Abstract
Background
Bronchiolitis is the leading cause of hospitalisation among infants in high‐income countries. Acute viral bronchiolitis is associated with airway obstruction and turbulent gas flow. Heliox, a mixture of oxygen and the inert gas helium, may improve gas flow through high‐resistance airways and decrease the work of breathing. In this review, we selected trials that objectively assessed the effect of the addition of heliox to standard medical care for acute bronchiolitis.
Objectives
To assess heliox inhalation therapy in addition to standard medical care for acute bronchiolitis in infants with respiratory distress, as measured by clinical endpoints (in particular the rate of endotracheal intubation, the rate of emergency department discharge, the length of treatment for respiratory distress) and pulmonary function testing (mainly clinical respiratory scores).
Search methods
We searched CENTRAL (2015, Issue 2), MEDLINE (1966 to March week 3, 2015), EMBASE (1974 to March 2015), LILACS (1982 to March 2015) and the National Institutes of Health (NIH) website (May 2009).
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs of heliox in infants with acute bronchiolitis.
Data collection and analysis
Two review authors independently extracted data and assessed trial quality.
Main results
We included seven trials involving 447 infants younger than two years with respiratory distress secondary to viral bronchiolitis. All children were recruited from a paediatric intensive care unit (PICU; 378 infants), except in one trial (emergency department; 69 infants). All children were younger than two (under nine months in two trials and under three months in one trial). Positive tests for respiratory syncytial virus (RSV) were required for inclusion in five trials. The two other trials were carried out in the bronchiolitis seasons. Seven different protocols were used for inhalation therapy with heliox.
When heliox was used in the PICU, we observed no significant reduction in the rate of intubation: risk ratio (RR) 2.73 (95% confidence interval (CI) 0.96 to 7.75, four trials, 408 infants, low quality evidence). When heliox inhalation was used in the emergency department, we observed no increase in the rate of discharge: RR 0.51 (95% CI 0.17 to 1.55, one trial, 69 infants, moderate quality evidence).
There was no decrease in the length of treatment for respiratory distress: mean difference (MD) ‐0.19 days (95% CI ‐0.56 to 0.19, two trials, 320 infants, moderate quality evidence). However, in the subgroup of infants who were started on nasal continuous positive airway pressure (nCPAP) right from the start, because of severe respiratory distress, heliox therapy reduced the length of treatment: MD ‐0.76 days (95% CI ‐1.45 to ‐0.08, one trial, 21 infants, low quality evidence). No adverse events related to heliox inhalation were reported.
We found that infants treated with heliox inhalation had a significantly lower mean clinical respiratory score in the first hour after starting treatment when compared to those treated with air or oxygen inhalation: MD ‐1.04 (95% CI ‐1.60 to ‐0.48, four trials, 138 infants, moderate quality evidence). This outcome had statistical heterogeneity, which remained even after removing the study using a standard high‐concentration reservoir mask. Several factors may explain this heterogeneity, including first the limited number of patients in each trial, and the wide differences in the baseline severity of disease between studies, with the modified Wood Clinical Asthma Score (m‐WCAS) in infants treated with heliox ranging from less than two to more than seven.
Authors' conclusions
Current evidence suggests that the addition of heliox therapy may significantly reduce a clinical score evaluating respiratory distress in the first hour after starting treatment in infants with acute RSV bronchiolitis. We noticed this beneficial effect regardless of which heliox inhalation protocol was used. Nevertheless, there was no reduction in the rate of intubation, in the rate of emergency department discharge, or in the length of treatment for respiratory distress. Heliox could reduce the length of treatment in infants requiring CPAP for severe respiratory distress. Further studies with homogeneous logistics in their heliox application are needed. Inclusion criteria must include a clinical severity score that reflects severe respiratory distress to avoid inclusion of children with mild bronchiolitis who may not benefit from heliox inhalation. Such studies would provide the necessary information as to the appropriate place for heliox in the therapeutic schedule for severe bronchiolitis.
Plain language summary
Heliox inhalation therapy for bronchiolitis in infants
Review question
We reviewed the evidence about the effect of heliox inhalation therapy in infants with airway obstruction due to winter viral pulmonary infection.
Background
Approximately 20% of all infants experience trouble breathing associated with viral infection in the first year of life, and 2% to 3% of these infants require hospitalisation for marked breathing difficulties. In this review, we wanted to asses the effect of the addition of heliox to standard medical care. Heliox is a mixture of oxygen and the gas helium. Helium inhalation decreases the work of breathing by increasing gas flow in small or partially obstructed airways by lowering resistance to gas flow.
Study characteristics
We retrieved seven trials involving 447 infants requiring hospitalisation for marked breathing difficulties. Six trials recruited infants from paediatric intensive care units and one trial from the emergency department. Four trials were supported in part by unrestricted grants from a manufacturer with a commercial interest in the results. The evidence is current to March 2015.
Key results
Pooled results failed to demonstrate a reduction in the rate of emergency department discharge, in the rate of intubation to achieve respiratory support, or in the length of respiratory support. However, four trials involving 138 infants used a clinical respiratory score system, with increased severity receiving a higher score. The pooled results show that infants treated with heliox inhalation had a reduction in this respiratory score in the first hour. In a small subgroup of infants who were started on a nasal device providing a continuous positive airway pressure right from the start, because of the severity of their disease, heliox inhalation could reduce length of treatment.
Quality of the evidence
Each trial included in this review used a different method for delivering heliox. They also used different thresholds of clinical respiratory score for inclusion, and were often underpowered for the major endpoints. Further studies using a valid method of heliox application in addition to standard medical care are needed. Inclusion criteria must include a clinical severity score that reflects severe respiratory distress to avoid the inclusion of children who are not very sick, who may not benefit from heliox inhalation. Such studies would provide necessary information about the appropriate place of heliox in the management of seasonal pulmonary infection in infants.
Summary of findings
for the main comparison.
| Heliox inhalation compared with air/oxygen for acute bronchiolitis | ||||||
|
Patient or population: infants with bronchiolitis Settings: paediatric intensive care unit and emergency department Intervention: heliox inhalation Comparison: air or oxygen inhalation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Heliox | |||||
| Need for mechanical ventilation (invasive or not) Follow‐up in the acute phase of bronchiolitis | 155 per 1000 | 148 per 1000 (94 to 235) | RR 0.95 (0.61 to 1.49) | 408 (4 trials) | ⊕⊕⊕⊕ high |
1, 2, 3, 4 |
| Rate of intubation (all reasons) Follow‐up in the acute phase of bronchiolitis | 19 per 1000 |
54 per 1000 (18 to 168) |
RR 2.73 (0.96 to 7.75) |
408 (4 trials) | ⊕⊕⊖⊖ low |
1, 5, 3, 6 |
| Rate of emergency department discharge Follow‐up at 240 min | 229 per 1000 | 118 per 1000 (39 to 355) |
RR 0.51 (0.17 to 1.55) |
69 (1 trial) |
⊕⊕⊕⊖ moderate |
7 |
| Length of treatment Follow‐up in the acute phase of bronchiolitis | The mean length of treatment ranged across control groups from 2.04 to 2.63 days | The mean length of treatment in the intervention groups was 0.19 days lower (0.56 lower to 0.19 higher) |
— | 320 (2 trials) |
⊕⊕⊕⊖ moderate |
1, 2, 8 |
| Length of treatment ‐ nCPAP Follow‐up in the acute phase of bronchiolitis | The mean length of treatment
ranged across the control
subgroup (with nCPAP) from 1.70 to 3.06 days |
The mean length of treatment in the intervention subgroup (with nCPAP) was 0.76 days lower (1.45 to 0.08 lower) |
— | 21 (1 trial) |
⊕⊕⊖⊖ low |
9 |
| Change in clinical respiratory scores (m‐WCAS) within the first hour after starting heliox treatment Follow‐up at the first hour after starting treatment | The mean change in m‐WCAS within the first hour after starting heliox treatment ranged across control groups from ‐0.35 to +0.04 | The mean change in m‐WCAS in the intervention groups was1.04 lower (1.60 to 0.48 lower) |
— | 138 (4 trials) | ⊕⊕⊕⊖ moderate |
10, 11, 12 |
| *Basis for the comparative risks: Theassumed risk is based on the Control group risk or the median control group risk (95% CI) across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio (Fixed effect); nCPAP: nasal continuous positive airway pressure; m‐WCAS: modified Wood Clinical Asthma Score | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1The largest trial was at unclear risk of bias for blinding but at low risk for other bias. 2No serious inconsistency: statistical heterogeneity was low: I2 statistic = 0%. 3No serious indirectness: the four trials were conducted in France, the United Kingdom and the USA. 4The 95% CI around the risk ratio is narrow and excludes clinically important effects. 5Downgraded by 1 for serious inconsistency: heterogeneity is present: I2 statistic = 20%.
6Downgraded by 1 for serious imprecision: the 95% CI around the risk ratio is wide. 7Downgraded by 1 for serious risk of bias: only one trial is included. 8Downgraded by 1: the 95% CI values around the mean change are under the unit (fraction of a day) and could be imprecise. 9Downgraded by 2: only results from a subgroup of one trial are included. 10The two trials are at low risk of bias. 11Downgraded by 1: statistical heterogeneity was high (I2 statistic = 80%) and remained when we kept only low risk of bias trials. 12No serious indirectness: the four trials were conducted in France, the USA, the United Kingdom and Spain.
Background
Description of the condition
Bronchiolitis, an acute inflammatory process of the small airways, is the leading cause of hospitalisation among infants in high‐income countries (Hall 2013; Welliver 2003). Common symptoms include a runny nose, cough and dyspnoea often with bronchospasm, mucus production and wheezing. Apnoea may be the initial manifestation before other respiratory signs are present and is most common in premature infants (Ralston 2009). Respiratory syncytial virus (RSV) is the most common pathogen isolated (Mansbach 2012). Approximately 20% of all infants have RSV‐associated wheezing in the first year of life and 2% to 3% require hospitalisation as part of the management strategy (AAP 2006). Young age (under six months of age) at the onset of the RSV season increases the risk of RSV hospitalisation (Carbonell‐Estrany 2000; Figueras‐Aloy 2008; Law 2004; Liese 2003). Analysis of representative studies over the last 30 years found that the risk ratio of boys to girls is 1.4:1 (Simoes 2003). More than 50% of hospitalisations caused by RSV infections are in infants and children with no known risk factors (Boyce 2000).
Even though there are many treatments, there is no evidence to endorse a specific treatment other than supportive care (Davison 2004). Current evidence suggests that nebulised 3% saline may significantly reduce the length of hospital stay among infants hospitalised with non‐severe acute viral bronchiolitis and improve the clinical severity score in both outpatient and inpatient populations (Zhang 2013). Supplemental oxygen and judicious fluid management remain the mainstays of therapy. Ventilatory support can be necessary in 7.5% of infants hospitalised for bronchiolitis with an overall clinical score of at least 4 on a scale of 0 to 10 (Skjerven 2013). The use of heated, humidified, high‐flow nasal cannula (HFNC) therapy may reduce the need for invasive respiratory support thus potentially lowering costs, with clinical advantages and fewer adverse effects (Beggs 2014; Bressan 2013; Hough 2014; Mayfield 2014; Milesi 2013).
Premature or low birth weight infants, infants with bronchopulmonary dysplasia and patients with haemodynamically significant congenital heart disease merit special attention. Recipients of hematopoetic stem cell transplants have had especially high rates of severe disease.The relatively smaller airways of select infant groups places them at higher risk of respiratory failure and need for specialised management. For example, the percentage of patients requiring endotracheal intubation or positive pressure ventilation (PPV) is higher in infants with congenital heart disease, immunocompromised status (Wang 1995), chronic lung disease or those born prematurely (Meert 1990). Mortality rates of infants hospitalised in a paediatric intensive care unit (PICU) for RSV‐bronchiolitis range from 0% to 3% if patients have no risk factors, and 2.5% to 6% if they have at least one risk factor (Chevret 2005; Prais 2003; Wang 1995). Mortality associated with RSV infection is relatively rare among young children in high‐income countries. The majority of deaths occur in infants with complex chronic conditions and in those with other acute conditions, such as sepsis, which could have contributed to their deaths (Byington 2015). It was estimated that between 66,000 and 199,000 children younger than five years died from RSV‐associated acute lower respiratory infection in 2005, with 99% of these deaths occurring in low‐income countries (Nair 2010).
Description of the intervention
In 1934, Barach first described the use of helium‐oxygen gas mixtures (heliox) for the treatment of upper airway obstruction (Barach 1934). Heliox has subsequently been shown to be a useful adjunctive therapy in children with asthma (Carter 1996; Kudukis 1997), bronchiolitis (Gross 2000; Hollmann 1998; Paret 1996), upper airway obstruction (Duncan 1979; Tobias 1997), acute respiratory distress (Winters 2000), and in children with post‐extubation stridor (Kemper 1991). Heliox may also increase the effectiveness of nasal continuous positive airway pressure (nCPAP) in the treatment of respiratory distress syndrome in premature infants (Colnaghi 2012). Although heliox can be an effective treatment option, the existing evidence does not provide support for the administration of heliox mixtures to all emergency department patients with acute asthma (Rodrigo 2010).
