Abstract
Background
Atherosclerotic peripheral arterial disease (PAD) can lead to disabling ischemia and limb loss. Treatment modalities have included risk factor optimization through life‐style modifications and medications, or operative approaches using both open and minimally invasive techniques, such as balloon angioplasty. Drug‐eluting balloon (DEB) angioplasty has emerged as a promising alternative to uncoated balloon angioplasty for the treatment of this difficult disease process. By ballooning and coating the inside of atherosclerotic vessels with cytotoxic agents, such as paclitaxel, cellular mechanisms responsible for atherosclerosis and neointimal hyperplasia are inhibited and its devastating complications are prevented or postponed. DEBs are considerably more expensive than uncoated balloons, and their efficacy in improving patient outcomes is unclear.
Objectives
To assess the efficacy of drug‐eluting balloons (DEBs) compared with uncoated, nonstenting balloon angioplasty in people with symptomatic lower‐limb peripheral arterial disease (PAD).
Search methods
The Cochrane Vascular Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched December 2015) and Cochrane Register of Studies (CRS) (2015, Issue 11). The TSC searched trial databases for details of ongoing and unpublished studies.
Selection criteria
We included all randomized controlled trials that compared DEBs with uncoated, nonstenting balloon angioplasty for intermittent claudication (IC) or critical limb ischemia (CLI).
Data collection and analysis
Two review authors (AK, TA) independently selected the appropriate trials and performed data extraction, assessment of trial quality, and data analysis. The senior review author (DKR) adjudicated any disagreements.
Main results
Eleven trials that randomized 1838 participants met the study inclusion criteria. Seven of the trials included femoropopliteal arterial lesions, three included tibial arterial lesions, and one included both. The trials were carried out in Europe and in the USA and all used the taxane drug paclitaxel in the DEB arm. Nine of the 11 trials were industry‐sponsored. Four companies manufactured the DEB devices (Bard, Bavaria Medizin, Biotronik, and Medtronic). The trials examined both anatomic and clinical endpoints. There was heterogeneity in the frequency of stent deployment and the type and duration of antiplatelet therapy between trials. Using GRADE assessment criteria, the quality of the evidence presented was moderate for the outcomes of target lesion revascularization and change in Rutherford category, and high for amputation, primary vessel patency, binary restenosis, death, and change in ankle‐brachial index (ABI). Most participants were followed up for 12 months, but one trial reported outcomes at five years.
There were better outcomes for DEBs for up to two years in primary vessel patency (odds ratio (OR) 1.47, 95% confidence interval (CI) 0.22 to 9.57 at six months; OR 1.92, 95% CI 1.45 to 2.56 at 12 months; OR 3.51, 95% CI 2.26 to 5.46 at two years) and at six months and two years for late lumen loss (mean difference (MD) ‐0.64 mm, 95% CI ‐1.00 to ‐0.28 at six months; MD ‐0.80 mm, 95% CI ‐1.44 to ‐0.16 at two years). DEB were also superior to uncoated balloon angioplasty for up to five years in target lesion revascularization (OR 0.28, 95% CI 0.17 to 0.47 at six months; OR 0.40, 95% CI 0.31 to 0.51 at 12 months; OR 0.28, 95% CI 0.18 to 0.44 at two years; OR 0.21, 95% CI 0.09 to 0.51 at five years) and binary restenosis rate (OR 0.44, 95% CI 0.29 to 0.67 at six months; OR 0.38, 95% CI 0.15 to 0.98 at 12 months; OR 0.26, 95% CI 0.10 to 0.66 at two years; OR 0.12, 95% CI 0.05 to 0.30 at five years). There was no significant difference between DEB and uncoated angioplasty in amputation, death, change in ABI, change in Rutherford category and quality of life (QoL) scores, or functional walking ability, although none of the trials were powered to detect a significant difference in these clinical endpoints. We carried out two subgroup analyses to examine outcomes in femoropopliteal and tibial interventions as well as in people with CLI (4 or greater Rutherford class), and showed no advantage for DEBs in tibial vessels at six and 12 months compared with uncoated balloon angioplasty. There was also no advantage for DEBs in CLI compared with uncoated balloon angioplasty at 12 months.
Authors' conclusions
Based on a meta‐analysis of 11 trials with 1838 participants, there is evidence of an advantage for DEBs compared with uncoated balloon angioplasty in several anatomic endpoints such as primary vessel patency (high‐quality evidence), binary restenosis rate (moderate‐quality evidence), and target lesion revascularization (low‐quality evidence) for up to 12 months. Conversely, there is no evidence of an advantage for DEBs in clinical endpoints such as amputation, death, or change in ABI, or change in Rutherford category during 12 months' follow‐up. Well‐designed randomized trials with long‐term follow‐up are needed to compare DEBs with uncoated balloon angioplasties adequately for both anatomic and clinical study endpoints before the widespread use of this expensive technology can be justified.
Keywords: Humans; Amputation, Surgical; Amputation, Surgical/statistics & numerical data; Angioplasty, Balloon; Angioplasty, Balloon/methods; Angioplasty, Balloon/mortality; Drug‐Eluting Stents; Femoral Artery; Lower Extremity; Lower Extremity/blood supply; Paclitaxel; Paclitaxel/therapeutic use; Peripheral Arterial Disease; Peripheral Arterial Disease/mortality; Peripheral Arterial Disease/therapy; Popliteal Artery; Randomized Controlled Trials as Topic; Tibial Arteries; Time Factors; Vascular Patency
Plain language summary
Uncoated balloon angioplasty versus drug‐eluting balloon angioplasty for peripheral arterial disease of the lower limbs
Background
Peripheral arterial disease (PAD) of the lower limbs is a widespread condition that affects many people. In its advanced form, PAD can lead to pain, infections, and amputation. People with PAD are usually first treated with medicines and lifestyle modifications including strategies to stop smoking and a walking program to optimize their general health. People who require an operation might have a traditional open surgery or a less invasive procedure known as angioplasty, which uses a balloon to open the blockages in the arteries. A new type of angioplasty, known as drug‐eluting balloon (DEB) angioplasty, has emerged as a promising alternative to traditional balloon angioplasty for the treatment of patients with PAD. By using DEBs to balloon and coat the inside of the blood vessels (tubes that carry blood around the body) with medicines to treat cancer (chemotherapy) such as paclitaxel, the hope is to halt the progression of PAD and prevent or postpone its devastating complications. The goal of this review was to determine how DEB angioplasty compares with traditional balloon angioplasty for the treatment of PAD of the lower limbs.
Study characteristics and key results
Our review included 11 clinical trials that randomized 1838 participants (current until December 2015). The trials included thigh and leg arteries above and below the knee. The trials were carried out in Europe and the USA, and all used DEBs that contained paclitaxel. Four companies manufactured the DEB devices: Bard, Bavaria Medizin, Biotronik, and Medtronic. Most participants were followed for 12 or more months (called follow‐up). At six and 12 months of follow‐up, DEBs were associated with improved primary vessel patency, which is an indicator of whether a vessel is still patent without any further interventions (blood flowing well), late lumen loss, which is the difference in millimeters between the angioplastied segment and how narrow it is on follow‐up, target lesion revascularization, which is an indicator of whether a person received more than one treatment to the same artery during the period covered by the study, and binary restenosis, which occurs when a treated artery becomes narrowed again after being previously treated.
Unfortunately, early anatomic (structural) advantages of DEBs were not accompanied by improvements in quality of life, functional walking ability, or in the occurrence of amputation or death. When we specifically examined arteries below the knee and people who had very advanced PAD, we found no clinical or angiographic advantage for DEBs at 12 months of follow‐up compared with uncoated balloon angioplasty. In summary, DEBs have several anatomic advantages over uncoated balloons for the treatment of lower limb PAD for up to 12 months after undergoing the procedure. However, more data are needed to assess the long‐term results of this treatment option adequately.
Quality of the evidence
All the trials had differences in the way in which they inserted the balloons, and in the type and duration of additional antiplatelet (anticlotting) therapy, leading to downgrading of the quality of the evidence. The quality of the evidence presented was moderate for target lesion revascularization and change in Rutherford category (a way of classifying PAD), and high for amputation, primary vessel patency, binary restenosis, death, and change in ankle‐brachial index (which is used to predict the severity of PAD).
Summary of findings
Summary of findings for the main comparison. Drug‐eluting balloon versus uncoated balloon angioplasty at 12 months.
| Drug‐eluting balloon compared to uncoated balloon angioplasty at 12 months | ||||||
| Patient or population: people with peripheral arterial disease of the lower limbs Setting: hospital Intervention: drug‐eluting balloon angioplasty Comparison: uncoated balloon angioplasty | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with uncoated balloon angioplasty at 12 months | Risk with drug‐eluting balloon angioplasty at 12 months | |||||
| Amputation | Study population | OR 1.56 (0.73 to 3.33) | 1649 (9 RCTs) | ⊕⊕⊕⊕ High 1 | ‐ | |
| 14 per 1000 | 22 per 1000 (10 to 46) | |||||
| Primary vessel patency | Study population | OR 1.92 (1.45 to 2.56) | 882 (3 RCTs) | ⊕⊕⊕⊕ High 1 | ‐ | |
| 479 per 1000 | 638 per 1000 (571 to 702) | |||||
| Moderate | ||||||
| 487 per 1000 | 645 per 1000 (579 to 708) | |||||
| Target lesion revascularization | Study population | OR 0.40 (0.31 to 0.51) | 1900 (11 RCTs) | ⊕⊕⊝⊝ Low 1, 2, 3 | ‐ | |
| 264 per 1000 | 126 per 1000 (100 to 155) | |||||
| Moderate | ||||||
| 368 per 1000 | 189 per 1000 (153 to 229) | |||||
| Binary restenosis | Study population | OR 0.38 (0.15 to 0.98) | 1094 (4 RCTs) | ⊕⊕⊕⊝ Moderate 1, 3 | ‐ | |
| 350 per 1000 | 170 per 1000 (75 to 346) | |||||
| Moderate | ||||||
| 477 per 1000 | 257 per 1000 (120 to 472) | |||||
| Death | Study population | OR 1.09 (0.64 to 1.85) | 1649 (9 RCTs) | ⊕⊕⊕⊕ High 1 | ‐ | |
| 43 per 1000 | 46 per 1000 (28 to 76) | |||||
| Moderate | ||||||
| 45 per 1000 | 49 per 1000 (29 to 80) | |||||
| Change in Rutherford category | ‐ | The mean change in Rutherford category in the intervention group was 0.1 lower (0.29 lower to 0.1 higher) | ‐ | 623 (3 RCTs) | ⊕⊕⊕⊝ Moderate 1 4 | A positive change in Rutherford category reflects a worsening clinical status, although in this case the difference was not statistically significant |
| Change in ankle‐brachial index | ‐ | The mean change in ankle‐brachial index in the intervention group was 0.03 lower (0.07 lower to 0.01 higher) | ‐ | 656 (3 RCTs) | ⊕⊕⊕⊕ High 1 | A negative change in the ankle‐brachial index reflects a worsening clinical status, although in this case the difference was not statistically significant |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomized controlled trial. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 All of the included trials were at a high risk of performance bias due to lack of blinding of operators. However, because this is an intrinsic limitation to intervention trials, we did not downgrade the GRADE class because of performance bias. 2 Funnel plot analysis indicated a likely publication bias, which resulted in downgrading of the GRADE class. 3 There was moderate heterogeneity between the included studies (P < 0.001), which resulted in downgrading of the GRADE class. 4 There was moderate heterogeneity between the included studies (P = 0.04), which resulted in downgrading of the GRADE class.
Background
Description of the condition
Peripheral arterial disease (PAD) is a major healthcare challenge resulting in significant age‐related patient morbidity. It is estimated to affect 4% of adults aged 40 years or older and 14.5% of adults aged 70 years or older in the USA (Selvin 2004). The presence of PAD does not always lead to symptoms, and asymptomatic PAD has been reported in 8% of Scottish adults (Fowkes 1991).
People with symptomatic lower‐limb PAD commonly present with intermittent claudication (IC). The prevalence of IC in people with PAD is estimated to be 3% in people aged 40 years and 6% in people aged 60 years (Norgren 2007). About 25% of people with IC will develop more severe claudication and a deterioration in functional status of their affected limb. Some of those people will also eventually develop critical limb ischemia (CLI) (Norgren 2007). Major amputation is required in less than 10% of people with IC (Aquino 2001).
Description of the intervention
The medical management of lower‐limb PAD is based on an appropriate diet and exercise regimen and vascular disease risk‐factor modification through smoking‐cessation, blood pressure control, tight control of blood sugar levels in people with diabetes, and the prescription of lipid‐lowering and antiplatelet medications (Hirsch 200). Surgical management is indicated when people develop CLI or debilitating IC that is refractory to nonoperative management.
While bypass with an autologous vein or prosthetic conduit is the mainstay of open surgical management of PAD, percutaneous endovascular interventions provide another treatment alternative. Percutaneous interventions use ultrasound and fluoroscopic guidance to access and cannulate the diseased artery. A balloon catheter is then employed to provide pneumatic dilation of the stenotic or occluded vessel segment. The first balloon catheters used for this purpose were not coated with any medications and showed excellent efficacy in treating these diseased arteries (Norgren 2007). More recently, drug‐eluting balloons (DEBs), in addition to stents, have been placed to provide additional support when balloon angioplasty results are not satisfactory.
Balloon angioplasty, with or without stent placement, has the advantage of a shorter hospital stay and fewer short‐term postinterventional complications compared with bypass surgery. It has also been shown to be similar to open surgery in overall and amputation‐free survival at two years (BASIL 2005). However, on long‐term follow‐up, bypass surgery with autologous venous conduit was associated with a seven‐month increase in overall survival while amputation‐free survival remained the same (Bradbury 2010). In cases where autologous venous conduit is not available for bypass, the differences between balloon angioplasty and bypass using a prosthetic graft are probably minimal in terms of outcome (Bradbury 2010).
How the intervention might work
Elastic recoil and vessel restenosis secondary to neointimal hyperplasia remain a challenge for all of the available lower‐limb PAD treatment interventions. Numerous strategies have sought to delay vessel restenosis, including the use of drug‐eluting stents and DEBs. To date, there is only one commercially available drug‐eluting stent for use in the superficial femoral artery (Dake 2013).
DEBs were developed to provide a complete and homogenous coating of an antiproliferative agent to the arterial wall (Seedial 2013). The most commonly used agent is paclitaxel, a highly lipophilic drug that has been shown to prevent neointimal hyperplasia after balloon angioplasty (Axel 1997). The immunosuppressant agent sirolimus has also been used to prevent neointimal hyperplasia (Seedial 2013). The advantages of DEBs compared with other percutaneous treatment modalities include the absence of stent thrombosis or scaffolding to disrupt patterns of flow, immediate drug release, and no residual foreign body (Seedial 2013). However, DEBs are more costly and carry the potential for long‐term negative vessel remodeling and elastic recoil.
Why it is important to do this review
Randomized controlled trials have demonstrated the safety and efficacy of angioplasty using DEBs compared with conventional uncoated balloon angioplasty. Our goal is to review the evidence for the use of DEBs in the management of lower‐limb PAD systematically. This will help guide decision‐making when considering whether to use this costly treatment modality and determine whether it is associated with improved clinical outcomes compared with conventional balloon angioplasty.
Objectives
To assess the efficacy of drug‐eluting balloons (DEBs) compared with uncoated, nonstenting balloon angioplasty in people with symptomatic lower‐limb peripheral arterial disease (PAD).
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) that compare DEBs with uncoated balloon angioplasty for IC or CLI.
Types of participants
People with IC or CLI undergoing drug‐eluting or uncoated balloon angioplasty for symptomatic lower‐limb PAD.
Types of interventions
We compared DEBs for PAD of the lower limbs with uncoated, nonstenting balloon angioplasty. Endovascular access in the included studies was established percutaneously or through a limited incision. We did not include studies of DEBs for the treatment of instent restenosis of the lower‐limb, as well as studies where DEBs were used simultaneously in combination with other angioplasty techniques (such as hybrid procedures involving surgery and DEBs). Our review focused on primary arterial interventions only. We excluded reinterventions and studies using cutting balloon angioplasty.
Types of outcome measures
Primary outcomes
Incidence of amputation.
Amputation‐free survival, defined as the probability of being alive without an amputation.
Amputation‐free rate, defined as the patency of the target vessels and freedom from amputation.
Vessel patency (primary and secondary), as determined by delayed arterial lumen loss, target lesion revascularization, and binary restenosis rate measured with duplex ultrasound or angiography.
Death.
Secondary outcomes
Change in Fontaine stage or Rutherford category of PAD (Norgren 2007).
Change in ankle‐brachial index (ABI).
Change in quality of life (QoL) scores.
Change in functional walking ability, as measured by the Walking Impairment Questionnaire (WIQ).
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Trials Search Co‐ordinator (TSC) searched the Specialised Register (December 2015). In addition, the TSC searched the Cochrane Register of Studies (CRS) (www.metaxis.com/CRSWeb/Index.asp; (CENTRAL) 2015, Issue 11). See Appendix 1 for details of the search strategy used to search the CRS. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, Embase, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals, and conference proceedings which have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Vascular module in theCochrane Library (www.cochranelibrary.com).
In addition, the TSC searched the following trial databases for details of ongoing and unpublished studies. See Appendix 2 for details of the search.
World Health Organization International Clinical Trials Registry (apps.who.int/trialsearch/).
ClinicalTrials.gov (clinicaltrials.gov/).
ISRCTN registry (www.controlled‐trials.com/).
Searching other resources
We examined the bibliographies of relevant papers found from the electronic searches to identify other studies. We also attempted to contact study authors for additional information when necessary.
Data collection and analysis
Selection of studies
Two review authors (AK and TA) independently selected trials for inclusion in this review. These trials were sent to a third review author (DR), who assessed and confirmed their suitability for inclusion and acted as an adjudicator in the event of disagreement. The Criteria for considering studies for this review section details the inclusion criteria used in this selection process.
Data extraction and management
Two review authors (AK and TA) extracted the data from each trial, including participant demographics (age, gender, comorbidities, Fontaine stage or Rutherford category of PAD, and ABI), interventions (DEBs and other balloon types, vessels treated, history of previous stent placement), and outcomes (as specified in the Criteria for considering studies for this review section). A third review author (DR), then cross‐checked the data and acted as an adjudicator in the event of disagreement. Statistical analysis complied with the standard methods of Cochrane Vascular. We used the computer software package Review Manager 5 to perform all statistical analyses and generate figures (RevMan 2014).
Assessment of risk of bias in included studies
Two review authors (AK and TA) assessed potential risks of bias for all included studies using the Cochrane's tool for assessing risk of bias (Higgins 2011). The tool assesses bias in six different domains: sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; incomplete outcome data; selective outcome reporting; and other sources of bias. Each domain receives a score of high, low, or unclear depending on each review author's judgment. A third review author (DR) acted as an adjudicator in the event of disagreement. Where doubt existed as to a potential risk of bias, we contacted the study authors for clarification.
Measures of treatment effect
We calculated and reported continuous outcome measures such as lumen loss using the mean difference (MD) and associated 95% confidence interval (CI) between the two treatment groups. We calculated and reported dichotomous outcome measures including the occurrence of a postprocedural complication such as death using the odds ratio (OR) and associated 95% CI, depending on the reported data. We based calculations on an intention‐to‐treat approach and all randomized participants were included in the analysis regardless of loss to follow‐up. In one study (FemPac 2008), where the late lumen loss and change in ABI were reported as medians, rather than means, we converted the median values to means as per the method described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Unit of analysis issues
The unit of analysis was the treated limb for outcomes in which repeat or additional procedures on the contralateral side were possible and reported. The unit of analysis was the individual participant when considering participant death, QoL scores, and change in functional walking ability.
Dealing with missing data
We contacted authors of the included studies to inquire about missing or incomplete data, such as information on participants who dropped out of the study, and missing statistics. We excluded no studies from the meta‐analysis due to concerns about missing data.
Assessment of heterogeneity
We assessed inter‐study heterogeneity visually using a forest plot. We also calculated the I2 statistic to measure the amount of inter‐study heterogeneity. We considered I2 values less than 50% as indicative of low heterogeneity, I2 values between 50% and 75% as indicative of moderate heterogeneity, and I2 values greater than 75% as indicative of significant heterogeneity (Higgins 2011).
Assessment of reporting biases
We constructed a funnel plot to test for reporting bias in meta‐analyses that included 10 or more studies (Higgins 2011).
Data synthesis
We used a fixed‐effect model to calculate the pooled treatment effect data and 95% CIs for continuous and dichotomous outcome variables, as detailed under Measures of treatment effect. We used a random‐effects model when we found significant heterogeneity (defined as I2 greater than 75%). We created a forest plot for each treatment effect, as per Cochrane Vascular guidelines.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses by the arterial segments treated (femoropopliteal versus tibial), and the severity of PAD (studies that only included participants with a Rutherford's class greater than 4). We were unable to perform subgroup analyses by type of DEB pharmacologic agent, as all the studies used paclitaxel.
Sensitivity analysis
We sequentially excluded studies with a high risk of bias in several domains (as described in the Assessment of risk of bias in included studies section) and performed a pooled sensitivity analysis in order to assess whether the included studies, deemed to be biased, impacted the final analysis.
'Summary of findings' table
We prepared 'Summary of findings' tables to present the evidence for DEB versus uncoated balloon angioplasty at 12 months of follow‐up in participants who underwent endovascular lower‐limb interventions for symptomatic PAD. We chose this time point because the greatest amount of data from the included trials was available at 12 months of follow‐up. We used no external information in generating the 'Assumed risk' column. We used the GRADE approach to evaluate the evidence and assign one of four levels of quality: high, moderate, low, or very low (Higgins 2011). No departures from the standard methods for generating these tables were required. We included the following primary and secondary endpoints described under the Types of outcome measures section: amputation, primary vessel patency, target lesion revascularization, binary restenosis, death, change in Rutherford category, and change in ABI. These endpoints were chosen because we deemed them to be the most clinically relevant.
Results
Description of studies
Results of the search
See Figure 1.
1.
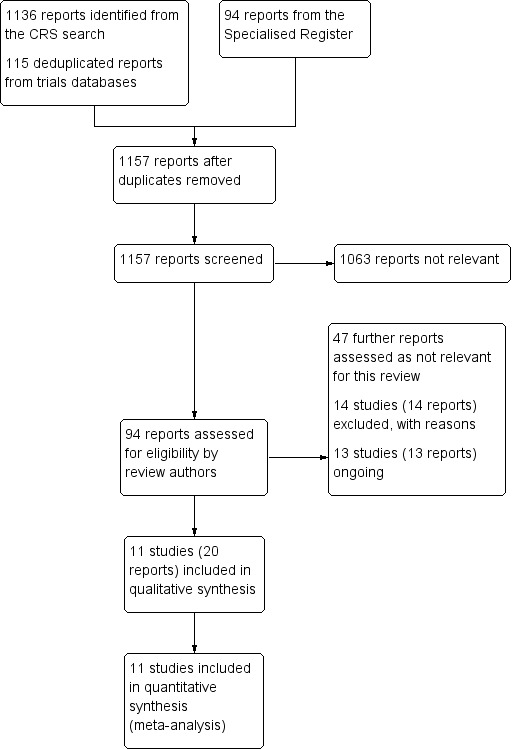
Study flow diagram.
