TRC‐11001323. Research on clinical efficacy of oral glucocorticoid in the treatment of eosinophilic nasal polyps and non‐eosinophilic nasal polyps. http://www.chictr.org.cn/showprojen.aspx?proj=8216.
NCT00841802 {published data only}
- Chronic rhinosinusitis with or without nasal polyps steroid study. http://clinicaltrials.gov/ct2/show/NCT00841802.
NCT02367118 {published data only}
- Prednisone in chronic rhinosinusitis without nasal polyps. http://clinicaltrials.gov/ct2/show/NCT02367118.
Additional references
Balk 2012
- Balk EM, Earley A, Patel K, Trikalinos TA, Dahabreh IJ. Empirical assessment of within‐arm correlation imputation in trials of continuous outcomes [Internet]. Report No.: 12(13)‐EHC141‐EF. AHRQ Methods for Effective Health Care. Rockville (MD): Agency for Healthcare Research and Quality, 2012. [PubMed] [Google Scholar]
Burton 2008
- Burton MJ, Harvey RJ. Idiopathic sudden sensorineural hearing loss. In: Gleeson M, Browning G, Burton MJ, Clarke R, Hibbert J, Jones NS, et al. editor(s). Scott‐Brown's Otorhinolaryngology, Head and Neck Surgery. 7th Edition. Vol. 3, London: Hodder Arnold, 2008:3577‐93. [Google Scholar]
Cho 2012
- Cho SH, Hong SJ, Han B, Lee SH, Suh L, Norton J, et al. Age‐related differences in the pathogenesis of chronic rhinosinusitis. Journal of Allergy and Clinical Immunology 2012;129(3):858‐60.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2016a
- Chong LY, Head K, Hopkins C, Philpott C, Burton MJ, Schilder AGM. Different types of intranasal steroids for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011993.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2016b
- Chong LY, Head K, Hopkins C, Philpott C, Schilder AGM, Burton MJ. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011996.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2016c
- Chong LY, Head K, Hopkins C, Philpott C, Glew S, Scadding G, et al. Saline irrigation for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011995.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1988. [Google Scholar]
Da Silva 2006
- Silva JA, Jacobs JW, Kirwan JR, Boers M, Saag KG, Inês LB, et al. Safety of low dose glucocorticoid treatment in rheumatoid arthritis: published evidence and prospective trial data. Annals of the Rheumatic Diseases 2006;65:285‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeMarcantonio 2011
- DeMarcantonio MA, Han JK. Nasal polyps: pathogenesis and treatment implications. Otolaryngologic Clinics of North America 2011;44(3):685‐95, ix. [DOI] [PubMed] [Google Scholar]
Ebbens 2010
- Ebbens FA, Toppila‐Salmi SK, Renkonen JA, Renkonen RL, Mullol J, Drunen CM, et al. Endothelial L‐selectin ligand expression in nasal polyps. Allergy 2010;65(1):95‐102. [DOI] [PubMed] [Google Scholar]
Ebbens 2011
- Ebbens FA, Toppila‐Salmi S, Groot EJ, Renkonen J, Renkonen R, Drunen CM, et al. Predictors of post‐operative response to treatment: a double blind placebo controlled study in chronic rhinosinusitis patients. Rhinology 2011;49(4):413‐9. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOS 2012
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology. Supplement 2012;50 Suppl 23:1‐298. [PubMed] [Google Scholar]
Fokkens 2007
- Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps 2007. Rhinology 2007;45(Suppl 20):1‐139. [PubMed] [Google Scholar]
Gliklich 1995
- Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngology ‐ Head and Neck Surgery 1995;113(1):104‐9. [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hastan 2011
- Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, et al. Chronic rhinosinusitis in Europe ‐ an underestimated disease. A GA2LEN study. Allergy 2011;66(9):1216‐23. [DOI] [PubMed] [Google Scholar]
Head 2016a
- Head K, Chong LY, Hopkins C, Philpott C, Schilder AGM, Burton MJ. Short‐course oral steroids as an adjunct therapy for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011992.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Head 2016b
- Head K, Chong LY, Piromchai P, Hopkins C, Philpott C, Schilder AGM, et al. Systemic and topical antibiotics for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011994.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hopkins 2016