How the intervention might work
In bronchiolitis, breathing becomes more difficult due to an increased end‐expiratory lung volume, decreased lung compliance and relative upper airway obstruction with increased airway resistance. Infection of bronchiolar respiratory and ciliated epithelial cells produces increased mucus secretion, cell death and sloughing. This is followed by a peribronchiolar lymphocytic infiltrate and submucosal oedema (AAP 2006; Piedimonte 2002). The combination of debris and oedema produces obstruction of the smaller airways. This critical narrowing results in turbulent flow and increased airway resistance. Decreased ventilation in affected areas causes ventilation/perfusion mismatching, resulting in hypoxia. During the expiratory phase of respiration, dynamic collapse of the airways produces a disproportionate decrease in airflow and resultant air trapping. Since bronchiolitis is associated with airway obstruction and turbulent gas flow, this disease process could theoretically be improved by heliox, which improves gas flow through high‐resistance airways because of the lower density of helium compared to air (Gupta 2005; Panitch 2003). Helium is an inert gas with no intrinsic bronchodilatory or anti‐inflammatory properties. Helium has the lowest density of any gas other than hydrogen, which is unfortunately not medically useful because of its flammable properties. Helium acts as a 'carrier gas', resulting in lower resistance to gas flow allowing for increased bulk flow, increased oxygen flow and decreased work of breathing (Wolfson 1984). Equally important is the fact that carbon dioxide diffuses through helium four to five times faster than through air, which aids ventilation and carbon dioxide removal. The effects of heliox are relatively rapid and therefore any significant clinical effects are expected to be seen within minutes. A one‐hour trial is usually enough to detect any beneficial effect of heliox (Martinon‐Torres 2015). Thus, the clinician quickly knows if heliox therapy will be beneficial for an individual patient or whether it should be abandoned for other possible therapies. For non‐ventilated patients, it is also essential to ensure that there is no accidental contamination of the heliox mixture with air or oxygen. For instance, heliox administration via a standard high‐concentration reservoir mask leads to significant dilution by room air (Standley 2008).
The use of premixed heliox tanks with at least a 21% oxygen concentration avoids accidental administration of a hypoxic gas mixture. Manufacturers supply pre‐mixed bottles at different concentrations of helium/oxygen for clinical use.
The administration of heliox during mechanical ventilation must be carried out with vigilance and accurate, continuous monitoring. Helium can interfere with the accuracy of pneumotachometer and ventilator function, which are typically calibrated for nitrogen instead of helium as the primary balance gas (Berkenbosch 2003). New generation helium/oxygen administration systems have been developed to help circumvent these issues. Even if helium‐oxygen can be safely delivered by modifying standard ventilators according to guidelines in the literature, caution must be applied when using this approach (Martinon‐Torres 2012).
Why it is important to do this review
The hypothesis of this review is that heliox inhalation is beneficial in the management of acute bronchiolitis as assessed by clinically relevant outcomes. In order to critically evaluate the clinical data, we undertook a systematic review of trials that use heliox for the treatment of bronchiolitis.
Objectives
To assess heliox inhalation therapy in addition to standard medical care for acute bronchiolitis in infants with respiratory distress, as measured by clinical endpoints (in particular the rate of endotracheal intubation, the rate of emergency department discharge, the length of treatment for respiratory distress) and pulmonary function testing (mainly clinical respiratory scores).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs. We considered both parallel‐group and cross‐over designs. We excluded studies where heliox was used as a vector for nebulisation (to improve aerosol drug delivery), or studies where helium was used to assess lung volumes.
Types of participants
Infants hospitalised for acute bronchiolitis. For the purpose of this review, acute bronchiolitis is defined by the presence of signs of respiratory distress secondary to respiratory syncytial virus (RSV) infection and/or those patients with respiratory distress and symptoms that occur within RSV epidemic periods and are not due to other medical conditions.
Types of interventions
Treatment with inhaled heliox versus a placebo (oxygen or air).
Types of outcome measures
Primary outcomes
In‐hospital mortality.
Need for mechanical ventilation (invasive or not).
Rate of endotracheal intubation.
Rate of emergency department discharge.
Length of treatment for respiratory distress.
Length of paediatric intensive care unit (PICU) stay.
Adverse effects.
Secondary outcomes
Gas exchange (effects on oxygenation and CO2 elimination) within the first 24 hours after starting heliox treatment.
Respiratory mechanics (effects on pulmonary compliance and resistance of airways) within the first 24 hours after starting heliox treatment.
Clinical respiratory scores within the first 24 hours after starting heliox treatment.
Total duration of hospitalisation (including duration in PICU).
Search methods for identification of studies
Electronic searches
For the 2015 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 2), which includes the Cochrane Acute Respiratory Infection Group's Specialised Register, MEDLINE (Ovid) (June 2009 to March week 3, 2015), Embase.com (June 2009 to March 2015), LILACS (BIREME) (2009 to March 2015) and the NIH website (May 2009). See Appendix 1 for details of previous searches.
We used the search strategy in Appendix 2 to search MEDLINE and CENTRAL. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying RCTs randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search terms to search EMBASE (see Appendix 3) and LILACS (see Appendix 4). We did not apply any date, language or publication limits.
Searching other resources
We searched the trials registries World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/default.aspx) and the US National Institutes of Health (www.clinicaltrials.gov) for completed and ongoing trials (latest search 30 March 2015). We checked the references of relevant systematic reviews and identified RCTs. Two review authors (VG, JML) contacted trial authors of all studies to locate other unpublished or in progress studies that met the inclusion criteria.
Data collection and analysis
Selection of studies
Two review authors (JML, GC) independently reviewed titles, abstracts and citations to assess potential relevance for full review. From the full text, both review authors independently assessed studies for inclusion based on the criteria for study design, population, intervention and outcomes. We excluded trials that did not meet our inclusion criteria and noted the reasons for their exclusion (see Characteristics of excluded studies table). We resolved any disagreement between the two review authors about study inclusion by discussion.
Data extraction and management
Two review authors (JML, GC) independently extracted data from the included trials using a standardised data extraction form. We resolved any disagreement between the two review authors by discussion. We entered results into the Cochrane Collaboration's software program (Review Manager 5.3) (RevMan 2014). Data extraction included the following items.
Methods (method of randomisation, allocation concealment, blinding, analysis by intention‐to‐treat (ITT), withdrawals).
Participants (age, gender, number of patients studied, location, RSV and other organisms, patient demographics, risk factors, withdrawals).
Interventions (fraction of inspired helium, duration of therapy, route of delivery, mechanical ventilation, intubation).
Control; concurrent treatments.
Outcomes. We extracted the results based upon the ITT population.
Assessment of risk of bias in included studies
Two review authors (JML, GC) assessed the methodological quality of the included trials. We assessed each study for validity using the Cochrane Collaboration's tool for assessing risk of bias (Higgins 2011). We classified six domains as low risk, unclear risk or high risk: random sequence generation, allocation concealment, blinding (participants and personnel, and outcome assessment), incomplete outcome data, selective reporting and other potential sources of bias. We resolved any disagreement between the two review authors by discussion.
Measures of treatment effect
We compared treatment with inhaled heliox to a placebo (oxygen or air) for primary and secondary outcomes. For endpoints with dichotomous measures (for example, mortality, need for endotracheal intubation), we measured effect size using the risk ratio (RR) and 95% confidence interval (CI). We calculated the mean difference (MD) and 95% CI for numerical outcomes.
Unit of analysis issues
We analysed studies with non‐standard designs, such as cross‐over trials, according to particular biases. The main concerns over risk of bias in cross‐over trials are whether the cross‐over design is suitable, whether there is a carry‐over effect, whether only first trial period data are available, incorrect analysis and comparability of results with those from parallel‐group trials. Since helium is an inert gas with no intrinsic bronchodilatory or anti‐inflammatory properties and could be useful only by improving gas flow, there is unlikely to be carry‐over of treatment effect across trial periods.
Dealing with missing data
In case of missing values, we contacted the original trial authors to request missing data. Otherwise, we assumed data to be missing at random. We also addressed the potential impact of missing data on the findings of the review in the Discussion section.
Assessment of heterogeneity
We assessed heterogeneity between studies by using the I2 statistic (Higgins 2003). We considered heterogeneity weak when the I2 statistic was less than 0.25.
Assessment of reporting biases
We assessed publication bias by funnel plots. Publication bias need not lead to asymmetry in funnel plots. In the absence of any intervention effect, selective publication based on the P value alone will lead to a symmetrical funnel plot in which studies on the extreme left or right are more likely to be published than those in the middle. This could bias the estimated between‐study heterogeneity variance.
Data synthesis
We used a random‐effects model to meta‐analyse data.
GRADE and 'Summary of findings' table
We created Table 1 using the following primary outcomes: need for mechanical ventilation (invasive or not); rate of endotracheal intubation; rate of emergency department discharge; and length of treatment for respiratory distress. We did not include the primary outcome 'in‐hospital mortality'. This primary outcome is unavoidable but it is not relevant to a clinician who uses heliox, which is an inert gas. This data would unnecessarily overburden the 'Summary of findings' table. We also included the secondary outcome clinical respiratory scores within the first 24 hours after starting heliox treatment. This outcome was the most investigated outcome and was the primary endpoint of four trials. Nevertheless, we classified this outcome in our protocol as a secondary outcome as it involves an element of subjectivity.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes (GRADE 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro GDT software (GRADEpro GDT 2015). We justified all decisions to downgrade or upgrade the quality of studies using footnotes and we made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We used RevMan 2014 to perform statistical analysis of extracted data. Planned subgroup analyses included infants younger than 12 months of age still hospitalised but not receiving mechanical ventilation, and infants younger than 12 months of age recruited receiving mechanical ventilation. The main endpoint in the first subgroup was the need for mechanical ventilation, and in the second subgroup the change in oxygenation index one hour after heliox administration. We did not undertake these because of the limited number of included trials.
Sensitivity analysis
We planned sensitivity analyses, but we did not undertake these because of the limited number of included trials.
Results
Description of studies
Results of the search
The 2009 MEDLINE search retrieved 227 citations, CENTRAL 273 citations, EMBASE 209 and LILACS a total of 41 citations.
The update on 25 March 2015 retrieved from the following databases: MEDLINE (Ovid) from 1 May 2009 to March week 3, 2015 (34 search results), Embase.com from 1 June 2009 to 25 March 2015 (103 search results), CENTRAL from 2009 Issue 2 to 2015 Issue 3 (41 search results), LILACS limited to year published 2009 to 2015 (17 search results); NB no filter for RCTs used. With duplicates removed there were 145 search results from the update.
Searches in registries (WHO ICTRP and clinicaltrials.gov) found two ongoing trials:
NCT02373683 'Helium‐oxygen gas mixtures delivered by a high flow nasal cannula in bronchiolitis' – date of registration 23 February 2015 – recruiting.
NCT00116584 'Heliox‐driven racemic epinephrine nebulization in treatment of moderate to severe bronchiolitis in pediatric emergency department patients' – date of registration 29 June 2005.
Included studies
Seven trials met the criteria for study selection for this review (see Characteristics of included studies table). Six trials were performed in non‐intubated children.
Four studies were parallel‐group trials (Cambonie 2006; Chowdhury 2013; Kim 2011; Liet 2005), and two studies used a cross‐over design (Hollmann 1998; Martinón‐Torres 2008). Two studies were multi‐centre trials, one involving three hospitals in Canada and one hospital in France (Liet 2005), and the other four centres in the UK and Australia (Chowdhury 2013). The other studies were conducted in France (Cambonie 2006), the USA (Hollmann 1998; Kim 2011), and Spain (Martinón‐Torres 2008). In one trial not all infants were randomised, but it was possible to recover data from the randomised infants separately (Hollmann 1998). We only extracted these data for this review.
One other trial (unblinded, cross‐over design) included 13 mechanically ventilated sedated and paralysed infants (Kneyber 2009).
Participants
All children were recruited from paediatric intensive care units (PICU) (n = 378), except in one trial conducted in an emergency department (n = 69) (Kim 2011). All children were under two years of age; they were all under nine months in two trials (Cambonie 2006; Liet 2005), and all under three months in one trial (Cambonie 2006). Infants were all treated for acute bronchiolitis. Positive tests for RSV were required for inclusion in five trials (Cambonie 2006; Hollmann 1998; Kneyber 2009; Liet 2005; Martinón‐Torres 2008). Kim 2011 enrolled infants from 1 October through 31 March (RSV+ 58%). Chowdhury 2013 was carried out in the bronchiolitis seasons (RSV+ 80%).
Interventions
All infants were treated with inhaled heliox versus a placebo (oxygen or air).
Seven different protocols were used for inhalation therapy with heliox:
by a standard non‐rebreather reservoir mask 7 L/min for two 20‐minute study periods (Hollmann 1998);
by inflatable head hood 9 to 15 L/min continuously (until ventilation or weaning after at least 24 hours of therapy) (Liet 2005);
by oxy hood 7 L/min for one hour (Cambonie 2006);
by non‐invasive ventilation equipment (nasal continuous positive airway pressure with minimum level of 5 cm H2O) for two 30‐minute study periods (Martinón‐Torres 2008);
through intratracheal tube with mechanical ventilation for three 30‐minute study periods (Kneyber 2009);
via high‐flow nasal cannula 6 L/min for four hours (Kim 2011);
by a tight‐fitting 3‐valve, non‐rebreathing facemask 10 L/min (nasal cannula 3 L/min, if facemask intolerant) or CPAP 6 cmH2O if severe respiratory distress (until recovery) (Chowdhury 2013).