Included studies
The review included 11 randomized controlled trials that compared DEB with uncoated balloon angioplasty for lower extremity IC or CLI (BIOLUX P‐I; BIOLUX P‐II; DEBATE‐BTK 2013; DEBELLUM 2012; FemPac 2008; IN.PACT DEEP 2014; IN.PACT SFA 2015; LEVANT I 2014; LEVANT II 2015; PACIFIER 2012; THUNDER 2008). The Characteristics of included studies table lists these in more detail.
All of the included studies were partially or entirely conducted in Europe. Three studies also enrolled people in the USA (IN.PACT SFA 2015; LEVANT I 2014; LEVANT II 2015). Most studies examined treatments for femoropopliteal arteries (BIOLUX P‐I; FemPac 2008; IN.PACT SFA 2015; LEVANT I 2014; LEVANT II 2015; PACIFIER 2012; THUNDER 2008), and three studies only examined tibial arteries (BIOLUX P‐II; DEBATE‐BTK 2013; IN.PACT DEEP 2014). The DEBELLUM 2012 trial examined both femoropopliteal and tibial arteries. Every included trial used paclitaxel as the balloon‐coating drug.
Medtronic manufactured the most‐frequently studied DEBs and sponsored three trials (IN.PACT DEEP 2014; IN.PACT SFA 2015; PACIFIER 2012). Two trials also used Medtronic devices but did not report receiving any industry sponsorship (DEBATE‐BTK 2013; DEBELLUM 2012). Bavaria Medizin (FemPac 2008; THUNDER 2008), Bard (LEVANT I 2014; LEVANT II 2015), and Biotronik (BIOLUX P‐I; BIOLUX P‐II) sponsored two trials each.
We also identified 13 trials that were either ongoing or awaiting publication (see Characteristics of ongoing studies table). We contacted authors of all ongoing studies to request study data.
Excluded studies
We excluded 14 trials from our review (COPA CABANA; DEBATE‐ISR; DEBATE SFA; DEFINITIVE AR; EURO CANAL; FAIR; Freeway Stent Study; IDEAS; ISAR‐PEBIS; ISAR‐STATH; PACUBA 1; PHOTOPAC; RAPID; SWEDEPAD). The reasons for exclusion are outlined in the Characteristics of excluded studies table. Studies were most commonly excluded for comparing DEB and bare‐metal or drug‐eluting stenting (DEBATE SFA; IDEAS; RAPID), DEB and uncoated balloon angioplasty for the management of instent restenosis (COPA CABANA; DEBATE‐ISR; FAIR; Freeway Stent Study; ISAR‐PEBIS; PACUBA 1), or DEB with or without atherectomy (DEFINITIVE AR; ISAR‐STATH). One trial compared DEB with or without photoablation therapy for the prevention of instent restenosis (PHOTOPAC). The SWEDEPAD trial compared drug‐eluting technologies (balloon or stents) with nondrug‐eluting technologies (balloons or stents). The EURO CANAL was terminated early before any data were collected because the manufacturer withdrew the product from the market.
Risk of bias in included studies
See 'Risk of bias' tables in the Characteristics of included studies table and summary results in Figure 2 and Figure 3.
2.
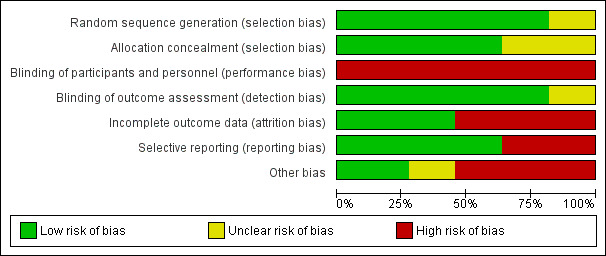
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
3.
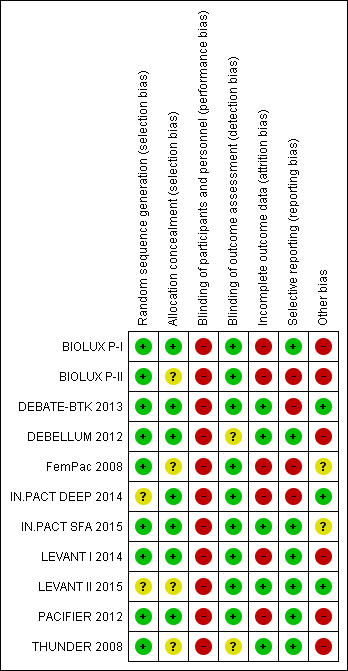
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Of the included studies, three were at high risk of bias and were excluded in sensitivity analyses to determine their impact (BIOLUX P‐I; BIOLUX P‐II; FemPac 2008). BIOLUX P‐I was at high risk of bias because of a low follow‐up compliance and a high rate of bailout stenting (26.7%) in the control arm. Furthermore, more than half of the participants in both arms had a history of peripheral vascular interventions, and it is unclear whether the lesions studied in the trial had been previously treated. BIOLUX P‐II did not blind the operators or the participants to the procedure. Approximately 20% of the participants in the intervention arm were lost to follow‐up, and the rate of technical success in the study was relatively low for both arms (54.5% for the DEB arm and 59.6% for the control arm). FemPac 2008 similarly did not have adequate follow‐up data on 27% of the DEB arm and 17% of the control arm. Furthermore, approximately 11% of the trial participants were stented, but the outcomes of those participants were not reported separately.
Allocation
Most of the included studies were at a low risk for sequence generation selection bias. IN.PACT DEEP 2014 randomized participants using "blocks of sealed envelopes" without specifying the methodology for generating those randomization blocks. LEVANT II 2015 did not specify how participants were randomized or allocated to either study arm.
Similarly, most studies were at a low risk for allocation concealment selection bias. However, four studies did not specify how allocation concealment bias was addressed (BIOLUX P‐II; FemPac 2008; LEVANT II 2015; THUNDER 2008).
Blinding
All of the included studies were at high risk for performance bias because the operators were not blinded to the procedure. In BIOLUX P‐II, neither the participants nor the operators were blinded.
Conversely, the risk of detection bias was low as study authors mostly ensured that outcome assessment was carried out by other blinded investigators. The DEBELLUM 2012 authors stated that "postoperative evaluation was deferred to different physicians not informed about the assigned intervention", but it was unclear what type of physicians performed those evaluations and what type of qualifications they had. The THUNDER 2008 authors stated that some of the operators also performed some of the poststudy evaluations.
Incomplete outcome data
Six trials were at high risk of attrition bias (BIOLUX P‐I; BIOLUX P‐II; FemPac 2008; IN.PACT DEEP 2014; LEVANT I 2014; PACIFIER 2012). In BIOLUX P‐I, four participants withdrew consent and five participants were lost to follow‐up, which equals a 15% attrition of the study population. Similarly, in BIOLUX P‐II, 19% of participants of the DEB arm either withdrew from the study or were lost to follow‐up at 12 months. FemPac 2008 had six‐month data available on only 73% of participants in the intervention arm and 83% of participants in the control arm. In IN.PACT DEEP 2014, the authors stated that low angiographic and wound imaging compliance may have limited the full assessment of the interventions. In LEVANT I 2014, six‐month angiographic follow‐up was available for 80% of the intervention arm and 69% of the control arm. The PACIFIER 2012 authors reported missing primary outcome data on 20.5% of the intervention arm and 27.3% of the control arm.
Selective reporting
Four trials were at high risk of bias due to incomplete reporting of data (BIOLUX P‐II; DEBATE‐BTK 2013; FemPac 2008; IN.PACT DEEP 2014). In BIOLUX P‐II, all prespecified outcomes were reported, but the results were not stratified by the type of treated infra‐popliteal vessels (i.e. anterior tibial, posterior tibial, or peroneal arteries). The authors also did not specify whether those participants with more than one target lesion (33.3% of DEB participants and 44.4% of percutaneous transluminal angioplasty (PTA) participants) had several target vessels and whether the outcomes differed by the number of treated lesions and vessels. DEBATE‐BTK 2013 was designed to assess infra‐popliteal arterial lesions but some of the participants also received treatment for femoropopliteal lesions. The study authors stated that inflow lesions located in the femoropopliteal segment were "treated by standard techniques during the same session" without elaborating on the nature or number of those techniques. FemPac 2008 reported that "no Doppler or angiographic information was obtained from 7 patients in the control and 9 patients in the coated balloon group", which amounts to approximately 18% of the study participant population. IN.PACT DEEP 2014 did not report several outcomes that were prespecified in the study protocol, such as change in Rutherford classification and QoL scores.
Other potential sources of bias
A major source of bias in many of the included studies was the concurrent use of stenting without separate reporting of the outcomes of stented participants. One quarter of the BIOLUX P‐I control arm (26.7%) required bailout stenting. LEVANT I 2014 randomized participants to the intervention or control arms after successful predilation or stenting "based on whether the interventionalist intended to use only balloon dilation of the lesion or intended concomitant stenting". Twenty‐six per cent of trial participants were stented prior to randomization, and a further 3% in the intervention and 16% in the control arm received "bailout stenting" after undergoing the intended therapy. The DEBELLUM 2012 authors reported that the "decision to implant a nitinol stent in the SFA [superficial femoral artery] territory was left to the judgment of the operator and typically driven by lesion length and presence of severe calcification". However, these stent‐deployment criteria were unclear, and approximately 37% of the treated lesions in the study were stented. The FemPac 2008 authors similarly reported that 11% of all participants received a stent, the PACIFIER 2012 authors reported that 21% of intervention and 34% of control participants received a stent, and in THUNDER 2008, 4% of intervention and 22% of control participants received a stent. The clinical outcomes of those stented participants were not reported separately. LEVANT II 2015 addressed this potential source of bias by only randomizing participants who did not require a stent after an initial angiogram.
In BIOLUX P‐I, predilation was performed more often in DEB than PTA participants (66.7% with DEB versus 30% with PTA, P = 0.01), and technical success was higher in the DEB group (76.7% with DEB versus 46.7% with PTA, P = 0.02). Most of the DEB (56.7%) and PTA (60%) participants had a history of previous peripheral interventions, although the type and location of those interventions was not specified.
In BIOLUX P‐II, while no bailout stenting was required in either treatment arm, the authors had a relatively low technical success rate (defined as less than 30% residual stenosis) in both arms (54.2% with DEB, 59.6% with PTA).
Device malfunction in LEVANT I 2014 was another potential source of bias. Eight DEB devices (16%) malfunctioned and failed to deploy. It was unclear how those participants were managed.
The approach to antiplatelet therapy also varied between trials. While FemPac 2008 did not specify the duration of antiplatelet therapy, participants in all the other trials received acetylsalicylic acid (ASA; aspirin) and at least four weeks of a second antiplatelet agent, most commonly clopidogrel. However, in IN.PACT DEEP 2014 and IN.PACT SFA 2015, the duration of the second antiplatelet agent depended on by whether a stent was deployed. Nonstented participants received a minimum of one month, while stented participants received a minimum of three months of a second antiplatelet agent.
While paclitaxel was used in the intervention arm of all the analyzed studies, there was variability in the paclitaxel dose and balloon drug carrier according to the type of DEB device used.
Finally, DEB device manufacturers sponsored nine of the 11 included studies (BIOLUX P‐I; BIOLUX P‐II; FemPac 2008; IN.PACT DEEP 2014; IN.PACT SFA 2015; LEVANT I 2014; LEVANT II 2015; PACIFIER 2012; THUNDER 2008). In the IN.PACT SFA 2015 study, every author listed in the published manuscript declared a financial relationship with the DEB device manufacturer.
Effects of interventions
See: Table 1
Primary outcomes
Amputation
The incidence of amputation was reported as a secondary endpoint in the included trials. While this outcome was not listed in the study protocol, we included it in the analysis because only one trial reported amputation‐free survival (IN.PACT DEEP 2014). There was no significant difference in the incidence of amputations between DEB and uncoated balloon angioplasty at six months (Analysis 1.1; OR 1.21, 95% CI 0.39 to 3.80; 541 participants; 7 studies); 12 months (Analysis 2.1; OR 1.56, 95% CI 0.73 to 3.33; 1649 participants; 9 studies); two years (Analysis 3.1; OR 0.65, 95% CI 0.11 to 3.88; 493 participants; 3 studies); or five years (Analysis 4.1; OR 4.82, 95% CI 0.52 to 44.70; 102 participants; 1 study).
1.1. Analysis.
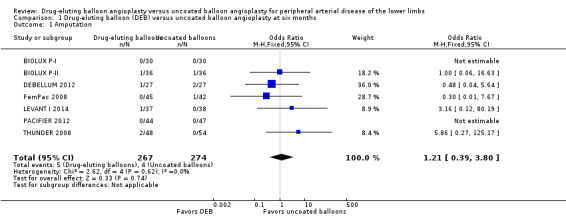
Comparison 1 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months, Outcome 1 Amputation.
2.1. Analysis.
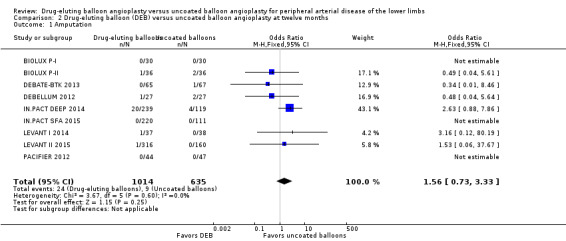
Comparison 2 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months, Outcome 1 Amputation.
3.1. Analysis.
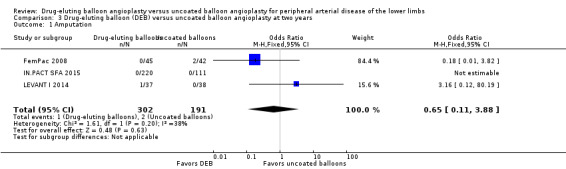
Comparison 3 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at two years, Outcome 1 Amputation.
4.1. Analysis.
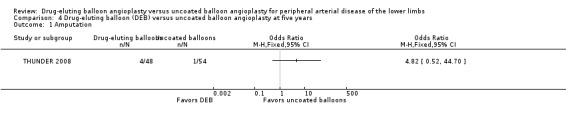
Comparison 4 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at five years, Outcome 1 Amputation.
Exclusion of BIOLUX P‐I, BIOLUX P‐II, and FemPac 2008 at six and 12 months and two years did not result in any significant differences in amputation outcomes (Analysis 1.11; OR 1.77, 95% CI 0.42 to 7.54; 322 participants; 4 studies at six months; Analysis 2.12; OR 1.78, 95% CI 0.79 to 4.04; 1517 participants; 7 studies at 12 months; Analysis 3.11; OR 3.16, 95% CI 0.12 to 80.19; 406 participants; 2 studies at two years). The sensitivity analysis was carried out due to concerns about participant follow‐up (BIOLUX P‐I; BIOLUX P‐II; FemPac 2008), and technical success rate (BIOLUX P‐II) in the study population.
1.11. Analysis.
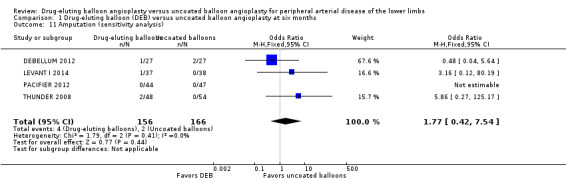
Comparison 1 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months, Outcome 11 Amputation (sensitivity analysis).
2.12. Analysis.
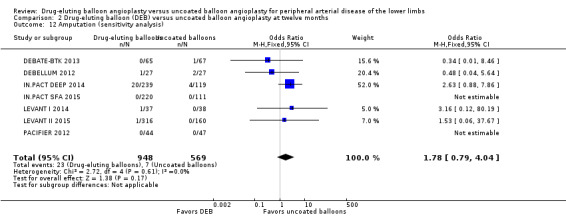
Comparison 2 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months, Outcome 12 Amputation (sensitivity analysis).
3.11. Analysis.
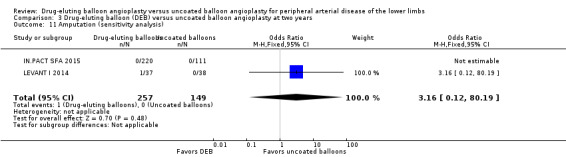
Comparison 3 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at two years, Outcome 11 Amputation (sensitivity analysis).
Amputation‐free survival
Only IN.PACT DEEP 2014 reported amputation‐free survival at 12 months in (Analysis 2.2; OR 0.68, 95% CI 0.38 to 1.19; 358 participants; 1 study). The trial showed a trend towards improved amputation‐free survival among control arm participants compared with the DEB arm, although this difference did not reach statistical significance.
2.2. Analysis.
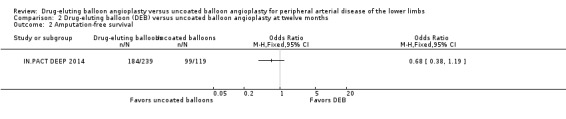
Comparison 2 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months, Outcome 2 Amputation‐free survival.
Amputation‐free rate
None of the included studies reported amputation‐free rate.
Vessel patency
Primary vessel patency
There was no significant difference in primary vessel patency between DEB and uncoated balloon angioplasty at six months (Analysis 1.2; OR 1.47, 95% CI 0.22 to 9.57; 162 participants; 2 studies). However, exclusion of FemPac 2008 resulted in a significant advantage for DEB compared with uncoated balloon angioplasty at six months (Analysis 1.12; OR 3.84, 95% CI 1.43 to 10.31; 75 participants; 1 study). FemPac 2008 was excluded due to concerns about incomplete participant follow‐up. DEBs were associated with significantly greater odds of primary vessel patency at 12 months (Analysis 2.3; OR 1.92, 95% CI 1.45 to 2.56; 882 participants; 3 studies) and two years (Analysis 3.2; OR 3.51, 95% CI 2.26 to 5.46; 406 participants; 2 studies).
1.2. Analysis.
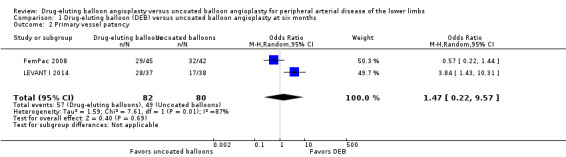
Comparison 1 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months, Outcome 2 Primary vessel patency.
1.12. Analysis.
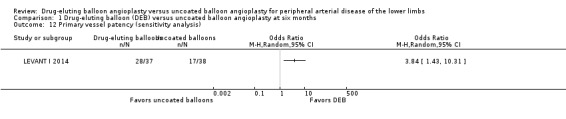
Comparison 1 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months, Outcome 12 Primary vessel patency (sensitivity analysis).
2.3. Analysis.
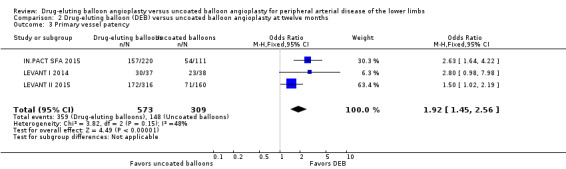
Comparison 2 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months, Outcome 3 Primary vessel patency.
3.2. Analysis.
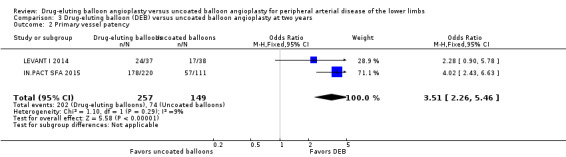
Comparison 3 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at two years, Outcome 2 Primary vessel patency.
Secondary vessel patency
None of the included trials reported secondary vessel patency rates.
Late lumen loss
Participants treated with DEB were less likely to develop late lumen loss at six months compared with the control participants (Analysis 1.3; MD ‐0.64 mm, 95% CI ‐1.00 to ‐0.28; 603 participants; 7 studies). However, this advantage was lost after 12 months, primarily due to the inclusion of IN.PACT DEEP 2014 (Analysis 2.4; MD ‐0.73 mm, 95% CI ‐1.59 to 0.13; 535 participants; 3 studies), which was limited to infra‐popliteal vessels that were smaller than the femoropopliteal vessels studied in the other trials. When the analysis excluded IN.PACT DEEP 2014, there was less late lumen loss with DEBs compared with uncoated balloon angioplasty (Analysis 2.15; MD ‐1.10 mm, 95% CI ‐1.41 to ‐0.79; 177 participants; 2 studies). There was an advantage for DEB compared with uncoated balloon angioplasty at two years (Analysis 3.3; MD ‐0.80 mm, 95% CI ‐1.44 to ‐0.16; 102 participants; 1 study).
1.3. Analysis.
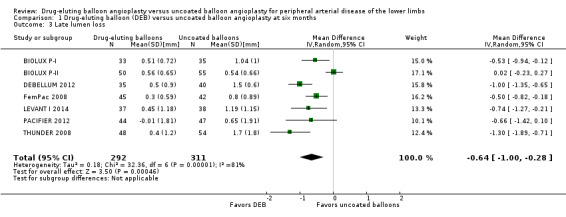
Comparison 1 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months, Outcome 3 Late lumen loss.
2.4. Analysis.
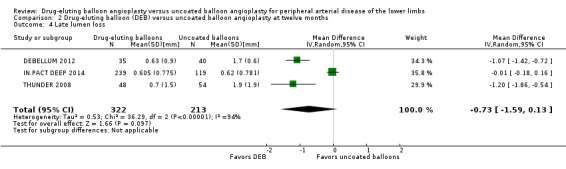
Comparison 2 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months, Outcome 4 Late lumen loss.
2.15. Analysis.
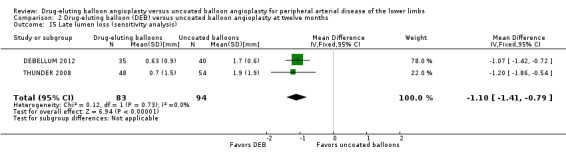
Comparison 2 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months, Outcome 15 Late lumen loss (sensitivity analysis).
3.3. Analysis.
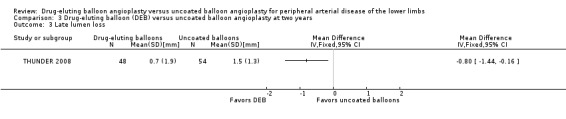
Comparison 3 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at two years, Outcome 3 Late lumen loss.
Exclusion of FemPac 2008, which reported late lumen losses as medians, and the BIOLUX P‐I and BIOLUX P‐II studies did not impact the late lumen loss advantage of DEB compared with uncoated balloon angioplasty at six months (Analysis 1.13; MD ‐0.96 mm, 95% CI ‐1.21 to ‐0.71; 343 participants; 4 studies).
1.13. Analysis.
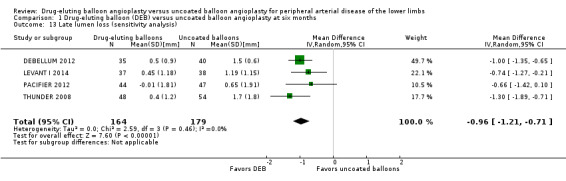
Comparison 1 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months, Outcome 13 Late lumen loss (sensitivity analysis).
Target lesion revascularization
The DEB arm of the trials had a clear advantage in target lesion revascularization compared with uncoated balloon angioplasty at six months (Analysis 1.4; OR 0.28, 95% CI 0.17 to 0.47; 603 participants; 7 studies), 12 months (Analysis 2.5; OR 0.40, 95% CI 0.31 to 0.51; 1900 participants; 11 studies), two years (Analysis 3.4; OR 0.28, 95% CI 0.18 to 0.44; 508 participants; 3 studies), and five years (Analysis 4.2; OR 0.21, 95% CI 0.09 to 0.51; 102 participants; 1 study).