- Hopkins C, Philpott C, Crowe S, Regan S, Degun A, Papachristou I, et al. Identifying the most important outcomes for systematic reviews of interventions for rhinosinusitis in adults: working with Patients, Public and Practitioners. Rhinology 2016;54(1):20‐6. [DOI] [PubMed] [Google Scholar]
Kern 2008
- Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi‐Peters A, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. American Journal of Rhinology 2008;22(6):549‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keswani 2012
- Keswani A, Chustz RT, Suh L, Carter R, Peters AT, Tan BK, et al. Differential expression of interleukin‐32 in chronic rhinosinusitis with and without nasal polyps. Allergy 2012;67(1):25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larsen 2004
- Larsen P, Tos M. Origin of nasal polyps: an endoscopic autopsy study. Laryngoscope 2004;114(4):710‐9. [DOI] [PubMed] [Google Scholar]
Martinez‐Devesa 2011
- Martinez‐Devesa P, Patiar S. Oral steroids for nasal polyps. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD005232.pub3] [DOI] [PubMed] [Google Scholar]
Mygind 1996
- Mygind N, Lildholdt T. Nasal polyps treatment: medical management. Allergy and Asthma Proceedings 1996;17:275‐82. [DOI] [PubMed] [Google Scholar]
Naber 1996
- Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8‐days' corticosteroid treatment. A prospective study. Psychoneuroendocrinology 1996;21:25‐31. [DOI] [PubMed] [Google Scholar]
Philpott 2015
- Philpott C, Hopkins C, Erskine S, Kumar N, Robertson A, Farboud A, et al. The burden of revision sinonasal surgery in the UK‐data from the Chronic Rhinosinusitis Epidemiology Study (CRES): a cross‐sectional study. BMJ Open 2015;5(4):e006680. [10.1136/bmjopen‐2014‐ 006680] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ragab 2004
- Ragab SM, Lund VJ, Scadding G. Evaluation of the medical and surgical treatment of chronic rhinosinusitis: a prospective, randomised, controlled trial. Laryngoscope 2004;114(5):923‐30. [DOI] [PubMed] [Google Scholar]
Ragab 2010
- Ragab SM, Lund VJ, Scadding G, Saleh HA, Khalifa MA. Impact of chronic rhinosinusitis therapy on quality of life: a prospective randomized controlled trial. Rhinology 2010;48(3):305‐11. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Stanbury 1998
- Stanbury RM, Graham EM. Systemic corticosteroid therapy‐‐side effects and their management. British Journal of Ophthalmology 1998;82:704‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2011
- Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. Journal of Allergy and Clinical Immunology 2011;128(6):1198‐206.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomassen 2011
- Tomassen P, Zele T, Zhang N, Perez‐Novo C, Bruaene N, Gevaert P, et al. Pathophysiology of chronic rhinosinusitis. Proceedings of the American Thoracic Society 2011;8(1):115‐20. [DOI] [PubMed] [Google Scholar]
van Drunen 2009
- Drunen CM, Reinartz SM, Wigman J, Fokkens W. Inflammation in chronic rhinosinusitis and nasal polyposis. Immunology and Allergy Clinics of North America 2009;29(4):621‐9. [DOI] [PubMed] [Google Scholar]
Venekamp 2014
- Venekamp RP, Thompson MJ, Hayward G, Heneghan CJ, Mar CB, Perera R, et al. Systemic corticosteroids for acute sinusitis. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD008115.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Venekamp 2015
- Venekamp RP, Thompson MJ, Rovers MM. Systemic corticosteroid therapy for acute sinusitis. JAMA 2015;313(12):1258‐9. [DOI] [PubMed] [Google Scholar]
Zhang 2008
- Zhang N, Zele T, Perez‐Novo C, Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T‐effector cells orchestrate mucosal inflammation in chronic sinus disease. Journal of Allergy and Clinical Immunology 2008;122(5):961‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2009
- Zhang XH, Lu X, Long XB, You XJ, Gao QX, Cui YH, et al. Chronic rhinosinusitis with and without nasal polyps is associated with decreased expression of glucocorticoid‐induced leucine zipper. Clinical and Experimental Allergy 2009;39(5):647‐54. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chong 2015
- Chong LY, Head K, Hopkins C, Philpott C, Burton MJ. Short‐course oral steroids alone for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD011991] [DOI] [PMC free article] [PubMed] [Google Scholar]