Outcome measures
In four trials the primary outcome measure was the effect on respiratory distress using a clinical scoring system, the modified Wood Clinical Asthma Score (m‐WCAS) (Cambonie 2006; Hollmann 1998; Kim 2011; Martinón‐Torres 2008). The m‐WCAS (initially described by Wood, Wood 1972) grades cyanosis, inspiratory breath sounds, accessory muscles used, expiratory wheezing and cerebral function from 0 to 2, with increased severity receiving a higher score. The respiratory distress assessment instrument (RDAI) was first described by Lowell (Lowell 1987), and grades wheezing and retractions with the maximum total points for wheezing being eight and for retractions nine. In Kim 2011, m‐WCAS and RDAI scores were recorded in the first four hours by video and recordable stethoscope with a standardised auscultatory examination covering eight positions for each time point and scores were assessed by the same masked investigator to eliminate interobserver variability.
In Liet 2005, the primary outcome measure was the rate of initiation of positive pressure, with a clinical score using the respiratory distress assessment instrument (RDAI).
In Chowdhury 2013, the primary endpoint was the length of treatment required to alleviate hypoxia and respiratory distress.
The cross‐over study that included already intubated children considered ventilatory mechanics parameters with data determined by electrical impedance tomography (Kneyber 2009).
Excluded studies
We excluded 22 trials. Many trials were case reports with no control groups (Duncan 1979; Gross 2000; Gupta 2004; Iglesias‐Fernandez 2007; Kneyber 2006; Martinon‐Torres 2005; Martinon‐Torres 2006; Mayordomo‐Colunga 2010; Montaguti 2011; Tobias 1999; Ulhoa 2000; Williams 2004). We also excluded paediatric publications reporting the effects of heliox therapy for conditions other than bronchiolitis (Abd‐Allah 2003; Colnaghi 2012; Dieperink 2007; Elleau 1993; Tobias 1997; Winters 2000). One study was not blinded and had an inadequate method of randomisation (alternate inclusion) (Martinon‐Torres 2002).
Risk of bias in included studies
Risk of bias in the included studies is summarised in Figure 1.
1.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Five trials described adequate allocation concealment (Cambonie 2006; Chowdhury 2013; Hollmann 1998; Kim 2011; Liet 2005). The method of randomisation was explicitly described and considered to be adequate in four trials (Chowdhury 2013; Hollmann 1998; Kim 2011; Liet 2005). In Cambonie 2006, the authors were requested to provide details regarding the method of randomisation and upon review we judged this study adequate. In Martinón‐Torres 2008, after assessing information provided by the trial authors, we judged the method of randomisation and allocation concealment adequate only for the first infant (coin tossing only for the first infant, then alternate inclusion in the treatment group or control group for the first cross‐over study period). We classified this trial as a quasi‐RCT and kept it in the review because of its cross‐over design and other research methodologies.
Blinding
We considered the methods for double‐blinding appropriate in five trials where only the respiratory therapists were unmasked to the type of gas because they controlled and monitored the mixing of gases at the blender (Cambonie 2006; Chowdhury 2013; Hollmann 1998; Kim 2011; Liet 2005). The labelling of intervention gases as cylinder A and B potentially exposed two trials to assessment bias (Cambonie 2006; Chowdhury 2013).
The two other trials were not blinded (Kneyber 2009; Martinón‐Torres 2008). This point is of concern as the main significant retrieved effect of heliox therapy is a respiratory score based on a clinical observation. Nevertheless, we kept these trials because of their cross‐over design and because of the sparse data available for this meta‐analysis.
Incomplete outcome data
We extracted all available data from the seven studies.
Selective reporting
All outcomes were available in the included studies. We cannot be certain that selective reporting did not occur, but have no reason to suspect this potential bias.
Other potential sources of bias
Diagnosis of bronchiolitis. Three trials included infants quite old (up to one year in Chowdhury 2013, and up to two years in Hollmann 1998 and Martinón‐Torres 2008) whether or not it was their first episode of acute bronchiolitis. Using this criterion, the inclusion of asthmatic infants was possible in these trials.
Aetiology of the respiratory distress. Two trials included, respectively, about 20% and 42% infants without established RSV contamination (Chowdhury 2013; Kim 2011).
Age of children. Results from infants less than one month old (often born prematurely) and results from older children were collected together.
The flow rate of heliox delivery should always be higher than the inspiratory peak flow rate of the infant to avoid dilution by room air.
The inspiratory peak flow rate can be very high for a brief time and so the inclusion of a large reservoir device is suggested for any investigation using a medical nebuliser driven by heliox, to prevent the dilution of the gas by the room air (Corcoran 2004). To obtain the full potential benefits of heliox in infants breathing spontaneously, heliox should be administered via a system that achieves a gas tight seal, without leakage between the dispenser and the ambient air (Standley 2008). Thus, different protocols used for delivering heliox (see above) could represent a potential bias. Liet 2005 used an inflatable head hood with a gas flow rate of 9 to 15 L/min. As there was no collapse of the non‐rigid plastic head hood, one can assume there was no room air dilution. This argument is not valid for Cambonie 2006, as a classic rigid head hood with a gas flow rate of 7 L/min was used. To deliver heliox, a standard high‐concentration reservoir mask was used in Hollmann 1998. However, significant dilution by room air has recently been well documented during heliox administration by this kind of reservoir mask (Roche‐Campo 2011; Standley 2008). Although Hollmann 1998 demonstrated a reduction in the clinical respiratory distress score following heliox inhalation, the benefit was lower compared to the other trials that used the same score to assess respiratory distress (Cambonie 2006; Kim 2011; Martinón‐Torres 2008). Martinón‐Torres 2008 used a device routinely used in neonatal units for non‐invasive ventilation, with specific nasal equipment, called nasal continuous positive airway pressure (nCPAP) with a minimum level of 5 cmH2O. A room air dilution of helium could theoretically occur if the infant breathes by the mouth. Nevertheless, this device, equipped with a heated humidifier, delivered a flow rate of 10 to 15 L/min, which limited this risk. Moreover, the humidifier included a reservoir system large enough to accommodate the increased tidal volumes that would be expected with heliox inhalation (Corcoran 2004). In Kim 2011, participants were randomised to the helium‐oxygen or oxygen group and received nebulised racaemic epinephrine via a face mask. Then, humidified helium‐oxygen or oxygen was delivered by high‐flow nasal cannula (HFNC) at 6 L/min for four hours. In addition to providing heated and humidified gases, the HFNC system provides a degree of continuous positive airway pressure better tolerated by the patient (McKiernan 2010; Schibler 2011). The exact level generated depends on the flow delivered relative to the size of the patient and on the leak around the nasal cannula. In Chowdhury 2013, children received heliox via by a tight‐fitting three‐valve, non‐rebreathing facemask 10 L/min, or by nasal cannula 3 L/min (for facemask intolerant children), or by nasal CPAP 6 cm H2O in case of severe respiratory distress.
5. Another concern is the dispatching of the gases in the hood. The lower density of helium relative to oxygen and nitrogen means that helium tends to concentrate at the top of the hood, which potentially increases the density of the mixture inhaled by the infant (Stillwell 1989). However, this concern can be alleviated. Firstly, infants subjected to the hood were in a supine position to allow the respiratory distress evaluation, thus placing the mouth and nose at least in a median position relative to the top and bottom of the hood. Secondly, some clinical studies, like Weber 2001, show the efficacy of helium‐oxygen mixtures administered by hood in patients with upper airways obstruction, suggesting that the activity of a dyspneic infant under the hood probably modifies the distribution of gases in the device. Thirdly, in Cambonie 2006 oxymetry was continuously monitored at the top of the hood and did not show any difference between the prescribed and the measured concentrations of oxygen in the hood (and the density of oxygen and nitrogen are very similar). The continuous flow of the delivered gas with constant renewal, and the spontaneous ventilation of the infants maintain a good mixing of heliox with other gas.
6. Accuracy of the heliox administration in mechanical ventilation. In Kneyber 2009, heliox was delivered by a ventilator with a time cycled, pressure‐limited ventilation mode. The accuracy of volumes delivered and monitored by the ventilator used for heliox administration has been previously studied.
Effects of interventions
See: Table 1
Seven randomised controlled trials involving 447 infants with acute bronchiolitis compared heliox inhalation to air or oxygen inhalation. The following clinical outcomes are representative of subgroups of these 447 participants.
Primary outcomes
1. In‐hospital mortality
Three trials considered in‐hospital mortality (Cambonie 2006; Kim 2011; Liet 2005). The authors of Chowdhury 2013 provided this outcome after a request. We reported all results in Table 2. One child died in the experimental group due to irreversible respiratory failure 34 days after stopping helium treatment (Liet 2005). The comparative effect of intervention on late mortality can not be taken in account in the cross‐over studies. No effects of helium can be related with mortality: risk ratio (RR) 3.47, 95% confidence interval (CI) 0.15 to 80.35, P value = 0.44, n = 408 (Analysis 1.1).
1. Mortality.
|
Experimental events |
Experimental total |
Control events |
Control total |
|
| Cambonie 2006 | 0 | 10 | 0 | 9 |
| Chowdhury 2013 | 0 | 140 | 0 | 141 |
| Kim 2011 | 0 | 34 | 0 | 35 |
| Liet 2005 | 1 | 18 | 0 | 21 |
1.1. Analysis.
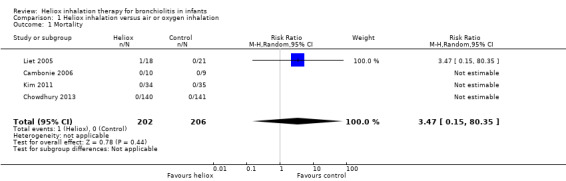
Comparison 1 Heliox inhalation versus air or oxygen inhalation, Outcome 1 Mortality.
2. Need for mechanical ventilation (invasive or not)
Four trials used the need for mechanical ventilation, invasive or not (Cambonie 2006; Chowdhury 2013; Kim 2011; Liet 2005). We reported this outcome measure in Table 3. The pooled results failed to demonstrate a reduction in the need for mechanical ventilation with heliox: RR 0.95 (95% CI 0.61 to 1.49, P value = 0.83, n = 408) (Analysis 1.2; Figure 2).
2. Need for mechanical ventilation (invasive or not).
|
Experimental events |
Experimental total |
Control events |
Control total |
|
| Cambonie 2006 | 1 | 10 | 1 | 9 |
| Chowdhury 2013 | 24 | 140 | 27 | 141 |
| Kim 2011 | 1 | 34 | 0 | 35 |
| Liet 2005 | 4 | 18 | 4 | 21 |
1.2. Analysis.
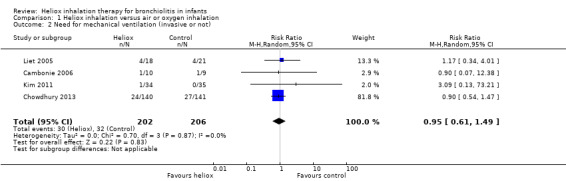
Comparison 1 Heliox inhalation versus air or oxygen inhalation, Outcome 2 Need for mechanical ventilation (invasive or not).
2.

Forest plot of comparison: 1 Heliox inhalation versus air or oxygen inhalation, outcome: 1.2 Need for mechanical ventilation (invasive or not).
3. Rate of endotracheal intubation
Three trials used the rate of intubation as an outcome measure (Cambonie 2006; Kim 2011; Liet 2005). Chowdhury 2013 provided this data after a request. We reported this outcome measure in Table 4. The pooled results failed to demonstrate a reduction in the rate of intubation with heliox use: RR 2.73 (95% CI 0.96 to 7.75, P value = 0.06, n = 408) (Analysis 1.3; Figure 3). The risk of being intubated is even greater in the experimental (heliox) group. When considering the causes of intubation in Chowdhury 2013, in which this ratio is the highest (6/140 infants intubated in the heliox group versus 0/141 in the control group), the cause of intubation does not seem to be related to treatment with heliox as they were technical problems (3/6) and undetected cardiopathy (1/6). The two other infants were intubated for apnoea. No significant trend was found when looking only at intubation for apnoea .
3. Rate of endotracheal intubation.
|
Experimental events |
Experimental total |
Control events |
Control total |
|
| Cambonie 2006 | 1 | 10 | 1 | 9 |
| Chowdhury 2013 | 6 | 140 | 0 | 141 |
| Kim 2011 | 0 | 34 | 0 | 35 |
| Liet 2005 | 4 | 18 | 3 | 21 |
1.3. Analysis.
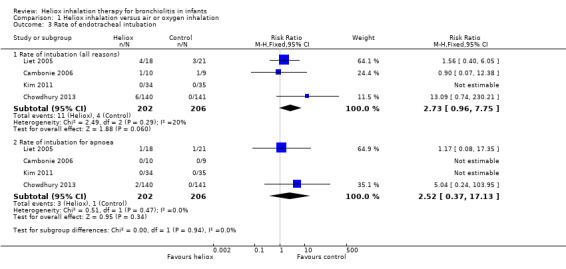
Comparison 1 Heliox inhalation versus air or oxygen inhalation, Outcome 3 Rate of endotracheal intubation.