1.4. Analysis.
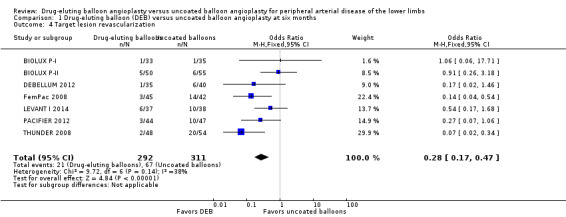
Comparison 1 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months, Outcome 4 Target lesion revascularization.
2.5. Analysis.
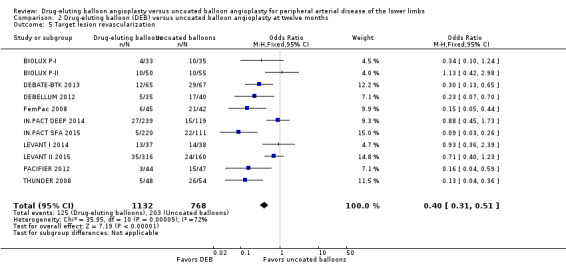
Comparison 2 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months, Outcome 5 Target lesion revascularization.
3.4. Analysis.
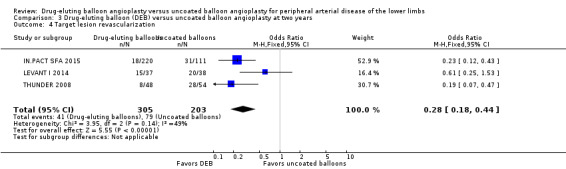
Comparison 3 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at two years, Outcome 4 Target lesion revascularization.
4.2. Analysis.
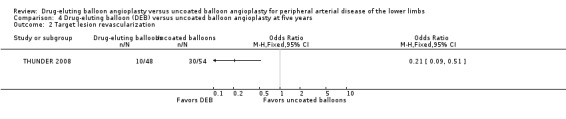
Comparison 4 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at five years, Outcome 2 Target lesion revascularization.
Exclusion of BIOLUX P‐I, BIOLUX P‐II, and FemPac 2008 at six and 12 months did not impact the target lesion revascularization advantage of DEB compared with uncoated balloon angioplasty (Analysis 1.14; OR 0.22, 95% CI 0.11 to 0.44; 343 participants; 4 studies at six months; Analysis 2.13; OR 0.33, 95% CI 0.18 to 0.61; 1640 participants; 8 studies at 12 months).
1.14. Analysis.
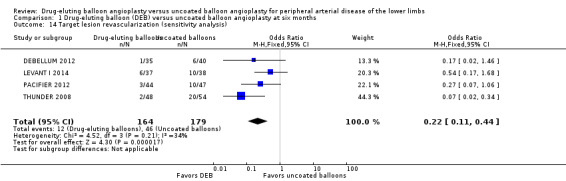
Comparison 1 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months, Outcome 14 Target lesion revascularization (sensitivity analysis).
2.13. Analysis.
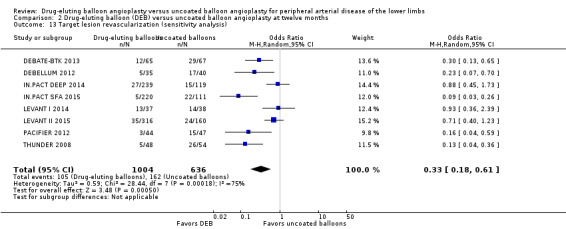
Comparison 2 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months, Outcome 13 Target lesion revascularization (sensitivity analysis).
A funnel plot constructed to test for publication bias in target lesion revascularization at 12 months demonstrated asymmetry, which was consistent with the heterogeneity observed in the analysis (Chi2 test for heterogeneity P < 0.0001) (Figure 4).
4.
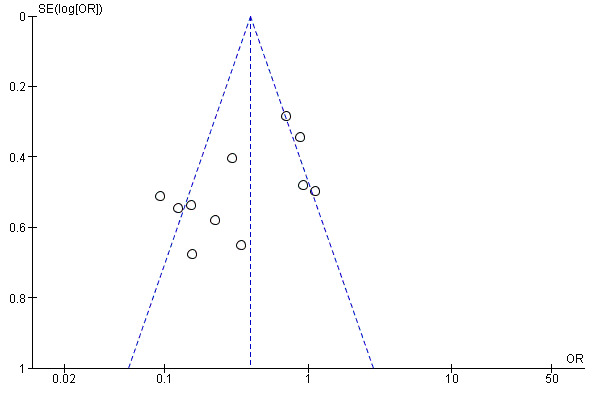
Funnel plot of comparison: 2 Drug‐eluting balloon versus uncoated balloon angioplasty at 12 months, outcome: 2.5 Target lesion revascularization.
Binary restenosis rate
Participants treated with DEBs had significantly lower odds of developing binary restenosis compared with participants in the control group at six months (Analysis 1.5; OR 0.44, 95% CI 0.29 to 0.67; 528 participants; 6 studies), 12 months (Analysis 2.6; OR 0.38, 95% CI 0.15 to 0.98; 1094 participants; 4 studies), two years (Analysis 3.5; OR 0.26, 95% CI 0.10 to 0.66; 87 participants; 1 stud), and five years (Analysis 4.3; OR 0.12, 95% CI 0.05 to 0.30; 102 participants; 1 study).
1.5. Analysis.
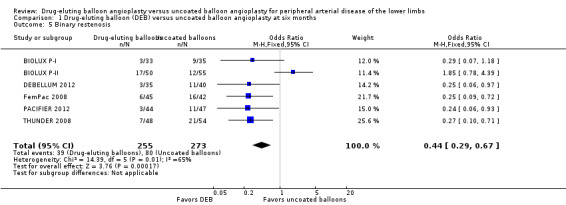
Comparison 1 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months, Outcome 5 Binary restenosis.
2.6. Analysis.
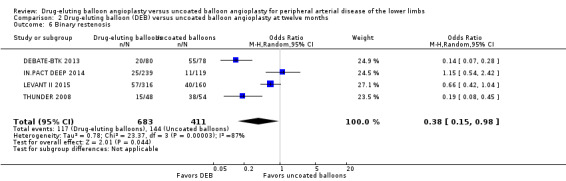
Comparison 2 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months, Outcome 6 Binary restenosis.
3.5. Analysis.
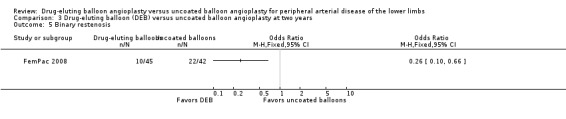
Comparison 3 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at two years, Outcome 5 Binary restenosis.
4.3. Analysis.
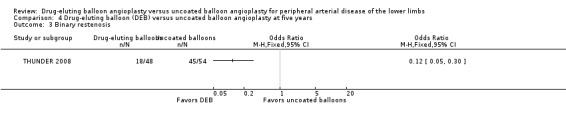
Comparison 4 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at five years, Outcome 3 Binary restenosis.
Exclusion of BIOLUX P‐I, BIOLUX P‐II, and FemPac 2008 at six months did not impact the binary restenosis rate advantage of DEB compared with uncoated balloon angioplasty (Analysis 1.15; OR 0.25, 95% CI 0.13 to 0.50; 268 participants; 3 studies).
1.15. Analysis.
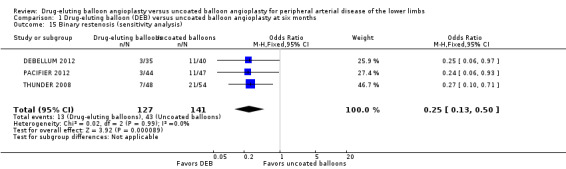
Comparison 1 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months, Outcome 15 Binary restenosis (sensitivity analysis).
Death
There was no significant difference in mortality between participants who underwent DEB or uncoated balloon angioplasty at six months (Analysis 1.6; OR 0.81, 95% CI 0.31 to 2.14; 541 participants; 7 studies), 12 months (Analysis 2.7; OR 1.04, 95% CI 0.64 to 1.71; 1649 participants; 9 studies), and five years (Analysis 4.4; OR 1.92, 95% CI 0.71 to 5.19; 102 participants; 1 study). At two years, uncoated balloon angioplasty had a slight mortality advantage compared with DEB (Analysis 3.6; OR 2.13, 95% CI 1.08 to 4.20; 595 participants; 4 studies).
1.6. Analysis.
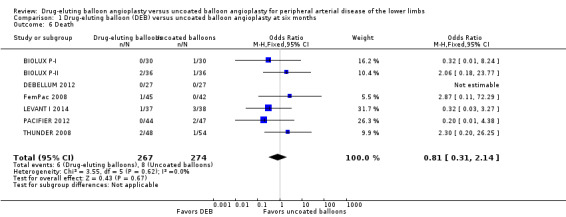
Comparison 1 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months, Outcome 6 Death.
2.7. Analysis.
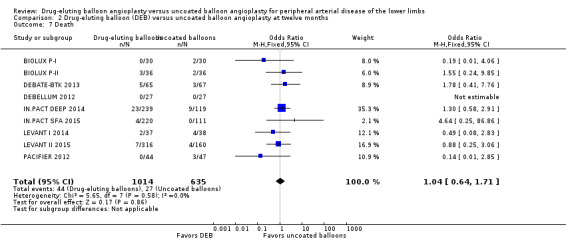
Comparison 2 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months, Outcome 7 Death.
4.4. Analysis.
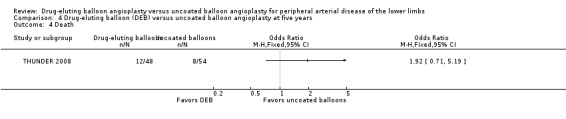
Comparison 4 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at five years, Outcome 4 Death.
3.6. Analysis.
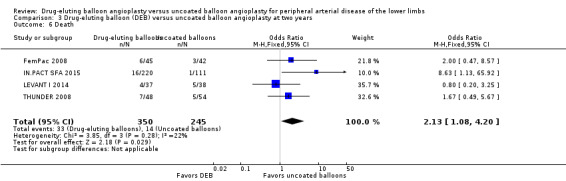
Comparison 3 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at two years, Outcome 6 Death.
Exclusion of BIOLUX P‐I, BIOLUX P‐II, and FemPac 2008 at six months, 12 months, and two years did not result in a significant difference in mortality between the treatment groups (Analysis 1.16; OR 0.57, 95% CI 0.15 to 2.11; 322 participants; 4 studies at six months; Analysis 2.14; OR 1.09, 95% CI 0.64 to 1.85; 1517 participants; 7 studies at 12 months; Analysis 3.12; OR 2.16, 95% CI 1.00 to 4.67; 508 participants; 3 studies at two years).
1.16. Analysis.
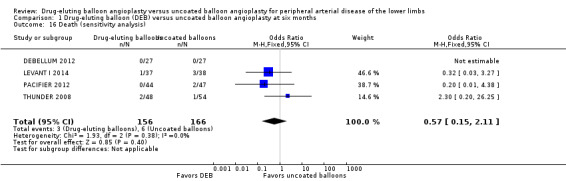
Comparison 1 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months, Outcome 16 Death (sensitivity analysis).
2.14. Analysis.
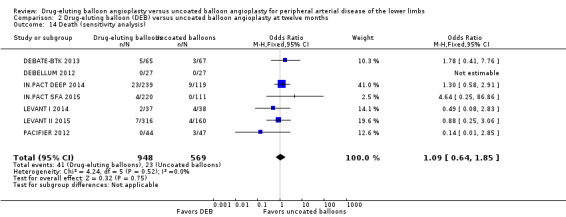
Comparison 2 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months, Outcome 14 Death (sensitivity analysis).
3.12. Analysis.
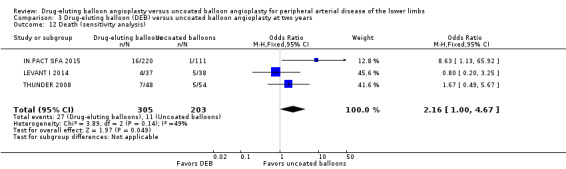
Comparison 3 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at two years, Outcome 12 Death (sensitivity analysis).
Secondary outcomes
Change in Fontaine stage or Rutherford category of peripheral arterial disease
There was no significant difference in the change in Rutherford category between the DEB and uncoated balloon angioplasty arms at six months (Analysis 1.7; MD ‐0.07, 95% CI ‐0.49 to 0.36; 249 participants; 3 studies), 12 months (Analysis 2.8; MD ‐0.10, 95% CI ‐0.29 to 0.10; 623 participants; 3 studies), and two years (Analysis 3.7; MD ‐0.30, 95% CI ‐0.80 to 0.20; 75 participants; 1 study).
1.7. Analysis.
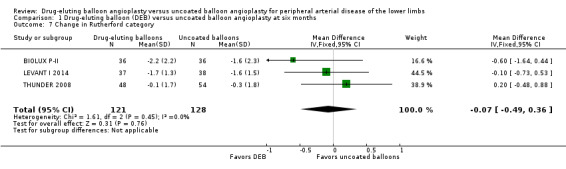
Comparison 1 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months, Outcome 7 Change in Rutherford category.
2.8. Analysis.
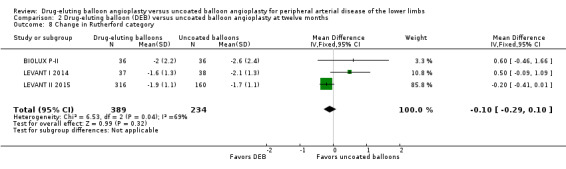
Comparison 2 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months, Outcome 8 Change in Rutherford category.
3.7. Analysis.
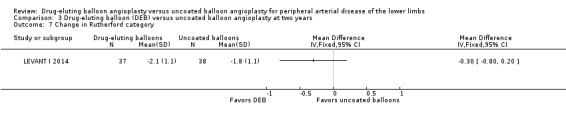
Comparison 3 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at two years, Outcome 7 Change in Rutherford category.
Exclusion of BIOLUX P‐II at six and 12 months did not result in a significant change in Rutherford category from baseline between the two treatment groups (Analysis 1.17; MD 0.04, 95% CI ‐0.42 to 0.50; 177 participants; 2 studies at six months; Analysis 2.16; MD 0.09, 95% CI ‐0.58 to 0.77; 551 participants; 2 studies at 12 months).
1.17. Analysis.
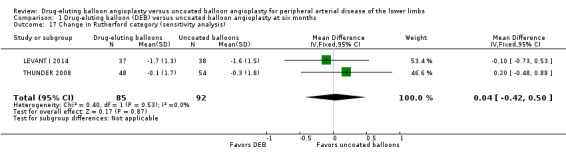
Comparison 1 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months, Outcome 17 Change in Rutherford category (sensitivity analysis).
2.16. Analysis.
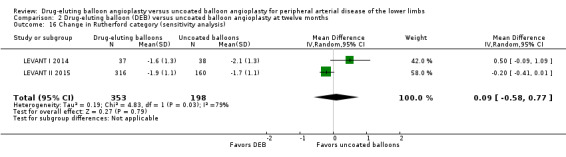
Comparison 2 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months, Outcome 16 Change in Rutherford category (sensitivity analysis).
One trial reported the Fontaine stage rather than the Rutherford classification system (DEBELLUM 2012). Our analysis did not include these data, however, because the authors reported the Fontaine class at six and 12 months rather than the change in Fontaine class from baseline. At six months, the DEB participants were 92% Fontaine class I, 8% class IIa, and 0% class IIb or III, versus uncoated balloon angioplasty participants who were 67% class I, 13% class IIa, 7% class IIb, and 13% class III. At 12 months, the DEB participants were 77% class I, 8% class IIa, 15% class IIb, and 0% class III, versus uncoated balloon angioplasty participants who were 60% class I, 13% class IIa, 13% class IIb, and 13% class III.
Change in ankle‐brachial index
There was no significant advantage for DEB when evaluating change in ABI at six months (Analysis 1.8; MD ‐0.00, 95% CI ‐0.19 to 0.18; 369 participants; 4 studies), 12 months (Analysis 2.9; MD ‐0.03, 95% CI ‐0.07 to 0.01; 656 participants; 3 studies), and two years (Analysis 3.8; MD 0.02, 95% CI ‐0.12 to 0.16; 83 participants; 1 study).
1.8. Analysis.
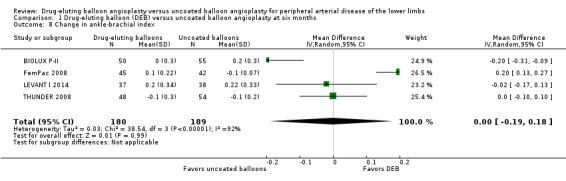
Comparison 1 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months, Outcome 8 Change in ankle‐brachial index.
2.9. Analysis.
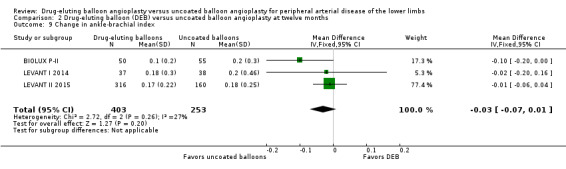
Comparison 2 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months, Outcome 9 Change in ankle‐brachial index.
3.8. Analysis.
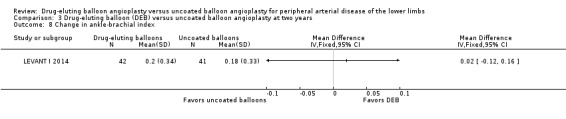
Comparison 3 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at two years, Outcome 8 Change in ankle‐brachial index.
Exclusion of FemPac 2008, which reported changes in ABI as medians, and BIOLUX P‐II demonstrated no advantage in ABI change for DEB compared with uncoated balloon angioplasty at six and 12 months (Analysis 1.18; MD ‐0.01, 95% CI ‐0.09 to 0.08; 177 participants; 2 studies at six months; Analysis 2.17; MD ‐0.01, 95% CI ‐0.05 to 0.03; 551 participants; 2 studies at 12 months).
1.18. Analysis.
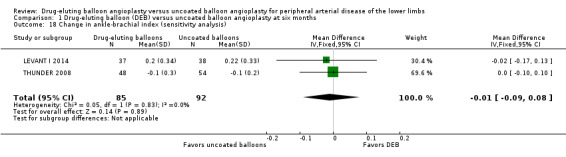
Comparison 1 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months, Outcome 18 Change in ankle‐brachial index (sensitivity analysis).
2.17. Analysis.
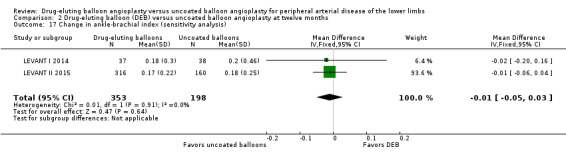
Comparison 2 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months, Outcome 17 Change in ankle‐brachial index (sensitivity analysis).
Change in quality of life scores
The Euro‐Qol Group 5‐Dimension Self‐Report Questionnaire (EQ‐5D) QoL scores were reported at six months (BIOLUX P‐II), 12 months (BIOLUX P‐II; IN.PACT SFA 2015; LEVANT II 2015) and two years (IN.PACT SFA 2015). There was no significant difference in the change of EQ‐5D scores between the DEB and uncoated balloon angioplasty arms (Analysis 1.9; MD ‐0.10, 95% CI ‐0.22 to 0.02; 72 participants; 1 study at six months, Analysis 2.10; MD 0.01, 95% CI ‐0.02 to 0.04; 879 participants; 3 studies at 12 months; Analysis 3.9; MD 0.04; 95% CI ‐0.01 to 0.09; 331 participants; 1 study at two years).
1.9. Analysis.
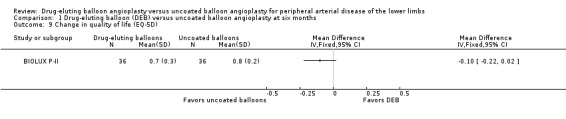
Comparison 1 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months, Outcome 9 Change in quality of life (EQ‐5D).
2.10. Analysis.
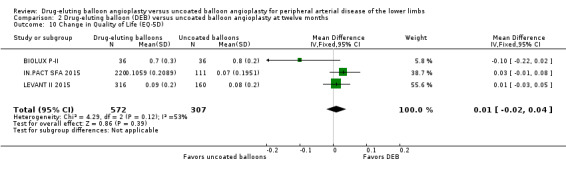
Comparison 2 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months, Outcome 10 Change in Quality of Life (EQ‐5D).
3.9. Analysis.
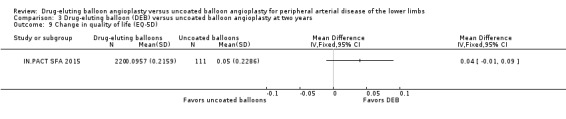
Comparison 3 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at two years, Outcome 9 Change in quality of life (EQ‐5D).
Exclusion of BIOLUX P‐II at 12 months did not result in a significant change in QoL scores from baseline between the two treatment groups (Analysis 2.18; MD 0.02, 95% CI ‐0.01 to 0.05; 807 participants; 2 studies).
2.18. Analysis.
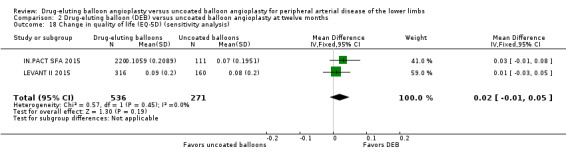
Comparison 2 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months, Outcome 18 Change in quality of life (EQ‐5D) (sensitivity analysis).
LEVANT II 2015 also reported 36‐item Short Form (SF‐36) Physical and Mental component scores at 12 months but demonstrated no significant difference. The MD and standard deviation (SD) in the Physical component score between the intervention and control arms was 0.6 ± 11 (95% CI ‐1.7 to 2.9). Similarly, the difference in the Mental component score between the intervention and control arms was ‐0.2 ± 12.8 (95% CI ‐2.9 to 2.5).
Change in functional walking ability
Two trials assessed the change in functional walking ability using the WIQ (LEVANT I 2014; LEVANT II 2015). There were no significant differences in WIQ scores between DEB and uncoated balloon angioplasty at six months (Analysis 1.10; MD ‐0.70, 95% CI ‐16.00 to 14.60; 75 participants; 1 study), 12 months (Analysis 2.11; MD 3.57, 95% CI ‐1.23 to 8.38; 513 participants; 2 studies), and two years (Analysis 3.10; MD 0.50, 95% CI ‐13.42 to 14.42; 75 participants; 1 study).
1.10. Analysis.
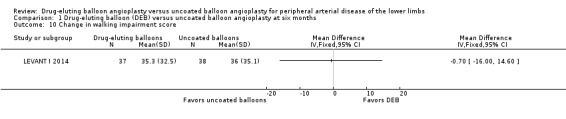
Comparison 1 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months, Outcome 10 Change in walking impairment score.
2.11. Analysis.
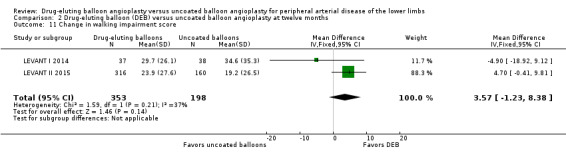
Comparison 2 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months, Outcome 11 Change in walking impairment score.
