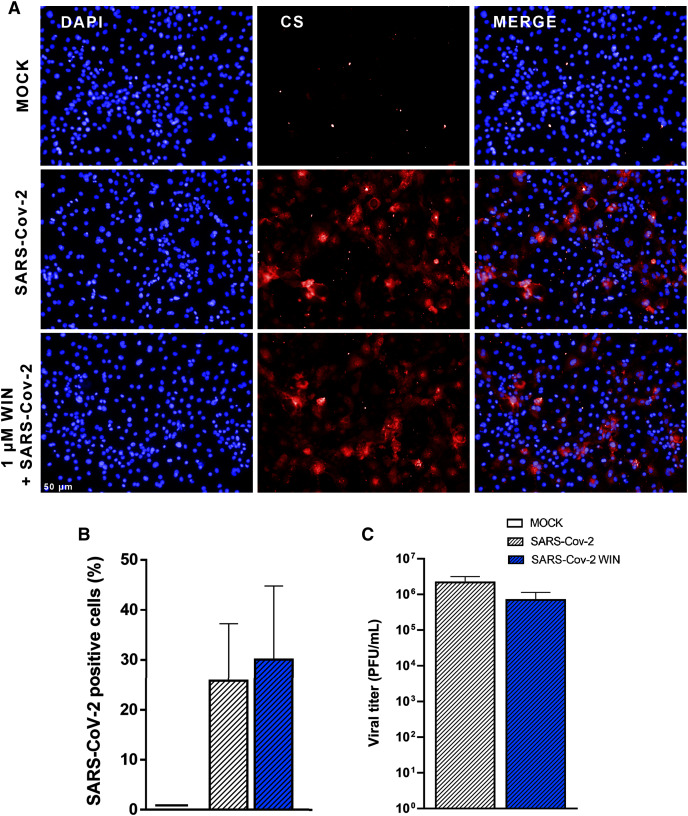
Figure 2. WIN does not reduce SARS-CoV-2 infection and replication in hiPSC-CMs.
(A) Representative micrographs of MOCK and SARS-CoV-2-infected hiPSC-CM pretreated or not with one μM WIN for 24 h. hiPSC-CM were immunostained with SARS-CoV-2 convalescent serum (CS) (red) and counterstained with DAPI (blue) at 48 h post-infection. Scale bar: 50 μm. (B) Percentage of CS positive cells. CS immunoreactivity was comparable between treated and untreated hiPSC-CM. (C) Viral titer quantification by plaque forming units assay using the supernatants of the SARS-CoV-2 infected hiPSC-CMs. Viral titer was comparable between treated and untreated hiPSC-CM. Error bars represent standard errors of the means (SEM) from three independent experiments (three cellular differentiations and three independent infections) from one iPSC line.