3.
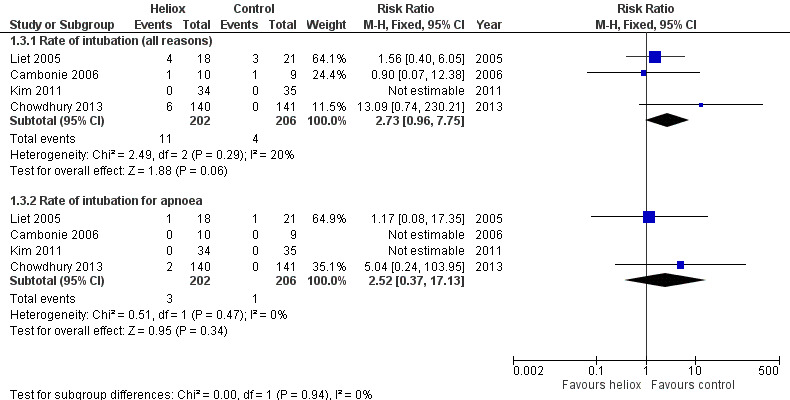
Forest plot of comparison: 1 Heliox inhalation versus air or oxygen inhalation, outcome: 1.3 Rate of endotracheal intubation.
4. Rate of emergency department discharge
Kim 2011 was conducted in the emergency department and considered the emergency department discharge (Table 5). No significant increase in the rate of emergency department discharge was observed between the two groups: RR 0.51, 95% CI 0.17 to 1.55, P value = 0.24, n = 69 (Analysis 1.4; Figure 4).
4. Rate of emergency department discharge.
|
Experimental events |
Experimental total |
Control events |
Control total |
|
| Kim 2011 | 4 | 34 | 8 | 35 |
1.4. Analysis.
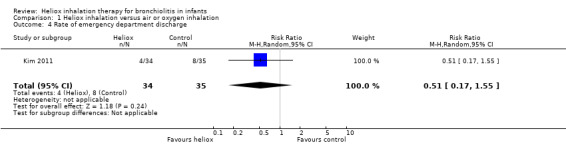
Comparison 1 Heliox inhalation versus air or oxygen inhalation, Outcome 4 Rate of emergency department discharge.
4.

Forest plot of comparison: 1 Heliox inhalation versus air or oxygen inhalation, outcome: 1.4 Rate of emergency department discharge.
5. Length of treatment for respiratory distress
Chowdhury 2013 and Liet 2005 used the length of treatment for respiratory distress as an outcome measure (Table 6). No significant reduction in the length of treatment was observed when heliox therapy was used: mean difference (MD) ‐0.19 days, 95% CI ‐0.56 to 0.19, P value = 0.33, n = 320 (Analysis 1.5; Figure 5). Chowdhury 2013 addressed a subgroup of infants with a respiratory distress severe enough to warrant nasal continuous positive airway pressure (nCPAP) right from the start. In this subgroup, a significant reduction was observed with heliox: MD ‐0.76 days, 95% CI ‐1.45 to ‐0.08; P value = 0.03, n = 21 (Analysis 1.5.2; Figure 5). This subgroup analysis was pre‐specified but not taken in account for the sample size calculation.
5. Length of treatment for respiratory distress.
|
Experimental n |
Experimental days (SD) |
Control n |
Control days (SD) |
|
| Chowdhury 2013 | 140 | 2.268 (1.66) | 141 | 2.487 (1.86) |
| Liet 2005 | 18 | 1.25 (0.87) | 21 | 1.29 (1.87) |
SD: standard deviation
1.5. Analysis.
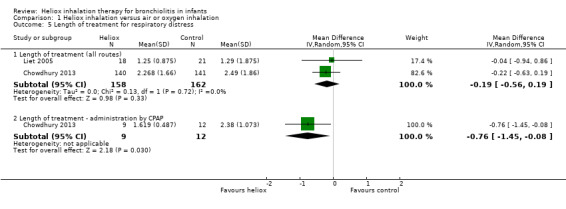
Comparison 1 Heliox inhalation versus air or oxygen inhalation, Outcome 5 Length of treatment for respiratory distress.
5.
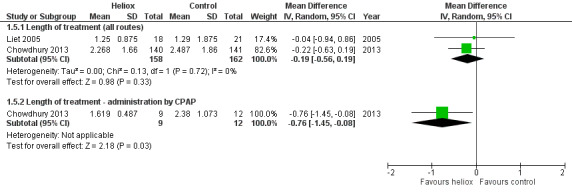
Forest plot of comparison: 1 Heliox inhalation versus air or oxygen inhalation, outcome: 1.5 Length of treatment for respiratory distress.
6. Length of paediatric intensive care unit (PICU) stay
Cambonie 2006 and Liet 2005 used PICU length of stay as an outcome measure (Table 7). These trials failed to demonstrate a reduction in the length of PICU stay with heliox use: MD 0.09 days, 95% CI ‐1.54 to 1.71, P value = 0.92, n = 58) (Analysis 1.6). In Liet 2005, a child included in the heliox group had contracted both respiratory syncytial virus (RSV) and adenoviral respiratory tract infection. He died 38 days after the beginning of mechanical ventilation due to irreversible respiratory failure. The weight of the results of this trial was 10.6% for this outcome and it does not result in a significant difference.
6. Length of PICU stay.
|
Experimental n |
Experimental days (SD) |
Control n |
Control days (SD) |
|
| Cambonie 2006 | 9 | 4.9 (0.9) | 10 | 5.1 (0.8) |
| Liet 2005 | 18 | 5.9 (13) | 21 | 3.4 (2.7) |
SD: standard deviation
1.6. Analysis.
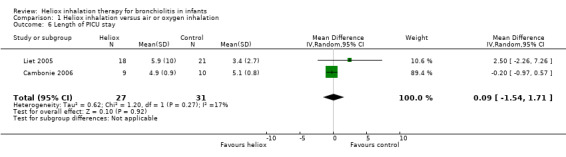
Comparison 1 Heliox inhalation versus air or oxygen inhalation, Outcome 6 Length of PICU stay.
7. Adverse effects
No adverse effects were reported relating to heliox inhalation in any of the included or excluded trials.
In Liet 2005, one child included in the heliox group contracted both RSV and adenoviral respiratory tract infection, and died 38 days after the beginning of mechanical ventilation due to irreversible respiratory failure. Helium therapy was discontinued four days after randomisation.
Secondary outcomes
1. Gas exchange (effects on oxygenation and CO2 elimination) within the first 24 hours after starting heliox treatment
Change in O2 needs after one hour of heliox treatment
Cambonie 2006 used change in O2 needs after one hour of heliox treatment as an outcome measure (Table 8). This trial failed to demonstrate a reduction in change in O2 needs after one hour of heliox treatment: MD 2.06%, 95% CI ‐2.86 to 6.98, P value = 0.41, n = 19 (Analysis 1.7).
7. Change in O2 needs after 1 hour of heliox treatment.
|
Experimental n |
Experimental mean (SD) |
Control n |
Control mean (SD) |
|
| Cambonie 2006 | 10 | +0.5 (3.98) | 9 | ‐1.56 (6.52) |
SD: standard deviation
1.7. Analysis.
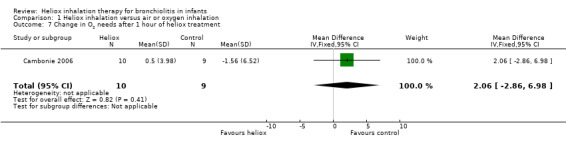
Comparison 1 Heliox inhalation versus air or oxygen inhalation, Outcome 7 Change in O2 needs after 1 hour of heliox treatment.
Change in SpO2 within the first hour after starting heliox treatment
Martinón‐Torres 2008 used change in oxygen saturation on pulse oximetry (SpO2) within the first hour after starting heliox treatment as an outcome measure (Table 9). This trial failed to demonstrate a reduction in change in SpO2 in the first hour after starting heliox treatment: MD 1.10%, 95% CI ‐1.90 to 4.10, P value = 0.47, n = 24 (Analysis 1.8).
8. Change in SpO2 in the first hour after starting heliox treatment.
|
Experimental n |
Experimental mean (SD) |
Control n |
Control mean (SD) |
|
| Martinón‐Torres 2008 | 12 | 8.0 (3.8) | 12 | 6.9 (3.7) |
SD: standard deviation
1.8. Analysis.
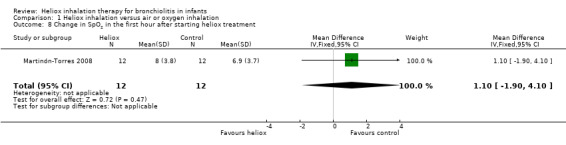
Comparison 1 Heliox inhalation versus air or oxygen inhalation, Outcome 8 Change in SpO2 in the first hour after starting heliox treatment.
Change in CO2 within the first hour after starting heliox treatment
Cambonie 2006 and Martinón‐Torres 2008 used change in CO2 within the first hour after starting treatment as an outcome measure (Table 10). These studies failed to demonstrate a reduction in change in CO2 within the first hour after starting heliox treatment: MD ‐2.09 mmHg, 95% CI ‐6.20 to 2.02, P value = 0.32, n = 43 (Analysis 1.9).
9. Change in CO2 within the first hour after starting heliox treatment.
|
Experimental n |
Experimental mmHg (SD) |
Control n |
Control mmHg (SD) |
|
| Cambonie 2006 | 10 | ‐2.2 (0.78) | 9 | ‐2.1 (0.93) |
| Martinón‐Torres 2008 | 12 | ‐9.7 (3.3) | 12 | ‐5.4 (1.6) |
SD: standard deviation
1.9. Analysis.
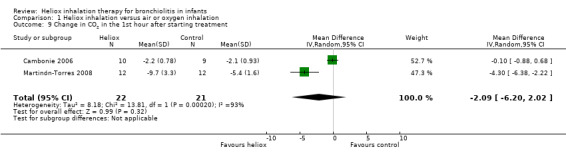
Comparison 1 Heliox inhalation versus air or oxygen inhalation, Outcome 9 Change in CO2 in the 1st hour after starting treatment.
2. Respiratory mechanics (effects on pulmonary compliance and resistance of airways) within the first 24 hours after starting heliox treatment
Kneyber 2009 studied the effects of heliox on pulmonary compliance and resistance of airways in 13 infants mechanically ventilated for acute bronchiolitis. Mechanical ventilation with heliox had an overall significant effect on respiratory system resistance. Respiratory system resistance decreased from 69.1 ± 6.9 cmH2O/L/sec to 50.2 ± 6.0 cmH2O/L/sec (P value = 0.02) after 30 minutes of ventilation with heliox. Peak expiratory flow rate (PEFR) was not significantly improved with heliox compared with nitrox (P value = 0.52). Static compliance was 1.9 ± 0.4 L/ cmH2O at T = 0 and not significantly different throughout the study (P value = 0.21).
3. Clinical respiratory scores within the first 24 hours after starting heliox treatment
Cambonie 2006, Hollmann 1998, Kim 2011, and Martinón‐Torres 2008 used as a clinical respiratory score the modified Wood Clinical Asthma Score (m‐WCAS). All data are reported in Table 11. All four trials, with a total of 138 infants, demonstrated a benefit of heliox inhalation in reducing clinical respiratory scores. The pooled results demonstrate that infants treated with heliox inhalation had a statistically significant reduction in clinical respiratory scores: MD ‐1.04, 95% CI ‐1.60 to ‐0.48, P value = 0.0003, n = 138 (Analysis 1.10; Figure 6). If only trials where room air dilution of heliox is less probable are included, the pooled data show a statistically significant reduction in m‐WCAS: MD ‐1.29, 95% CI ‐1.92 to ‐0.66, P value < 0.0001, n = 112 (Cambonie 2006; Kim 2011; Martinón‐Torres 2008).
10. Change in clinical respiratory scores (m‐WCAS) within the first hour after starting heliox treatment.
|
Experimental n |
Experimental mean (SD) |
Control n |
Control mean (SD) |
|
| Cambonie 2006 | 10 | ‐2.35 (0.81) | 9 | ‐0.06 (0.98) |
| Hollmann 1998 | 13 | ‐0.46 (0.19) | 13 | ‐0.04 (0.19) |
| Kim 2011 | 34 | ‐0.77 (0.77) | 35 | +0.1 (0.79) |
| Martinón‐Torres 2008 | 12 | ‐2.12 (0.6) | 12 | ‐1.08 (0.4) |
SD: standard deviation
1.10. Analysis.
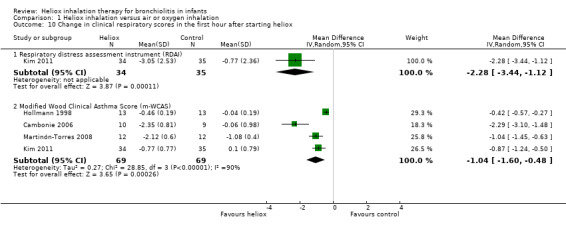
Comparison 1 Heliox inhalation versus air or oxygen inhalation, Outcome 10 Change in clinical respiratory scores in the first hour after starting heliox.
6.