3.10. Analysis.
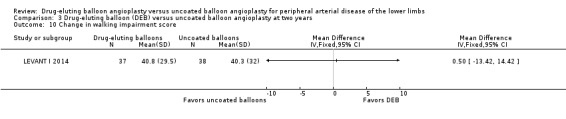
Comparison 3 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at two years, Outcome 10 Change in walking impairment score.
Subgroup analysis
Arterial segments
Seven trials included only femoropopliteal arterial lesions (BIOLUX P‐I; FemPac 2008; IN.PACT SFA 2015; LEVANT I 2014; LEVANT II 2015; PACIFIER 2012; THUNDER 2008), and three trials included only tibial arterial lesions (BIOLUX P‐II; DEBATE‐BTK 2013; IN.PACT DEEP 2014). DEBELLUM 2012 included both femoropopliteal and tibial lesions, but did not stratify the outcomes by type of arterial segment and as such was excluded from this subgroup analysis. Data were available to permit subgroup analysis by arterial segment at six and 12 months for the following outcomes: amputation, late lumen loss, target lesion revascularization, binary restenosis, death, change in Rutherford category, and change in ABI.
Amputation
There was no significant difference in the incidence of amputation by arterial segment at six months (Analysis 5.1; OR 1.87, 95% CI 0.40 to 8.75; 415 participants; 5 studies for femoropopliteal vessels versus OR 1.00, 95% CI 0.06 to 16.63; 72 participants; 1 study for tibial vessels; P = 0.70) or 12 months (Analysis 6.1; OR 2.21, 95% CI 0.23 to 21.51; 1033 participants; 5 studies for femoropopliteal vessels versus OR 1.95, 95% CI 0.78 to 4.89; 562 participants; 3 studies for tibial vessels; P = 0.92).
5.1. Analysis.
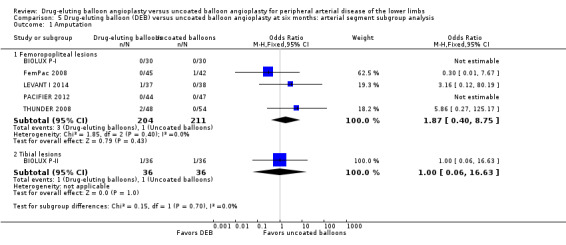
Comparison 5 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months: arterial segment subgroup analysis, Outcome 1 Amputation.
6.1. Analysis.
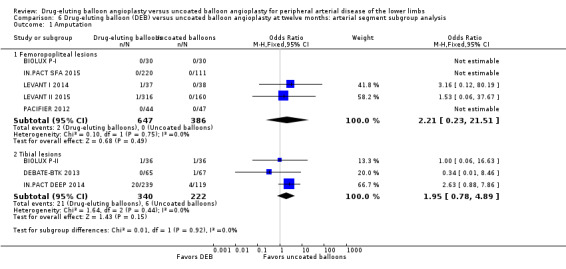
Comparison 6 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months: arterial segment subgroup analysis, Outcome 1 Amputation.
Exclusion of BIOLUX P‐I and FemPac 2008 did not result in a significant change in the incidence of amputation for DEB compared with uncoated balloon angioplasty at six months (Analysis 5.8; OR 4.47, 95% CI 0.49 to 40.67; 268 participants; 3 studies for femoropopliteal vessels) and 12 months (Analysis 6.9; OR 2.21, 95% CI 0.23 to 21.51; 973 participants; 4 studies for femoropopliteal vessels versus OR 2.10, 95% CI 0.79 to 5.59; 490 participants; 2 studies for tibial vessels).
5.8. Analysis.
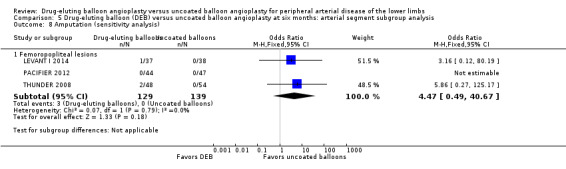
Comparison 5 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months: arterial segment subgroup analysis, Outcome 8 Amputation (sensitivity analysis).
6.9. Analysis.
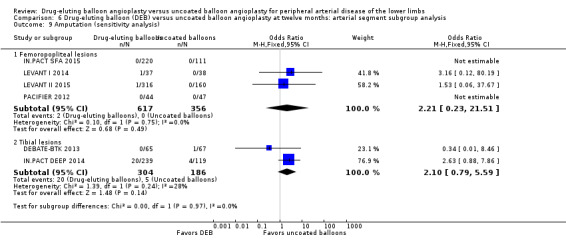
Comparison 6 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months: arterial segment subgroup analysis, Outcome 9 Amputation (sensitivity analysis).
Late lumen loss
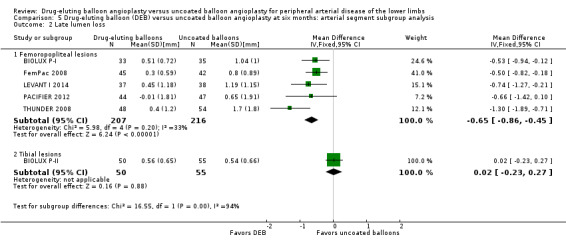
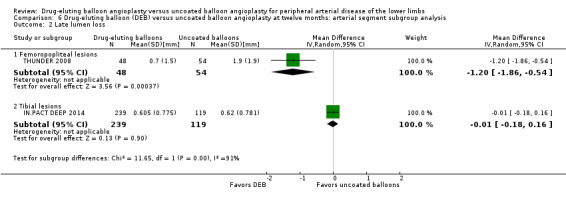
Comparison of the arterial segment subgroups demonstrated a significant difference in late lumen loss favoring the DEB arm of the femoropopliteal group at six months (Analysis 5.2; MD ‐0.65 mm, 95% CI ‐0.86 to ‐0.45; 423 participants; 5 studies for femoropopliteal vessels versus MD 0.02 mm, 95% CI ‐0.23 to 0.27; 105 participants; 1 study tibial vessels; P < 0.0001) and 12 months (Analysis 6.2; MD ‐1.20 mm, 95% CI ‐1.86 to ‐0.54; 102 participants; 1 study for femoropopliteal vessels versus MD ‐0.01 mm, 95% CI ‐0.18 to 0.16; 358 participants; 1 study for tibial vessels; P = 0.0006).
5.2. Analysis.
Comparison 5 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months: arterial segment subgroup analysis, Outcome 2 Late lumen loss.
6.2. Analysis.
Comparison 6 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months: arterial segment subgroup analysis, Outcome 2 Late lumen loss.
Exclusion of BIOLUX P‐I and FemPac 2008 did not result in a significant change in the late lumen loss advantage of DEB compared with uncoated balloon angioplasty at six months (Analysis 5.9; MD ‐0.92 mm, 95% CI ‐1.27 to ‐0.57; 268 participants; 3 studies for femoropopliteal vessels).
5.9. Analysis.
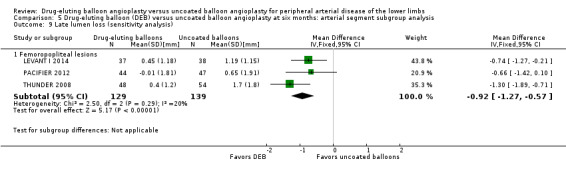
Comparison 5 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months: arterial segment subgroup analysis, Outcome 9 Late lumen loss (sensitivity analysis).
Given the intrinsic lumen diameter differences between femoropopliteal and tibial vessels, the implications of this analysis are limited.
Target lesion revascularization
Comparison of the arterial segment subgroups demonstrated a significant advantage in target lesion revascularization for the DEB arm of the femoropopliteal subgroup, but there was no significant difference between the DEB and control arms of the tibial subgroup at six months (Analysis 5.3; OR 0.22, 95% CI 0.12 to 0.41; 423 participants; 5 studies for femoropopliteal vessels versus OR 0.91, 95% CI 0.26 to 3.18; 105 participants; 1 study for tibial vessels; P = 0.05) and 12 months (Analysis 6.3; OR 0.26, 95% CI 0.13 to 0.56; 1230 participants; 7 studies for femoropopliteal vessels versus OR 0.65, 95% CI 0.30 to 1.44; 595 participants; 3 studies for tibial vessels; P = 0.10).
5.3. Analysis.
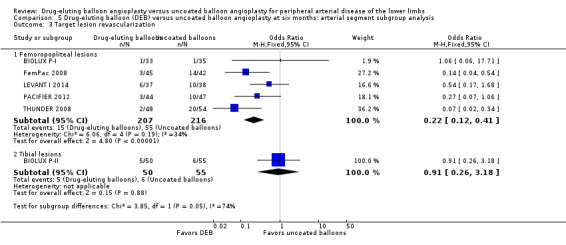
Comparison 5 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months: arterial segment subgroup analysis, Outcome 3 Target lesion revascularization.
6.3. Analysis.
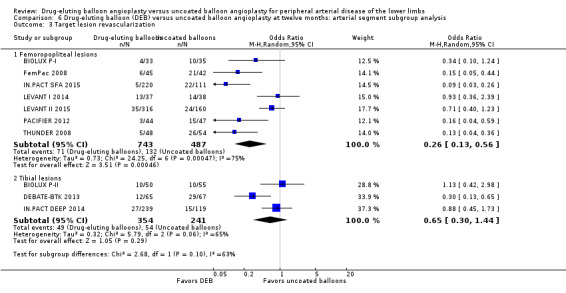
Comparison 6 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months: arterial segment subgroup analysis, Outcome 3 Target lesion revascularization.
Exclusion of BIOLUX P‐I, BIOLUX P‐II, and FemPac 2008 did not result in a significant change in the target lesion revascularization advantage of DEB compared with uncoated balloon angioplasty at six months (Analysis 5.10; OR 0.23, 95% CI 0.11 to 0.48; 268 participants; 3 studies for femoropopliteal vessels) and 12 months (Analysis 6.10; OR 0.28, 95% CI 0.10 to 0.73; 1075 participants; 5 studies for femoropopliteal vessels versus OR 0.52, 95% CI 0.18 to 1.52; 490 participants; 2 studies for tibial vessels; P = 0.23).
5.10. Analysis.
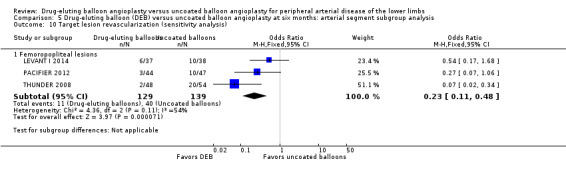
Comparison 5 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months: arterial segment subgroup analysis, Outcome 10 Target lesion revascularization (sensitivity analysis).
6.10. Analysis.
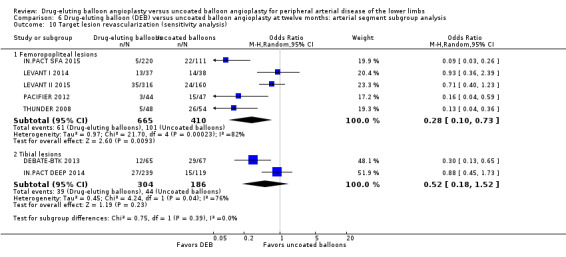
Comparison 6 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months: arterial segment subgroup analysis, Outcome 10 Target lesion revascularization (sensitivity analysis).
Binary restenosis
There was a significant advantage for DEB in the incidence of binary femoropopliteal vessel restenosis compared with uncoated balloon angioplasty at six months (Analysis 5.4; OR 0.26, 95% CI 0.15 to 0.46; 348 participants; 4 studies for femoropopliteal vessels versus OR 1.85, 95% CI 0.78 to 4.39; 105 participants; 1 study for tibial vessels; P = 0.0002). However, this effect was not sustained at 12 months (Analysis 6.4; OR 0.37, 95% CI 0.11 to 1.26; 578 participants; 2 studies for femoropopliteal vessels versus OR 0.40, 95% CI 0.05 to 3.14; 516 participants; 2 studies for tibial vessels; P = 0.96).
5.4. Analysis.
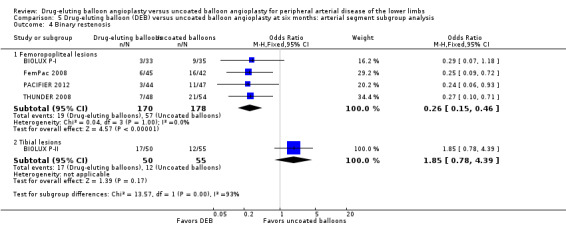
Comparison 5 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months: arterial segment subgroup analysis, Outcome 4 Binary restenosis.
6.4. Analysis.
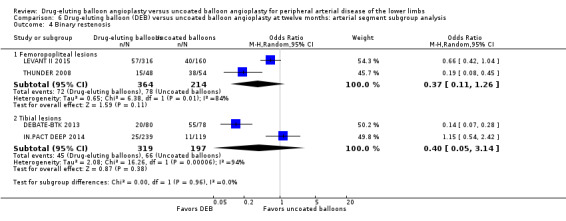
Comparison 6 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months: arterial segment subgroup analysis, Outcome 4 Binary restenosis.
Exclusion of BIOLUX P‐I and FemPac 2008 did not result in a significant change in the binary restenosis advantage of DEB compared with uncoated balloon angioplasty at six months (Analysis 5.11; OR 0.26, 95% CI 0.12 to 0.57; 193 participants; 2 studies).
5.11. Analysis.
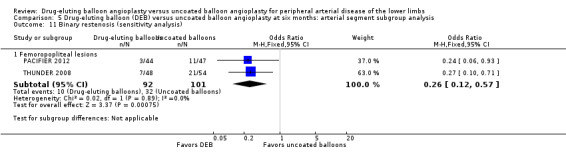
Comparison 5 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months: arterial segment subgroup analysis, Outcome 11 Binary restenosis (sensitivity analysis).
Death
Comparison of the arterial segment subgroups demonstrated no difference in mortality between DEB and uncoated balloon angioplasty at six months (Analysis 5.5; OR 0.66, 95% CI 0.22 to 1.97; 415 participants; 5 studies for femoropopliteal vessels versus OR 2.06, 95% CI 0.18 to 23.77; 72 participants; 1 study for tibial vessels; P = 0.41) and 12 months (Analysis 6.5; OR 0.67, 95% CI 0.31 to 1.46; 1033 participants; 5 studies for femoropopliteal vessels versus OR 1.41, 95% CI 0.73 to 2.74; 562 participants; 3 studies for tibial vessels; P = 0.15).
5.5. Analysis.
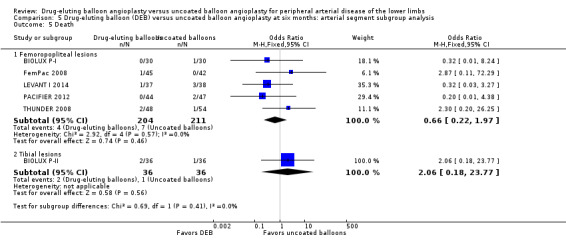
Comparison 5 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months: arterial segment subgroup analysis, Outcome 5 Death.
6.5. Analysis.
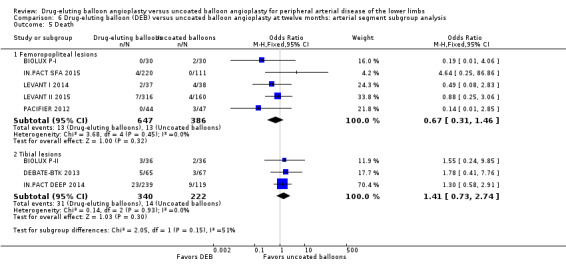
Comparison 6 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months: arterial segment subgroup analysis, Outcome 5 Death.
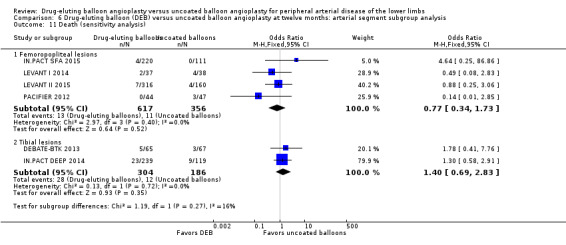
Exclusion of BIOLUX P‐I, BIOLUX P‐II, and FemPac 2008 did not result in a significant change in mortality for DEB compared with uncoated balloon angioplasty at six months (Analysis 5.12; OR 0.57, 95% CI 0.15 to 2.11; 268 participants; 3 studies for femoropopliteal vessels) and 12 months (Analysis 6.11; OR 0.77, 95% CI 0.34 to 1.73; 973 participants; 4 studies for femoropopliteal vessels versus OR 1.40, 95% CI 0.69 to 2.83; 490 participants; 2 studies for tibial vessels; P = 0.27).
5.12. Analysis.
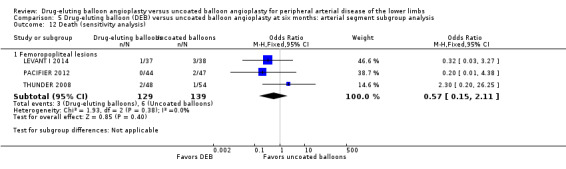
Comparison 5 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months: arterial segment subgroup analysis, Outcome 12 Death (sensitivity analysis).
6.11. Analysis.
Comparison 6 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months: arterial segment subgroup analysis, Outcome 11 Death (sensitivity analysis).
Change in Rutherford category
Comparison of the arterial segment subgroups demonstrated no difference in Rutherford category between DEB and uncoated balloon angioplasty at six months (Analysis 5.6; MD 0.04, 95% CI ‐0.42 to 0.50; 177 participants; 2 studies for femoropopliteal vessels versus MD ‐0.60, 95% CI ‐1.64 to 0.44; 72 participants; 1 study for tibial vessels; P = 0.27) and 12 months (Analysis 6.6; MD 0.09, 95% CI ‐0.58 to 0.77; 551 participants; 2 studies for femoropopliteal vessels versus MD 0.60, 95% CI ‐0.46 to 1.66; 72 participants; 1 study for tibial vessels; P = 0.43).
5.6. Analysis.
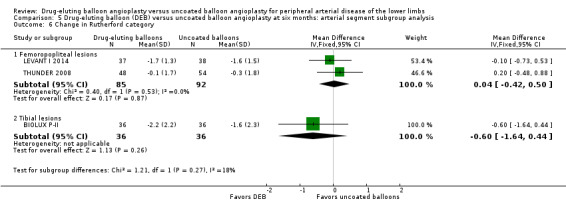
Comparison 5 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months: arterial segment subgroup analysis, Outcome 6 Change in Rutherford category.
6.6. Analysis.
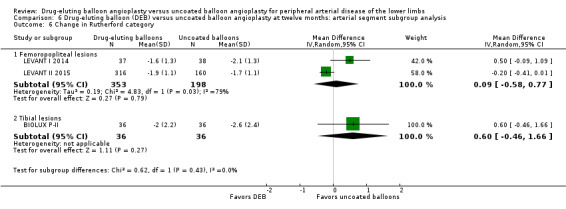
Comparison 6 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months: arterial segment subgroup analysis, Outcome 6 Change in Rutherford category.
Exclusion of FemPac 2008 did not result in a significant change in Rutherford category between DEB and uncoated balloon angioplasty at six months (Analysis 5.13; MD ‐0.01, 95% CI ‐0.09 to 0.08; 177 participants; 2 studies for femoropopliteal vessels).
5.13. Analysis.
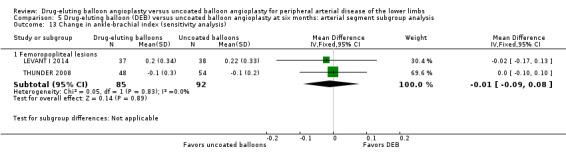
Comparison 5 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months: arterial segment subgroup analysis, Outcome 13 Change in ankle‐brachial index (sensitivity analysis).
Change in ankle‐brachial index
There was a significant change in ABI advantage for uncoated balloon angioplasty compared with DEB in tibial vessels, but this advantage was not seen in femoropopliteal vessels at six months (Analysis 5.7; MD 0.07, 95% CI ‐0.09 to 0.22; 264 participants; 3 studies for femoropopliteal vessels versus MD ‐0.20, 95% CI ‐0.31 to ‐0.09; 105 participants; 1 study for tibial vessels; P = 0.007) and 12 months (Analysis 6.7; MD ‐0.01, 95% CI ‐0.05 to 0.03; 551 participants; 2 studies for femoropopliteal vessels versus MD ‐0.20, 95% CI ‐0.31 to ‐0.09; 105 participants; 1 study for tibial vessels; P = 0.003).
5.7. Analysis.
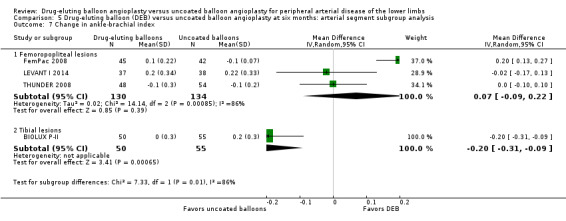
Comparison 5 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months: arterial segment subgroup analysis, Outcome 7 Change in ankle‐brachial index.
6.7. Analysis.
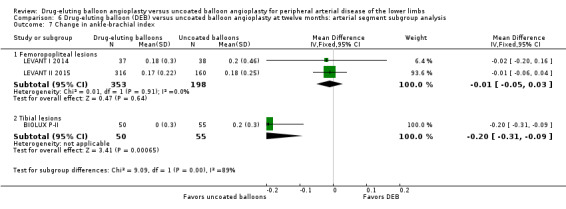
Comparison 6 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months: arterial segment subgroup analysis, Outcome 7 Change in ankle‐brachial index.
Exclusion of FemPac 2008 did not result in a significant change in ABI between DEB and uncoated balloon angioplasty for femoropopliteal vessels at six months (Analysis 5.13; MD ‐0.01, 95% CI ‐0.09 to 0.08; 177 participants; 2 studies for femoropopliteal vessels).
Severity of peripheral arterial disease
Two trials included only participants with CLI (Rutherford class 4 or greater) (DEBATE‐BTK 2013; IN.PACT DEEP 2014). The other trials included participants with varying degrees of PAD but did not stratify the outcomes by severity of PAD and as such were excluded from this subgroup analysis. Data were only available to permit subgroup analysis for participants with CLI at 12 months for the following outcomes: amputation, late lumen loss, target lesion revascularization, binary restenosis, and death.