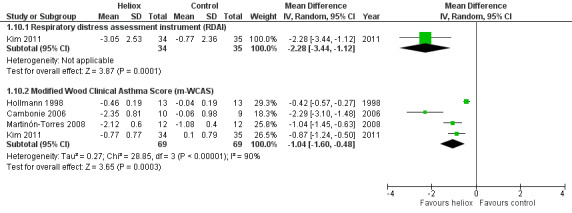
Forest plot of comparison: 1 Heliox inhalation versus air or oxygen inhalation, outcome: 1.10 Change in clinical respiratory scores in the first hour after starting heliox.
In Chowdhury 2013, heliox reduced the clinical score for respiratory distress across all time points and was statistically significant at eight hours onwards: MD ‐0.13, 95% CI ‐0.20 to ‐0.06, P value < 0.001.
The respiratory distress assessment instrument (RDAI) within the first hour after starting heliox treatment is available only in Kim 2011, with a statistically significant reduction: MD ‐2.28, 95% CI ‐3.44 to ‐1.12, P value = 0.0001, n = 69 (Analysis 1.10; Figure 6).
4. Total duration of hospitalisation (including duration in PICU)
No available data.
Discussion
Summary of main results
In this review we retrieved seven trials, which objectively assessed the effect of the addition of heliox to standard medical care on the course of acute bronchiolitis in 447 infants.
We examined six primary outcomes. For five of them (in‐hospital mortality, need for mechanical ventilation (invasive or not), rate of endotracheal intubation, rate of emergency department discharge and length of paediatric intensive care stay), the pooled results failed to demonstrate a reduction in these outcomes. Length of treatment for respiratory distress was assessed in two trials (n = 320) and was not reduced by heliox therapy. However, in a subgroup of infants who were started on nasal continuous positive airway pressure (nCPAP) right from the start because of severe respiratory distress (n = 21; Chowdhury 2013), a significant reduction of length of treatment was observed when adding a heliox therapy treatment (‐0.76 days lower; P value = 0.03). Secondary outcomes incorporated gas exchange effects and variation in clinical respiratory scores. Five trials used changes in the modified Wood Clinical Asthma Scores as outcomes (Cambonie 2006; Chowdhury 2013; Hollmann 1998; Kim 2011; Martinón‐Torres 2008). This score, initially described by Wood 1972, grades cyanosis, inspiratory breath sounds, accessory muscles used, expiratory wheezing and cerebral function from zero to two, with increased severity receiving a higher score. All trials demonstrated a benefit of heliox inhalation in reducing clinical respiratory scores. The pooled results show that after one hour, infants treated with heliox inhalation had a statistically significant reduction in clinical respiratory scores: mean difference (MD) ‐1.04, 95% confidence interval (CI) ‐1.60 to ‐0.48, P value = 0.0003, n = 138. This benefit of heliox inhalation on clinical scores in the first hour after starting heliox treatment was not accompanied by an improvement in gas exchange: no reduction in CO2, needs in oxygen, or SpO2. The method of delivering heliox might alter the validity and the relevance of these results. Seven different protocols for delivering heliox were used. When removing the trial that used a standard non‐rebreather reservoir mask, a method of delivery where there is a proven room air dilution (Roche‐Campo 2011; Standley 2008), the benefit of heliox administration is observed in the clinical score only during the first hour after starting heliox therapy. Nevertheless, all these trials provide consistent results. Chowdhury 2013 showed that heliox is effective if delivered via a tight‐fitting non‐rebreathing face mask or nCPAP, but not via a nasal cannula at 3 L/minute because of insufficient flow rate.
Kneyber 2009 assessed the effect of the addition of heliox in 13 mechanically ventilated, sedated and paralysed infants. Mechanical ventilation significantly decreased respiratory system resistance. This was not accompanied by an improved CO2 elimination, or a reduced air‐trapping.
The available data suggest that heliox inhalation could be useful in addition to standard medical care in the management of acute bronchiolitis. Of note, this benefit is observed on clinical score only during the first hour after starting heliox therapy, and is not confirmed by gas exchange effects. No benefits were observed in terms of need for mechanical ventilation, rate of intubation or in the length of stay in the paediatric intensive care unit (PICU). The significant reduction of length of treatment observed when adding a heliox therapy treatment in a subgroup of infants directly treated by nCPAP because of severe respiratory distress is promising. It suggests that heliox therapy could be useful especially for the most severely affected children.
Overall completeness and applicability of evidence
Martinón‐Torres 2008 was considered a quasi‐RCT (where there is alternate allocation to treatment and control groups) but we kept it in the review because of its cross‐over design with brief periods. Data from this trial affect the results of the effects of heliox inhalation on clinical respiratory scores in the first hour after starting heliox treatment but the data provided were similar to those of the three other integrated trials with a comparable weight (25.8%).
Quality of the evidence
The only outcome for which a benefit of heliox therapy in bronchiolitis was demonstrated (change in clinical respiratory scores within the first hour after starting heliox treatment) was found to have statistical heterogeneity (Figure 6). This statistical heterogeneity remained even after removing the study that used a standard high‐concentration reservoir mask.
Several factors may explain this heterogeneity, including the limited number of participants in each trial and wide differences in baseline severity of disease between studies, with m‐WCAS in infants treated with heliox ranging from 3 in Chowdhury 2013 to 6.7 in Martinón‐Torres 2008. Different versions of m‐WCAS were used: Hollmann 1998 included a 'mild' category of 0.5 points for accessory muscle use and expiratory wheezing to better define the clinical response to heliox inhalation. This modification provided four scores for each of these two variables, i.e. 0 for none, 0.5 for mild, 1 for moderate and 2 for maximum. Cambonie 2006 used a visual analogue scale to standardise the scoring of these two score variables. Martinón‐Torres 2008 slightly modified this scoring and also adopted a 'mild' category of 0.5 points for cyanosis, inspiratory breath sounds and cerebral function. Kim 2011 standardised auscultatory examination on four positions on the chest and used a recordable stethoscope and a video camcorder to assess later the score on videotape with a masked scorer. Chowdhury 2013 defined a maximum value for m‐WCAS of 11, instead of 10 in the others studies, by the introduction of a supplementary variable to grade cyanosis. Finally, only two studies reported inter‐observer agreement regarding the use of this score (Cambonie 2006; Kim 2011).
Potential biases in the review process
The funnel plots performed are symmetric on the median line (Figure 7; Figure 8). Nevertheless, only few studies were included in this review.
7.
Funnel plot of comparison: 1 Heliox inhalation versus air or oxygen inhalation, outcome: 1.2 Need for mechanical ventilation (invasive or not).
8.

Funnel plot of comparison: 1 Heliox inhalation versus air or oxygen inhalation, outcome: 1.10 Change in clinical respiratory scores in the first hour after starting heliox.
Agreements and disagreements with other studies or reviews
We found no disagreements between the few included studies.
Authors' conclusions
Implications for practice.
Heliox inhalation produces a significant reduction in the clinical respiratory score in unintubated infants with respiratory distress due to acute bronchiolitis. Nevertheless, this therapy does not reduce the need for mechanical ventilation, intubation or the length of stay in the paediatric intensive care unit (PICU). Given its good safety profile, heliox therapy could be used in addition to standard medical care to treat infants who are hospitalised in PICUs for acute bronchiolitis.
Implications for research.
Large randomised controlled trials, preferably multi‐centred, are required to evaluate the effectiveness of heliox inhalation in infants with acute bronchiolitis. Only infants less than 12 months old with severe respiratory distress should be included. Inclusion criteria must also include a clinical severity score that reflects severe respiratory distress (for example, a modified Wood Clinical Asthma Score (m‐WCAS) of more than 3/10) to avoid the inclusion of children with mild bronchiolitis who may not benefit from heliox inhalation.
An additional consideration would be the potential benefit of heliox therapy in association with nasal continuous positive airway pressure (nCPAP) or high‐flow nasal cannula (HFNC), to reduce the length of treatment for respiratory distress. In a small subgroup of infants with respiratory distress severe enough to warrant CPAP from the start, Chowdhury 2013 suggested a relevant reduction in length of treatment with heliox. To be adequately blinded such studies would have to compare the use of nCPAP with heliox and nCPAP with air/oxygen, bearing in mind that the different sound made by a CPAP device using helium may alert the investigators to the study intervention. HFNC could be a good alternative.
What's new
| Date | Event | Description |
|---|---|---|
| 25 March 2015 | New citation required but conclusions have not changed | The conclusions have not changed. Recommendations for further studies are appropriate. |
| 25 March 2015 | New search has been performed | We re‐wrote the meta‐analysis. We updated the searches in March 2015 and included two new studies (Chowdhury 2013; Kim 2011) and excluded three new studies (Colnaghi 2012; Mayordomo‐Colunga 2010; Montaguti 2011). |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 4, 2010
| Date | Event | Description |
|---|---|---|
| 17 October 2013 | Amended | Additional tables moved to the correct section of the review. |
| 14 May 2008 | Amended | Converted to new review format. |
Acknowledgements
The review authors thank the following referees for reviewing the 2015 meta‐analysis: Jennifer LaRosa, Robert Ware, Federico Martinon‐Torres and Allen Cheng. We also thank the referees who commented on the draft review: Anne Lyddiatt, Sree Nair and Allen Cheng. They raised important points and we think that they helped us to improve the review and to clarify its content.
Appendices
Appendix 1. Previous search
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2009, Issue 2), which includes the Cochrane Acute Respiratory Infections (ARI) Group's Specialised Register, MEDLINE (1966 to June Week 3 2009), EMBASE (1974 to June 2009), LILACS (1982 to May 2009) and the NIH website (ClinicalTrials.gov) (May 2009).
We used the following search terms to search MEDLINE and CENTRAL. The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying RCTs in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). The search terms were adapted to search EMBASE and LILACS.
MEDLINE (Ovid)
1 Helium/ 2 helium.tw,nm. 3 heliox.tw,nm. 4 heo2.tw. 5 he‐o2.tw. 6 or/1‐5
Embase.com
1. 'helium'/exp 2. helium:ti,ab OR heliox:ti,ab OR heo2:ti,ab OR 'he‐o2':ti,ab 3. #1 OR #2 4. random*:ti,ab OR placebo:ti,ab,de OR 'double blind':ti,ab 5. #3 AND #4
LILACS
The LILACS search (heliox OR helium) retrieved a total of 41 citations.
Appendix 2. MEDLINE (Ovid) search strategy
MEDLINE (Ovid)
1 Helium/ 2 helium.tw,nm. 3 heliox.tw,nm. 4 heo2.tw. 5 he‐o2.tw. 6 or/1‐5
Appendix 3. Embase search strategy
#10. #6 AND #9 499 25 March 2015 #9. #7 OR #8 878,114 25 March 2015 #8. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross‐over':ab,ti OR 'cross over':ab,ti OR volunteer*:ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR ((singl* OR doubl*) NEAR/1 blind*):ab,ti AND [embase]/lim 838,011 25 March 2015 #7. 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp AND [embase]/lim 245,411 25 March 2015 #6. #1 OR #2 OR #3 OR #4 OR #5 6,546 25 March 2015 #5. 'he‐o2':ab,ti OR heo2:ab,ti AND [embase]/lim 1 25 March 2015 #4. heliox:ab,ti AND [embase]/lim 410 25 March 2015 #3. helium:ab,ti AND [embase]/lim 4,794 25 March 2015 #2. 'helium'/de AND [embase]/lim 3,672 25 March 2015 #1. 'helium oxygen breathing'/exp AND [embase]/lim 219 25 March 2015
Appendix 4. LILACS search strategy
"HELIUM" [Subject descriptor] or helium OR heliox OR helium‐oxygen OR heo2 OR he‐o2 [Words]
Data and analyses
Comparison 1. Heliox inhalation versus air or oxygen inhalation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 4 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 3.47 [0.15, 80.35] |
| 2 Need for mechanical ventilation (invasive or not) | 4 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.61, 1.49] |
| 3 Rate of endotracheal intubation | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Rate of intubation (all reasons) | 4 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.96, 7.75] |
| 3.2 Rate of intubation for apnoea | 4 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [0.37, 17.13] |
| 4 Rate of emergency department discharge | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.17, 1.55] |
| 5 Length of treatment for respiratory distress | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Length of treatment (all routes) | 2 | 320 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.56, 0.19] |
| 5.2 Length of treatment ‐ administration by CPAP | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.45, ‐0.08] |
| 6 Length of PICU stay | 2 | 58 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐1.54, 1.71] |
| 7 Change in O2 needs after 1 hour of heliox treatment | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 2.06 [‐2.86, 6.98] |
| 8 Change in SpO2 in the first hour after starting heliox treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐1.90, 4.10] |
| 9 Change in CO2 in the 1st hour after starting treatment | 2 | 43 | Mean Difference (IV, Random, 95% CI) | ‐2.09 [‐6.20, 2.02] |
| 10 Change in clinical respiratory scores in the first hour after starting heliox | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Respiratory distress assessment instrument (RDAI) | 1 | 69 | Mean Difference (IV, Random, 95% CI) | ‐2.28 [‐3.44, ‐1.12] |
| 10.2 Modified Wood Clinical Asthma Score (m‐WCAS) | 4 | 138 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.60, ‐0.48] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cambonie 2006.