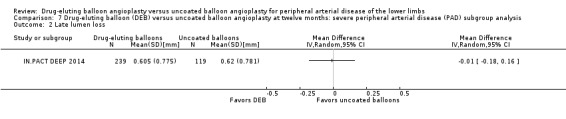
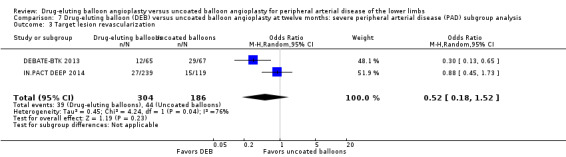
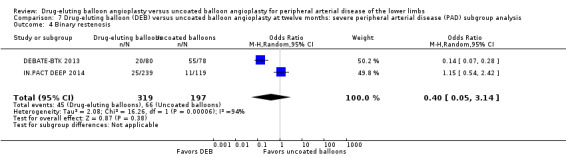
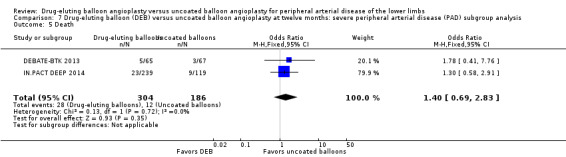
There was no significant difference in the incidence of amputation between the DEB and control arms in participants with CLI (Analysis 7.1; OR 2.10, 95% CI 0.79 to 5.59; 490 participants; 2 studies). Similarly, there was no difference in late lumen loss (Analysis 7.2; MD ‐0.01 mm, 95% CI ‐0.18 to 0.16; 358 participants; 1 study), target lesion revascularization (Analysis 7.3; OR 0.52, 95% CI 0.18 to 1.52; 490 participants; 2 studies); binary restenosis (Analysis 7.4; OR 0.40, 95% CI 0.05 to 3.14; 516 participants; 2 studies); or death (Analysis 7.5; OR 1.40, 95% CI 0.69 to 2.83; 490 participants; 2 studies), between the DEB and control arms in participants with CLI.
7.1. Analysis.
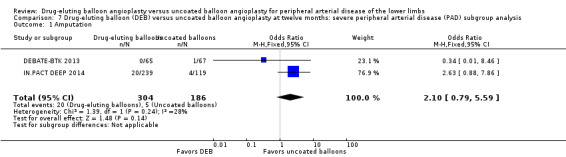
Comparison 7 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months: severe peripheral arterial disease (PAD) subgroup analysis, Outcome 1 Amputation.
7.2. Analysis.
Comparison 7 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months: severe peripheral arterial disease (PAD) subgroup analysis, Outcome 2 Late lumen loss.
7.3. Analysis.
Comparison 7 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months: severe peripheral arterial disease (PAD) subgroup analysis, Outcome 3 Target lesion revascularization.
7.4. Analysis.
Comparison 7 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months: severe peripheral arterial disease (PAD) subgroup analysis, Outcome 4 Binary restenosis.
7.5. Analysis.
Comparison 7 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months: severe peripheral arterial disease (PAD) subgroup analysis, Outcome 5 Death.
Discussion
Summary of main results
DEB angioplasty was associated with improved primary vessel patency and late lumen loss for up to two years and target lesion revascularization and binary restenosis rates for up to five years. However, there was no advantage for DEB in clinical endpoints such as amputation, change in Rutherford class, QoL scores, functional walking ability, or mortality compared with uncoated balloon angioplasty. On subgroup analysis, DEB angioplasty showed improved late lumen loss, target lesion revascularization, and binary restenosis for up to six months, and late lumen loss and target lesion revascularization for up to 12 months in femoropopliteal vessels. However, DEB angioplasty of tibial vessels was not superior to uncoated balloon angioplasty in any domains. Furthermore, DEB angioplasty was not superior to uncoated balloon angioplasty in people with CLI.
Overall completeness and applicability of evidence
We included 11 prospective randomized trials that were designed to compare clinical differences after DEB versus uncoated balloon angioplasty. The trial endpoints in all of the included studies were clinically relevant, patient‐oriented, and included a widely utilized mix of anatomic and clinical endpoints, which makes our findings clinically applicable.
While the trials had several limitations (listed in Assessment of risk of bias in included studies), most were conducted at high‐volume centers with experience in device‐efficacy trials. Three trials were at a high risk of bias and their impact was assessed with serial sensitivity analyses, but in most instances their exclusion had no significant impact (BIOLUX P‐I; BIOLUX P‐II; FemPac 2008).
A limitation of our subgroup analysis was that there were only three trials available for the tibial vessel analysis (BIOLUX P‐II; DEBATE‐BTK 2013; IN.PACT DEEP 2014), and only two trials for the CLI subgroup analysis (DEBATE‐BTK 2013; IN.PACT DEEP 2014). As such, it is unclear whether the subgroup analysis results were due to the effects of tibial arteries or CLI. Furthermore, we were unable to carry out any subgroup analyses by severity of PAD because none of the trials reported their outcomes by degree of severity.
Finally, several of the ongoing trials (listed in Characteristics of ongoing studies table) have not published their results in peer‐reviewed publications despite having completed enrolment for several years. Two of those trials started enrolling participants in 2008 (Advance 18PTX Balloon Catheter Study; PICCOLO). This is concerning for possible reporting bias if those privately sponsored trials were not published because of unfavorable results. We have attempted to contact the authors of all ongoing trials with limited success.
We have identified several trials that are still enrolling participants or have not published their results, and several of the studies included in our analysis have not yet published their long‐term results. As such, future versions of this review will hopefully be able to provide better evidence for the efficacy of DEB in managing lower‐limb ischemia.
Quality of the evidence
There was heterogeneity in the frequency of stent deployment and the type and duration of antiplatelet therapy between trials. Using GRADE assessment criteria, the quality of the evidence presented was low for the outcome of target lesion revascularization; moderate for binary restenosis and change in Rutherford category; and high for amputation, primary vessel patency, death, and change in ABI.
The included studies were powered to detect anatomic endpoints that were measured according to angiographic and ultrasound imaging criteria. As such, caution must be taken in interpreting the clinical endpoint results, since the included studies were unlikely to be powered to specifically assess those endpoints.
Risk of selection bias was low in most of the studies. Conversely, performance bias was uniformly high because of the difficulty in blinding operators to the interventions. However, this, is a limitation intrinsic to most surgical and procedural trials. The included studies mostly took adequate steps to address this limitation by implementing measures, such as independent core lab evaluation, to avoid detection bias. While follow‐up compliance was good in half of the included studies, most studies were at low risk for reporting bias.
There was a substantial amount of heterogeneity in the studies that may have biased the analysis. First, while the DEB in all of the included studies employed the mitotic inhibitor paclitaxel, the medication doses varied and were not consistently reported. Second, the DEB devices in the studies employed different drug carriers, as described in Schnorr 2013. The effect of different drug carriers on the results was not clear. Third, the type and duration of antiplatelet therapy differed between trials. Finally, the wide use of stenting in most of the included trials invariably affected the outcomes, but few data were available on the stented subgroups of participants.
It is unclear what criteria were used to determine the need for target lesion revascularization, resulting in heterogeneity between studies reporting this outcome. While most studies stated that target lesion revascularization was carried out for clinically driven indications (BIOLUX P‐I; DEBATE‐BTK 2013; DEBELLUM 2012; IN.PACT DEEP 2014; IN.PACT SFA 2015; LEVANT II 2015; PACIFIER 2012), those indications were not defined or explained.
Those limitations notwithstanding, our analysis demonstrates an advantage for DEB compared with uncoated balloon angioplasty in the management of PAD for up to two years of follow‐up in several anatomic endpoints. There is insufficient evidence to support an advantage for either treatment modality beyond two years. However, with many other ongoing or unreported studies, short‐ and long‐term data will hopefully become available to assess the efficacy of this treatment modality better in the near future.
Potential biases in the review process
We carried out our review in a transparent manner consistent with Cochrane guidelines. We conducted intention‐to‐treat analysis on all of the data in this study, and used the initial number of randomized participants in each study arm for all subsequent calculations regardless of participant death or loss to follow‐up. We encountered difficulty in obtaining unpublished information from some study authors (IN.PACT SFA 2015; LEVANT I 2014; LEVANT II 2015). Despite several attempts to contact the authors with requests for information, only three authors provided us with data for this analysis (BIOLUX P‐I; BIOLUX P‐II; DEBELLUM 2012). One trial reported medians instead of means for late lumen loss and change in ABI (FemPac 2008). We converted the median values to means and included them in the analysis. A subsequent sensitivity analysis did not demonstrate a significant impact of this study's inclusion on our results. Finally, our analysis at five years was based on only one study (THUNDER 2008).
Agreements and disagreements with other studies or reviews
In the past few years, several meta‐analyses have compared DEB with uncoated balloon angioplasty for femoropopliteal and tibial arterial lesions.
Baerlocher 2015 reported an advantage for DEB in the anatomic endpoints of late lumen loss, binary restenosis, and target lesion revascularization, and no advantage in clinical endpoints at 12 months. However, their analysis included IDEAS 2014, which compared DEB to drug‐eluting stents. The authors also carried out a tibial vessel analysis using DEBATE‐BTK 2013 and DEBELLUM 2012 and reported an advantage for DEBs in target lesion revascularization and binary restenosis at 12 months. We did not include DEBELLUM 2012 in our tibial vessel subgroup analysis because the authors of that study included both femoropopliteal and tibial lesions but did not report the results separately.
Canaud 2014 also reported an advantage for DEB in target lesion revascularization and binary restenosis. However, the authors included fewer randomized trials and included two case series (Micari 2012; Schmidt 2011).
Cassese 2012 analyzed four randomized controlled trials, which were also included in our analysis, and similarly reported an advantage for target lesion revascularization, binary restenosis, and late lumen loss compared with uncoated balloon angioplasty for femoropopliteal lesions.
Finally, Jens and colleagues reported an advantage for DEB compared with uncoated balloon angioplasty in target lesion revascularization of femoropopliteal arterial lesions but no difference in clinical endpoints (Jens 2014a). The authors also reported an advantage for DEB in wound healing, target lesion revascularization, binary restenosis, and change in Rutherford classification in treating tibial arterial lesions based on the DEBATE BTK trial (Jens 2014b). However, our tibial subgroup analysis included both DEBATE‐BTK 2013 and IN.PACT DEEP 2014 and demonstrated no advantage for DEB compared with uncoated balloon angioplasty in anatomic or clinical endpoints. Furthermore, the change in Rutherford classification data reported by Jens and colleagues was not included in the manuscript published by Liistro and colleagues when DEBATE‐BTK 2013 was published.
Authors' conclusions
Implications for practice.
Our analysis has demonstrated an advantage for drug‐eluting balloon (DEB) angioplasty compared with uncoated balloon angioplasty in treating lower extremity peripheral arterial disease (PAD) for up to five years in several domains. The quality of the evidence was low to high depending on the outcome. The advantage of DEB is limited to anatomic endpoints such as primary vessel patency, late lumen loss, target lesion revascularization, and binary restenosis. However, there is insufficient evidence to compare DEB and uncoated balloon angioplasty adequately in clinical outcomes such as change in quality of life (QoL) and walking impairment score.
Given the high re‐intervention rates in people with PAD, the superiority of DEB compared with uncoated balloon angioplasty in the anatomic endpoints reported in our analysis is encouraging. However, rigorous long‐term clinical outcome data are needed before one can conclude that DEB are superior to uncoated balloon angioplasty, especially given the considerably increased costs currently associated with DEB.
Implications for research.
Future well‐designed, publicly funded trials that are adequately powered to detect meaningful clinical endpoints, such as amputation and change in QoL, are needed to assess the utility of DEBs in the management of people with lower‐extremity PAD better, and to allow for the stratification of outcomes by arterial segment and indication for the intervention. However, given the small effect size differences in clinical endpoints between DEB and uncoated balloon angioplasty, and the rapidly evolving endovascular treatment modalities for PAD, it is unlikely that there will be any future randomized controlled trials powered to detect clinical endpoints because of the substantial cost and logistic challenges involved. We identified 13 studies that are either ongoing or are pending publication, in addition to the 11 studies included in our analysis. The eventual publication of short‐ and long‐term data from those studies will hopefully allow for a more rigorous analysis in future versions of this review. Furthermore, as data from more studies becomes available, we plan to perform more subgroup analyses by paclitaxel dose and type of carrier used in the DEB.
Acknowledgements
The review authors would like to thank Drs Cathryn Broderick, Marlene Stewart, and Karen Welch of Cochrane Vascular for their guidance in creating this review and developing the search strategy.
Appendices
Appendix 1. Cochrane Register of Studies (CRS) search strategy
| #1 | MESH DESCRIPTOR Arteriosclerosis | 863 |
| #2 | MESH DESCRIPTOR Arteriolosclerosis EXPLODE ALL TREES | 0 |
| #3 | MESH DESCRIPTOR Arteriosclerosis Obliterans | 69 |
| #4 | MESH DESCRIPTOR Atherosclerosis | 494 |
| #5 | MESH DESCRIPTOR Arterial Occlusive Diseases | 695 |
| #6 | MESH DESCRIPTOR Intermittent Claudication | 669 |
| #7 | MESH DESCRIPTOR Ischemia | 722 |
| #8 | MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES | 2085 |
| #9 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD ):TI,AB,KY | 8107 |
| #10 | ((arter* or vascular or vein* or veno* or peripher*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 6888 |
| #11 | (peripheral near3 dis*):TI,AB,KY | 2934 |
| #12 | (claudic* or IC):TI,AB,KY | 2695 |
| #13 | (isch* or CLI):TI,AB,KY | 20738 |
| #14 | arteriopathic:TI,AB,KY | 7 |
| #15 | dysvascular*:TI,AB,KY | 10 |
| #16 | (leg near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 82 |
| #17 | (limb near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 120 |
| #18 | ((lower near3 extrem*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 71 |
| #19 | ((iliac or femoral or popliteal or femoro* or fempop* or crural) near3(occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 826 |
| #20 | MESH DESCRIPTOR Leg EXPLODE ALL TREES WITH QUALIFIERS BS | 1062 |
| #21 | MESH DESCRIPTOR Iliac Artery | 135 |
| #22 | MESH DESCRIPTOR Popliteal Artery | 249 |
| #23 | MESH DESCRIPTOR Femoral Artery | 728 |
| #24 | MESH DESCRIPTOR Tibial Arteries | 30 |
| #25 | (((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) )):TI,AB,KY | 960 |
| #26 | restenosis:TI,AB,KY | 2235 |
| #27 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 | 40028 |
| #28 | MESH DESCRIPTOR Angioplasty EXPLODE ALL TREES | 3980 |
| #29 | MESH DESCRIPTOR Endovascular Procedures | 101 |
| #30 | (angioplas* or percutan* or PTA):TI,AB,KY | 11608 |
| #31 | valvuloplasty:TI,AB,KY | 105 |
| #32 | (recanali* or revascular*):TI,AB,KY | 6256 |
| #33 | dilat*:TI,AB,KY | 6732 |
| #34 | (balloon or baloon):TI,AB,KY | 6321 |
| #35 | endovascular:TI,AB,KY | 1093 |
| #36 | #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 | 23334 |
| #37 | MESH DESCRIPTOR Paclitaxel | 1316 |
| #38 | (drug and (elut* or releas*)):TI,AB,KY | 18150 |
| #39 | DEB:TI,AB,KY | 68 |
| #40 | paclitax*:TI,AB,KY | 3786 |
| #41 | sirolimus:TI,AB,KY | 1746 |
| #42 | zotarolimus:TI,AB,KY | 241 |
| #43 | rapalog:TI,AB,KY | 0 |
| #44 | (IN.PACT or MOXY or PANTERA or ELUTAX or DIOR or FREEWAY or SeQuent or GENIE):TI,AB,KY | 61 |
| #45 | #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 | 22498 |
| #46 | #27 AND #36 AND #45 | 1136 |
Appendix 2. Trials databases
Clinicaltrials.gov
82 studies found for: peripheral arterial disease and drug eluting | Interventional Studies
23 studies found for: drug eluting and ischemia and critical| Interventional Studies
47 studies found for: drug eluting and restenosis and peripheral| Interventional Studies
WHO
41 records for 40 trials found for: drug eluting and peripheral
31 records for 30 trials found for: drug eluting and ischemia
4 records for 4 trials found for: drug eluting and restenosis and peripheral
ISRCTN
drug eluting and peripheral 6
drug eluting and ischemia 4
drug eluting and restenosis 14
Data and analyses
Comparison 1. Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amputation | 7 | 541 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.39, 3.80] |
| 2 Primary vessel patency | 2 | 162 | Odds Ratio (M‐H, Random, 95% CI) | 1.47 [0.22, 9.57] |
| 3 Late lumen loss | 7 | 603 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.00, ‐0.28] |
| 4 Target lesion revascularization | 7 | 603 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.17, 0.47] |
| 5 Binary restenosis | 6 | 528 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.29, 0.67] |
| 6 Death | 7 | 541 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.31, 2.14] |
| 7 Change in Rutherford category | 3 | 249 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.49, 0.36] |
| 8 Change in ankle‐brachial index | 4 | 369 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.19, 0.18] |
| 9 Change in quality of life (EQ‐5D) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Change in walking impairment score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Amputation (sensitivity analysis) | 4 | 322 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.42, 7.54] |
| 12 Primary vessel patency (sensitivity analysis) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13 Late lumen loss (sensitivity analysis) | 4 | 343 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.21, ‐0.71] |
| 14 Target lesion revascularization (sensitivity analysis) | 4 | 343 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.11, 0.44] |
| 15 Binary restenosis (sensitivity analysis) | 3 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.13, 0.50] |
| 16 Death (sensitivity analysis) | 4 | 322 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.15, 2.11] |
| 17 Change in Rutherford category (sensitivity analysis) | 2 | 177 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.42, 0.50] |
| 18 Change in ankle‐brachial index (sensitivity analysis) | 2 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.09, 0.08] |
Comparison 2. Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amputation | 9 | 1649 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.73, 3.33] |
| 2 Amputation‐free survival | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Primary vessel patency | 3 | 882 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.45, 2.56] |
| 4 Late lumen loss | 3 | 535 | Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.59, 0.13] |
| 5 Target lesion revascularization | 11 | 1900 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.31, 0.51] |
| 6 Binary restenosis | 4 | 1094 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.15, 0.98] |
| 7 Death | 9 | 1649 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.64, 1.71] |
| 8 Change in Rutherford category | 3 | 623 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.29, 0.10] |
| 9 Change in ankle‐brachial index | 3 | 656 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.07, 0.01] |
| 10 Change in Quality of Life (EQ‐5D) | 3 | 879 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.02, 0.04] |
| 11 Change in walking impairment score | 2 | 551 | Mean Difference (IV, Fixed, 95% CI) | 3.57 [‐1.23, 8.38] |
| 12 Amputation (sensitivity analysis) | 7 | 1517 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.79, 4.04] |
| 13 Target lesion revascularization (sensitivity analysis) | 8 | 1640 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.18, 0.61] |
| 14 Death (sensitivity analysis) | 7 | 1517 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.64, 1.85] |
| 15 Late lumen loss (sensitivity analysis) | 2 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐1.41, ‐0.79] |
| 16 Change in Rutherford category (sensitivity analysis) | 2 | 551 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.58, 0.77] |
| 17 Change in ankle‐brachial index (sensitivity analysis) | 2 | 551 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 18 Change in quality of life (EQ‐5D) (sensitivity analysis) | 2 | 807 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.01, 0.05] |
Comparison 3. Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at two years.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amputation | 3 | 493 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.11, 3.88] |
| 2 Primary vessel patency | 2 | 406 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.51 [2.26, 5.46] |
| 3 Late lumen loss | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Target lesion revascularization | 3 | 508 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.18, 0.44] |
| 5 Binary restenosis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Death | 4 | 595 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.08, 4.20] |
| 7 Change in Rutherford category | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Change in ankle‐brachial index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Change in quality of life (EQ‐5D) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Change in walking impairment score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Amputation (sensitivity analysis) | 2 | 406 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.12, 80.19] |
| 12 Death (sensitivity analysis) | 3 | 508 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.16 [1.00, 4.67] |
Comparison 4. Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at five years.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amputation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Target lesion revascularization | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Binary restenosis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Death | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at six months: arterial segment subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amputation | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Femoropopliteal lesions | 5 | 415 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.40, 8.75] |
| 1.2 Tibial lesions | 1 | 72 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.63] |
| 2 Late lumen loss | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Femoropopliteal lesions | 5 | 423 | Mean Difference (IV, Fixed, 95% CI) | ‐0.65 [‐0.86, ‐0.45] |
| 2.2 Tibial lesions | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.23, 0.27] |
| 3 Target lesion revascularization | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Femoropopliteal lesions | 5 | 423 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.12, 0.41] |
| 3.2 Tibial lesions | 1 | 105 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.26, 3.18] |
| 4 Binary restenosis | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Femoropopliteal lesions | 4 | 348 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.15, 0.46] |
| 4.2 Tibial lesions | 1 | 105 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.78, 4.39] |
| 5 Death | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Femoropopliteal lesions | 5 | 415 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.22, 1.97] |
| 5.2 Tibial lesions | 1 | 72 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.18, 23.77] |
| 6 Change in Rutherford category | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Femoropopliteal lesions | 2 | 177 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.42, 0.50] |
| 6.2 Tibial lesions | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.64, 0.44] |
| 7 Change in ankle‐brachial index | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Femoropopliteal lesions | 3 | 264 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.09, 0.22] |
| 7.2 Tibial lesions | 1 | 105 | Mean Difference (IV, Random, 95% CI) | ‐0.2 [‐0.31, ‐0.09] |
| 8 Amputation (sensitivity analysis) | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Femoropopliteal lesions | 3 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.47 [0.49, 40.67] |
| 9 Late lumen loss (sensitivity analysis) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Femoropopliteal lesions | 3 | 268 | Mean Difference (IV, Fixed, 95% CI) | ‐0.92 [‐1.27, ‐0.57] |
| 10 Target lesion revascularization (sensitivity analysis) | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Femoropopliteal lesions | 3 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.11, 0.48] |
| 11 Binary restenosis (sensitivity analysis) | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Femoropopliteal lesions | 2 | 193 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.12, 0.57] |
| 12 Death (sensitivity analysis) | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Femoropopliteal lesions | 3 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.15, 2.11] |
| 13 Change in ankle‐brachial index (sensitivity analysis) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 Femoropopliteal lesions | 2 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.09, 0.08] |
Comparison 6. Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months: arterial segment subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amputation | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Femoropopliteal lesions | 5 | 1033 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.23, 21.51] |
| 1.2 Tibial lesions | 3 | 562 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.78, 4.89] |
| 2 Late lumen loss | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Femoropopliteal lesions | 1 | 102 | Mean Difference (IV, Random, 95% CI) | ‐1.2 [‐1.86, ‐0.54] |
| 2.2 Tibial lesions | 1 | 358 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.18, 0.16] |
| 3 Target lesion revascularization | 10 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Femoropopliteal lesions | 7 | 1230 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.13, 0.56] |
| 3.2 Tibial lesions | 3 | 595 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.30, 1.44] |
| 4 Binary restenosis | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Femoropopliteal lesions | 2 | 578 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.11, 1.26] |
| 4.2 Tibial lesions | 2 | 516 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.05, 3.14] |
| 5 Death | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Femoropopliteal lesions | 5 | 1033 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.31, 1.46] |
| 5.2 Tibial lesions | 3 | 562 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.73, 2.74] |
| 6 Change in Rutherford category | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Femoropopliteal lesions | 2 | 551 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.58, 0.77] |
| 6.2 Tibial lesions | 1 | 72 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.46, 1.66] |
| 7 Change in ankle‐brachial index | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Femoropopliteal lesions | 2 | 551 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 7.2 Tibial lesions | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐0.2 [‐0.31, ‐0.09] |
| 8 Change in quality of life (EQ‐5D) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Femoropopliteal lesions | 2 | 807 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.01, 0.05] |
| 8.2 Tibial lesions | 1 | 72 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.22, 0.02] |
| 9 Amputation (sensitivity analysis) | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Femoropopliteal lesions | 4 | 973 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.23, 21.51] |
| 9.2 Tibial lesions | 2 | 490 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.79, 5.59] |
| 10 Target lesion revascularization (sensitivity analysis) | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Femoropopliteal lesions | 5 | 1075 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.10, 0.73] |
| 10.2 Tibial lesions | 2 | 490 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.18, 1.52] |
| 11 Death (sensitivity analysis) | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Femoropopliteal lesions | 4 | 973 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.34, 1.73] |
| 11.2 Tibial lesions | 2 | 490 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.69, 2.83] |
6.8. Analysis.
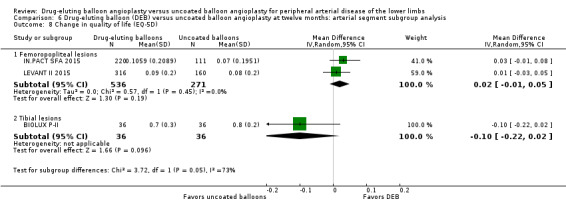
Comparison 6 Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months: arterial segment subgroup analysis, Outcome 8 Change in quality of life (EQ‐5D).
Comparison 7. Drug‐eluting balloon (DEB) versus uncoated balloon angioplasty at twelve months: severe peripheral arterial disease (PAD) subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amputation | 2 | 490 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.79, 5.59] |
| 2 Late lumen loss | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Target lesion revascularization | 2 | 490 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.18, 1.52] |
| 4 Binary restenosis | 2 | 516 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.05, 3.14] |
| 5 Death | 2 | 490 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.69, 2.83] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
BIOLUX P‐I.
| Methods | Study design: randomized controlled trial Method of randomization: computer‐generated randomization lists Blinding: participants were blinded but the operators were not blinded Exclusions postrandomization: none Losses to follow‐up: 5 Study enrollment period: October 2010 to August 2011 Cross‐over: 0 |
|
| Participants | Country: Austria and Germany Setting: 4 German hospitals and 1 Austrian hospital Number of participants: 60 (68 lesions) Age (mean ± SD): 71 ± 10 years Gender: male 57% Rutherford class: DEB: class 2: 23.3%, class 3: 56.7%, class 4: 13.3%, class 5: 6.7%; PTA: class 2: 30%, class 3: 56.7%, class 4: 6.7%, class 5: 6.7% ABI (± SD): 0.7 ± 0.2 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Uncoated balloon angioplasty: 30 participants, 35 lesions Uncoated balloon angioplasty device: Passeo‐18 PTA catheter DEB: 30 participants, 33 lesions DEB device: Passeo‐18 Lux drug‐releasing balloon catheter Drug used: paclitaxel 3 μg/mm2 balloon surface Vessels treated: femoropopliteal arteries Anticoagulation/platelets: dual antiplatelet therapy recommended for 1 month postprocedure and for 3 months in case of bailout stenting with a bare metal stent Predilation before DEB: yes: 66.7% of DEB and 30% of PTA procedures |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Clinical and angiographic follow‐up was scheduled at 6 months ± 30 days and clinical follow‐up at 12 months ± 30 days 6% of participants were treated in the anterior and posterior tibial arteries Sponsor: Biotronik AG |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization lists were computer generated" |
| Allocation concealment (selection bias) | Low risk | "Sealed envelopes with the randomization group included were provided to the sites" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The operators could not be blinded to the assigned treatment, which might have affected the rate of predilation and bailout stenting" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "QVA analysis was performed by an independent core laboratory (MedStar Health Research Institute, Washington, DC, USA), and the study was supervised by an independent clinical events committee and data safety monitoring board" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Follow‐up compliance was low, with 4 subjects withdrawing consent and 5 patients lost to follow‐up" |
| Selective reporting (reporting bias) | Low risk | The authors reported all prespecified outcomes |
| Other bias | High risk | Predilation was performed more often in people receiving DEB than PTA (66.7% with DEB vs. 30% with PTA, P = 0.010), and technical success was higher in the DEB group (76.7% with DEB vs. 46.7% with PTA, P = 0.017). 26.7% of the control arm required bailout stenting. Most of the people receiving DEB (56.7%) and PTA (60%) had a history of previous peripheral interventions, although the type and location of those interventions was not specified |
BIOLUX P‐II.
| Methods | Study design: randomized controlled trial Method of randomization: electronic case report form after successful wire passage through the target lesion Blinding: neither the participants nor the operators were blinded to the procedure Exclusions postrandomization: 0 exclusions after randomization Losses to follow‐up: 3 lost to follow‐up and 4 withdrew (DEB), 2 lost to follow‐up (PTA) Study enrollment period: July 2012 to June 2013 Cross‐over: 0 |
|
| Participants | Country: Austria, Belgium, and Germany. Setting: 1 hospital in Austria, 2 in Belgium, and 3 in Germany Number of participants: 72 Age (mean ± SD): 72.9 ± 10.3 years (DEB), 69.6 ± 8.9 years (PTA) Gender: males: DEB 27 (75%), PTA 30 (83%) Rutherford class: DEB: class 2: 2.8%, class 3: 19.4%, class 4: 5.6%, class 5: 72.2%; PTA: class 2: 8.3%, class 3; 13.9%, class 4: 5.6%, class 5: 72.2% ABI (± SD): DEB: 0.8 ± 0.3, PTA: 0.7 ± 0.3 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Uncoated balloon angioplasty: 36 participants (55 lesions) Uncoated balloon angioplasty device: uncoated Passeo‐18 PTA balloon catheter DEB: 36 participants (50 lesions) DEB device: Passeo‐18 LUX drug‐releasing PTA balloon catheter Drug used: paclitaxel 3 μg/mm2 balloon surface Vessels treated: infrapopliteal arteries Anticoagulation/platelets: dual antiplatelet therapy with ASA 100 mg/day to 325 mg/day and clopidogrel 75 mg/day for 1 month postprocedure and for 3 months in case of bailout stenting with a bare‐metal stenting Predilation before DEB: yes for DEB but not PTA |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Clinical follow‐up scheduled at 30 days, 6 months, and 12 months, and angiographic follow‐up at 6 months Sponsor: Biotronik AG |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed after successful wire passage through the lesion via the electronic case report form. Patients were allocated to DEB and PTA in a 1:1 ratio, with block sizes of 4 and 6" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details provided to assess allocation concealment bias adequately |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither the participants nor the operators were blinded to the procedure |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Quantitative vascular angiography (QVA) analysis was performed by an independent core laboratory that was blinded to the treatment, and all adverse events were adjudicated by an independent clinical events committee" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 19% of the DEB arm either withdrew from the study or were lost to follow‐up at 12 months |
| Selective reporting (reporting bias) | High risk | All prespecified outcomes were reported in the paper or provided by the authors through electronic correspondence. However, the results were not stratified by the type of treated infrapopliteal vessels (i.e. anterior tibial, posterior tibial, or peroneal arteries). The authors also did not specify whether those participants with more than 1 target lesion (33.3% of people with DEB and 44.4% with PTA) had several target vessels and whether the outcomes differed by the number of treated lesions and vessels |
| Other bias | High risk | While no bailout stenting was required in either treatment arm, the authors had a relatively low technical success rate (defined as < 30% residual stenosis) in both arms (54.2% DEB, 59.6% PTA) |
DEBATE‐BTK 2013.
| Methods | Study design: randomized controlled trial Method of randomization: concealed randomization in blocks of 10 using computer software and sealed envelopes after successful passage of the guidewire Blinding: participants and outcomes assessors were blinded Exclusions postrandomization: 24 Losses to follow‐up: 0 Study enrollment period: November 2010 to October 2011 Cross‐over: 0 |
|
| Participants | Country: Italy Setting: hospital ‐ single center Number of participants: 132 (158 lesions in 143 limbs) Age (mean): 75 years Gender: 106 men, 26 women Rutherford class: DEB: class 4: 2.8%, class 5: 78.9%, class 6: 18.3%; PTA: class 4: 4.2%, class 5: 81.9%, class 6: 13.9% ABI (± SD): DEB: 0.31 ± 0.2, PTA: 0.29 ± 0.3 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Uncoated balloon angioplasty: 67 (78 lesions in 72 limbs) Uncoated balloon angioplasty device: Amphirion Deep, Medtronic DEB: 65 (80 lesions in 71 limbs) DEB device: IN.PACT Amphirion, Medtronic Drug used: paclitaxel (dose unknown) Vessels treated: BTK vessels Anticoagulation/platelets: heparin 70 IU/kg after sheath insertion, ASA 100 mg and clopidogrel 75 mg ≥ 4 weeks then ASA 100 mg alone daily Predilation before DEB: yes |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Clinic visits twice weekly for 2 months, then once weekly for third month, then every 2 weeks for duration of study Doppler ultrasound within 12 months Angiogram at 12 months or during target‐lesion revascularization Sponsor: no industry support |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed in blocks of 10 with the use of computer‐generated random digits" |
| Allocation concealment (selection bias) | Low risk | "The assignments were placed in sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Double blind (subject, outcomes assessor)". The authors suggest that those performing the procedure were not blinded, although this was not addressed in the manuscript |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Acquired angiograms and DUS [duplex ultrasound] scans were reviewed by 2 blinded investigators who did not actively participate in recruitment […] and had no knowledge of clinical status and randomization group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "No patient was lost to follow‐up." Causes of participant mortality and lack of follow‐up imaging were reported |
| Selective reporting (reporting bias) | High risk | While all prespecified outcomes were reported, the authors state that, "inflow lesions located in the femoropopliteal segment were treated by standard techniques during the same session." The authors did not clarify what those techniques were and how many such lesions were treated |
| Other bias | Low risk | The authors state that 1 participant in each group required a DES at the end of the procedure |
DEBELLUM 2012.
| Methods | Study design: randomized controlled trial Method of randomization: participants were randomized (1:1) without stratification using computer‐generated assignments when they entered the angiographic suite Blinding: participants but not operators were blinded to the assigned intervention Exclusions postrandomization: 4 Losses to follow‐up: 0 Study enrollment period: September 2010 to March 2011 Cross‐over: 0 |
|
| Participants | Country: Italy Setting: hospital ‐ single center Number of participants: 54 randomized, 50 analyzed, 28 participants meeting study criteria Age (mean ± SD): 66 ± 4 years Gender: male 74% Fontaine class: DEB: class IIb (64%), class III (28%), class IV (8%); PTA: class IIb (60%), class III (28%), class IV (12%) ABI (± SD): DEB: 0.55 ± 0.06, PTA: 0.57 ± 0.05 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Uncoated balloon angioplasty: 27 participants Uncoated balloon angioplasty device: noncoated IN.PACT Admiral (SFA) and noncoated IN.PACT Amphirion (BTK), Medtronic DEB: 27 participants DEB device: IN.PACT Admiral (SFA) and IN.PACT Amphirion (BTK), Medtronic Drug used: paclitaxel (dose unknown) Vessels treated: femoropopliteal (75.4%) and BTK (24.6%) vessels Anticoagulation/platelets: ASA 100 mg/day and clopidogrel 75 mg/day for 3 days preprocedure. If participant was not receiving antiplatelet therapy preoperatively then clopidogrel 300 mg loading dose was administered. Heparin 5000 U administered after sheath insertion. Clopidogrel continued for 4 weeks Predilation before DEB: yes |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Late lumen loss was the difference in millimeters between the MLD immediately after the procedure and the MLD during follow‐up One third of participants received stents Substantial number of participants with TASC II C (33.6%) and D (10.6%) lesions Participants were followed‐up at 6, 12, and 24 months Duplex follow‐up in femoropopliteal region and angiography in the BTK arterial region Sponsor: no industry support |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized (1:1) without stratification using computer‐generated assignments when they entered the angiographic suite" |
| Allocation concealment (selection bias) | Low risk | "Patients were randomized (1:1) without stratification using computer‐generated assignments when they entered the angiographic suite" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Patients, but not operators, were blinded to the assigned intervention" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Postoperative evaluation was deferred to different physicians not informed about the assigned intervention". Unclear what type of physicians performed those evaluations and what type of qualifications they had |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0 participants reported as lost to follow‐up and no missing data |
| Selective reporting (reporting bias) | Low risk | Restenosis rate not reported at 1 year. While this omission was concerning, the remaining outcomes were reported and as such this single omission does not, in our opinion, place the study at a high risk of reporting bias |
| Other bias | High risk | "Patients requiring provisional or bailout stenting […] were excluded from the study". "The decision to implant a nitinol stent in the SFA territory was left to the judgment of the operator and typically driven by lesion length and presence of severe calcification" The stent deployment criteria are unclear and approximately 37% of the treated lesions were also stented |
FemPac 2008.
| Methods | Study design: randomized controlled trial Method of randomization: central randomization in advance for all participants without stratification. Random list portions assigned to participating centers Blinding: attempt at blinding operators but success unclear because of differences in appearance between the coated and uncoated balloons Exclusions postrandomization: 0 Losses to follow‐up: 19 (withdrew consent or declined angiography because of another reason) Study enrollment period: July 2004 to January 2006 Cross‐over: 0 |
|
| Participants | Country: Germany Setting: 2 academic hospitals Number of participants: 87 Age (median): 67.3 years (DEB), 70.2 years (PTA) Gender: males: DEB 27 (60%), PTA 25 (60%) Rutherford class: DEB: class 1: 4%, class 2: 22%, class 3: 69%, class 4: 4%; PTA: class 1: 2%, class 2: 17%, class 3: 74%, class 4: 7% ABI (median): DEB: 0.7, PTA: 0.7 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Uncoated balloon angioplasty: 42 participants Uncoated balloon angioplasty device: Bavaria Medizin Technologie GmbH (Oberpfaffenhofen, Germany) DEB: 45 participants DEB device: Bavaria Medizin Technologie GmbH (Oberpfaffenhofen, Germany) Drug used: paclitaxel 3 μg/mm2 balloon surface (mean ± SD: 3.7 ± 2.5 μg per participant) Vessels treated: femoropopliteal arteries Anticoagulation/platelets: clopidogrel 75 mg/day and ASA 100 mg/day were started as long‐term medication on the day of angioplasty. After common femoral sheath placement, all participants received an initial bolus of heparin 2500 IU to 5000 IU. Further concomitant medication was documented by the investigator Predilation before DEB: no |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Stents were placed in 6 PTA and 4 DEB participants (overall 11%) Late lumen loss defined as the difference between the minimal luminal diameter after the procedure and at 6 months by quantitative angiography Sponsor: balloon catheters provided by Bavaria Medizin Technologie (Oberpfaffenhofen, Germany) and financial support for the study provided by Bayer‐Schering‐Pharma AG (Berlin, Germany) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done centrally in advance for all patients without any stratification. Portions of the random list (eg, numbers 1 to 30) were assigned to a centre that enrolled the patients in the sequence of the randomization list" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details provided in the study to assess allocation concealment bias adequately |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Blinding of the investigators was attempted but not guaranteed because of differences in the appearance of coated and uncoated balloons" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Quantitative evaluation of six‐month angiographic control was performed by an independent core laboratory blinded to the type of treatment" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6‐month primary outcome data available on 73% of participants in intervention arm and 83% in control arm |
| Selective reporting (reporting bias) | High risk | "No Doppler or angiographic information was obtained from 7 patients in the control and 9 patients in the coated balloon group" |
| Other bias | Unclear risk | 11% of participants received a stent but clinical outcomes of those participants not reported separately |
IN.PACT DEEP 2014.
| Methods | Study design: randomized‐controlled trial Method of randomization: performed using blocks of sealed envelopes Blinding: participant‐blinded Exclusions postrandomization: 0 Losses to follow‐up: 4 (3 in DEB arm, 1 in PTA arm) Study enrollment period: September 2009 to July 2012 Cross‐over: 0 |
|
| Participants | Country: 13 European centers Setting: hospital, multicenter Number of participants: 358 (DEB 239, PTA 119) Age (mean ± SD): 73.3 ± 8.2 years (DEB), 71.7 ± 9.9 years (PTA) Gender: male 74% Rutherford class: DEB: class 3: 0%, class 4: 14.2%, class 5: 84.1%, class 6: 1.7%; PTA: class 3: 0.8%, class 4: 17.6%, class 5: 77.3%, class 6: 4.2% ABI: DEB: 0.75 ± 0.4, PTA: 0.81 ± 0.44 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Uncoated balloon angioplasty: 119 participants Uncoated balloon angioplasty device: standard PTA, unspecified DEB: 239 participants DEB device: IN.PACT Amphirion (Medtronic) Drug used: paclitaxel (dose unknown) Vessels treated: infrapopliteal arteries Anticoagulation/platelets: preprocedure: ASA 100 mg at least 4 days prior to the index intervention, alternatively at least 500 mg loading dose prior to or within 2 hours postprocedure; clopidogrel 75 mg/day at least 4 days prior to the index intervention, alternatively at least 300 mg loading dose prior to or within 2 hours postprocedure (or ticlopidine, if required); the use of bivalirudin was allowed as an alternative to heparin Postprocedure: ASA 100 mg indefinitely and daily clopidogrel 75 mg (or ticlopidine, if required) for at least 1 month following the procedure. Prolonged antiplatelet therapy could be given at the discretion of the physician and should be considered after placement of stents Predilation before DEB: yes: 90.5% of procedures |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | It is unclear which vessels were treated and whether any participants required more than 1 treatment per limb Sponsor: Medtronic, Santa Rosa California |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The randomization process was performed using blocks of sealed envelopes". The methodology for generating those randomization blocks was not specified |
| Allocation concealment (selection bias) | Low risk | "The randomization process was performed using blocks of sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "This trial is a 2:1 randomized, controlled, patient‐blinded multicentre trial". This implies that the operators were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Both wound and angiographic core laboratories were blinded to the assigned treatment" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "The low angiographic and wound imaging compliance may have limited the full assessment of this therapy" |
| Selective reporting (reporting bias) | High risk | The published protocol listed several unreported outcomes such as change in Rutherford category and QoL scores. The number of inflow lesions treated and how those lesions were managed not reported |
| Other bias | Low risk | The outcomes of the 5% of participants who received a stent not reported |
IN.PACT SFA 2015.
| Methods | Study design: randomized controlled trial Method of randomization: participants were randomly assigned by an Interactive Voice Response System with the use of a method of permuted blocks to ensure that a 2:1 ratio was maintained across sites Blinding: single‐blinded Exclusions postrandomization: DEB: 13 participants, PTA: 4 participants Losses to follow‐up: DEB: 3 participants, PTA: 3 participants Study enrollment period: September 2010 to April 2011 and April 2012 to January 2013 Cross‐over: 0 The trial included 2 cohorts: European (IN.PACT SFA I) and North American (IN.PACT SFA II) |
|
| Participants | Country: Europe, USA, and Canada Setting: hospital (13 sites in Europe, 44 in the USA and Canada) Number of participants: 331 Age (mean ± SD): 67.5 ± 9.5 years (DEB), 68.0 ± 9.2 years (PTA) Gender: males: DEB 65%, PTA: 67.6% Rutherford class: DEB: class 2: 37.7%, class 3: 57.3%, class 4: 5%, class 5: 0%; PTA: class 2: 37.8%, class 3: 55.9%, class 4: 5.4%, class 5: 0.9% ABI: DEB: 0.769 ± 0.228, PTA: 0.744 ± 0.189 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Uncoated balloon angioplasty: 111 Uncoated balloon angioplasty device: unspecified "standard PTA balloon" DEB: 220 DEB device: IN.PACT Admiral (Medtronic, Santa Rosa, CA) Drug used: paclitaxel 3.5 μg/mm2 balloon surface Vessels treated: femoropopliteal arteries Anticoagulation/platelets: periprocedural: loading dose of ASA 300 mg to 325 mg and clopidogrel 300 mg within 24 hours of the index procedure or 2 hours postprocedure. Heparin administered at the time of the procedure to maintain an activated clotting time ≥ 250 seconds Postprocedural: ASA 81 mg/day to 325 mg/day (for a minimum of 6 months) and clopidogrel 75 mg/day for a minimum duration of 1 month for nonstented participants and 3 months for participants who received stents Predilation before DEB: yes |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Primary patency defined as freedom from clinically driven TLV and restenosis as determined by a duplex ultrasonography‐derived peak systolic velocity ratio of ≤ 2.4 Participants were followed by the treating physician at 30 days, 6 months, and 12 months, including office visits with duplex ultrasonography functional testing and adverse event assessment Sponsor: Medtronic, Santa Rosa, CA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly assigned by an Interactive Voice Response System with the use of a method of permuted blocks to ensure that a 2:1 ratio was maintained across sites" |
| Allocation concealment (selection bias) | Low risk | "Subjects were randomly assigned by an Interactive Voice Response System with the use of a method of permuted blocks to ensure that a 2:1 ratio was maintained across sites" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Because of the visual difference between the IN.PACT DCB and standard PTA balloon, treating physicians, research coordinators, and catheterization laboratory staff were not blinded to the treatment assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Independent core laboratories analyzed all images, including duplex ultrasonography", "Each component of the primary efficacy end point was independently adjudicated by the blinded Clinical Events Committee" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 94% of DEB participants and 96% of PTA participants analyzed at 1 year. "Multiple imputation was performed by using the logistic regression approach for patients with missing primary end point data (29 DCB, 7 PTA)" |
| Selective reporting (reporting bias) | Low risk | Restenosis rate not reported. While this omission was concerning, the remaining outcomes were reported and as such this single omission does not, in our opinion, place the study at a high risk of reporting bias |
| Other bias | Unclear risk | The outcomes of the 9% of stented participants were not reported. However, the authors stated that, "when stented patients were excluded from the analysis, there were no changes in any of the conclusions". The majority of study authors declared a financial relationship with the study sponsor |
LEVANT I 2014.
| Methods | Study design: randomized controlled trial Method of randomization: sequentially numbered sealed envelopes in blocks of 4 via computer‐generated random numbers Blinding: single‐blind (participants) Exclusions postrandomization: 0 Losses to follow‐up: 5 (at 6‐month follow‐up) Study enrollment period: June 2009 to December 2009 Cross‐over: 0 |
|
| Participants | Country: Europe and USA Setting: 9 hospitals Number of participants: 101 Age (mean ± SD): 67 ± 8 (DEB), 70 ± 10 (PTA) Gender: male: DEB 34 (69%), PTA 30 (58%) Rutherford class: DEB: class 2: 22%, class 3: 72%, class 4: 2%, class 5: 4%; PTA: class 2: 21%, class 3: 71%, class 4: 4%, class 5: 4% ABI (± SD): DEB: 0.69 ± 0.23, PTA: 0.60 ± 0.36 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Uncoated balloon angioplasty: 52 (of whom 38 did not receive a stent) Uncoated balloon angioplasty device: unspecified "standard PTA balloon" DEB: 49 (of whom 37 did not receive a stent) DEB device: Lutonix DEB (C.R. Bard, New Hope, MN) Drug used: paclitaxel 2 μg/mm2 balloon surface Vessels treated: femoropopliteal arteries Anticoagulation/platelets: according to local clinical practice. ASA 100 mg/day to 325 mg/day indefinitely and clopidogrel loading dose (75 mg or 300 mg) with maintenance for 1 month in balloon‐only participants and 3 months in stented participants Predilation before DEB: yes |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | 25% of enrolled participants received a stent Clinical follow‐up at 1, 6, 12, and 24 months after the procedure Angiography of the treated limb performed at 6 months Duplex ultrasound, Rutherford classification, ABI, and WIQ evaluated at baseline, 6, 12, and 24 months Sponsor: C.R. Bard (New Hope, MN) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects in each stratum (intended balloon‐only or intended stenting) were randomized 1:1 to Lutonix DCB or uncoated balloon (control group) using sequentially numbered sealed envelopes in blocks of 4 via computer‐generated random numbers" |
| Allocation concealment (selection bias) | Low risk | "Subjects in each stratum (intended balloon‐only or intended stenting) were randomized 1:1 to Lutonix DCB or uncoated balloon (control group) using sequentially numbered sealed envelopes in blocks of 4 via computer‐generated random numbers" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was "single blind" (to participant) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome assessed by "independent, blinded angiographic core lab analysis". "Major adverse events were independently adjudicated by a Clinical events committee" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Six‐month angiographic follow‐up for the primary endpoint was available for 39 patients (80%) in the Lutonix DCB group and 36 (69%) in the uncoated balloon group, due in part to 4 deaths and 5 withdrawals" |
| Selective reporting (reporting bias) | Low risk | All primary and secondary endpoint data reported |
| Other bias | High risk | 8 DEB devices (16%) malfunctioned and failed to deploy properly. Antiplatelet therapy regimens varied across sites and anticoagulation protocol with heparin not specified |
LEVANT II 2015.