| Methods | Prospective, randomised, double‐blind study | |
| Participants | 20 infants (all < 3 months old) admitted to the PICU with moderate‐to‐severe RSV bronchiolitis Criteria for inclusion: bronchiolitis RSV+, m‐WCAS > 5, PaCO2 > 42 mmHg Group characteristics after randomisation, mean ± SD: Age, d 29 ± 5 versus 34 ± 9 Weight, kg 3.5 ± 0.46 versus 3.83 ± 0.24 Clinical score, (m‐WCAS, maximum score of 10): 5.4 ± 0.2 versus 5.6 ± 0.2 Respiratory rate, breaths/min 59.4 ± 6.4 versus 64.9 ± 5.7 FiO2, % 31.4 ± 4.0 versus 34.0 ± 2.6 PCO2, mmHg 56.5 ± 4.9 versus 53.6 ± 2.3 | |
| Interventions | Inhalation of either heliox OR air‐oxygen, for 1 hour under a 7 L/min oxy hood | |
| Outcomes | Primary goal: to assess the effect on respiratory distress evaluated using the modified Wood Clinical Asthma Score (m‐WCAS) at H1 Secondary endpoints: need for mechanical ventilation, rate of intubation, length of mechanical ventilation, length of stay in PICU, mortality, wheezing score evaluated at H1 and H24, pCO2 at H1, respiratory rate at H1 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random listing |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | 1 investigator was not blinded: the physiologist The labelling of intervention gases as cylinder A and B potentially exposed the study to assessment bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study and there were no losses to follow‐up, no treatment withdrawals 31 infants with the diagnosis of respiratory distress attributable to RSV bronchiolitis were admitted to the PICU during the study period. Among them, 11 were excluded for clinical scores below 5. 20 infants were thus randomised into the 2 groups, with 10 in each group. One infant randomised into the control group was excluded for an oxygen haemoglobin saturation < 90% that persisted under the maximum 40% Fio2 authorised by the protocol |
| Selective reporting (reporting bias) | Low risk | All results for outcomes were reported |
| Other bias | Low risk | |
Chowdhury 2013.
| Methods | Prospective, double‐blind, multi‐centre, randomised controlled trial | |
| Participants | 281 infants (< 1 year old) with clinical bronchiolitis requiring admission for hypoxia or respiratory distress. The study was carried out in the bronchiolitis seasons Criteria for inclusion: diagnosis of bronchiolitis and need of hospitalisation for respiratory distress or hypoxia (SpO2 < 93% in room air) Baseline characteristics of subjects at presentation, median (IQRs): Age, wk 10.90 (5.85 to 25.50) versus 17.70 (6.80 to 29.40) Weight, kg 5.65 (4.34 to 7.70) versus 5.70 (4.40 to 7.70) RSV+, % 79.3 versus 82.3 Modified Woods Clinical Asthma Score (m‐WCAS, maximum score of 11, median interquartile): 3.0 (2.0 to 3.0) versus 3.0 (2.0 to 4.0) Respiratory rate, breaths per min 56.0 (44.0 to 62.0) versus 53.0 (47.0 to 63.5) SpO2 in room air, % 92.0% (89.0 to 95.0) versus 91.0% (89.0 to 94.0) | |
| Interventions | Patients were randomised to receive via facemask 10 L/min (nasal cannula 3L/min, if facemask intolerant) or nCPAP 6 cm H2O if severe respiratory distress: HeOx = Heliox21 (79% He + 21% O2) ± additional O2OR AirOx = Medical Air (79% N2 + 21% O2) ± additional O2, with humidification of the gases | |
| Outcomes | Primary endpoint was length of treatment (LoT) required to alleviate hypoxia and respiratory distress Secondary endpoints were proportion of participants needing nCPAP; nCPAP (LoT); and change in respiratory distress score | |
| Notes | In the clinical score used, 2 variables are redundant: pulse oxymetry and cyanosis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐stratified block randomisation |
| Allocation concealment (selection bias) | Low risk | Patients were enrolled and allocated by telephone to Gas A or B |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The labelling of intervention gases as cylinder A and B potentially exposed the study to assessment bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Consent was obtained for 319 participants who were randomised and enrolled into the study. Authors reported that analysis was performed by intention‐to‐treat, but provided results from a subgroup of 281 infants 3 participants were excluded (2 withdrawal of consent and 1 screening failure). An additional 35 participants were excluded because of protocol violation, consent withdrawal, screening failure, hospital clinician's decision or premature disruption of therapy |
| Selective reporting (reporting bias) | Low risk | All outcome results were reported |
| Other bias | Low risk | |
Hollmann 1998.
| Methods | Randomised, double‐blind, controlled, cross‐over study | |
| Participants | 13 infants (3 weeks to 23 months old) admitted to the PICU with RSV bronchiolitis RSV+, FiO2 < 50%, Criteria for inclusion: Bronchiolitis RSV+, with signs of lower respiratory tract disease Baseline characteristics, median (range): Age, mo 2.5 (3 wks to 24 mos) Clinical Asthma Score (m‐WCAS, maximum score of 10): 3.04 (1 to 7.5). | |
| Interventions | Inhalation in a random order of heliox AND air‐oxygen, by non‐rebreather standard reservoir mask for two 20‐minute study periods | |
| Outcomes | Primary endpoint: Clinical Asthma Score at 20 min Secondary endpoints: respiratory rate, heart rate and SpO2 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin tossing |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | 1 investigator was not blinded: the respiratory therapists; blinding was maintained by covering the on‐off valves to the air and helium sources with tape |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study and there were no losses to follow‐up, no treatment withdrawals 21 eligible patients were admitted during the study period. 3 patients were excluded from the study because of technical difficulties in setting up the gas delivery system (n = 1) and because of marked agitation (n = 2). 1 of the patients with agitation was intubated shortly after admission to the ICU. A total of 18 patients were studied, 13 of whom were randomised. Of the 13 randomised patients, 12 patients had Clinical Asthma Scores of < 6 and 1 patient had a Clinical Asthma Score of 7.5 but only mild signs of expiratory obstruction |
| Selective reporting (reporting bias) | Low risk | All results for outcomes were reported |
| Other bias | Low risk | |
Kim 2011.
| Methods | Prospective, randomised, single‐blind study | |
| Participants | 69 infants (2 to 12 months old) with bronchiolitis and m‐WCAS > 3 in the Emergency Department (ED) A convenience sample of infants was enrolled from 1 October through 31 March each year when bronchiolitis had a high incidence Criteria for inclusion: Bronchiolitis with Modified Woods Clinical Asthma Score (m‐WCAS) of 3 or higher Baseline characteristics, median: Age, mo 3.78 versus 5.03 RSV+, % 68 versus 49 m‐WCAS, (maximum score of 10): 3.84 versus 3.67 RDAI, (maximum score of 17): 10 |
|
| Interventions | Patients initially received nebulised albuterol treatment driven by 100% oxygen Patients were randomised to the helium‐oxygen OR oxygen group and received nebulised racaemic epinephrine via a face mask After nebulisation, humidified helium‐oxygen OR oxygen, was delivered by high‐flow nasal cannula (HFNC) started at 6 L/min with humidified gases After 60 minutes of inhalation therapy, patients with m‐WCAS of 2 or higher received a second delivery of nebulised racaemic epinephrine followed by helium‐oxygen or oxygen delivered by HFNC | |
| Outcomes | The primary outcome measure was the degree of improvement of m‐WCAS for 240 minutes (at 60‐minute intervals) or until ED discharge (if < 240 minutes) | |
| Notes | Family history of asthma: 50% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was predetermined using a random number generator and occurred in blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Assignments were kept in sealed, opaque envelopes and opened immediately after informed consent by the study investigator |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The respiratory therapists were unmasked to the type of gas because they controlled and monitored the mixing of gases at the blender |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study and there were no losses to follow‐up and no treatment withdrawals A total of 2580 patients were ineligible due to a m‐WCAS lower than 3. Eligibility criteria were met by 256 infants. Of these 256 infants, 187 met the exclusion criteria. Of these 187 excluded patients, 141 were excluded for the following criteria: vapotherm issue (44 patients), direct admission (41 patients), corticosteroid use within 72 hours (28 patients), declined to participate (15 patients) and chronic lung disease (13 patients). The remaining 46 patients met other exclusion criteria. 34 infants completed treatment with helium‐oxygen therapy and 35 with oxygen therapy |
| Selective reporting (reporting bias) | Low risk | All results for outcomes were reported |
| Other bias | Low risk | |
Kneyber 2009.
| Methods | Prospective, cross‐over study | |
| Participants | 13 infants mechanically ventilated (AVEA® ventilator, Cardinal Health), sedated and paralysed Criteria for inclusion: RSV+ bronchiolitis admitted in PICU for mechanical ventilation Baseline, range: Age, wk 4 to 23 Weight, kg 3.2 to 10 PCO2, mmHg 35 to 75 | |
| Interventions | Three 30‐minute periods: data collected at T0 and T60 (ventilation with nitrox), AND at T30 and T90 (ventilation with heliox) | |
| Outcomes | Respiratory system resistance, peak expiratory flow rate, lung resistance, static compliance, change in end‐expiratory lung volume Response to heliox (electrical impedance tomography measurements: EIT) Oxygenation index, alveolo‐arterial oxygen gradient, ventilation index, dead‐space/tidal volume ratio | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomisation |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study and there were no losses to follow‐up, no treatment withdrawals |
| Selective reporting (reporting bias) | Low risk | All results for outcomes were reported |
| Other bias | Low risk | |
Liet 2005.
| Methods | Multi‐centre, randomised, double‐blind, placebo‐controlled trial | |
| Participants | 39 infants (all < 9 months old) admitted to PICU with first episode of RSV bronchiolitis and signs of respiratory failure Criteria for inclusion: infants admitted to PICU with a first episode of RSV bronchiolitis and with criteria for respiratory failure (pulse oximetry saturation < 92% in room air or PaO2 < 40 mmHg) Baseline characteristics, mean ± SEM: Age, mo 1.0 ± 0.2 versus 1.1 ± 0.2 Weight, kg 4.2 ± 0.3 versus 4.3 ± 0.3 Respiratory rate, per min 53 ± 1 versus 59 ± 1 FiO2, % 40 ± 1 versus 39 ± 1 pCO2, mmHg 65 ±1 versus 62 ± 1 RDAI (maximum score of 17): 8 ± 0 versus 9 ± 0 | |
| Interventions | Inhalation of either heliox OR air‐oxygen, under an inflatable head hood with a gas flow of 9 to 15 L/min | |
| Outcomes | Primary outcome was initiation of positive pressure ventilation Secondary endpoints: need for invasive mechanical ventilation, rate of intubation, length of mechanical ventilation, length of stay in PICU, mortality, FiO2 at H24, pCO2 at H24, respiratory distress assessment instrument (RDAI) at H24 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random listing with stratification by centre |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | 1 investigator was not blinded: the respiratory therapists; identical tanks at the bedside of all patients and gas flow meters covered with opaque bags to hide the dials |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study and there were no losses to follow‐up, no treatment withdrawals. 157 infants were screened for inclusion and 39 met the eligibility criteria. Reasons for non‐enrollment included intubation and institution of mechanical ventilation before PICU entry (83), age > 9 months or weight > 10 kg (16), negative RSV test (10), PICU stay > 8 hours (8), uncorrected cyanotic heart disease or cardiac failure (8), unavailable equipment (6), history of a previous bronchiolitis episode (5), parental refusal of consent (4), bronchopulmonary dysplasia (4) and pneumothorax (1). 1 child in the helium group was removed from the study from the first day at the parents' request and was not included in the final analyses |
| Selective reporting (reporting bias) | Low risk | All results for outcomes were reported |
| Other bias | Low risk | |
Martinón‐Torres 2008.