| Methods | Study design: randomized controlled trial Method of randomization: 2:1 randomization Blinding: single‐blind (participants) Exclusions postrandomization: 0 Losses to follow‐up: 74 of 476 participants (16%) at 12 months Study enrollment period: July 2011 to July 2012 Cross‐over: 0 |
|
| Participants | Country: Europe and USA Setting: 54 hospitals Number of participants: 476 Age (mean ± SD): 68 ± 10 years (DEB), 69 ± 9 years (PTA) Gender: male: DEB 193 (61%), PTA 107 (67%) Rutherford class: DEB: class 2: 29% class 3: 63%, class 4: 8%; PTA: class 2: 34%, class 3: 58%, class 4: 8% ABI (± SD): DEB: 0.74 ± 0.20, PTA: 0.73 ± 0.18 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Uncoated balloon angioplasty: 160 Uncoated balloon angioplasty device: unspecified "standard PTA balloon" DEB: 316 DEB device: Lutonix DEB (C.R. Bard, New Hope, MN) Drug used: paclitaxel 2 μg/mm2 balloon surface Vessels treated: femoropopliteal arteries Anticoagulation/platelets: according to local clinical practice ASA loading dose 75 mg to 325 mg before the procedure and 75 mg/day to 100 mg/day indefinitely, and clopidogrel or prasugrel loading dose (clopidogrel 75 mg or 300 mg and prasugrel 10 mg or 60 mg) before the procedure and clopidogrel 75 mg/day or prasugrel 5 mg to 10 mg (depending on bodyweight) for at least 1 month postoperatively Predilation before DEB: yes |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Primary patency defined as the absence of evidence of binary restenosis and freedom from TLR Procedural success defined as technical success without periprocedural complications Participants were followed up at 1, 3, and 12 months postoperatively Sponsor: C.R. Bard (New Hope, MN) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomized 2:1 to the intervention and control arms, but the methodology of randomization not explained in the manuscript, supplementary materials, or study protocol |
| Allocation concealment (selection bias) | Unclear risk | Methodology of allocation not explained in the manuscript, supplementary materials, or study protocol |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The clinician was aware of the index treatment because the drug‐coated balloon looked different from a standard balloon" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Investigators who completed follow‐up, vascular‐laboratory personnel, core laboratory evaluators, and members of the clinical‐events committee were unaware of the treatment received" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 16% of participants were lost to follow‐up over the 12‐month study period |
| Selective reporting (reporting bias) | Low risk | All primary and secondary endpoint data reported |
| Other bias | Low risk | Participants randomized after a lesion was predilated so people requiring stent placement were excluded. More challenging or dissection‐prone lesions were thus excluded from this study, which may not be reflective of existing clinical practices |
PACIFIER 2012.
| Methods | Study design: randomized controlled trial Method of randomization: computer generated, in blocks of 10 participants Blinding: single‐blind (participants) Exclusions postrandomization: 0 Losses to follow‐up: 4 participants at 6 months, 10 participants at 12 months Study enrollment period: 2010 to 2011 Cross‐over: 0 |
|
| Participants | Country: Germany Setting: hospital (3 centers) Number of participants: 85 (91 intervention procedures, 1 target lesion per procedure) Age (mean ± SD): 71 ± 7 years (DEB), 71 ± 9 years (PTA) Gender: males: DEB 26 (59%), PTA 30 (64%) Rutherford class: DEB: class 2: 9.1%, class 3: 86.4%, class 4: 0%, class 5: 4.5%; PTA: class 2: 12.8%, class 3: 83%, class 4: 4.3%, class 5: 0% ABI: DEB: 0.73 ± 0.30, PTA: 0.65 ± 0.26 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Uncoated balloon angioplasty: 47 procedures in 44 participants Uncoated balloon angioplasty device: Pacific Xtreme (Medtronic, Santa Rosa, CA) DEB: 44 procedures in 41 participants DEB device: IN.PACT Pacific (Medtronic, Santa Rosa, CA) Drug used: paclitaxel 3 μg/mm2 balloon surface Vessels treated: femoropopliteal arteries Anticoagulation/platelets: all participants were pretreated with ASA and thienopyridines, which were continued for > 2 months after PTA Predilation before DEB: yes: DEB 6 cases (13.6%), PTA 3 cases (6.4%) |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Follow‐up to 24 months reported Stents provisionally implanted in 9 (20.5%) DEB cases and 16 (34%) PTA cases Sponsor: Medtronic (Santa Rosa, CA) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization sequence was computer generated, in blocks of 10 patients each" |
| Allocation concealment (selection bias) | Low risk | "Allocation concealment was guaranteed by the use of numbered, opaque, sealed envelopes, which were only opened after the decision was made that the patient had to be treated according to the protocol" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Blinding of investigators after assignment of a patient to a treatment is not possible due to differences in the appearance of coated and uncoated catheters" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary endpoint "assessed by blinded angiographic core lab quantitative analyses" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing primary outcome data on 20.5% of DEB arm and 27.3% of control arm |
| Selective reporting (reporting bias) | Low risk | All primary and secondary endpoint data reported |
| Other bias | High risk | 21% of DEB and 34% of control participants were stented but little discussion of outcomes in those participants. Baseline risk characteristics such as smoking and diabetes were unevenly distributed between DEB and control arms. Dosing and duration of antiplatelet therapies were unclear |
THUNDER 2008.
| Methods | Study design: randomized controlled trial Method of randomization: participants were assigned to different treatment groups according to a lot‐generated random list Blinding: participant blinded Exclusions postrandomization: 0 Losses to follow‐up: 3 Study enrollment period: June 2004 to June 2005 Cross‐over: 0 |
|
| Participants | Country: Germany Setting: 3 hospitals Number of participants: 154 Age (mean ± SD): 69 ± 8 years (DEB), 68 ± 9 years (PTA) Gender: males: DEB 31 (65%), PTA 34 (63%) Rutherford class (mean ± SD): DEB: 3.4 ± 0.8, PTA 3.1 ± 0.8 ABI (± SD): DEB: 0.5 ± 0.3, PTA 0.5 ± 0.3 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Uncoated balloon angioplasty: 54 Uncoated balloon angioplasty device: Bavaria Medizintechnologie DEB: 48 DEB device: Bavaria Medizintechnologie Drug used: paclitaxel 3 μg/mm2 balloon surface, mean dose (± SD) 4.7 ± 3.μg Vessels treated: femoropopliteal arteries Anticoagulation/platelets: participants not already taking ASA and clopidogrel were administered loading doses of 300 mg of each drug 12 hours before the procedure. All participants received ASA 100 mg/day indefinitely and clopidogrel 75 mg/day for 4 weeks after the intervention. In addition, participants were given an intra‐arterial bolus of heparin 3000 U to 5000 U) at the time of the procedure Predilation before DEB: yes |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Study had 3 arms: balloons coated with paclitaxel, uncoated balloons with paclitaxel dissolved in the contrast medium, and uncoated balloons Clinical evaluations were performed at baseline, at 24 to 72 hours after intervention, and at 6 months after intervention Angiographic evaluation of restenosis was performed at 6 months with the same projections as those used during intervention Sponsor: Bavaria Medizintechnologie and Schering, Germany |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to different treatment groups according to a lot‐generated random list |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment strategy |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The paclitaxel‐coated balloons had a distinctive appearance that could be recognized by the investigators, who also performed some of the poststudy evaluations |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The paclitaxel‐coated balloons had a distinctive appearance that could be recognized by the investigators, who also performed some of the poststudy evaluations". "All angiograms were assessed in a blinded fashion by an independent angiographic core laboratory (C2RM, Lille, France)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 DEB participants and 1 PTA participant did not undergo clinical follow‐up and angiography at 6 months |
| Selective reporting (reporting bias) | Low risk | All primary and secondary endpoint data reported |
| Other bias | High risk | 4% of the intervention and 22% of control participants were stented but little discussion was provided of the outcomes in those participants |
ABI: ankle‐brachial index; ASA: acetylsalicylic acid (aspirin); BTHC: n‐butyryl tri‐nhexyl citrate; BTK: below the knee; CLI: critical limb ischemia; DCB: drug‐coated balloon; DEB: drug‐eluting balloon; DES: drug‐eluting stent; DS: diameter stenosis; EQ‐5D: Euro‐Qol Group 5‐Dimension Self‐Report Questionnaire; IU: international unit; MAE: major adverse events; MDRD: modification of diet in renal disease; MI: myocardial infarction; MLD: minimum lumen diameter; PTA: percutaneous transluminal angioplasty; QoL: quality of life; QVA: quantitative vascular angiography; RVD: reference vessel diameter; SD: standard deviation; SFA: superficial femoral artery; SF‐36: Medical Outcomes Study 36‐Item Short‐Form Health Survey; TASC: Trans‐Atlantic Inter‐Society Consensus Document on Management of Peripheral Arterial Disease; TLR: target lesion revascularization; TVR: target vessel revascularization; U: unit; WIQ: Walking Impairment Questionnaire.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| COPA CABANA | Randomized controlled trial comparing DEB with uncoated balloon angioplasty for the treatment of instent restenosis |
| DEBATE SFA | Randomized controlled trial comparing stenting and DEB with stenting and uncoated balloon angioplasty for the treatment of peripheral arterial disease |
| DEBATE‐ISR | Randomized controlled trial comparing DEB with uncoated balloon angioplasty for the prevention of instent restenosis in people with diabetes |
| DEFINITIVE AR | Randomized controlled trial comparing atherectomy and DEB angioplasty with DEB angioplasty alone for the treatment of peripheral arterial disease |
| EURO CANAL | The trial was terminated early by the DEB manufacturer before any data were collected. The reason for early termination was that the manufacturer withdrew the DEB from the market |
| FAIR | Randomized controlled trial comparing DEB with uncoated balloon angioplasty for the prevention of instent restenosis |
| Freeway Stent Study | Randomized controlled trial of primary nitinol stenting followed by DEB or uncoated balloon angioplasty for the prevention of instent restenosis |
| IDEAS | Randomized controlled trial comparing DEB angioplasty with drug‐eluting stenting for the treatment of peripheral arterial disease |
| ISAR‐PEBIS | Randomized controlled trial comparing drug‐eluting with uncoated balloon angioplasty for the prevention of instent restenosis |
| ISAR‐STATH | Randomized controlled trial comparing DEB angioplasty, stenting, and atherectomy for the treatment of lower‐extremity peripheral arterial disease |
| PACUBA 1 | Randomized controlled trial comparing DEB with uncoated balloon angioplasty for the treatment of instent restenosis |
| PHOTOPAC | Randomized controlled trial comparing photoablation and DEB angioplasty with DEB angioplasty alone for the prevention of instent restenosis |
| RAPID | Randomized controlled trial comparing stenting and DEB with stenting and uncoated balloon angioplasty for the treatment of peripheral arterial disease |
| SWEDEPAD | Randomized controlled trial comparing drug‐eluting technologies (DEB or drug‐eluting stenting) with nondrug‐eluting technologies (uncoated balloon angioplasty or stenting) for the treatment of peripheral arterial disease |
DEB: drug‐eluting balloon.
Characteristics of ongoing studies [ordered by study ID]
AcoArt I.
| Trial name or title | Prospective, Multi‐center and Randomized Controlled Clinical Study to Verify Effectiveness and Safety of Drug‐Eluting Balloon in PTA Procedure (AcoArt I Study) |
| Methods | Randomized controlled trial |
| Participants | Country: China Setting: 7 hospitals Number of participants: 200 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Uncoated balloon angioplasty device: Orchid catheter DEB device: Admiral catheter Vessels treated: femoropopliteal vessels |
| Outcomes | Primary:
Secondary:
|
| Starting date | April 2013 |
| Contact information | Acotec Scientific Company, China |
| Notes | Sponsor: Acotec Scientific Company |
AcoArt II.
| Trial name or title | Prospective, Multi‐center and Randomized Controlled Clinical Study to Verify Effectiveness and Safety of Drug‐Eluting Balloon in PTA Procedure of the Infrapopliteal Artery |
| Methods | Randomized controlled trial |
| Participants | Country: China Setting: single hospital Number of participants: 180 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Uncoated balloon angioplasty device: Lotus/Tulip catheter DEB device: Amphirion Deep catheter Vessels treated: below‐knee vessels |
| Outcomes | Primary:
Secondary:
|
| Starting date | May 2014 |
| Contact information | Qianqian Wei, weiqianqian@mrbc‐nccd.com |
| Notes | Sponsor: Acotec Scientific Company |
ACOART‐BTK.
| Trial name or title | Evaluation of the Use of ACOTEC Drug‐Eluting Balloon Litos in Below‐The‐Knee Arteries to Treat Critical Limb Ischemia (ACOART‐BTK). |
| Methods | Randomized controlled trial |
| Participants | Country: Italy Setting: single hospital Number of participants: 140 Inclusion Criteria:
Exclusion criteria:
|
| Interventions | Uncoated balloon angioplasty device: unspecified conventional balloon catheter DEB device: Litos drug‐eluting balloon catheter Vessels treated: infrapopliteal arteries |
| Outcomes | Primary:
Secondary:
|
| Starting date | September 2015 |
| Contact information | Dr Leonardo Bolognese, l.bolognese@usl8.toscana.it |
| Notes | Sponsor: Ospedale San Donato No industry support declared |
Advance 18PTX Balloon Catheter Study.
| Trial name or title | Advance® 18PTX® Balloon Catheter Study: Treatment of Lesions in Superficial Femoral Artery/Popliteal Artery with a Paclitaxel‐coated Balloon. |
| Methods | Randomized controlled trial |
| Participants | Country: Germany and Russia Setting: 3 hospitals in Germany and 1 hospital in Russia Number of participants: 150 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Uncoated balloon angioplasty device: Advance® 18LP catheter DEB device: Advance 18PTX catheter Drug used: paclitaxel |
| Outcomes | Primary: Late lumen loss. Secondary: No information provided on secondary outcomes |
| Starting date | October 2008 |
| Contact information | None provided |
| Notes | Sponsor: Cook |
EFFPac.
| Trial name or title | Phase III Multicenter Randomized Controlled Trial to Assess the Effectiveness of Paclitaxel‐coated Luminor Balloon Catheter versus Uncoated Balloon Catheter in the Superficial Femoral and Popliteal Arteries to Prevent Vessel Restenosis or Reocclusion |
| Methods | Randomized controlled trial |
| Participants | Country: Germany Setting: 9 German hospitals Number of participants: 172 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Uncoated balloon angioplasty device: unspecified DEB device: Luminor 35 Paclitaxel Eluting Peripheral Balloon catheter Drug used: paclitaxel Vessels treated: femoropopliteal arteries |
| Outcomes | Primary:
Secondary:
|
| Starting date | April 2015 |
| Contact information | Dr Ulf Teichgräber, Jena University Hospital, Germany Sponsor: University of Jena, Germany |
| Notes | ‐ |
FREERIDE Study.
| Trial name or title | Phase III FREERIDE STUDY Freeway Randomized Angioplasty Study |
| Methods | Randomized controlled trial |
| Participants | Country: Europe and Colombia Setting: 16 European hospitals and 1 Colombian hospital Number of participants: 280 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Uncoated balloon angioplasty device: unspecified DEB device: Freeway balloon catheter Drug used: paclitaxel 3 µg/mm2 Vessels treated: femoropopliteal arteries |
| Outcomes | Primary:
Secondary:
|
| Starting date | May 2011 |
| Contact information | Beatriz Fernandez, fernandez@eurocor.de Rembert Pogge von Strandmann, pogge@eurocor.de |
| Notes | Sponsor: Eurocor GmbH |
ILLUMENATE.
| Trial name or title | Pivotal Trial of a Novel Paclitaxel‐Coated Percutaneous Angioplasty Balloon |
| Methods | Randomized controlled trial |
| Participants | Country: USA and Austria Setting: 44 hospitals and endovascular centers Number of participants: 360 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Uncoated balloon angioplasty device: EverCross catheter DEB device: Cardiovascular Ingenuity (CVI) catheter Vessels treated: femoropopliteal vessels |
| Outcomes | Primary:
Secondary:
|
| Starting date | June 2013 |
| Contact information | Dr Prakash Krishnan, prakash.krishnan@mssm.edu Dr Sean Lyden, lydens@ccf.org |
| Notes | Sponsor: Spectranetics Corporation |
LEVANT Japan.
| Trial name or title | A Prospective, Multicenter, Single Blind, Randomized, Controlled Japanese Population Trial Comparing MD02‐LDCB Versus Standard Balloon Angioplasty for Treatment of Femoropopliteal Arteries |
| Methods | Randomized controlled trial |
| Participants | Country: Japan Setting: single hospital Number of participants: 150 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Uncoated balloon angioplasty device: unspecified DEB device: MD02‐LDCB balloon catheter Drug used: paclitaxel Vessels treated: femoropopliteal arteries |
| Outcomes | Primary:
Secondary:
|
| Starting date | March 2013 |
| Contact information | Dr Osamu lida, Kansai Rosai Hospital Cardiovascular Internal Medicine |
| Notes | Sponsors: C.R. Bard and Medicon, Inc |
Lutonix BTK.
| Trial name or title | A Prospective, Multicenter, Single Blind, Randomized, Controlled Trial Comparing the Lutonix Drug Coated Balloon Versus Standard Balloon Angioplasty for Treatment of Below‐the‐Knee (BTK) Arteries (Lutonix BTK Trial) |
| Methods | Randomized controlled trial |
| Participants | Country: Europe, Japan, and North America Setting: 59 hospitals in Europe, USA, Canada, and Japan Number of participants: 480 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Uncoated balloon angioplasty device: unspecified DEB device: Lutonix drug‐coated balloon catheter Drug used: paclitaxel Vessels treated: below‐the‐knee arteries |
| Outcomes | Primary:
Secondary:
|
| Starting date | May 2013 |
| Contact information | Robert M Jardin, lutonixresearch@crbard.com |
| Notes | Sponsor: C.R. Bard |
MDT‐2113 SFA.
| Trial name or title | Randomized Trial of MDT‐2113 Drug‐Eluting Balloon (DEB) vs. Standard PTA for the Treatment of Atherosclerotic Lesions in the Superficial Femoral Artery and/or Proximal Popliteal Artery |
| Methods | Randomized controlled trial |
| Participants | Country: Japan Setting: single hospital Number of participants: 100 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Uncoated balloon angioplasty device: unspecified DEB device: MDT‐2113 drug‐eluting balloon catheter Drug used: paclitaxel Vessels treated: femoropopliteal arteries |
| Outcomes | Primary: Freedom from clinically driven TLR and freedom from restenosis as determined by duplex ultrasound Secondary: Freedom from device‐ and procedure‐related death through 30 days postprocedure, and freedom from target limb major amputation and clinically driven TVR |
| Starting date | September 2013 |
| Contact information | Unspecified |
| Notes | Sponsor: Medtronic Endovascular |
PICCOLO.
| Trial name or title | Paclitaxel Coated Balloons for Prevention of Restenosis in Small Arteries Below the Knee Compared to Angioplasty Using Uncoated Balloons |
| Methods | Randomized controlled trial |
| Participants | Country: Germany Setting: 5 hospitals Number of participants: 114 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Uncoated balloon angioplasty device: Submarine, Ampherion Deep by Invatec DEB device: Submarine, Ampherion Deep by Invatec coated with paclitaxel 3 µg/mm2 Drug used: paclitaxel Vessels treated: below‐knee arteries |
| Outcomes | Primary:
Secondary:
|
| Starting date | April 2008 |
| Contact information | Dr Gunnar Tepe, gunnar.tepe@med.uni‐tuebingen.de |
| Notes | Sponsor: University of Tuebingen, Germany. Industrial support unspecified The status of this trial is unclear. The trial information has not been updated on ClinicalTrials.gov since 2008. Attempts to reach the trial researchers have not been successful |
RANGER‐SFA.
| Trial name or title | Prospective, Randomized, Multicentre Clinical Study of the Hemoteq Ranger™ Paclitaxel‐Coated PTA Balloon Catheter (Ranger DCB) in Comparison to Uncoated PTA Balloons in Femoropopliteal Lesions |
| Methods | Randomized controlled trial |
| Participants | Country: Europe Setting: 2 hospitals in Austria, 7 in Germany, 4 in France Number of participants: 105 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Uncoated balloon angioplasty device: unspecified DEB device: Ranger drug‐coated balloon Drug used: paclitaxel Vessels treated: femoropopliteal arteries |
| Outcomes | Primary:
Secondary:
|
| Starting date | January 2014 |
| Contact information | Dr Dierk Scheinert, Park‐Krankenhaus Leipzig GmbH |
| Notes | Sponsor: Hemoteq AG, Ceres GmbH Evaluation and Research, CoreLab Bad Krozingen GmbH |
SINGA‐PACLI.
| Trial name or title | Singapore INfra‐Genicular Angioplasty with PAclitaxel‐eluting Balloon for Critical Limb Ischaemia (SINGA‐PACLI) Trial |
| Methods | Randomized controlled trial |
| Participants | Country: Singapore Setting: 2 hospitals Number of participants: 136 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Uncoated balloon angioplasty device: unspecified DEB device: unspecified Drug used: paclitaxel Vessels treated: infrapopliteal arteries |
| Outcomes | Primary:
Secondary:
|
| Starting date | December 2013 |
| Contact information | Dr Bien Soo Tan, tan.bien.soo@sgh.com.sg Dr Farah Gillan Irani, farah.gillan.irani@sgh.com.sg |
| Notes | Sponsor: Singapore General Hospital No industrial support has been specified |
ABI: ankle‐brachial index; CLI: critical limb ischemia; DEB: drug‐eluting balloon; DES: drug‐eluting stent; DS: diameter stenosis; EQ‐5D: Euro‐Qol Group 5‐Dimension Self‐Report Questionnaire; PA: popliteal artery; PAD: peripheral arterial disease; PSVR: peak systolic velocity ratio; PTA: percutaneous transluminal angioplasty; QoL: quality of life; MAE: major adverse events; MI: myocardial infarction; SFA: superficial femoral artery; STEMI: ST‐elevation myocardial infarction; TLR: target lesion revascularization; TVR: target vessel revascularization; WIQ: Walking Impairment Questionnaire.