| Methods | Prospective, interventional, single‐centre, cross‐over study | |
| Participants | 12 infants (< 2 years old) admitted to the PICU with RSV bronchiolitis RSV+, m‐WCAS > 5, SpO2 < 92% or tcPCO2 > 50 mmHg despite optimised supportive therapy Criteria for inclusion: RSV + bronchiolitis with Modified Woods Clinical Asthma Score > 5, arterial oxygen saturation < 92%, or transcutaneous CO2 pressure > 50 mmHg Baseline, mean (SD): Age, mo 5 (2.9) versus 4.5 (2.2) m‐WCAS, maximum score of 10, mean standard deviation: 7.6 (1.2) versus 7.7 (1.0) | |
| Interventions | Inhalation in a random order of heliox AND air‐oxygen, by non‐invasive ventilation equipment (nasal continuous positive airway pressure with minimum level of 5 cmH2O) for 2 30‐minute study periods The heliox mixture was delivered to the patient, warmed, and humidified by means of a standard device | |
| Outcomes | Main outcome measures: modified Woods Clinical Asthma Score (m‐WCAS), and pCO2 at 30 minutes Secondary outcomes: rate of intubation, length of mechanical ventilation, length of stay in PICU, mortality, m‐WCAS at H24 and H48, pCO2 at H24 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Coin tossing only for the first infant; then alternately |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | Non‐blinded study: obvious changes in ventilator noise because of the expiratory branch of the flow generator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study and there were no losses to follow‐up and no treatment withdrawals During the study period, 40 infants with acute bronchiolitis were admitted to the PICU; 12 of them (7 boys and 5 girls) fulfilled the inclusion criteria and were enrolled in the study |
| Selective reporting (reporting bias) | Low risk | All results for outcomes were reported |
| Other bias | Low risk | |
ED: emergency department FiO2: fraction of inspired oxygen H1: first hour H24: twenty‐fourth hour IQR: interquartile range m‐WCAS: modified Wood Clinical Asthma Score min: minute mo: month nCPAP: nasal continuous positive airway pressure pCO2: carbon dioxide pressure PICU: paediatric intensive care unit RDAI: respiratory distress assessment instrument RSV: respiratory syncytial virus SD: standard deviation SEM: standard error of the mean SpO2: oxygen saturation on pulse oximetry wk: week
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abd‐Allah 2003 | No infants, no bronchiolitis |
| Colnaghi 2012 | No bronchiolitis |
| Dieperink 2007 | No bronchiolitis |
| Duncan 1979 | Case report |
| Elleau 1993 | No infants, no bronchiolitis |
| Gross 2000 | Series of case reports |
| Grosz 2001 | Series of case reports |
| Gupta 2004 | Case report |
| Iglesias‐Fernandez 2007 | Series of case reports |
| Isakov 1970 | No bronchiolitis |
| Kneyber 2006 | Case report |
| Martinon‐Torres 2002 | Inadequate method of randomisation (alternate inclusion), unblinded study |
| Martinon‐Torres 2005 | Case report |
| Martinon‐Torres 2006 | Series of case reports |
| Mayordomo‐Colunga 2010 | Series of case reports |
| Montaguti 2011 | Series of case reports |
| Paret 1996 | Case report |
| Tobias 1997 | Not about bronchiolitis |
| Tobias 1999 | Case report |
| Ulhoa 2000 | Series of case reports |
| Williams 2004 | Series of case reports |
| Winters 2000 | No bronchiolitis |
Characteristics of ongoing studies [ordered by study ID]
Rotta 2015.
| Trial name or title | ClinicalTrials.gov identifier: NCT02373683 |
| Methods | Prospective pilot study |
| Participants | •Children 0 to 24 months
•Admission to the Paediatric Intensive Care Unit
•Diagnosis of bronchiolitis, with respiratory syncytial virus infection confirmed by laboratory testing •Mechanical ventilation |
| Interventions | Heliox delivered via a heated and humidified high flow nasal cannula (HFNC) system |
| Outcomes | The aim of this prospective pilot study is to determine the effect of heliox delivered via a proprietary calibrated heated and humidified high flow nasal cannula (HFNC) system (Vapotherm Precision Flow Heliox) in children ages 0 to 24 months with severe bronchiolitis |
| Starting date | February 2015 |
| Contact information | Katherine N Slain, DO |
| Notes | — |
Differences between protocol and review
Not only first episodes of acute bronchiolitis were included. Not all infants were under 12 months of age.
This review was first published in 2010 (Liet 2010). Two new trials were included in this 2015 update (Chowdhury 2013; Kim 2011). We modified the outcomes to include more results and to clarify others. Primary outcomes: (2) For purposes of clarity, the outcome 'Need for mechanical ventilation' became 'Need for mechanical ventilation (invasive or not)'. (4) The outcome 'Admission rate' was never investigated, in contrast to the 'Rate of emergency discharge', which replaced it advantageously. (5) 'Length of treatment for respiratory distress' replaced 'Duration of mechanical ventilation'. This new primary outcome is a more relevant outcome to the extent that very few children received mechanical ventilation.
Contributions of authors
Dr. Jean‐Michel Liet (JML) had primary responsibility for protocol development and wrote the draft protocol and review. Dr. Gilles Cambonie (GC) participated in the development of the protocol and was responsible for study selection, quality assessment and data collection. Dr. Vineet Gupta (VG) contacted trial authors of all studies to locate other unpublished or in progress studies, and provided input for writing the protocol and review. Dr. Thierry Ducruet (TD) read the protocol and the review, and checked the statistical aspects. The final version of the review was approved by all review authors.
Sources of support
Internal sources
-
University of Wisconsin General Clinical Research Center, USA.
Holmann 1998 was supported, in part, by grant M01 RR03186‐07 from the University of Wisconsin General Clinical Research Center
-
Fonds de la Recherche en Santé du Quebec (FRSQ), Canada.
Liet 2005 was supported in part by a grant from the Fonds de la Recherche en Santé du Quebec (FRSQ)
External sources
-
British Oxygen Company Medical, UK.
Chowdhury 2012 funding: supported by a research grant from British Oxygen Company (BOC) Medical, and equipment support from Fisher and Paykel Healthcare, EME (Electro‐Medical Equipment) Carefusion and Covidien Nellcor
-
Praxair corporation, USA.
Kim 2011 was funded in part by an unrestricted research grant from Praxair Corporation
-
Air Liquide Santé International, France.
Liet 2005 was supported in part by Air Liquide Santé International
-
Air Liquide Santé, France.
Air Liquide Santé provided the heliox and air tanks, set up the study equipment and was involved in the study design of Cambonie 2006
-
None, Other.
Authors of this meta analysis did not receive any financial support for this work
Declarations of interest
Jean‐Michel Liet: I was investigator in one trial, supported by Air Liquide Santé International. Air Liquide provided the heliox and air tanks, set up the study equipment and was involved in the study design. Air Liquide Santé had no role in data management, data analysis or data interpretation, or writing of the report and decision to submit it for publication. I had no financial relationship with Air Liquide Santé. Currently I am a local investigator in a multicentre observational study promoted by Air Liquide Healthcare. My institution receives money when a child is included.
Gilles Cambonie et al performed a study with heliox referenced as Cambonie G, Milési C, Fournier‐Favre S, Counil F, Jaber S, Picaud J‐C, Matecki S. Clinical effects of heliox administration for acute bronchiolitis in young infants. Chest 2006; 129: 676‐82. As indicated in the published article, for this study Air Liquide Santé provided the heliox and air tanks, set up the study equipment, and was involved in the study design; it had no role in data management, data analysis or data interpretation, or in the writing of the report and the decision to submit it for publication. The review author has no financial relationship with Air Liquide Santé. Thierry Ducruet: none known.
Vineet Gupta: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Cambonie 2006 {published data only}
- Cambonie G, Milési C, Fournier‐Favre S, Counil F, Jaber S, Picaud JC, et al. Clinical effects of heliox administration for acute bronchiolitis in young infants. Chest 2006;129(3):676‐82. [DOI] [PubMed] [Google Scholar]
Chowdhury 2013 {published data only}
- Chowdhury MM, McKenzie SA, Pearson CC, Carr S, Pao C, Shah AR, et al. Heliox therapy in bronchiolitis: phase III multicenter double‐blind randomized controlled trial. Pediatrics 2013;131(4):661‐9. [DOI] [PubMed] [Google Scholar]
Hollmann 1998 {published data only}
- Hollman G, Shen G, Zeng L, Yngsdal‐Krenz R, Perloff W, Zimmerman J, et al. Helium‐oxygen improves clinical asthma scores in children with acute bronchiolitis. Critical Care Medicine 1998;26(10):1731‐6. [DOI] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim IK, Phrampus E, Sikes K, Pendleton J, Saville A, Corcoran T, et al. Helium‐oxygen therapy for infants with bronchiolitis: a randomized controlled trial. Archives of Pediatrics and Adolescent Medicine 2011;165(12):1115‐22. [DOI] [PubMed] [Google Scholar]
Kneyber 2009 {published data only}
- Kneyber MC, Heerde M, Twisk JW, Plötz FB, Markhors DG. Heliox reduces respiratory system resistance in respiratory syncytial virus induced respiratory failure. Critical Care 2009;13(3):R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liet 2005 {published data only}
- Liet JM, Millotte B, Tucci M, Laflammme S, Hutchison J, Creery D, et al. Noninvasive therapy with helium‐oxygen for severe bronchiolitis. Journal of Pediatrics 2005;147(6):812‐7. [DOI] [PubMed] [Google Scholar]
Martinón‐Torres 2008 {published data only}
- Martinón‐Torres F, Rodríguez‐Núñez A, Martinón‐Sánchez JM. Nasal continuous positive airway pressure with heliox versus air oxygen in infants with acute bronchiolitis: a crossover study. Pediatrics 2008;121(5):e1190‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abd‐Allah 2003 {published data only}
- Abd‐Allah SA, Rogers MS, Terry M, Gross M, Perkin RM. Helium‐oxygen therapy for pediatric acute severe asthma requiring mechanical ventilation. Pediatric Critical Care Medicine 2003;4(3):353‐7. [DOI] [PubMed] [Google Scholar]
Colnaghi 2012 {published data only}
- Colnaghi M, Pierro M, Migliori C, Ciralli F, Matassa PG, Vendettuoli V, et al. Nasal continuous positive airway pressure with heliox in preterm infants with respiratory distress syndrome. Pediatrics 2012;129(2):e333‐8. [DOI] [PubMed] [Google Scholar]
Dieperink 2007 {published data only}
- Dieperink W, Knol JA, Boersma HJ, Eindhoven GB, Aarts LP, Goorhuis JF, et al. Combination of heliox and CPAP without a ventilator: bench test and clinical observations. European Journal of Anaesthesiology 2007;24(10):889‐91. [DOI] [PubMed] [Google Scholar]
Duncan 1979 {published data only}
- Duncan PG. Efficacy of helium‐oxygen mixtures in the management of severe viral and post‐intubation croup. Canadian Anaesthetists' Society Journal 1979;26(3):206‐12. [DOI] [PubMed] [Google Scholar]
Elleau 1993 {published data only}
- Elleau C, Galperine RI, Guenard H, Demarquez JL. Helium‐oxygen mixture in respiratory distress syndrome: a double‐blind study. Journal of Pediatrics 1993;122(1):132‐6. [DOI] [PubMed] [Google Scholar]
Gross 2000 {published data only}
- Gross MF, Spear RM, Peterson BM. Helium‐oxygen mixture does not improve gas exchange in mechanically ventilated children with bronchiolitis. Critical Care 2000;4(3):188‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grosz 2001 {published data only}
- Grosz AH, Jacobs IN, Cho C, Schears GJ. Use of helium‐oxygen mixtures to relieve upper airway obstruction in a pediatric population. Laryngoscope 2001;111(9):1512‐4. [DOI] [PubMed] [Google Scholar]
Gupta 2004 {published data only}
- Gupta VK, Grayck EN, Cheifetz IM. Heliox administration during high‐frequency jet ventilation augments carbon dioxide clearance. Respiratory Care 2004;49(9):1038‐44. [PubMed] [Google Scholar]
Iglesias‐Fernandez 2007 {published data only}
- Iglesias Fernández C, López‐Herce Cid J, Mencía Bartolomé S, Santiago Lozano MJ, Moral Torrero R, Carrillo Alvarez A. Efficacy of heliox therapy in respiratory insufficiency in infants and children [Eficacia del tratamiento con heliox en ninos con insuficiencia respiratoria]. Anales de Pediatría 2007;66(3):240‐7. [DOI] [PubMed] [Google Scholar]
Isakov 1970 {published data only}
- Isakov IuF, Mikhel'son VA, Anokhin MI, Ostrekov IF, Dianov AG. Inhalation of helium in the complex treatment of children with respiratory insufficiency. Khirurgiia 1970;46(4):145‐9. [PubMed] [Google Scholar]
Kneyber 2006 {published data only}
- Kneyber MC, Heerde M, Markhorst DG, Plötz FB. Mechanical ventilation with heliox decreases respiratory system resistance and facilitates CO2 removal in obstructive airway disease. Intensive Care Medicine 2006;32(10):1676‐7. [DOI] [PubMed] [Google Scholar]
Martinon‐Torres 2002 {published data only}
- Martinon‐Torres F, Rodriguez‐Nunez A, Martinon‐Sanchez JM. Heliox therapy in infants with acute bronchiolitis. Pediatrics 2002;109:68‐73. [DOI] [PubMed] [Google Scholar]
Martinon‐Torres 2005 {published data only}
- Martinón‐Torres F, Crespo Suárez PA, Silvia Barbàra C, Castelló Muñoz A, Rodríguez Núñez A, Martinón Sánchez JM. Noninvasive ventilation with heliox in an infant with acute respiratory distress syndrome [Ventilacion no invasiva con heliox en un lactante con sindrome de dificultad respiratoria aguda]. Anales de Pediatría 2005;62(1):64‐7. [DOI] [PubMed] [Google Scholar]
Martinon‐Torres 2006 {published data only}
- Martinón‐Torres F, Rodríguez‐Núñez A, Martinón‐Sánchez JM. Nasal continuous positive airway pressure with heliox in infants with acute bronchiolitis. Respiratory Medicine 2006;100(8):1458‐62. [DOI] [PubMed] [Google Scholar]
Mayordomo‐Colunga 2010 {published data only}
- Mayordomo‐Colunga J, Medina A, Rey C, Concha A, Los Arcos M, Menéndez S. Helmet‐delivered continuous positive airway pressure with heliox in respiratory syncytial virus bronchiolitis. Acta Paediatrica 2010;99(2):308‐11. [DOI] [PubMed] [Google Scholar]
Montaguti 2011 {published data only}
- Montaguti A, Frontalini S, Carleo A, Deha A, Franceschi A, Lampugnani E, et al. Noninvasive ventilation for acute respiratory failure. Pediatric Critical Care Medicine 2011;12(Suppl 3):A83. [Google Scholar]
Paret 1996 {published data only}
- Paret G, Dekel B, Vardi A, Szeinberg A, Lotan D, Barzilay Z. Heliox in respiratory failure secondary to bronchiolitis: a new therapy. Pediatric Pulmonology 1996;22(5):322‐3. [DOI] [PubMed] [Google Scholar]
Tobias 1997 {published data only}
- Tobias JD. Heliox in children with airway obstruction. Pediatric Emergency Care 1997;13(1):29‐32. [DOI] [PubMed] [Google Scholar]
Tobias 1999 {published data only}
- Tobias JD, Grueber RE. High‐frequency jet ventilation using a helium‐oxygen mixture. Paediatric Anaesthesia 1999;9(5):451‐5. [DOI] [PubMed] [Google Scholar]
Ulhoa 2000 {published data only}
- Ulhôa CA, Larner L. Helium‐oxigen (Heliox) mixture in airway obstruction. Jornal de Pediatria 2000;76(1):73‐8. [DOI] [PubMed] [Google Scholar]
Williams 2004 {published data only}
- Williams J, Stewart K, Tobias JD, Berkenbosch JW. Therapeutic benefits of helium‐oxygen delivery to infants via nasal cannula. Pediatric Emergency Care 2004;20(9):574‐78. [DOI] [PubMed] [Google Scholar]
Winters 2000 {published data only}
- Winters JW, Willing MA, Sanfilippo D. Heliox improves ventilation during high‐frequency oscillatory ventilation in pediatric patients. Pediatric Critical Care Medicine 2000;1(1):33‐7. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Rotta 2015 {unpublished data only}
- ClinicalTrials.gov identifier: NCT02373683. Ongoing study February 2015.
Additional references
AAP 2006
- American Academy of Pediatrics. Diagnosis and management of bronchiolitis. Pediatrics 2006;118(4):1774‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Barach 1934
- Barach AL. The therapeutic use of helium. Journal of the American Medical Association 1936;107:1273‐80. [Google Scholar]
Beggs 2014
- Beggs S, Wong ZH, Kaul S, Ogden KJ, Walters JA. High‐flow nasal cannula therapy for infants with bronchiolitis. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD009609.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berkenbosch 2003
- Berkenbosch JW, Grueber RE, Dabbagh O, McKibben AW. Effect of helium‐oxygen (heliox) gas mixtures on the function of four pediatric ventilators. Critical Care Medicine 2003;31(7):2052‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boyce 2000
- Boyce TG, Mellen BG, Mitchel EF Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. Journal of Pediatrics 2000;137(6):865‐70. [DOI] [PubMed] [Google Scholar]
Bressan 2013
- Bressan S, Balzani M, Krauss B, Pettenazzo A, Zanconato S, Baraldi E. High‐flow nasal cannula oxygen for bronchiolitis in a pediatric ward: a pilot study. European Journal of Pediatrics 2013;172(12):1649‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Byington 2015
- Byington CL, Wilkes J, Korgenski K, Sheng X. Respiratory syncytial virus‐associated mortality in hospitalized infants and young children. Journal of Pediatrics 2015;135(1):e24‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carbonell‐Estrany 2000
- Carbonell‐Estrany X, Quero J, Bustos G, Cotero A, Doménech E, Figueras‐Aloy J, et al. Rehospitalization because of respiratory syncytial virus infection in premature infants younger than 33 weeks of gestation: a prospective study. IRIS Study Group. Pediatric Infectious Disease Journal 2000;19(7):592‐7. [DOI] [PubMed] [Google Scholar]
Carter 1996
- Carter ER, Webb CR, Moffitt DR. Evaluation of heliox in children hospitalized with acute severe asthma. A randomized crossover trial. Chest 1996;109(5):1256‐61. [DOI] [PubMed] [Google Scholar]
Chevret 2005
- Chevret L, Mbieleu B, Essouri S, Durand P, Chevret S, Devictor D. Bronchiolitis treated with mechanical ventilation: prognosis factors and outcome in a series of 135 children. Archives de Pédiatrie 2005;12(4):385‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Corcoran 2004
- Corcoran TE, Gamard S. Development of aerosol drug delivery with helium oxygen gas mixtures. Journal of Aerosol Medicine 2004;17(4):299‐309. [DOI] [PubMed] [Google Scholar]
Davison 2004
- Davison C, Ventre KM, Luchetti M, Randolph AG. Efficacy of interventions for bronchiolitis in critically ill infants: a systematic review and meta‐analysis. Pediatric Critical Care Medicine 2004;5(5):482‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Figueras‐Aloy 2008
- Figueras‐Aloy J, Carbonell‐Estrany X, Quero‐Jiménez J, Fernández‐Colomer B, Guzmán‐Cabañas J, Echaniz‐Urcelay I, et al. FLIP‐2 Study: risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born in Spain at a gestational age of 32 to 35 weeks. Pediatric Infectious Disease Journal 2008;27(9):788‐93. [DOI] [PubMed] [Google Scholar]
GRADE 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT: GRADEpro Guideline Development Tool. Available from www.gradepro.org. Hamilton: McMaster University (developed by Evidence Prime, Inc.), 2015.
Gupta 2005
- Gupta VK, Cheifetz IM. Heliox administration in the pediatric intensive care unit: an evidence‐based review. Pediatric Critical Care Medicine 2005;6:204‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hall 2013
- Hall CB, Simőes EA, Anderson LJ. Clinical and epidemiologic features of respiratory syncytial virus. Current Topics in Microbiology and Immunology 2013;372:39‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hough 2014
- Hough JL, Pham TM, Schilber A. Physiologic effects of high‐flow nasal cannula in infants with bronchiolitis. Pediatric Critical Care Medicine 2014;15(5):e214‐9. [DOI] [PubMed] [Google Scholar]
Kemper 1991
- Kemper KJ, Ritz RH, Benson MS, Bishop MS. Helium‐oxygen mixture in the treatment of postextubation stridor in pediatric trauma patients. Critical Care Medicine 1991;19(3):356‐9. [DOI] [PubMed] [Google Scholar]
Kudukis 1997
- Kudukis TM, Manthous CA, Schmidt GA, Hall JB, Wylam ME. Inhaled helium‐oxygen revisited: effect of inhaled helium‐oxygen during the treatment of status asthmaticus in children. Journal of Pediatrics 1997;130(2):217‐24. [DOI] [PubMed] [Google Scholar]
Law 2004
- Law BJ, Langley JM, Allen U, Paes B, Lee DS, Mitchell I, et al. The Pediatric Investigators Collaborative Network on Infections in Canada study of predictors of hospitalization for respiratory syncytial virus infection for infants born at 33 through 35 completed weeks of gestation. Pediatric Infectious Disease Journal 2004;23(9):806‐14. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Liese 2003
- Liese JG, Grill E, Fischer B, Roeckl‐Wiedmann I, Carr D, Belohradsky BH, et al. Incidence and risk factors of respiratory syncytial virus‐related hospitalizations in premature infants in Germany. European Journal of Pediatrics 2003;162(4):230‐6. [DOI] [PubMed] [Google Scholar]
Lowell 1987
- Lowell DI, Lister G, Koss H, McCarthy P. Wheezing in infants: the response to epinephrine. Pediatrics 1987;76(6):939‐45. [PubMed] [Google Scholar]
Mansbach 2012
- Mansbach JM, Piedra PA, Teach SJ, Sullivan AF, Forgey T, Clark S, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Archives of Pediatrics and Adolescent Medicine 2012;166(8):769‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinon‐Torres 2012
- Martinon‐Torres F. Noninvasive ventilation with helium‐oxygen in children. Journal of Critical Care 2012;27(2):e1‐9. [DOI] [PubMed] [Google Scholar]
Martinon‐Torres 2015
- Martinón‐Torres F. What's weighing down heliox?. Lancet Respiratory Medicine 2015;3(1):14‐5. [DOI] [PubMed] [Google Scholar]
Mayfield 2014
- Mayfield S, Bogossian F, O'Malley L, Schibler A. High‐flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. Journal of Paediatrics and Children Health 2014;50(5):373‐8. [DOI] [PubMed] [Google Scholar]
McKiernan 2010
- McKiernan C, Chua LC, Visintainer PF, Allen H. High flow nasal cannulae therapy in infants with bronchiolitis. Journal of Pediatrics 2010;156:634‐8. [DOI] [PubMed] [Google Scholar]
Meert 1990
- Meert K, Heidemann S, Abella B, Sarnaik A. Does prematurity alter the course of respiratory syncytial virus infection?. Critical Care Medicine 1990;18(12):1357‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Milesi 2013
- Milési C, Baleine J, Matecki S, Durand S, Combes C, Novais AR, et al. Is treatment with a high flow nasal cannula effective in acute viral bronchiolitis? A physiologic study. Intensive Care Medicine 2013;39(6):1088‐94. [DOI] [PubMed] [Google Scholar]
Nair 2010
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta‐analysis. Lancet 2010;375(9725):1545‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Panitch 2003
- Panitch HB. Respiratory syncytial virus bronchiolitis: supportive care and therapies designed to overcome airway obstruction. Pediatric Infectious Disease Journal 2003;22(Suppl):83‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Piedimonte 2002
- Piedimonte G. Pathophysiological mechanisms for the respiratory syncytial virus‐reactive airway disease link. Respiratory Research 2002;3(Suppl 1):21‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prais 2003
- Prais D, Schonfeld T, Amir J, Israeli Respiratory Syncytial Virus Monitoring Group. Admission to the intensive care unit for respiratory syncytial virus bronchiolitis: a national survey before palivizumab use. Pediatrics 2004;113(3 Pt 1):629. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ralston 2009
- Ralston S, Hill V. Incidence of apnea in infants hospitalized with respiratory syncytial virus bronchiolitis: a systematic review. Journal of Pediatrics 2009;155(5):728‐33. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roche‐Campo 2011
- Roche‐Campo F, Vignaux L, Galia F, Lyazidi A, Vargas F, Texereau J, et al. Delivery of helium oxygen mixture during spontaneous breathing: evaluation of three high‐concentration face masks. Intensive Care Medicine 2011;37:1787‐92. [DOI] [PubMed] [Google Scholar]
Rodrigo 2010
- Rodrigo G, Pollack C, Rodrigo C, Rowe BH. Heliox for non‐intubated acute asthma patients. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD002884.pub2] [DOI] [Google Scholar]
Schibler 2011
- Schibler A, Pham TM, Dunster KR, Foster K, Barlow A, Gibbons K, et al. Reduced intubation rates for infants after introduction of high‐flow nasal prong oxygen delivery. Intensive Care Medicine 2011;37:847‐52. [DOI] [PubMed] [Google Scholar]
Simoes 2003
- Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. Journal of Pediatrics 2003;143(5 Suppl):118‐26. [DOI] [PubMed] [Google Scholar]
Skjerven 2013
- Skjerven HO, Hunderi JO, Brügmann‐Pieper SK, Brun AC, Engen H, Eskedal L, et al. Racemic adrenaline and inhalation strategies in acute bronchiolitis. New England Journal of Medicine 2013;368(24):2286‐93. [DOI] [PubMed] [Google Scholar]
Standley 2008
- Standley TD, Smith HL, Brennan LJ, Wilkins IA, Bradley PG, Barrera Groba C, et al. Room air dilution of heliox given by facemask. Intensive Care Medicine 2008;34(8):1469‐76. [DOI] [PubMed] [Google Scholar]
Stillwell 1989
- Stillwell PC, Quick JD, Munro PR, Mallory GB Jr. Effectiveness of open‐circuit and oxyhood delivery of helium‐oxygen. Chest 1989;95(6):1222‐4. [DOI] [PubMed] [Google Scholar]
Wang 1995
- Wang EE, Law BJ, Stephens D. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. Journal of Pediatrics 1995;126(2):212‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weber 2001
- Weber JE, Chudnofsky CR, Younger JG, Larkin GL, Boczar M, Wilkerson MD, et al. A randomized comparison of helium‐oxygen mixture (Heliox) and racemic epinephrine for the treatment of moderate to severe croup. Pediatrics 2001;107(6):E96. [DOI] [PubMed] [Google Scholar]
Welliver 2003
- Welliver RC. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. Journal of Pediatrics 2003;143(Suppl):112‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wolfson 1984
- Wolfson MR, Bhutani VK, Shaffer TH, Bowen FW Jr. Mechanics and energetics of breathing helium in infants with bronchopulmonary dysplasia. Journal of Pediatrics 1984;104(5):752‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wood 1972
- Wood DW, Downes JJ, Lecks HI. A clinical scoring system for the diagnosis of respiratory failure. Preliminary report on childhood status asthmaticus. American Journal of Diseases of Children 1972;123(3):227‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2013
- Zhang L, Mendoza‐Sassi RA, Wainwright C, Klassen TP. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD006458.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Liet 2008
- Liet JM, Ducruet T, Gupta V, Cambonie G. Heliox inhalation therapy for bronchiolitis in infants. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006915] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liet 2010
- Liet JM, Ducruet T, Gupta V, Cambonie G. Heliox inhalation therapy for bronchiolitis in infants. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD006915.pub2] [DOI] [PubMed] [Google Scholar]