Differences between protocol and review
We did not include trials that examined the use of DEB for the treatment of instent restenosis. Our team believes that the disease process underlying instent restenosis differs from stenosis in unstented vessels. Change in functional walking ability was assessed by the Walking Impairment Questionnaire rather than treadmill walking distance, which was not reported in any of the included trials. Finally, the incidence of amputation was added as an outcome and amputation‐free rate was not used in the analysis because it was not reported by any of the included studies.
Contributions of authors
AK: drafting the proposal, study selection, data extraction and analysis, analysis interpretation, and final review drafting; will carry out updating. TA: drafting the proposal, study selection, data extraction and analysis, analysis interpretation, and final review drafting. GO: drafting the proposal, analysis interpretation, and final review drafting. GR: drafting the proposal, analysis interpretation, and final review drafting. KT: drafting the proposal, analysis interpretation, and final review drafting. DR: drafting the proposal, analysis interpretation, and final review drafting; will carry out updating.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
AK: none known. TA: none known. GO: reports having received consultancy fees from Cordis Medical (provision of services for lectures around abdominal aortic aneurysm (AAA) and peripheral vascular disease (PVD) with no requirement to endorse Cordis products). GR: reports having received consultancy fees from Cordis Medical and Cook Medical related to giving lectures/proctoring on AAA and PVD with no exclusive relationship to promote their products. KT: reports that his institution is involved in many multicenter clinical trials and he is a principal investigator for: The IN.PACT Global Clinical Study for the Treatment of Comprehensive Superficial Femoral and/or Popliteal Artery Lesions using Drug‐Eluting Balloon; BIOFLEX‐I clinical study: The Treatment of Iliac and Femoral Atherosclerotic Lesions Using the Self‐expanding Astron and Astron Pulsar stents; Local Delivery of Paclitaxel for Prevention of Restenosis in Hemodialysis Access. Funds from Biotronic Inc and Medtronic Endovascular are managed independently by the department research board. KT declares payment for procuring and teaching of clinical specialists in advance aortic aneurysm stent graft (Cook Medical). Funds were also received by his institution for live course consultancy (Cordis), teaching and research activities from Cook Medical for the Advance EVAR [endovascular aneurysm repair] registry, Covidien grant for tumor ablation, and Gore Medical Teaching grants for fellows. These were also managed by the research department. DR: reports having received consultancy fees from TVA Medical (no commercially available products and the startup company (percutaneous creation of dialysis fistulas) has no interests within peripheral arterial disease); and Cordis Medical (provision of services for lectures around AAA and PVD with no requirement to endorse Cordis products).
New
References
References to studies included in this review
BIOLUX P‐I {published and unpublished data}
- A prospective, multi‐centre, randomized controlled, first in man study to assess the safety and performance of the Passeo‐18 Lux paclitaxel releasing PTA balloon catheter vs. the uncoated Passeo 18 balloon catheter in patients with stenosis and occlusion of the femoropopliteal arteries (BIOLUX P‐I). clinicaltrials.gov/ct2/show/NCT01221610 (accessed 15 August 2015).
- Scheinert D, Schulte KL, Zeller T, Lammer J, Tepe G. Paclitaxel‐releasing balloon in femoropopliteal lesions using a BTHC excipient: twelve‐month results from the BIOLUX P‐I randomized trial. Journal of Endovascular Therapy 2015;22:14‐21. [DOI] [PubMed] [Google Scholar]
- Tepe G, Schulte K‐L, Zeller T, Lammer J, Pfaffinger P, Schmidt A, et al. Six‐month results of the BIOLUX P‐I first in man study comparing a paclitaxel‐releasing balloon catheter versus an uncoated balloon catheter in femoropopliteal lesions. Cardiovascular and Interventional Radiology 2012; Vol. 35:S264‐5.
- Zeller T, Lammer J, Rastan A, Schulte K, Tepe G, Scheinert D. Twelve‐month results of the BIOLUX P‐I first‐in‐man study comparing a paclitaxel‐releasing balloon catheter versus an uncoated catheter in femoropopliteal lesions. Vasa ‐ Journal of Vascular Diseases 2013;42(Suppl 84):FV 9.7. [Google Scholar]
BIOLUX P‐II {published and unpublished data}
- BIOTONIK's ‐ first in men study of the Passeo‐18 LUX drug releasing PTA balloon catheter vs. the uncoated Passeo 18 balloon catheter in subjects requiring revascularization of infrapopliteal arteries (BIOLUX P‐II). clinicaltrials.gov/ct2/show/NCT01867736 (accessed 15 August 2015). [DOI] [PubMed]
- Deloose K, Zeller T, Scheinert D, Beschorner U. BIOLUX P‐II: a randomized clinical trial of Passeo‐18 Lux DRB vs POBA for the treatment of infrapopliteal artery lesions. Journal of the American College of Cardiology 2014; Vol. 64, issue Suppl B.
- Scheinert D, Brodmann M, Zeller T, Bosiers M, Peeters P, Karl‐Ludwig S, et al. BIOLUX P‐II: a randomized clinical trial of Passeo‐18 lux drug releasing balloon versus plain old balloon angioplasty for the treatment of infrapopliteal artery lesions. Journal of the American College of Cardiology 2014;64(11 Suppl 1):B79. [Google Scholar]
DEBATE‐BTK 2013 {published data only}
- Liistro F, Porto I, Angioli P, Grotti S, Ricci L, Ducci K, et al. Drug‐eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE‐BTK): a randomized trial in diabetic patients with critical limb ischemia. Circulation 2013;128(6):615‐21. [DOI] [PubMed] [Google Scholar]
DEBELLUM 2012 {published and unpublished data}
- Fanelli F, Cannavale A, Boatta E, Corona M, Lucatelli P, Wlderk A, et al. Lower limb multilevel treatment with drug‐eluting balloons: 6‐month results from the DEBELLUM randomized trial. Journal of Endovascular Therapy 2012;19(5):571‐80. [DOI] [PubMed] [Google Scholar]
- Fanelli F, Cannavale A, Corona M, Lucatelli P, Wlderk A, Salvatori FM. The "DEBELLUM" ‐ lower limb multilevel treatment with drug eluting balloon ‐ randomized trial: 1‐year results. Journal of Cardiovascular Surgery 2014;55(2):207‐16. [PubMed] [Google Scholar]
FemPac 2008 {published and unpublished data}
- Werk M, Langner S, Reinkensmeier B, Boettcher HF, Tepe G, Dietz U, et al. Inhibition of restenosis in femoropopliteal arteries: paclitaxel‐coated versus uncoated balloon: femoral paclitaxel randomized pilot trial. Circulation 2008;118(13):1358‐65. [DOI] [PubMed] [Google Scholar]
IN.PACT DEEP 2014 {published data only}
- Zeller T, Baumgartner I, Scheinert D, Brodmann M, Bosiers M, Micari A, et al. Drug‐eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia: 12‐month results from the IN.PACT DEEP randomized trial. Journal of the American College of Cardiology 2014;64(15):1568‐76. [DOI] [PubMed] [Google Scholar]
- Zeller T, Baumgartner I, Scheinert D, Brodmann M, Bosiers M, Micari A, et al. IN.PACT Amphirion paclitaxel eluting balloon versus standard percutaneous transluminal angioplasty for infrapopliteal revascularization of critical limb ischemia: rationale and protocol for an ongoing randomized controlled trial. Trials 2014;15:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
IN.PACT SFA 2015 {published data only (unpublished sought but not used)}
- Laird JR, Schneider P, Tepe G, Brodmann M, Zeller T, Metzger C, et al. Durability of treatment effect using a drug‐coated balloon for femoropopliteal lesions 24‐month results of IN.PACT SFA. Journal of the American College of Cardiology 2015;66(21):2329‐38. [DOI] [PubMed] [Google Scholar]
- Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, et al. Drug‐coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12‐month results from the IN.PACT SFA randomized trial. Circulation 2015;131(5):495‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
LEVANT I 2014 {published data only (unpublished sought but not used)}
- Scheinert D, Duda S, Zeller T, Krankenberg H, Ricke J, Bosiers M, et al. The LEVANT I (Lutonix paclitaxel‐coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first‐in‐human randomized trial of low‐dose drug‐coated balloon versus uncoated balloon angioplasty. JACC. Cardiovascular Interventions 2014;7(1):10‐9. [DOI] [PubMed] [Google Scholar]
LEVANT II 2015 {published data only}
- Rosenfield K, Jaff MR, White CJ, Rocha‐Singh K, Mena‐Hurtado C, Metzger DC, et al. Trial of a paclitaxel‐coated balloon for femoropopliteal artery disease. New England Journal of Medicine 2015;373(2):145‐53. [DOI] [PubMed] [Google Scholar]
PACIFIER 2012 {published data only}
- Werk M, Albrecht T, Meyer DR, Ahmed MN, Behne A, Dietz U, et al. Paclitaxel‐coated balloons reduce restenosis after femoro‐popliteal angioplasty: evidence from the randomized PACIFIER trial. Circulation. Cardiovascular Interventions 2012;5(6):831‐40. [DOI] [PubMed] [Google Scholar]
THUNDER 2008 {published data only}
- Tepe G, Schnorr B, Albrecht T, Brechtel K, Claussen CD, Scheller B, et al. Angioplasty of femoral‐popliteal arteries with drug‐coated balloons: 5‐year follow‐up of the THUNDER trial. JACC. Cardiovascular Interventions 2015;8(1):102‐8. [DOI] [PubMed] [Google Scholar]
- Tepe G, Zeller T, Albrecht T, Heller S, Schwarzwälder U, Beregi JP, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. New England Journal of Medicine 2008;358(7):689‐99. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
COPA CABANA {published data only}
- Cotavance™ Paclitaxel‐coated balloon versus uncoated balloon angioplasty for treatment of in‐stent restenosis in SFA and popliteal arteries COPA CABANA Study. clinicaltrials.gov/ct2/show/NCT01594684 (accessed 15 August 2015).
DEBATE‐ISR {published data only}
- Liistro F, Angioli P, Porto I, Ricci L, Ducci K, Grotti S, et al. Paclitaxel‐eluting balloon vs. standard angioplasty to reduce recurrent restenosis in diabetic patients with in‐stent restenosis of the superficial femoral and proximal popliteal arteries: the DEBATE‐ISR study. Journal of Endovascular Therapy 2014;21(1):1‐8. [DOI] [PubMed] [Google Scholar]
DEBATE SFA {published data only}
- Liistro F, Grotti S, Porto I, Angioli P, Ricci L, Ducci K, et al. Drug‐eluting balloon in peripheral intervention for the superficial femoral artery: the DEBATE‐SFA randomized trial (drug eluting balloon in peripheral intervention for the superficial femoral artery). JACC. Cardiovascular Interventions 2013;6(12):1295‐302. [DOI] [PubMed] [Google Scholar]
DEFINITIVE AR {published data only}
- Directional atherectomy followed by a paclitaxel‐coated balloon to inhibit restenosIs and maintain vessel patency: a pilot study of anti‐restenosis treatment. clinicaltrials.gov/ct2/show/NCT01366482 (accessed 15 August 2015).
EURO CANAL {published data only}
- European study of POBA versus Cotavance paclitaxel coated balloon for the treatment of infrapopliteal lesions in critical limb ischemia. clinicaltrials.gov/ct2/show/NCT01260870 (accessed 15 August 2015).
FAIR {published data only}
- Femoral artery in‐stent restenosis (FAIR) trial. clinicaltrials.gov/ct2/show/record/NCT01305070 (accessed 15 August 2015).
Freeway Stent Study {published data only}
- Tacke J, Kieselbach D, Schulte KL. [The Freeway stent study]. Presented at the Transcatheter Cardiovascular Therapeutics Conference in Washington, D.C. September 2014.
IDEAS {published data only}
- Siablis D, Kitrou PM, Spiliopoulos S, Katsanos K, Karnabatidis D. Paclitaxel‐coated balloon angioplasty versus drug‐eluting stenting for the treatment of infrapopliteal long‐segment arterial occlusive disease: the IDEAS randomized controlled trial. JACC. Cardiovascular Interventions 2014;7(9):1048‐56. [DOI] [PubMed] [Google Scholar]
ISAR‐PEBIS {published data only}
- Randomized trial of paclitaxel eluting balloon or conventional balloon for treatment of in‐stent restenosis of the superficial femoral artery in patients with symptomatic peripheral artery disease (ISAR‐PEBIS). clinicaltrials.gov/ct2/show/NCT01083394 (accessed 15 August 2015).
ISAR‐STATH {published data only}
- Randomized trial of stenting after dilation with or without paclitaxel eluting balloon or atherectomy in patients with symptomatic peripheral artery disease. clinicaltrials.gov/ct2/show/NCT00986752 (accessed 15 August 2015).
PACUBA 1 {published data only}
- A monocenter randomized clinical trial of PAClitaxel drUg‐eluting BAlloon versus standard percutaneous transluminal angioplasty to reduce restenosis in patients with in‐stent stenoses in the superficial femoral and proximal popliteal artery. clinicaltrials.gov/ct2/show/NCT01247402 (accessed 15 August 2015).
PHOTOPAC {published data only}
- Photoablative atherectomy followed by a paclitaxel‐coated balloon to inhibit restenosis in instent femoro‐popliteal obstructions. clinicaltrials.gov/ct2/show/NCT01298947 (accessed 15 August 2015). [DOI] [PubMed]
RAPID {published data only}
- Karimi A, Boer SW, Heuvel D, Fioole B, Vroegindeweij D, Heyligers JMM, et al. Randomized trial of Legflow® paclitaxel eluting balloon and stenting versus standard percutaneous transluminal angioplasty and stenting for the treatment of intermediate and long lesions of the superficial femoral artery (RAPID trial): study protocol for a randomized controlled trial. Trials 2013;14:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
SWEDEPAD {published data only}
- Swedish drug‐elution trial in peripheral arterial disease ‐ a multicenter, prospective randomized controlled clinical trial based on the Swedish Vascular Registry (SWEDVASC) Platform. clinicaltrials.gov/ct2/show/NCT02051088 (accessed 15 August 2015).
References to ongoing studies
ACOART‐BTK {published data only (unpublished sought but not used)}
- Evaluation of the use of ACOTEC drug‐eluting balloon Litos in below‐the‐knee arteries to treat critical limb ischemia (ACOART‐BTK). clinicaltrials.gov/ct2/show/NCT02563535 (accessed 25 December 2015).
AcoArt I {published data only (unpublished sought but not used)}
- Prospective, multi‐center and randomized controlled clinical study to verify effectiveness and safety of drug‐eluting balloon in PTA procedure (AcoArt I Study). clinicaltrials.gov/ct2/show/NCT01850056 (accessed 15 August 2015).
AcoArt II {published data only (unpublished sought but not used)}
- Prospective, multi‐center and randomized controlled clinical study to verify effectiveness and safety of drug‐eluting balloon in PTA procedure of the infrapopliteal artery. clinicaltrials.gov/ct2/show/NCT02137577 (accessed 15 August 2015).
Advance 18PTX Balloon Catheter Study {published data only (unpublished sought but not used)}
- Advance® 18PTX® Balloon Catheter Study: treatment of lesions in superficial femoral artery/popliteal artery with a paclitaxel‐coated balloon. clinicaltrials.gov/ct2/show/NCT00776906 (accessed 15 August 2015).
EFFPac {published data only (unpublished sought but not used)}
- Teichgräber U. Phase III multicenter randomized controlled trial to assess the effectiveness of paclitaxel‐coated Luminor balloon catheter versus uncoated balloon catheter in the superficial femoral and popliteal arteries to prevent vessel restenosis or reocclusion. Presented at the Leipzig Interventional Course; Leipzig, Germany January 2015.
FREERIDE Study {published data only (unpublished sought but not used)}
- Phase III FREERIDE study Freeway randomized angioplasty study. clinicaltrials.gov/ct2/show/NCT01960647 (accessed 15 August 2015).
ILLUMENATE {published data only (unpublished sought but not used)}
- Pivotal trial of a novel paclitaxel‐coated percutaneous angioplasty balloon. clinicaltrials.gov/ct2/show/NCT01858428 (accessed 20 December 2015).
LEVANT Japan {published data only (unpublished sought but not used)}
- A prospective, multicenter, single blind, randomized, controlled Japanese population trial comparing MD02‐LDCB versus standard balloon angioplasty for treatment of femoropopliteal arteries. clinicaltrials.gov/ct2/show/NCT01816412 (accessed 15 August 2015).
Lutonix BTK {published data only (unpublished sought but not used)}
- A prospective, multicenter, single blind, randomized, controlled trial comparing the Lutonix drug coated balloon versus standard balloon angioplasty for treatment of below‐the‐knee (BTK) arteries (Lutonix BTK trial). clinicaltrials.gov/ct2/show/NCT01870401 (accessed 15 August 2015).
MDT‐2113 SFA {published data only (unpublished sought but not used)}
- Randomized trial of MDT‐2113 drug‐eluting balloon (DEB) vs. standard PTA for the treatment of atherosclerotic lesions in the superficial femoral artery and/or proximal popliteal artery. clinicaltrials.gov/ct2/show/NCT01947478 (accessed 15 August 2015).
PICCOLO {published data only (unpublished sought but not used)}
- Paclitaxel coated balloons for prevention of restenosis in small arteries below the knee compared to angioplasty using uncoated balloons. clinicaltrials.gov/ct2/show/NCT00696956 (accessed 15 August 2015).
RANGER‐SFA {published data only (unpublished sought but not used)}
- Prospective, randomized, multicentre clinical study of the Hemoteq Ranger™ paclitaxel‐coated PTA balloon catheter (Ranger DCB) in comparison to uncoated PTA balloons in femoropopliteal lesions. clinicaltrials.gov/ct2/show/NCT02013193 (accessed 15 August 2015).
SINGA‐PACLI {published data only (unpublished sought but not used)}
- Singapore infra‐genicular angioplasty with paclitaxel‐eluting balloon for critical limb ischaemia (SINGA‐PACLI) trial. clinicaltrials.gov/ct2/show/NCT02129634 (accessed 15 August 2015).
Additional references
Aquino 2001
- Aquino R, Johnnides C, Makaroun M, Whittle JC, Muluk VS, Kelley ME, et al. Natural history of claudication: long‐term serial follow‐up study of 1244 claudicants. Journal of Vascular Surgery 2001;34(6):962‐70. [DOI] [PubMed] [Google Scholar]
Axel 1997
- Axel DI, Kunert W, Goggelmann C, Oberhoff M, Herdeg C, Kuttner A, et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation 1997;96(2):636‐45. [DOI] [PubMed] [Google Scholar]
Baerlocher 2015
- Baerlocher MO, Kennedy SA, Rajebi MR, Baerlocher FJ, Misra J, Liu D, et al. Meta‐analysis of drug‐eluting balloon angioplasty and drug‐eluting stent placement for infrainguinal peripheral arterial disease. Journal of Vascular and Interventional Radiology 2015;26(4):459‐73. [DOI] [PubMed] [Google Scholar]
BASIL 2005
- Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. BASIL trial participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005;366(9501):1925‐34. [DOI] [PubMed] [Google Scholar]
Bradbury 2010
- Bradbury AW, BASIL trial investigators and participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL) trial in perspective. Journal of Vascular Surgery 2010;51(5 Suppl):1S‐4S. [DOI] [PubMed] [Google Scholar]
Canaud 2014
- Canaud L, Ozdemir BA, Belli AM, Loftus IM, Thompson MM, Hinchliffe RJ. Infrainguinal angioplasty with drug‐eluting stents and balloons. Journal of Vascular Surgery 2014;59:1721‐36. [DOI] [PubMed] [Google Scholar]
Cassese 2012
- Cassese S, Byrne RA, Ott I, Ndrepepa G, Nerad M, Kastrati A, et al. Paclitaxel‐coated versus uncoated balloon angioplasty reduces target lesion revascularization in patients with femoropopliteal arterial disease: a meta‐analysis of randomized trials. Circulation. Cardiovascular Interventions 2012;5:582‐9. [DOI] [PubMed] [Google Scholar]
Dake 2013
- Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, et al. Zilver PTX Investigators. Sustained safety and effectiveness of paclitaxel‐eluting stents for femoropopliteal lesions: 2‐year follow‐up from the Zilver PTX randomized and single‐arm clinical studies. Journal of the American College of Cardiology 2013;61(24):2417‐27. [DOI] [PubMed] [Google Scholar]
Fowkes 1991
- Fowkes FGR, Housley E, Cawood EHH, Macintyre CCA, Ruckley CV, Prescott RJ. Edinburgh artery study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. International Journal of Epidemiology 1991;20(2):384‐92. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirsch 200
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter‐Society Consensus; and Vascular Disease Foundation. Circulation 2006;113(11):e463‐654. [DOI] [PubMed] [Google Scholar]
IDEAS 2014
- Siablis D, Kitrou PM, Spiliopoulos S, Katsanos K, Karnabatidis D. Paclitaxel‐coated balloon angioplasty versus drug‐eluting stenting for the treatment of infrapopliteal long‐segment arterial occlusive disease: the IDEAS randomized controlled trial. JACC. Cardiovascular Interventions 2014;7:1048‐56. [DOI] [PubMed] [Google Scholar]
Jens 2014a
- Jens S, Conijn AP, Koelemay MJW, Bipat S, Reekers JA. Randomized trials for endovascular treatment of infrainguinal arterial disease: systematic review and meta‐analysis (part 1: above the knee). European Journal of Vascular and Endovascular Surgery 2014;47(5):524‐35. [DOI] [PubMed] [Google Scholar]
Jens 2014b
- Jens S, Conijn AP, Koelemay MJW, Bipat S, Reekers JA. Randomized trials for endovascular treatment of infrainguinal arterial disease: systematic review and meta‐analysis (part 2: below the knee). European Journal of Vascular and Endovascular Surgery 2014;47(5):536‐44. [DOI] [PubMed] [Google Scholar]
Micari 2012
- Micari A, Cioppa A, Vadala G, Castriota F, Liso A, Marchese A, et al. Clinical evaluation of a paclitaxel‐eluting balloon for treatment of femoropopliteal arterial disease: 12‐month results from a multicenter Italian registry. JACC. Cardiovascular Interventions 2012;5:331‐8. [DOI] [PubMed] [Google Scholar]
Norgren 2007
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, TASC II Working Group. Inter‐society consensus for the management of peripheral arterial disease (TASC II). Journal of Vascular Surgery 2007;45(Suppl S):S5‐67. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schmidt 2011
- Schmidt A, Piorkowski M, Werner M, Ulrich M, Bausback Y, Braeunlich S, et al. First experience with drug‐eluting balloons in infrapopliteal arteries: restenosis rate and clinical outcome. Journal of the American College of Cardiology 2011;58:1105‐9. [DOI] [PubMed] [Google Scholar]
Schnorr 2013
- Schnorr B, Albrecht T. Drug‐coated balloons and their place in treating peripheral arterial disease. Expert Review of Medical Devices 2013;10(1):105‐14. [DOI] [PubMed] [Google Scholar]
Seedial 2013
- Seedial SM, Ghosh S, Saunders RS, Suwanabol PA, Shi X, Liu B, et al. Local drug delivery to prevent restenosis. Journal of Vascular Surgery 2013;57(5):1403‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Selvin 2004
- Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999‐2000. Circulation 2004;110(6):738‐43. [DOI] [PubMed] [Google Scholar]


